Timbe (Acaciella angustissima) as an Alternative Source of Compounds with Biological Activity: Antidiabetic
Abstract
1. Introduction
2. Results
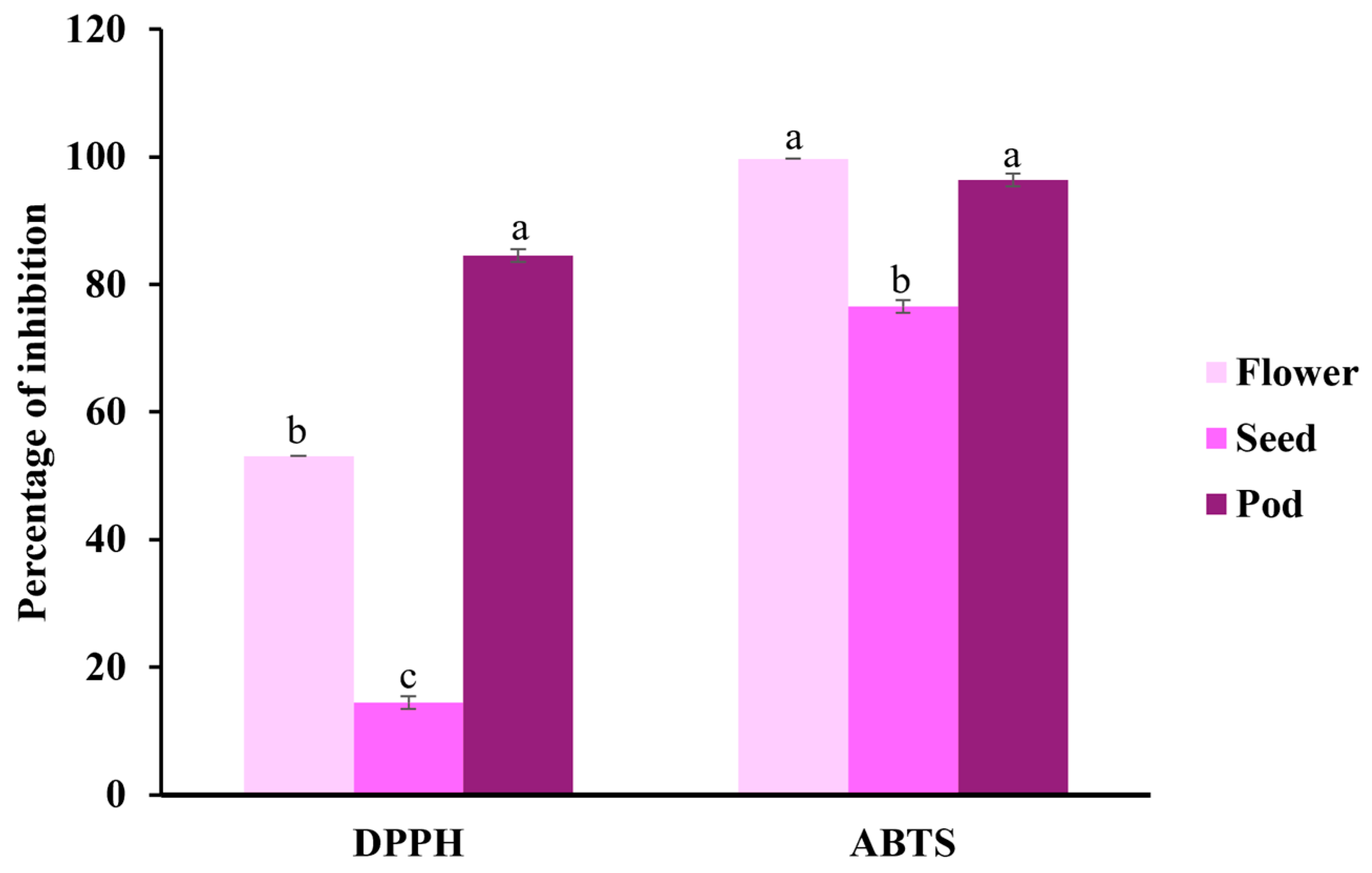
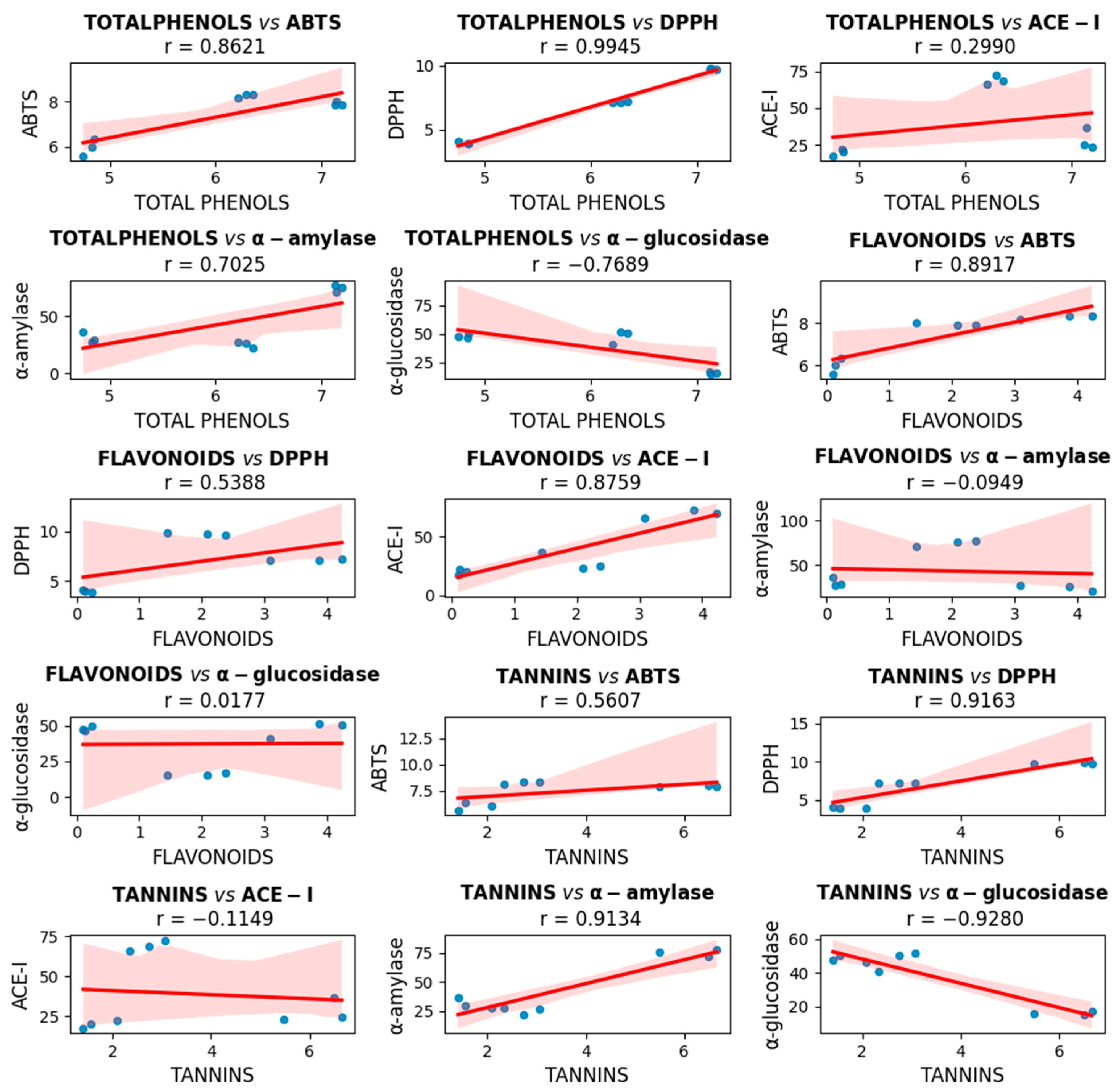
2.1. Phenolic Compounds and Antioxidant Capacity
2.2. Metabolic Composition and Fatty Acid Content
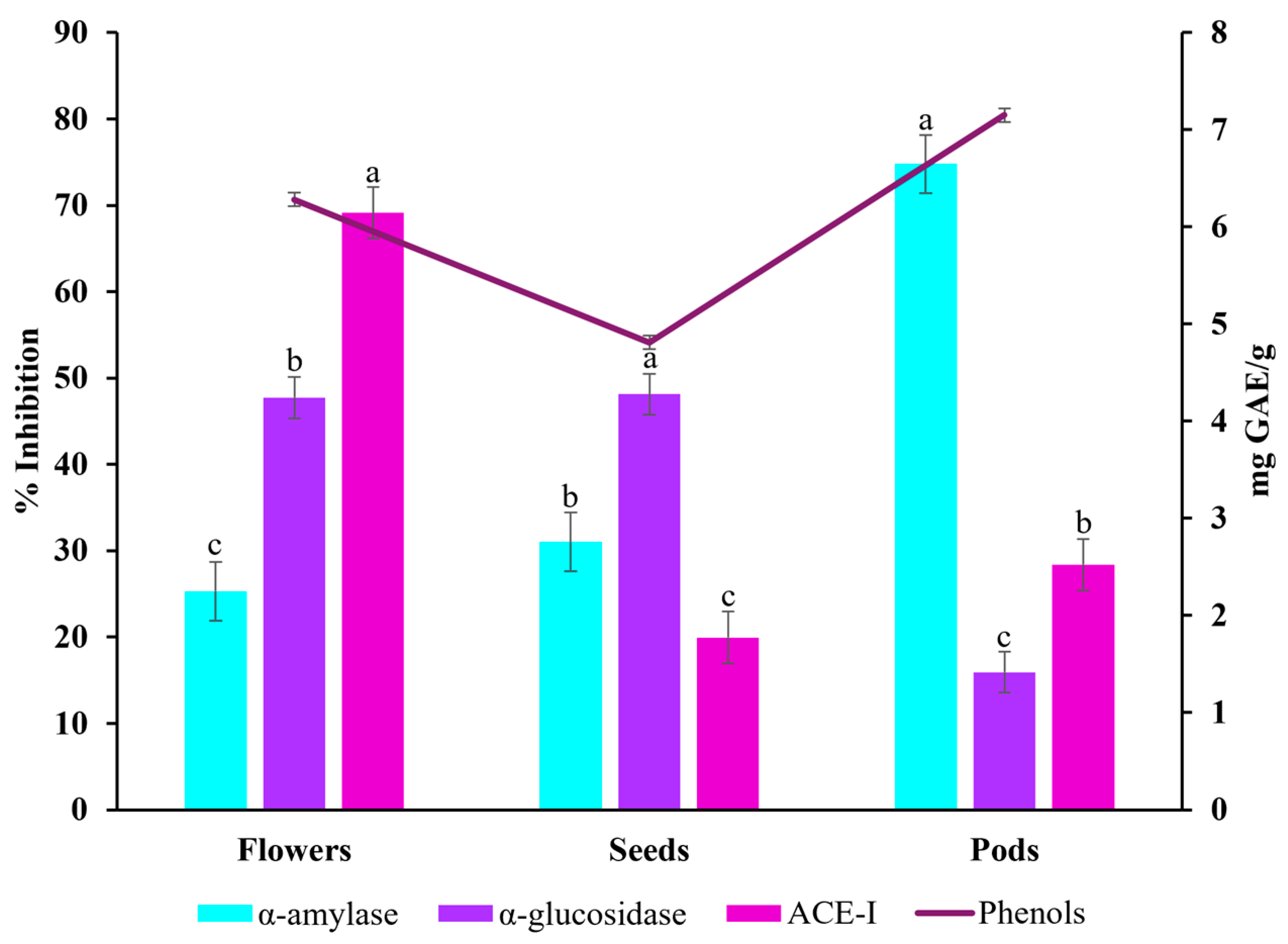
2.3. α-glucosidase, α-amylase, and ACE-I Inhibitory Activities
2.4. Antimicrobial Activity
3. Discussion
3.1. Antioxidant Capacity by Phenolic Compounds
3.2. Metabolic Profile and Fatty Acid Profile
3.3. α-glucosidase, α-amylase and ACE-I Inhibitory Activities
3.4. Antimicrobial Testing
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Content of Total Phenols, Flavonoids, and Condensed Tannins
4.3. Antioxidant Capacity: DPPH Y ABTS
4.3.1. DPPH
4.3.2. ABTS
4.4. Analysis by Gas Chromatography–Mass Spectrometry (GC-MS)
4.4.1. Fatty Acid Profile
4.4.2. Metabolic Profile
4.5. Evaluation of Enzymatic Activity
4.5.1. Extract Preparation
4.5.2. α-amylase Inhibition Assay
4.5.3. α-glucosidase Inhibition Assay
4.5.4. ACE-I Assay
4.6. Assessment Antimicrobial Activity
4.6.1. Extract Preparation
4.6.2. Microorganisms and Growing Conditions
4.6.3. Broth Microdilution Method
4.7. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Traditional, Complementary and Integrative Medicine. Available online: https://www.who.int/ (accessed on 17 January 2025).
- Madariaga-Mazón, A.; Naveja, J.J.; Medina-Franco, J.L.; Noriega-Colima, K.O.; Martinez-Mayorga, K. DiaNat-DB: A Molecular Database of Antidiabetic Compounds from Medicinal Plants. RSC Adv. 2021, 11, 5172–5178. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Ullah, R.; Shahat, A.A. Bioactive Constituents and Toxicological Evaluation of Selected Antidiabetic Medicinal Plants of Saudi Arabia. eCAM 2022, 2022, 7123521. [Google Scholar] [CrossRef]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid. Med. Cell. Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Hussein, M.M.; Ahmed, O.M.; Al-Jameel, S.S.; Al-Muzafar, H.M.; Amin, K.A.; Abdou, H.M. Oleuropein Ameliorates Hyperlipidemia, Oxidative Stress, Inflammatory and Liver Dysfunction Biomarkers, in Streptozotocin-Induced Diabetic Rats. J. Appl. Pharm. Sci. 2024, 14, 227–234. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Abd El-Twab, S.M.; Al-Muzafar, H.M.; Adel Amin, K.; Abdel Aziz, S.M.; Abdel-Gabbar, M. Musa paradisiaca L. Leaf and Fruit Peel Hydroethanolic Extracts Improved the Lipid Profile, Glycemic Index and Oxidative Stress in Nicotinamide/Streptozotocin-Induced Diabetic Rats. Vet. Med. Sci. 2021, 7, 500–511. [Google Scholar] [CrossRef]
- Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15, 65. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, T.; Li, S.; Liu, J.; Li, M.; Lu, J.; Zhang, M.; Chen, H. Updated Progress on Polysaccharides with Anti-Diabetic Effects through the Regulation of Gut Microbiota: Sources, Mechanisms, and Structure–Activity Relationships. Pharmaceuticals 2024, 17, 456. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Cockram, C.S.; Ma, R.C.W.; Luk, A.O.Y. Diabetes and Infection: Review of the Epidemiology, Mechanisms and Principles of Treatment. Diabetologia 2024, 67, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, A.; Cassani, G.; Puggioni, A.; Rossi, A.; Colombo, A.; Onodera, T.; Ferrannini, E. The Diabetes Pandemic and Associated Infections: Suggestions for Clinical Microbiology. Rev. Res. Med. Microbiol. 2019, 30, 1–17. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. [Google Scholar] [CrossRef]
- Singhal, P.; Kaushik, G.; Mathur, P. Antidiabetic Potential of Commonly Consumed Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 655–672. [Google Scholar] [CrossRef]
- Musara, C.; Bosede Aladejana, E. Acaciella angustissima (Mill.) Brit. & Rose: Botanical Features, Distribution, Medicinal and Pharmacological Properties. J. Pharm. Nutr. Sci. 2020, 10, 325–330. [Google Scholar] [CrossRef]
- Alonso-Herrada, J.; Rico-Reséndiz, F.; Campos-Guillén, J.; Guevara-González, R.G.; Torres-Pacheco, I.; Cruz-Hernández, A. Establishment of in Vitro Regeneration System for Acaciella angustissima (Timbe) a Shrubby Plant Endemic of México for the Production of Phenolic Compounds. Ind. Crops Prod. 2016, 86, 49–57. [Google Scholar] [CrossRef]
- Rico Arce, M.d.L.; Bachman, S. A Taxonomic Revision of Acaciella (Leguminosae, Mimosoideae). An. Jardín Botánico Madrid 2006, 63, 189–244. [Google Scholar] [CrossRef]
- Vargas-Hernández, M.; Munguía-Fragozo, P.V.; Cruz-Hernández, A.; Guerrero, B.Z.; Gonzalez-Chavira, M.M.; Feregrino-Pérez, A.A.; Mendoza-Díaz, S.O.; Loarca-Piña, G.; Torres-Pacheco, I.; Hernández-Salazar, M.; et al. Bioactivity and Gene Expression Studies of an Arbustive Mexican Specie Acaciella angustissima (Timbe). Ind. Crops Prod. 2014, 52, 649–655. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, A.J.; Carmen-Sandoval, W.; Lomas-Soria, C.; Guevara-González, R.G.; Reynoso-Camacho, R.; Villagran-Herrera, M.E.; Salazar-Olivo, L.; Torres-Pacheco, I.; Feregrino-Pérez, A.A. Timbe (Acaciella angustissima) Pods Extracts Reduce the Levels of Glucose, Insulin and Improved Physiological Parameters, Hypolipidemic Effect, Oxidative Stress and Renal Damage in Streptozotocin-Induced Diabetic Rats. Molecules 2018, 23, 2812. [Google Scholar] [CrossRef] [PubMed]
- Odenyo, A.A.; Osuji, P.O.; Reed, J.D.; Smith, A.H.; Mackie, R.I.; Mcsweeney, C.S.; Hanson, J. Acacia angustissima: Its Anti-Nutrients Constituents, Toxicity and Possible Mechanisms to Alleviate the Toxicity-a Short Review. Agrofor. Syst. 2003, 5, 141–147. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abolibda, T.Z.; Alruwaili, A.H.; Farag, B.; Boraie, W.E.; Al-Hussain, S.A.; Zaki, M.E.A.; Hussein, A.M. Efficient Green Synthesis of Hydrazide Derivatives Using L-Proline: Structural Characterization, Anticancer Activity, and Molecular Docking Studies. Catalysts 2024, 14, 489. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline Metabolism and Redox; Maintaining a Balance in Health and Disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Lange, K.M.; Nakamura, Y.; Reissmann, A. Nutrition in the Management of ADHD: A Review of Recent Research. Curr. Nutr. Rep. 2023, 12, 383–394. [Google Scholar] [CrossRef]
- Poongothai, G.; Sripathi, S.K. A Review on Insulinomimetic Pinitol from Plants. Int. J. Pharm. Bio. Sci. 2013, 4, 992–1009. [Google Scholar]
- Azab, A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M.; Chandrashekar, N. Effects of D-Pinitol on Diabetes Mellitus: An Updated Review. Bull. Natl. Res. Cent. 2022, 46, 130. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on Its Anti-Tumor Effect and Mechanism of Action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef] [PubMed]
- Ngenge Tamfu, A.; Mfifen Munvera, A.; Veronica Dediu Botezatu, A.; Talla, E.; Ceylan, O.; Tagatsing Fotsing, M.; Tanyi Mbafor, J.; Shaheen, F.; Mihaela Dinica, R. Synthesis of Benzoyl Esters of β-Amyrin and Lupeol and Evaluation of Their Antibiofilm and Antidiabetic Activities. Results Chem. 2022, 4, 100322. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Wang, Y.; Yuan, X.; Liu, L.; Dong, H.; Wang, Q.; Zhang, Z. Antimicrobial and Antitumor Activity of Peptidomimetics Synthesized from Amino Acids. Bioorg. Chem. 2021, 106, 104506. [Google Scholar] [CrossRef] [PubMed]
- Umumararungu, T.; Gahamanyi, N.; Mukiza, J.; Habarurema, G.; Katandula, J.; Rugamba, A.; Kagisha, V. Proline, a Unique Amino Acid Whose Polymer, Polyproline II Helix, and Its Analogues Are Involved in Many Biological Processes: A Review. Amino Acids 2024, 56, 50. [Google Scholar] [CrossRef] [PubMed]
- Hope, H.C.; Salmond, R.J. The Role of Non-Essential Amino Acids in T Cell Function and Anti-Tumour Immunity. Arch. Immunol. Ther. Exp. 2021, 69, 29. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B.; Szablińska-Piernik, J.; Lahuta, L.B.; Gromadziński, L.; Majewski, M.S. Antioxidant and Anticoagulant Properties of Myo-Inositol Determined in an Ex Vivo Studies and Gas Chromatography Analysis. Sci. Rep. 2024, 14, 25633. [Google Scholar] [CrossRef]
- Abbas, A.H.; Mahmood, A.A.R.; Tahtamouni, L.H.; Al-Mazaydeh, Z.A.; Rammaha, M.S.; Alsoubani, F.; Al-Bayati, R.I. A Novel Derivative of Picolinic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Human Non-Small Cell Lung Cancer Cells: Synthesis, Docking Study, and Anticancer Activity. Pharmacia 2021, 68, 679–692. [Google Scholar] [CrossRef]
- Borawska, M.H.; Czechowska, S.K.; Markiewicz, R.; Pałka, J.; Świsłocka, R.; Lewandowski, W. Antimicrobial Activity and Cytotoxicity of Picolinic Acid and Selected Picolinates as New Potential Food Preservatives. Pol. J. Food Nutr. Sci. 2008, 58, 415–418. [Google Scholar]
- Kim, S.; Jang, S.H.; Kim, M.J.; Lee, J.J.; Kim, K.M.; Kim, Y.H.; Lee, J.H.; Jung, S.K. Hybrid Nutraceutical of 2-Ketoglutaric Acid in Improving Inflammatory Bowel Disease: Role of Prebiotics and TAK1 Inhibitor. Biomed. Pharmacother. 2024, 171, 116126. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Bioly. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; van der Kooi, C.J. Coloration of Flowers by Flavonoids and Consequences of PH Dependent Absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; Morales, D.; Miguel-Castro, M. Potential Role of Bioactive Proteins and Peptides Derived from Legumes towards Metabolic Syndrome. Nutrients 2022, 14, 5271. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Gaber, N.B.; El-Dahy, S.I.; Shalaby, E.A. Comparison of ABTS, DPPH, Permanganate, and Methylene Blue Assays for Determining Antioxidant Potential of Successive Extracts from Pomegranate and Guava Residues. Biomass. Convers. Biorefin. 2023, 13, 4011–4020. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by Abts and Dpph Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-Amylase, α-Glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Park, S.; Park, S.-Y. Can Antioxidants Be Effective Therapeutics for Type 2 Diabetes? Yeungnam Univ. J. Med. 2021, 38, 83–94. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef]
- Sekowski, S.; Veiko, A.; Olchowik-Grabarek, E.; Dubis, A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zavodnik, I.B.; Lapshina, E.; Dobrzynska, I.; Abdulladjanova, N.; et al. Hydrolysable Tannins Change Physicochemical Parameters of Lipid Nano-Vesicles and Reduce DPPH Radical—Experimental Studies and Quantum Chemical Analysis. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183778. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First Evidence of C- and O-Glycosyl Flavone in Blood Orange (Citrus sinensis (L.) Osbeck) Juice and Their Influence on Antioxidant Properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimisation of Aqueous Extraction Conditions for the Recovery of Phenolic Compounds and Antioxidants from Lemon Pomace. Int. J. Food Sci. Technol. 2016, 51, 2009–2018. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Yan, Z.; Liu, Z.; Ma, Q.; Zhang, Z.; Wang, Y.; Su, Y. The Relationship between Erythrocytes and Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 6656062. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cao, S.; Duan, Y.; Xu, C.; Gan, D.; He, W. Dietary Antioxidant Indices in Relation to All-Cause and Cause-Specific Mortality Among Adults with Diabetes: A Prospective Cohort Study. Front. Nutr. 2022, 9, 849727. [Google Scholar] [CrossRef]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Oliveira, J.; Pinho, A.; Carvalho, E. The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites 2022, 12, 184. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and Their Effects on Diabetes Management: A Review. Med. J. Islam. Repub. Iran 2017, 31, 886–892. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef]
- Gać, P.; Poręba, M.; Januszewska, L.; Prokopowicz, A.; Martynowicz, H.; Mazur, G.; Poręba, R. The Total Antioxidant Status, Serum Selenium Concentrations and the Ultrasound Assessment Carotid Intima Media Thickness in Patients with Arterial Hypertension. Antioxidants 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Serina, J.J.C.; Castilho, P.C.M.F. Using Polyphenols as a Relevant Therapy to Diabetes and Its Complications, a Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8355–8387. [Google Scholar] [CrossRef]
- Taghi-Gharibzahedi, S.M.; Mousavi, S.M.; Jafari, M.S.; Faraji, K. Proximate Composition, Mineral Content, and Fatty Acids Profile of Two Varieties of Lentil Seeds Cultivated in Iran. Chem. Nat. Compd. 2012, 47, 976–978. [Google Scholar] [CrossRef]
- Lastras, C.; Revilla, I.; González-Martín, M.I.; Vivar-Quintana, A.M. Prediction of Fatty Acid and Mineral Composition of Lentils Using near Infrared Spectroscopy. J. Food Compos. Anal. 2021, 102, 104023. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Mathur, A. Study on Nutritional and Phytochemical Profile of Seven Edible Food Supplements of Uttarakhand (Garhwal himalaya). Vegetos 2021, 34, 678–683. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S. Vegetable Soybean (Glycine max (L.) Merr.) Leaf Extracts: Functional Components and Antioxidant and Anti-Inflammatory Activities. J. Food Sci. 2021, 86, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Razavi, S.H. Therapeutic Effects of Polyphenols in Fermented Soybean and Black Soybean Products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A.; Bobrecka-Jamro, D.; Jańczak-Pieniązėk, M.; Kotecki, A.; Kozak, M. Effect of Nitrogen Fertilisation and Inoculation with Bradyrhizobium Japonicum on the Fatty Acid Profile of Soybean (Glycine max (L.) Merrill) Seeds. Agronomy 2021, 11, 941. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Ferreira Fernández, J.J. Fatty Acids in Dry Beans (Phaseolus vulgaris L.): A Contribution to Their Analysis and the Characterization of a Diversity Panel. Foods 2024, 13, 2023. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-I.I.; Vattem, D.A.; Shetty, K. Evaluation of Clonal Herbs of Lamiaceae Species for Management of Diabetes and Hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Sevilla-Asencio, A.; Dublán-García, O.; Gómez-Oliván, L.M.; López-Martínez, L.X. Actividad α-glucosidasa y α-amilasa de extractos acuosos de algunas especias en la mexicana. Ciencia UAT 2013, 8, 42–47. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Mendoza, S.; Ramos-Gómez, M.; Reynoso, R. Antioxidant, Antimutagenic, and Antidiabetic Activities of Edible Leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef]
- Darwitz, B.P.; Genito, C.J.; Thurlow, L.R. Triple Threat: How Diabetes Results in Worsened Bacterial Infections. Infect. Immun. 2024, 92, e00509-23. [Google Scholar] [CrossRef]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Vargas-Hernández, M.; Munguía-Fragozo, P.V.; Loarca-Piña, G.F.; Mendoza-Díaz, S.O.; Ocampo-Velázquez, R.V.; Rico-García, E.; Guevara-Gónzalez, R.G. Antioxidant and Antimutagenic Activities of Acacia Pennatula Pods. J. Sci. Ind. Res. 2011, 70, 859–864. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Araújo, M.G.D.F.; Hilário, F.; Nogueira, L.G.; Vilegas, W.; Dos Santos, L.C.; Bauab, T.M. Chemical Constituents of the Methanolic Extract of Leaves of Leiothrix Spiralis Ruhland and Their Antimicrobial Activity. Molecules 2011, 16, 10479–10490. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, X.; Zhen, L.; Ares, J.N.; Vackier, T.; Lange, H.; Crestini, C.; Steenackers, H.P. Effect of Chemical Modifications of Tannins on Their Antimicrobial and Antibiofilm Effect against Gram-Negative and Gram-Positive Bacteria. Front. Microbiol. 2023, 13, 987164. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Timmermann, B.N.; Mclaughlin, S.P.; Punnapayak, H. Potential Antimicrobial Activity of Plants from the Southwest-Ern United States. Int. J. Pharmacog 1993, 31, 101–115. [Google Scholar] [CrossRef]
- Martínez Sánchez, A.K. Evaluación de Actividad Antimicrobiana de Extractos Metanólicos de Timbe (Acaciella Mill.), En Ajo Contra Sclerotium Cepivorum Berk. Bachelor’s Thesis, UAQ, Querétaro, México, 2013. [Google Scholar]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant Extracts for Type 2 Diabetes: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum Inhibitory Concentrations of Medicinal Plants Used in Northern Peru as Antibacterial Remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef]
- Nielsen, T.R.H.; Kuete, V.; Jäger, A.K.; Meyer, J.J.M.; Lall, N. Antimicrobial Activity of Selected South African Medicinal Plants. BMC Complement. Altern. Med. 2012, 12, 74. [Google Scholar] [CrossRef]
- Dalmarco, J.; Dalmarco, E.; Koelzer, J.; Pizzolatti, M.; Fröde, T. Isolation and Identification of Bioactive Compounds Responsible for the Anti-Bacterial Efficacy of Lotus Corniculatus Var. So Gabriel. Int. J. Green Pharm. 2010, 4, 108–114. [Google Scholar] [CrossRef]
- Tamokou, J.d.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Wamba, B.E.N.; Mbaveng, A.T.; Kuete, V. Fighting Gram-Positive Bacteria with African Medicinal Plants: Cut-off Values for the Classification of the Activity of Natural Products. Adv. Bot. Res. 2023, 106, 413–522. [Google Scholar] [CrossRef]
- Razafindrakoto, R.; Lovarintsoa, J.R.; Eliane, R.V.; Hanitra, R.R.; Danielle, A.D.R.; Victor, L.J. Antimicrobial Activity of the Extracts of Albizia Masikororum R.Vig., a Fabaceae from Madagascar. Afr. J. Microbiol. Res. 2018, 12, 464–469. [Google Scholar] [CrossRef]
- Mahamat Djamalladine, D.; Mabou, F.D.; Feugap Tsamo, D.L.; Tamokou, J.D.D.; Voutquenne-Nazabadioko, L.; Tsopmo, A.; Ngnokam, D. New Triterpenoid Saponin from the Aerial Part of Abrus canescens Welw Ex. Bak. (Fabaceae) and Their Antibacterial Activities. Nat. Prod. Res. 2025, 39, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Mpude, L.; Yendze, A.W.B.; Assonfack, D.J.; Cadet, E.; Matieta, V.Y.; Megaptche, J.F.; Mbaveng, A.T.; Kuete, V. Antibacterial Activity of Sarcocephalus latifolius and Acacia sieberiana, and the Effect of Their Association with Antibiotics against Multidrug-Resistant Staphylococcus Aureus. Investig. Med. Chem. Pharmacol. 2024, 7, 96. [Google Scholar] [CrossRef]
- Morton, K.E.; Coghill, S.H. Staphylococcus Aureus Is the Predominant Pathogen in Hospitalised Patients with Diabetes-Related Foot Infections: An Australian Perspective. Antibiotics 2024, 13, 594. [Google Scholar] [CrossRef]
- Sonkoue Lambou, J.C.; Noubom, M.; Djoumsie Gomseu, B.E.; Takougoum Marbou, W.J.; Tamokou, J.D.D.; Gatsing, D. Multidrug-Resistant Escherichia Coli Causing Urinary Tract Infections among Controlled and Uncontrolled Type 2 Diabetic Patients at Laquintinie Hospital in Douala, Cameroon. Genet. Res. 2022, 2022, 1250264. [Google Scholar] [CrossRef]
- Thapliyal, S.; Dandriyal, A. A Literature Review on Urinary Tract Infection Due to Diabetes Mellitus and Development of Antimicrobial Resistance. Int. J. Sci. Res. 2023, 12, 2461–2465. [Google Scholar] [CrossRef]
- Arfaoui, A.; Rojo-Bezares, B.; Fethi, M.; López, M.; Toledano, P.; Sayem, N.; Ben Khelifa Melki, S.; Ouzari, H.-I.; Klibi, N.; Sáenz, Y. Molecular Characterization of Pseudomonas aeruginosa from Diabetic Foot Infections in Tunisia. J. Med. Microbiol. 2024, 73, 001851. [Google Scholar] [CrossRef]
- Wang, H.P.; He, Z.G. Treatment with Incomplete Freund’s Adjuvant and Listeria Monocytogenes Delays Diabetes via an Interleukin-17-Secretion-Independent Pathway. Exp. Ther. Med. 2015, 9, 1934–1938. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Castaño-Tostado, E.; Loarca-Piña, G. Antimutagenic Activity of Natural Phenolic Compounds Present in the Common Bean (Phaseolus vulgaris) against Aflatoxin B1. Food Addit. Contam. 2002, 19, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and Antioxidative Activities in Common Beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M. Determination of Phenolic Compounds of Dry Beans Using Vanillin, Redox and Precipitation Assays. J. Food Sci. 1987, 52, 332–334. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Velázquez, H.D.J.; Aparicio-Fernández, X.; Escobar-Ortiz, A.; Feregrino-Pérez, A.A.; Reynoso-Camacho, R.; Pérez-Ramírez, I.F. Phytochemical Fingerprint of Chia Sprouts Grown Under Chemical Elicitation with Salicylic Acid and Hydrogen Peroxide. Plant Foods Hum. Nutr. 2024, 79, 127–136. [Google Scholar] [CrossRef]
- Rico-Chávez, A.K.; Pérez-Ramírez, I.F.; Escobar-Ortíz, A.; Feregrino-Pérez, A.A.; Torres-Pacheco, I.; Guevara-González, R.G. Hormetic Elicitation of Phthalides in Celery Seeds (Apium graveolens L. Var Dulce) and Its Effect on Seedling Development. Ind. Crops Prod. 2023, 202, 117022. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; de la Torre-Rodríguez, Y.C.; Alicia Alanís-Garza, B.; Alejandro Pérez-López, L.; Waksman-de-Torres, N. Evaluación de La Actividad Biológica de Productos Herbolarios Comerciales. Med. Univ. 2009, 11, 156–164. [Google Scholar]
- Aguirre-Becerra, H.; Pineda-Nieto, S.A.; García-Trejo, J.F.; Guevara-González, R.G.; Feregrino-Pérez, A.A.; Álvarez-Mayorga, B.L.; Rivera Pastrana, D.M. Jacaranda Flower (Jacaranda mimosifolia) as an Alternative for Antioxidant and Antimicrobial Use. Heliyon 2020, 6, e05802. [Google Scholar] [CrossRef]
- Mccue, P.; Kwon, Y.-I.; Shetty, K. All Rights Reserved. 278 Blackwell Science, LtdOxford. J. Food Biochem. 2005, 29, 278–294. [Google Scholar] [CrossRef]
- Worthington Biochemical Corp (a). Alpha amylase. In Worthington Enzyme Manual; Worthington, V., Ed.; Worthington Biochemical Corp.: Lakewood, NJ, USA, 1993; pp. 36–41. [Google Scholar]
- Abdulmunem, O.; Makky, E.A.; Tawfiq, A.A.; Mahendran, K.; Zamri, N. Evaluating the Effect of Combined Plant Extracts on α-Amylase and α-Glucosidase Inhibition Activity as Antidiabetic Agents. Pakistan J. Biol. Sci. 2020, 23, 287–294. [Google Scholar] [CrossRef]
- Worthington Biochemical Corp (b). Maltase-α-glucosidase. In Worthington Enzyme Manual; Worthington, V., Ed.; Worthington Biochemical Corp.: Lakewood, NJ, USA, 1993; p. 261. [Google Scholar]
- Hou, W.C.; Lee, M.H.; Hsu, F.L.; Lin, Y.H. Inhibitory Activities of Semicarbazide-Sensitive Amine Oxidase and Angiotensin Converting Enzyme of Pectin Hydroxamic Acid. J. Agric. Food Chem. 2003, 51, 6362–6366. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of Solvent Evaporation Method on Phenolic Compounds and the Antioxidant Activity of Moringa Oleifera Cultivated in Southern Tunisia. S. Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M100: Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI: Malvern, PA, USA, 2023. [Google Scholar]
- Swebocki, T.; Barras, A.; Maria Kocot, A. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays Using Broth Microdilution Method. JUN 2023, 23. [Google Scholar]
- Vanegas, D.; Novillo, A.A.; Khachatryan, A.; Jerves Andrade, L.; Peñaherrera, E.; Cuzco, N.; Wilches, I.; Calle, J.; León-Tamariz, F. Validation of a Method of Broth Microdilution for the Determination of Antibacterial Activity of Essential Oils. BMC Res. Notes 2021, 14, 439. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In Vitro Evaluation of Antimicrobial Property of Silver Nanoparticles and Chlorhexidine against Five Different Oral Pathogenic Bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
| Analysis | Flowers | Seeds | Pods |
|---|---|---|---|
| Total Phenols (mg GAE/g) | 6.281 ± 0.07 b | 4.810 ± 0.05 c | 7.151 ± 0.04 a |
| Flavonoids (mg RE/g) | 4.052 ± 0.26 a | 0.121 ± 0.02 c | 2.235 ± 0.20 b |
| Condensed Tannins (mg CE/g) | 2.714 ± 0.36 b | 1.677 ± 0.36 b | 6.213 ± 0.64 a |
| DPPH (mg Trolox/g) | 7.160 ± 0.02 b | 3.979 ± 0.09 c | 9.745 ± 0.07 a |
| ABTS (mg Trolox/g) | 8.261 ± 0.08 a | 5.989 ± 0.37 b | 7.931 ± 0.08 a |
| Sample | Retention Time (min) | Name | Area (%) | Associated Biological Activity |
|---|---|---|---|---|
| Flowers | 7.200 | L-Proline | 2.724 | Precursor to the synthesis of compounds with antioxidant and antidiabetic potential [24,25]. |
| 12.491 | L-Threonic acid | 1.607 | In the form of magnesium salt, it could have beneficial neurofunctional effects in the treatment of Attention-deficit hyperactivity disorder [26]. | |
| 17.489 | D-Pinitol | 5.528 | It has been found in other fabaceous, such as soybeans and tamarind, and is credited with antidiabetic, anti-inflammatory, antioxidant, and immunosuppressive properties [27,28,29]. | |
| 39.682 | Stigmasterol | 6.905 | It has anticancer, anti-inflammatory, antioxidant, neuroprotective, antidiabetic, and antiparasitic effects, as well as anti-osteoarthritis, immunomodulatory, antifungal, and antibacterial properties [30,31,32]. | |
| 40.441 | β-Amyrin | 11.324 | It has antioxidant potential, anti-inflammatory effects, antimicrobial activity, and antidiabetic properties [33,34]. | |
| Seeds | Amino acid | Amino acids can act as precursors in the synthesis of antibacterial and anticancer agents, while non-essential amino acids can modulate and enhance antitumor function [35,36,37]. | ||
| 5.759 | L-Valine | 0.694 | ||
| 6.741 | L-Leucine | 0.126 | ||
| 7.202 | L-Proline | 0.187 | ||
| 8.456 | Serine | 0.107 | ||
| 8.987 | L-Threonine | 0.115 | ||
| 9.595 | L-Aspartic acid | 0.216 | ||
| 19.652 | D-pinitol | 0.502 | It is the same compound as in flowers. | |
| 21.697 | Myo-Inositol | 1.289 | It shows protective effects against oxidative damage in proteins and lipids, improving vascular function and blood coagulation [38]. | |
| 39.678 | Stigmasterol | 0.247 | It is the same compound as in flowers. | |
| Pods | 11.515 | Picolinic acid | 2.565 | Picolinic acid derivatives have demonstrated antitumor, antiangiogenic, and antimicrobial effects [39,40]. |
| D-pinitol | 8.083 | It is the same compound as in flowers. | ||
| 19.367 | 2-Ketoglutaric acid | 1.953 | It acts as a prebiotic that reduces inflammation, improves the function of the intestinal barrier, and restores the balance of the intestinal microbiota [41]. | |
| 39.678 | Stigmasterol | 13.063 | It is the same compound as in flowers. | |
| 40.438 | β-Amyrin | 3.282 | It is the same compound as in flowers. |
| Sample | Retention Time (min) | Name | Area (%) | Concentration (µg/g Sample) |
|---|---|---|---|---|
| Flowers | 14.011 | Hexadecanoic acid | 58.54 | 334.329 |
| 16.711 | Stearic acid | 13.69 | 125.154 | |
| 20.451 | Linolenic acid | 25.99 | 215.076 | |
| 22.739 | Popenoic acid | 1.78 | NQ | |
| Seeds | 14.017 | Palmitic acid | 23.07 | 843.249 |
| 16.676 | Stearic acid | 4.02 | 237.454 | |
| 17.408 | Oleic acid | 17.10 | 2424.105 | |
| 18.700 | Linoleic acid | 55.31 | 3404.042 | |
| 20.490 | Eicosanoic acid | 0.48 | 50.888 | |
| Pods | 8.858 | Ethanedioic acid | 0.76 | NQ |
| 9.439 | Propanedioic acid | 0.32 | NQ | |
| 10.194 | Butanedioic acid | 0.54 | NQ | |
| 12.130 | Tetradecanoate acid | 1.67 | ND | |
| 13.997 | Palmitic acid | 37.87 | 498.944 | |
| 14.436 | Butanoic acid | 0.64 | NQ | |
| 15.712 | Nonanedioic acid | 0.60 | ND | |
| 16.640 | Stearic acid | 12.05 | 260.789 | |
| 17.313 | 6-Octadecenoic acid | 20.17 | 1033.730 | |
| 18.667 | Linoleic acid | 23.06 | 511.773 | |
| 20.532 | 1,3,14,16-Nonadecatetraene | 2.29 | 42.122 |
| % Inhibition | |||
|---|---|---|---|
| Assay | Flowers | Seeds | Pods |
| α-amylase | 25.31 ± 3 b | 31.01 ± 4 b | 74.77 ± 3 a |
| α-glucosidase | 47.72 ± 6 a | 48.12 ± 2 a | 15.92 ± 1 b |
| ACE-I | 69.14 ± 3 a | 19.95 ± 2 b | 28.39 ± 7 b |
| Bacteria | Minimum Inhibitory Concentration (mg/mL) | |||
|---|---|---|---|---|
| Gentamicin | Flowers | Seeds | Pods | |
| S. typhimurium | 0.039 | 5 | >20 | 5 |
| E. coli | 20 | >20 | >20 | 1.25 |
| P. aeruginosa | 0.039 | 5 | 20 | 5 |
| S. aureus | 0.039 | 2.5 | 1.25 | 0.625 |
| K. pneumoniae | 0.039 | 10 | >20 | 0.625 |
| L. monocytogenes | 0.039 | 5 | >20 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel-Sandoval, D.K.; Guerrero-Becerra, L.; Lomas-Soria, C.; Rico-Chávez, A.K.; Cervantes-Chávez, J.A.; Reyes-Castro, L.A.; Morales-Miranda, A.; Feregrino-Pérez, A.A. Timbe (Acaciella angustissima) as an Alternative Source of Compounds with Biological Activity: Antidiabetic. Pharmaceuticals 2025, 18, 593. https://doi.org/10.3390/ph18040593
Rangel-Sandoval DK, Guerrero-Becerra L, Lomas-Soria C, Rico-Chávez AK, Cervantes-Chávez JA, Reyes-Castro LA, Morales-Miranda A, Feregrino-Pérez AA. Timbe (Acaciella angustissima) as an Alternative Source of Compounds with Biological Activity: Antidiabetic. Pharmaceuticals. 2025; 18(4):593. https://doi.org/10.3390/ph18040593
Chicago/Turabian StyleRangel-Sandoval, Diana Karina, Lucia Guerrero-Becerra, Consuelo Lomas-Soria, Amanda Kim Rico-Chávez, José Antonio Cervantes-Chávez, Luis Antonio Reyes-Castro, Angélica Morales-Miranda, and Ana Angélica Feregrino-Pérez. 2025. "Timbe (Acaciella angustissima) as an Alternative Source of Compounds with Biological Activity: Antidiabetic" Pharmaceuticals 18, no. 4: 593. https://doi.org/10.3390/ph18040593
APA StyleRangel-Sandoval, D. K., Guerrero-Becerra, L., Lomas-Soria, C., Rico-Chávez, A. K., Cervantes-Chávez, J. A., Reyes-Castro, L. A., Morales-Miranda, A., & Feregrino-Pérez, A. A. (2025). Timbe (Acaciella angustissima) as an Alternative Source of Compounds with Biological Activity: Antidiabetic. Pharmaceuticals, 18(4), 593. https://doi.org/10.3390/ph18040593






