Endoplasmic Reticulum-Targeted Phototherapy Remodels the Tumor Immunopeptidome to Enhance Immunogenic Cell Death and Adaptive Anti-Tumor Immunity
Abstract
1. Introduction
2. Results
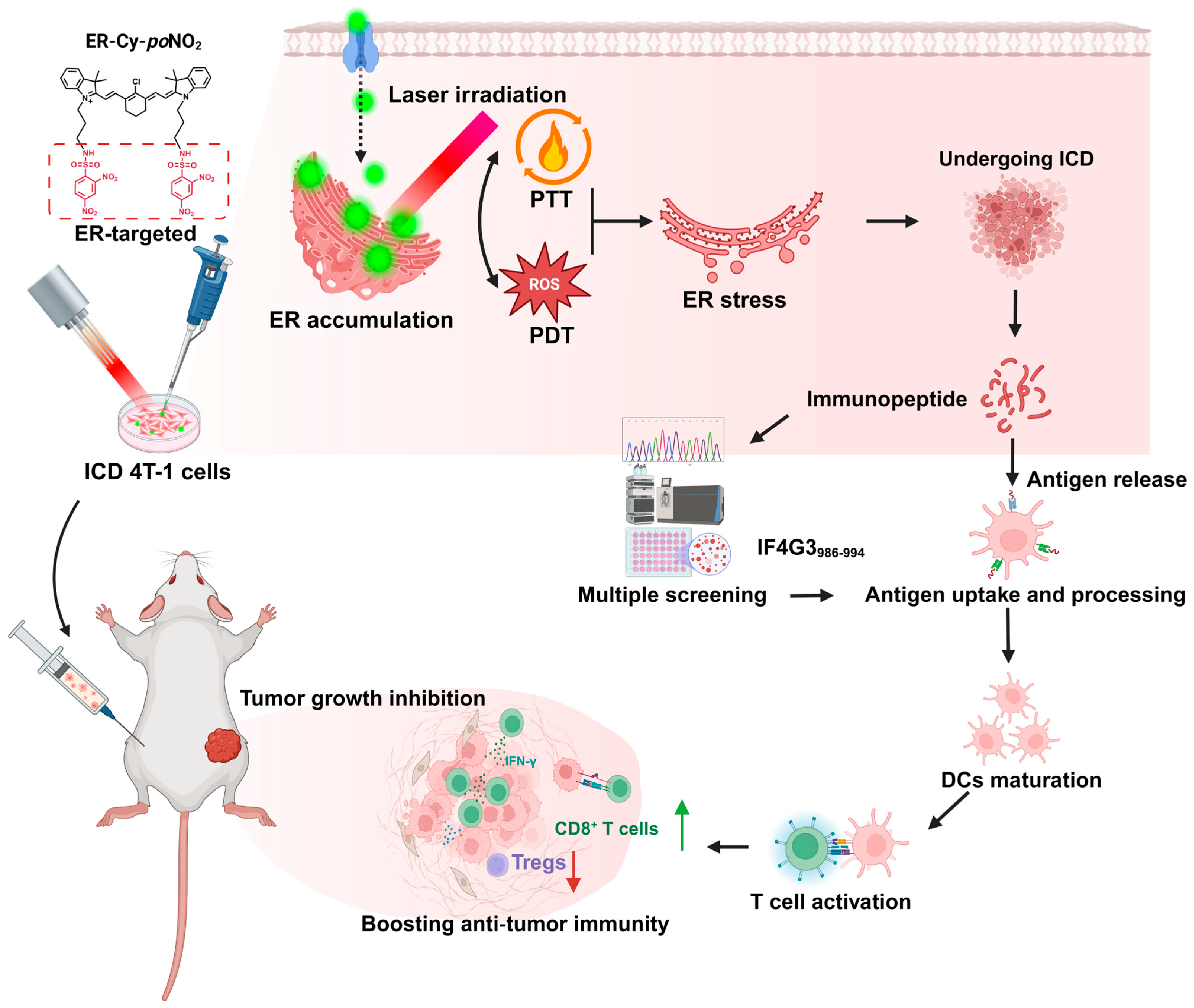
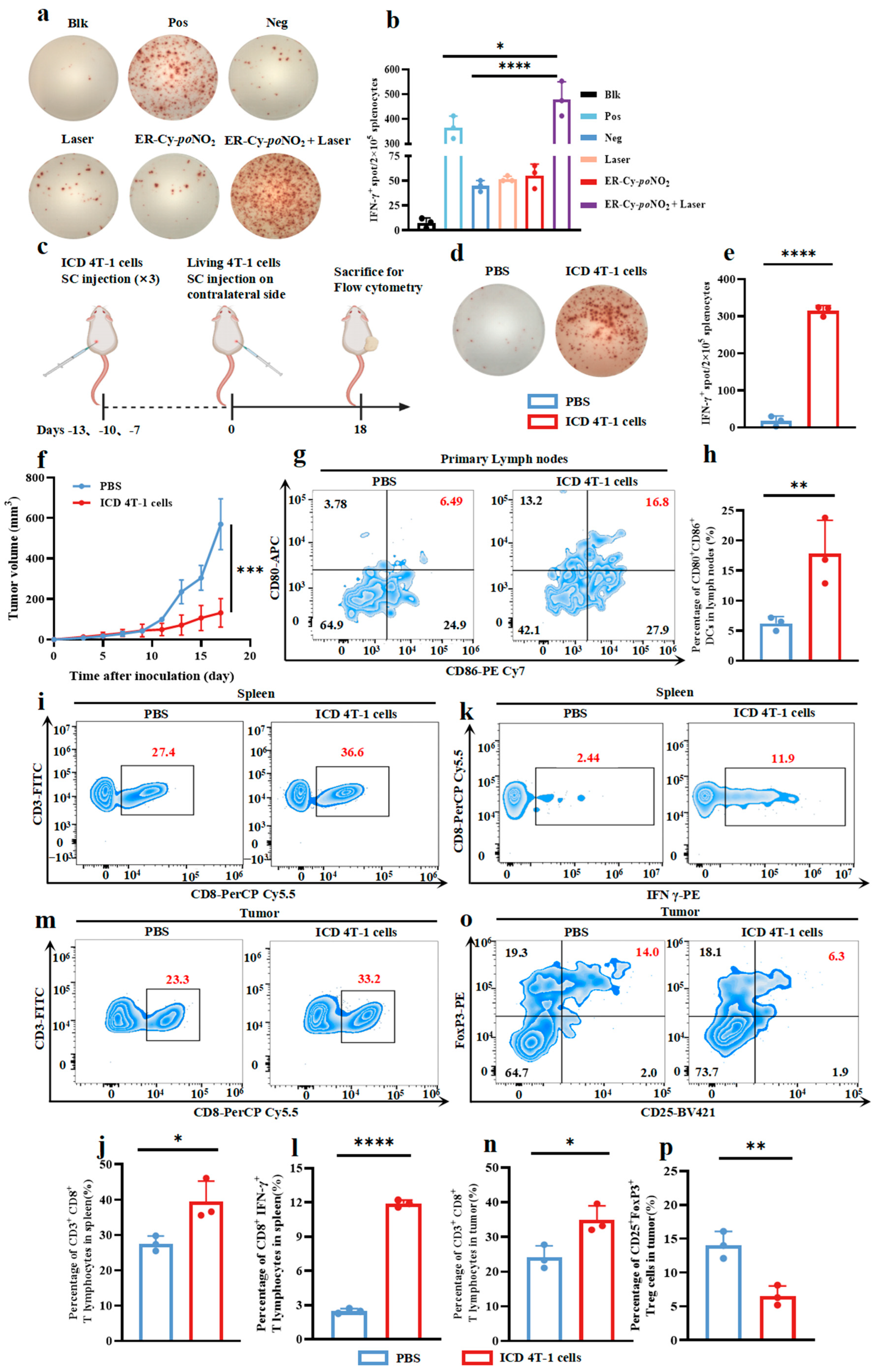
2.1. ER-Targeted Phototherapy Enhanced the Immunogenicity of 4T-1 Tumor Cells Undergoing ICD
2.2. ICD Tumor Cells Induced by ER-Targeted Phototherapy Triggered a Robust Anti-Tumor Immunity in 4T-1-Bearing Mice
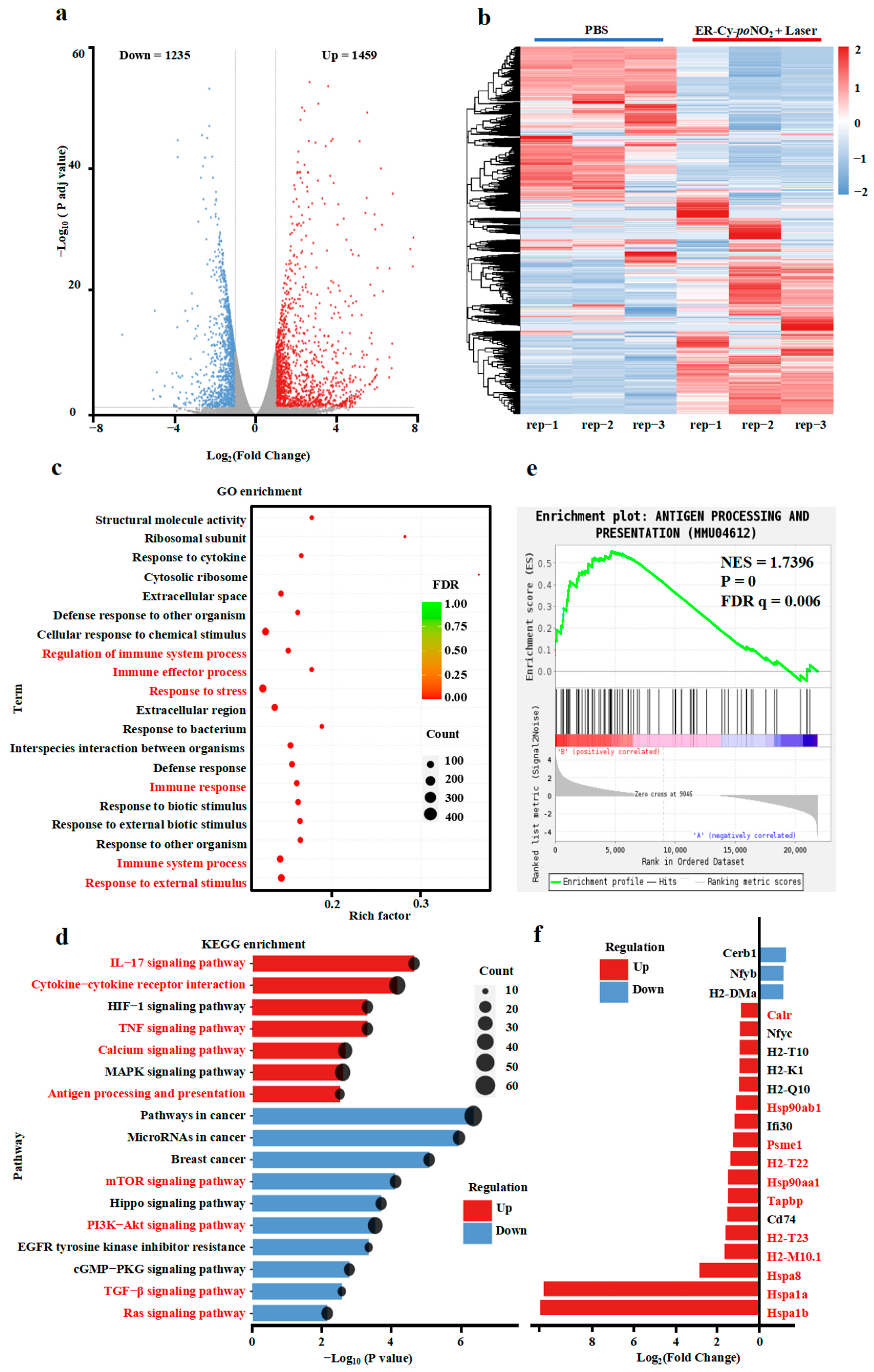
2.3. ER-Targeted Phototherapy Upregulated Antigen Processing and Immune Signaling Pathways in 4T-1 Cells
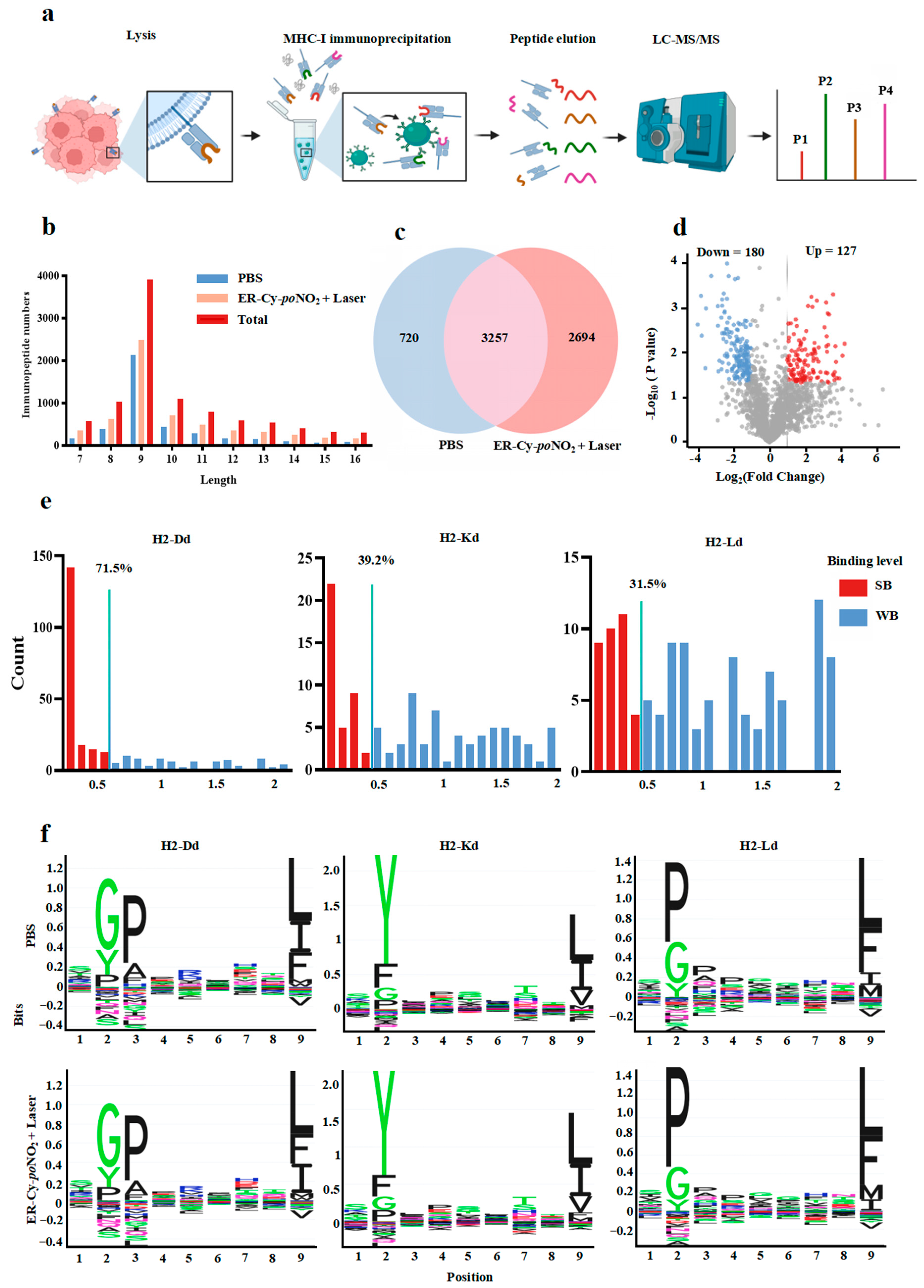
2.4. ER-Targeted Phototherapy Remodeled Immunopeptidome of 4T-1 Cells, Generating High-Affinity MHC-I Ligands
2.5. The IF4G3986–994 Peptide Demonstrated a Pronounced Immunogenicity Advantage over the Other Tested Candidates
2.6. The Highly Immunogenic Peptide IF4G3986–994 Could Effectively Activate the Anti-Tumor Immune Response and Subsequently Inhibit Tumor Growth in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of ER-Cy-poNO2
4.3. Cell Culture and Treatment
4.4. Immunogenicity Evaluation of 4T-1 Cells
4.5. Transcriptome Sequencing Analysis
4.6. Immunopeptidomics, Peptide MHC-I-Binding Affinity, and Immunogenicity Prediction
4.7. Tumor Vaccine Experiments
4.8. Tumor Treatment Experiments
4.9. In Vivo Immunogenicity Assessment of Candidate Peptides
4.10. Flow Cytometry Analysis
4.11. Candidate Peptide Synthesis and In Vitro Immunogenicity Assessment
4.12. In Vitro BMDC Maturation Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, X.; Zhou, J.-L.; Yuan, L.; Fan, J.; Yoon, J.; Zhang, X.-B.; Peng, X.; Tan, W. Phototherapy: Progress, challenges, and opportunities. Sci. China Chem. 2024, 68, 865. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.-G.; Jin, H.; Kim, J.; Oh, Y.-K. Claudin 4-targeted nanographene phototherapy using a Clostridium perfringens enterotoxin peptide-photosensitizer conjugate. Acta Pharmacol. Sin. 2017, 38, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, K.; Kang, M.; Yan, D.; Niu, N.; Yan, S.; Sun, P.; Zhang, L.; Sun, L.; Wang, D.; et al. A new era of cancer phototherapy: Mechanisms and applications. Chem. Soc. Rev. 2024, 53, 12014–12042. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and photothermal therapies: Synergy opportunities for nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, M.; Wang, W.; Hong, L.; Wu, Z.; Luo, G.; Lu, S.; Tang, Y.; Li, J.; Wang, J.; et al. 5-ALA mediated photodynamic therapy with combined treatment improves anti-tumor efficacy of immunotherapy through boosting immunogenic cell death. Cancer Lett. 2023, 554, 216032. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, M.; Dotse, E.; Chow, K.T.; Lo, P.C. Inducing immunogenic cancer cell death through oxygen-economized photodynamic Therapy with Nitric oxide-releasing photosensitizers. Angew. Chem. 2024, 63, e202404561. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.L.; Lang, T.Q.; Yuan, W.H.; Yin, Q.; Li, Y.P. Nanosized drug delivery systems modulate the immunosuppressive microenvironment to improve cancer immunotherapy. Acta Pharmacol. Sin. 2022, 43, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.A.; Okamura, S.M.; De Magalhaes Filho, C.D.; Bergeron, D.M.; Rodriguez, A.; West, M.; Yadav, D.; Heim, R.; Fong, J.J.; Garcia-Guzman, M. Cancer-targeted photoimmunotherapy induces antitumor immunity and can be augmented by anti-PD-1 therapy for durable anticancer responses in an immunologically active murine tumor model. Cancer Immunol. Immunother. 2023, 72, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Veld, R.V.H.I.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current challenges and opportunities of photodynamic therapy against cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy-current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, Y.; Xu, L.; Wang, S.; Li, Z.; Liu, Y.; Kwok, R.T.K.; Sun, J.; Lam, J.W.Y.; He, G.; et al. Aggregation-induced emission luminogen based wearable visible-light penetrator for deep photodynamic Therapy. ACS Nano 2024, 18, 29930–29941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, H.; Meng, X.; Li, W.; Qi, J. A hypoxia-activated and microenvironment-remodeling nanoplatform for multifunctional imaging and potentiated immunotherapy of cancer. Nat. Commun. 2024, 15, 10395. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, G.L.; Li, N.; Wang, M.; Wu, P.; He, Y.; Zhou, W.; Xiao, H.; Tan, X.; Tang, L.; et al. A mitochondria-targeted molecular phototheranostic platform for NIR-II imaging-guided synergistic photothermal/photodynamic/immune therapy. J. Nanobiotechnol. 2022, 20, 475. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Peng, Z.; Zhang, X.; Lin, Y.; Hu, L.; Zheng, L.; Tang, B.Z.; Zhang, J. Strategically engineered Au(I) complexes for orchestrated tumor eradication via chemo-phototherapy and induced immunogenic cell death. Nat. Commun. 2024, 15, 8187. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- English, A.R.; Voeltz, G.K. Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb. Perspect. Biol. 2013, 5, a013227. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic reticulum-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Gao, D.S. Endoplasmic reticulum proteins quality control and the unfolded protein response: The regulative mechanism of organisms against stress injuries. BioFactors 2014, 40, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Tameire, F.; Verginadis, I.I.; Koumenis, C. Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy. Semin. Cancer Biol. 2015, 33, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Sisinni, L.; Pietrafesa, M.; Lepore, S.; Maddalena, F.; Condelli, V.; Esposito, F.; Landriscina, M. Endoplasmic reticulum stress and unfolded protein response in breast cancer: The balance between apoptosis and autophagy and its role in drug resistance. Int. J. Mol. Sci. 2019, 20, 857. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, Z.; Liang, M.X.; Fei, Y.J.; Zhang, W.; Wu, Y.; Tang, J.H. Endoplasmic reticulum stress targeted therapy for breast cancer. Cell Commun. Signal. 2022, 20, 174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, G.; Huang, L.; Yuan, Y.; Wu, C.; Li, Y. Transmissible endoplasmic reticulum stress: A novel perspective on tumor immunity. Front. Cell Dev. Biol. 2020, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, M.; Xing, H.; Du, Z.; Wu, Z.; Liu, J.; Li, T.; Cao, J.; Yang, X.; Li, R.; et al. Rationally designed heptamethine cyanine photosensitizers that amplify tumor-specific endoplasmic reticulum stress and boost antitumor immunity. Small 2022, 18, e2202728. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, M.; Xie, Y.; Sun, Q.; Gao, M.; Li, C. Rationally integrated precise er-targeted and oxygen-compensated photodynamic immunostimulant for immunogenicity-boosted tumor therapy. Adv. Health Mater. 2023, 12, e2301728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qian, J.; Yang, Z.; Song, Y.; Pan, W.; Ye, Y.; Qin, X.; Yan, X.; Huang, X.; Wang, X.; et al. Photodynamic modulation of endoplasmic reticulum and mitochondria network boosted cancer immunotherapy. Adv. Mater. 2024, 36, e2310964. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ni, Z.; Xu, Y.; Zhang, L.; Liu, Y.; Zeng, F.; Zhang, M.; Liu, L.; Feng, G.; Tang, B.Z. Multi-functional aie phototheranostic agent enhancing αpd-l1 response for oral squamous cell carcinoma immunotherapy. Small 2024, 20, e2405470. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Wang, X.; Zhang, Z.; Cao, L.; Fan, X.; Wan, B.; Liu, F.; Zhang, X.; He, Z.; et al. Gas therapy potentiates aggregation-induced emission luminogen-based photoimmunotherapy of poorly immunogenic tumors through cGAS-STING pathway activation. Nat. Commun. 2023, 14, 2950. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nikolos, F.; Lee, Y.C.; Jain, A.; Tsouko, E.; Gao, H.; Kasabyan, A.; Leung, H.E.; Osipov, A.; Jung, S.Y.; et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat. Commun. 2020, 11, 6299. [Google Scholar] [CrossRef] [PubMed]
- Konda, P.; Roque Iii, J.A.; Lifshits, L.M.; Alcos, A.; Azzam, E.; Shi, G.; Cameron, C.G.; McFarland, S.A.; Gujar, S. Photodynamic therapy of melanoma with new, structurally similar, NIR-absorbing ruthenium (II) complexes promotes tumor growth control via distinct hallmarks of immunogenic cell death. Am. J. Cancer Res. 2022, 12, 210–228. [Google Scholar] [PubMed]
- Wan, J.; Ren, L.; Li, X.; He, S.; Fu, Y.; Xu, P.; Meng, F.; Xian, S.; Pu, K.; Wang, H. Photoactivatable nanoagonists chemically programmed for pharmacokinetic tuning and in situ cancer vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2210385120. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, A.M.; Duzagac, F.; Deng, N.; Reyes-Uribe, L.; Chang, K.; Wu, W.; Bowen, C.M.; Taggart, M.W.; Thirumurthi, S.; Lynch, P.M.; et al. Genomic Landscape of lynch syndrome colorectal neoplasia identifies shared mutated neoantigens for immunoprevention. Gastroenterology 2024, 166, 787–801.e711. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Patton, K.; Kasprzyk, T.; Long, B.; Gupta, S.; Zoog, S.J.; Tracy, K.; Vettermann, C. Validation of an IFN-gamma ELISpot assay to measure cellular immune responses against viral antigens in non-human primates. Gene Ther. 2022, 29, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Y.; Jin, F.; Hu, K.; Lv, M.; Zhou, Y.; Zhao, W.; Hu, Y.; Wu, J.; Yang, Y.; et al. Multifunctional nanoparticles potentiate in-situ tumor vaccines via reversing insufficient Photothermal therapy by disrupting tumor vasculature. J. Control Release 2024, 376, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- MacNabb, B.W.; Tumuluru, S.; Chen, X.; Godfrey, J.; Kasal, D.N.; Yu, J.; Jongsma, M.L.M.; Spaapen, R.M.; Kline, D.E.; Kline, J. Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing. Immunity 2022, 55, 982–997.e988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, H.; Wang, P.; Que, W.; Zhong, L.; Li, X.K.; Du, F. Different subpopulations of regulatory T cells in human autoimmune disease, transplantation, and tumor immunity. MedComm 2022, 3, e137. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, Y.; Qiao, S.; Liu, M.; Ji, Q.; Zhang, B.L.; Mei, Q.B.; Zhou, S. Nano-Codelivery of Temozolomide and siPD-L1 to Reprogram the Drug-Resistant and Immunosuppressive Microenvironment in Orthotopic Glioblastoma. ACS Nano 2022, 16, 7409–7427. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Qiao, R.; Xin, L.; Chen, X.; Shan, B.; Li, M. Personalized deep learning of individual immunopeptidomes to identify neoantigens for cancer vaccines. Nat. Mach. Intell. 2020, 2, 764–771. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Molina, C.; De Andrea, C.E.; Fernandez-Sendin, M.; Villalba, M.; Gonzalez-Gomariz, J.; Ochoa, M.C.; Teijeira, A.; Glez-Vaz, J.; Aranda, F.; et al. Intratumoral co-injection of the poly I:C-derivative BO-112 and a STING agonist synergize to achieve local and distant anti-tumor efficacy. J. Immunother. Cancer 2021, 9, e002953. [Google Scholar] [CrossRef] [PubMed]
- Sales Conniff, A.; Encalada, G.; Patel, S.; Bhandary, M.; Al-Takrouri, F.; Heller, L. Poly(I:C) transfection induces a pro-inflammatory cascade in murine mammary carcinoma and fibrosarcoma cells. RNA Biol. 2022, 19, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, C.; Shan, W.; Wang, Z.; Chen, X.; Deng, H. Nanomedicines for an enhanced immunogenic cell death-based in situ cancer vaccination response. Acc. Chem. Res. 2024, 57, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, J.; Zhou, F.; Hoover, A.R.; Murray, C.; Zhou, B.; Wang, L.; Qu, J.; Chen, W.R. NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv. Sci. 2019, 6, 1802157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, W.; Lin, B.; Yuan, Y.; Ning, P.; Tao, X.; Lv, R. NIR-II imaging-guided photothermal cancer therapy combined with enhanced immunogenic death. Biomater. Sci. 2023, 11, 5177–5185. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, D.; Liu, D. Targeted Nanophotoimmunotherapy potentiates cancer treatment by enhancing tumor immunogenicity and improving the immunosuppressive tumor microenvironment. Bioconjugate Chem. 2023, 34, 283–301. [Google Scholar] [CrossRef]
- Chen, P.; Rong, J.; Chen, K.; Huang, T.; Shen, Q.; Sun, P.; Tang, W.; Fan, Q. Photo-amplified plasma membrane rupture by membrane-anchoring nir-ii small molecule design for improved cancer photoimmunotherapy. Angew. Chem. 2025, 64, e202418081. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Chen, K.; Yan, J.; Chen, P.; Gao, W.Q.; Ma, B. Targeting the mevalonate pathway potentiates NUAK1 inhibition-induced immunogenic cell death and antitumor immunity. Cell Rep. Med. 2025, 6, 101913. [Google Scholar] [CrossRef] [PubMed]
- Mandula, J.K.; Chang, S.; Mohamed, E.; Jimenez, R.; Sierra-Mondragon, R.A.; Chang, D.C.; Obermayer, A.N.; Moran-Segura, C.M.; Das, S.; Vazquez-Martinez, J.A.; et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell 2022, 40, 1145–1160.e1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Y.; Ding, Y.; Zhang, X.; Dong, X.; Xie, L.; Yang, Z.; Hu, Z.W. Dual-enzyme-instructed peptide self-assembly to boost immunogenic cell death by coordinating intracellular calcium overload and chemotherapy. ACS Nano 2025, 19, 488–503. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Xu, X.; Wu, Y.; Lei, Y.; Liu, H.; Qian, X.; Li, Y.; Zhang, Z. Microvesicle-inspired oxygen-delivering nanosystem potentiates radiotherapy-mediated modulation of tumor stroma and antitumor immunity. Biomaterials 2022, 290, 121855. [Google Scholar] [CrossRef] [PubMed]
- De Nagel, D.C.; Pierce, S.K. Heat shock proteins implicated in antigen processing and presentation. Semin. Immunol. 1991, 3, 65–71. [Google Scholar]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef]
- Chen, A.C.Y.; Jaiswal, S.; Martinez, D.; Yerinde, C.; Ji, K.; Miranda, V.; Fung, M.E.; Weiss, S.A.; Zschummel, M.; Taguchi, K.; et al. The aged tumor microenvironment limits T cell control of cancer. Nat. Immunol. 2024, 25, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, C.; Wang, Y.; Liu, G.; Wang, N.; Liang, N.; Zhang, L.; Tu, Q.; Lv, J.; Jiang, H.; et al. ALDOB/KAT2A interactions epigenetically modulate TGF-β expression and T cell functions in hepatocellular carcinogenesis. Hepatology 2025, 81, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Liu, J.; Xing, W.; Mu, F.; Wu, Y.; Zhao, J.; Liu, X.; Wang, D.; Wang, J.; Li, X.; et al. Hypoxic glioma-derived exosomal miR-25-3p promotes macrophage M2 polarization by activating the PI3K-AKT-mTOR signaling pathway. J. Nanobiotechnol. 2024, 22, 628. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, Y.; Gao, W.; Wang, J.; Hu, Y.; Yang, H.; Xie, Y.; Lv, Y.; Zhang, H.; Wu, D.; et al. CSF1R inhibition reprograms tumor-associated macrophages to potentiate anti-PD-1 therapy efficacy against colorectal cancer. Pharmacol. Res. 2024, 202, 107126. [Google Scholar] [CrossRef] [PubMed]
- Sirois, I.; Isabelle, M.; Duquette, J.D.; Saab, F.; Caron, E. Immunopeptidomics: Isolation of mouse and human MHC class I- and II-associated peptides for mass spectrometry analysis. J. Vis. Exp. 2021, 176, e63052. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Fenoy, E.; Harndahl, M.; Kristensen, A.B.; Nielsen, I.K.; Nielsen, M.; Buus, S. Pan-specific prediction of peptide-MHC Class I complex stability, a correlate of T cell immunogenicity. J. Immunol. 2016, 197, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Dalchau, N.; Phillips, A.; Goldstein, L.D.; Howarth, M.; Cardelli, L.; Emmott, S.; Elliott, T.; Werner, J.M. A peptide filtering relation quantifies MHC class I peptide optimization. PLoS Comput. Biol. 2011, 7, e1002144. [Google Scholar] [CrossRef] [PubMed]
- Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved peptide-MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC class I antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, S.; Zhu, G.; Li, J.; Meng, G.; Chen, X.; Zhang, M.; Wang, S.; Li, X.; Pan, Y.; et al. Immunopeptidome mining reveals a novel ERS-induced target in T1D. Cell Mol. Immunol. 2024, 21, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhang, T.; Wang, Q.; Wu, X.; Li, L.; Lin, R.; Zhou, Y.; Qu, S. Photocatalytic carbon dots-triggered pyroptosis for whole cancer cell vaccines. Adv. Mater. 2024, 36, e2408685. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Alföldi, R.; Nagy, L.I.; Neuperger, P.; Gémes, N.; Balog, J.; Tiszlavicz, L.; Puskás, L.G. Introduction of an ultraviolet C-irradiated 4t1 murine breast cancer whole-cell vaccine model. Vaccines 2023, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, H.; Zhou, Y.; Liu, X.; Huang, S.; Zhu, S. Effectiveness analysis of three-drug combination therapies for refractory focal epilepsy. Neurother. J. Am. Soc. Exp. Neurother. 2024, 21, e00345. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, H.; Ke, S.; Mo, L.; Qiu, M.; Zhu, G.; Zhu, W.; Liu, L. Identifying potential biomarkers of idiopathic pulmonary fibrosis through machine learning analysis. Sci. Rep. 2023, 13, 16559. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.L.; Verbeke, R.; Asselman, C.; Aernout, I.; Gul, A.; Eggermont, D.; Boucher, K.; Thery, F.; Maia, T.M.; Demol, H.; et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 2022, 13, 6075. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, M.; Hoppe, S.; Winter, J.; Tichy, D.; Zeller, C.; Küpper, M.D.; Schitter, E.C.; Blatnik, R.; Riemer, A.B. Performance evaluation of MHC Class-I binding prediction tools based on an experimentally validated mhc-peptide binding data set. Cancer Immunol. Res. 2019, 7, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mahajan, S.; Ma, S.; Jeffery, E.D.; Zhang, X.; Bhattacharjee, A.; Venkatasubramanian, M.; Weirauch, M.T.; Miraldi, E.R.; Grimes, H.L.; et al. Splicing neoantigen discovery with SNAF reveals shared targets for cancer immunotherapy. Sci. Transl. Med. 2024, 16, eade2886. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Kaemmerer, U.; Illert, B.; Muehling, B.; Pfetzer, N.; Wittig, R.; Voelker, H.U.; Thiede, A.; Coy, J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Nechama, M.; Kwon, J.; Wei, S.; Kyi, A.T.; Welner, R.S.; Ben-Dov, I.Z.; Arredouani, M.S.; Asara, J.M.; Chen, C.H.; Tsai, C.Y.; et al. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Gao, M.; Mo, B.; Huang, X.; Du, Z.; Wang, S.; Chen, J.; Luo, S.; Xing, H. Endoplasmic Reticulum-Targeted Phototherapy Remodels the Tumor Immunopeptidome to Enhance Immunogenic Cell Death and Adaptive Anti-Tumor Immunity. Pharmaceuticals 2025, 18, 491. https://doi.org/10.3390/ph18040491
Xiao W, Gao M, Mo B, Huang X, Du Z, Wang S, Chen J, Luo S, Xing H. Endoplasmic Reticulum-Targeted Phototherapy Remodels the Tumor Immunopeptidome to Enhance Immunogenic Cell Death and Adaptive Anti-Tumor Immunity. Pharmaceuticals. 2025; 18(4):491. https://doi.org/10.3390/ph18040491
Chicago/Turabian StyleXiao, Weidong, Mingquan Gao, Banghui Mo, Xie Huang, Zaizhi Du, Shufeng Wang, Jianhong Chen, Shenglin Luo, and Haiyan Xing. 2025. "Endoplasmic Reticulum-Targeted Phototherapy Remodels the Tumor Immunopeptidome to Enhance Immunogenic Cell Death and Adaptive Anti-Tumor Immunity" Pharmaceuticals 18, no. 4: 491. https://doi.org/10.3390/ph18040491
APA StyleXiao, W., Gao, M., Mo, B., Huang, X., Du, Z., Wang, S., Chen, J., Luo, S., & Xing, H. (2025). Endoplasmic Reticulum-Targeted Phototherapy Remodels the Tumor Immunopeptidome to Enhance Immunogenic Cell Death and Adaptive Anti-Tumor Immunity. Pharmaceuticals, 18(4), 491. https://doi.org/10.3390/ph18040491






