Evaluation of Chitosan-Based Axiostat as Hemostatic Dressing for Endovascular Procedures in Patients with Leriche Syndrome on Anticoagulant Therapy
Abstract
1. Introduction
2. Results
2.1. Population Study
2.2. Procedure Data
2.3. Outcomes E Follow-Up
2.4. Hemostasis Evaluation
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection and Procedure
4.3. Outcomes and Follow-Up
4.4. Hemostasis Evaluation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, H.L.; Li, M.F.; Hsiao, C.C.; Wu, C.J.; Wu, T.H. Endovascular management of aorto-iliac occlusive disease (Leriche syndrome). J. Formos. Med. Assoc. 2021, 120, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Setacci, C.; Galzerano, G.; Setacci, F.; De Donato, G.; Sirignano, P.; Kamargianni, V.; Cannizzaro, A.; Cappelli, A. Endovascular approach to Leriche syndrome. J. Cardiovasc. Surg. 2012, 53, 301–306. [Google Scholar]
- Matsuura, H.; Honda, H. Leriche syndrome. Cleve Clin. J. Med. 2021, 88, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, T.; Usai, M.V.; Taneva, G.T.; Pitoulias, G.A.; Torsello, G.; Donas, K.P. The role of open and endovascular treatment of patients with chronic aortoiliac Leriche syndrome. Vascular 2020, 28, 68–73. [Google Scholar] [CrossRef]
- Kholmurodov, S.F.; Kuchkorov, S.B.; Bakhronov, J.J. Leriche’s Syndrome. Gold. Brain 2023, 1, 104–109. [Google Scholar]
- Beyaz, M.O.; Urfalı, S.; Kaya, S.; Oruç, D.; Fansa, İ. Is Surgery the Only Fate of the Patient with Leriche Syndrome? Our Endovascular Therapy Results Early Follow-Up Outcomes. Heart Surg. Forum 2022, 25, E721–E725. [Google Scholar] [CrossRef]
- King, J.R.; Maijub, J.G.; Motaganahalli, R.L. Aortoiliac Occlusive Disease: Endovascular Management. In Vascular Reconstructions; Hoballah, J.J., Bechara, C.F., Eds.; Springer: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Li, X.F.; Lu, P.; Jia, H.R.; Li, G.; Zhu, B.; Wang, X.; Wu, F.G. Emerging materials for hemostasis. Coord. Chem. Rev. 2023, 475, 214823. [Google Scholar] [CrossRef]
- Curcio, F.; Perri, P.; Piro, P.; Galassi, S.; Sole, R.; Trombino, S.; Cassano, R. Synthetic Haemostatic Sealants: Effectiveness, Safety, and In Vivo Applications. Pharmaceuticals 2024, 17, 288. [Google Scholar] [CrossRef]
- Cassano, R.; Perri, P.; Scarcello, E.; Piro, P.; Sole, R.; Curcio, F.; Trombino, S. Chitosan Hemostatic Dressings: Properties and Surgical Applications. Polymers 2024, 16, 1770. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Roberts, J.S.; Niu, J.; Pastor-Cervantes, J.A. Comparison of hemostasis times with a chitosan-based hemostatic pad (Clo-SurPlus Radial™) vs. mechanical compression (TR Band®) following transradial access: A pilot study. Cardiovasc. Revascularization Med. 2019, 20, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhong, W. Hemostatic materials in wound care. Burn. Trauma 2021, 9, tkab019. [Google Scholar] [CrossRef] [PubMed]
- Mecwan, M.; Li, J.; Falcone, N.; Ermis, M.; Torres, E.; Morales, R.; Hassani, A.; Haghniaz, R.; Mandal, K.; Sharma, S.; et al. Recent advances in biopolymer-based hemostatic materials. Regen. Biomater. 2022, 9, rbac063. [Google Scholar] [CrossRef]
- Gheorghiță, D.; Moldovan, H.; Robu, A.; Bița, A.I.; Grosu, E.; Antoniac, A.; Corneschi, I.; Antoniac, I.; Bodog, A.D.; Băcilă, C.I. Chitosan-Based Biomaterials for Hemostatic Applications: A Review of Recent Advances. Int. J. Mol. Sci. 2023, 24, 10540. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Liu, C.-C.; Cherng, J.-H.; Lin, C.-S.; Chang, S.-J.; Hong, Z.-J.; Liu, C.-C.; Chiu, Y.-K.; Hsu, S.-D.; Chang, H. Biological effects of chitosan-based dressing on hemostasis mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Cao, S.; Xu, G.; Li, Q.; Zhang, S.; Yang, Y.; Chen, J. Double cross linking chitosan sponge with antibacterial and hemostatic properties for accelerating wound repair. Compos. Part B Eng. 2022, 234, 109746. [Google Scholar] [CrossRef]
- Ciarlantini, C.; Lacolla, E.; Francolini, I.; Fernández-García, M.; Muñoz-Núñez, C.; Muñoz-Bonilla, A.; Piozzi, A. Development of antioxidant and antimicrobial membranes based on functionalized and crosslinked chitosan for tissue regeneration. Int. J. Mol. Sci. 2024, 25, 1961. [Google Scholar] [CrossRef]
- Yoshimura, G.; Kamidani, R.; Miura, T.; Oiwa, H.; Mizuno, Y.; Yasuda, R.; Kitagawa, Y.; Fukuta, T.; Miyake, T.; Okamoto, H.; et al. Leriche syndrome diagnosed due to polytrauma: A case report. Int. J. Emerg. Med. 2022, 15, 8. [Google Scholar] [CrossRef]
- Dattilo, P.B.; Tsai, T.T.; Garcia, J.A.; Allshouse, A.; Casserly, I.P. Clinical outcomes with contemporary endovascular therapy of iliac artery occlusive disease. Catheter. Cardiovasc. Interv. 2012, 80, 644–654. [Google Scholar] [CrossRef]
- Kumar, A.; Agrawal, A.; Bharani, K.K.; Mavely, L. Evaluation of Hemostatic Effectiveness in a Standard Swine Hemorrhage Model of Severe Bleeding: A Comparative Study of Chitosan Gauze and Kaolin Gauze. Int. J. Health Technol. Innov. 2024, 3, 38–47. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d’Ayala, G.G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Mawazi, S.M.; Kumar, M.; Ahmad, N.; Ge, Y.; Mahmood, S. Recent applications of chitosan and its derivatives in antibacterial, anticancer, wound healing, and tissue engineering fields. Polymers 2024, 16, 1351. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Applications of chitosan in surgical and post-surgical materials. Mar. Drugs 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Archer, J. The seldinger technique: A revolution in medicine. ANZ J. Surg. 2023, 93, 184–214. [Google Scholar]
- Farber, A.; Angle, N.; Avgerinos, E.; Dubois, L.; Eslami, M.; Geraghty, P.; Haurani, M.; Jim, J.; Ketteler, E.; Pulli, R.; et al. The Society for Vascular Surgery clinical practice guidelines on popliteal artery aneurysms. J. Vasc. Surg. 2022, 75, 109S–120S. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Spatari, C.; Ragno, G. A single MCR-ALS model for drug analysis in different formulations: Application on diazepam commercial preparations. J. Pharm. Biomed. Anal. 2017, 134, 346–351. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chemometric methods for spectroscopy-based pharmaceutical analysis. Front. Chem. 2018, 6, 576. [Google Scholar] [CrossRef]






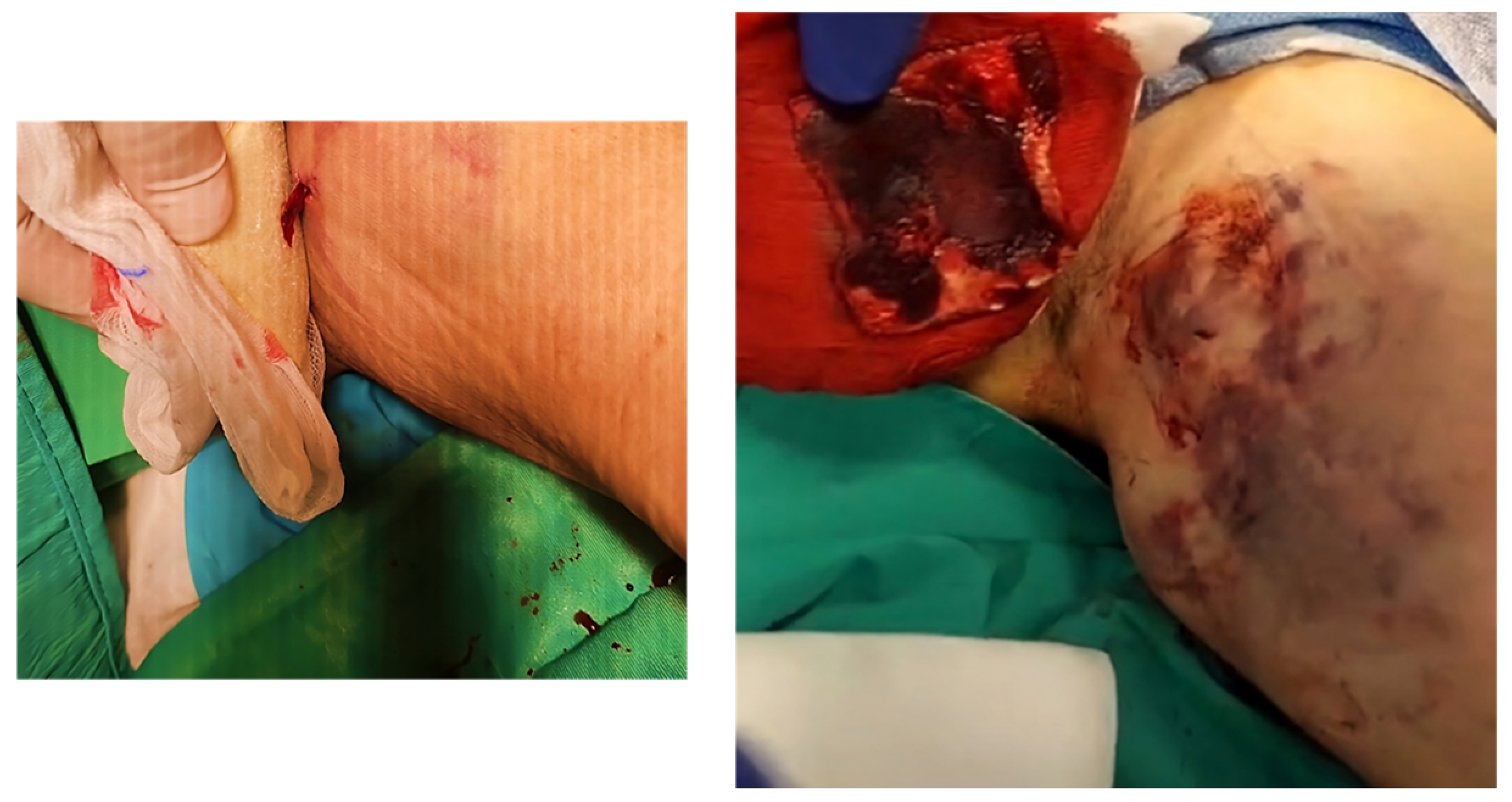
| VARIABLES | ALL PATIENTS (N = 60) |
|---|---|
| Ages (Years) | 67 ± 2.8 (67.4) |
| Sex (%M/%F) | 40% M/60 F% |
| BMI * | 27 ± 0.7 (27.1) |
| aPTT ** (S) | 33 ± 0.8 (33.2) |
| Platelet Count (×106/Ml) | 330 ± 8.7 (330.6) |
| Diabetes Mellitus | 40 (66%) |
| Coronary Artery Disease | 8 (13%) |
| Congestive Heart Failure | 11 (18%) |
| Cerebrovascular Disease | 2 (3%) |
| Smoking History | 45 (75%) |
| Hypertension | 33 (56%) |
| Hyperlipidemia | 37 (62%) |
| Chronic Renal Failure (Egfr < 60 Ml/Min) | 6 (10%) |
| VARIABLES | ALL PATIENTS (N = 60) |
|---|---|
| Mono-Antiaggregant Therapy | 15 (25%) |
| Dual-Antiaggregant Therapy | 33 (55%) |
| Anticoagulant Therapy | 9 (15%) |
| Anticoagulant + Antiaggregant Therapy | 4 (7%) |
| OUTCOMES | MONO-ANTIAGGREGANT THERAPY | DUAL-ANTIAGGREGANT THERAPY | ANTICOAGULANT THERAPY | ANTIAGGREGANT + ANTICOAGULANT THERAPY |
|---|---|---|---|---|
| PRIMARY SUCCES (%) | 100 | 100 | 98 | 95.5 |
| SECONDARY TECHINCAL SUCCES (%) | 100 | 100 | 100 | 100 |
| CLINICAL SUCCES (%) | 100 | 100 | 98 | 96 |
| SURGICAL SUCCES (%) | 100 | 100 | 100 | 100 |
| AMBULATION AFTER 24H (%) | 100 | 100 | 100 | 100 |
| RECOVERY OF FUNCTION (%) | 100 | 100 | 98.8 | 96 |
| Outcomes | Mono-Antiaggregant Therapy (n° Control = 15) | Dual-Antiaggregant Therapy (n° Control = 33) | Anticoagulant Therapy (n° Control = 9) | Antiaggregant + Anticoagulant Therapy (n° Control = 4) |
|---|---|---|---|---|
| Hematoma | 0 | 0 | 1 | 2 |
| Pseudoaneurysm | 0 | 5 | 2 | 0 |
| Dissection | 0 | 0 | 0 | 0 |
| Arteriovenous fistula | 0 | 0 | 0 | 0 |
| Arterial thrombosis | 1 | 0 | 0 | 1 |
| Arterial stenosis | 2 | 0 | 1 | 0 |
| Hemorrhage | 0 | 1 | 1 | 1 |
| Infection | 0 | 0 | 1 | 0 |
| Neuropathy | 0 | 2 | 0 | 0 |
| Surgery | 0 | 0 | 2 | 1 |
| Drain removal after 24 h | 0 | 0 | 2 | 1 |
| Antibiotic therapy | 0 | 0 | 2 | 3 |
| All Patients (n = 60) | Hemostatic Success (%) | Hemostasis Time (min) | Hemostatic Insuccess (%) |
|---|---|---|---|
| Mono-Antiaggregant Therapy | 100 | 5 | 0 |
| Dual-Antiaggregant Therapy | 100 | 6 | 0 |
| Anticoagulant Therapy | 99.5 | 5 | 0 |
| Antiaggregant + Anticoagulant Therapy | 98.2 | 7 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, P.; Curcio, F.; De Luca, M.; Piro, P.; Trombino, S.; Cassano, R. Evaluation of Chitosan-Based Axiostat as Hemostatic Dressing for Endovascular Procedures in Patients with Leriche Syndrome on Anticoagulant Therapy. Pharmaceuticals 2025, 18, 584. https://doi.org/10.3390/ph18040584
Perri P, Curcio F, De Luca M, Piro P, Trombino S, Cassano R. Evaluation of Chitosan-Based Axiostat as Hemostatic Dressing for Endovascular Procedures in Patients with Leriche Syndrome on Anticoagulant Therapy. Pharmaceuticals. 2025; 18(4):584. https://doi.org/10.3390/ph18040584
Chicago/Turabian StylePerri, Paolo, Federica Curcio, Michele De Luca, Paolo Piro, Sonia Trombino, and Roberta Cassano. 2025. "Evaluation of Chitosan-Based Axiostat as Hemostatic Dressing for Endovascular Procedures in Patients with Leriche Syndrome on Anticoagulant Therapy" Pharmaceuticals 18, no. 4: 584. https://doi.org/10.3390/ph18040584
APA StylePerri, P., Curcio, F., De Luca, M., Piro, P., Trombino, S., & Cassano, R. (2025). Evaluation of Chitosan-Based Axiostat as Hemostatic Dressing for Endovascular Procedures in Patients with Leriche Syndrome on Anticoagulant Therapy. Pharmaceuticals, 18(4), 584. https://doi.org/10.3390/ph18040584











