Volatile Organic Compounds in Biological Matrices as a Sensitive Weapon in Cancer Diagnosis
Abstract
1. Introduction
2. Volatile Organic Compound and Available Detection Techniques
3. Olfactory Network Assessment for Odorant Detection in Animals
Animals as Bio-Detectors
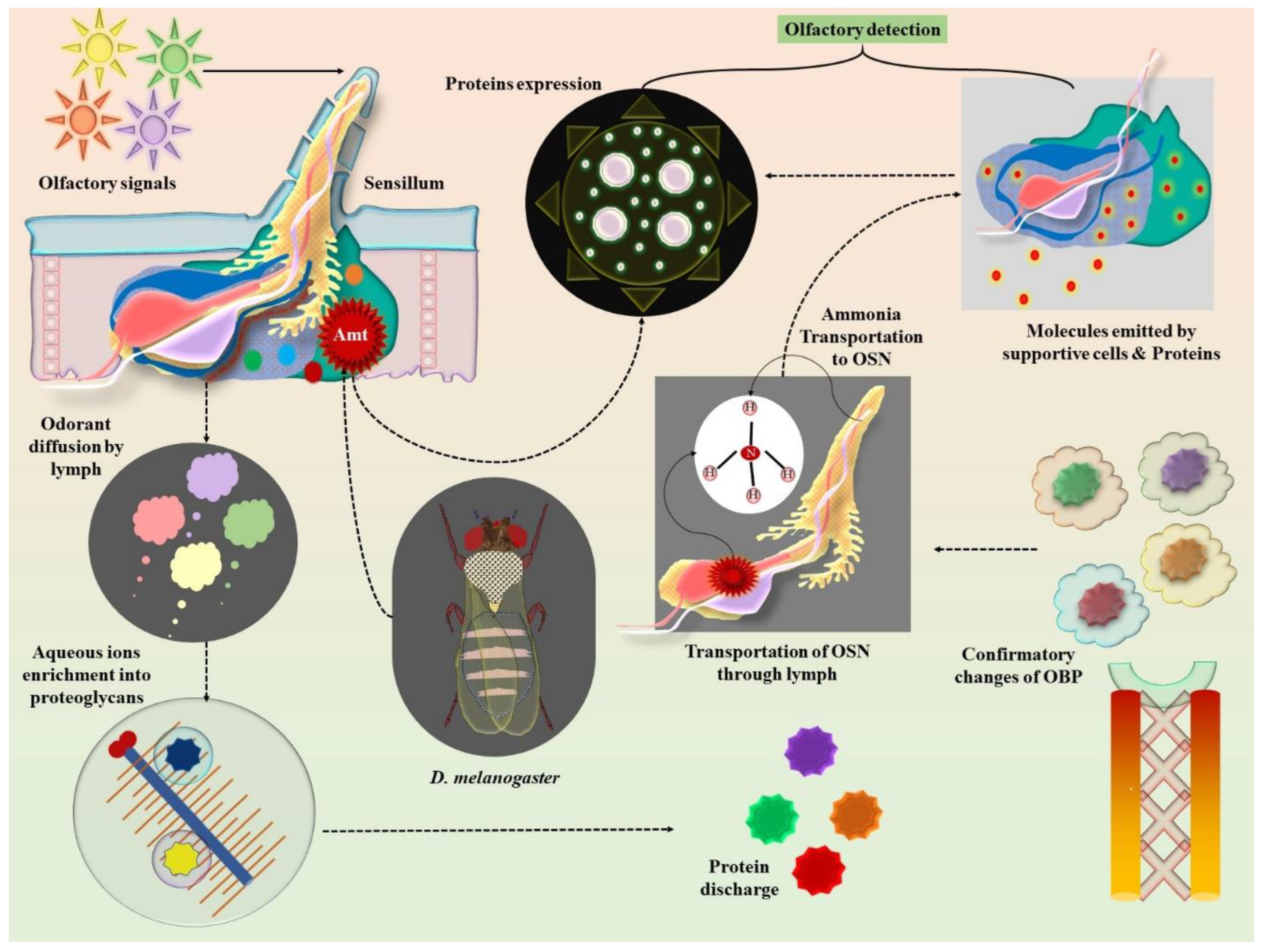
4. Insect Olfactory Neurobiology: Molecular Insights into Pheromone Detection
5. Olfactory Biochemistry and Impulse Sensitivity of Regulatory Receptors
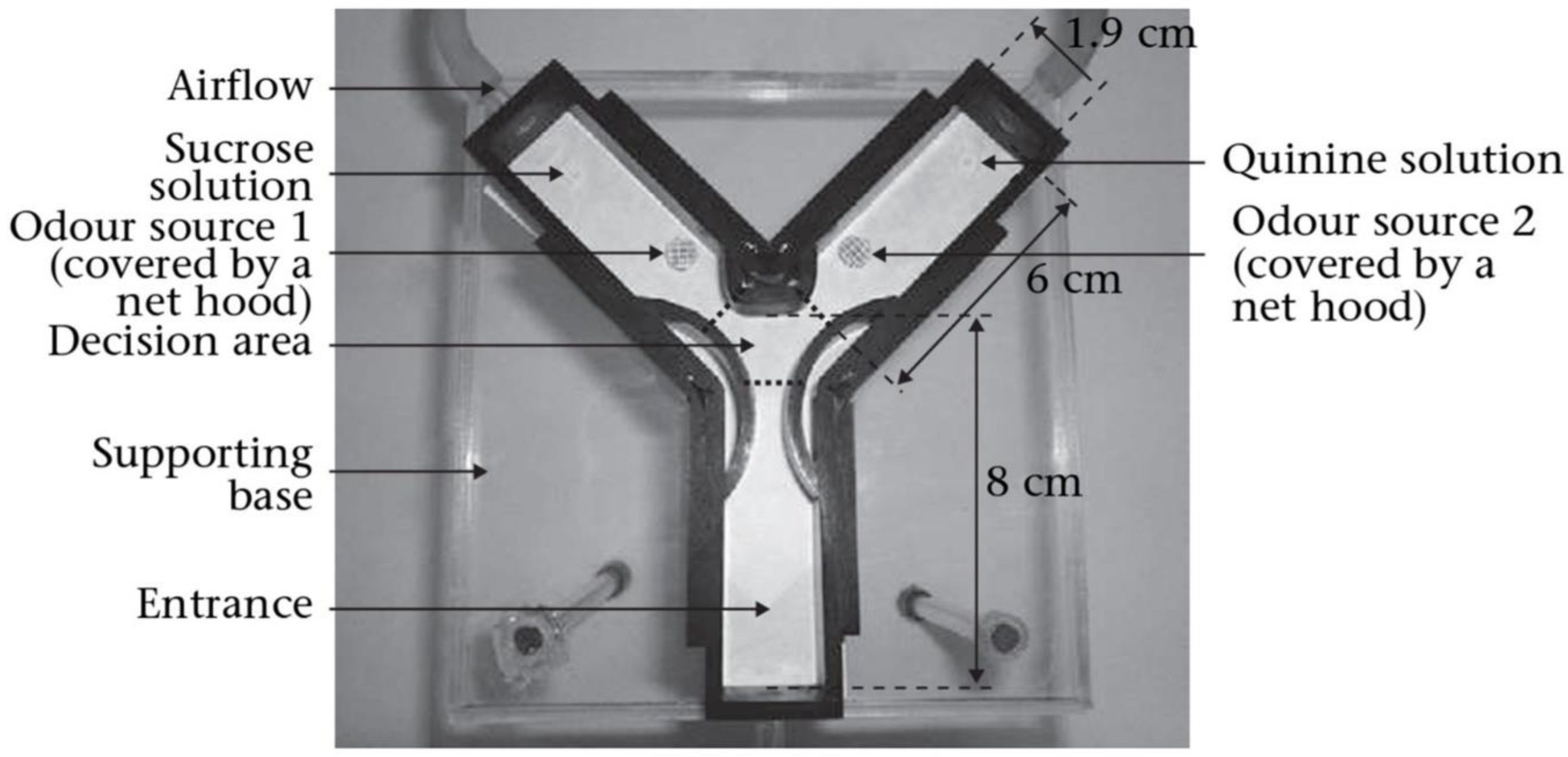
6. Olfactory Morphology of Ants and Its Potential Applications
Exploiting Insect Neurobiology for Early Cancer Sensing
7. Challenges and Advanced Strategies to Overcome Cancer Diagnosis via the Detection of Volatile Organic Compounds from Biological Matrices
7.1. Complexity of Biological Matrices
7.2. Diminished Concentration of Volatile Organic Compounds
7.3. Benign Interference
7.4. Instability Concerns
7.5. Biological Diversity, Matrix Complexity, and the Chemistry of Non-Cancerous VOCs
7.6. Regulatory Hurdles
8. The Development of Modern Electronic Nose Applications for Identifying Cancer VOCs
8.1. Metal-Oxide Semiconductor (MOS) Sensors
8.2. Conducting Polymer Sensors
8.3. Quartz Crystal Microbalance (QCM) Devices
8.4. Surface Acoustic Wave Sensors
8.5. Carbon Nanotube (CNT)-Based Detectors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18F-FDG PET-CT | 18F-fluorodeoxyglucose- positron emission tomography-computed tomography |
| AAs | advanced adenomas |
| Amt | ammonium/methylammonium transport |
| AUROC | area under the receiver operating characteristic |
| CD36 | cluster of differentiation 36 |
| CNT | carbon nanotube |
| COPD | chronic obstructive pulmonary disease |
| CP | chronic pancreatitis |
| CRC | colorectal cancer |
| cVA | cis-vaccenyl acetate |
| DMEM | Dulbecco’s modified eagle medium |
| DT | decision tree |
| e-nose | electronic nose |
| FAIMS | high field asymmetric waveform ion mobility spectrometry |
| GC-IMS | gas chromatography-ion mobility spectrometry |
| GC-MS | gas chromatography-mass spectrometry |
| GC-TOF-MS | gas chromatography coupled to time-of-flight mass spectrometry |
| GIT | gastrointestinal tract |
| HCC | hepatocellular carcinoma |
| HPPI-TOFMS | high-pressure photon ionization time-of-flight mass spectrometry |
| HS-SPME-GC-MS | headspace solid-phase microextraction-gas chromatography-mass spectrometry |
| IBD | inflammatory bowel disease |
| IBS | irritable bowel syndrome |
| IR | ionotropic receptor |
| IR84a | ionotropic receptor 84a |
| kNN | k-nearest neighbors |
| LC-MS/MS | liquid Chromatography-mass spectrometry/mass spectrometry |
| LFP | local field potential |
| LIMP2 | lysosomal integral membrane protein 2 |
| LR | logistic regression |
| Mag-HSAE-TD-GC-MS | magnetic headspace adsorptive extraction-thermal desorption-gas chromatography-mass spectrometry |
| MOS | metal oxide semiconductors |
| mRMR | maximum relevance minimum redundancy |
| NB | naive Bayes |
| NN | neural network |
| OBP | olfactory binding protein |
| OR | olfactory receptor |
| ORCO | olfactory receptor co-receptor |
| ORN | olfactory receptor neuron |
| OSN | olfactory sensory neuron |
| PDAC | pancreatic ductal adenocarcinoma |
| PEN3 | portable electronic nose |
| PVOIDs | potential volatile organic and inorganic derivatives |
| PPK25 | pickpocket 25 |
| PTR-TOF-MS | proton-transfer-reaction mass spectrometry |
| QCM | quartz crystal microbalance |
| RF | random forest |
| RS | resistant strain |
| SAW | surface acoustic wave |
| SIFT-MS | selected ion flow tube mass spectrometry |
| SNMP1 | sensory neuron membrane protein 1 |
| SPME | solid phase microextraction |
| SVM | support vector machine |
| TOF-MS | time-of-flight-mass spectrometry |
| VOCs | volatile organic compounds |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Gong, D.H.; Wu, H.; Gao, Y.Y.; Hao, G.F. Grasping Cryptic Binding Sites to Neutralize Drug Resistance in the Field of Anticancer. Drug Discov. Today 2023, 28, 103705. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of Cancer through Exhaled Breath: A Systematic Review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Baghernajad Salehi, L.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs Associated with Different Characteristics of Breast Cancer Cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid Volatolomics and Disease Detection. Angew. Chem. Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, X. A Simple, Rapid and Sensitive Method for Determination of Aldehydes in Human Blood by Gas Chromatography/Mass Spectrometry and Solid-Phase Microextraction with on-Fiber Derivatization. Rapid Commun. Mass Spectrom. 2004, 18, 1715–1720. [Google Scholar] [CrossRef]
- Ahmed, I.; Greenwood, R.; de Costello, B.L.; Ratcliffe, N.M.; Probert, C.S. An Investigation of Fecal Volatile Organic Metabolites in Irritable Bowel Syndrome. PLoS ONE 2013, 8, e58204. [Google Scholar] [CrossRef]
- Gallagher, M.; Wysocki, C.J.; Leyden, J.J.; Spielman, A.I.; Sun, X.; Preti, G. Analyses of Volatile Organic Compounds from Human Skin. Br. J. Dermatol. 2008, 159, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Thaler, H.T.; Kornblith, A.B.; McCarthy Lepore, J.; Friedlander-Klar, H.; Coyle, N.; Smart-Curley, T.; Kemeny, N.; Norton, L.; Hoskins, W.; et al. Symptom Prevalence, Characteristics and Distress in a Cancer Population. Qual. Life Res. 1994, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Toretsky, J.A.; Campbell, A.B.; Eskenazi, A.E. Recognition of Common Childhood Malignancies. Am. Fam. Physician 2000, 61, 2144–2154. [Google Scholar] [PubMed]
- Barros, J.A.; Valladares, G.; Faria, A.R.; Fugita, E.M.; Ruiz, A.P.; Vianna, A.G.D.; Trevisan, G.L.; Oliveira, F.A.M. de Early Diagnosis of Lung Cancer: The Great Challenge. Epidemiol. Var. Clin. Var. Staging Treat. 2006, 32, 221–227. [Google Scholar]
- Gupta, A.K.; Brenner, D.E.; Turgeon, D.K. Early Detection of Colon Cancer: New Tests on the Horizon. Mol. Diagn. Ther. 2008, 12, 77–85. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Eng. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Brooks, J.D. Translational Genomics: The Challenge of Developing Cancer Biomarkers. Genome Res. 2012, 22, 183–187. [Google Scholar] [CrossRef]
- Beger, R.D. A Review of Applications of Metabolomics in Cancer. Metabolites 2013, 3, 552. [Google Scholar] [CrossRef]
- Tiss, A.; Timms, J.; Menon, U.; Gammerman, A.; Cramer, R. Proteomics Approaches towards Early Detection and Diagnosis of Ovarian Cancer. J. Immunother. Cancer 2014, 2, O5. [Google Scholar] [CrossRef]
- Durán-Acevedo, C.M.; Jaimes-Mogollón, A.L.; Gualdrón-Guerrero, O.E.; Welearegay, T.G.; Martinez-Marín, J.D.; Caceres-Tarazona, J.M.; Sánchez-Acevedo, Z.C.; Beleño-Saenz, K.d.J.; Cindemir, U.; Österlund, L.; et al. Exhaled Breath Analysis for Gastric Cancer Diagnosis in Colombian Patients. Oncotarget 2018, 9, 28805. [Google Scholar] [CrossRef]
- Rössler, W.; Stengl, M. Insect Chemoreception: A Tribute to John G. Hildebrand. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2013, 199, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How Many Species Are There on Earth and in the Ocean? PLoS Biol. 2011, 9, 1001127. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S. A Bug’s Smell--Research into Insect Olfaction. Trends Neurosci. 2002, 25, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jafari, S.; Pask, G.; Zhou, X.; Reinberg, D.; Desplan, C. Evolution, Developmental Expression and Function of Odorant Receptors in Insects. J. Exp. Biol. 2020, 223, jeb208215. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach. Front. Cell. Neurosci. 2018, 12, 400763. [Google Scholar] [CrossRef]
- Robertson, H.M. Molecular Evolution of the Major Arthropod Chemoreceptor Gene Families. Annu. Rev. Entomol. 2019, 64, 227–242. [Google Scholar] [CrossRef]
- Grabe, V.; Sachse, S. Fundamental Principles of the Olfactory Code. Biosystems 2018, 164, 94–101. [Google Scholar] [CrossRef]
- Amin, H.; Lin, A.C. Neuronal Mechanisms Underlying Innate and Learned Olfactory Processing in Drosophila. Curr. Opin. Insect Sci. 2019, 36, 9–17. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Freeman, M.R.; Lessing, D.; Kim, J.; Carlson, J.R. A Novel Family of Divergent Seven-Transmembrane Proteins: Candidate Odorant Receptors in Drosophila. Neuron 1999, 22, 327–338. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A Spatial Map of Olfactory Receptor Expression in the Drosophila Antenna. Cell 1999, 96, 725–736. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.A. Comparative Morphogenesis of Sensilla: A Review. Int. J. Insect Morphol. Embryol. 1997, 26, 151–160. [Google Scholar] [CrossRef]
- Strauch, M.; Lüdke, A.; Münch, D.; Laudes, T.; Giovanni Galizia, C.; Martinelli, E.; Lavra, L.; Paolesse, R.; Ulivieri, A.; Catini, A.; et al. More than Apples and Oranges—Detecting Cancer with a Fruit Fly’s Antenna. Sci. Rep. 2014, 4, 3576. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Nowotny, T.; D’Ettorre, P.; Giurfa, M. Olfactory Experience Shapes the Evaluation of Odour Similarity in Ants: A Behavioural and Computational Analysis. Proc. Biol. Sci. 2016, 283, 20160551. [Google Scholar] [CrossRef]
- Guerrieri, F.J.; D’Ettorre, P. The Mandible Opening Response: Quantifying Aggression Elicited by Chemical Cues in Ants. J. Exp. Biol. 2008, 211, 1109–1113. [Google Scholar] [CrossRef]
- di Mauro, G.; Perez, M.; Lorenzi, M.C.; Guerrieri, F.J.; Millar, J.G.; D’Ettorre, P. Ants Discriminate between Different Hydrocarbon Concentrations. Front. Ecol. Evol. 2015, 3, 133. [Google Scholar] [CrossRef]
- Gouzerh, F.; Bessière, J.M.; Ujvari, B.; Thomas, F.; Dujon, A.M.; Dormont, L. Odors and Cancer: Current Status and Future Directions. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188644. [Google Scholar] [CrossRef]
- Ache, B.W.; Young, J.M. Olfaction: Diverse Species, Conserved Principles. Neuron 2005, 48, 417–430. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and Animal Behavior: Chemical Signals and Signatures, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 1–405. [Google Scholar] [CrossRef]
- Paschke, K.M.; Mashir, A.; Dweik, R.A. Clinical Applications of Breath Testing. F1000 Med. Rep. 2010, 2, 56. [Google Scholar] [CrossRef]
- Sani, S.N.; Zhou, W.; Ismail, B.B.; Zhang, Y.; Chen, Z.; Zhang, B.; Bao, C.; Zhang, H.; Wang, X. LC-MS/MS Based Volatile Organic Compound Biomarkers Analysis for Early Detection of Lung Cancer. Cancers 2023, 15, 1186. [Google Scholar] [CrossRef]
- Chapman, E.A.; Baker, J.; Aggarwal, P.; Hughes, D.M.; Nwosu, A.C.; Boyd, M.T.; Mayland, C.R.; Mason, S.; Ellershaw, J.; Probert, C.S.; et al. GC-MS Techniques Investigating Potential Biomarkers of Dying in the Last Weeks with Lung Cancer. Int. J. Mol. Sci. 2023, 24, 1591. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.Z.; Gashimova, E.M.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V.; Dmitrieva, E.V. Non-Invasive Lung Cancer Diagnostics through Metabolites in Exhaled Breath: Influence of the Disease Variability and Comorbidities. Metabolites 2023, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, S.M.; Pirrone, F.; Sedda, G.; Gasparri, R.; Romano, R.; Spaggiari, L.; Mariangela, A. Two-Step Investigation of Lung Cancer Detection by Sniffer Dogs. J. Breath Res. 2020, 14, 026011. [Google Scholar] [CrossRef]
- Ding, X.; Lin, G.; Wang, P.; Chen, H.; Li, N.; Yang, Z.; Qiu, M. Diagnosis of Primary Lung Cancer and Benign Pulmonary Nodules: A Comparison of the Breath Test and 18F-FDG PET-CT. Front. Oncol. 2023, 13, 1204435. [Google Scholar] [CrossRef]
- Shaffie, A.; Soliman, A.; Eledkawy, A.; Fu, X.A.; Nantz, M.H.; Giridharan, G.; van Berkel, V.; El-Baz, A. Lung Cancer Diagnosis System Based on Volatile Organic Compounds (VOCs) Profile Measured in Exhaled Breath. Appl. Sci. 2022, 12, 7165. [Google Scholar] [CrossRef]
- Sukaram, T.; Apiparakoon, T.; Tiyarattanachai, T.; Ariyaskul, D.; Kulkraisri, K.; Marukatat, S.; Rerknimitr, R.; Chaiteerakij, R. VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma. Diagnostics 2023, 13, 257. [Google Scholar] [CrossRef]
- Nazir, N.U.; Abbas, S.R. Identification of Phenol 2,2-Methylene Bis, 6 [1,1-D] as Breath Biomarker of Hepatocellular Carcinoma (HCC) Patients and Its Electrochemical Sensing: E-Nose Biosensor for HCC. Anal. Chim. Acta 2023, 1242, 340752. [Google Scholar] [CrossRef]
- Sukaram, T.; Tansawat, R.; Apiparakoon, T.; Tiyarattanachai, T.; Marukatat, S.; Rerknimitr, R.; Chaiteerakij, R. Exhaled Volatile Organic Compounds for Diagnosis of Hepatocellular Carcinoma. Sci. Rep. 2022, 12, 5326. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Tyagi, H.; Daulton, E.; Covington, J.A.; Arasaradnam, R.P. Exploratory Study Using Urinary Volatile Organic Compounds for the Detection of Hepatocellular Carcinoma. Molecules 2021, 26, 2447. [Google Scholar] [CrossRef]
- Boulind, C.E.; Gould, O.; Costello, B.d.L.; Allison, J.; White, P.; Ewings, P.; Wicaksono, A.N.; Curtis, N.J.; Pullyblank, A.; Jayne, D.; et al. Urinary Volatile Organic Compound Testing in Fast-Track Patients with Suspected Colorectal Cancer. Cancers 2022, 14, 2127. [Google Scholar] [CrossRef]
- Cheng, H.R.; van Vorstenbosch, R.W.; Pachen, D.M.; Meulen, L.W.; Straathof, J.W.; Dallinga, J.W.; Jonkers, D.M.; Masclee, A.A.; van Schooten, F.J.; Mujagic, Z.; et al. Detecting Colorectal Adenomas and Cancer Using Volatile Organic Compounds in Exhaled Breath: A Proof-of-Principle Study to Improve Screening. Clin. Transl. Gastroenterol. 2022, 13, e00518. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Non-Invasive Detection and Staging of Colorectal Cancer Using a Portable Electronic Nose. Sensors 2021, 21, 5440. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Zhou, J.; Chu, Y.; Lu, Y.; Zou, X.; Xia, L.; Liu, Y.; Huang, C.; Shen, C.; Zhang, L.; et al. Distinguish Oral-Source VOCs and Control Their Potential Impact on Breath Biomarkers. Anal. Bioanal. Chem. 2022, 414, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Seo, H.S.; Kim, J.H.; Song, K.Y.; Park, C.H.; Lee, H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Time Analysis Analysis from Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021, 11, 560591. [Google Scholar] [CrossRef]
- Teränen, V.; Nissinen, S.; Roine, A.; Antila, A.; Siiki, A.; Vaalavuo, Y.; Kumpulainen, P.; Oksala, N.; Laukkarinen, J. Bile-Volatile Organic Compounds in the Diagnostics of Pancreatic Cancer and Biliary Obstruction: A Prospective Proof-of-Concept Study. Front. Oncol. 2022, 12, 918539. [Google Scholar] [CrossRef]
- Daulton, E.; Wicaksono, A.N.; Tiele, A.; Kocher, H.M.; Debernardi, S.; Crnogorac-Jurcevic, T.; Covington, J.A. Volatile Organic Compounds (VOCs) for the Non-Invasive Detection of Pancreatic Cancer from Urine. Talanta 2021, 221, 121604. [Google Scholar] [CrossRef]
- Lett, L.; George, M.; Slater, R.; De Lacy Costello, B.; Ratcliffe, N.; García-Fiñana, M.; Lazarowicz, H.; Probert, C. Investigation of Urinary Volatile Organic Compounds as Novel Diagnostic and Surveillance Biomarkers of Bladder Cancer. Br. J. Cancer 2022, 127, 329–336. [Google Scholar] [CrossRef]
- Woollam, M.; Siegel, A.P.; Munshi, A.; Liu, S.; Tholpady, S.; Gardner, T.; Li, B.Y.; Yokota, H.; Agarwal, M. Canine-Inspired Chemometric Analysis of Volatile Organic Compounds in Urine Headspace to Distinguish Prostate Cancer in Mice and Men. Cancers 2023, 15, 1352. [Google Scholar] [CrossRef]
- Giró Benet, J.; Seo, M.; Khine, M.; Gumà Padró, J.; Pardo Martnez, A.; Kurdahi, F. Breast Cancer Detection by Analyzing the Volatile Organic Compound (VOC) Signature in Human Urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef]
- Piqueret, B.; Bourachot, B.; Leroy, C.; Devienne, P.; Mechta-Grigoriou, F.; D’Ettorre, P.; Sandoz, J.C. Ants Detect Cancer Cells through Volatile Organic Compounds. iScience 2022, 25, 103959. [Google Scholar] [CrossRef]
- Díaz de León-Martínez, L.; Flores-Ramírez, R.; López-Mendoza, C.M.; Rodríguez-Aguilar, M.; Metha, G.; Zúñiga-Martínez, L.; Ornelas-Rebolledo, O.; Alcántara-Quintana, L.E. Identification of Volatile Organic Compounds in the Urine of Patients with Cervical Cancer. Test Concept for Timely Screening. Clin. Chim. Acta 2021, 522, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic Nose: A Non-Invasive Technology for Breath Analysis of Diabetes and Lung Cancer Patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F.; Magan, N. Electronic Noses and Disease Diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef]
- Provecho, Y.; Josens, R. Olfactory Memory Established during Trophallaxis Affects Food Search Behaviour in Ants. J. Exp. Biol. 2009, 212, 3221–3227. [Google Scholar] [CrossRef]
- D’ettorre, P. Genomic and Brain Expansion Provide Ants with Refined Sense of Smell. Proc. Natl. Acad. Sci. USA 2016, 113, 13947–13949. [Google Scholar] [CrossRef]
- Hildebrand, J.G.; Shepherd, G.M. Mechanisms of Olfactory Discrimination: Converging Evidence for Common Principles across Phyla. Annu. Rev. Neurosci. 1997, 20, 595–631. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Xie, J.; Deng, C.; Sun, X.; Hao, C. Glomerular Organization of the Antennal Lobes of the Diamondback Moth, Plutella xylostella L. Front. Neuroanat. 2019, 13, 430656. [Google Scholar] [CrossRef]
- Anton, S.; Rössler, W. Plasticity and Modulation of Olfactory Circuits in Insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef]
- Pirrone, F.; Albertini, M. Olfactory Detection of Cancer by Trained Sniffer Dogs: A Systematic Review of the Literature. J. Vet. Behav. Clin. Appl. Res. 2017, 19, 105–117. [Google Scholar] [CrossRef]
- Feil, C.; Staib, F.; Berger, M.R.; Stein, T.; Schmidtmann, I.; Forster, A.; Schimanski, C.C. Sniffer Dogs Can Identify Lung Cancer Patients from Breath and Urine Samples. BMC Cancer 2021, 21, 917. [Google Scholar] [CrossRef]
- Oh, Y.; Kwon, O.S.; Min, S.S.; Shin, Y.B.; Oh, M.K.; Kim, M. Olfactory Detection of Toluene by Detection Rats for Potential Screening of Lung Cancer. Sensors 2021, 21, 2967. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M. Cancer Detection by Fruit Fly Olfaction. Lab Anim. 2014, 43, 76. [Google Scholar] [CrossRef] [PubMed]
- Piqueret, B.; Sandoz, J.C.; D’Ettorre, P. Ants Learn Fast and Do Not Forget: Associative Olfactory Learning, Memory and Extinction in Formica Fusca. R. Soc. Open Sci. 2019, 6, 190778. [Google Scholar] [CrossRef] [PubMed]
- Parnas, M.; McLane-Svoboda, A.K.; Cox, E.; McLane-Svoboda, S.B.; Sanchez, S.W.; Farnum, A.; Tundo, A.; Lefevre, N.; Miller, S.; Neeb, E.; et al. Precision Detection of Select Human Lung Cancer Biomarkers and Cell Lines Using Honeybee Olfactory Neural Circuitry as a Novel Gas Sensor. Biosens. Bioelectron. 2024, 261, 116466. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Ions and Mucoid Substances in Sensory Organs--Microanalytical Data from Insect Sensilla. Symp. Soc. Exp. Biol. 1989, 43, 131–138. [Google Scholar]
- Su, C.Y.; Martelli, C.; Emonet, T.; Carlson, J.R. Temporal Coding of Odor Mixtures in an Olfactory Receptor Neuron. Proc. Natl. Acad. Sci. USA 2011, 108, 5075–5080. [Google Scholar] [CrossRef]
- Martelli, C.; Carlson, J.R.; Emonet, T. Intensity Invariant Dynamics and Odor-Specific Latencies in Olfactory Receptor Neuron Response. J. Neurosci. 2013, 33, 6285. [Google Scholar] [CrossRef]
- Sun, J.S.; Xiao, S.; Carlson, J.R. The Diverse Small Proteins Called Odorant-Binding Proteins. R. Soc. Open Biol. 2018, 8, 180208. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Xu, P.; Atkinson, R.; Jones, D.N.M.; Smith, D.P. Drosophila OBP LUSH Is Required for Activity of Pheromone-Sensitive Neurons. Neuron 2005, 45, 193–200. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Reina, J.H.; Cambillau, C.; Benton, R. Ligands for Pheromone-Sensing Neurons Are Not Conformationally Activated Odorant Binding Proteins. PLoS Biol. 2013, 11, e1001546. [Google Scholar] [CrossRef] [PubMed]
- Larter, N.K.; Sun, J.S.; Carlson, J.R. Organization and Function of Drosophila Odorant Binding Proteins. eLife 2016, 5, e20242. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, E.A.; Smith, D.P. Odor-Specific Deactivation Defects in a Drosophila Odorant-Binding Protein Mutant. Genetics 2019, 213, 897–909. [Google Scholar] [CrossRef]
- Menuz, K.; Larter, N.K.; Park, J.; Carlson, J.R. An RNA-Seq Screen of the Drosophila Antenna Identifies a Transporter Necessary for Ammonia Detection. PLoS Genet. 2014, 10, e1004810. [Google Scholar] [CrossRef]
- Cifarelli, V.; Abumrad, N.A. Intestinal CD36 and Other Key Proteins of Lipid Utilization: Role in Absorption and Gut Homeostasis. Compr. Physiol. 2018, 8, 493–507. [Google Scholar] [CrossRef]
- Rogers, M.E.; Sun, M.; Lerner, M.R.; Vogt, R.G. Snmp-1, a Novel Membrane Protein of Olfactory Neurons of the Silk Moth Antheraea Polyphemus with Homology to the CD36 Family of Membrane Proteins. J. Biol. Chem. 1997, 272, 14792–14799. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An Essential Role for a CD36-Related Receptor in Pheromone Detection in Drosophila. Nature 2007, 450, 289–293. [Google Scholar] [CrossRef]
- Vogt, R.G.; Miller, N.E.; Litvack, R.; Fandino, R.A.; Sparks, J.; Staples, J.; Friedman, R.; Dickens, J.C. The Insect SNMP Gene Family. Insect Biochem. Mol. Biol. 2009, 39, 448–456. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Bargeton, B.; Abuin, L.; Bukar, N.; Reina, J.H.; Bartoi, T.; Graf, M.; Ong, H.; Ulbrich, M.H.; Masson, J.F.; et al. A CD36 Ectodomain Mediates Insect Pheromone Detection via a Putative Tunnelling Mechanism. Nat. Commun. 2016, 7, 11866. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xu, W.; Chen, Q.M.; Sun, L.N.; Anderson, A.; Xia, Q.Y.; Papanicolaou, A. A Phylogenomics Approach to Characterizing Sensory Neuron Membrane Proteins (SNMPs) in Lepidoptera. Insect Biochem. Mol. Biol. 2020, 118, 103313. [Google Scholar] [CrossRef]
- Jin, X.; Tal, S.H.; Smith, D.P. SNMP Is a Signaling Component Required for Pheromone Sensitivity in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 10996–11001. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ni, J.D.; Huang, J.; Montell, C. Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity. PLoS Genet. 2014, 10, e1004600. [Google Scholar] [CrossRef] [PubMed]
- German, P.F.; van der Poel, S.; Carraher, C.; Kralicek, A.V.; Newcomb, R.D. Insights into Subunit Interactions within the Insect Olfactory Receptor Complex Using FRET. Insect Biochem. Mol. Biol. 2013, 43, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Reisert, J.; Zhao, H. Perspectives on: Information and Coding in Mammalian Sensory Physiology: Response Kinetics of Olfactory Receptor Neurons and the Implications in Olfactory Coding. J. Gen. Physiol. 2011, 138, 303. [Google Scholar] [CrossRef]
- Ng, R.; Salem, S.S.; Wu, S.T.; Wu, M.; Lin, H.H.; Shepherd, A.K.; Joiner, W.J.; Wang, J.W.; Su, C.Y. Amplification of Drosophila Olfactory Responses by a DEG/ENaC Channel. Neuron 2019, 104, 947–959. [Google Scholar] [CrossRef]
- Dweck, H.K.M.; Ebrahim, S.A.M.; Thoma, M.; Mohamed, A.A.M.; Keesey, I.W.; Trona, F.; Lavista-Llanos, S.; Svatoš, A.; Sachse, S.; Knaden, M.; et al. Pheromones Mediating Copulation and Attraction in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, E2829–E2835. [Google Scholar] [CrossRef]
- Lin, H.H.; Cao, D.S.; Sethi, S.; Zeng, Z.; Chin, J.S.R.; Chakraborty, T.S.; Shepherd, A.K.; Nguyen, C.A.; Yew, J.Y.; Su, C.Y.; et al. Hormonal Modulation of Pheromone Detection Enhances Male Courtship Success. Neuron 2016, 90, 1272–1285. [Google Scholar] [CrossRef]
- Grosjean, Y.; Rytz, R.; Farine, J.P.; Abuin, L.; Cortot, J.; Jefferis, G.S.X.E.; Benton, R. An Olfactory Receptor for Food-Derived Odours Promotes Male Courtship in Drosophila. Nature 2011, 478, 236–240. [Google Scholar] [CrossRef]
- Kaissling, K.E.; Strausfeld, C.Z.; Rumbo, E.R. Adaptation Processes in Insect Olfactory Receptors. Mechanisms and Behavioral Significance. Ann. N. Y. Acad. Sci. 1987, 510, 104–112. [Google Scholar] [CrossRef]
- Nagel, K.I.; Wilson, R.I. Biophysical Mechanisms Underlying Olfactory Receptor Neuron Dynamics. Nat. Neurosci. 2011, 14, 208–218. [Google Scholar] [CrossRef]
- Kaissling, K.E. Chemo-Electrical Transduction in Insect Olfactory Receptors. Annu. Rev. Neurosci. 1986, 9, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Gorur-Shandilya, S.; Demir, M.; Long, J.; Clark, D.A.; Emonet, T. Olfactory Receptor Neurons Use Gain Control and Complementary Kinetics to Encode Intermittent Odorant Stimuli. eLife 2017, 6, e27670. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, J. Multiple Sites of Adaptation Lead to Contrast Encoding in the Drosophila Olfactory System. Physiol. Rep. 2016, 4, e12762. [Google Scholar] [CrossRef] [PubMed]
- Clyne, P.; Grant, A.; O’Connell, R.; Carlson, J.R. Odorant Response of Individual Sensilla on the Drosophila Antenna. Invertebr. Neurosci. 1997, 3, 127–135. [Google Scholar] [CrossRef]
- Getahun, M.N.; Olsson, S.B.; Lavista-Llanos, S.; Hansson, B.S.; Wicher, D. Insect Odorant Response Sensitivity Is Tuned by Metabotropically Autoregulated Olfactory Receptors. PLoS ONE 2013, 8, e58889. [Google Scholar] [CrossRef]
- Bahk, S.; Jones, W.D. Insect Odorant Receptor Trafficking Requires Calmodulin. BMC Biol. 2016, 14, 83. [Google Scholar] [CrossRef]
- Cao, L.H.; Jing, B.Y.; Yang, D.; Zeng, X.; Shen, Y.; Tu, Y.; Luo, D.G. Distinct Signaling of Drosophila Chemoreceptors in Olfactory Sensory Neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E902–E911. [Google Scholar] [CrossRef]
- Guo, H.; Kunwar, K.; Smith, D. Odorant Receptor Sensitivity Modulation in Drosophila. J. Neurosci. 2017, 37, 9465–9473. [Google Scholar] [CrossRef]
- Sargsyan, V.; Getahun, M.N.; Llanos, S.L.; Olsson, S.B.; Hansson, B.S.; Wicher, D. Phosphorylation via PKC Regulates the Function of the Drosophila Odorant Co-Receptor. Front. Cell. Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef]
- Getahun, M.N.; Thoma, M.; Lavista-Llanos, S.; Keesey, I.; Fandino, R.A.; Knaden, M.; Wicher, D.; Olsson, S.B.; Hansson, B.S. Intracellular Regulation of the Insect Chemoreceptor Complex Impacts Odour Localization in Flying Insects. J. Exp. Biol. 2016, 219, 3428–3438. [Google Scholar] [CrossRef]
- Guo, H.; Smith, D.P. Odorant Receptor Desensitization in Insects. J. Exp. Neurosci. 2017, 11, 1179069517748600. [Google Scholar] [CrossRef] [PubMed]
- Abuin, L.; Prieto-Godino, L.L.; Pan, H.; Gutierrez, C.; Huang, L.; Jin, R.; Benton, R. In Vivo Assembly and Trafficking of Olfactory Ionotropic Receptors. BMC Biol. 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 1469–1658. [Google Scholar] [CrossRef]
- Getahun, M.N.; Wicher, D.; Hansson, B.S.; Olsson, S.B. Temporal Response Dynamics of Drosophila Olfactory Sensory Neurons Depends on Receptor Type and Response Polarity. Front. Cell. Neurosci. 2012, 6, 54. [Google Scholar] [CrossRef]
- Su, C.Y.; Menuz, K.; Reisert, J.; Carlson, J.R. Non-Synaptic Inhibition between Grouped Neurons in an Olfactory Circuit. Nature 2012, 492, 66–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Tsang, T.K.; Bushong, E.A.; Chu, L.A.; Chiang, A.S.; Ellisman, M.H.; Reingruber, J.; Su, C.Y. Asymmetric Ephaptic Inhibition between Compartmentalized Olfactory Receptor Neurons. Nat. Commun. 2019, 10, 1560. [Google Scholar] [CrossRef]
- Miriyala, A.; Kessler, S.; Rind, F.C.; Wright, G.A. Burst Firing in Bee Gustatory Neurons Prevents Adaptation. Curr. Biol. 2018, 28, 1585–1594. [Google Scholar] [CrossRef]
- Mohapatra, P.; Menuz, K. Molecular Profiling of the Drosophila Antenna Reveals Conserved Genes Underlying Olfaction in Insects. G3 2019, 9, 3753–3771. [Google Scholar] [CrossRef]
- Piqueret, B.; Montaudon, É.; Devienne, P.; Leroy, C.; Marangoni, E.; Sandoz, J.C.; D’Ettorre, P. Ants Act as Olfactory Bio-Detectors of Tumours in Patient-Derived Xenograft Mice. Proc. R. Soc. B Biol. Sci. 2023, 290, 20221962. [Google Scholar] [CrossRef]
- Walter, F.; Fletcher, D.J.C.; Chautems, D.; Cherix, D.; Keller, L.; Francke, W.; Fortelius, W.; Rosengren, R.; Vargo, E.L. Identification of the Sex Pheromone of an Ant, Formica Lugubris (Hymenoptera, Formicidae). Naturwissenschaften 1993, 80, 30–34. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; d’Ettorre, P. Nestmate Recognition in Social Insects: What Does It Mean to Be Chemically Insignificant? Front. Ecol. Evol. 2020, 7, 480942. [Google Scholar] [CrossRef]
- Mizunami, M.; Yamagata, N.; Nishino, H. Alarm Pheromone Processing in the Ant Brain: An Evolutionary Perspective. Front. Behav. Neurosci. 2010, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Monnin, T.; Peeters, C. Dominance Hierarchy and Reproductive Conflicts among Subordinates in a Monogynous Queenless Ant. Behav. Ecol. 1999, 10, 323–332. [Google Scholar] [CrossRef]
- Rossi, N.; Pereyra, M.; Moauro, M.A.; Giurfa, M.; D’Ettorre, P.; Josens, R. Trail Pheromone Modulates Subjective Reward Evaluation in Argentine Ants. J. Exp. Biol. 2020, 223, jeb230532. [Google Scholar] [CrossRef]
- Rossi, N.; Baracchi, D.; Giurfa, M.; D’ettorre, P. Pheromone-Induced Accuracy of Nestmate Recognition in Carpenter Ants: Simultaneous Decrease in Type I and Type II Errors. Am. Nat. 2019, 193, 267–278. [Google Scholar] [CrossRef]
- Guerrieri, F.J.; D’Ettorre, P. Associative Learning in Ants: Conditioning of the Maxilla-Labium Extension Response in Camponotus Aethiops. J. Insect Physiol. 2010, 56, 88–92. [Google Scholar] [CrossRef]
- Seid, M.A.; Junge, E. Social Isolation and Brain Development in the Ant Camponotus floridanus. Sci. Nat. 2016, 103, 42. [Google Scholar] [CrossRef]
- Guerrieri, F.J.; D’Ettorre, P.; Devaud, J.M.; Giurfa, M. Long-Term Olfactory Memories Are Stabilised via Protein Synthesis in Camponotus Fellah Ants. J. Exp. Biol. 2011, 214, 3300–3304. [Google Scholar] [CrossRef]
- Menzel, R. Searching for the Memory Trace in a Mini-Brain, the Honeybee. Learn. Mem. 2001, 8, 53–62. [Google Scholar] [CrossRef]
- Dupuy, F.; Sandoz, J.C.; Giurfa, M.; Josens, R. Individual Olfactory Learning in Camponotus Ants. Anim. Behav. 2006, 72, 1081–1091. [Google Scholar] [CrossRef]
- Josens, R.; Eschbach, C.; Giurfa, M. Differential Conditioning and Long-Term Olfactory Memory in Individual Camponotus Fellah Ants. J. Exp. Biol. 2009, 212, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Bos, N.; Dreier, S.; Jørgensen, C.G.; Nielsen, J.; Guerrieri, F.J.; D’Ettorre, P. Learning and Perceptual Similarity among Cuticular Hydrocarbons in Ants. J. Insect Physiol. 2012, 58, 138–146. [Google Scholar] [CrossRef]
- Bos, N.; Guerrieri, F.J.; d’Ettorre, P. Significance of Chemical Recognition Cues Is Context Dependent in Ants. Anim. Behav. 2010, 80, 839–844. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging Deeper into Volatile Organic Compounds Associated with Cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Schmidt, K.; Podmore, I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Tsou, P.H.; Lin, Z.L.; Pan, Y.C.; Yang, H.C.; Chang, C.J.; Liang, S.K.; Wen, Y.F.; Chang, C.H.; Chang, L.Y.; Yu, K.L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. [Google Scholar] [CrossRef]
- Dragonieri, S.; Annema, J.T.; Schot, R.; van der Schee, M.P.C.; Spanevello, A.; Carratú, P.; Resta, O.; Rabe, K.F.; Sterk, P.J. An Electronic Nose in the Discrimination of Patients with Non-Small Cell Lung Cancer and COPD. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef]
- Angioli, R.; Santonico, M.; Pennazza, G.; Montera, R.; Luvero, D.; Gatti, A.; Zompanti, A.; Finamore, P.; Incalzi, R.A. Use of Sensor Array Analysis to Detect Ovarian Cancer through Breath, Urine, and Blood: A Case-Control Study. Diagnostics 2024, 14, 561. [Google Scholar] [CrossRef]
- Raspagliesi, F.; Bogani, G.; Benedetti, S.; Grassi, S.; Ferla, S.; Buratti, S. Detection of Ovarian Cancer through Exhaled Breath by Electronic Nose: A Prospective Study. Cancers 2020, 12, 2408. [Google Scholar] [CrossRef]
- Amal, H.; Shi, D.Y.; Ionescu, R.; Zhang, W.; Hua, Q.L.; Pan, Y.Y.; Tao, L.; Liu, H.; Haick, H. Assessment of Ovarian Cancer Conditions from Exhaled Breath. Int. J. Cancer 2015, 136, E614–E622. [Google Scholar] [CrossRef] [PubMed]
- Hintzen, K.F.H.; Eussen, M.M.M.; Neutel, C.; Bouvy, N.D.; van Schooten, F.J.; Hooijmans, C.R.; Lubbers, T. A Systematic Review on the Detection of Volatile Organic Compounds in Exhaled Breath in Experimental Animals in the Context of Gastrointestinal and Hepatic Diseases. PLoS ONE 2023, 18, e0291636. [Google Scholar] [CrossRef] [PubMed]
- Mezmale, L.; Leja, M.; Lescinska, A.M.; Pčolkins, A.; Kononova, E.; Bogdanova, I.; Polaka, I.; Stonans, I.; Kirsners, A.; Ager, C.; et al. Identification of Volatile Markers of Colorectal Cancer from Tumor Tissues Using Volatilomic Approach. Molecules 2023, 28, 5990. [Google Scholar] [CrossRef]
- Zheng, W.; Min, Y.; Pang, K.; Wu, D. Sample Collection and Processing in Volatile Organic Compound Analysis for Gastrointestinal Cancers. Diagnostics 2024, 14, 1563. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Lian, A.; Sun, B.; Wang, X.; Guo, L.; Chi, C.; Liu, S.; Zhao, W.; Luo, S.; et al. Blood Volatile Compounds as Biomarkers for Colorectal Cancer. Cancer Biol. Ther. 2014, 15, 200–206. [Google Scholar] [CrossRef]
- van Liere, E.L.S.A.; van Dijk, L.J.; Bosch, S.; Vermeulen, L.; Heymans, M.W.; Burchell, G.L.; de Meij, T.G.J.; Ramsoekh, D.; de Boer, N.K.H. Urinary Volatile Organic Compounds for Colorectal Cancer Screening: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2023, 186, 69–82. [Google Scholar] [CrossRef]
- Nardi-Agmon, I.; Peled, N. Exhaled Breath Analysis for the Early Detection of Lung Cancer: Recent Developments and Future Prospects. Lung Cancer Targets Ther. 2017, 8, 31–38. [Google Scholar] [CrossRef]
- Keogh, R.J.; Riches, J.C. The Use of Breath Analysis in the Management of Lung Cancer: Is It Ready for Primetime? Curr. Oncol. 2022, 29, 7355–7378. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef]
- Allard-Coutu, A.; Singh, K.; David, D.; Dobson, V.; Dahmer, L.; Heller, B. Volatile Organic Compounds: A Promising New Frontier for Cancer Screening. Tumor Discov. 2024, 3, 2061. [Google Scholar] [CrossRef]
- Grizzi, F.; Bax, C.; Hegazi, M.A.A.A.; Lotesoriere, B.J.; Zanoni, M.; Vota, P.; Hurle, R.F.; Buffi, N.M.; Lazzeri, M.; Tidu, L.; et al. Early Detection of Prostate Cancer: The Role of Scent. Chemosensors 2023, 11, 356. [Google Scholar] [CrossRef]
- Suthat Na Ayutaya, V.; Tantisatirapoon, C.; Aekgawong, S.; Anakkamatee, W.; Danjittrong, T.; Kreepala, C. Urinary Cancer Detection by the Target Urine Volatile Organic Compounds Biosensor Platform. Sci. Rep. 2024, 14, 3551. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, H.P.; Chang, K.H.; Lee, M.S.; Lee, C.F.; Lin, C.Y.; Lin, Y.C.; Huang, W.J.; Liao, C.H.; Yu, C.C.; et al. Predicting Clinically Significant Prostate Cancer Using Urine Metabolomics via Liquid Chromatography Mass Spectrometry. World J. Mens. Health 2024, 42, 376. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic Nose: Current Status and Future Trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K.K.; Kang, M.S.; Shin, D.M.; Oh, J.W.; Lee, C.S.; Han, D.W. Artificial Olfactory Sensor Technology That Mimics the Olfactory Mechanism: A Comprehensive Review. Biomater. Res. 2022, 26, 40. [Google Scholar] [CrossRef]
- Hajivand, P.; Carolus Jansen, J.; Pardo, E.; Armentano, D.; Mastropietro, T.F.; Azadmehr, A. Application of Metal-Organic Frameworks for Sensing of VOCs and Other Volatile Biomarkers. Coord. Chem. Rev. 2024, 501, 215558. [Google Scholar] [CrossRef]
- Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors 2021, 21, 4976. [Google Scholar] [CrossRef]
- Jin, H. Volatile Organic Compound Sensors—the Future of Cancer Detection? Res. Features 2019, 22–25. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Surface Acousticwave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A Review on Carbon Nanotubes in Biosensor Devices and Their Applications in Medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Rabehi, A.; Helal, H.; Zappa, D.; Comini, E. Advancements and Prospects of Electronic Nose in Various Applications: A Comprehensive Review. Appl. Sci. 2024, 14, 4506. [Google Scholar] [CrossRef]
- Scheepers, M.H.M.C.; Al-Difaie, Z.; Brandts, L.; Peeters, A.; Van Grinsven, B.; Bouvy, N.D. Diagnostic Performance of Electronic Noses in Cancer Diagnoses Using Exhaled Breath: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, E2219372. [Google Scholar] [CrossRef] [PubMed]


| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/ Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| Lung Cancer | Exhaled Breath | Clinical Study | 3-hydroxy-2-butanone (TG-4) | LC-MS/MS | 70% | 76% | [41] |
| 3-hydroxy-2-butanone (TG-4), glycolaldehyde (TG-7), 2-pentanone (TG-8), acrolein (TG-11), nonaldehyde (TG-19), decanal (TG-20), and crotonaldehyde (TG-22) | -- | -- | |||||
| Lung Cancer | Urine | Clinical Study | Nonan-2-one or 5-methylhexan-2-one, heptan-2-one, Benzaldehyde, 5-ethyl-5-methyloxolan-2-one, propan-2-one, 2-methyl-5-methylsulfanylfuran, 3,4-dimethylhexan-2-one, hexane-3,4-dione, Cyclohexanone, Phenol, 4-methylpent-3-enoic acid, 1,2,4-triazole-3,4-diamine, (E)-non-3-en-2-one, (E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one, p-Cimene, 5-(3,3-dimethyloxiran-2-yl)-3-methylpent-1- en-3-ol, 2-buta-1,3-dienyl-1,3,5-trimethylbenzene, Pulegone, 2,6,6,10-tetramethyl-1-oxaspiro[4.5]dec-9-ene 3,7-dimethyloctan-3-ol or 3-methylpentan-3-ol, hydroperoxyhexane, 1-(furan-2-yl)ethenone, 2-methoxyphenol, (1,4-dimethylpent-2-enyl)benzene, 1-methyl-4-propan-2-ylcyclohexa-1,4-diene, 1-methyl-4-prop-1-en-2-ylcyclohexa-1,3-diene, 1-methyl-4-prop-1-en-2-ylbenzene or 1-methyl-2-prop-1-en-2-ylbenzene, Carvone, 4,7,7-trimethylbicyclo[4.1.0]hept-2-ene((+)- 4-Carene), 1-methyl-4-propan-2-yl-7- oxabicyclo[2.2.1]heptane, 3,4-dimethylthiophene, 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol, 2,6-dimethyloct-7-en-2-ol, Menthol | HS-SPME-GC-MS | -- | -- | [42] |
| Lung Cancer | Exhaled Breath | Clinical Study | Isoprene, Acetone, Dimethylsulfid, 1-Methylthiopropene, Allyl methyl sulfide, 1-Methylthiopropane, 2-Pentanone, Dimethyl disulfide, 2.3-Butandione, Acetonitrile, 2-Butanone, Dimethyl trisulfide, Benzaldehyde, 1-Pentanol, 2-Heptanone, Heptane, Nonanal, Hexane, 3-Heptanone, Octanal, Octane, Toluene, Pentanal, 31 Hexanal, Decane, Dodecane, Undecane, Propylbenzene, Decanal, Heptanal, Butanal Nonane, Benzene, 1.3-pentadiene, Ethylbenzene, 1-Butanol, 1.4-pentadiene, Butyl acetate, o-Xylene, M + p-Xylene | GC-MS | 82–88% (ANN Model) | 80–86% (ANN Model) | [43] |
| Lung Cancer | Breath Sample Lung Cancer Tissue Urine | Clinical and Preclinical Studies | -- | Statistical software—IBM SPSS version 25.0 (Armonk, NY, USA: IBM Corp) | 91.7% | 85.1% | [44] |
| 3(4H)-dibenzofuranone, 4a,9b-dihydro-8,9b-dimethyl-(3(4H)-DBZ) | 50.4% | 50.1% | |||||
| p-Cresol + 3(4H)-dibenzofuranone, 4a,9b-dihydro-8,9b-dimethyl-(3(4H)-DBZ) | -- | -- | |||||
| Lung Cancer | Exhaled Breath | Clinical Study | Acetaldehyde, Ethanol, Propionaldehyde, Propanol, 2-Hydroxyacetaldehyde, Dimethyl sulfide, Isoprene, Butanal, Benzene, Pentanal, Butyric acid, Toluene, Phenol, Cyclohexanone, Hexanal, Propyl, Styrene, Benzaldehyde, Heptanal;4-hydroxyhexanal, Acetophenone, Propyl cyclohexane, Octanal, Benzothiazole, Nonanal, Decanal, 2,2-Dimethyldecane | HPPI-TOFMS, 18F-FDG PET-CT | 82.1% | 92.3% | [45] |
| Lung Cancer | Exhaled Breath | Clinical Study | Butyraldehyde (C4H8O) | mRMR | SVM 80% | SVM 90% | [46] |
| LR 74% | LR 90% | ||||||
| kNN 70% | kNN 90% | ||||||
| NB 32% | NB 86% | ||||||
| DT 58% | DT 78% | ||||||
| RF 88% | RF 86% | ||||||
| Bagging 82% | Bagging 86% | ||||||
| AdaBoost 74% | AdaBoost 76% | ||||||
| NN 66% | NN 73% | ||||||
| Formaldehyde, Acetaldehyde, Acetone, Isoprenol, Hexanal, 4-Heptanone, Octanal, Hexamethylacetone, Menthol, Undecanal, Dodecyl aldehyde, Tridecanal, Butyric acid, Acetic acid, Cyclopropanone, Ethyl butyrate, Chalcogran, Methylglyoxal, Methyl acrylate, Crotonaldehyde, Methyl propiolate, Cyclopentanone, Benzaldehyde, Cyclohexylmethanone, Geranylacetone, Anthracene-9-carbaldehyde | -- | -- |
| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/ Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| HCC | Exhaled Breath | Clinical Study | Ethanol, Acetone monomer, Dimethyl sulfide, 1,4-pentadiene, Benzene, Isopropyl alcohol, Acetone dimer, Acetonitrile, Toluene | 70.0% | 88.6% | [47] | |
| Acetone dimer | 95.7% | 73.3% | |||||
| HCC | Breath Sample | Clinical Study | Phenol 2,2 methylene bis [6-(1,1-dimethyl ethyl)-4-methyl] (MBMBP) | GC-MS | -- | -- | [48] |
| HCC | Exhaled Breath | Clinical Study | Acetone; 1,4-pentadiene; methylene chloride; benzene; phenol; allyl methyl sulfide | SPME-GC-MS, SVM | 76.5% | 82.7% | [49] |
| Acetic acid; methyl ester; methylene chloride; phenol; benzene; cyclopentane; pentane | 98% | 56% | |||||
| Camphene; cyclopentane; methyl; 2-pentanone; dimethyl sulfde; acetonitrile; cyclopentane; 1,3-dimethyl | 9.35% | 100% | |||||
| HCC HCC vs. Fibrosis | Urine | Clinical Study | 4-methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (2TMS derivative); 2-butanone; 2-hexanone; Benzene, 1-ethyl-2-methyl-; 3-Butene- 1,2-diol, 1-(2-furanyl)-; Bicyclo[4.1.0]heptane, 3,7,7-trimethyl-, [1S-(1a,3ß,6a)]-; Sulpiride | GC-IMS, GC-TOF-MS | 43% | 95% | [50] |
| HCC vs. Non-Fibrosis | 60% | 74% | |||||
| Fibrosis vs. Non-Fibrosis | 29% | 90% |
| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/ Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| CRC | Urine | Clinical Study | Carbon disulphide; Acetone; Ethanol; 2,2,6,6-tetramethyl-4-ethyl-heptane; Dimethyldisulphide; m-xylene; 4-heptanone; Benzenethiol; Pyrrole; 1,6-dichloro-1,5-cyclooctadiene; Biphenyl; Phenol; dibenzofuran | GC-MS | 87.8% | 88.2% | [51] |
| FAIMS | 89.9% | 77.8% | |||||
| SIFT-MS | 77.8% | 78.0% | |||||
| CRC AAs- Negative Control | Exhaled Breath | Clinical Study | Propyl pyruvate; 2-methylfuran; 2,2,4-tetramethylpentane; p-meth-3-ene; 6-methyl heptane; 2,4-dimethyl pyrrole; Lactic acid; 2-propenoic acid ethenyl ester | GC-MS | 79% | 70% | [52] |
| (CRC + AA) − Control | 77% | 70% | |||||
| CRC − Control | 80% | 70% | |||||
| CRC vs. Non-Cancerous (Neural Network) | Urine | Clinical Study | Octanal; Nonanal; Decanal; 2,4-Di-tert-butylphenol; Heptanal; Heptadecane; Undecanal; 3,4-Dimethylcyclohexanol; 5-Hepten-2-ol, 6-methyl-; Hexanal; Acetone; 2-Pentanone; Biphenyl; 2-Heptanone; Cyclopentanone, 2-methyl-; Ethylbenzene; Methane, isocyanato-; Acetophenone; 1-Undecanol; p-Xylene; Benzene; 1-methyl-3-(1-methylethyl)-; Naphthalene; Octane, 2,2,6-trimethyl- | 86% | 81% | ||
| CRC vs. Non-Cancerous (Random Network) | GC-TOF- MS | 89% | 75% | [53] | |||
| CRC vs. Non-Cancerous (Neural Network) | 91% | 55% | |||||
| CRC vs. Non-Cancerous (Random Network) | PEN3 | 82% | 55% |
| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/ Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| Esophageal Cancer | Breath | Clinical Study | Indole; Phenol; 1-Propanol; P-cresol; Dimethyl disulfide | SPME-GC-MS | -- | -- | [54] |
| Gastric Cancer | Exhaled Breath | Clinical Study | Propanal | MS, PTR- TOF-MS | 53.8% | 100.0% | [55] |
| Aceticamide | 61.5% | 88.2% | |||||
| Isoprene | 84.6% | 64.7% | |||||
| 1,3-propanediol | 73.1% | 76.5% | |||||
| Ethylene; Methyl isobutyl ketone; Acetic acid; m-Tolualdehyde; 1,3,5-trimethylbenzene | 61.5% | 94.1% | |||||
| Pancreatic Cancer | Bile | Clinical Study | Bile Samples | FAIMS | 100% | 77.8% | [56] |
| Pancreatic Cancer PDAC vs. Healthy | - | - | 2-pentanone; Nonanal; 4-ethyl-1,2-dimethyl-Benzene; 2,6-dimethyl-octane; Benzene, 1-ethenyl-2-methyl- | GC-IMS, GC-TOF-MS | 72% | 96% | [57] |
| PDAC vs. CP | - | - | 38% | 88% | |||
| CP vs. Healthy | - | - | 38% | 96% |
| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/ Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| Urinary Bladder Cancer | Urine | Clinical Study | Butyrolactone, 2-methoxyphenol, 3-methoxy-5-methylphenol, 1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)-2-buten 1-one, nootkatone and 1-(2,6,6-trimethyl-1-cyclohexenyl)-2-buten-1-one | SPME, GC × GC-TOF-MS | -- | -- | [58] |
| Urinary Bladder Cancer | Urine | Clinical Study | Nonanal; phenol; 5-ethyl-3-methyloxolan-2-one; 2-ethylhexan-1-ol; 1,1,4a-trimethyl4,5,6,7-tetrahydro-3H-naphthalen-2-one; 1-methyl-4-propan-2-ylcyclohexan-1-ol; benzaldehyde; 2,6-dimethyloct-7-en-2-ol | SPME-GC-MS | AUROC (0.77) 71% | AUROC (0.77) 72% | [58] |
| Nonanal; 2-ethylhexan-1-ol; 1,1,4a-trimethyl-4,5,6,7-tetrahydro 3H-naphthalen-2-on; 5-ethyl-3-methyloxolan-2-one; 4-methylpent-3-enoic acid; Heptan-2-one | AUROC (0.80) 71% | AUROC (0.80) 80% | |||||
| Prostate Cancer | Urine | Clinical and Preclinical Studies | p-Menth-1-en-3-one, 2-Ethyl-1-hexanol, Carvone, 2,4-Di-tert-butyl-phenol, 2,5-Dimethylbenzaldehyde, 4-Heptanone | SPME, GC-MS | 75% | 69% | [59] |
| Cancer Type | Medium | Study Type | VOCs Biomarker | Method/Technique | Sensitivity | Specificity | Source |
|---|---|---|---|---|---|---|---|
| Breast Cancer | Urine | Clinical Study | 2-nonanone, 4-methil-2-heptanone, Isobutyric acid allyl ester, 1,3-dis-ter-butylbenzene, Benzaldehyde | GC-MS | 100.00% | 85.71% | [60] |
| e-Nose | 100.00% | 50.00% | |||||
| Breast Cancer (MCF-7 (Luminal-A); MCF-10A; MCF-7; MDA-MD-231) | Urine | Preclinical Study | Styrene; oxime-, methoxy-phenyl; benzaldehyde; phenol; aromatic compound; decane; 1-hexanol, 2-ethyl-; benzyl-alcohol; benzeneacetaldehyde; hydrocarbon; decane, 4-methyl-; hydrocarbon; acetophenone; undecane; hydrocarbon; nonanal; dodecane; decanal; benzaldehyde, 3,4-dimethyl; benzene, 1,3-bis(1,1-dimethylethyl)-; decanol; 2-undecanone. | SPME-GC-MS | -- | -- | [61] |
| Cervical Cancer | Urine | Clinical Study | 4,7,7-Trimethylbicyclo[2.2.1] hepta-2,5-diene; Androst-5-en-3-ol, 4,4-dimethyl-; (3beta)-; Azulene 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethyl)-; Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl); Humulane-1,6-dien-3-ol; Isocyclocitral; Octadecane; Tridecane, 4,8-dimethyl-, between the control group and CC are: 2-Methyl-4-(2,6,6-trimethylcyclohex- 1-enyl)but-2-en-1-ol; 6-Azaestra-1,3,5(10),6,8-pentaen-17-one, 3-methoxy-; Caryophyllene; Cyclopentanol; 3-methyl-2-(2-pentenyl)-, Hexadecane, 1-bromo-; Nonadecane; Thunbergol Neoclovene-(I), dihydro-; (2,6,6-Trimethylcyclohex-1-enyl) acetic acid; 1-Heptatriacotanol; 1-Hydroxy-1,7-dimethyl-4-isopropyl-2,7-cyclodecadiene; 1-Naphthalenepropanol; alphaethenyldecahydro-alpha,5,5,8a-tetramethyl-2-methylene-; 2(1H)-Naphthalenone; octahydro-4a-phenyl-, trans-, 3,5,24-Trimethyltetracontane; Cyclohexanone 3-ethenyl-3-methyl-2-(1-methylethenyl)-6-(1-methylethylidene)-; trans-, Phenol, 4,4′-(1,1-dimethyl-3-methylene-1,3-propanediyl)bis. | GC-MS | 91.6% | 100% | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Karmakar, V.; Nair, A.B.; Jacob, S.; Shinu, P.; Aldhubiab, B.; Almuqbil, R.M.; Gorain, B. Volatile Organic Compounds in Biological Matrices as a Sensitive Weapon in Cancer Diagnosis. Pharmaceuticals 2025, 18, 638. https://doi.org/10.3390/ph18050638
Ghosh A, Karmakar V, Nair AB, Jacob S, Shinu P, Aldhubiab B, Almuqbil RM, Gorain B. Volatile Organic Compounds in Biological Matrices as a Sensitive Weapon in Cancer Diagnosis. Pharmaceuticals. 2025; 18(5):638. https://doi.org/10.3390/ph18050638
Chicago/Turabian StyleGhosh, Arya, Varnita Karmakar, Anroop B. Nair, Shery Jacob, Pottathil Shinu, Bandar Aldhubiab, Rashed M. Almuqbil, and Bapi Gorain. 2025. "Volatile Organic Compounds in Biological Matrices as a Sensitive Weapon in Cancer Diagnosis" Pharmaceuticals 18, no. 5: 638. https://doi.org/10.3390/ph18050638
APA StyleGhosh, A., Karmakar, V., Nair, A. B., Jacob, S., Shinu, P., Aldhubiab, B., Almuqbil, R. M., & Gorain, B. (2025). Volatile Organic Compounds in Biological Matrices as a Sensitive Weapon in Cancer Diagnosis. Pharmaceuticals, 18(5), 638. https://doi.org/10.3390/ph18050638







