Cancer and Traditional Medicine: An Integrative Approach
Abstract
1. Introduction
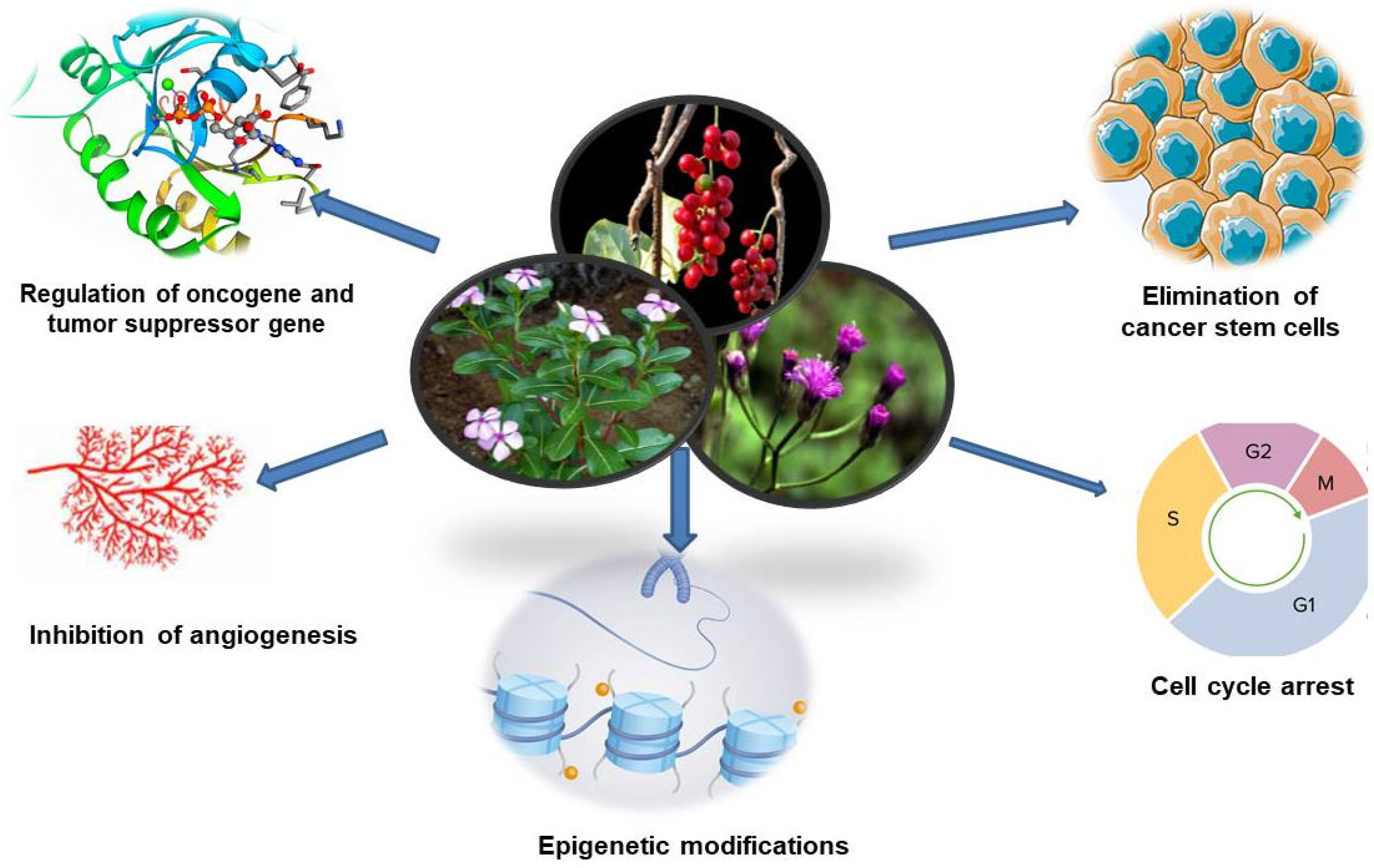
2. Traditional Medicines and Cancer Care
3. Advantages of Herbal Drugs
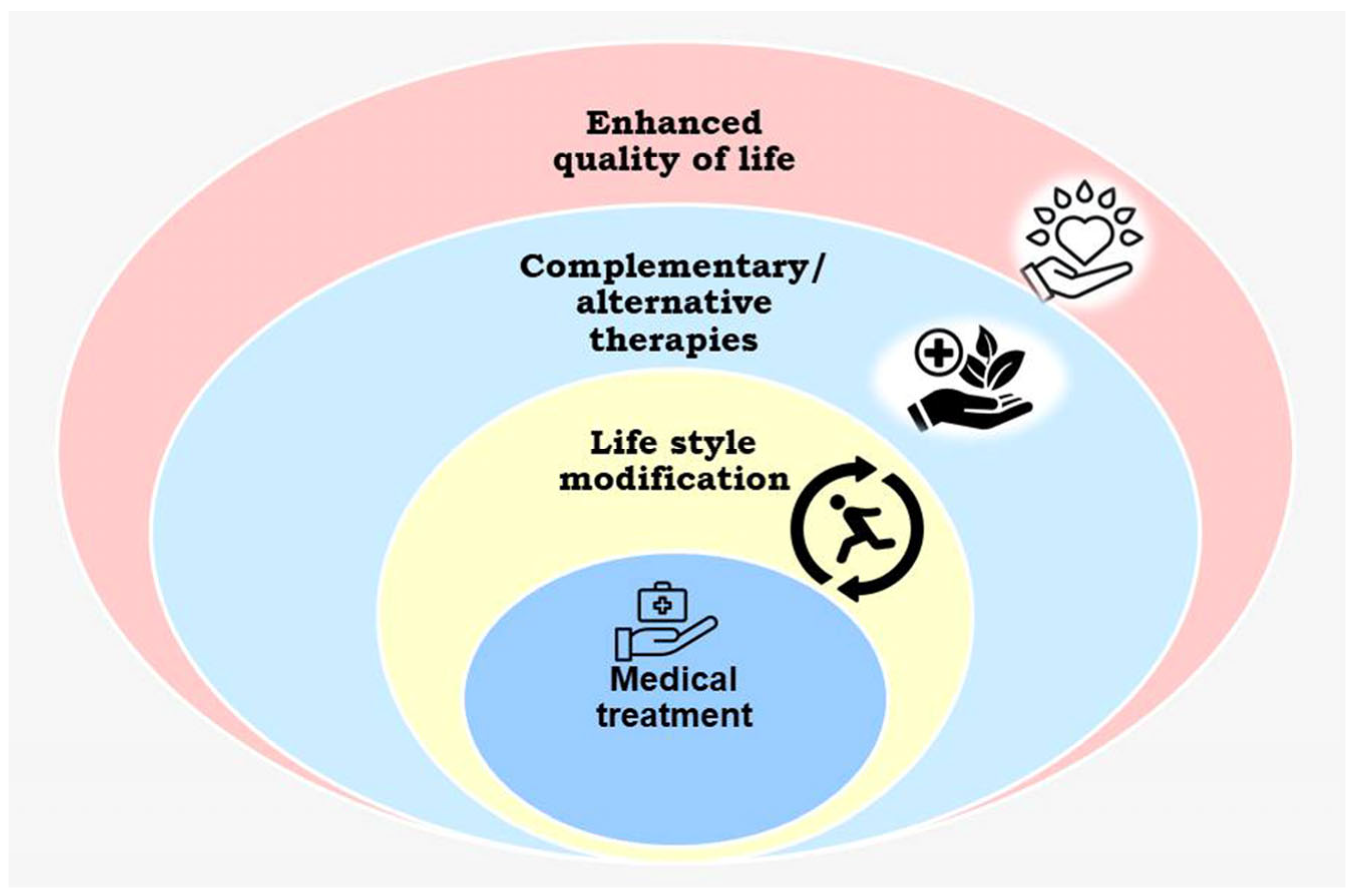
4. Integrative Clinical Oncology
5. Global Status of Integrative Oncology
6. Status of Integrative Oncology in South Africa
7. Anti-Inflammatory and Antioxidant Activities of Traditional Medicines
8. Disadvantages of Traditional Medicines
9. Conclusions and Scope for Future Research
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lee, K.-H.; Xiao, Z. Podophyllotoxins and analogs. In Anticancer Agents from Natural Products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 71–87. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hu, Q.; Kasagi, Y.; Oki, E.; Mori, M. Recent developments in cancer research: Expectations for a new remedy. Ann. Gastroenterol. Surg. 2021, 5, 419–426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdalla, Y.O.A.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Motlhatlego, K.; Netshia, V. Traditionally used polyherbals in a southern African therapeutic context. J. Ethnopharmacol. 2022, 288, 114977. [Google Scholar] [CrossRef]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef] [PubMed]
- Scharman, E.J. Reserpine. In Encyclopedia of Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 661–662. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Jyothi, C.K.; Unnithan, J.K.; Prabhukumar, K.M.; Balachandran, I. Identification of suitable substitute for Sarpagandha (Rauvolfia serpentina (L.) Benth. ex Kurz) by phytochemical and pharmacological evaluation. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 42. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anti-cancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Wang, J.; He, C.Z.; Dang, C.L.; Huang, R.F. Genetic diversity and relationship of Allium tchongchanense and A. wallichii based on AFLP analysis. Guihaia 2011, 31, 295–299. [Google Scholar]
- Bhandari, J.; Muhammad, B.; Thapa, P.; Shrestha, B.G. Shrestha Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement. Altern. Med. 2017, 17, 102. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Shahida, V.; Balachandran, I. Effect of Extraction Solvent on the Phytoconstituents of Aegle marmelos (L.) Correa. J. Nat. Remedies 2015, 15, 58–64. [Google Scholar] [CrossRef]
- Chockalingam, V.; Kadali, S.S.; Gnanasambantham, P. Antiproliferative and antioxidant activity of Aegle marmelos (Linn.) leaves in Dalton’s Lymphoma Ascites transplanted mice. Indian J. Pharmacol. 2012, 44, 225–229. [Google Scholar] [CrossRef]
- Akhouri, V.; Kumari, M.; Kumar, A. Therapeutic effect of Aegle marmelos fruit extract against DMBA induced breast cancer in rats. Sci. Rep. 2020, 10, 18016. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Luanratana, O.; Jongsomboonkusol, S.; Pongpan, N. Antiproliferative activity of Thai medicinal plant extracts on human breast adenocarcinoma cell line. Fitoterapia 2004, 75, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S. Review of the phytochemical, pharmacological and toxicological properties of Alstonia Scholaris Linn. R. Br (Saptaparna). Chin. J. Integr. Med. 2012; 1–14. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Shi, H.; Yi, M.; Xiong, B.; Li, T. Tailoring traditional Chinese medicine in cancer therapy. Mol. Cancer 2025, 24, 27. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, G.; Yang, X.; Wang, M.; Wang, R.; Wan, M.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway. J. Ethnopharmacol. 2023, 303, 115933. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Zhang, Y.; Fan, H.; Lin, H. Traditional Chinese medicine and cancer: History, present situation, and development. Thorac. Cancer 2015, 6, 561–569. [Google Scholar] [CrossRef]
- Hu, B.; An, H.M.; Shen, K.P. Documents research of TCM cancer treatment of SCI journals. Chin. J. Ethnomed. Ethnopharm. 2009, 20, 26–27. [Google Scholar]
- Hou, W.; Liu, J.; Shi, W.G.; Lin, H.S. Multi-center, randomized, controlled clinical study of radiation pneumonitis treated with compound matrine injection in primary lung cancer patients. Chin. J. New Drugs 2013, 17, 2065–2068. [Google Scholar]
- Lin, H.; Sun, G.; Qin, F.; Cao, Y.; Wang, X.; Chen, J.; Wang, X.; Huang, H. A randomized, double-blinded, drug-controlled and multicentre clinical trial of chemotherapy assisted with Jinlong capsule on gastric cancer. Cancer Res. Prev. Treat. 2013, 1, 12–15. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Venkatesh, P.; Debnath, P.K. Changing scenario for promotion and development of Ayurveda—Way forward. J. Ethnopharmacol. 2012, 143, 424–434. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2016, 7, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Bhishagratha, K.L. Sushruta Samhita; Choukhamba Orientalia: Varanasi, India, 1991. [Google Scholar]
- Balachandran, P.; Govindarajan, R. Cancer: An ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Liu, X.S.; Struhl, K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Lum, P.Y.; Yang, X.; Pinto, S.; MacNeil, D.J.; Zhang, C.; Lamb, J.; Edwards, S.; Sieberts, S.K.; et al. Variations in DNA elucidate molecular networks that cause disease. Nature 2008, 452, 429–435. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sumantran, V.N.; Tillu, G. Cancer, Inflammation, and Insights from Ayurveda. Evid.-Based Complement. Altern. Med. 2012, 2012, 306346. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Wallace, R.K. Ayurveda and Epigenetics. Medicina 2020, 56, 687. [Google Scholar] [CrossRef]
- Mondal, P.; Natesh, J.; Penta, D.; Meeran, S.M. Progress and Promises of Epigenetic Drugs and Epigenetic Diets in Cancer Prevention and Therapy: A clinical update. Semin. Cancer Biol. 2022, 83, 503–522. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Li, Y.; Shukla, S.; Tollefsbol, T.O. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS ONE 2012, 7, e37748. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.B.; Lee, S.; Kim, J.Y.; Earm, Y.E. Physiome and sasang constitutional medicine. J. Physiol. Sci. 2008, 58, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Song, S.-Y.; More, S.V.; Kang, S.-M.; Kim, B.-W.; Kim, I.-S.; Choi, D.-K. Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules 2013, 18, 14670–14693. [Google Scholar] [CrossRef]
- Yoon, S.W.; Jeong, J.S.; Kim, J.H.; Aggarwal, B.B. ancer Prevention and Therapy: Integrating Traditional Korean Medicine Into Modern Cancer Care. Integr. Cancer Ther. 2013, 13, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Seki, T.; Tsutani, K. Traditional Japanese medicine, Kampo: Its history and current status. Chin. J. Integr. Med. 2011, 17, 85–87. [Google Scholar] [CrossRef]
- Bashir, F.; Akhtar, J.; Anjum, N.; Alam, S.; Khan, A.A. Concept of Sartān (Cancer) and Anti-cancerous drugs in Unani System of Medicine. Int. J. Curr. Sci. Multidiscip. Res. 2020, 3, 159–169. [Google Scholar]
- Mustehasan; Naushin, S.; Alam, M. Role of Diet in the Prevention and Management of Cancer (Saraöän) in Unani Medicine. Hippocrat. J. Unani Med. 2019, 14, 1–13. [Google Scholar]
- Zohar, A.M.A.M.I. Kitabul Taiseer, (Urdu Translation by CCRUM), 1st ed.; Ministry of Health and Family Welfare, Government of India: New Delhi, India, 1986; p. 217. [Google Scholar]
- Sowmyalakshmi, S.; Nur-e-Alam, M.; Akbarsha, M.A.; Thirugnanam, S.; Rohr, J.; Chendil, D. Investigation on Semecarpus Lehyam—A Siddha medicine for breast cancer. Planta 2005, 220, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Jada, S.R.; Subur, G.S.; Matthews, C.; Hamzah, A.S.; Lajis, N.H.; Saad, M.S.; Stevens, M.F.; Stanslas, J. Semisynthesis and in vitro anticancer activities of andrographolide analogues. J. Phytochem. 2007, 68, 904–912. [Google Scholar] [CrossRef]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- Streffer, J.R.; Schabet, M.; Dichgans, J.; Weller, M. Response of radiochemotherapy-associated cerebral edema to a phytotherapeutic agent, H15. Neurology 2001, 56, 1219–1221. [Google Scholar] [CrossRef]
- Panthong, A.; Norkaew, P.; Kanjanapothi, D.; Taesotikul, T.; Anantachoke, N.; Reutrakul, V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J. Ethnopharmacol. 2007, 111, 335–340. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Guo, Q.-L.; You, Q.-D.; Zhao, L.; Gu, H.-Y. Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol. Pharm. Bull. 2004, 27, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Gu, H.; Yang, Y.; Lu, N.; Zhao, J.; Liu, W.; Ling, H.; You, Q.-D.; Wang, X.; Guo, Q. Involvement of matrix metalloproteinase 2 and 9 in gambogic acid induced suppression of MDA-MB-435 human breast carcinoma cell lung metastasis. J. Mol. Med. 2008, 86, 1367–1377. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S103–S127. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Velmurugan, B.; Kaur, M.; Singh, R.P.; Agarwal, R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res. 2010, 70, 2368–2378. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.-W.; Hu, R.; Yang, Y.; Qi, Q.; Lu, N.; Liu, W.; Chu, Y.-Y.; You, Q.-D.; Guo, Q.-L. Wogonin induces G1 phase arrest through inhibiting Cdk4 and cyclin D1 concomitant with an elevation in p21Cip1 in human cervical carcinoma HeLa cells. Biochem. Cell Biol. 2009, 87, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Raina, K.; Deep, G.; Chan, D.; Agarwal, R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin. Cancer Res. 2009, 15, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ge, C.-M.; Meng, Q.-H.; Cao, J.-P.; Tong, J.; Fan, S.-J. Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol. Sin. 2007, 28, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J.; Shen, X.-L.; An, J.; Sun, H.; Wang, L.; Hu, Y.-J.; Sun, Q.; Fu, L.-C.; Sheikh, M.S.; et al. Dihydroartemisinin upregulates death receptor 5 expression and cooperates with TRAIL to induce apoptosis in human prostate cancer cells. Cancer Biol. Ther. 2010, 9, 819–824. [Google Scholar] [CrossRef]
- Mogami, S.; Hattori, T. Beneficial effects of rikkunshito, a Japanese kampo medicine, on gastrointestinal dysfunction and anorexia in combination with Western drug: A systematic review. Evid.-Based Complement. Altern. Med. 2014, 2014, 519035. [Google Scholar] [CrossRef]
- Motoo, Y.; Cameron, S. Kampo medicines for supportive care of patients with cancer: A brief review. Integr. Med. Res. 2022, 11, 100839. [Google Scholar] [CrossRef]
- Lee, J.; Son, Y.H.; Kwon, Y.; Park, S.Y.; Koo, B.; Jung, S.H. Anticancer Effects of a Korean Herbal Medicine Formula (H9) via AMPK and HER2-PI3K/Akt Signaling in Breast Cancer Cells. Phytother. Res. 2017, 31, 1765–1775. [Google Scholar] [CrossRef]
- Shimizu, M.; Takayama, S.; Kikuchi, A.; Arita, R.; Ono, R.; Ishizawa, K.; Ishii, T. Kampo Medicine Treatment for Advanced Pancreatic Cancer: A Case Series. Front. Nutr. 2021, 8, 702812. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rao, S.K. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol. Pharm. Bull. 2006, 29, 460–466. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.; Rao, A.S.; Nandal, A.; Kumar, S.; Ganaie, S.; Narasihman, B. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J. Ethnopharmacol. 2021, 270, 113704. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Patra, D.; Kundu, R. Lignan enriched fraction (LRF) of Phyllanthus amarus promotes apoptotic cell death in human cervical cancer cells in vitro. Sci. Rep. 2019, 9, 14950. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.T.; Ramesh, P.R.; Mahesh, K.; Anandan, E.M.; Praveen, M.; Balachandran, I. Metabolite profiling of Cyanthillium cinereum (L.) H. Rob. and its herbal formulation by tandem mass spectroscopic analysis. Nat. Prod. Res. 2022, 36, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.; Deepak, M.; Praveen, T.; Lijini, K.; Salman, M.; Sadheeshnakumari, S.; Balachandran, I. Metabolite profiling and anti-cancer activity of two medicinally important Euphorbia species. Med. Omics 2022, 7, 100018. [Google Scholar] [CrossRef]
- Mugomeri, E.; Chatanga, P.; Chakane, N. Medicinal herbs used by HIV positive people in Lesotho. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 123–131. [Google Scholar] [CrossRef]
- Malangu, N. Self-reported use of traditional, complementary and over-the-counter medicines by HIV-infected patients on antiretroviral therapy in Pretoria, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Langlois-Klassen, D.; Kipp, W.; Jhangri, G.S.; Rubaale, T. Use of traditional herbal medicine by AIDS patients in Kabarole District, western Uganda. Am. J. Trop. Med. Hyg. 2007, 77, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Illamola, S.M.; Amaeze, O.U.; Krepkova, L.V.; Birnbaum, A.K.; Karanam, A.; Job, K.M.; Bortnikova, V.V.; Sherwin, C.M.; Enioutina, E.Y. Use of herbal medicine by pregnant women: What physicians need to know. Front. Pharmacol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Jain, S.; Dwivedi, J.; Jain, P.K.; Satpathy, S.; Patra, A. Medicinal Plants for Treatment of Cancer: A Brief Review. Pharmacogn. J. 2016, 8, 87–102. [Google Scholar] [CrossRef]
- Sasidharan, S.; Saravanan, D.; Chen, Y.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakshmi Priya, M.; Bhanu Priya, K.; Kotakadi, V.S.; Josthna, P. Herbal and Medicinal Plants Molecules Towards Treatment of Cancer: A Mini Review. Am. J. Ethnomedicine 2015, 2, 136–142. [Google Scholar]
- Umadevi, M.; Sampath Kumar, K.P.; Bhowmik, D.; Duraivel, S. Traditionally Used Anticancer Herbs in India. J. Med. Plants Stud. 2013, 1, 56–74. [Google Scholar]
- Gordon, M.C.; David, J. Plants as a source of anticancer agents. J. Ethnopharmacol. 2005, 100, 72. [Google Scholar]
- Kuo, Y.-T.; Chang, T.-T.; Muo, C.-H.; Wu, M.Y.; Sun, M.F.; Yeh, C.C.; Yen, H.R. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: A nationwide population-based study. Integr. Cancer Ther. 2017, 17, 531–541. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.; Im, E.; Kim, N.D. Etoposide as a Key Therapeutic Agent in Lung Cancer: Mechanisms, Efficacy, and Emerging Strategies. Int. J. Mol. Sci. 2025, 26, 796. [Google Scholar] [CrossRef]
- Jermini, M.; Dubois, J.; Rodondi, P.-Y.; Zaman, K.; Buclin, T.; Csajka, C.; Orcurto, A.; Rothuizen, L.E. Complementary medicine use during cancer treatment and potential herb-drug interactions from a cross-sectional study in an academic centre. Sci. Rep. 2019, 9, 5078. [Google Scholar] [CrossRef] [PubMed]
- Dhanoa, A.; Yong, T.L.; Yeap, S.J.L.; Lee, I.S.Z.; Singh, V.A. Complementary and alternative medicine use amongst Malaysian orthopaedic oncology patients. BMC Complement. Altern. Med. 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.G.; Xiong, S.Q.; Yan, Y.; Zhu, H.; Yi, C. Use of Chinese herb medicine in cancer patients: A survey in southwestern China. Evid.-Based Complement. Altern. Med. 2012, 2012, 769042. [Google Scholar] [CrossRef]
- Karadeniz, C.; Pinarli, F.G.; Oguz, A.; Gursel, T.; Canter, B. Complementary/alternative medicine use in a pediatric oncology unit in Turkey. Pediatr. Blood Cancer 2007, 48, 540–543. [Google Scholar] [CrossRef]
- Nazik, E.; Nazik, H.; Api, M.; Kale, A.; Aksu, M. Complementary and alternative medicine use by gynecologic oncology patients in Turkey. Asian Pac. J. Cancer Prev. 2012, 13, 21–25. [Google Scholar] [CrossRef]
- Molassiotis, A.; Fernandez-Ortega, P.; Pud, D.; Ozden, G.; Platin, N.; Hummerston, S.; Scott, J.A.; Panteli, V.; Gudmundsdottir, G.; Selvekerova, S.; et al. Complementary and alternative medicine use in colorectal cancer patients in seven European countries. Complement. Ther. Med. 2005, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, O.; Muslu, U.; Somali, I.; Erten, C.; Alacacioglu, A.; Varol, S.; Aslan, L. An analysis of the use of complementary and alternative therapies in patients with breast cancer. Breast Care 2009, 4, 301–307. [Google Scholar] [CrossRef]
- Ezeome, E.R.; Anarado, A.N. Use of complementary and alternative medicine by cancer patients at the University of Nigeria Teaching Hospital, Enugu, Nigeria. BMC Complement. Altern. Med. 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Anouti, B.; Shatila, H.; Akel, R.; Haibe, Y.; Tfayli, A. Prevalence and correlates of complementary and alternative medicine use among patients with lung cancer: A cross-sectional study in Beirut, Lebanon. Evid.-Based Complement. Altern. Med. 2017, 2017, 8434697. [Google Scholar] [CrossRef]
- Puataweepong, P.; Sutheechet, N.; Ratanamongkol, P. A survey of complementary and alternative medicine use in cancer patients treated with radiotherapy in Thailand. Evid.-Based Complement. Altern. Med. 2012, 2012, 670408. [Google Scholar] [CrossRef]
- Wong, L.C.; Chan, E.; Tay, S.; Lee, K.M.; Back, M. Complementary and alternative medicine practices among Asian radiotherapy patients. Asia-Pac. J. Clin. Oncol. 2010, 6, 357–363. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Wei, W.-C.; Jian, F.-Y.; Yang, N.-S. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid.-Based Complement. Altern. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Z.-P.; Yang, X.-X.; Huang, M.; Gao, Y.; Tang, W.; Chan, S.Y.; Dai, X.; Ye, J.; Ho, P.C.-L.; et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006, 6, 499–508. [Google Scholar] [CrossRef]
- Lam, M. Natural medicine. In Beating Cancer with Natural Medicine; United States of America Bloomington: Bloomington, IN, USA, 2003; pp. 80–85. [Google Scholar]
- Rees, R.W.; Feigel, I.; Vickers, A.; Zollman, C.; Mc Gurk, R.; Smith, C. Prevalence of complementary therapy use by women with breast cancer: A population based survey. Eur. J. Cancer. 2000, 36, 1354–1364. [Google Scholar] [CrossRef]
- Gray, R.E.; Fitch, M.; Goel, V.; Franssen, E.; Labrecque, M. Utilization of complementary/alternative services by women with breast cancer. J Health Soc Policy 2003, 16, 75–84. [Google Scholar] [CrossRef]
- Henderson, J.W.; Donattele, R.J. Complementary and alternative medicine use by women after completion of allopatice treatment for breast cancer. Altern. Ther. Health Med. 2004, 10, 1052–1057. [Google Scholar]
- Morris, K.T.; Johnson, N.; Homer, L.; Walts, D. A comparison of complementary therapy use between breast cancer patients and with other primary tumor sites. Am. J. Surg. 2000, 179, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, V.; Davis, J.; Djamgoz, M.B.A. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers 2020, 12, 3096. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Jentzsch, V. Integrative Management of Pancreatic Cancer (PDAC): Emerging Complementary Agents and Modalities. Nutr. Cancer 2022, 74, 1139–1162. [Google Scholar] [CrossRef]
- Ramamoorthy, A. Integrative oncology in Indian subcontinent: An overview. J. Clin. Diagn. Res. 2015, 9, XE01–XE03. [Google Scholar] [CrossRef]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; Dewitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.-S.; Nair, M.G. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother Res. 2009, 23, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Riyasdeen, A.; Periasamy, V.S.; Paul, P.; Alshatwi, A.A.; Akbarsha, M.A. Chloroform Extract of Rasagenthi Mezhugu, a Siddha Formulation, as an Evidence-Based Complementary and Alternative Medicine for HPV-Positive Cervical Cancers. Evid. Based Complement. Altern. Med. 2012, 2012, 136527. [Google Scholar] [CrossRef]
- Rocha, V.; Ladas, E.J.; Lin, M.; Cacciavillano, W.; Ginn, E.; Kelly, K.M.; Chantada, G.; Castillo, L. Beliefs and determinants of use of traditional complementary/alternative medicine in pediatric patients who undergo treatment for cancer in South America. J. Glob. Oncol. 2017, 3, 701–710. [Google Scholar] [CrossRef]
- Samano, E.S.T.; Goldenstein, P.T.; Ribeiro, L.d.M.; Lewin, F.; Filho, E.S.V.; Soares, H.P.; del Giglio, A. Praying correlates with higher quality of life: Results from a survey on complementary/alternative medicine use among a group of Brazilian cancer patients. Sao Paulo Med. J. 2004, 122, 60–63. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, K.; Zheng, Y.; Zheng, W.; Lu, W.; Shu, X.O. The use of complementary and alternative medicine among Chinese women with breast cancer. J. Altern. Complement. Med. 2008, 14, 1049–1055. [Google Scholar] [CrossRef]
- McQuade, J.L.; Meng, Z.; Chen, Z.; Wei, Q.; Zhang, Y.; Bei, W.; Palmer, J.L.; Cohen, L. Utilization of and attitudes towards traditional Chinese medicine therapies in a Chinese cancer hospital: A survey of patients and physicians. Evid.-Based Complement. Altern. Med. 2012, 2012, 504507. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiao, T.-T.; Ding, H.; Li, C.-X.; Zheng, H.-L.; Chen, X.-L.; Hu, S.-M.; Yu, S.-Y. Use of Chinese herbal medicine therapies in comprehensive hospitals in central China: A parallel survey in cancer patients and clinicians. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2015, 35, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Ichikawa, H.; Garodia, P.; Weerasinghe, P.; Sethi, G.; Bhatt, I.D.; Pandey, M.K.; Shishodia, S.; Nair, M.G. From traditional Ayurvedic medicine to modern medicine: Identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin. Ther. Targets 2006, 10, 87–118. [Google Scholar] [CrossRef] [PubMed]
- Coopoosamy, R.M. An ethnobotanical study of medicinal plants used by traditional healers in Durban, South Africa. Afr. J. Pharm. Pharmacol. 2012, 6, 818–823. [Google Scholar] [CrossRef]
- Koduru, S.; Grierson, D.S.; Afolayan, A.J. Ethnobotanical information of medicinal plants used for treatment of cancer in the Eastern Cape Province, South Africa. Curr. Sci. 2007, 92, 906–908. [Google Scholar]
- Thring, T.; Weitz, F. Medicinal plant use in the bredasdorp/elim region of the southern overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- Semenya, S.; Maroyi, A.; Potgieter, M.; Erasmus, L. Herbal medicines used by Bapedi traditional healers to treat reproductive ailments in the Limpopo Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 331–339. [Google Scholar] [CrossRef]
- Twilley, D.; Rademan, S.; Lall, N. A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Otang-Mbeng, W. Plants Used for the Traditional Management of Cancer in the Eastern Cape Province of South Africa: A Review of Ethnobotanical Surveys, Ethnopharmacological Studies and Active Phytochemicals. Molecules 2021, 26, 4639. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- Banerjee, S.; Nau, S.; Hochwald, S.N.; Xie, H.; Zhang, J. Anticancer properties and mechanisms of botanical derivatives. Phytomed. Plus 2022, 3, 100396. [Google Scholar] [CrossRef]
- Joshi, P.; Yadaw, G.; Joshi, S.; Semwal, R.; Semwal, D. Antioxidant and anti-inflammatory activities of selected medicinal herbs and their polyherbal formulation. S. Afr. J. Bot. 2020, 130, 440–447. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chiou, Y.-S.; Tsai, M.-L.; Ho, C.-T. Anti-inflammatory activity of traditional Chinese medicinal herbs. J. Tradit. Complement. Med. 2011, 1, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Hodzic, Z.; Pasalic, H.; Memisevic, A.; Srabovic, M.; Saletovic, M.; Poljakovic, M. The influence of total phenols content on antioxidant capacity in the whole grain extract. Eur. J. Sci. Res. 2009, 28, 471–477. [Google Scholar]
- Nam, S.-Y.; Kim, K.-Y.; Kim, M.H.; Jang, J.-B.; Rah, S.-Y.; Lee, J.-M.; Kim, H.-M.; Jeong, H.-J. Anti-inflammatory effects of a traditional Korean medicine: Ojayeonjonghwan. Pharm. Biol. 2017, 55, 1856–1862. [Google Scholar] [CrossRef]
- Sunagawa, M.; Yamaguchi, K.; Tsukada, M.; Ebihara, N.; Ikemoto, H.; Hisamitsu, T. Kampo (Traditional Japanese Herbal) Formulae for Treatment of Stomatitis and Oral Mucositis. Medicines 2018, 5, 130. [Google Scholar] [CrossRef]
- Hecht, F.; Zocchi, M.; Alimohammadi, F.; Harris, I.S. Regulation of antioxidants in cancer. Mol. Cell 2024, 84, 23–33. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Vlietinck, A.; Pieters, L.; Apers, S. Legal requirements for the quality of herbal substances and herbal preparations for the manufacturing of herbal medicinal products in the European union. Planta Medica 2009, 75, 683–688. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Medicinal Plant/ Formulation | Traditional System of Medicine | Major Active Compounds/ Ingredient Drugs | Type of Cancer | References |
|---|---|---|---|---|
| Andrographis paniculata | Ayurveda | Andrographolide | Leukemia, breast cancer, colon cancer | [47,48,49] |
| Boswellia serrata | Ayurveda | Triterpenic acids | Brain tumors | [50] |
| Garcinia hanburyi Hook.f. | TCM | Gambogic acid | Glioblastoma, breast cancer, lung, and liver | [51,52,53] |
| Curcuma longa | Ayurveda/TCM | Curcumin | Leukemia, lymphoma, melanoma colon, and gastric cancers | [54,55,56] |
| Scutellaria baicalensis | TCM | Wogonin | Cervical carcinoma | [57] |
| Silybum marianum | TCM | Silibinin | Prostate, colon, bladder, and lung | [58] |
| Artemisia annua | TCM | Artemisinin and its derivatives | Colon, ovarian, prostate | [59,60] |
| Rikkunshito | Kampo medicine | Hesperidin, isoliquiritigenin, atractylodin, glycycoumarin | Chemotherapy-induced dyspepsia, cancer cachexia | [61,62] |
| H9 | Korean medicine | Psoraleae semen, evodia fruit, fennel, nutmeg, ginseng, alpiniae officinarum rhizome, sparganium rhizome, curcuma root, and cinnamon bark | Breast cancer | [63] |
| Juzentaihoto | Kampo | Astragalus root, cinnamon bark, rehmannia root, peony root, cnidium rhizome, atractylodes lancea rhizome, angelica root, ginseng, poria sclerotium, glycyrrhiza | Pancreatic cancer | [64] |
| Tinospora cordifolia | Ayurveda | 20β-hydroxyecdysterone, cordioside, columbin | Ascites carcinoma | [65] |
| Withania somnifera | Ayurveda | Withanolides, withaferin | Colon, mammary, lung, prostate, skin, blood, liver, and kidney | [66] |
| Phyllanthus amarus | Ayurveda | Phyllanthin, niranthrin, phyltetralin, nirtetralin | Lung, cervical cancer | [67] |
| Cynodon dactylon | Ayurveda | Delphinidin-3-O-monoglucoside, cyanidin-3-O-monoglucoside | Lung, breast cancer | [68] |
| Cyanthillium cinereum | Ayurveda | Luteolin 7-O-glucuronide, Kaempferol 3-O-(6-O-acetyl) glycoside, apigenein-6-C-pentosyl-8-C-hexoside | Lung, breast cancer | [69] |
| Euphorbia thymifolia | Ayurveda | p-Coumaric acid, ferulic acid, kaempferol-3-glucuronide | Ascites carcinoma | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Cancer and Traditional Medicine: An Integrative Approach. Pharmaceuticals 2025, 18, 644. https://doi.org/10.3390/ph18050644
Sulaiman C, George BP, Balachandran I, Abrahamse H. Cancer and Traditional Medicine: An Integrative Approach. Pharmaceuticals. 2025; 18(5):644. https://doi.org/10.3390/ph18050644
Chicago/Turabian StyleSulaiman, Cheruthazhakkat, Blassan P. George, Indira Balachandran, and Heidi Abrahamse. 2025. "Cancer and Traditional Medicine: An Integrative Approach" Pharmaceuticals 18, no. 5: 644. https://doi.org/10.3390/ph18050644
APA StyleSulaiman, C., George, B. P., Balachandran, I., & Abrahamse, H. (2025). Cancer and Traditional Medicine: An Integrative Approach. Pharmaceuticals, 18(5), 644. https://doi.org/10.3390/ph18050644







