Unlocking the Antidiabetic Potential of CBD: In Vivo Preclinical Studies
Abstract
1. Introduction
2. Results
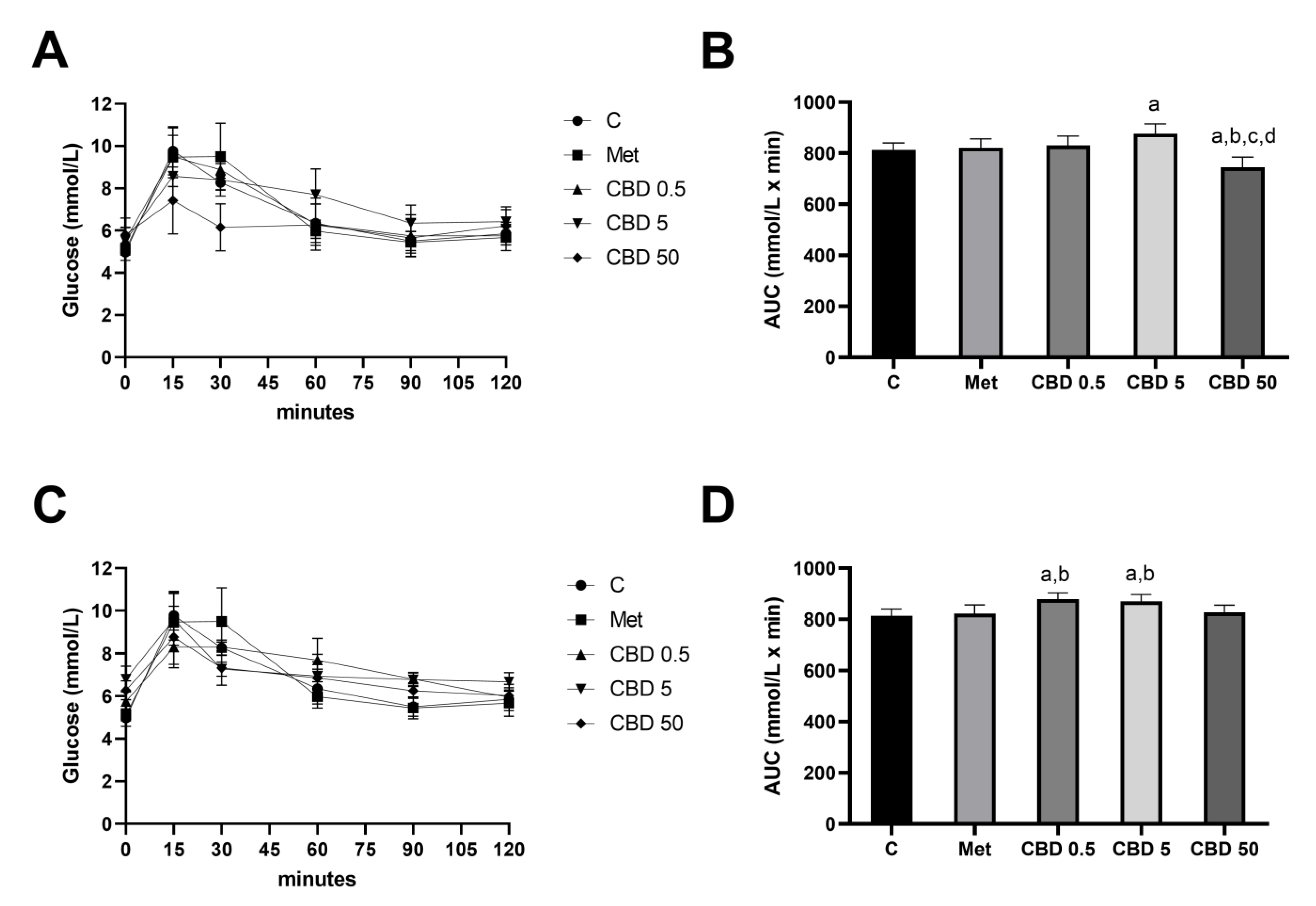
2.1. Oral Glucose Tolerance Test (OGTT)—Intragastric vs. Intraperitoneal Application of CBD
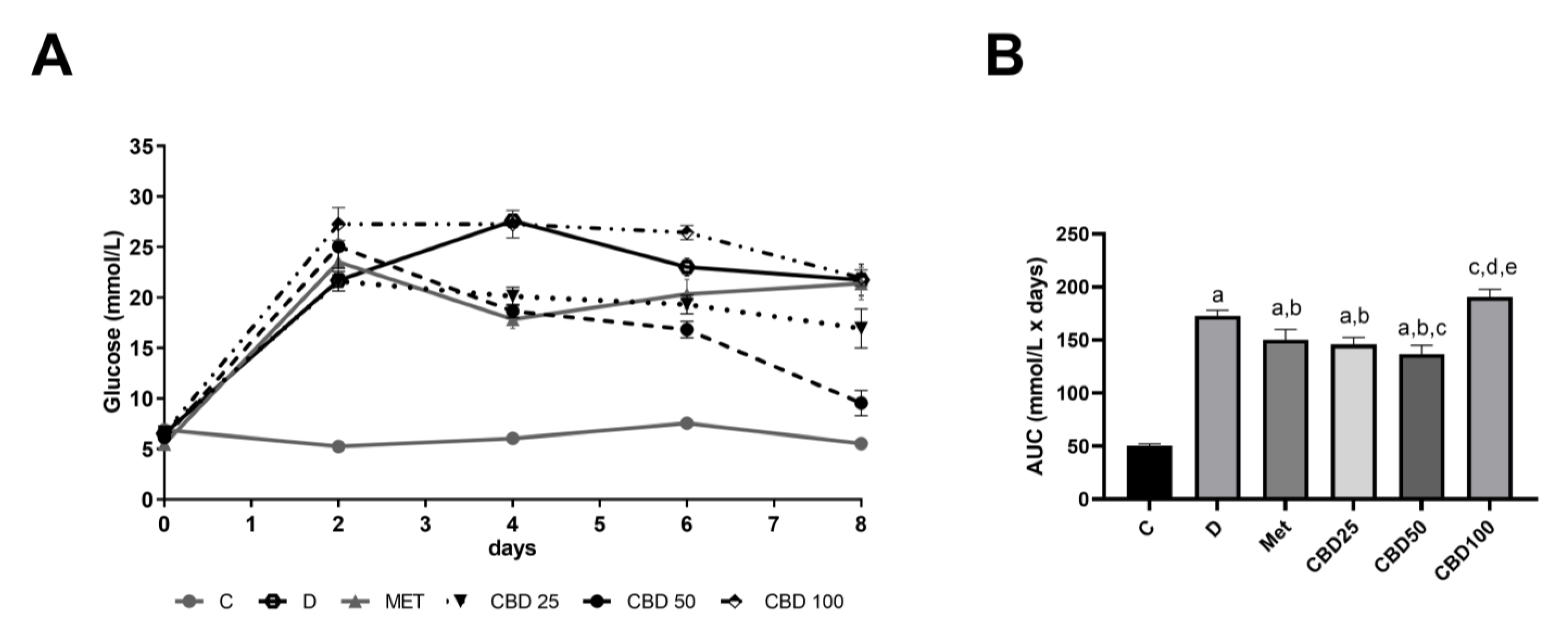
2.2. Daily Glucose Regulation During Multiple-Dose Study
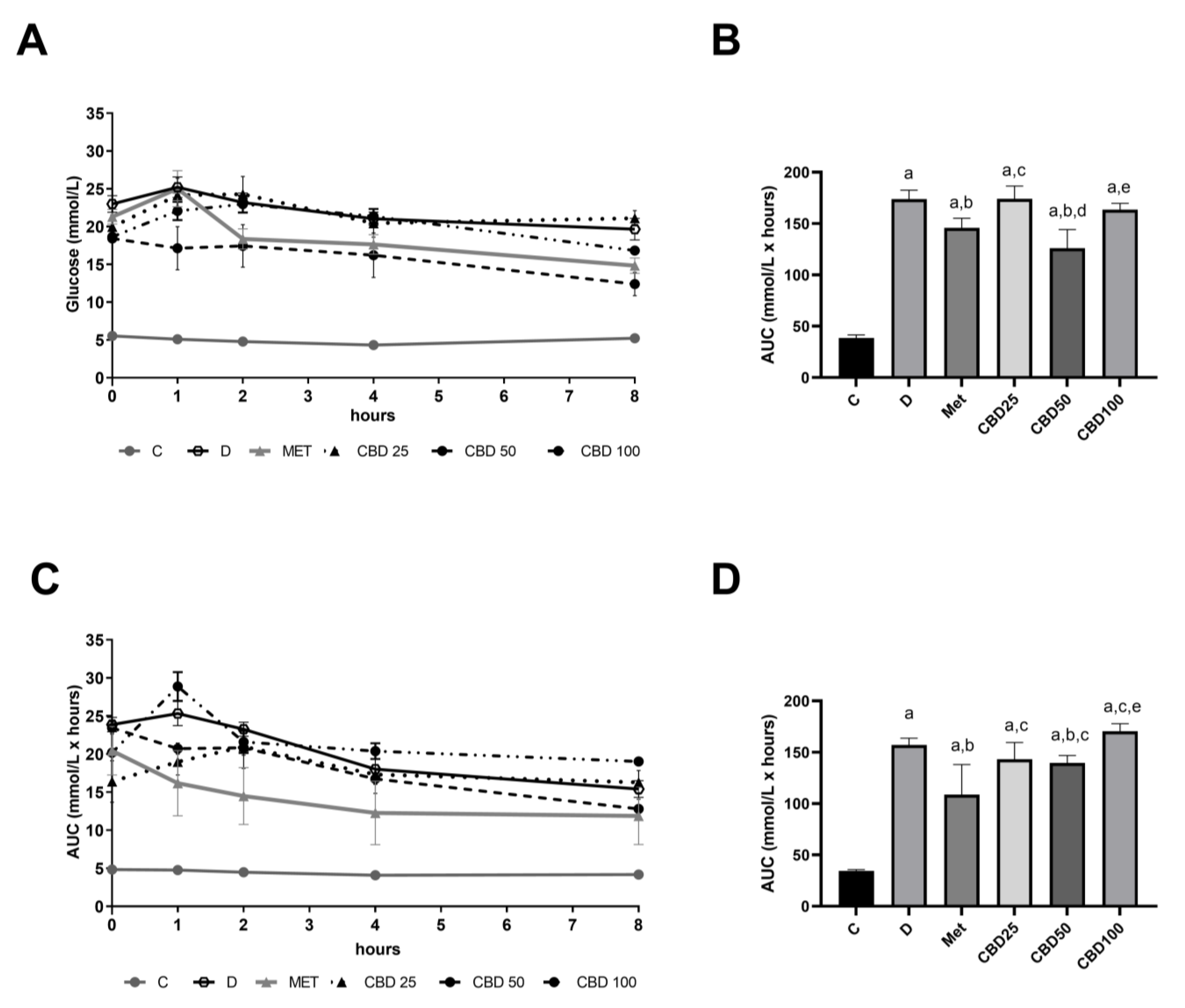
2.3. Hourly Profile of Blood Glucose Levels During Multiple-Dose Study on Days 2 and 6
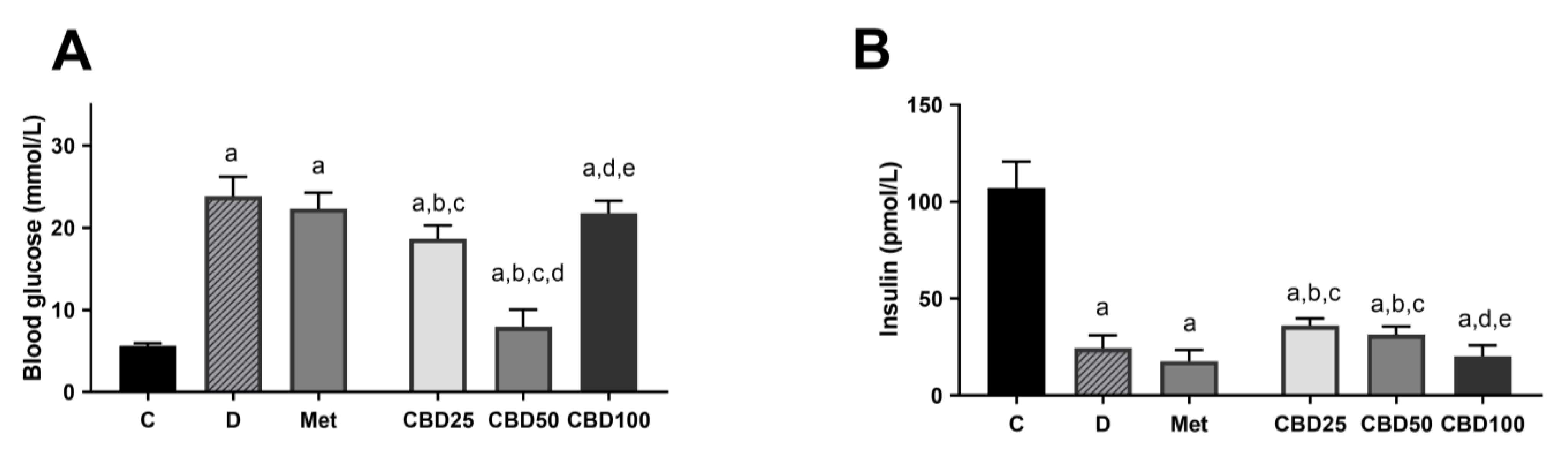
2.4. Blood Glucose and Insulin Concentration
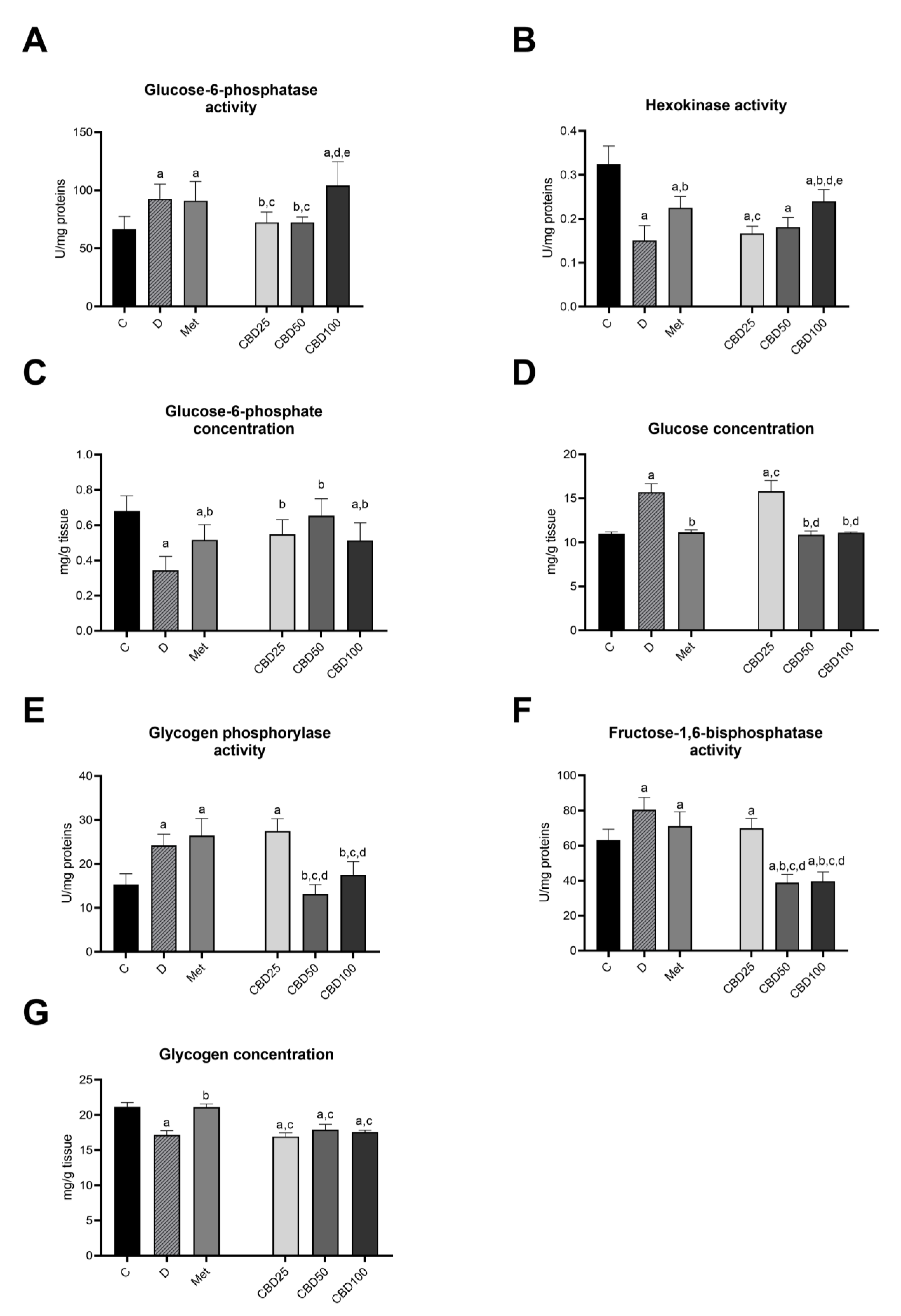
2.5. Carbohydrate Metabolism in the Liver
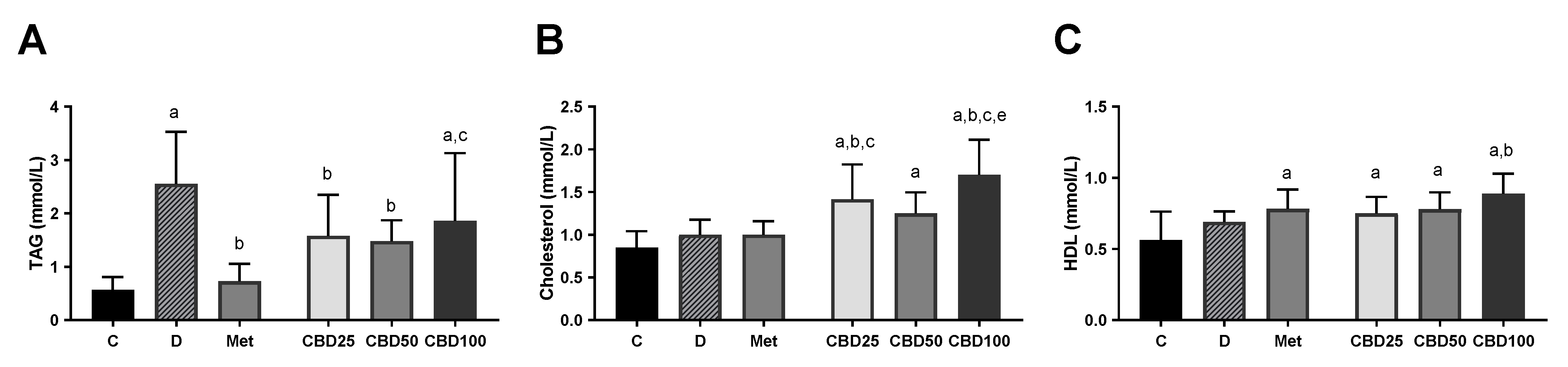
2.6. Blood Lipid Parameters
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Procedures
4.2. Treatment of Animals
4.3. Oral Glucose Tolerance Test (OGTT)
4.4. Multiple-Dose Study in Streptozotocin-Induced Diabetic Rats
4.5. Biochemical Analysis
4.6. Enzymes Activities and Substrates Concentration
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid Pharmacology: The First 66 Years. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alatriste, C.A.; Alarcón-Aguilar, F.J. Cannabis and Cannabinoids as an Alternative Remedy in Metabolic Syndrome. Braz. J. Pharm. Sci. 2022, 58, e20161. [Google Scholar] [CrossRef]
- Devsi, A.; Kiyota, B.; Ouellette, T.; Hegle, A.P.; Rivera-Acevedo, R.E.; Wong, J.; Dong, Y.; Pugsley, M.K.; Fung, T. A Pharmacological Characterization of Cannabis sativa Chemovar Extracts. J. Cannabis Res. 2020, 2, 17. [Google Scholar] [CrossRef]
- Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Gryczka, K.; Kurant, D.; Szambelan, M.; Malinowski, B.; Falkowski, M.; Zabrzyński, J.; Słupski, M. The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing? J. Clin. Med. 2023, 12, 4620. [Google Scholar] [CrossRef]
- Bilbao, A.; Spanagel, R. Medical Cannabinoids: A Pharmacology-Based Systematic Review and Meta-Analysis for All Relevant Medical Indications. BMC Med. 2022, 20, 259. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Zhang, J.S.; Lin, C.; Jiang, S.; Wang, H.; Wang, Y.; Du, X.; Hutchinson, M.R.; Zhao, H.; Fang, L.; Wang, X. The Pharmacology and Therapeutic Role of Cannabidiol in Diabetes. Exploration 2023, 3, 20230047. [Google Scholar] [CrossRef]
- CBD & Diabetes | American Diabetes Association. Available online: https://diabetes.org/health-wellness/cbd-and-diabetes (accessed on 6 April 2024).
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef]
- Afshar, S.; Khalili, S.; Amin, G.; Abbasinazari, M. A Phase I Randomized, Double-Blind, Placebo-Controlled Study on Efficacy and Safety Profile of a Sublingually Administered Cannabidiol /Delta 9-Tetrahydrocannabidiol (10: 1) Regimen in Diabetes Type 2 Patients. Iran. J. Pharm. Res. 2023, 21, e132647. [Google Scholar] [CrossRef]
- Kaufmann, R.; Aqua, K.; Lombardo, J.; Lee, M. Observed Impact of Long-Term Consumption of Oral Cannabidiol on Liver Function in Healthy Adults. Cannabis Cannabinoid Res. 2023, 8, 148–154. [Google Scholar] [CrossRef]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two Non-Psychoactive Cannabinoids Reduce Intracellular Lipid Levels and Inhibit Hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Chaves, Y.C.; Genaro, K.; Crippa, J.A.; da Cunha, J.M.; Zanoveli, J.M. Cannabidiol Induces Antidepressant and Anxiolytic-like Effects in Experimental Type-1 Diabetic Animals by Multiple Sites of Action. Metab. Brain Dis. 2021, 36, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, H.; Liu, C.; Seeram, N.P. Evaluation of Cannabidiol’s Inhibitory Effect on Alpha-Glucosidase and Its Stability in Simulated Gastric and Intestinal Fluids. J. Cannabis Res. 2021, 3, 20. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol Lowers Incidence of Diabetes in Non-Obese Diabetic Mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef]
- Molehin, O.R.; Fakayode, A.E.; Olaoye, A.B.; Teibo, J.O.; Adeola, O.A. Biochemical Pathways Involved in Diabetes Mellitus. In Biochemical Immunology of Diabetes and Associated Complications; Tripathi, P., Tripathi, R.P., Kaushik, M.P., Eds.; Academic Press: New York, NY, USA, 2024. [Google Scholar]
- Raddatz, D.; Ramadori, G. Carbohydrate Metabolism and the Liver: Actual Aspects from Physiology and Disease. Z. Gastroenterol. 2007, 45, 51–62. [Google Scholar] [CrossRef]
- Cherkasova, V.; Ilnytskyy, Y.; Kovalchuk, O.; Kovalchuk, I. Transcriptome Analysis of Cisplatin, Cannabidiol, and Intermittent Serum Starvation Alone and in Various Combinations on Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 14743. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Kajero, J.A.; Seedat, S.; Ohaeri, J.; Akindele, A.; Aina, O. Investigation of the Effects of Cannabidiol on Vacuous Chewing Movements, Locomotion, Oxidative Stress and Blood Glucose in Rats Treated with Oral Haloperidol. World J. Biol. Psychiatry 2020, 21, 612–626. [Google Scholar] [CrossRef]
- Kajero, J.A.; Seedat, S.; Ohaeri, J.U.; Akindele, A.J.; Aina, O. Effects of Cannabidiol on Vacuous Chewing Movements, Plasma Glucose and Oxidative Stress Indices in Rats Administered High Dose Risperidone. Sci. Rep. 2022, 12, 19718. [Google Scholar] [CrossRef]
- de Morais, H.; Chaves, Y.C.; Waltrick, A.P.F.; Jesus, C.H.A.; Genaro, K.; Crippa, J.A.; da Cunha, J.M.; Zanoveli, J.M. Sub-Chronic Treatment with Cannabidiol but Not with URB597 Induced a Mild Antidepressant-like Effect in Diabetic Rats. Neurosci. Lett. 2018, 682, 62–68. [Google Scholar] [CrossRef]
- Ten Ham, M.; De Yong, Y. Effects of Δ9 Tetrahydrocannabinol and Cannabidiol on Blood Glucose Concentrations in Rabbits and Rats. Pharm. Weekbl. 1975, 110, 1157–1161. [Google Scholar]
- Reyes-Cuapio, E.; Coronado-Álvarez, A.; Quiroga, C.; Alcaraz-Silva, J.; Ruíz-Ruíz, J.C.; Imperatori, C.; Murillo-Rodríguez, E. Juvenile Cannabidiol Chronic Treatments Produce Robust Changes in Metabolic Markers in Adult Male Wistar Rats. Eur. J. Pharmacol. 2021, 910, 174463. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.N.; Mori, M.A.; Guimarães, F.S.; Milani, H.; Weffort, M. Effects of Cannabidiol on Diabetes Outcomes and Chronic Cerebral Hypoperfusion Comorbidities in Middle-Aged Rats. Neurotox. Res. 2019, 35, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Zorzenon, M.R.T.; Santiago, A.N.; Mori, M.A.; Piovan, S.; Jansen, C.A.; Perina Padilha, M.E.; Ciotta, S.R.; Cezar de Freitas Mathias, P.; Guimarães, F.S.; Weffort de Oliveira, R.M.; et al. Cannabidiol Improves Metabolic Dysfunction in Middle-Aged Diabetic Rats Submitted to a Chronic Cerebral Hypoperfusion. Chem. Biol. Interact. 2019, 312, 108819. [Google Scholar] [CrossRef]
- Chaves, Y.C.; Genaro, K.; Stern, C.A.; de Oliveira Guaita, G.; de Souza Crippa, J.A.; da Cunha, J.M.; Zanoveli, J.M. Two-Weeks Treatment with Cannabidiol Improves Biophysical and Behavioral Deficits Associated with Experimental Type-1 Diabetes. Neurosci. Lett. 2020, 729, 135020. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and Drug Metabolizing Enzymes: Potential for Drug-Drug Interactions and Implications for Drug Safety and Efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor Oxygenated Cannabinoids from High Potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef]
- Rock, E.M.; Parker, L.A. Constituents of Cannabis sativa. In Cannabinoids and Neuropsychiatric Disorders; Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Jones, P.G.; Falvello, L.; Kennard, O.; Sheldrick, G.M.; Mechoulam, R. Cannabidiol. Acta Crystallogr. Sect. B 1977, 33, 3211–3214. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Sztolsztener, K.; Harasim-Symbor, E.; Berk, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. Cannabidiol—A Phytocannabinoid That Widely Affects Sphingolipid Metabolism under Conditions of Brain Insulin Resistance. Biomed. Pharmacother. 2021, 142, 112057. [Google Scholar] [CrossRef] [PubMed]
- Berk, K.; Konstantynowicz-Nowicka, K.; Charytoniuk, T.; Harasim-Symbor, E.; Chabowski, A. Distinct Effects of Cannabidiol on Sphingolipid Metabolism in Subcutaneous and Visceral Adipose Tissues Derived from High-Fat-Diet-Fed Male Wistar Rats. Int. J. Mol. Sci. 2022, 23, 5382. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol Presents an Inverted U-Shaped Dose-Response Curve in a Simulated Public Speaking Test. Braz. J. Psychiatry 2019, 41, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (CBD), Cannabidivarine (CBDV), Δ9-Tetrahydrocannabivarin (THCV) and Cannabigerol (CBG) in Rats and Mice Following Oral and Intraperitoneal Administration and CBD Action on Obsessive–Compulsive Behaviour. Psychopharmacology 2011, 219, 859–873. [Google Scholar]
- Cinar, R.; Iyer, M.R.; Kunos, G. The Therapeutic Potential of Second and Third Generation CB1R Antagonists. Pharmacol. Ther. 2020, 208, 107477. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-EnrichedCannabisextracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Colsoul, B.; Nilius, B.; Vennekens, R. Transient Receptor Potential (TRP) Cation Channels in Diabetes. Curr. Top. Med. Chem. 2013, 13, 258–269. [Google Scholar] [CrossRef]
- Szallasi, A.; Di Marzo, V. New Perspectives on Enigmatic Vanilloid Receptors. Trends Neurosci. 2000, 23, 491–497. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and Molecular Mechanisms of Metformin: An Overview. Clin. Sci. 2011, 122, 253–270. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Li, Y.R.; Danelisen, I. Molecular Mechanisms of Action of Metformin: Latest Advances and Therapeutic Implications. Clin. Exp. Med. 2023, 23, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Ehud, Z.; Lola, W.; Itamar, R.; Natan, P.; Zhanna, Y.; Ruth, G. Islet Protection and Amelioration of Diabetes Type 2 in Psammomys Obesus by Treatment with Cannabidiol. J. Diabetes Mellit. 2012, 2, 27–34. [Google Scholar] [CrossRef][Green Version]
- McGrath, S.; Bartner, L.R.; Rao, S.; Packer, R.A.; Gustafson, D.L. Randomized Blinded Controlled Clinical Trial to Assess the Effect of Oral Cannabidiol Administration in Addition to Conventional Antiepileptic Treatment on Seizure Frequency in Dogs with Intractable Idiopathic Epilepsy. J. Am. Vet. Med. Assoc. 2019, 254, 1301–1308. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.; Wang, K.; Qian, Y.; Cai, L. Diabetic-Induced Alterations in Hepatic Glucose and Lipid Metabolism: The Role of Type 1 and Type 2 Diabetes Mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Agius, L. Glucokinase and Molecular Aspects of Liver Glycogen Metabolism. Biochem. J. 2008, 414, 1–18. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting Hepatic Glucose Output in the Treatment of Type 2 Diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.J.; Rines, A.K.; Puigserver, P. Molecular Pathophysiology of Hepatic Glucose Production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef]
- Halse, R.; Bonavaud, S.M.; Armstrong, J.L.; McCormack, J.G.; Yeaman, S.J. Control of Glycogen Synthesis by Glucose, Glycogen, and Insulin in Cultured Human Muscle Cells. Diabetes 2001, 50, 720–726. [Google Scholar] [CrossRef]
- Agius, L. Role of Glycogen Phosphorylase in Liver Glycogen Metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef]
- Rajas, F.; Gautier-Stein, A.; Mithieux, G. Glucose-6 Phosphate, a Central Hub for Liver Carbohydrate Metabolism. Metabolites 2019, 9, 282. [Google Scholar] [CrossRef]
- Wajngot, A.; Chandramouli, V.; Schumann, W.C.; Ekberg, K.; Jones, P.K.; Efendic, S.; Landau, B.R. Quantitative Contributions of Gluconeogenesis to Glucose Production during Fasting in Type 2 Diabetes Mellitus. Metabolism 2001, 50, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Bernroider, E. Hepatic Glucose Metabolism in Humans—Its Role in Health and Disease. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Olofinsan, K.A.; Oyedemi, S.O.; Chukwuma, C.I.; Nde, A.L.; Islam, M.S. Cannabidiol Improves Glucose Utilization and Modulates Glucose-Induced Dysmetabolic Activities in Isolated Rats’ Peripheral Adipose Tissues. Biomed. Pharmacother. 2022, 149, 112863. [Google Scholar] [CrossRef]
- Sargsyan, A.; Herman, M.A. Regulation of Glucose Production in the Pathogenesis of Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Hoek, J.B. Hexokinase II: The Integration of Energy Metabolism and Control of Apoptosis. Curr. Med. Chem. 2003, 10, 1535–1551. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Kostrzewa, M.K.; Marolda, V.; Cerasuolo, M.; Maccarinelli, F.; Coltrini, D.; Rezzola, S.; Giacomini, A.; Mollica, M.P.; Motta, A.; et al. Cannabidiol Alters Mitochondrial Bioenergetics via VDAC1 and Triggers Cell Death in Hormone-Refractory Prostate Cancer. Pharmacol. Res. 2023, 189, 106683. [Google Scholar] [CrossRef]
- Minassian, C.; Tarpin, S.; Mithieux, G. Role of Glucose-6 Phosphatase, Glucokinase, and Glucose-6 Phosphate in Liver Insulin Resistance and Its Correction by Metformin. Biochem. Pharmacol. 1998, 55, 1213–1219. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Hexokinase-2-Linked Glycolytic Overload and Unscheduled Glycolysis—Driver of Insulin Resistance and Development of Vascular Complications of Diabetes. Int. J. Mol. Sci. 2022, 23, 2165. [Google Scholar] [CrossRef]
- Villar-Palasí, C.; Guinovart, J.J. The Role of Glucose 6-Phosphate in the Control of Glycogen Synthase. FASEB J. 1997, 11, 544–558. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evid.-Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Su, F.; Wang, G.; Peng, Z.; Xu, Y.; Zhang, Y.; Xu, N.; Hou, K.; Hu, Z.; Chen, Y.; et al. Glucose-Lowering Effect of Berberine on Type 2 Diabetes: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 1015045. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Wheal, A.J.; Randall, M.D.; O’Sullivan, S.E. Cannabinoids Alter Endothelial Function in the Zucker Rat Model of Type 2 Diabetes. Eur. J. Pharmacol. 2013, 720, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F.; Müller-Vahl, K. Cannabis Und Cannabinoide in Der Medizin: Fakten Und Ausblick. Suchttherapie 2016, 17, 71–76. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; Honkakoski, P.; Hukkanen, J.; Raunio, H. Inhibition and Induction of Human Cytochrome P450 Enzymes: Current Status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Guan, J.; Zhou, L.; Chen, B. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules 2023, 13, 250. [Google Scholar] [CrossRef]
- Nakakaawa, L.; Gbala, I.D.; Bargul, J.L.; Cheseto, X.; Wesonga, J.M. Therapeutic Potential of Brassica Carinata Microgreens Extract in Alleviating Symptoms of Type 2 Diabetes in Wistar Rats. Food Sci. Nutr. 2025, 13, e4635. [Google Scholar] [CrossRef]
- Igbayilola, Y.D.; Gujja, M.G. Alpha-Amylase and Alpha-Glucosidase Upregulated Glucose Homeostasis in High-Fat Fed Wistar Rats Supplemented with Cocoa Flavonoid-Rich Aqueous. Food Biosci. 2024, 59, 104070. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Decker, K. Glycogen Determination with Amyloglucosidase. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 1127–1131. [Google Scholar]
- Hers, H.G. Glycogen Storage Disease. In Advances in Metabolic Disorders; Levine, R., Luft, R., Eds.; Academic Press: New York, NY, USA, 1959; pp. 36–85. [Google Scholar]
- Freedland, R.A.; Harper, A.E. Metabolic Adaptations in Higher Animals. The Study of Metabolic Pathways by Means of Metabolic Adaptations. J. Biol. Chem. 1959, 234, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, W.; Wulf, H.D.; Hue, L.; Hers, H.-G. The Sequential Inactivation of Glycogen Phosphorylase and Activation of Glycogen Synthetase in Liver after the Administration of Glucose to Mice and Rats. Eur. J. Biochem. 1974, 41, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, F.; Hue, L.; Hers, H.-G. Phosphorylation of Glucose in Isolated Rat Hepatocytes. Sigmoidal Kinetics Explained by the Activity of Glucokinase Alone. Biochem. J. 1978, 174, 603–611. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The Colorimetric Determination of Phosphorus. J. Biol. Chem. 1978, 66, 357–400. [Google Scholar] [CrossRef]
| Code | Treatment | Dose |
|---|---|---|
| C | Water | 1 mL/100 g body weight |
| Met | Metformin | 70 mg/kg body weight |
| CBD 0.5 i.g. | CBD oil extract | 0.5 mg/kg |
| CBD 5 i.g. | CBD oil extract | 5 mg/kg |
| CBD 50 i.g. | CBD oil extract | 50 mg/kg |
| CBD 0.5 i.p. | CBD oil extract | 0.5 mg/kg |
| CBD 5 i.p. | CBD oil extract | 5 mg/kg |
| CBD 50 i.p. | CBD oil extract | 50 mg/kg |
| Code | Type of Rats | Treatment | Dose |
|---|---|---|---|
| C | Normal control | Water | 1 mL/100 g body weight |
| D | Diabetic control | Olive oil | 1 mL/100 g body weight |
| Met | Diabetic rats | Metformin | 70 mg/kg body weight |
| CBD25 | Diabetic rats | CBD oil extract | 25 mg/kg body weight |
| CBD50 | Diabetic rats | CBD oil extract | 50 mg/kg body weight |
| CBD100 | Diabetic rats | CBD oil extract | 100 mg/kg body weight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafailovska, E.; Xhemaili, E.; Naumovska, Z.; Gigopulu, O.; Miova, B.; Suturkova, L.; Stefkov, G. Unlocking the Antidiabetic Potential of CBD: In Vivo Preclinical Studies. Pharmaceuticals 2025, 18, 446. https://doi.org/10.3390/ph18040446
Rafailovska E, Xhemaili E, Naumovska Z, Gigopulu O, Miova B, Suturkova L, Stefkov G. Unlocking the Antidiabetic Potential of CBD: In Vivo Preclinical Studies. Pharmaceuticals. 2025; 18(4):446. https://doi.org/10.3390/ph18040446
Chicago/Turabian StyleRafailovska, Elena, Elona Xhemaili, Zorica Naumovska, Olga Gigopulu, Biljana Miova, Ljubica Suturkova, and Gjoshe Stefkov. 2025. "Unlocking the Antidiabetic Potential of CBD: In Vivo Preclinical Studies" Pharmaceuticals 18, no. 4: 446. https://doi.org/10.3390/ph18040446
APA StyleRafailovska, E., Xhemaili, E., Naumovska, Z., Gigopulu, O., Miova, B., Suturkova, L., & Stefkov, G. (2025). Unlocking the Antidiabetic Potential of CBD: In Vivo Preclinical Studies. Pharmaceuticals, 18(4), 446. https://doi.org/10.3390/ph18040446






