Abstract
Background: Eugenia involucrata DC., a Cerrado native plant, is recognized for its medicinal properties. However, its bioactive compounds remain inadequately explored. Objectives: This study investigated bioactive compounds from a standardized liquid extract from E. involucrata leaves that can act with antioxidant, cytogenotoxic, cytoprotective, and genoprotective effects. Methods: The phenolic compounds in the standardized liquid extract from E. involucrata leaves were screened by HPLC-DAD. The capture of the free radicals DPPH, ABTS+, and the metal reduction power FRAP determined the antioxidant potential. Cytotoxicity was evaluated in RAW 264.7 macrophages (MTT assay), and (anti)cytotoxic and (anti)genotoxic effects were assessed in human lymphocytes using the Trypan blue exclusion method and comet assay, respectively. Results: The extracts present key phenolic compounds, such as ellagic acid, myricitrin, and epicatechin gallate. The standardized extract demonstrated antioxidant capacity, evidenced by its ability to reduce iron and scavenge free radicals. The liquid extract from E. involucrata leaves exhibited cytotoxic effects on RAW 264.7 macrophages at higher concentrations, while demonstrating (anti)cytotoxic activity on human lymphocytes from all tested concentrations. The highest concentration tested of the standardized liquid extract from E. involucrata leaves (250 µg/mL) showed genotoxicity against human lymphocytes compared to the negative control. In contrast, the lowest concentration (62.5 µg/mL) exhibited an antigenotoxic effect on human lymphocytes, reducing the genotoxicity of doxorubicin by approximately 27%. Conclusions: The bioactive compounds in the standardized liquid extract from E. involucrata leaves exhibited antioxidant and antigenotoxic properties, suggesting potential value for nutraceutical and pharmaceutical applications, particularly those related to oxidative stress associated withaging and disease progression.
1. Introduction
Eugenia involucrata DC. is a native Brazilian species of the Myrtaceae family, known as “pitanga-do-cerrado” in the Cerrado biome. The leaves of this plant are popularly used as a therapeutic agent to treat diabetes mellitus [1]. Previous studies have demonstrated the presence of flavonoids, tannins, and terpenoids in the leaves, fruits, and seeds of E. involucrata [1,2]. The biological activity of essential oils and extract fractions from this species has also been described, indicating its therapeutic potential for various pathological conditions [1,2,3]. However, this species remains understudied regarding its phytochemical and pharmaceutical properties.
The leaves of E. involucrata and its extract showed the highest content of phenolic compounds, with ellagic acid, myricitrin, and epicatechin gallate being the most prominent [4,5]. These compounds exhibit various biological activities, including anti-inflammatory, hypoglycemic, antimicrobial, and antioxidant effects. Plant extracts with antioxidant properties can protect the body against oxidative stress in different cellular processes, such as delaying aging and preventing or reducing damage caused by disease mechanisms [6].
Oxidative stress is generated by the production of free radicals (reactive oxygen and nitrogen species), which are chemical entities that, in excess, cause cellular damage, including the destruction of the cell membrane and DNA [7]. Many diseases, such as obesity, hypertension, and cancer, involve oxidative stress due to excessive free radical production, which accelerates disease progression [8]. Species of the Eugenia genus, such as Eugenia uniflora L., have antiproliferative activity against gastric adenocarcinoma and modulate both cell viability and migration in melanoma tumor cells [9,10], highlighting the antitumor potential of the Eugenia genus.
Furthermore, the literature reports that compounds with antioxidant activity may reduce tumor cells’ incidence and progression by mitigating oxidative stress and/or delaying abnormal cell division [11,12,13]. According to the Pan American Health Organization, cancer is one of the leading causes of death in the Americas. Additionally, it is estimated that by 2045, there will be six million cases, alarming data for public health [14]. Species of the Eugenia genus show significant antioxidant potential due to their phenolic compounds, such as flavonoids and tannins. These compounds effectively scavenge free radicals, thereby reducing oxidative stress associated with aging and various diseases. Supported by studies, the antioxidant properties of Eugenia spp. suggest their use in developing natural therapies to prevent cellular damage and enhance health, with potential applications in traditional and modern medicine [15,16].
The safety of using medicinal plants and herbal products is essential for ensuring consumer health and well-being. While many plants have traditional therapeutic benefits, they may also pose risks, including toxicity, drug interactions, and adverse effects. Rigorous safety evaluations help identify safe dosages, potential toxicities, and contraindications, enabling informed and responsible use by healthcare professionals and patients. Promoting safe practices in herbal medicine builds public trust and encourages the informed application of these alternatives in modern healthcare in harmony with cultural traditions [17,18].
Therefore, this study aimed to investigate the biological properties of Eugenia involucrata leaves by characterizing and standardizing the liquid extract, evaluating its antioxidant activity through DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS+, and FRAP (ferric reducing antioxidant power) assays, and determining its cytotoxicity in RAW 264.7 murine macrophages, as well as its cytogenotoxic, cytoprotective, and genoprotective effects on human lymphocytes.
2. Results and Discussion
2.1. Physicochemical Characterization of the Liquid Extract
Characterizing the liquid extract is essential to establishing process standardization controls, ensuring medicinal properties’ quality, safety, and reproducibility [19]. These parameters also serve as a foundation for technological development in herbal products [20]. The liquid extract exhibited a pH of 5.35 ± 0.01, a solid content of 4.59 ± 0.30%, and a density of 1.03 ± 0.02. The acidic pH enhances the stability of bioactive compounds and inhibits microbial growth [21], indicating the presence of acidic constituents. Density and solid content are critical for quality assessment and guiding downstream technological processes, such as drying [22].
Phenolic compounds are key markers for evaluating plant extracts’ biological potential. The liquid extract contained 1.5 ± 0.11 mg/mL of phenolics and 0.63 ± 0.01 mg/mL of flavonoids. Using the modified Rufino and Alves (2007) method [23], polyphenol content was quantified at 700.9 ± 6 mg gallic acid/100 g, corroborating prior results via an alternative methodology. This value surpassed those reported for other Brazilian species: Eugenia dysenterica (201.1 ± 10.7 mg/100 g), Byrsonima crassifolia (259.2 ± 13.8 mg/100 g), and Annona montana (192.1 ± 17.3 mg/100 g) [23,24].
2.2. Screening of Phenolic Compounds in Liquid Extract Obtained by Ultrasound from Eugenia involucrata Leaf Powder and Liquid Extract by HPLC-DAD
The chromatographic profile of the plant material (Eugenia involucrata leaves) and analytical standards are shown in Figure 1 and Supplementary Figure S1, respectively. The chromatographic analysis of the plant material indicated the presence of two phenolic compounds: epicatechin, detected at 280 nm with a retention time (RT) of 13.487 min, and rutin, detected at 340 nm with an RT of 14.528 min (Figure 1A,C). These findings were confirmed by comparing the retention times with those of the analytical standards for epicatechin (RT: 13.495 min) and rutin (RT: 14.529 min) (Supplementary Figure S1A,C). The liquid extract also contained rutin and epicatechin, as shown in Figure 2 and Figure 3.
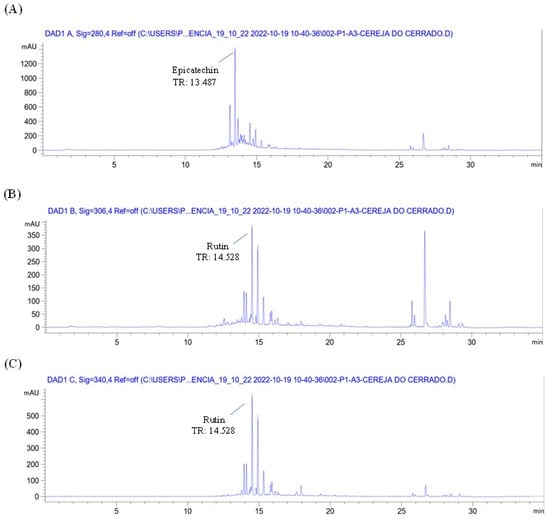
Figure 1.
Chromatogram of the plant drug (dried and powdered leaves) of Eugenia involucrata at 280 nm (A), 306 nm (B), and 340 nm (C) by HPLC-DAD. Extract obtained by ultrasound-assisted extraction. Chromatographic conditions: The analysis used a mobile phase of acetonitrile and water acidified with 0.2% acetic acid in a gradient elution system. The method employed an injection volume of 5 μL, a 1 mL/min flow rate, and maintained the column temperature at 30 °C. Detection was achieved using a diode array detector (DAD) with a C18 column. Peak heights were recorded in milliabsorbance units (mAU), and retention times in minutes (min).
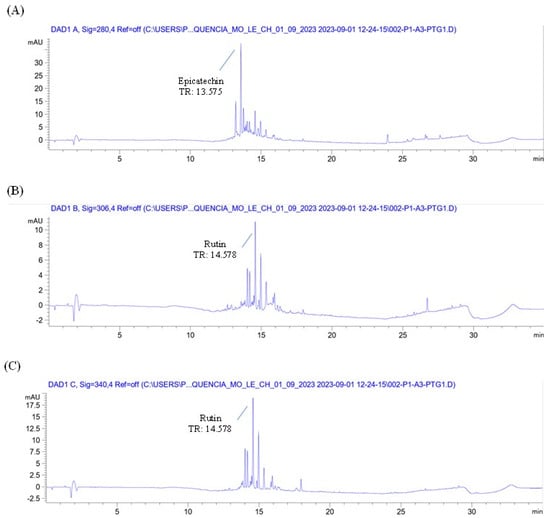
Figure 2.
Chromatogram of the liquid extract from Eugenia involucrata leaves at 280 nm (A), 306 nm (B), and 340 nm (C) by HPLC-DAD. Chromatographic conditions: Mobile phase consisting of acetonitrile and water acidified with 0.2% acetic acid, using gradient elution. Injection volume: 5 μL; flow rate: 1 mL/min; column temperature: 30 °C. Diode array detector (DAD); C18 column. Peak height (mAU); time in minutes (min); retention time (RT).
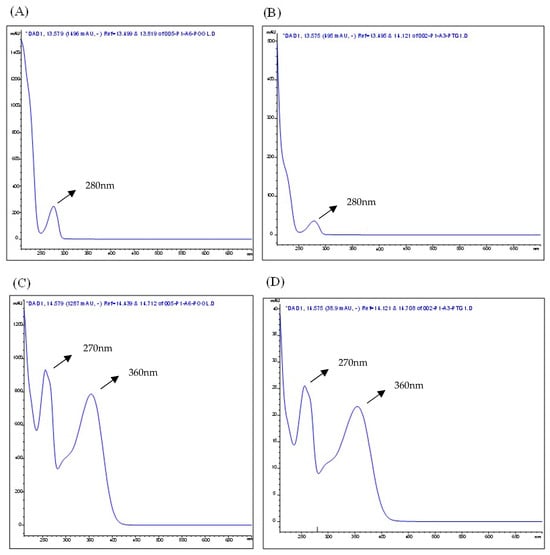
Figure 3.
The absorption spectra in the Ultraviolet–Visible (UV–Vis) region correspond to the peaks of the epicatechin standard (A) and epicatechin in the liquid extract from Eugenia involucrata leaves (B), as well as the rutin standard (C) and rutin in the liquid extract from E. involucrata leaves (D), as analyzed by HPLC-DAD. Chromatographic conditions: The analysis used a mobile phase composed of acetonitrile and water acidified with 0.2% acetic acid, with gradient elution. The method employed an injection volume of 5 μL, a 1 mL/min flow rate, and maintained the column temperature at 30 °C. Detection was performed using a diode array detector (DAD), with separation achieved on a C18 column. *DAD: Diode Array Detection.
A previous study also detected the presence of epicatechin and rutin in E. involucrata leaves [25]. However, those authors reported additional compounds, such as ellagic acid, gallic acid, quercetin, and catechin, which were not detected in our study. The absence of these compounds may be attributed to several factors, including the detection limit of the equipment and co-elution with other compounds. Thus, modifications in sample preparation techniques or analytical methods could enhance their detectability [26]. The absence of ellagic acid and other related compounds in our results may be attributed to our study’s specific extraction method and chromatographic conditions. Different extraction techniques, solvents, and chromatographic parameters can influence the profile of detected compounds, potentially leading to the non-detection of certain phenolics such as ellagic acid, which are commonly reported in other Eugenia species [27,28,29].
Epicatechin is a flavonoid belonging to the flavan-3-ol class, characterized by hydroxyl groups at positions 3′ and 4′ on the B ring. It has been associated with various biological activities, including antioxidant, antimicrobial, anti-inflammatory, antitumor, and cardioprotective effects [30]. Rutin (quercetin-3-rutinoside) is another flavonoid, classified as a flavonol, consisting of an aglycone (quercetin) bound to a rutinoside disaccharide. The literature reports highlight its multiple biological activities, such as analgesic, antiarthritic, antibacterial, cardioprotective, antioxidant, and antidiabetic properties [31]. Both compounds have been previously reported in species of the Eugenia genus [25].
2.3. Determination of the Antioxidant Activity of the Liquid Extract
The antioxidant activity of the E. involucrata leaf extract was evaluated by DPPH, ABTS+, and the metal reduction power FRAP (Table 1). The ABTS+ method, applicable to hydrophilic and lipophilic matrices [32,33], showed the highest activity (2701.67 μM Trolox/g extract), followed by FRAP (1490.79 μM Fe2+/g extract), which evaluates the electron-donating capacity with high reproducibility [33]. The DPPH assay, based on hydrogen or electron transfer to a stable radical [32], indicated moderate activity (75.0 ± 11 µg extract/µg DPPH). These differences highlight the extract’s broad antioxidant profile and the relevance of combining multiple assays for a more comprehensive evaluation.

Table 1.
Antioxidant activity using the DPPH, FRAP, and ABTS+ methods on the liquid extract from the leaves of Eugenia involucrata DC. Capture of the free radical 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+); capacity to reduce the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH); power of iron reduction (FRAP); relative standard deviation (%).
In this context, relying on a single method to determine antioxidant activity may yield insufficient information due to multiple factors, including the type of matrix used and the different mechanisms/sites of antioxidant action [34]. Therefore, employing various methods, as presented in this study, becomes essential to provide a more comprehensive understanding of the potential mechanisms of the analyzed material.
Compared with other species of the same genus evaluated in previous studies, the leaf extract of E. involucrata demonstrates a promising antioxidant profile. For instance, in the study by Ferreira et al. (2023) [35], Eugenia uniflora exhibited a lower DPPH IC50 value (1.46 µg/mL), indicating more vigorous radical scavenging activity in that specific assay. Conversely, its ferric reducing antioxidant power (FRAP) value was significantly lower (78.45 µM TE/g), suggesting that E. involucrata possesses superior ferric ion-reducing capacity.
Barbosa et al. (2025) [15] reported that Eugenia dysenterica showed FRAP values between 1633 and 1692 µM FeSO4/g and DPPH activity between 73 and 83 g sample/g DPPH. These values are close to those obtained for E. involucrata, indicating a similar antioxidant capacity. Notably, the ABTS+ activity of E. involucrata (2701.67 μM Trolox/g extract) exceeds the values observed for E. dysenterica (1379 to 1633 μM Trolox/g), suggesting a higher capacity for radical scavenging through electron transfer mechanisms [15]. Moreover, phenolic compounds, including epicatechin and rutin identified in this extract, exhibit potent antioxidant activity due to their ability to scavenge free radicals, donate hydrogen/electrons, and chelate transition metals [33]. Their presence substantiates the extract’s potential for antioxidant applications.
Other species of the genus Eugenia demonstrated antioxidant activity using the DPPH, ABTS+, and FRAP methods. The essential oil from the leaves of Eugenia uniflora L. exhibited antioxidant activity in DPPH, ABTS+, and FRAP assays [36]. The antioxidative activity of essential oils from the stem (0.82 ± 0.15 μg/mL), bud (1.18 ± 0.56 μg/mL), and leaf (1.16 ± 0.74 μg/mL) of Eugenia caryophylata was also demonstrated by the DPPH method and ABTS+ method with values for the stem (0.81 ± 0.16 μg/mL), bud (0.54 ± 0.77 μg/mL) (0.81 ± 0.16 μg/mL), and leaves (0.66 ± 0.67 μg/mL) [37]. The leaf extract of Eugenia punicifolia was also able to demonstrate antioxidant activity by DPPH (28.84 ± 0.54 μg/mL) and ABTS (10.5 ± 1.2 μg/mL) [38]. The species Eugenia stipitata also showed radical scavenging activity, with a DPPH IC50 of 0.69 ± 0.23 μg/mL [39]. The standard of rutin was employed as a positive control in all the methodologies, and the values obtained were 228.4 g standard/g DPPH, 2047.3 μM ferrous sulfate/g rutin, and 1410.4 μM Trolox/g extract. Regarding the positive control rutin, the standardized liquid extract of E. involucrata demonstrated superiority compared to the DPPH and ABTS+ methods. This suggests that the extract contains a complex mixture of phenolic compounds and flavonoids that may synergistically enhance its radical scavenging capacity. These findings support the possible application of this extract in protecting against oxidative stress-related conditions and warrant further investigation into the specific constituents responsible for this activity.
2.4. Cytotoxic Investigation in Murine Macrophages
The MTT colorimetric assay assessed the viability of RAW 264.7 murine macrophages incubated with E. involucrata extract. Cells were treated with different concentrations of the extract to determine potential cytotoxicity. The liquid extract from E. involucrata leaves demonstrated cytotoxicity at higher concentrations, ranging from 1110 to 9200 µg/mL, in RAW 264.7 murine macrophages. The calculated IC50 value for the liquid extract of E. involucrata was 1468.51 ± 143.34 µg/mL. The cell viability data at each concentration are shown in Table S1. Gupta et al. (2002) showed that polyphenols can initiate auto-oxidation and produce toxicity in high concentrations [40]. However, no toxic effects were observed at a 575 µg/mL concentration (cell viability above 80%) (Figure 4), suggesting that the extract is safe at this concentration. Moreover, an increase in cell viability was observed at this concentration. Similarly, the methanol extract of Calophyllum brasiliense has been shown to promote increased cell proliferation using the MTT assay [41]. Furthermore, it has been demonstrated that plant extracts can stimulate cell proliferation due to their antioxidant activity [42].
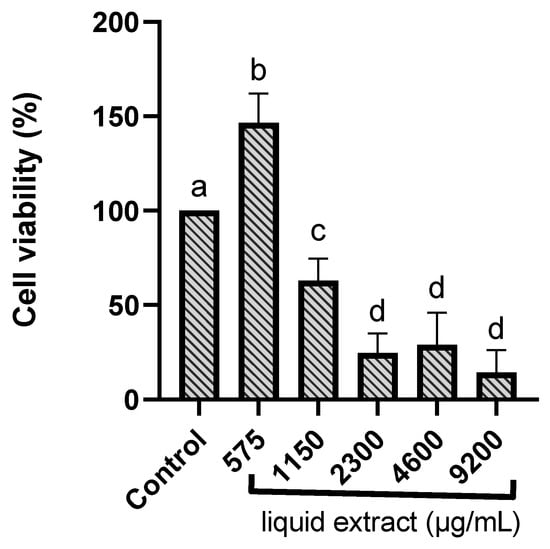
Figure 4.
Cytotoxicity of the standardized liquid extract from Eugenia involucrata leaves on RAW 264.7 murine macrophages. Quantification of RAW 264.7 murine macrophage viability in the presence of different concentrations of Eugenia involucrata (575; 1150; 2300; 4600; and 9200 μg/mL) and negative control (non-treated cells) using an MTT assay. Data are shown as mean ± standard deviation (SD) and were evaluated by one-way ANOVA. After multiple comparisons of means using the Tukey test, all treatments were statistically different, except for the treatments 2300 vs. 4600, 2300 vs. 9200, and 4600 vs. 9200; a, b, c, and d represent the different groups formed after multiple comparisons. Significant difference in comparison with the control (p < 0.05).
These results highlight the importance of cytotoxicity assays in evaluating the safety of plant-derived extracts. Macrophages, as the primary resident phagocytic cells in all tissues, play a vital role in clearing waste materials from the blood and maintaining homeostasis, making them a relevant model for toxicity studies [34]. Cytotoxicity assays represent a valuable tool for the preliminary safety assessment of medicinal plants as they reveal potential toxic effects on various cell types, including human cells. This information guides using plant-based remedies, ensuring therapeutic benefits without health risks. These evaluations support the development of effective and safe natural therapies for modern healthcare [43,44].
2.5. Cytogenotoxic Evaluation of the Liquid Extract from Eugenia involucrata Leaves
Based on the results obtained in the RAW 264.7 macrophage model, the concentrations selected for evaluation in human lymphocytes were lower than the highest non-toxic dose identified in the murine macrophage assay. This approach was adopted to ensure cellular integrity in primary human cells, which may be more sensitive than immortalized cell lines, and to avoid potential false-positive results in genotoxicity testing due to cytotoxic interference [45,46]. Moreover, using sub-toxic concentrations is recommended in genotoxic and genoprotective assays to distinguish better direct DNA effects from secondary damage caused by cytotoxicity [47].
In the present study, the Trypan blue assay was employed to assess the cytotoxic potential of the liquid extract of E. involucrata leaves. The results demonstrated that the extract exhibited no cytotoxicity against human lymphocytes at the concentrations tested in this study (Figure 5A, p > 0.05). When co-treated with doxorubicin (DXR), the liquid extract did not exhibit cytoprotective effects (p > 0.05), nor did it enhance the cytotoxic effect of DXR on human lymphocytes at the tested concentrations (Figure 5B, p > 0.05). As expected, DXR caused a significant decrease in cell viability compared to the control group (Figure 5A,B, p < 0.05) [48]. Similarly, cytotoxicity was absent for the leaf extract of Eugenia uniflora, a species of the same genus of E. involucrata (Cunha et al., 2016 [49]). However, the crude ethanolic extract of E. involucrate seeds showed cytotoxicity (1000 mg·mL−1) in non-cancerous cells (human umbilical vein endothelial cells—HUVEC, and monkey kidney epithelial cells—VERO). The fruit extract was also investigated but did not exhibit such activity [50].
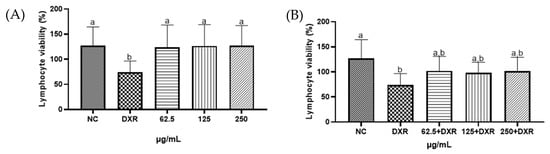
Figure 5.
Viability of human lymphocytes treated with the standardized liquid extract from Eugenia involucrata leaves (62.5; 125; and 250 µg/mL) in the absence (A) or presence (B) of doxorubicin (DXR). A negative control group (NC; sterile water) was also included. Different letters indicate statistically significant differences between groups (p < 0.05), whereas the same letters indicate no significant difference (p > 0.05); a and b represent the different groups formed after multiple comparisons.
Regarding the genotoxicity assessment, the standardized liquid extract of E. involucrata leaves exhibited genotoxicity against human lymphocytes at the highest concentration tested (250 µg/mL) when compared to the negative control, as detected by the comet assay (Figure 6A, p < 0.05). On the other hand, a genoprotective effect was observed at the lowest concentration tested of the standardized liquid extract of E. involucrata (62.5 µg/mL), which reduced the genotoxicity of doxorubicin by approximately 27% (Figure 6B, p < 0.05). As expected, DXR (positive control) caused significant DNA damage compared to the negative control cells (Figure 6A,B; p < 0.05).
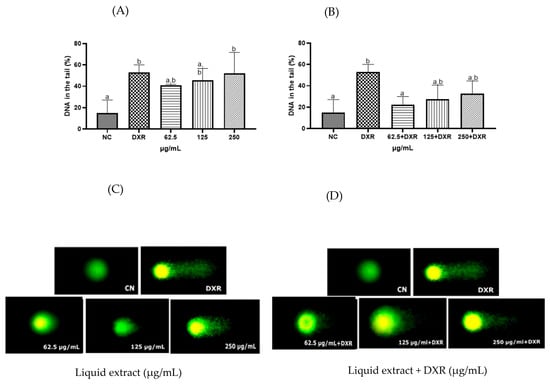
Figure 6.
Effect of the standardized liquid extract from Eugenia involucrata leaves on DNA of human lymphocytes in the absence (A,C) or presence (B,D) of doxorubicin. Negative control (NC, sterile water); doxorubicin (DXR, 50 µg/mL). The values are expressed as the mean (± standard deviation) of three independent experiments, p < 0.05; a and b represent the groups formed after multiple comparisons. Cells were stained with the nucleic acid dye Diamond™, and images were captured using a fluorescence microscope (Axioplan-ImagingVR), with Zen Blue Software 2.3, using an excitation filter of 510–560 nm and a barrier filter of 590 nm (10× objective).
The genotoxicity of our species differs from the results obtained by Cunha et al. (2016) [49] for E. uniflora, whose leaf extract showed the absence of both cytotoxic and genotoxic effects even at higher concentrations (240 and 480 µg/mL). This result was attributed to compounds such as quercetin, quercitrin, isoquercitrin, luteolin, and ellagic acid [49], which were not detected in our extract using the methods employed. Furthermore, other unidentified compounds in the extract (due to the techniques used in this study) may lead to a genotoxic effect at higher concentrations, as observed at 250 µg/mL. The results presented by Neri-Numa et al. (2013) demonstrated that the ethanolic extract of Eugenia stipitata, a species of the same genus, presented antimutagenic and genoprotective properties at the highest concentration tested (300 mg/kg of body weight) [39].
On the other hand, under the conditions of this study, we identified the presence of epicatechin and rutin in the liquid extract of E. involucrata. These flavonoids are known for their antioxidant potential, which helps capture and eliminate radicals. They are among medicinal plants’ most widely found secondary metabolites [51,52]. Evidence has shown that compounds with antioxidant properties can remove ROS before these species interact with the DNA molecule, resulting in permanent damage [53]. Studies suggest chemoprotective activities are associated with the antioxidant capacity of these metabolites [54]. Khan et al. (2018) found that rutin solutions at 20 µg/mL and 40 µg/mL could reduce in vitro genetic damage induced by methyl methanesulfonate in human lymphocytes by 20.57% and 47.14%, respectively [55].
In general, rutin, epicatechin, and phenolic compounds are known for their powerful antioxidant properties, which are crucial in protecting cells from oxidative damage and genotoxic stress. These bioactive molecules can neutralize free radicals and reactive oxygen species, reducing oxidative stress that can lead to DNA damage, mutations, and cell death. Additionally, phenolic compounds such as antioxidant enzymes may enhance the activity of cellular defense systems and inhibit pathways involved in inflammation and apoptosis. Their ability to modulate cellular responses helps provide genoprotective effects, contributing to the maintenance of genomic stability and overall cell integrity. This protective mechanism is critical in conditions associated with oxidative and genotoxic stress, highlighting the potential therapeutic benefits of these compounds in preventing cellular damage [56,57,58].
Using the comet assay, Haza and Morales (2011) [59] evaluated the protective effect of epicatechin (10–50 µM) against oxidative DNA damage induced by carcinogens in HepG2 cells. Their findings indicated that epicatechin significantly reduced lesions caused by N-nitrosodibutylamine and N-nitrosopiperidine. Moreover, the authors observed that epicatechin attenuated oxidative lesions promoted by N-nitrosodibutylamine and N-nitrosopiperidine in the genetic material of the cell line [48,59].
In line with these findings, the standardized extract of E. involucrata exhibited a significant protective effect against DXR genotoxicity at 62.5 µg/mL against DXR-induced damage. DXR, a recognized antitumor drug, induces DNA breaks and generates free radicals, producing cellular damage [60,61]. The DXR molecule can also directly interact with iron to form a DXR–Fe complex, facilitating iron cycling between Fe2+ and Fe3+ forms, producing significant ROS, and causing cellular damage [62].
The metal-chelating capacity of several phenolic compounds and their antioxidant activities may account for the genoprotective effects of the liquid extract observed in this study. Supporting this hypothesis, the extract demonstrated significant antioxidant activity, as evidenced by its free radical scavenging and ferric-reducing capabilities. These properties underscore its potential to attenuate oxidative stress and DNA damage caused by genotoxic agents. Furthermore, antioxidant compounds in the extract, such as epicatechin and rutin, may have played a protective role against genetic damage induced by doxorubicin.
Compounds lacking genotoxicity might be used for therapeutic purposes with reduced concern for DNA-related adverse effects. Further, the genoprotective properties of a substance indicate its potential for preventing DNA damage, which may be particularly relevant in treatments to reduce the side effects of chemotherapeutic agents [63,64]. The phenolic compounds, including rutin and epicatechin, possess notable antioxidant and genoprotective properties that could significantly contribute to developing novel therapies for genotoxic conditions. By effectively neutralizing reactive oxygen species and reducing oxidative stress, these compounds may protect DNA integrity and prevent mutations induced by environmental agents, radiation, or chemotherapeutic drugs. Their ability to modulate cellular defense mechanisms suggests they could be used as adjuncts alongside conventional treatments to minimize collateral DNA damage in healthy cells, thereby reducing side effects and enhancing overall treatment efficacy [65,66]. Future therapeutic strategies might involve using the E. involucrata extract or its bioactive compounds to develop protective agents or supplements that shield normal tissues during genotoxic exposures. Thus, future studies are necessary to investigate the compounds involved in DNA damage and the protective effects of E. involucrata extract in the present study.
3. Materials and Methods
3.1. Plant Material
The leaves of Eugenia involucrata were collected in the municipality of Hidrolândia (latitude 16°54′01.0″ S, longitude 49°15′32.5″ W, 832 m altitude) located in the State of Goiás, Brazil. Prof. Dr. José Realino de Paula identified the specimen from the Federal University of Goiás, and the herbarium material was deposited as a voucher at the Herbarium of the State University of Goiás (UEG-GO) under the voucher number 15,366 (Figure S1). After collection, the leaves were dried in an oven at 40 °C with air circulation, and the resulting material was ground in a knife mill, weighed, and stored in a container protected from light and moisture. The nomenclature of the species was reviewed in Index Plant Names. The post-taxonomic revision nomenclature of the section Phyllocalyx (homotypic) was adopted in this research, from Eugenia calycina Cambess to Eugenia involucrata DC., as stated in the Taxonomic Catalog Flora and Funga of Brazil (2020) and the description of the original type specimen in Fl. bras. Merid. 2: 352 (1832) [67].
3.2. Screening of Phenolic Compounds in Liquid Extract Obtained by Ultrasound from Eugenia involucrata Leaf Powder by HPLC-DAD
In a 25 mL volumetric flask, 1 g of the plant sample (powdered) and 10 mL of 60% ethanol (w/w) were added. The flask was sonicated in an ultrasonic bath (Ultronique model Q5.9/40A, frequency 40 kHz, power 200 W) at 60 °C for 30 min. After this period, the solution was subjected to simple filtration using filter paper. From the filtrate, 2 mL was transferred to an Eppendorf tube and centrifuged (IKA® mini G) at 6000 rpm for 15 min. Subsequently, the supernatant was filtered again, using a PTFE membrane (0.45 μm), and collected in a 2 mL amber vial before being used for phenolic compound screening.
The screening was performed using HPLC-DAD (model G7115A) with the OpenLab CDS software 2.8 and an autosampler (Agilent Technologies, Inc. 1290 Infinity II, Santa Clara, CA, USA). Chromatographic separations were carried out using an Agilent InfinityLab Poroshell 120 EC-C18 column (4.6 × 100 mm, 2.7 µm) with a mobile phase consisting of acetonitrile and water acidified with 0.2% acetic acid in a gradient elution (Table 1). The analysis was conducted at a 1 mL/min flow rate with an injection volume of 5 µL and a column temperature maintained at 30 °C. Detection was performed at three different wavelengths (280 nm, 306 nm, and 340 nm) to identify phenolic compounds by comparing their retention times with analytical standards. For this study, ten reference standards from Sigma-Aldrich (St. Louis, MO, USA) were used, all prepared in HPLC-grade methanol: resveratrol (0.2% w/v), gallic acid (0.2% w/v), caffeic acid (0.2% w/v), ellagic acid (0.2% w/v), quercetin (0.2% w/v), catechin (0.2% w/v), epicatechin (0.2% w/v), rutin (0.2% w/v), apigenin (0.2% w/v), and kaempferol (0.08% w/v) (Supplementary Figure S2).
The powdered plant material (50 g) was placed in a suitable container and uniformly moistened with 54% (w/w) ethanol. The container was then kept at rest, protected from light, for 24 h, to allow swelling of the material. After this period, the sample was transferred to a stainless-steel percolator and left for maceration for 3 days. The percolation was performed exhaustively for 5 days using 750 mL of 57% (w/w) ethanol. The extract was then concentrated by rotary evaporation (IKA® RV10) under controlled conditions of a 40 °C temperature, 25 rpm rotation speed, and 80 bar pressure to obtain the final concentrated extract.
3.3. Characterization of the Concentrated Liquid Extract from Eugenia involucrata Leaves
The physicochemical characterization of the concentrated liquid extract from Eugenia involucrata leaves was performed through three key analyses, pH determination, solid content measurement, and density evaluation, all conducted in triplicate according to standardized methodologies [19]. The pH was measured using a calibrated Tecnopon pH meter, with prior calibration using pH 4.0 and 7.0 buffer solutions to ensure accuracy. For solid content determination, 0.5 g samples of the extract were evenly distributed on the balance plate of a MOC-63U SHIMADZU (Kyoto, Japan) instrument equipped with a halogen heating lamp and heated at 105 °C until reaching constant weight, with results calculated as 100% minus the moisture percentage. Density was assessed through a 10 mL pycnometer, where the empty, clean, and dry pycnometer was first weighed, then filled with distilled water at 20 °C and weighed again, followed by the same procedure with the extracted sample. The mass density was calculated using the formula that accounts for the sample’s relative density compared to water and a standard correction factor of 0.0012, providing precise density values in g/mL. These comprehensive analyses established fundamental quality parameters for the extract, ensuring reproducibility and standardization for potential applications.
3.4. Quantification of Total Phenolics and Flavonoids
The quantitative analysis of total phenolic compounds was conducted according to the spectrophotometric method of Hagerman and Butler (1978), as modified by Mole and Waterman (1987) [68,69]. A stock standard solution at 0.1 mg/mL in distilled water was prepared, from which a series of dilutions (0.050, 0.100, 0.150, 0.200, 0.250, and 0.300 mg/mL) were made to establish the calibration curve. The assay mixture consisted of 2 mL of sodium lauryl sulfate–triethanolamine solution (SLS 1% w/v–TEA 5% v/v), 1 mL of ferric chloride solution (1.62 g/L in 0.001 M hydrochloric acid), and 1 mL of sample or standard solution. After a 15 min incubation period, absorbance readings were taken at 510 nm using a Metash ESPEC-UV-5100 UV–Vis (Shanghai, China) spectrophotometer. The plant extract was diluted 50-fold in distilled water for sample analysis, and the same reaction conditions were applied. All measurements were performed in triplicate, with results expressed as mean values ± standard deviation in mg/mL.
A modified version of the Rolim et al. (2005) method was employed for flavonoid quantification [70]. A rutin standard solution (0.1 mg/mL in methanol–0.02 M acetic acid 99:1) served as the reference, with subsequent dilutions ranging from 0.005 to 0.025 mg/mL prepared for calibration. The plant extract was diluted 200-fold and mixed with the methanol–acetic acid solvent to a final volume of 4 mL. Absorbance measurements were conducted at 361 nm using the exact spectrophotometer, with all analyses performed in triplicate. The blank consisted of the methanol–acetic acid solvent alone. Final flavonoid concentrations were calculated based on the rutin standard curve and reported as mean ± standard deviation in mg/mL. Both analytical procedures included appropriate blank controls and were conducted under standardized conditions to ensure the reproducibility of results.
3.5. Determination of Phenolic Compounds in Liquid Extract of Eugenia involucrata by HPLC-DAD
The phenolic compounds present in the concentrated liquid extract of Eugenia involucrata were analyzed using HPLC-DAD (Agilent Technologies 1290 Infinity II system). Chromatographic separation was achieved using an Agilent InfinityLab Poroshell 120 EC-C18 column (4.6 × 100 mm, 2.7 µm) with the following parameters: a mobile phase flow rate of 1 mL/min, column temperature maintained at 30 °C, and injection volume of 5 µL. The mobile phase consisted of acetonitrile and water acidified with 0.2% acetic acid, applied in a gradient elution (Table 2). Detection was performed at three wavelengths (280 nm, 306 nm, and 340 nm) for comprehensive compound identification.

Table 2.
Gradient composition used as a mobile phase during phenolic compound screening by HPLC-DAD in the leaf material of Eugenia involucrata.
Analytical standards from Sigma-Aldrich were prepared in HPLC-grade methanol at the following concentrations: resveratrol (0.2% w/v), gallic acid (0.2% w/v), caffeic acid (0.2% w/v), ellagic acid (0.2% w/v), quercetin (0.2% w/v), catechin (0.2% w/v), epicatechin (0.2% w/v), rutin (0.2% w/v), apigenin (0.2% w/v), and kaempferol (0.08% w/v).
For sample preparation, 1 mL of concentrated liquid extract was transferred to a 10 mL volumetric flask and diluted to volume with HPLC-grade methanol. The solution was sonicated for 15 min at room temperature using an ultrasonic bath (Ultronique model Q5.9/40A, 40 kHz frequency, 200 W power), followed by filtration through filter paper. A 2 mL aliquot of the filtrate was centrifuged at 6000 rpm (IKA mini G; Staufen, Germany) for 15 min, and the supernatant was filtered through a 0.45 µm PTFE membrane into a 2 mL amber vial before analysis.
Compound identification was based on retention time matching and comparison of UV–Visible absorption spectra between the analytical standards and corresponding peaks in the plant extract. This dual confirmation approach (retention time and spectral data) ensured the accurate identification of phenolic compounds in the sample. The analytical process was conducted under controlled conditions to maintain method reproducibility and reliability.
The analytical method was validated for the simultaneous quantification of rutin and epicatechin, evaluating the parameters of selectivity, linearity, matrix effect, precision, accuracy, robustness, limit of detection (LOD), and limit of quantification (LOQ), in compliance with the requirements of Resolution RDC No. 166/2017 from the Brazilian Health Regulatory Agency (Anvisa) and the Association of Official Analytical Chemists International [71,72].
3.6. Determination of Antioxidant Activity of the Liquid Extract
3.6.1. Sample Preparation
The sample preparation for the following methods (FRAP, DPPH, and ABTS•+) was performed according to the methodology adapted from Larrauri, Rupérez, and Saura-Calixto [73]. The positive control used for all the analyses was the standard of rutin (Sigma Aldrich®).
3.6.2. Ferric Reducing Antioxidant Power (FRAP) Method
The total antioxidant activity was determined using the FRAP assay according to the modified method of Rufino et al. (2006) [23]. A standard solution was prepared by dissolving 27.8 mg of ferrous sulfate in distilled water and adjusting the volume to 50 mL in a volumetric flask. This solution was homogenized and transferred to a labeled amber glass vial. From this stock solution, standard curve concentrations were prepared: 500 μM, 1000 μM, 1500 μM, and 2000 μM [23]
For the assay, 90 μL aliquots of each ferrous sulfate solution (in duplicate) were transferred to test tubes, followed by adding 270 μL of distilled water and 2.7 mL of FRAP reagent. The tubes were homogenized and kept in the dark for 30 min. The blank consisted of the FRAP reagent alone.
For the liquid extract evaluation, five dilutions were prepared from Solution A (4000 mg/L, described in Section 3.6.1.), yielding concentrations of 1000 mg/L, 760 mg/L, 500 mg/L, 248 mg/L, and 124 mg/L. Aliquots (90 μL) of each dilution (in duplicate) were mixed with 270 μL of distilled water and 2.7 mL of FRAP reagent. After 30 min of incubation in the dark, absorbance was measured at 595 nm using a UV–Vis spectrophotometer (Metash, model ESPEC-UV-5100; Shanghai, China). Results were expressed as μM ferrous sulfate/g extract.
3.6.3. ABTS+ Radical Scavenging Method
The total antioxidant activity was determined using the ABTS+ radical method, following the modified protocol of Rufino et al. (2007) [74]. A standard solution was prepared by dissolving 25 mg of Trolox in ethanol and adjusting the volume to 50 mL in a volumetric flask. This solution was homogenized and stored in a labeled amber vial. Standard curve concentrations (100 μM, 500 μM, 1000 μM, 1500 μM, and 2000 μM) were prepared from this stock [74].
For the assay, 30 μL aliquots of each Trolox dilution (in duplicate) were mixed with 3 mL of ABTS•+ radical solution. After homogenization, absorbance was measured at 734 nm after 6 min of incubation in the dark. The blank consisted of ethanol.
For the liquid extract, five dilutions were prepared from Solution A (4000 mg/L), yielding concentrations of 1000 mg/L, 760 mg/L, 500 mg/L, 248 mg/L, and 124 mg/L. Absorbance was measured at 734 nm (Metash ESPEC-UV-5100; (Shanghai, China). Results were expressed as μM Trolox equivalents/g extract.
3.6.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Method
The total antioxidant activity was determined using the DPPH radical method, following the modified protocol of Rufino et al. (2007) [74]. A standard solution was prepared by dissolving 2.4 mg of DPPH in methanol and adjusting the volume to 100 mL in a volumetric flask. This solution was homogenized and stored in a labeled amber vial. Standard curve concentrations (10 μM, 20 μM, 30 μM, 40 μM, 50 μM, and 60 μM) were prepared from this stock [75].
For the assay, 4 mL aliquots of each DPPH solution were transferred to test tubes, homogenized, and measured at 515 nm (Metash ESPEC-UV-5100; Shanghai, China). The blank consisted of methanol.
For the liquid extract, five dilutions were prepared from Solution A (4000 mg/L), yielding concentrations of 500 mg/L, 248 mg/L, 124 mg/L, 60 mg/L, and 30 mg/L. After kinetic testing, a 22 min incubation time was established. Aliquots (100 μL) of each dilution (in duplicate) were mixed with 3.9 mL of DPPH radical solution. Absorbance was measured at 515 nm after 22 min. All procedures were performed in the dark. Results were expressed as g extract/g DPPH.
3.7. Cytotoxicity Investigation in Murine Macrophage Cells RAW 264.7
RAW 264.7 cells were grown in RPMI-1640 (Sigma Chemical Co., St. Louis, MO, USA) supplemented with 10% FBS (Cripion, São Paulo, Brazil). Cells were inactivated by incubation at 56 °C for 30 min in RPMI containing 2 mML-glutamine (Sigma Chemical Co.), 50 mM2-mercaptoethanol (Sigma-Aldrich), 100 U/mL penicillin, 100 mg/mL streptomycin (Sigma-Aldrich), and 2 mM Hepes (Sigma-Aldrich). Cells (2 × 105) were grown in 2 mL of RPMI in a well of 6-well culture plates (Costar, New York, NY, USA) in a 37 °C incubator at 5% CO2. The RAW 264.7 cells (1 × 106 cells/mL) were plated in triplicate in 96-well plates in RPMI-1640 medium supplemented with 10% FBS and treated with one of five concentrations of the Eugenia involucrata leaf extract (575, 1150, 2300, 4600, or 9200 µg/mL) dissolved in dimethyl sulfoxide solution (DMSO; 10 mg/mL). After 48 h of incubation, 5 mL of MTT (5 mg/mL) was added to each well, and the plates were incubated for an additional 3 h. The blue MTT formed a precipitate, which was then dissolved in DMSO. The absorbance was measured using a microplate reader (Biolisa Reader, Bioclin, Belo Horizonte, MG, Brazil) at 450 to 630 nm. Cell viability was expressed as a percentage of the control (not treated cells). Analysis of Variance (ANOVA) was used to compare treatments with the Tukey post-test (p < 0.05).
3.8. Determination of Cytotoxic and Genotoxic Activity in Human Lymphocytes
3.8.1. Blood Samples
Human blood samples for in vitro cytotoxic and genotoxic evaluation were obtained from healthy volunteers (aged 18–30 years) with no history of chronic diseases, smoking, or alcoholism. The study was approved by the Research Ethics Committee of Universidade Estadual de Goiás (CEP No. CAAE 66860023.1.0000.8113).
3.8.2. Human Lymphocyte Culture
Lymphocyte cultures were prepared using an adapted method from Rocha et al. (2020) [76]. Venous blood (20 mL) was collected in heparinized tubes, mixed with 20 mL RPMI medium, and centrifuged (120× g, 4 min, 18 °C). After plasma removal, the leukocyte layer was isolated using Ficoll-Paque PLUS (200× g, 10 min, 18 °C). Pellets were resuspended in RPMI medium supplemented with 10% fetal bovine serum, 0.5% phytohemagglutinin, and 0.2% penicillin–streptomycin antibiotic solution, then incubated (37 °C, 24 h) in a BOD incubator.
3.8.3. Cytotoxicity and Cytoprotective Assay
Cell viability was assessed using 0.4% Trypan blue staining. Lymphocytes (4 × 105 viable cells/well) in 24-well plates were treated with E. involucrata leaf extract (62.5–250 µg/mL in water) with/without 50 µg/mL doxorubicin (DXR) for 3 h (37 °C). Sterile water and DXR served as the negative and positive controls, respectively. Viable cells were recounted post-treatment. All tests were performed in triplicate.
3.8.4. Genotoxicity and Genoprotective Effects (Comet Assay)
Treated lymphocytes (100 µL) were mixed with 120 µL low-melting-point agarose (1%), layered on agarose-coated slides, and lysed (Triton X-100 lysis buffer, 24 h). Electrophoresis was conducted in an alkaline buffer (300 mM NaOH, 4 °C, 25 V/cm, 30 min). Slides were neutralized (0.4 M Tris-HCl, pH 7.5), stained with Diamond™ Nucleic Acid Dye (1:10,000 in PBS), and analyzed via fluorescence microscopy (Axioplan-Imaging, 10× objective). DNA damage (% tail DNA) was quantified using CometScore™ (v1.5), with 100 cells analyzed per treatment. Statistical significance (p < 0.05) was determined by one-way ANOVA with Tukey’s test (GraphPad Prism 8).
4. Conclusions
The liquid extract of Eugenia involucrata leaves demonstrated the presence of phenolic compounds, namely, rutin and epicatechin. This extract holds significant importance due to its antioxidant effects, as evidenced by its iron-reducing capacity and free radical scavenging activity, which can lead to chemoprotective and antitumoral consequences. The liquid extract was not cytotoxic but was genotoxic to human lymphocytes at the highest tested concentration (250 µg/mL). Conversely, it demonstrated a genoprotective effect at the lowest tested concentration (62.5 µg/mL). This role as a protective agent against oxidative stress and a potential therapeutic alternative emphasizes the value of Eugenia involucrata in developing natural health products aimed at cancer prevention and treatment, thereby contributing to advancements in phytomedicine. Future perspectives include identifying the remaining compounds, conducting additional cytogenotoxic tests in systemic models (such as murine), and investigating its antitumoral potential in cancer cell cultures.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18050764/s1: Figure S1. Image of the herbarium specimen deposited at the Herbarium of the State University of Goiás (code number 15366) of the Eugenia involucrate species. Figure S2. Chromatogram of the analytical standards at 280 nm (A), 306 nm (B), and 340 nm (C) by HPLC-DAD. Table S1. Cell viability (%) and standard deviation (SD) after 48 h of treatment of RAW 264.7 macrophages with the hydroalcoholic extract of Eugenia involucrata DC. Leaves.
Author Contributions
Conceptualization, M.N.G.T. and L.L.B.; methodology, M.N.G.T., G.G.d.M., C.M.A.M., J.D.R., L.G.C. and F.G.B.; software, M.N.G.T.; validation, A.M.d.M., M.N.G.T., L.G.C. and F.G.B.; formal analysis, M.N.G.T. and J.D.R.; investigation, M.N.G.T., A.S.F. and L.B.R.d.C.; resources, L.L.B.; data curation, C.M.G., M.A.P.d.O. and J.A.M.d.P.; writing—original draft preparation, M.N.G.T., C.M.G., M.A.P.d.O., E.F.L.C.B., J.A.M.d.P. and L.L.B.; writing—review and editing, M.N.G.T., A.S.F., L.B.R.d.C. and L.L.B.; visualization, M.N.G.T.; supervision, L.L.B.; project administration, M.N.G.T. and L.L.B.; funding acquisition, L.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank FAPEG (Fundação de Amparo à Pesquisa no Estado de Goiás) for granting the scholarship and to the Institutional Program for Support to Research of PUC Goiás (PAP/PUC Goiás) for the financial support, as well as to the Program for Support of Scientific Publication (Ordinance n. 179/2014-GR), also from PUC Goiás.
Institutional Review Board Statement
The study involved blood collection from human volunteers and was approved by the human research ethics committee of the State University of Goiás under the number CEP No. CAAE 66860023.1.0000.8113 (approved on 1 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in this study’s design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Ferreira, F.P.S.; Morais, S.R.; Bara, M.T.F.; Conceição, E.C.; Paula, J.R.; Carvalho, T.C.; Vaz, B.G.; Costa, H.B.; Romão, W.; Rezende, M.H. Eugenia calycina Cambess Extracts and Their Fractions: Their Antimicrobial Activity and the Identification of Major Polar Compounds Using Electrospray Ionization FT-ICR Mass Spectrometry. J. Pharm. Biomed. Anal. 2014, 99, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.S.G.; Silva, S.A.; Teixera, T.L.; De Oliveira, A.; Morais, S.A.L.; Da Silva, C.V.; Espindola, L.S.; Sousa, R.M.F. Essential Oil from Leaves of Eugenia calycina Cambes: Natural Larvicidal against Aedes aegypti. J. Sci. Food Agric. 2021, 101, 1202–1208. [Google Scholar] [CrossRef]
- Sousa, R.M.F.; de Morais, S.A.L.; Vieira, R.B.K.; Napolitano, D.R.; Guzman, V.B.; Moraes, T.S.; Cunha, L.C.S.; Martins, C.H.G.; Chang, R.; de Aquino, F.J.T.; et al. Chemical Composition, Cytotoxic, and Antibacterial Activity of the Essential Oil from Eugenia calycina Cambess. Leaves against Oral Bacteria. Ind. Crops Prod. 2015, 65, 71–78. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Silva, E.K.; Arruda, H.S.; Rodrigues de Morais, D.; Angela, A.; Meireles, M.; Pereira, G.A.; Pastore, G.M. Recovering Phenolic Compounds from Eugenia calycina Cambess Employing High-Intensity Ultrasound Treatments: A Comparison among Its Leaves, Fruit Pulp, and Seed as Promising Sources of Bioactive Compounds. Sep. Purif. Technol. 2021, 272, 118920. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Arruda, H.S.; de Paulo Farias, D.; Molina, G.; Pereira, G.A.; Pastore, G.M. Plants from the Genus Eugenia as Promising Therapeutic Agents for the Management of Diabetes Mellitus: A Review. Food Res. Int. 2021, 142, 110182. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Betteridge, D.J. What Is Oxidative Stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- de Oliveira, F.M.G.; Rodrigues Pereira de Oliveira Borlot, J.; Kitagawa, R.R.; Gonçalves, R.d.C.R.; Batista de Oliveira Filpo, R.C.; Kuster, R.M. Characterization of Phenolic Compounds in Eugenia uniflora Leaves by ESI(-) FT-ICR MS, Analysis of Cytotoxic Activity on Gastric Adenocarcinoma (AGS Cells), and Anti- Helicobacter pylori Activity. Nat. Prod. Res. 2024, 38, 4297–4301. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Bonadiman, B.d.S.R.; Marafon, F.; Fischer, D.L.d.O.; Bagatini, M.D.; Kempka, A.P. Eugenia uniflora L. Seed and Pulp Extracts: Phytochemical Profile, Cytotoxic Potential, Antitumoral Activity, and α-Amylase and α-Glucosidase Inhibition Capacity. Nat. Prod. Res. 2023, 37, 3862–3867. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, W.M.; El-Sayed, M.A.A.; Abdel-Aziz, N.I.; El-Azab, A.S.; Ahmed, E.R.; Abdel-Aziz, A.A.M. Synthesis, Antitumour and Antioxidant Activities of Novel α,β-Unsaturated Ketones and Related Heterocyclic Analogues: EGFR Inhibition and Molecular Modelling Study. J. Enzym. Inhib. Med. Chem. 2018, 33, 507–518. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, A.S.N.; Da Costa Rodrigues, L.L.G.; Rocha, J.D.; Guimarães, L.M.M.; Ramos, L.C.C.; Napolitano, H.B.; Bailão, E.F.L.C.; Borges, L.L. Theoretical Exploration of the Antioxidant Activity, Chemopreventive, and Antineoplastic Potentials of Molecules Present in Morinda lucida, Momordica charantia, and Vernonanthura polyanthes. J. Comput. Biophys. Chem. 2024, 23, 657–677. [Google Scholar] [CrossRef]
- PAHO/WHO PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/cancer (accessed on 5 April 2025).
- Barbosa, F.G.; Silva, G.F.; de Oliveira, V.L.P.; Kubijan, L.A.C.; Costa, L.G.; de Melo, A.M.; Teófilo, M.N.G.; Morgado, C.M.A.; de Campos, A.J.; Peixoto, J.d.C.; et al. Bioinputs from Eugenia dysenterica DC. (Myrtaceae): Optimization of Ultrasound-Assisted Extraction and Assessment of Antioxidant, Antimicrobial, and Antibiofilm Activities. Molecules 2025, 30, 1115. [Google Scholar] [CrossRef]
- Smaniotto, F.A.; Dluzniewski, L.T.; Bortolazzo, P.C.; Monteiro, C.S.A.; Baranzelli, J.; da Silva, D.T.; Somacal, S.; Conterato, G.M.M.; Emanuelli, T. In Vitro Assessment of Antidiabetic, Anti-Obesogenic, and Antioxidant Potential of Pulp and Seed Extracts from Eugenia involucrata Fruits. Food Res. Int. 2025, 202, 115693. [Google Scholar] [CrossRef]
- Harmouzi, A.; Ammari, Y. EL Quality Control in the Primary Processing of Aromatic and Medicinal Plants. Sci. Afr. 2025, 27, e02519. [Google Scholar] [CrossRef]
- Kanama, S.K.; Vermaak, I.; van de Venter, M.; Koekemoer, T.C.; Viljoen, A. In Vitro Cytotoxic, Genotoxic, and Multi-Parameter Hepatotoxic Evaluation of Selected South African Medicinal Plant Extracts. S. Afr. J. Bot. 2025, 180, 625–635. [Google Scholar] [CrossRef]
- Brasil Farmacopeia Brasileira. 2024. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2024/publicada-a-atualizacao-da-farmacopeia-brasileira-6a-edicao (accessed on 17 May 2025).
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of Herbal Medicines: Concerns and Future Prospects. J. Ethnopharmacol. 2023, 309, 116383. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing Herbal Medicine: Enhancing Product Quality and Safety through Robust Quality Control Practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Moreira, G.G.; Paz, A.T.S.; Oliveira, T.L.S.; Silva, L.A.D.; da Conceição, E.C.; Borges, L.L.; da Silva, V.B.; de Paula, J.R. Process Optimization of Physicochemical Properties of Spray-Dried Hydrocotyle umbellata L. Extract. Braz. J. Pharm. Sci. 2023, 59, e21211. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total Em Frutas Pelo Método de Redução Do Ferro (FRAP); Comunicado Técnico; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2006. [Google Scholar]
- Fidelis, A.A.G.; de Oliveira Fernandes, G.; Melo, F.R.; de Oliveira Leme, L.; Adona, P.R.; Kawamoto, T.S.; Dode, M.A.N. Ethanolic Extract of Dried Leaves from the Cerrado Biome Increases the Cryotolerance of Bovine Embryos Produced In Vitro. Oxid. Med. Cell Longev. 2020, 2020, 6046013. [Google Scholar] [CrossRef] [PubMed]
- Peixoto Araujo, N.M.; Arruda, H.S.; dos Santos, F.N.; de Morais, D.R.; Pereira, G.A.; Pastore, G.M. LC-MS/MS Screening and Identification of Bioactive Compounds in Leaves, Pulp and Seed from Eugenia calycina Cambess. Food Res. Int. 2020, 137, 109556. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.G.; Laquete de Barros, G.; Pegoraro, C.; Vizzotto, M.; Soldi, C.; Conterato, G.; Heiden, G.; dos Santos, K.L.; Barbieri, R.L. Phytochemistry and Pharmacology of the Brazilian Cherry (Eugenia involucrata, Myrtaceae): A Systematic Review. Phytochem. Rev. 2025, 1, 1–21. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Zhang, L.; Han, B.; Yao, X.; Chen, W.; Hu, Y. Preparation of Ellagic Acid Molecularly Imprinted Polymeric Microspheres Based on Distillation–Precipitation Polymerization for the Efficient Purification of a Crude Extract. J. Sep. Sci. 2016, 39, 3098–3104. [Google Scholar] [CrossRef]
- Xie, J.; Chen, M.; Ren, T.; Zheng, Q. Optimization of Ellagic Acid Extraction from Blueberry Pulp through Enzymatic Hydrolysis Combined with Ultrasound-Assisted Organic Solvent. Environ. Technol. Innov. 2023, 31, 103147. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Chidambara Murthy, K.N. Biological Functions of Epicatechin: Plant Cell to Human Cell Health. J. Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of Molecular Structure on Kinetics and Dynamics of the Trolox Equivalent Antioxidant Capacity Assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant Evaluation Protocols: Food Quality or Health Effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Ferreira, M.R.A.; Lima, L.B.; Santos, E.C.F.; Machado, J.C.B.; Silva, W.A.V.; Paiva, P.M.G.; Napoleão, T.H.; Soares, L.A.L. Eugenia uniflora: A Promising Natural Alternative against Multidrug-Resistant Bacteria. Braz. J. Biol. 2023, 83, e274084. [Google Scholar] [CrossRef]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; da Silva, W.P.; da Motta, A.d.S.; Nascente, P.d.S. Essential Oil of the Leaves of Eugenia uniflora L.: Antioxidant and Antimicrobial Properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef]
- Julianus Sohilait, H.; Kainama, H. Free Radical Scavenging Activity of Essential Oil of Eugenia caryophylata from Amboina Island and Derivatives of Eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Galeno, D.M.L.; Carvalho, R.P.; De Araújo Boleti, A.P.; Lima, A.S.; De Almeida, P.D.O.; Pacheco, C.C.; De Souza, T.P.; Lima, E.S. Extract from Eugenia punicifolia Is an Antioxidant and Inhibits Enzymes Related to Metabolic Syndrome. Appl. Biochem. Biotechnol. 2014, 172, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the Antioxidant, Antiproliferative and Antimutagenic Potential of Araçá-Boi Fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Gupta, S.; Saha, B.; Giri, A.K. Comparative Antimutagenic and Anticlastogenic Effects of Green Tea and Black Tea: A Review. Mutat. Res. Rev. Mutat. Res. 2002, 512, 37–65. [Google Scholar] [CrossRef] [PubMed]
- Bueno, E.C.; Hermes Zandonai, R.; Coelho, F.; Ferreira, J.; Karla, A.; Mendes, B.; Biavatti, M.W.; Niero, R.; Filho, V.C.; Casagranda Bueno, E.; et al. Evaluation of the Proliferative Activity of Methanol Extracts from Six Medicinal Plants in Murine Spleen Cells. Braz. J. Pharm. Sci. 2010, 46, 323–333. [Google Scholar] [CrossRef]
- Ghica, A.; Tănase, M.L.; Niculițe, C.M.; Tocilă, A.; Popescu, L.; Luță, E.A.; Olaru, O.T.; Popovici, V.; Balaci, T.D.; Duțu, L.E.; et al. In Vitro Toxicity Evaluation of Some Plant Extracts and Their Potential Application in Xerosis Cutis. Cosmetics 2024, 11, 124. [Google Scholar] [CrossRef]
- Madić, V.; Stojanović-Radić, Z.; Jušković, M.; Jugović, D.; Žabar Popović, A.; Vasiljević, P. Genotoxic and Antigenotoxic Potential of Herbal Mixture and Five Medicinal Plants Used in Ethnopharmacology. S. Afr. J. Bot. 2019, 125, 290–297. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, S.M.; Choi, M.S.; Oh, J.H.; Kim, S.K.; Yoon, S.; Park, H.R.; Han, H.Y. Enhancing Toxicity Prediction for Natural Products in Herbal Medicine and Dietary Supplements: Integrating (Q)STR Models and in Vitro Assays. Toxicol. Appl. Pharmacol. 2025, 495, 117220. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Priyadarshini, T.; Aravindhababu, N.; Masthan, K.M.K. Cell Culture: An Insight View. Biomed. Pharmacol. J. 2015, 8, 27–31. [Google Scholar] [CrossRef]
- Azqueta, A.; Stopper, H.; Zegura, B.; Dusinska, M.; Møller, P. Do Cytotoxicity and Cell Death Cause False Positive Results in the in Vitro Comet Assay? Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 881, 503520. [Google Scholar] [CrossRef] [PubMed]
- Silva Fernandes, A.; Hollanda Véras, J.; Silva, L.S.; Puga, S.C.; Luiz Cardoso Bailão, E.F.; de Oliveira, M.G.; Cardoso, C.G.; Carneiro, C.C.; Da Costa Santos, S.; Chen-Chen, L. Pedunculagin Isolated from Plinia cauliflora Seeds Exhibits Genotoxic, Antigenotoxic and Cytotoxic Effects in Bacteria and Human Lymphocytes. J. Toxicol. Environ. Health A 2022, 85, 353–363. [Google Scholar] [CrossRef]
- da Cunha, F.A.B.; Waczuk, E.P.; Duarte, A.E.; Barros, L.M.; Elekofehinti, O.O.; Matias, E.F.F.; da Costa, J.G.M.; Sanmi, A.A.; Boligon, A.A.; da Rocha, J.B.T.; et al. Cytotoxic and Antioxidative Potentials of Ethanolic Extract of Eugenia uniflora L. (Myrtaceae) Leaves on Human Blood Cells. Biomed. Pharmacother. 2016, 84, 614–621. [Google Scholar] [CrossRef]
- Girardelo, J.R.; Munari, E.L.; Dallorsoleta, J.C.S.; Cechinel, G.; Goetten, A.L.F.; Sales, L.R.; Reginatto, F.H.; Chaves, V.C.; Smaniotto, F.A.; Somacal, S.; et al. Bioactive Compounds, Antioxidant Capacity and Antitumoral Activity of Ethanolic Extracts from Fruits and Seeds of Eugenia involucrata DC. Food Res. Int. 2020, 137, 109615. [Google Scholar] [CrossRef]
- Korga, A.; Józefczyk, A.; Zgórka, G.; Homa, M.; Ostrowska, M.; Burdan, F.; Dudka, J. Evaluation of the Phytochemical Composition and Protective Activities of Methanolic Extracts of Centaurea borysthenica and Centaurea daghestanica (Lipsky) Wagenitz on Cardiomyocytes Treated with Doxorubicin. Food Nutr. Res. 2017, 61, 1344077. [Google Scholar] [CrossRef]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of Antioxidant and Antibacterial Activities of the Stems of Flammulina velutipes and Hypsizygus tessellatus (White and Brown Var.) Extracted with Different Solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Ayoub, M.; De Camargo, A.C.; Shahidi, F. Antioxidants and Bioactivities of Free, Esterified and Insoluble-Bound Phenolics from Berry Seed Meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef]
- Sanz-Serrano, J.; Vettorazzi, A.; Muruzabal, D.; López de Cerain, A.; Azqueta, A. In Vitro Genotoxicity Assessment of Functional Ingredients: DHA, Rutin and α-Tocopherol. Food Chem. Toxicol. 2021, 153, 112237. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Abul Qais, F.; Ahmad, I.; Hussain, A.; Alajmi, M.F. Genotoxicity Inhibition by Syzygium cumini (L.) Seed Fraction and Rutin: Understanding the Underlying Mechanism of DNA Protection. Toxicol. Res. 2018, 7, 156. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; de Freitas, A.F.S.; Costa, W.K.; Machado, J.C.B.; Bezerra, I.C.F.; Ferreira, M.R.A.; Paiva, P.M.G.; Napoleão, T.H.; Soares, L.A.L. Flavonoid-Rich Fraction of Croton blanchetianus Baill. (Euphorbiaceae) Leaves: Chemical Profile, Acute and Subacute Toxicities, Genotoxicity and Antioxidant Potential. South. Afr. J. Bot. 2022, 144, 238–249. [Google Scholar] [CrossRef]
- Sanzovo, T.O.R.; Lima, N.M.; Marqui, S.R.; Andrade, T.J.A.S.; Navegante, G.; Serafim, R.B.; Sorbo, J.M.; Valente, V.; Silva, D.H.S.; Soares, C.P. Chemoprevention Assessment, Genotoxicity and Cytotoxicity of Flavonoids from Inga laurina Leaves (FABACEAE). Nat. Prod. Res. 2019, 35, 3089–3094. [Google Scholar] [CrossRef]
- Cristina Marcarini, J.; Ferreira Tsuboy, M.S.; Cabral Luiz, R.; Regina Ribeiro, L.; Beatriz Hoffmann-Campo, C.; Ségio Mantovani, M. Investigation of Cytotoxic, Apoptosis-Inducing, Genotoxic and Protective Effects of the Flavonoid Rutin in HTC Hepatic Cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef]
- Haza, A.I.; Morales, P. Effects of (+)Catechin and (−)Epicatechin on Heterocyclic Amines-Induced Oxidative DNA Damage. J. Appl. Toxicol. 2011, 31, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Recalcati, S.; Menna, P.; Salvatorelli, E.; Corna, G.; Cairo, G. Doxorubicin Cardiotoxicity and the Control of Iron Metabolism: Quinone-Dependent and Independent Mechanisms. Methods Enzymol. 2004, 378, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Elfadadny, A.; Ragab, R.F.; Hamada, R.; Al Jaouni, S.K.; Fu, J.; Mousa, S.A.; El-Far, A.H. Natural Bioactive Compounds-Doxorubicin Combinations Targeting Topoisomerase II-Alpha: Anticancer Efficacy and Safety. Toxicol. Appl. Pharmacol. 2023, 461, 116405. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, Á.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Tinitana, F.; Martínez-Espinosa, R.; Jaramillo-Velez, A.; Palacio-Arpi, A.; Aguilar-Hernandez, J.; Romero-Benavides, J.C. Cytotoxic, Antioxidative, Genotoxic and Antigenotoxic Effects of Horchata, Beverage of South Ecuador. BMC Complement. Altern. Med. 2017, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Wu, H.; Wang, J.; Liu, F.; Wang, W. Natural Flavonoids Function as Chemopreventive Agents from Gancao (Glycyrrhiza inflata Batal). J. Funct. Foods 2015, 19, 563–574. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Li, N.; Zhang, X.; Zhao, W.; Li, J.; Si, Y. Chemopreventive Flavonoids from Millettia pulchra Kurz Var-Laxior (Dunn) Z.Wei (Yulangsan) Function as Michael Reaction Acceptors. Bioorg Med. Chem. Lett. 2015, 25, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 17 May 2025).
- Waterman, P.G.; Mole, S. A Critical Analysis of Techniques for Measuring Tannins in Ecological Studies I: Techniques for Chemically Defining Tannins. Oecologia 1987, 72, 137–147. [Google Scholar]
- Hagerman, A.E.; Butler, L.G. Protein Precipitation Method for the Quantitative Determination of Tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Rolim, A.; Maciel, C.P.M.; Kaneko, T.M.; Consiglieri, V.O.; Salgado-Santos, I.M.N.; Velasco, M.V.R. Validation Assay for Total Flavonoids, as Rutin Equivalents, from Trichilia catigua Adr. Juss (Meliaceae) and Ptychopetalum olacoides Bentham (Olacaceae) Commercial Extract. J. AOAC Int. 2005, 88, 1015–1019. [Google Scholar] [CrossRef]
- Guidelines for Dietary Supplements and Botanicals; AOAC International: Rockville, MD, USA, 2019.
- Brasil Resolução Da Diretoria Colegiada-RDC No 166; Ministério da Saúde Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2017.
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total Em Frutas Pela Captura Do Radical Livre ABTS+; Comunicado Técnico; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2007. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Totalem Frutaspela Captura do Radical Livre DPPH; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2007. [Google Scholar]
- Rocha, J.D.; da Silva Ferreira, J.; Vieira Silva, J.G.; Silva Fernandes, A.; Hollanda Véras, J.; Madureira de Almeida, L.; Magalhães Teles, A.; Luiz Borges, L.; Chen-Chen, L.; Luiz Cardoso Bailão, E.F. In vitro hematotoxicity of Vernonanthura polyanthes leaf aqueous extract and its fractions. Drug Chem Toxicol. 2022, 45, 1026–1034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).