Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment
Abstract
1. Introduction
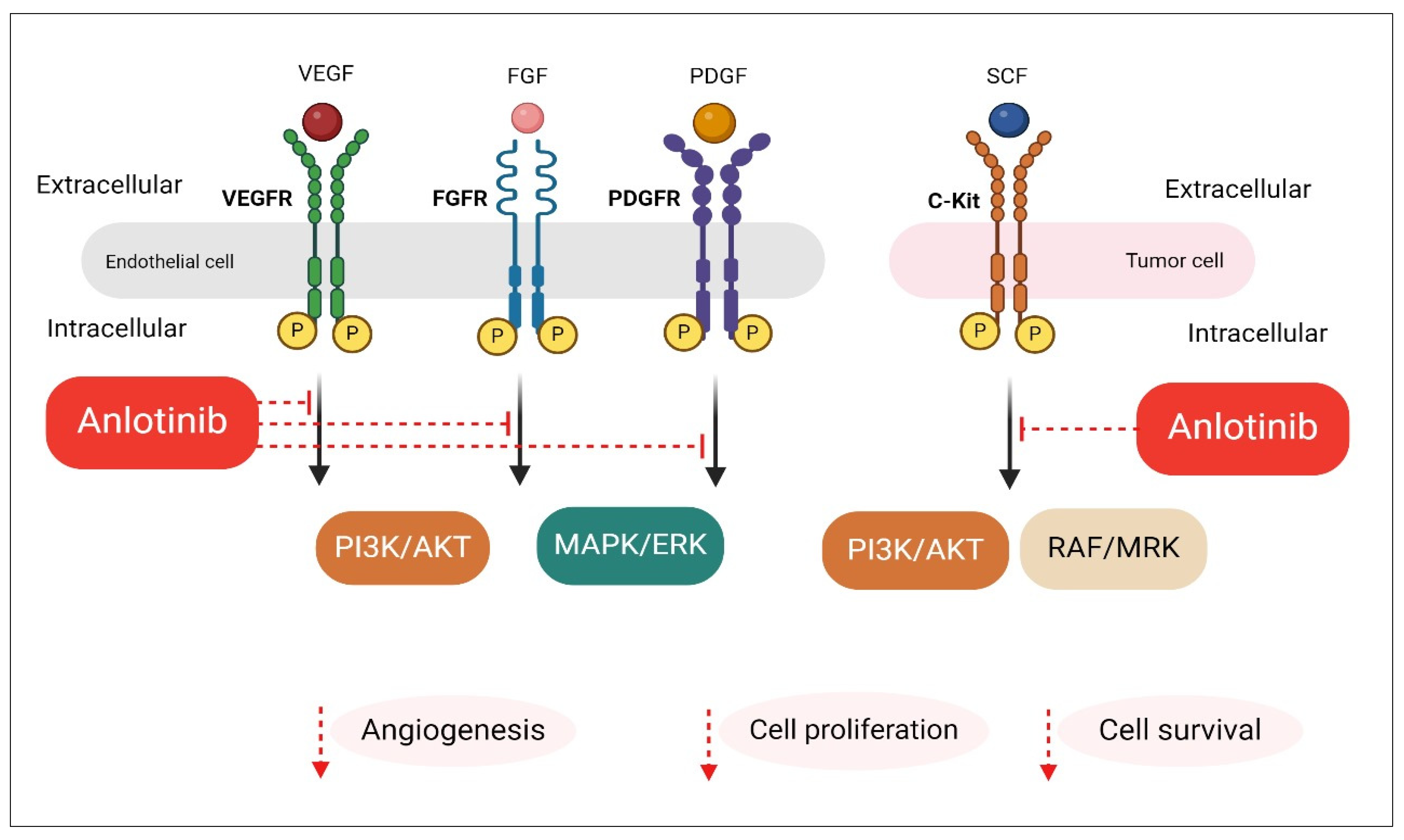
2. Anlotinib Mechanism of Action in Advanced NSCLC
3. Anlotinib Monotherapy: Clinical Insights and Therapeutic Efficacy
4. Clinical Evidence and Trials of Anlotinib-Based Combination Regimens
5. Anlotinib and Chemotherapy Combinations for NSCLC
6. Anlotinib and Immunotherapy Combinations for NSCLC
7. Addressing Challenges That Hinder the Use of Anlotinib
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Scagliotti, G.; Tsao, M.S.; Yatabe, Y.; Travis, W.D. 2021 WHO Classification of Lung Cancer: A Globally Applicable and Molecular Biomarker-Relevant Classification. J. Thorac. Oncol. 2022, 17, e80–e83. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Li, Y.; He, Z.-C.; Zhang, X.-N.; Liu, Q.; Chen, C.; Zhu, Z.; Chen, Q.; Shi, Y.; Yao, X.-H.; Cui, Y.-H. Stanniocalcin-1 augments stem-like traits of glioblastoma cells through binding and activating NOTCH1. Cancer Lett. 2018, 416, 66–74. [Google Scholar]
- Islami, F.; Torre, L.A.; Jemal, A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015, 4, 327. [Google Scholar]
- Shao, J.; Xue, J.; Dai, Y.; Liu, H.; Chen, N.; Jia, L.; Huang, J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur. J. Cancer 2012, 48, 2086–2096. [Google Scholar]
- Funai, K.; Yokose, T.; Ishii, G.; Araki, K.; Yoshida, J.; Nishimura, M.; Nagai, K.; Nishiwaki, Y.; Ochiai, A. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am. J. Surg. Pathol. 2003, 27, 978–984. [Google Scholar] [CrossRef]
- Clinical Lung Cancer Genome Project (CLCGP) and Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci. Transl. Med. 2013, 5, 209ra153. [Google Scholar] [CrossRef]
- Fischer, B.M.; Mortensen, J.; Hansen, H.; Vilmann, P.; Larsen, S.S.; Loft, A.; Bertelsen, A.K.; Ravn, J.; Clementsen, P.; Høegholm, A.; et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: A randomised trial. Thorax 2011, 66, 294–300. [Google Scholar] [CrossRef]
- Saettele, T.M.; Ost, D.E. Multimodality systematic approach to mediastinal lymph node staging in non-small cell lung cancer. Respirology 2014, 19, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Darling, G.E.; Maziak, D.E.; Inculet, R.I.; Gulenchyn, K.Y.; Driedger, A.A.; Ung, Y.C.; Gu, C.S.; Kuruvilla, M.S.; Cline, K.J.; Julian, J.A.; et al. Positron emission tomography-computed tomography compared with invasive mediastinal staging in non-small cell lung cancer: Results of mediastinal staging in the early lung positron emission tomography trial. J. Thorac. Oncol. 2011, 6, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Rekhtman, N.; Riley, G.J.; Geisinger, K.R.; Asamura, H.; Brambilla, E.; Garg, K.; Hirsch, F.R.; Noguchi, M.; Powell, C.A.; et al. Pathologic diagnosis of advanced lung cancer based on small biopsies and cytology: A paradigm shift. J. Thorac. Oncol. 2010, 5, 411–414. [Google Scholar] [CrossRef]
- Yang, X.; Ye, X.; Zheng, A.; Huang, G.; Ni, X.; Wang, J.; Han, X.; Li, W.; Wei, Z. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: Clinical evaluation of 47 cases. J. Surg. Oncol. 2014, 110, 758–763. [Google Scholar]
- De Baere, T.; Tselikas, L.; Catena, V.; Buy, X.; Deschamps, F.; Palussière, J. Percutaneous thermal ablation of primary lung cancer. Diagn. Interv. Imaging 2016, 97, 1019–1024. [Google Scholar]
- Healey, T.T.; March, B.T.; Baird, G.; Dupuy, D.E. Microwave ablation for lung neoplasms: A retrospective analysis of long-term results. J. Vasc. Interv. Radiol. 2017, 28, 206–211. [Google Scholar]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015, 5, 2892. [Google Scholar]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R. Erlotinib in previously treated non–small-cell lung cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar]
- Fukuoka, M.; Wu, Y.-L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.-S.; Sriuranpong, V.; Chao, T.-Y.; Nakagawa, K.; Chu, D.-T.; Saijo, N. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [PubMed]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar]
- Zhou, M.; Chen, X.; Zhang, H.; Xia, L.; Tong, X.; Zou, L.; Hao, R.; Pan, J.; Zhao, X.; Chen, D.; et al. China National Medical Products Administration approval summary: Anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun 2019, 39, 36. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Zhao, Y.; et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients with Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1569–1575. [Google Scholar] [CrossRef]
- Xie, C.; Wan, X.; Quan, H.; Zheng, M.; Fu, L.; Li, Y.; Lou, L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018, 109, 1207–1219. [Google Scholar] [CrossRef]
- Taurin, S.; Yang, C.-H.; Reyes, M.; Cho, S.; Jarboe, E.; Werner, T.; Coombs, D.; Chen, P.; Janát-Amsbury, M. Abstract 3244: Treatment of endometrial cancer cells with a new small tyrosine kinase inhibitor targeting mutated fibroblast growth factor receptor-2. Cancer Res. 2017, 77, 3244. [Google Scholar] [CrossRef]
- Taurin, S.; Yang, C.H.; Reyes, M.; Cho, S.; Coombs, D.M.; Jarboe, E.A.; Werner, T.L.; Peterson, C.M.; Janát-Amsbury, M.M. Endometrial Cancers Harboring Mutated Fibroblast Growth Factor Receptor 2 Protein Are Successfully Treated With a New Small Tyrosine Kinase Inhibitor in an Orthotopic Mouse Model. Int. J. Gynecol. Cancer 2018, 28, 152–160. [Google Scholar] [CrossRef]
- Lin, B.; Song, X.; Yang, D.; Bai, D.; Yao, Y.; Lu, N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018, 654, 77–86. [Google Scholar] [PubMed]
- Han, B.; Li, K.; Wang, Q.; Zhao, Y.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; et al. Third-line treatment: A randomized, double-blind, placebo-controlled phase III ALTER-0303 study—Efficacy and safety of anlotinib treatment in patients with refractory advanced NSCLC. J. Clin. Oncol. 2017, 35, 9053. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Wang, Q.; Zhao, Y.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Yuankai, S. Efficiency of anlotinib as 3rd line treatment in patients with different EGFR gene status, an exploratory subgroup analysis of ALTER0303 trial. J. Thorac. Oncol. 2017, 12, S2275. [Google Scholar]
- Li, K.; Han, B.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Chen, W. OS outcomes to anlotinib in patients (pts) with refractory NSCLC of both wild-type (WT) and mutant EGFR. J. Clin. Oncol. 2018, 36, e21013. [Google Scholar]
- Cheng, Y.; Han, B.; Li, K.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; He, J.; Shi, Y.; Chen, W.; et al. Effect of anlotinib as a third- or further-line therapy in advanced non-small cell lung cancer patients with different histologic types: Subgroup analysis in the ALTER0303 trial. Cancer Med. 2020, 9, 2621–2630. [Google Scholar] [CrossRef]
- Shi, J.; Han, B.; Li, K.; Wang, Q.; Zhang, L.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Chen, W. Subgroup analysis of elderly patients (pts) in ALTER0303: Anlotinib hydrochloride as 3rd-line and further line treatment in refractory advanced NSCLC pts from a randomized, double-blind, placebo-controlled phase III ALTER0303 trial. J. Clin. Oncol. 2018, 36, e21181. [Google Scholar]
- Wang, Q.; Han, B.; Li, K.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Chen, W. Efficiency of anlotinib hydrochloride as 3rd line treatment in patients (pts) from a randomized, double-blind, placebo-controlled phase III trial, an exploratory subgroup analysis of ALTER0303 trial for the previous therapy strategy effect. J. Clin. Oncol. 2018, 36, e21182. [Google Scholar]
- Tan, Y.; Xie, Y.; Qiao, X.; Bai, S. The China Food and Drug Administration (CFDA). In Approaching China’s Pharmaceutical Market; Springer: Cham, Switzerland, 2015; pp. 239–284. [Google Scholar] [CrossRef]
- Li, J. Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Primary Lung Cancer; People’s Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for Advanced Non-Small Cell Lung Cancer: A Decade of Progress. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2021; Volume 41, pp. 1–23. [Google Scholar] [CrossRef]
- Oronsky, B.; Abrouk, N.; Caroen, S.; Lybeck, M.; Guo, X.; Wang, X.; Yu, Z.; Reid, T. A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). J. Cancer 2022, 13, 2945–2953. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Früh, M.; Gubens, M.A.; et al. Management of Stage III Non-Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 40, 1356–1384. [Google Scholar] [CrossRef]
- Carril-Ajuria, L.; Desnoyer, A.; Meylan, M.; Dalban, C.; Naigeon, M.; Cassard, L.; Vano, Y.; Rioux-Leclercq, N.; Chouaib, S.; Beuselinck, B.; et al. Baseline circulating unswitched memory B cells and B-cell related soluble factors are associated with overall survival in patients with clear cell renal cell carcinoma treated with nivolumab within the NIVOREN GETUG-AFU 26 study. J. Immunother. Cancer 2022, 10, e004885. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Cohen, V.; Kasymjanova, G.; Small, D.; Novac, K.; Peterson, J.; Levit, A.; Agulnik, J. An exploratory comparative analysis of tyrosine kinase inhibitors or docetaxel in second-line treatment of EGFR wild-type non-small-cell lung cancer: A retrospective real-world practice review at a single tertiary care centre. Curr. Oncol. 2015, 22, e157–e163. [Google Scholar] [CrossRef] [PubMed]
- Caponi, S.; Vasile, E.; Ginocchi, L.; Tibaldi, C.; Borghi, F.; D’Incecco, A.; Lucchesi, M.; Caparello, C.; Andreuccetti, M.; Falcone, A. Second-line treatment for non-small-cell lung cancer: One size does not fit all. Clin. Lung Cancer 2010, 11, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Auliac, J.B.; Chouaid, C.; Greillier, L.; Monnet, I.; Le Caer, H.; Falchero, L.; Corre, R.; Descourt, R.; Bota, S.; Berard, H.; et al. Randomized open-label non-comparative multicenter phase II trial of sequential erlotinib and docetaxel versus docetaxel alone in patients with non-small-cell lung cancer after failure of first-line chemotherapy: GFPC 10.02 study. Lung Cancer 2014, 85, 415–419. [Google Scholar] [CrossRef]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Q.; Wang, Y.; Wang, Y.; Zheng, M.; Ding, X.; Miao, L. Efficacy and Safety of Anlotinib-Containing Regimens in Advanced Non-Small Cell Lung Cancer: A Real-World Study. Int. J. Gen. Med. 2023, 16, 4165–4179. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar]
- Ts, M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar]
- Hsu, W.-H.; Yang, J.-H.; Mok, T.; Loong, H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018, 29, i3–i9. [Google Scholar]
- Rocco, D.; Della Gravara, L.; Palazzolo, G.; Gridelli, C. The role of antiangiogenic monoclonal antibodies combined to EGFR-TKIs in the treatment of advanced non-small cell lung cancer with activating EGFR mutations: Acquired resistance mechanisms and strategies to overcome them. Cancer Drug Resist. 2022, 5, 1016. [Google Scholar]
- Choi, S.H.; Yoo, S.S.; Lee, S.Y.; Park, J.Y. Anti-angiogenesis revisited: Reshaping the treatment landscape of advanced non-small cell lung cancer. Arch. Pharmacal Res. 2022, 45, 263–279. [Google Scholar]
- Grande, E.; Díez, J.J.; Zafon, C.; Capdevila, J. Thyroid cancer: Molecular aspects and new therapeutic strategies. J. Thyroid. Res. 2012, 2012, 847108. [Google Scholar] [PubMed]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar]
- Tian, S.; Quan, H.; Xie, C.; Guo, H.; Lü, F.; Xu, Y.; Li, J.; Lou, L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011, 102, 1374–1380. [Google Scholar]
- Chu, T.; Zhang, W.; Zhang, B.; Zhong, R.; Zhang, X.; Gu, A.; Shi, C.; Wang, H.; Xiong, L.; Lu, J. Efficacy and safety of first-line anlotinib-based combinations for advanced non-small cell lung cancer: A three-armed prospective study. Transl. Lung Cancer Res. 2022, 11, 1394–1404. [Google Scholar] [CrossRef]
- Chu, T.; Zhong, R.; Zhong, H.; Zhang, B.; Zhang, W.; Shi, C.; Qian, J.; Zhang, Y.; Chang, Q.; Zhang, X. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J. Thorac. Oncol. 2021, 16, 643–652. [Google Scholar]
- Zhou, H.-Q.; Zhang, Y.-X.; Chen, G.; Yu, Q.-T.; Zhang, H.; Wu, G.-W.; Wu, D.; Lin, Y.-C.; Zhu, J.-F.; Chen, J.-H. Gefitinib (an EGFR tyrosine kinase inhibitor) plus anlotinib (an multikinase inhibitor) for untreated, EGFR-mutated, advanced non-small cell lung cancer (FL-ALTER): A multicenter phase III trial. Signal Transduct. Target. Ther. 2024, 9, 215. [Google Scholar]
- Han, B.; Chu, T.; Zhang, X.; Zhong, H.; Zhang, B.; Wang, H.; Gu, A.; Zhang, W.; Shi, C.; Zhong, R. p1. 01-95 efficacy and safety of anlotinib in combination with chemotherapy as first-line therapy in advanced non-small cell lung cancer (NSCLC) patients. J. Thorac. Oncol. 2019, 14, S398. [Google Scholar]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016, 316, 401–409. [Google Scholar]
- Soon, Y.Y.; Tham, I.W.K.; Lim, K.H.; Koh, W.Y.; Lu, J.J. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst. Rev. 2014, 3, CD009454. [Google Scholar] [CrossRef]
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [PubMed]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388, 2004–2014. [Google Scholar] [PubMed]
- Levy, C.; Allouache, D.; Lacroix, J.; Dugue, A.; Supiot, S.; Campone, M.; Mahe, M.; Kichou, S.; Leheurteur, M.; Hanzen, C. REBECA: A phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann. Oncol. 2014, 25, 2351–2356. [Google Scholar]
- Jiang, S.; Liang, H.; Liu, Z.; Zhao, S.; Liu, J.; Xie, Z.; Wang, W.; Zhang, Y.; Han, B.; He, J. The impact of anlotinib on brain metastases of non-small cell lung cancer: Post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist 2020, 25, e870–e874. [Google Scholar]
- Brun, L.; Dupic, G.; Chassin, V.; Chautard, E.; Moreau, J.; Dedieu, V.; Khalil, T.; Verrelle, P.; Lapeyre, M.; Biau, J. Hypofractionated stereotactic radiotherapy for large brain metastases: Optimizing the dosimetric parameters. Cancer/Radiothérapie 2021, 25, 1–7. [Google Scholar]
- Kahrom, A.; Grimley, R.; Jeffree, R.L. A case of delayed cyst formation post brain AVM stereotactic radiosurgery for arteriovenous malformation: Case report. J. Clin. Neurosci. 2021, 87, 17–19. [Google Scholar]
- Li, J.; Tian, Y.; Zheng, M.; Ge, J.; Zhang, J.; Kong, D.; Chen, M.; Yu, P. Anlotinib plus chemotherapy for T790M-negative EGFR-mutant non-sqNSCLC resistant to TKIs: A multicenter phase 1b/2 trial. Thorac. Cancer 2022, 13, 3496–3503. [Google Scholar] [CrossRef]
- He, Z.; Yang, X.; Ma, T.; Yang, Q.; Zhang, C.; Chen, Y.; Wang, P.; D’Incecco, A.; Metro, G.; Uematsu, S.; et al. Efficacy and safety of anlotinib combined with carboplatin and pemetrexed as first-line induction therapy followed by anlotinib plus pemetrexed as maintenance therapy in EGFR/ALK wild-type advanced non-squamous non-small cell lung cancer in China: A multicenter, single-arm trial. Transl. Lung Cancer Res. 2022, 11, 1657–1666. [Google Scholar] [CrossRef]
- Xiang, M.; Yang, X.; Ren, S.; Du, H.; Geng, L.; Yuan, L.; Wen, Y.; Lin, B.; Li, J.; Zhang, Y.; et al. Anlotinib Combined with S-1 in Third- or Later-Line Stage IV Non-Small Cell Lung Cancer Treatment: A Phase II Clinical Trial. Oncologist 2021, 26, e2130–e2135. [Google Scholar] [CrossRef]
- Pu, X.; Xiao, Z.; Li, J.; Wu, Z.; Ma, Z.; Weng, J.; Xiao, M.; Chen, Y.; Cao, Y.; Cao, P.; et al. Anlotinib plus docetaxel vs. docetaxel alone for advanced non-small-cell lung cancer patients who failed first-line treatment: A multicenter, randomized phase II trial. Lung Cancer 2024, 191, 107538. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Zhang, L.; Mai, W.; Jiang, W.; Geng, Q. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front. Oncol. 2020, 10, 594558. [Google Scholar] [CrossRef]
- National Medical Products Administration. Drug Approval Documents Released on 3 June 2021. Available online: https://english.nmpa.gov.cn/ (accessed on 28 March 2025).
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Ying, J.; Wang, S.; Tao, X.; Zhao, J.; Mao, Y.; Wang, B.; et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 2020, 15, 816–826. [Google Scholar] [CrossRef]
- Gao, S.; Li, N.; Gao, S.; Xue, Q.; Wang, S.; Lv, F.; Zhao, L.; Zhang, F.; Zhao, Z.; Su, K.; et al. Two-year follow-up of single PD-1 blockade in neoadjuvant resectable NSCLC. J. Clin. Oncol. 2021, 39, 8522. [Google Scholar] [CrossRef]
- Han, B.; Chu, T.; Yu, Z.; Wang, J.; Zhao, Y.; Mu, X.; Yu, X.; Shi, X.; Shi, Q.; Guan, M. LBA57 Sintilimab plus anlotinib versus platinum-based chemotherapy as first-line therapy in metastatic NSCLC (SUNRISE): An open label, multi-center, randomized, phase II study. Ann. Oncol. 2022, 33, S1423–S1424. [Google Scholar]
- Dou, X.J.; Ma, R.Y.; Ren, D.W.; Liu, Q.; Yan, P. Effectiveness and Safety of Anlotinib Combined with PD-1 Blockades in Patients with Previously Immunotherapy Treated Advanced Non-Small Cell Lung Cancer: A Retrospective Exploratory Study. Lung Cancer 2024, 15, 29–40. [Google Scholar] [CrossRef]
- Chen, H.-J.; Tu, H.-Y.; Hu, Y.; Fan, Y.; Wu, G.; Cang, S.; Yang, Y.; Yang, N.; Ma, R.; Jin, G. A phase II trial of anlotinib plus EGFR-TKIs in advanced non-small cell lung cancer with gradual, oligo, or potential progression after EGFR-TKIs treatment (CTONG-1803/ALTER-L001). J. Hematol. Oncol. 2025, 18, 3. [Google Scholar] [PubMed]
- Duan, H.; Shao, C.; Luo, Z.; Wang, T.; Tong, L.; Liu, H.; Yao, X.; Lei, J.; Zhao, J.; Gao, Y.J. Perioperative sintilimab and neoadjuvant anlotinib plus chemotherapy for resectable non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 2 trial (TD-NeoFOUR trial). Signal Transduct. Target. Ther. 2024, 9, 296. [Google Scholar] [PubMed]
- Han, X.; Guo, J.; Li, L.; Huang, Y.; Meng, X.; Wang, L.; Zhu, H.; Meng, X.; Shao, Q.; Li, X.J.S.T.; et al. Sintilimab combined with anlotinib and chemotherapy as second-line or later therapy in extensive-stage small cell lung cancer: A phase II clinical trial. Signal Transduct. Target. Ther. 2024, 9, 241. [Google Scholar] [PubMed]
- Le, X.; Puri, S.; Negrao, M.V.; Nilsson, M.B.; Robichaux, J.; Boyle, T.; Hicks, J.K.; Lovinger, K.L.; Roarty, E.; Rinsurongkawong, W. Landscape of EGFR-dependent and-independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin. Cancer Res. 2018, 24, 6195–6203. [Google Scholar]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Giuliano, S.; Pagès, G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie 2013, 95, 1110–1119. [Google Scholar]
- Bonanno, L.; Jirillo, A.; Favaretto, A. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors and new therapeutic perspectives in non small cell lung cancer. Curr. Drug Targets 2011, 12, 922–933. [Google Scholar]
- Chan, C.Y.-K.; Yuen, V.W.-H.; Wong, C.C.-L. Hypoxia and the metastatic niche. In Hypoxia Cancer Metastasis; Springer Nature: Cham, Switzerland, 2019; pp. 97–112. [Google Scholar]
- Santaniello, A.; Napolitano, F.; Servetto, A.; De Placido, P.; Silvestris, N.; Bianco, C.; Formisano, L.; Bianco, R. Tumour microenvironment and immune evasion in EGFR addicted NSCLC: Hurdles and possibilities. Cancers 2019, 11, 1419. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar]
- Zhang, C.; Cao, H.; Cui, Y.; Jin, S.; Gao, W.; Huang, C.; Guo, R. Concurrent use of anlotinib overcomes acquired resistance to EGFR-TKI in patients with advanced EGFR-mutant non-small cell lung cancer. Thorac. Cancer 2021, 12, 2574–2584. [Google Scholar] [CrossRef]
- Li, T.; Qian, Y.; Zhang, C.; Uchino, J.; Provencio, M.; Wang, Y.; Shi, X.; Zhang, Y.; Zhang, X. Anlotinib combined with gefitinib can significantly improve the proliferation of epidermal growth factor receptor-mutant advanced non-small cell lung cancer in vitro and in vivo. Transl. Lung Cancer Res. 2021, 10, 1873–1888. [Google Scholar] [CrossRef]
- Gu, G.; Hu, C.; Hui, K.; Zhang, H.; Chen, T.; Zhang, X.; Jiang, X. Exosomal miR-136-5p Derived from Anlotinib-Resistant NSCLC Cells Confers Anlotinib Resistance in Non-Small Cell Lung Cancer Through Targeting PPP2R2A. Int. J. Nanomed. 2021, 16, 6329–6343. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, W.; Du, F.; Du, C.; Li, S.; Wang, J.; Li, L.; Wang, F.; Hao, Y.; Li, C.; et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 2016, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, S.; Ren, J.; Jiang, T.; Zhang, C.; Yan, Z. Anlotinib affects systemic lipid metabolism and induces lipid accumulation in human lung cancer cells. Lipids Health Dis. 2023, 22, 134. [Google Scholar]
- Yang, X.; Yang, B.; Li, D.; Pan, W.; Tong, Q.; Wang, L.; Chen, D.; Fu, C. Thromboembolic events associated with epidermal growth factor receptor tyrosine kinase inhibitors: A pharmacovigilance analysis of the US FDA adverse event reporting system (FAERS) database. Clin. Drug Investig. 2024, 44, 199–207. [Google Scholar]
- Beck, J.; Procopio, G.; Bajetta, E.; Keilholz, U.; Negrier, S.; Szczylik, C.; Bokemeyer, C.; Bracarda, S.; Richel, D.J.; Staehler, M.; et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: A large open-label study in diverse community settings. Ann. Oncol. 2011, 22, 1812–1823. [Google Scholar] [CrossRef]
- Gore, M.E.; Szczylik, C.; Porta, C.; Bracarda, S.; Bjarnason, G.A.; Oudard, S.; Hariharan, S.; Lee, S.H.; Haanen, J.; Castellano, D.; et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: An expanded-access trial. Lancet. Oncol. 2009, 10, 757–763. [Google Scholar] [CrossRef]
- Stadler, W.M.; Figlin, R.A.; McDermott, D.F.; Dutcher, J.P.; Knox, J.J.; Miller, W.H., Jr.; Hainsworth, J.D.; Henderson, C.A.; George, J.R.; Hajdenberg, J.; et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer 2010, 116, 1272–1280. [Google Scholar] [CrossRef]
- Qi, W.X.; Shen, Z.; Tang, L.N.; Yao, Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: An up-to-date meta-analysis. Crit. Rev. Oncol./Hematol. 2014, 92, 71–82. [Google Scholar] [CrossRef]
| Types of Combination | Intervention | Study ID | Type of the Study | Number of Patients | Efficacy | Safety TRAEs (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mPFS (Month) (95 % CI) | OS (Months) | OSR (Percentage) | ORR (%) | DCR (%) | ||||||
| Anlotinib and chemotherapy combinations | Anlotinib with platinum-based Chemotherapy and pemetrexed | Li et al. 2022 [71] | A multicenter phase 1b/2 trial | 19 | 5.75 (4.37–7.52) | NA | 47.4% | NA * | NA | Hypertension (50.0%). Decreased platelet count (16.7%). Hypertriglyceridemia (8.3%). |
| Anlotinib and carboplatin combined with pemetrexed | He et al. 2022 [72] | A multicenter, single-arm trial | 38 | 10.5 (4.1–17.0) | NA | 23.4% | 60.5% | 94.7% | Hypertension (23.7%). Neutropenia (19.4%). Bone marrow toxicity (10.5%). | |
| Anlotinib Combined with S-1 | Xiang et al. 2021 [73] | A Phase II Clinical Trial | 29 | 5.8 | 16.7 | NA | 30% | NA | Fatigue (55%) Hypertension (38%) Liver dysfunction/failure (clinical) (34%) Hypothyroidism (31%) | |
| Anlotinib plus docetaxel | Pu et al. 2024 [74] | A multicenter, randomized phase II trial | 40 | 4.4 | 12 | NA | 32.5% | 87.5% | TRAEs (82.5%) include Fatigue Anemia Leukopenia Grade ≥3 TRAEs occur in 30.0 %. | |
| Anlotinib + Immunochemotherapy | Sintilimab and anlotinib | Han et al. 2022 [83] | An open-label, multicenter, randomized, phase II study | 41 | 10.8 (0.25–0.74) | NA | NA | 50% | 85% | Grade 3-4 TRAEs (11.6 %) include: Hypothyroidism Hyponatremia AST elevation. |
| Anlotininb combined with PD-1 blockades (sintilimab, ocrelizumab, tislelizumab, and pembrolizumab) | Dou et al. 2024 [84] | A Retrospective Exploratory Study | 67 | 6 (2.37–9.83) | 16.5 | NA | 23.9% | 85.1% | ||
| EGFR-TKI + Anti-angiogenesis (Gefitinib + Anlotinib) | Zhou et al. 2024 [60] | Multicenter, double-blind, randomized Phase 3 trial | 157 | 14.8 (12.9–15.4) | 31.2 (25.7–NE) | NA | 76.1 (68.6–82.6) | NA | Grade ≥ 3 (49.7%) Hypertension (29.7%) Diarrhea (66.5%) Rash (65.8%) | |
| Anlotinib + Immunochemotherapy | Anlotinib (antiangiogenic TKI) + EGFR-TKIs (1st/2nd/3rd generation) | Chen et al. 2025 [85] | Open-label, single-arm, multicenter, phase II trial. | 120 | 9.1 (6.8–11.7) | 81.1 (71.8–87.5) | NA | 6.7 | 87.5 | All-grade: 96.7% (116/120). Grade ≥ 3: 52.5% (63/120). Most common: Hypertension (19.2%) Diarrhea (5.0%) Weight loss (4.2%). Discontinuations due to AEs: 12.5% (15/120). Serious AEs: Hemoptysis (4 cases), interstitial lung disease (1 case). |
| Perioperative immunotherapy (sintilimab) + neoadjuvant antiangiogenic therapy (anlotinib) + chemotherapy (platinum-based doublet). | Duan et al. 2024 [86] | Open-label, single-arm, phase 2 trial. | 45 | 81.5 (64.5–90.9) (24 months). | 97.7 (84.6–99.7) (12 months) | NA | 71.1 | 97.8 | All-grade: 100% (45/45). Grade 3/4: 55.6% (25/45). Most common: White blood cell decrease (11.1%). Neutrophil count decrease (11.1%). Vomiting (8.9%). Immune-Related AEs (irAEs): Neoadjuvant phase: 15.6% (7/45). Adjuvant phase: 34.1% (14/41), with grade 3 in 17.1%. | |
| immunotherapy (sintilimab), antiangiogenic therapy (anlotinib), and chemotherapy (nab-paclitaxel) | Han et al. 2024 [87] | Open-label, single-arm, phase II clinical trial. | 25 | 6.0 (5.4–9.7) | 62.2 (12 months) | NA | 60 | 76 | All-grade: 92% (23/25). Most common: Leukopenia (56%), Anemia (52%) Elevated GGT (48%). Grade ≥ 3: 16% (4/25). Elevated AST (12%) Rash (4%). Serious AEs: 12% (3/25). Discontinuations due to AEs: 8% (2/25). irAEs: 44% (11/25), most frequently hypertriglyceridemia (16%). | |
| Protocol ID | Clinical Phase | Study Status | Combination Partners | Key Endpoints |
|---|---|---|---|---|
| NCT02388919 | Phase 2 Phase 3 | Completed | Anlotinib used as monotherapy. | Evaluate the efficacy and safety of anlotinib as the 3-line treatment of patients with advanced non-small lung cancer, with placebo control. |
| NCT04967079 | Phase 1 | Completed | MEK inhibitor trametinib (2 mg) in combination with anlotinib (6 mg, 8 mg, 10 mg, 12 mg). | In part A, the primary endpoint is the determination of the recommended RP2D. Secondary endpoint for phase Ia includes evaluating the ORR, DCR, PFS, and AEs. Following the establishment of the RP2D, the expansion cohort will be initiated. Transitioning to part B, 20 patients will be enrolled to further evaluate the ORR. |
| NCT06188650 | NA | Recruiting | DEB-BACE combined with anlotinib and adebelimumab. | The goal of this clinical trial is to learn about DEB-BACE combined with anlotinib and adalimumab in patients with advanced NSCLC after second-line treatment. |
| NCT03765775 | Phase 2 | Unknown | Anlotinib plus sintilimab. | This is an efficacy and safety study of anlotinib combined with sintilimab (IBI 308) in participants with advanced or metastatic NSCLC who have resistance against first-generation EGFR-TKIs, along with T790M negative. |
| NCT04211896 | Phase 2 | Unknown | Anlotinib combined with nivolumab. | This study evaluates the safety and efficacy of anlotinib in combination with nivilumab as a second-line treatment in advanced NSCLC patients. The primary endpoint of the study is PFS; the secondary endpoints are DCR, ORR, OS, and safety. |
| NCT05460481 | Phase 2 | Unknown | Anlotinib plus penpulimab. | The investigation of the efficacy and safety of anlotinib plus docetaxel in advanced NSCLC patients who have progressed following prior PD-1 or PD-L1 inhibitor treatment. |
| NCT01924195 | Phase 2 | Completed | Anlotinib used as monotherapy. | The trial is to explore anlotinib for the effectiveness and safety of advanced non-small cell lung cancer patients who have failed two lines of chemotherapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetta, H.F.; Aljohani, H.M.; Sirag, N.; Elfadil, H.; Salama, A.; Al-Twalhy, R.; Alanazi, D.; Al-johani, M.D.; Albalawi, J.H.; Al-Otaibi, R.M.; et al. Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment. Pharmaceuticals 2025, 18, 585. https://doi.org/10.3390/ph18040585
Hetta HF, Aljohani HM, Sirag N, Elfadil H, Salama A, Al-Twalhy R, Alanazi D, Al-johani MD, Albalawi JH, Al-Otaibi RM, et al. Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment. Pharmaceuticals. 2025; 18(4):585. https://doi.org/10.3390/ph18040585
Chicago/Turabian StyleHetta, Helal F., Hashim M. Aljohani, Nizar Sirag, Hassabelrasoul Elfadil, Ayman Salama, Rand Al-Twalhy, Danah Alanazi, Manal D. Al-johani, Jumanah H. Albalawi, Rinad M. Al-Otaibi, and et al. 2025. "Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment" Pharmaceuticals 18, no. 4: 585. https://doi.org/10.3390/ph18040585
APA StyleHetta, H. F., Aljohani, H. M., Sirag, N., Elfadil, H., Salama, A., Al-Twalhy, R., Alanazi, D., Al-johani, M. D., Albalawi, J. H., Al-Otaibi, R. M., Alsharif, R. A., & Sayad, R. (2025). Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment. Pharmaceuticals, 18(4), 585. https://doi.org/10.3390/ph18040585







