1. Introduction
Bixa orellana L., commonly known as annatto, is a tropical plant renowned for its carotenoid-rich seeds, particularly bixin, which constitutes approximately 80% of its carotenoid content [
1]. Bixin exhibits potent antioxidant properties, effectively neutralizing free radicals and oxidative agents. However, its hydrophobic nature presents significant challenges regarding solubility and bioavailability, limiting its therapeutic applications [
2]. Annatto seed extracts have been investigated for their potential in treating and preventing various health conditions in animal models, including low-density lipoprotein (LDL) oxidation [
3], hepatocarcinoma [
4], diabetes [
5], skin disorders [
6], lung injury [
7], metabolic syndrome [
8], and cardiac injury [
9]. These benefits are generally attributed to the extract’s ability to combat oxidative stress. Bixin is highly effective at neutralizing and deactivating free radicals and non-radical oxidants, exhibiting a significant scavenging effect [
10]. However, bixin’s extensive carbon chain renders it highly hydrophobic, which limits its bioavailability and poses challenges for developing pharmaceutical formulations and in vivo applications [
11].
In prior research, bixin was tested in the form of polymeric nanoparticles in an in vivo murine model of acute lung inflammation (ALI) induced by cigarette smoke (CS) exposure [
2,
7]. The results were promising and provided a foundation for further investigation into bixin using alternative approaches to improve its production and viability. To address these limitations, medicinal chemistry has explored various strategies, including the formation of ionic salts to enhance solubility. CS contains a variety of free radicals and oxidants, including alkyl, peroxyl, nitric oxide, and superoxide anion radicals, as well as semiquinone-derived compounds in its particulate matter [
12]. Exposure to these substances can activate alveolar macrophages and recruit neutrophils, which then prompt both resident and infiltrated leukocytes to generate reactive oxygen species (ROS) and inflammatory mediators [
13]. This process contributes to lung injury, partly due to the disruption of redox balance [
14]. Oxidative stress leads to cellular damage, including tyrosine nitration of proteins [
15], apoptosis through lipid peroxidation and caspase activation [
16], and increased leukocyte recruitment to the lung parenchyma by enhancing the expression of cytokines such as tumor necrosis factor alpha (TNF-α) [
17].
The formation of salts from lipophilic compounds involves the reaction between an ionizable site within an apolar molecule and an acid or base [
18]. This process yields a salt, resulting in the formation of positively and negatively charged ions. The resulting salt improves solubility in polar solvents due to enhanced hydrogen bonding between the salt molecules and the solvent [
19]. Beyond solubility enhancement, salt formation can modulate, indirectly, other critical pharmacokinetic and pharmacodynamic properties, including chemical stability and molecular target interactions [
20]. In summary, this research aims to enhance the therapeutic potential of bixin through the development of potassium bixinate, addressing its solubility issues and evaluating its efficacy against oxidative and inflammatory stimuli. By combining chemical innovation with biological evaluation, this study seeks to provide new insights into the therapeutic applications of annatto-derived compounds.
3. Discussion
This study aimed to synthesize potassium bixinate to improve its solubility and evaluate its potential to combat oxidative stress and inflammation caused by cigarette smoke and LPS. In vitro experiments on macrophages exposed to cigarette smoke extract assessed the antioxidant, anti-inflammatory, and anti-chemotactic properties of potassium bixinate. In vivo studies on mice evaluated its anti-inflammatory effects. Mechanistic studies, including molecular docking, investigated how bixinate activates the Nrf2 pathway and interacts with HDAC6, providing insights into its potential biological activity and mechanisms of action.
Bixin, a natural pigment, typically exists in the more stable E (all-trans) form [
25]. However, this study detected the presence of the less common Z (all-cis) isomer. This finding suggests potential differences in processing conditions or environmental factors that favored the formation of the Z isomer. The Z isomer may possess unique physicochemical properties and biological properties. All subsequent experiments in this study were conducted exclusively with the Z-bixin isomer, marking the first reported use of Z-bixin in biological assays.
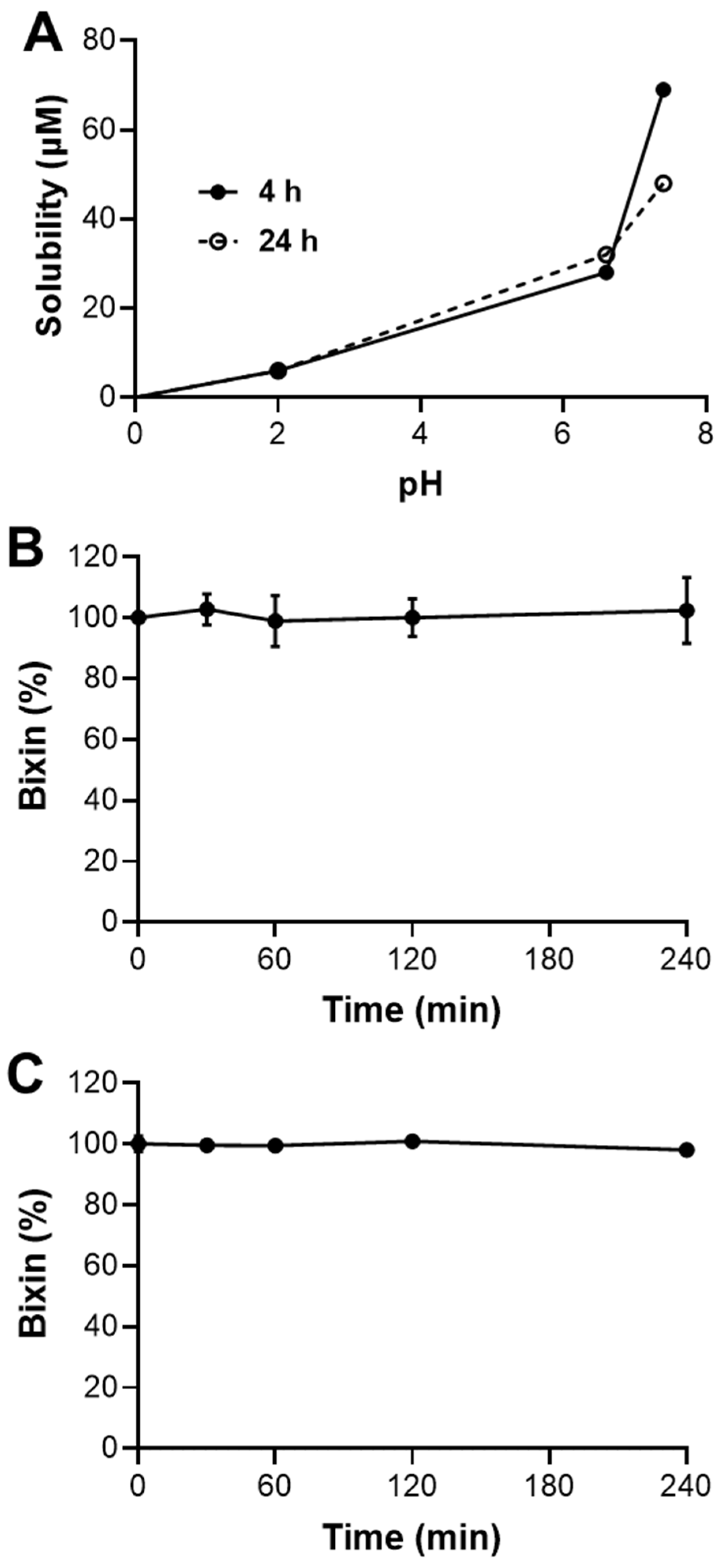
This study shows that HBx has limited solubility in acidic conditions (pH 2.0), but its solubility improves at neutral to slightly basic pH levels (6.6 and 7.4). This suggests that bixin is more likely to be absorbed in the upper small intestine than in the stomach [
26]. However, its solubility decreases over 24 h at pH 7.4, indicating potential precipitation and reduced bioavailability. The low permeability and absorbed fraction suggest limited oral bioavailability for HBx. Enhancements or alternative delivery methods may be needed to improve absorption. Despite these challenges, bixin shows high plasmatic and microsomal hydrolysis stability, supporting its potential as a therapeutic agent. To improve solubility and bioavailability, the synthesis of bixin’s potassium salt (KBx) is a promising approach, as salt formation typically enhances solubility and absorption [
19,
27]. KBx could provide better pharmacokinetic properties, overcoming HBx’s limitations and improving its oral delivery and therapeutic efficacy. This study aimed to produce bixin potassium salt and assess its ability to retain HBx’s anti-inflammatory and antioxidant properties, with in vitro and in vivo models using RAW 264.7 cells and intranasal LPS administration. The synthesis of the KBx was performed using the well-established solvent evaporation method [
19,
20]. Solubility tests confirmed a significant increase in the aqueous solubility of the product, and carotenoid tests indicated no degradation of bixin during synthesis. Mass spectrometry revealed peaks corresponding to bixinate (395.2220
m/
z) and its potassium adduct (433.1778
m/
z), confirming the expected formation of KBx without evidence of bixin hydrolysis. Additionally, the absence of the broadband corresponding to a hydroxyl group in the KBx FTIR further supports the successful salt formation [
28]. The melting point of KBx was approximately 10 °C lower than HBx (187–191 °C vs. 199–204 °C), enhancing its solubility due to reduced intermolecular interactions [
29,
30,
31]. TEM images showed KBx as an amorphous solid with irregular edges and little crystalline organization, a form known to improve solubility by reducing the energetic barrier for molecular solvation. These findings support the successful synthesis of KBx with properties that enhance its potential for improved solubility and therapeutic application.
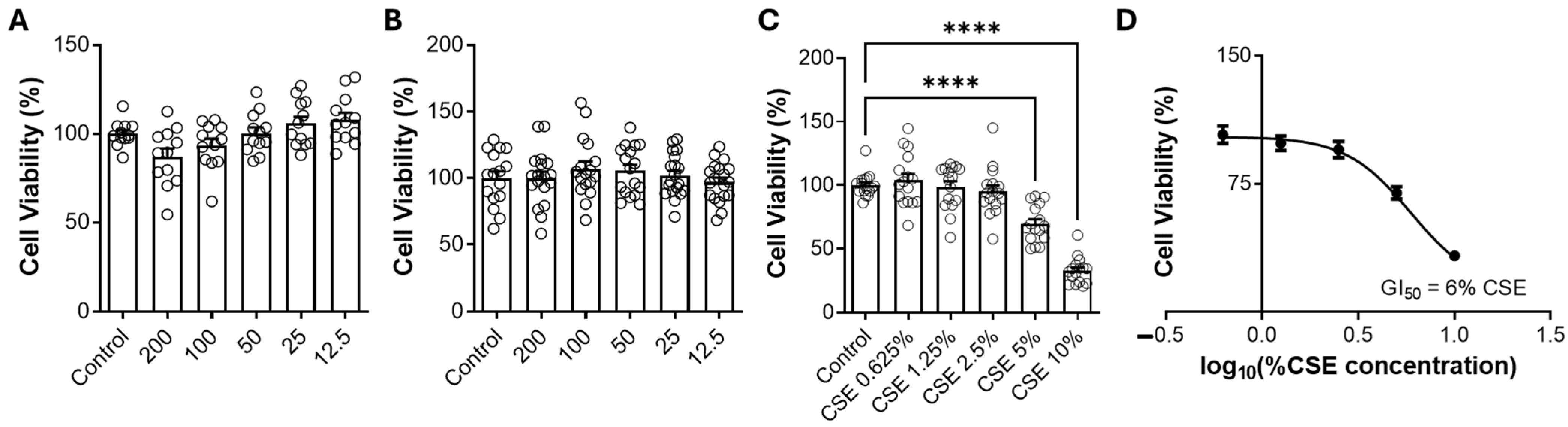
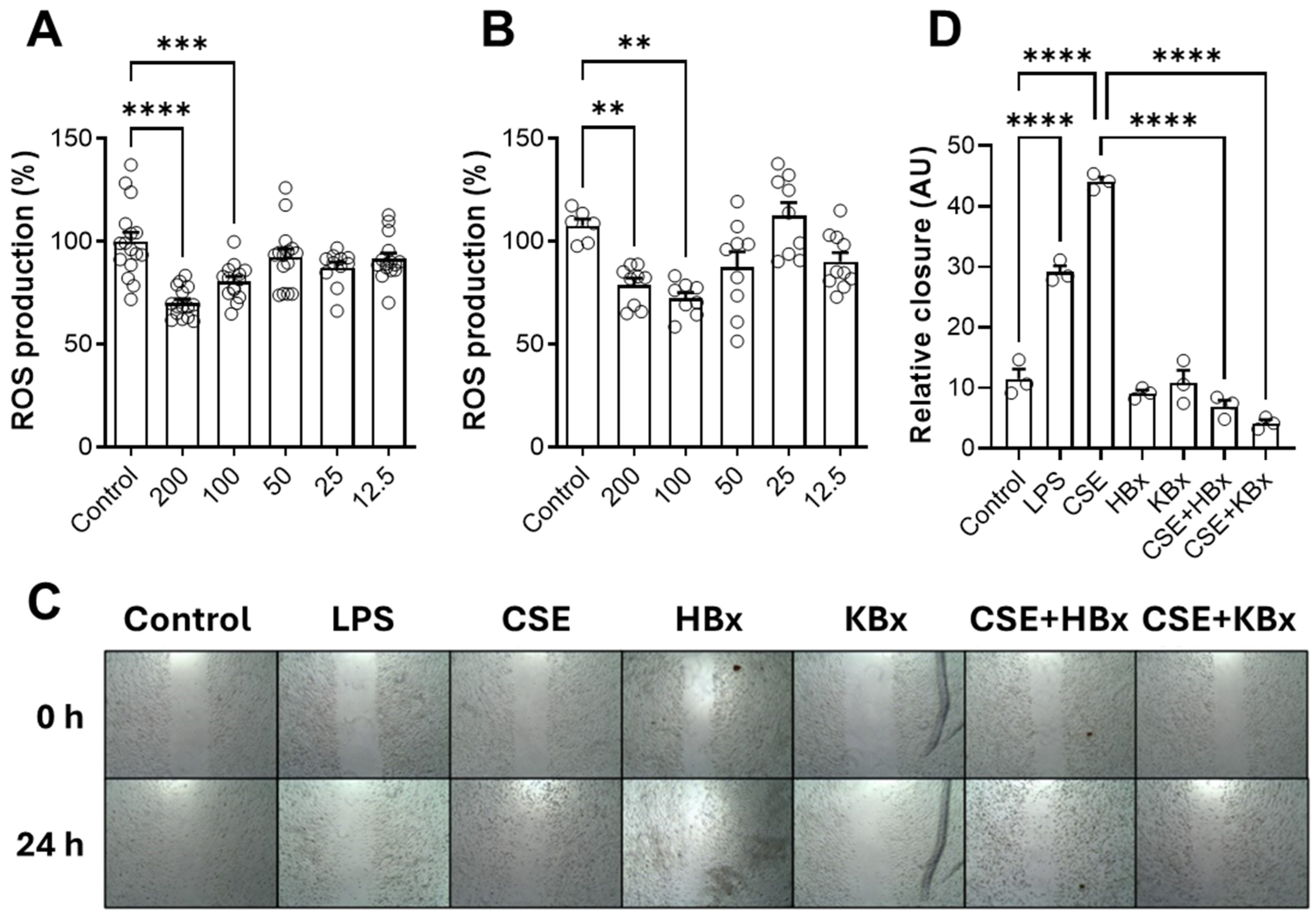
Bixin shows promise in modulating oxidative stress and inflammation, with Nrf2 activation being a key mechanism. Both HBx and its potassium derivative (KBx) demonstrated no cytotoxicity in vitro, maintaining cell viability up to 200 µM. At this concentration, both compounds effectively reduced ROS formation, supporting its selection for further experiments. Cells exposed to CSE showed decreased viability at 5% concentration, which was used in subsequent studies due to the high content of toxic components like nicotine, PAHs, and electrophilic substances [
32].
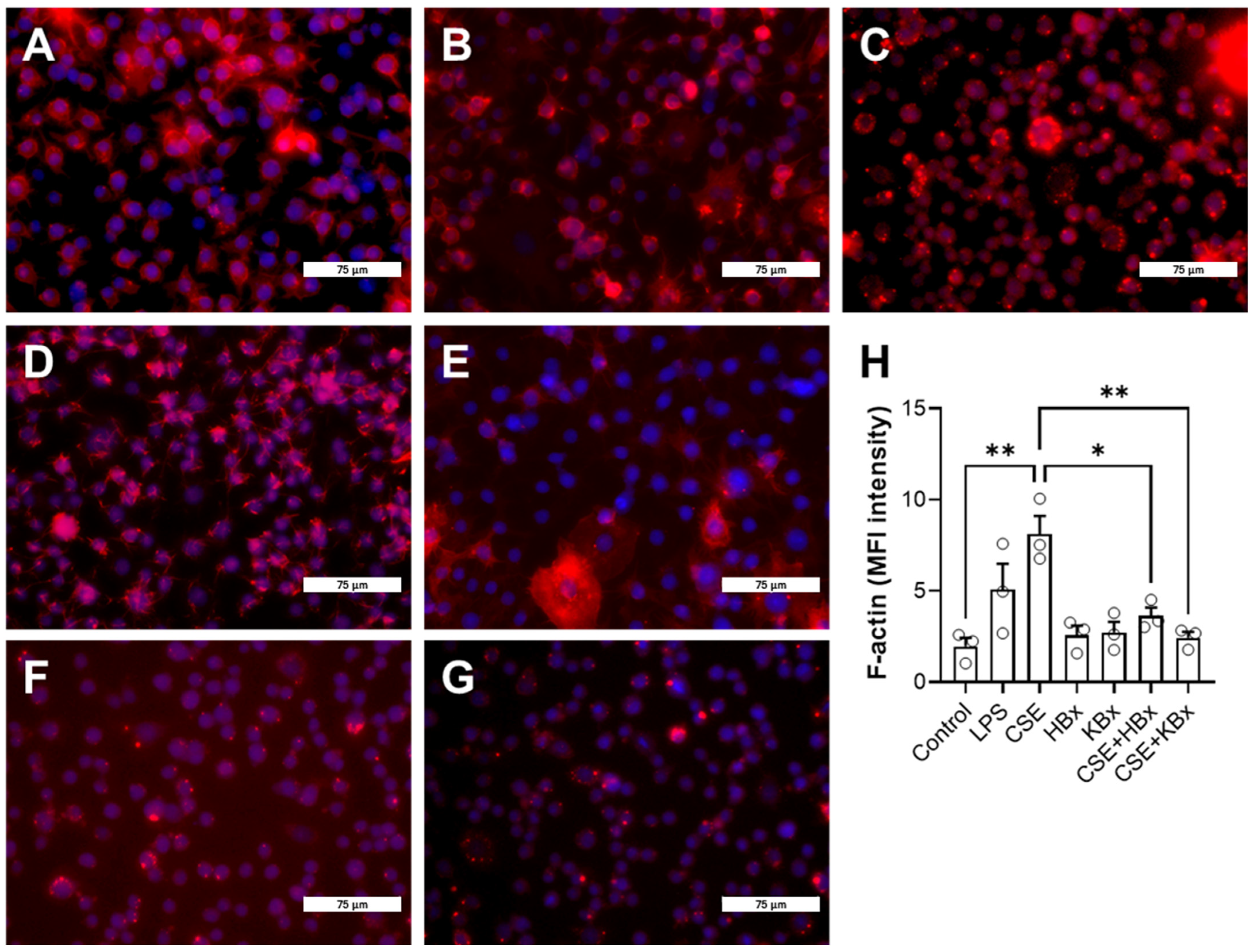
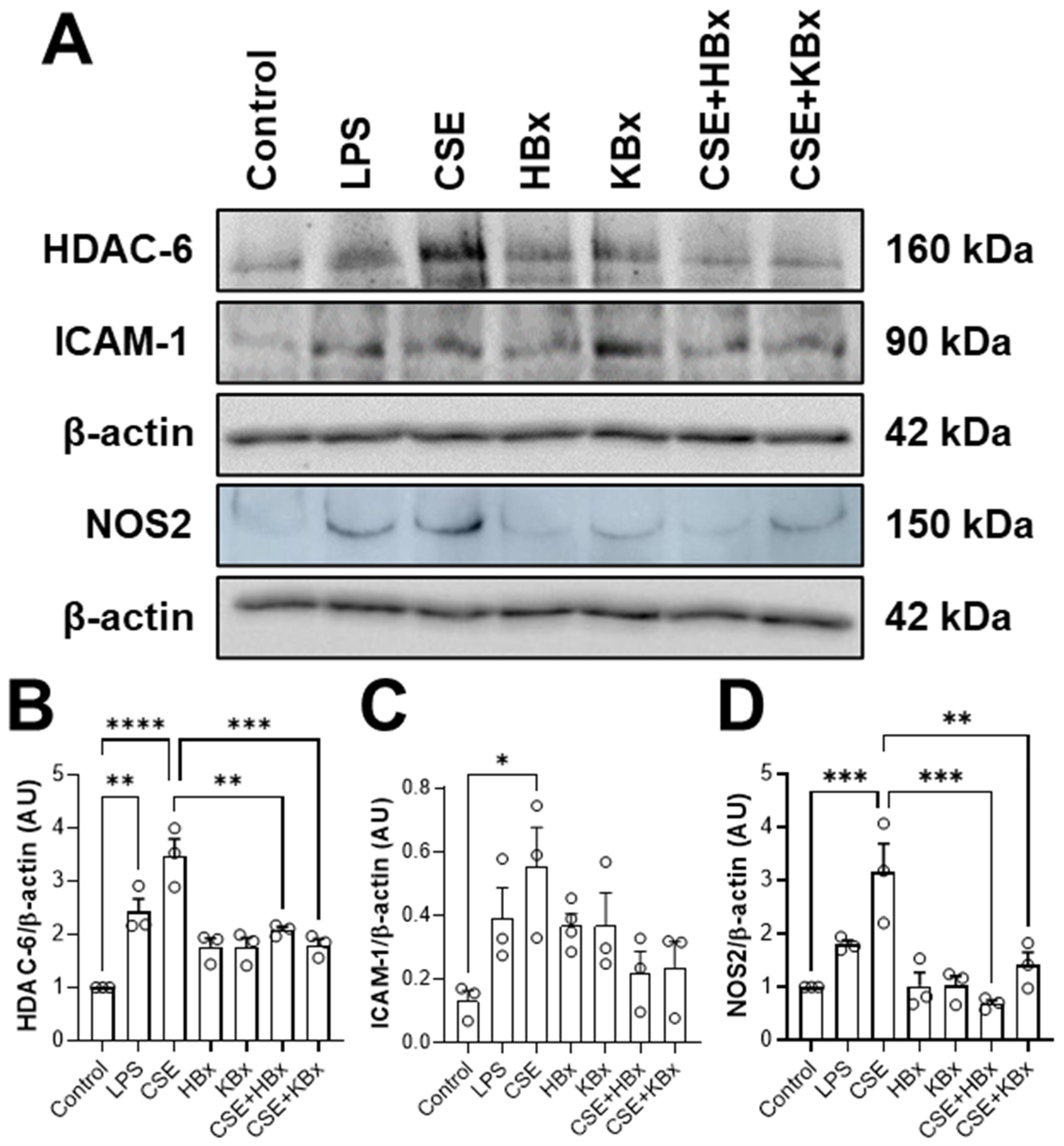
CSE exposure increased ICAM-1 expression, contributing to immune cell recruitment and inflammation. However, treatment with HBx or KBx reversed ICAM-1 expression to baseline levels, reducing cell migration and macrophage presence in the BAL [
33], corroborating our previous study with polymeric nanoparticles of bixin, although this mechanism was not investigated on this occasion [
2]. This effect is likely mediated by HBx and KBx, which modulate ICAM-1 and reduce NF-κB activation by limiting p-p65 nuclear translocation [
34,
35,
36,
37]. Additionally, both compounds inhibited actin filament polymerization, crucial for cell migration, further reducing migratory capacity in the experimental model. HDAC6, a key enzyme in actin polymerization during inflammation [
38,
39], may also play a role in this process, since molecular docking shows bixinate to chelate the zinc atom in catalytic site of the enzyme. The production of ROS and reactive nitrogen species (RNS) during inflammation is closely linked to proteins such as NOS2 (inducible nitric oxide synthase) [
17], which marks the M1 pro-inflammatory macrophage profile. Elevated NOS2 expression and activity lead to excessive nitric oxide (NO) production [
40]. While physiological levels of NO support tissue homeostasis, excessive NO reacts with superoxide to form peroxynitrite, causing nitrative protein damage [
41]. In cells exposed to CSE, NOS2 levels were increased, but it was reversed by HBx and KBx, demonstrating their ability to prevent nitrative protein damage.
Nicotine, a key CSE component, activates the NF-κB pathway, leading to the nuclear translocation of the p65 subunit and the transcription of pro-inflammatory genes like KC, TNF-α, NOS2, and ICAM-1 [
34,
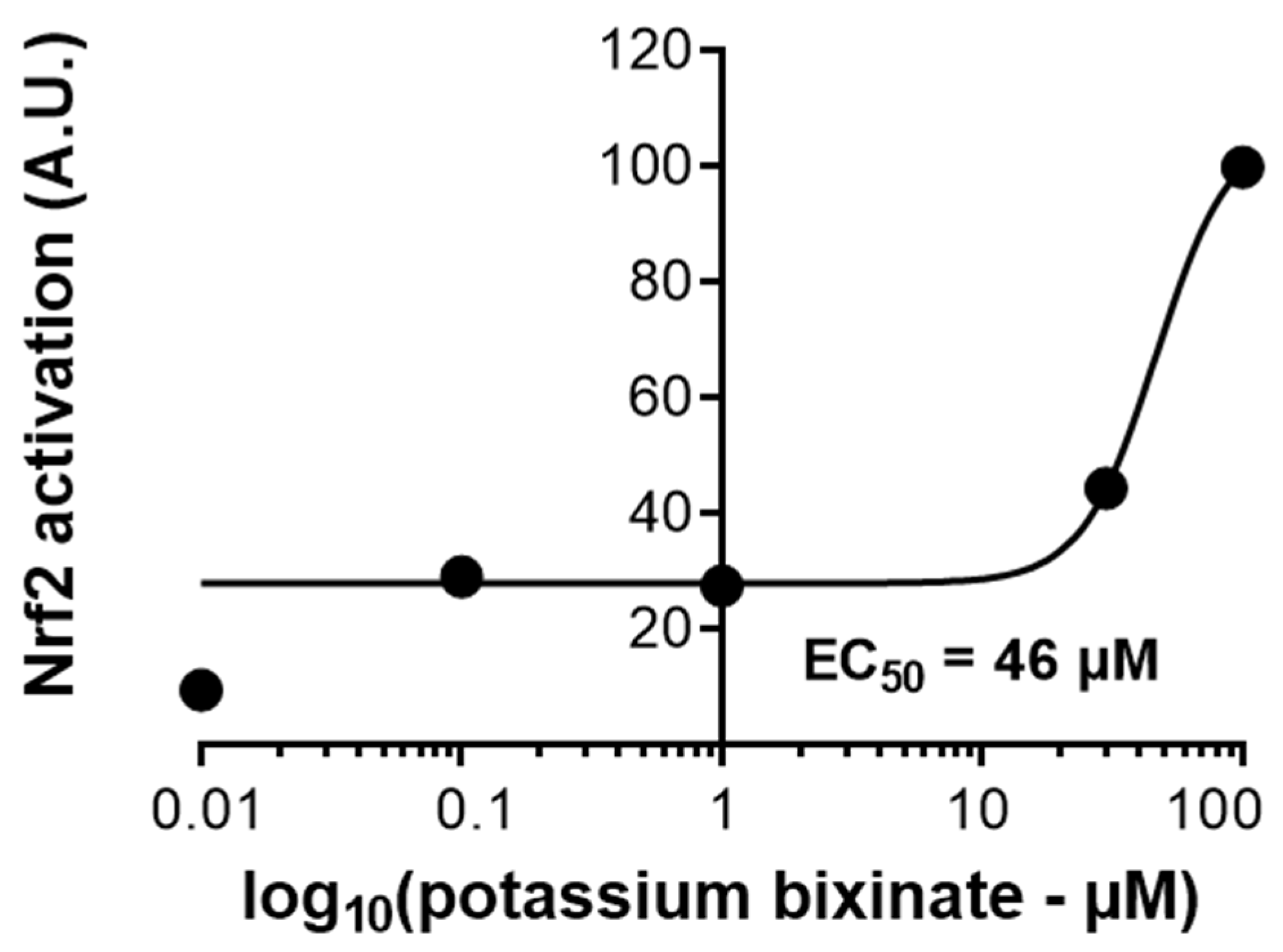
42]. CSE exposure heightened nuclear p-p65 levels, which were reduced by HBx and KBx, highlighting their role in inhibiting this pro-inflammatory pathway. To confirm KBx’s antioxidant capacity, a luciferase assay showed that it maintained Nrf2/ARE pathway activation and scavenged ROS effectively. Molecular docking analysis revealed that bixinate interacts with Keap1 through a protein–protein interaction inhibition mechanism rather than classical electrophilic activation, effectively inhibiting Nrf2-Keap1 interaction [
23]. These findings underscore HBx and KBx’s potential to modulate oxidative stress and inflammation via Nrf2 and NF-κB pathways.
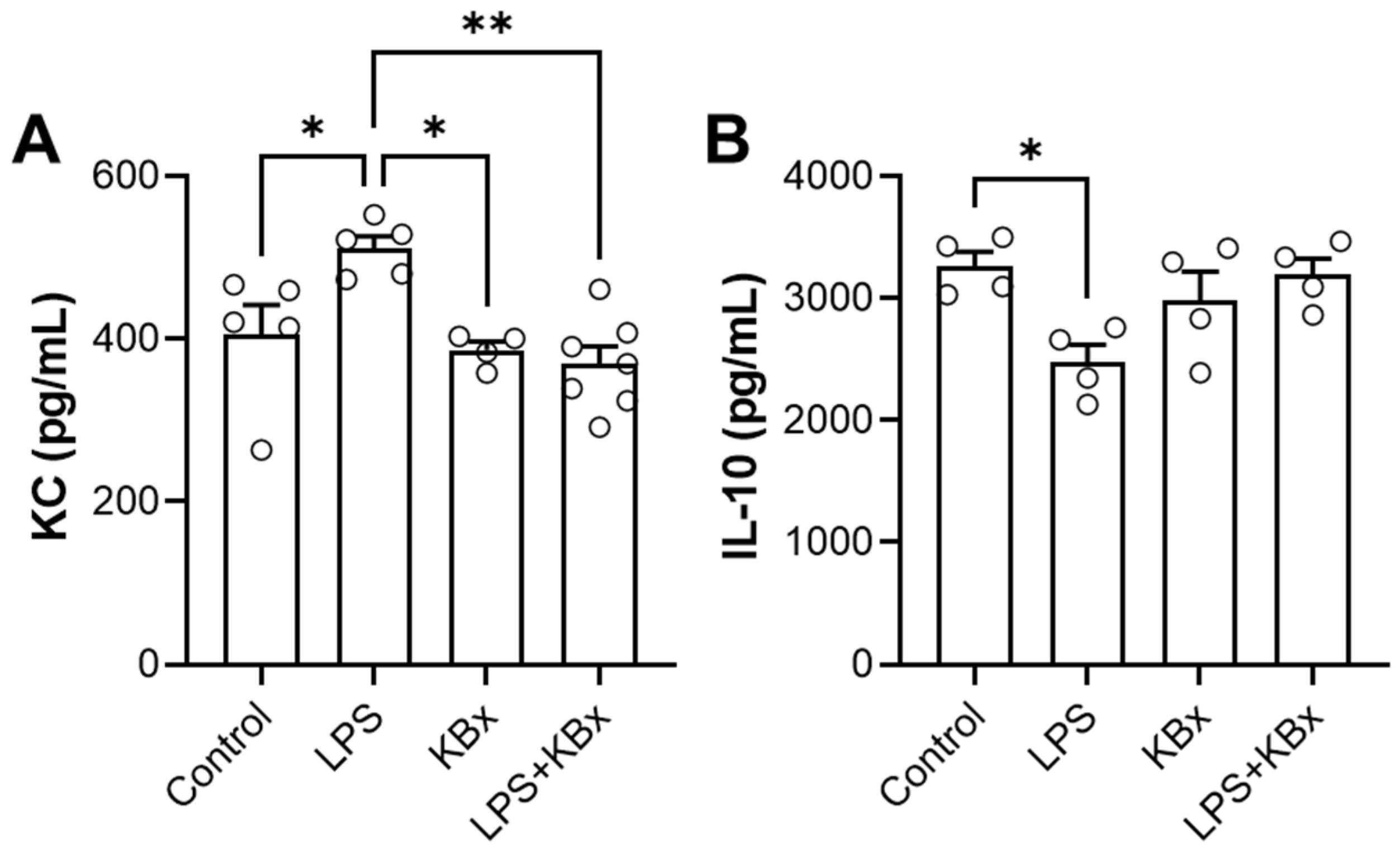
The in vivo experiment demonstrated that KBx, the potassium salt derivative of bixin, is absorbed via the gastrointestinal tract and preserves its anti-inflammatory properties. Mice pretreated with KBx showed reduced KC levels and increased IL-10 in BAL fluid after LPS-induced lung inflammation, confirming KBx’s therapeutic potential. Bixin and KBx also reduced markers of inflammation such as TNF-α, IL-6, and neutrophil influx in lung tissue, primarily through Nrf2 activation, which prevents its degradation and promotes nuclear translocation, reducing ROS levels and oxidative stress.
Nrf2 plays a critical role in modulating immune response in COPD, with reduced expression linked to chronic inflammation and oxidative stress [
41,
42]. RAW 264.7 macrophages were used as a model to study Nrf2 activation due to their relevance in inflammatory and oxidative stress responses, including their polarization to an M2 phenotype that aids in inflammation resolution. Single-cell analyses from the COPD Cell Atlas revealed heterogeneous Nrf2 expression across immune cell types, emphasizing its potential as a therapeutic target [
24]. The Nrf2/ARE signaling pathway is essential for defense against oxidative stress, and bixin has been shown to modulate this pathway by releasing Nrf2 from Keap1, allowing for antioxidant gene activation.
Previous studies in mice using bixin-loaded nanoparticles showed promising anti-inflammatory effects [
2,
7], but the compound’s low solubility limited its application. The development of KBx addresses this limitation by enhancing solubility and enabling effectiveness in vivo absorption without nanotechnology, making long-term treatments more feasible and cost-effective. These findings underscore the therapeutic promise of HBx and KBx for chronic inflammatory diseases like COPD. Future research should focus on optimizing dosages, ensuring long-term safety, and further elucidating the mechanisms of Nrf2 activation and its effects on immune cell function and inflammation.
In conclusion, this study is the first to report the use of the Z-isomer of HBx in both in vivo and in vitro experiments, as well as the development of its potassium salt derivative for treatment purposes. This approach successfully addressed the challenges associated with HBx’s chemical structure that hinder its oral absorption, making it a viable candidate for therapeutic use. KBx demonstrated bioavailability and effectiveness while retaining the anti-inflammatory and antioxidant properties previously described for HBx, offering potential as a treatment targeting macrophage and lung tissue stressors.
4. Materials and Methods
4.1. Reagents
Annatto’s seeds were purchased from Florien Fitoativos (São Paulo, SP, Brazil). Hexane, chloroform, methanol, ethanol, isopropyl alcohol, dodecane, dimethyl sulfoxide (DMSO), acetonitrile for HPLC, methanol for HPLC, pH 2.0 buffered solution, L-α-soy phosphatidylcholine, acyclovir, ceftriaxone, coumarin, ofloxacin, verapamil, nitro-tetrazolium blue chloride (NBT), lipopolysaccharide (LPS), SIGMAFAST™ Protease Inhibitor, sodium orthovanadate, bovine serum albumin (BSA), mitomycin C, RIPA buffer, ethylenediaminetetraacetic acid (EDTA), methylthiazolyldiphenyl-tetrazolium bromide (MTT), hydrochloric acid (HCl), sodium hydroxide (NaOH), synthetic poly-vinylidene fluoride (PVDF) membrane, potassium hydroxide (KOH), sodium chloride (NaCl), sodium dihydrogen phosphate (NaH2PO4), sodium hydrogen phosphate (Na2HPO4), potassium phosphate dibasic (K2HPO4), potassium phosphate monobasic (KH2PO4), sodium formate, Tween-20, and heparin were obtained from Sigma-Aldrich (Saint Louis, MO, USA). ActinRed, DAPI (4′,6-diamidino-2-phenylindole), SuperSignal West Femto Maximum Sensitivity Substrate is an ultra-sensitive enhanced chemiluminescent (ECL) substrate, BCA Protein Assay Kit, Opti-MEM, phosphate-buffered saline (PBS) at pH 7.4, Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), DMEM FluoroBrite, and Lipofectamine 2000 were obtained from ThermoFisher Scientific (Waltham, MA, USA). DMSO-d6 containing 0.05% v/v TMS was obtained from Cambridge Isotope Laboratories, Inc. (Cambridge, MA, USA). Immun-Blot® PVDF membrane, glycine, Tris-HCl, sodium dodecyl sulfate (SDS), 10× Tris/Glycine/SDS buffer, 10× Tris/Glycine buffer, and 30% Acrylamide/Bis Solution (37.5:1) were obtained from Bio-Rad Laboratórios do Brasil (São Paulo, Brazil). Isoflurane was obtained from Cristália Produtos Químicos e Farmacêuticos (Rio de Janeiro, Brazil).
4.2. Bixin Purification, Characterization, and Salt Synthesis
The annatto’s seeds underwent a two-step Soxhlet extraction process. First, hexane was used for 8 h, followed by chloroform extraction for another 8 h. The red organic phase obtained from the chloroform extraction was concentrated using a rotary evaporator. The remaining residue was then dissolved in a 5% (
w/
v) methanolic NaOH solution under magnetic stirring for 45 min. The resulting alkaline solution was filtered to remove insoluble impurities. Bixin was precipitated by adding diluted HCl solution until the pH reached 4. The precipitate was then filtered using a vacuum apparatus. The solid material retained on the filter was washed with distilled water until a neutral pH was achieved. Finally, it was dried at 70 °C until all residual solvents evaporated completely [
2,
7].
Bixin purification was achieved by recrystallizing a hot 9:1 ethanol:water mixture. The extracted powder was dissolved in hot ethanol under magnetic stirring, filtered, and mixed with hot water in the appropriate ratio. The solution was left at 25 °C for 24 h to allow for bixin recrystallization. Analytical HPLC was used to determine the compound’s purity. The HPLC system employed an LC-20AD Shimadzu (Kyoto, Japan) equipped with a 100-5C18 Kromasil (Bohus, Sweden) analytical column (4.6 mm × 250 mm, 5 μm). Detection was performed with a SPD-M20A Shimadzu (Kyoto, Japan) detector set at 254 nm and 457 nm wavelengths. The isocratic mobile phase for HPLC analysis consisted of 85% acetonitrile and 15% methanol at 22 °C with a 1 mL/min flow rate.
Bixin was characterized using an externally calibrated electrospray ionization spectrometer, amaZon SL model Esquire 6000—ESI Ion Trap Msn System Bruker (Billerica, MA, USA). Sodium formate was used for external calibration in a range from 75 to 1200 m/z. Infrared (IR) spectra (cm−1) were obtained using a Nicolet iS10 FTIR Spectrometer (ThermoFisher Scientific, Waltham, MA, USA) in a range from 400 to 4000 cm−1. The melting point of bixin was determined using a manual fusiometer Q340.23 Quimis (São Paulo, Brazil) with a glass capillary method.
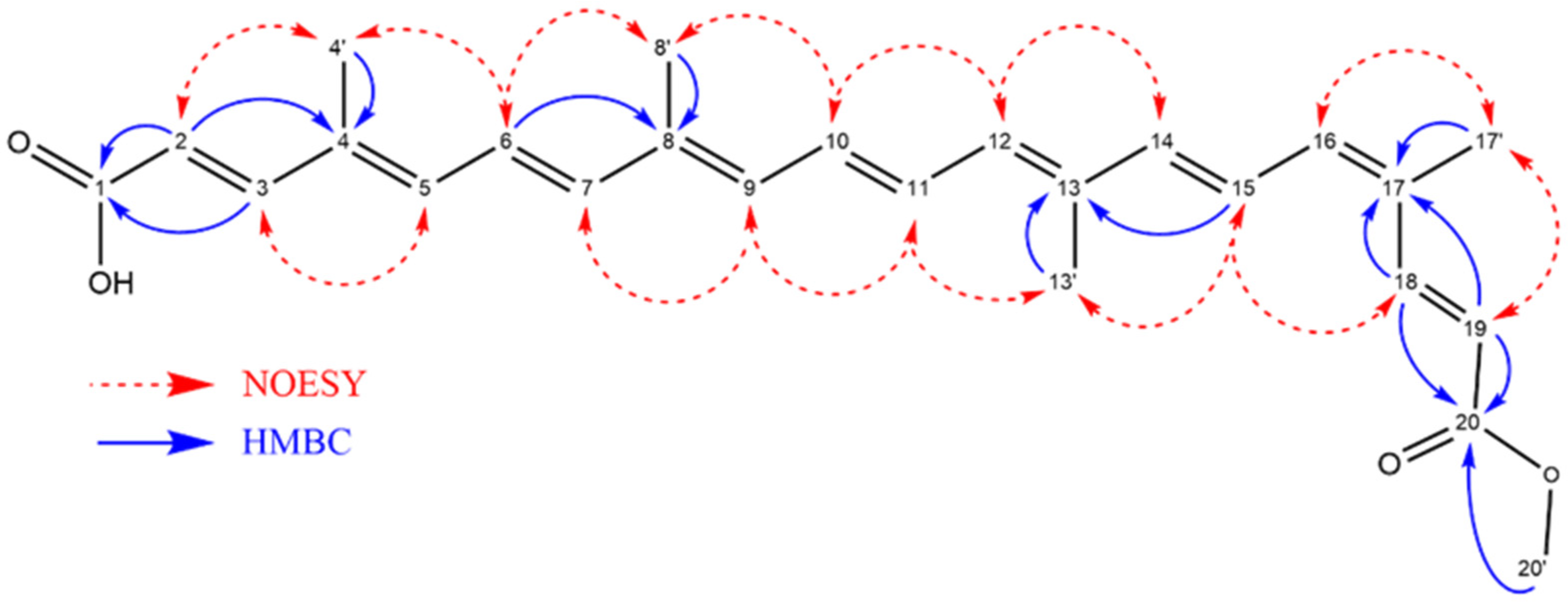
Nuclear magnetic resonance (NMR) analyses were performed on a 2.0 mg sample of bixin dissolved in 600 μL of DMSO-d6 containing 0.05% v/v TMS. All spectra were acquired on an Agilent VNMRS-500 spectrometer (1H at 499.79 MHz and 13C at 125.68 MHz) (Santa Clara, CA, USA) at 25 °C and processed using MestReNova software v. 14.3.3. The following parameters were used for spectral acquisition:
1H NMR (64 scans);
1H–13C HSQC pure shift (16 scans, 1500 points in f2 and 400 in f1);
1H–13C HMBC (32 scans, 901 points in f2 and 512 in f1);
1H–1H COSY (16 scans, 901 points in f2 and 512 in f1);
1H–1H NOESY (200 ms mixing time, 16 scans, 901 points in f2 and 512 in f1).
Potassium bixinate was prepared using a solvent evaporation method. The reaction involved bixin and KOH crystals in a 1:1 stoichiometric ratio within an aqueous medium. The reaction was carried out in a round-bottomed flask in an ice bath. After complete solubilization of the bixin, the solution was transferred to an oil bath for complete solvent evaporation, resulting in a potassium bixinate precipitate. Potassium bixinate was characterized using the same methods employed for bixin, including thermal analysis (melting point), physical analysis (MS), and physicochemical analysis (IR). For transmission electron microscopy (TEM) analysis, 10 μL of a 1 mg/mL solution was dropped onto a 300-mesh copper grid without staining, and images were obtained using a HT7800 HITACHI (Tokyo, Japan) microscope at 100 kV.
4.2.1. Bixin
1H NMR (500 MHz, DMSO-d6): δ 7.89 (d, J = 15.4 Hz, 1H), 7.27 (d, J = 15.5 Hz, 1H), 6.93–6.83 (m, 1H), 6.84–6.78 (m, 2H), 6.71 (dd, J = 14.7, 11.2 Hz, 1H), 6.62 (t, J = 14.2 Hz, 2H), 6.57–6.42 (m, 4H), 5.95 (d, J = 15.4 Hz, 1H), 5.83 (d, J = 15.5 Hz, 1H), 3.70 (s, 3H), 1.96 (dd, 12H). 13C NMR, (DMSO-d6, 125 MHz): δ 167.66 (C1), 148.05 (C3), 141.31 (C7), 140.43 (C14), 139.75 (C18), 138.87 (C5), 137.89 (C16), 136.62 (C8), 134.38 (C12), 133.22 (C4), 131.25 (C11), 130.99 (C17), 124.61 (C6), 122.95 (C15), 117.48 (C19), 116.99 (C2), 51.27 (C20), 19.73 (C17′), 12.50 (C13′), 12.30 (C4′). HPLC457nm: 97.78% Melting point: 199–204 °C. MS: 395.22 m/z. FTIR: 727 cm−1, 964 cm−1, 1159 cm−1, 1379 cm−1, 1431 cm−1, 1608 cm−1, 1717 cm−1, 2853 cm−1, 2922 cm−1 and 3426 cm−1.
4.2.2. Potassium Bixinate
HPLC457nm: 95.56% Melting point: 187–191 °C. MS: 433.1778 m/z. FTIR: 1172 cm−1, 1421 cm−1, 1557 cm−1, 1692 cm−1 and 2851 cm−1.
4.3. Drug-like Properties Determination
For the kinetic solubility assay, a 10 mM stock solution of bixin was analytically prepared in DMSO and then diluted in different buffered solutions at pH 2.0 (commercial), 6.8 (potassium-phosphate-buffered saline solution (KPE) buffer—100 mM K2HPO4, 100 mM KH2PO4 and 5 mM EDTA), and 7.4 (PBS buffer) to a final concentration of 200 μM. These solutions were incubated in a shaking water bath at 37 °C for 4 and 24 h. Immediately after each incubation period, the samples were centrifuged at 21,000× g Universal Centrifuge 320R Hettich (Tuttlingen, Germany) at 22 °C. The supernatant was collected, filtered through a 0.44 μm Millipore filter (Sigma-Aldrich—Saint Louis, MO, USA) into a vial, and analyzed using a HPLC LC-20AD Shimadzu (Kyoto, Japan) coupled with a DAD detector set at 457 nm. The mobile phase consisted of acetonitrile and methanol (85:15 v/v) using a 100-5C18 Kromasil (Bohus, Sweden) analytical column (4.6 mm × 250 mm, 5 μm) as the stationary phase in isocratic mode at 22 °C with a flow rate of 1 mL/min. A calibration curve was prepared using bixin in acetonitrile at concentrations ranging from 0 to 200 μM and analyzed under the same HPLC conditions. LogS was calculated as the base-10 logarithm of the solubility (in μM) for each time point.
The parallel artificial membrane permeability assay (PAMPA) utilizes a 96-well plate system in a “sandwich” format, where one plate is positioned above another (PAMPA) [
43]. The upper plate, known as the donor compartment, contains the compounds (test or control) dissolved in a buffered solution. This compartment features a PVDF membrane impregnated with a lipid solution, creating a barrier that allows for the compounds to diffuse to the lower plate, termed the receptor compartment [
44]. The lipid composition of the PVDF filter varies depending on the type of permeability test. For gastrointestinal tract (GIT) permeability assessments, L-α-soy phosphatidylcholine in dodecane is used. Optical density values are measured at specified wavelengths for each compound and compared against controls. For GIT permeability, the absorbed fraction (Fa%) and permeability (Pe) are determined. PAMPA-GIT results categorize compounds based on their absorbed fraction as having high (70–100%), medium (30–69%), or low (0–29%) intestinal permeability. Compounds for these assays were diluted from a 10 mM stock solution. These assays were conducted using a 1 mg/mL stock solution per compound. The PAMPA’s validity was confirmed by comparing experimental data with the literature reports on the artificial membrane permeability of various drugs (acyclovir, ceftriaxone, coumarin, norfloxacin, and verapamil) [
22].
4.4. Rat Plasma and Microsomal Stability
Two- to three-month-old healthy male Wistar rats (with an average weight of 385 g) were obtained from the Graduation Program in Pharmacology and Medicinal Chemistry Bioterium (CCS/UFRJ) (Rio de Janeiro, Brazil). The rats were fed ad libitum with Purina chow (Nuvilab, Curitiba, Brazil) and had unrestricted access to water. They were housed in a controlled environment maintained at 22 ± 2 °C, 50–70% relative humidity and a 12 h light/dark cycle. The local Animal Ethics Committee approved all experimental procedures (046/21).
A 10 mM stock solution of bixin was prepared in DMSO. Rat blood was collected with heparin and centrifuged. The plasma fraction was separated and diluted to 80% in PBS at pH 7.4. Then, 50 μL of the 80% rat plasma solution was added to a final volume of 250 μL of a KPE at pH 7.4 containing 50 μM of bixin. The bixin was incubated with rat plasma under these conditions for 0, 30, 60, 120, and 240 min with shaking in a water bath at 37 °C. Immediately after the incubation time, proteins were precipitated, and bixin was extracted by adding 1000 μL of cold acetonitrile: methanol (1:1) mixture containing 5 μM of an internal standard. The resulting solution was centrifuged at 20,000× g in a Universal Centrifuge 320R Hettich (Tuttlingen, Germany) at 4 °C for 15 min. The supernatant was collected, filtered through a 0.22 μm filter into a vial, and then analyzed by HPLC using an LC-20AD Shimadzu (Kyoto, Japan) system equipped with a 100-5C18 Kromasil (Bohus, Sweden) analytical column (4.6 mm × 250 mm, 5 μm) and an SPD M20A diode array detector Shimadzu (Kyoto, Japan) set to wavelengths of 271 nm and 457 nm. The solvent system for the HPLC analysis consisted of acetonitrile and methanol in an 85:15 ratio in isocratic mode at 22 °C with a flow rate of 1 mL/min.
A final solution of microsomes (1 mg/mL protein) in 250 μL of PBS at pH 7.4 containing 50 μM of bixin was prepared in the absence of an NADPH-regenerating metabolic cofactor system. The bixin was incubated with rat microsomes under these conditions for 0, 30, 60, 120, and 240 min with shaking in a water bath at 37 °C. The subsequent steps for protein precipitation, bixin extraction, and HPLC analysis were identical to those described for the rat plasma stability assay.
4.5. Cigarette Smoke Extract (CSE) Preparation
CSE was prepared immediately before each experiment. The extract was obtained by collecting the smoke from a burning cigarette (Marlboro®, 10 mg tar, 0.8 mg nicotine, and 10 mg carbon monoxide—Richmond, VA, USA) and transferring it into 10 mL of DMEM at a rate of 1/2 cigarette per minute. The pH was adjusted to 7.4, when necessary, immediately after the procedure, and the resulting solution was filtered using a 0.22 μm Millipore filter (Sigma-Aldrich—Saint Louis, MO, USA). The final concentration of this solution was expressed as a percentage (100% corresponds to 1 cigarette per 1 mL of medium). A serial dilution of the CSE was performed to achieve concentrations of 0.65%, 1.25%, 2.5%, 5%, and 10%. CSE quality control was conducted by measuring the absorbance at 320 nm.
4.6. RAW 264.7 Macrophage Cell Line and Experimental Design
The RAW 264.7 macrophage cell lines were cultured in DMEM supplemented with 10% (v/v) FBS, pH 7.2, at 37 °C in a humidified atmosphere with 5% CO2. RAW 264.7 cells were plated in a 24-well plate (3.5 × 105 cells/well) in DMEM with 1% (v/v) FBS and left overnight to adhere. Subsequently, 5% CSE in DMEM was added to each well for 2 h. After incubation, the medium was removed, and a 100 μM solution of bixin or potassium bixinate (diluted in DMEM; bixin was first prepared as a stock solution in DMSO) was added for an additional 1 h.
4.7. MTT Assay
RAW 264.7 cells were plated (5 × 104 cells/well) in a 96-well plate with DMEM and 10% FBS. When cells reached 90% confluence, the 10% (v/v) FBS DMEM was discarded and replaced with the stimuli. The plate was incubated at 37 °C and 5% CO2 for 4 h, after which MTT solution (5 mg/mL) in DMEM 1% (v/v) FBS was added. The metabolism of MTT by the cells was monitored by the formation of insoluble salts, which were then dissolved in isopropyl alcohol. Absorbance was read at 570 nm using a Varioskan LUX (ThermoFisher Scientific, Waltham, MA, USA). Cell viability was assessed at different concentrations of CSE (0–10%), bixin, and potassium bixinate (0–200 μM). Cell viability was normalized in all groups by the control (1% (v/v) FBS DMEM), with 10% (v/v) FBS in DMEM used as the positive growth control.
4.8. ROS Scavenger Activity Assay
ROS levels were determined using the NBT method [
45]. RAW 264.7 cells were incubated for 1 h with 10 μg/mL LPS and different concentrations of bixin or potassium bixinate (0–200 μM) to evaluate their ability to intercept superoxide anions induced by LPS. After incubation, a 10% (
w/
v) NBT solution was added to each well and incubated for 1 h at 37 °C. Cellular membranes were permeabilized with 2 M KOH, and the resulting formazan was solubilized in DMSO. Optical density (OD) was measured at 630 nm using a Varioskan LUX spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA).
4.9. Cell Lysate, Protein Content Determination, and Western Blotting
Cells were lysed in RIPA buffer containing SIGMAFAST™ Protease Inhibitor and 1 mM sodium orthovanadate. Samples were then analyzed using the bicinchoninic acid (BCA) method (BCA Protein Assay Kit). Standard curves with known albumin concentrations (0–2 mg/mL) were used. The working solution was prepared according to the manufacturer’s instructions. For protein quantification, 10 μL of the sample was mixed with 100 μL of the working solution, and the same procedure was performed for each point on the standard curve in a 96-well plate. The plate was incubated at 37 °C, protected from light, to allow for the reaction to occur and the colorimetric complex to form. Absorbance was read at 565 nm using a Varioskan LUX spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA).
Cell lysates (10–20 μg of protein) were separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel for electrophoresis) (8 or 12% polyacrylamide) and transferred (25 V, 1.0 A, 30 min—Trans-Blot Turbo Transfer System, Bio-Rad—São Paulo, Brazil) to a PVDF membrane. The proteins were incubated overnight at 4 °C with the following primary antibodies: anti-ICAM-1 (gt5811-goat-1:250; gel of 10%) (Sigma-Aldrich, Saint Louis, MO, USA), anti-NOS2 (ab178945-rabbit-1:1000; gel of 8%) (Abcam—Cambridge, United Kingdom), anti-HDAC6 (#7612-rabbit-1:1000; gel of 8%) (Cell Signaling Technology—Danvers, MA, USA), and anti-β-actin (a5441-mouse-1:5000; gel of 12%) (Sigma-Aldrich—Saint Louis, MO, USA). Membranes were then incubated for at least 1 h with peroxidase-conjugated secondary antibodies (ab6789-mouse-1:10,000, ab6721-rabbit-1:10,000, or ab6741-goat-1:10,000) (Abcam, Cambridge, UK). Immunoreactive proteins were visualized using chemiluminescence with the assistance of an ECL solution, and membranes were developed and photographed using a ImageQuant™ LAS500, LAS4000 (GE Healthcare, Chicago, IL, USA) or a ChemiDoc (Bio-Rad, São Paulo, Brazil). Densitometry was quantified using ImageJ software v. 1.54p. Results are expressed as densitometric values for each protein normalized to β-actin.
4.10. Wound Healing Migration Assay
RAW 264.7 cells were seeded in a 24-well plate (4.0 × 105 cells/well) and cultured until maximum confluence. Cells were then treated with mitomycin C (5 μg/mL) in FBS-free DMEM for 2 h, followed by washing with PBS. A linear scratch was made with a 200 μL pipette tip in each well. Cells were then stimulated for 24 h with the stimuli diluted in DMEM supplemented with 1% (v/v) FBS. Wound closure was monitored by capturing images at 0 and 24 h. The area of wound closure in each image was measured at five different points using a ruler tool, and an average was calculated for each image and condition. Cell migration was quantified as the percentage of wound closure at 24 h compared to the area at 0 h for each group.
4.11. Immunocytochemistry for p-p65
RAW 264.7 cells were seeded in a 24-well plate (2.5 × 105 cells/well) and stimulated as previously described. After reaching 70% confluence, cells were blocked with 5% (w/v) BSA for 1 h, then washed three times in PBS-Tween 20 (0.1% (v/v)). The supernatants were replaced with a solution containing the primary antibody against p-p65 (#3033-rabbit-1:500) (Cell Signaling Technology, Danvers, MA, USA) diluted in PBS-T with 1% (w/v) BSA and incubated overnight. After incubation, cells were incubated for 1 h with a secondary antibody conjugated to Alexa Fluor-488 (1:1000; ThermoFisher Scientific, Waltham, MA, USA). Cells were then washed three times with PBS-T and incubated with DAPI for 5 min. After the final wash, DMEM FluoroBrite was added, and fluorescence was analyzed using the EVOS inverted microscope (ThermoFisher Scientific, Waltham, MA, USA). Images were captured at 20× magnification from at least 10 fields for each experimental condition, and fluorescence intensity was quantified.
4.12. F-Actin Polymerization Assay
RAW 264.7 cells were seeded in a 24-well plate (2.5 × 105 cells/well) and stimulated as previously described. After reaching 70% confluence, cells were blocked with 5% (w/v) BSA for 1 h and washed three times in PBS-Tween 20 (0.1% (v/v)). The supernatants were replaced with ActinRed (ThermoFisher Scientific, Waltham, MA, USA) for 30 min, followed by washing and incubation with DAPI for 5 min. After the final wash, DMEM FluoroBrite was added, and fluorescence was analyzed using the EVOS inverted microscope (ThermoFisher Scientific, Waltham, MA, USA).
4.13. Luciferase ARE (Antioxidant Response Element) Reporter Assay
RAW 264.7 cells (3.5 × 105 cells/well) were seeded into 24-well plates in FBS-free DMEM. Next day, cells were transfected with the ARE-responsive luciferase reporter kit (#60514, BPS Bioscience, San Diego, CA, USA) in Opti-MEM containing Lipofectamine 2000 and incubated for 24 h. After stimulation with different concentrations of potassium bixinate (0–200 μM), the luciferase-containing medium was collected and incubated with luciferin substrate (Dual Luciferase Assay System) (#60683-1, BPS Bioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Luminescence emitted by luciferin cleavage was measured using the Varioskan LUX plate reader (ThermoFisher Scientific, Waltham, MA, USA).
4.14. Mouse Experimental Design
Eight-week-old male C57BL/6 mice were obtained from the Multidisciplinary Center for Biological Research in Laboratory Animal Science (CEMIB/UNICAMP, Campinas, Brazil). The mice were provided with Purina chow ad libitum (Nuvilab, Curitiba, Brazil) and had unrestricted access to water in a controlled environment maintained at 22 ± 2 °C, 50–70% relative humidity, and a 12 h light/dark cycle (EB-273B, Insight Equipamentos Ltd., São Paulo, Brazil). They were acclimatized for two weeks before the experimental procedures. All experimental protocols were approved by the local Animal Ethics Committee (096/19).
To evaluate the pharmacological effects of potassium bixinate, the animals were divided into four groups (n = 5–7): (1) Control, (2) KBx, (3) LPS, and (4) LPS + KBx. For five consecutive days, animals in groups 2 and 4 were treated by oral gavage with 200 mg/kg of potassium bixinate diluted in PBS in a final volume of 100 μL, while animals in groups 1 and 3 were treated with 100 μL of PBS. On the sixth day, animals in groups 3 and 4 were challenged intranasally with 60 μg of LPS (extracted from Escherichia coli O55:B5) diluted in PBS (10 μL), while animals in groups 1 and 2 received 10 μL of PBS intranasally. Sixteen hours after LPS/PBS instillation, the animals were euthanized by cervical dislocation under isoflurane sedation.
4.15. Bronchoalveolar Lavage (BAL) Collection
Immediately following euthanasia, BAL was collected using an intratracheal cannula. The lavage was performed with 1.5 mL of saline solution (3 × 0.5 mL). The collected lavage fluid was centrifuged at 600× g for 10 min, and the supernatant was stored at −80 °C for subsequent enzyme-linked immunosorbent assay (ELISA) analysis.
4.16. ELISA for BAL Cytokine Measurement
Cytokine levels (IL-10 and KC; #900-K53K and #900-K127K) were quantified in BAL samples using an ELISA kit, following the manufacturer’s instructions (PeproTech, Neuilly Sur-Seine, France). A sample volume of 25 μL was used without dilution. The enzymatic reaction was initiated with peroxidase and a specific substrate, and the reaction was stopped before measuring absorbance at 450 nm and 570 nm using a Varioskan LUX spectrometer (ThermoFisher Scientific, Waltham, MA, USA). A calibration curve was generated using standard concentrations of the target protein, with the method showing linearity across a range of 2000–0 pg/mL.
4.17. In Silico Study
Molecular docking simulations were conducted using the Genetic Optimization for Ligand Docking (GOLD) software v. 5.6. The crystallographic structure of Keap1, identified by the PDB code 4IFN (resolution 2.40 Å), was obtained from the Protein Data Bank (PDB;
http://www.rcsb.org, accessed on 13 March 2025). The co-crystallized ligand associated with this structure served as a reference for validating the docking methodology and defining the binding site. Hydrogen atoms were added to the protein, and the binding pocket was delineated using the co-crystallized ligand and all amino acid residues within a 6 Å radius. Redocking of the co-crystallized ligand was performed to assess the reliability of the docking protocol. The validation of the methodology was determined by calculating the root-mean-square deviation (RMSD) between the experimentally observed pose and the redocked pose. The RMSD values obtained using the ASP (6.687), ChemPLP (5.938), ChemScore (6.922), and GoldScore (2.159) scoring functions were analyzed, and the scoring function with the best performance was selected for subsequent docking studies.
The structure of potassium bixinate was constructed, and its protonation state was determined using the Percepta software v. 2012. The molecule’s geometry was then optimized using the PM6 (Parametric Method 6) semi-empirical approach to generate the lowest-energy conformer for use in docking studies. Additionally, another docking was performed within the bixinate and HDAC6 crystal structure (PDB: 6UO2—resolution: 1.65 Å), following the same parameters used for the 4IFN. The redocking scoring functions results for validation were as follows: ASP (RMSD: 1.05684), ChemPLP (RMSD: 1.00982), ChemScore (RMSD: 1.88182), and GoldScore (RMSD: 1.08718).
4.18. COPD Cell Atlas Single-Cell Gene Expression Analysis
A search for the NFE2L2 gene was conducted in the COPD Cell Atlas database (
http://www.copdcellatlas.com, accessed on 23 April 2024) [
24] using the UMAP (Uniform Manifold Approximation and Projection) model across all cell subtype categories. An empirical analysis of its gene expression distribution was performed.
4.19. Statistical Analysis
All experiments were performed in triplicate, and the results are expressed as the mean ± SEM (Standard Error of the Mean). Statistical analyses were conducted using one-way ANOVA followed by Bonferroni’s post hoc test. GraphPad Prism software v. 8.0 was used for all statistical calculations. Statistical significance was set at p < 0.05.