Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oils’ Chemical Composition
2.2. Antioxidant Activities
2.3. Antibacterial Activity
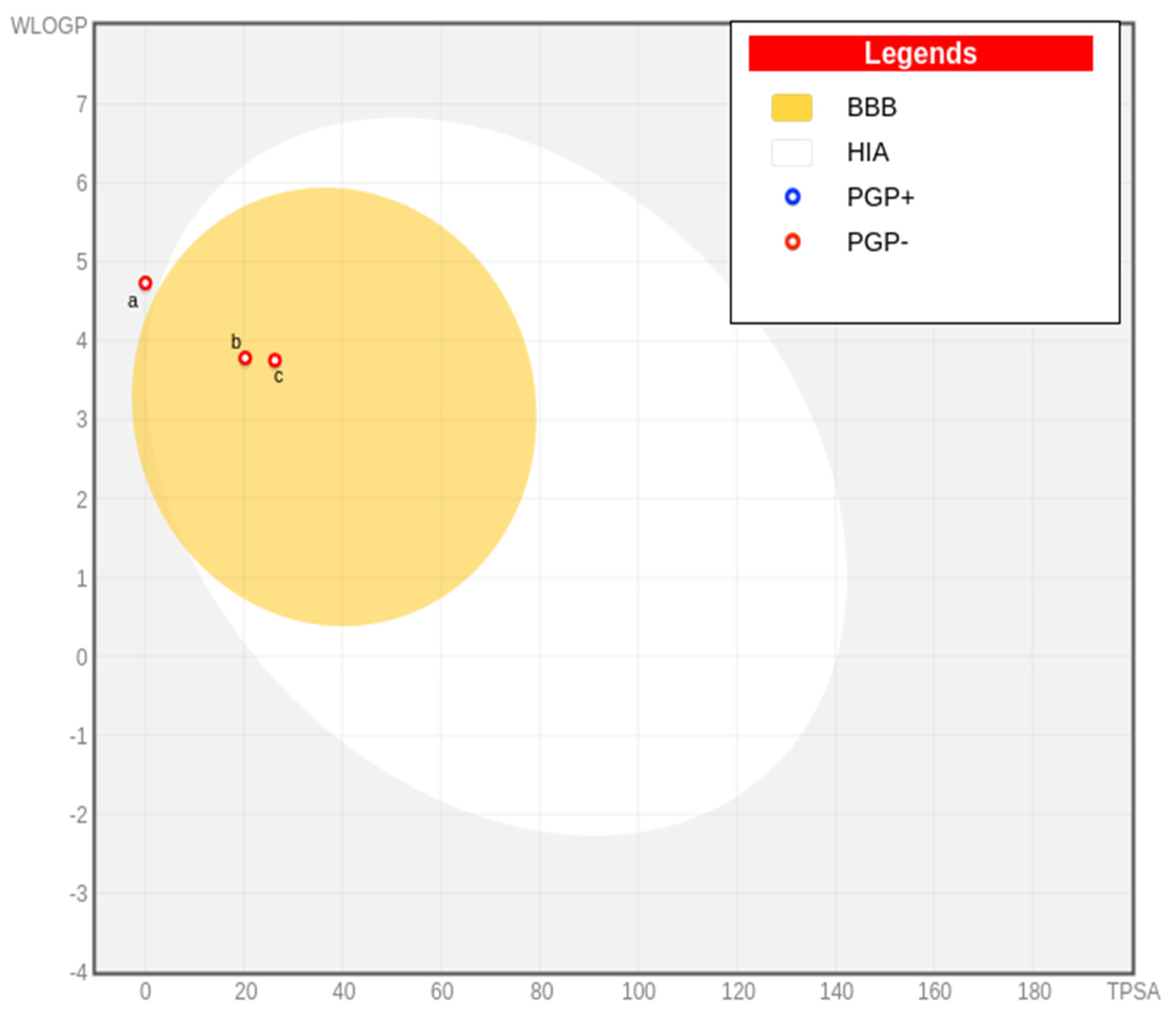
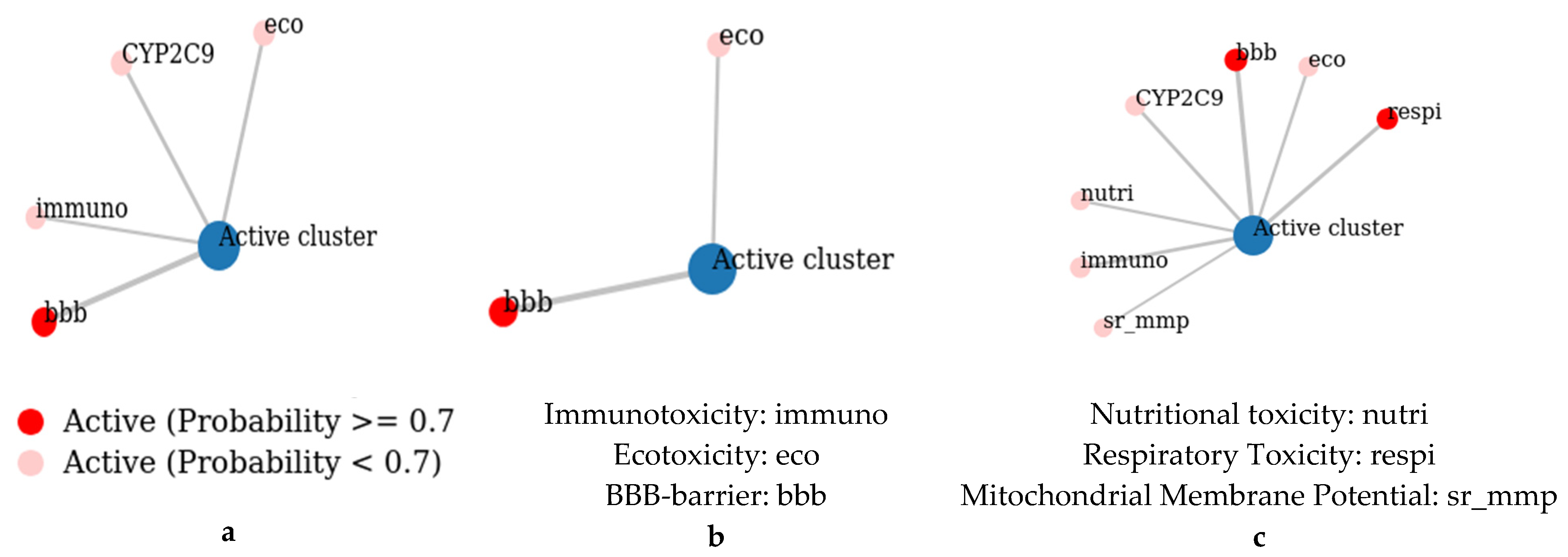
2.4. ADMET Study
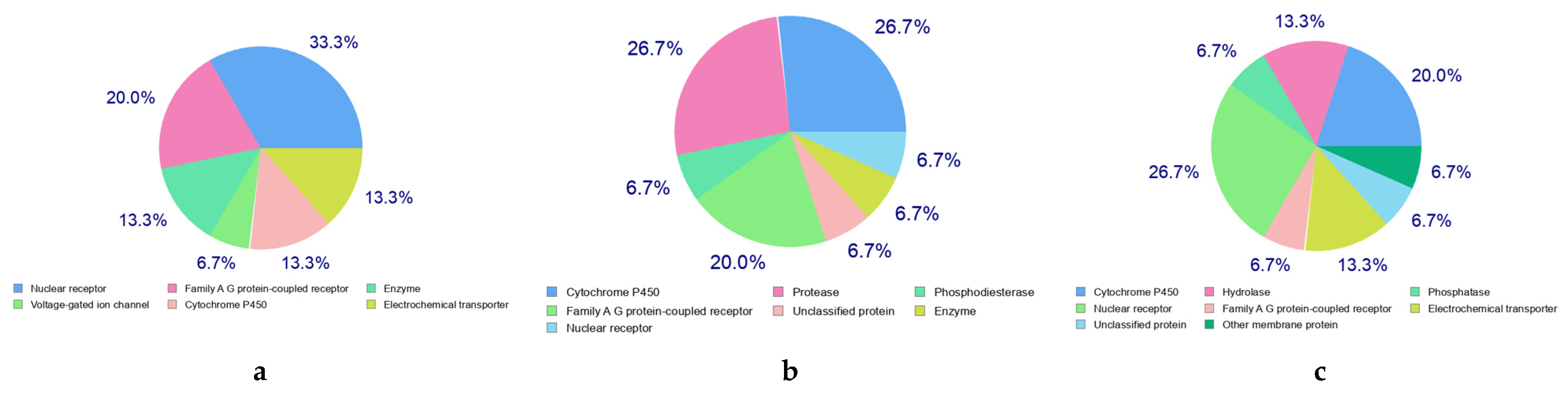
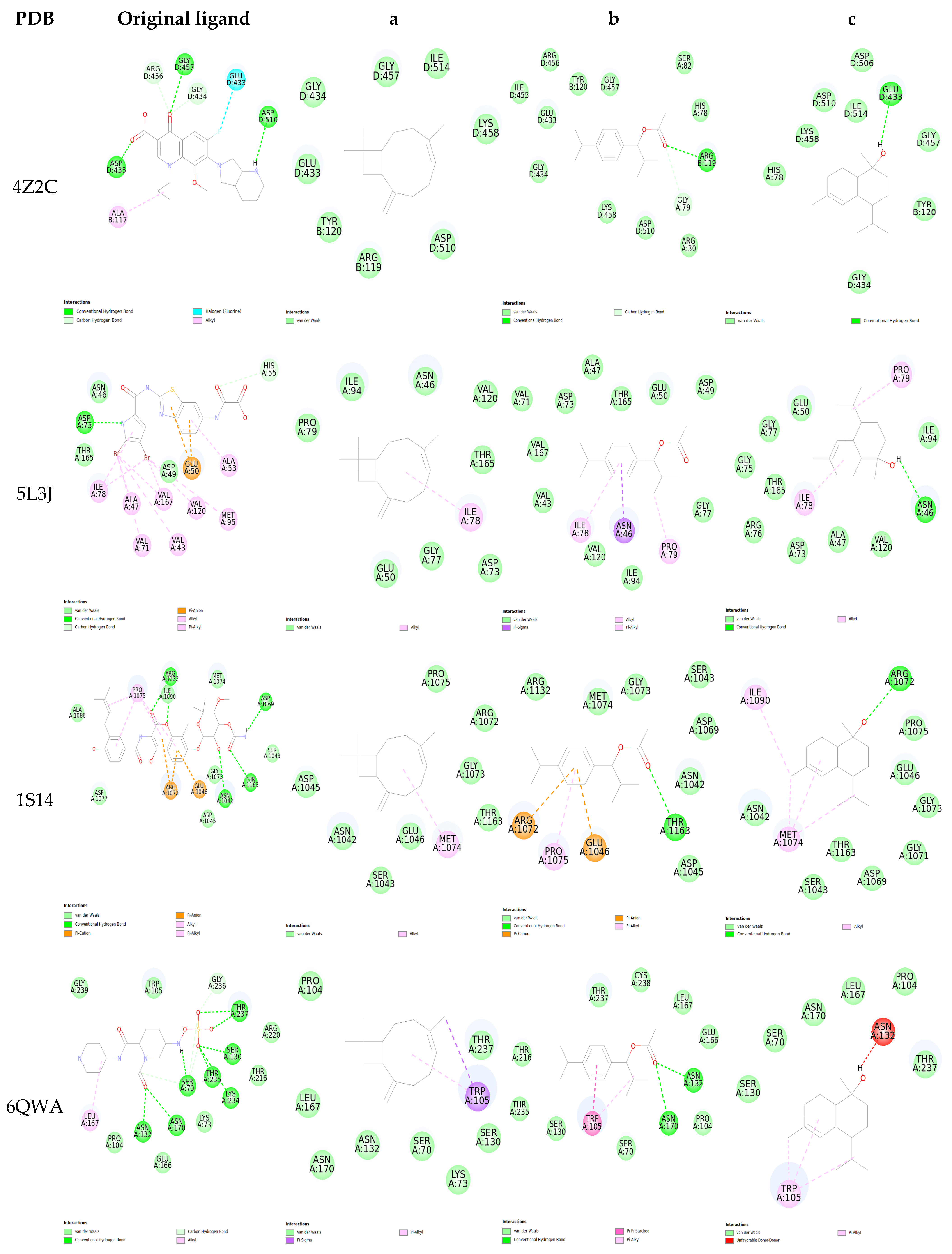
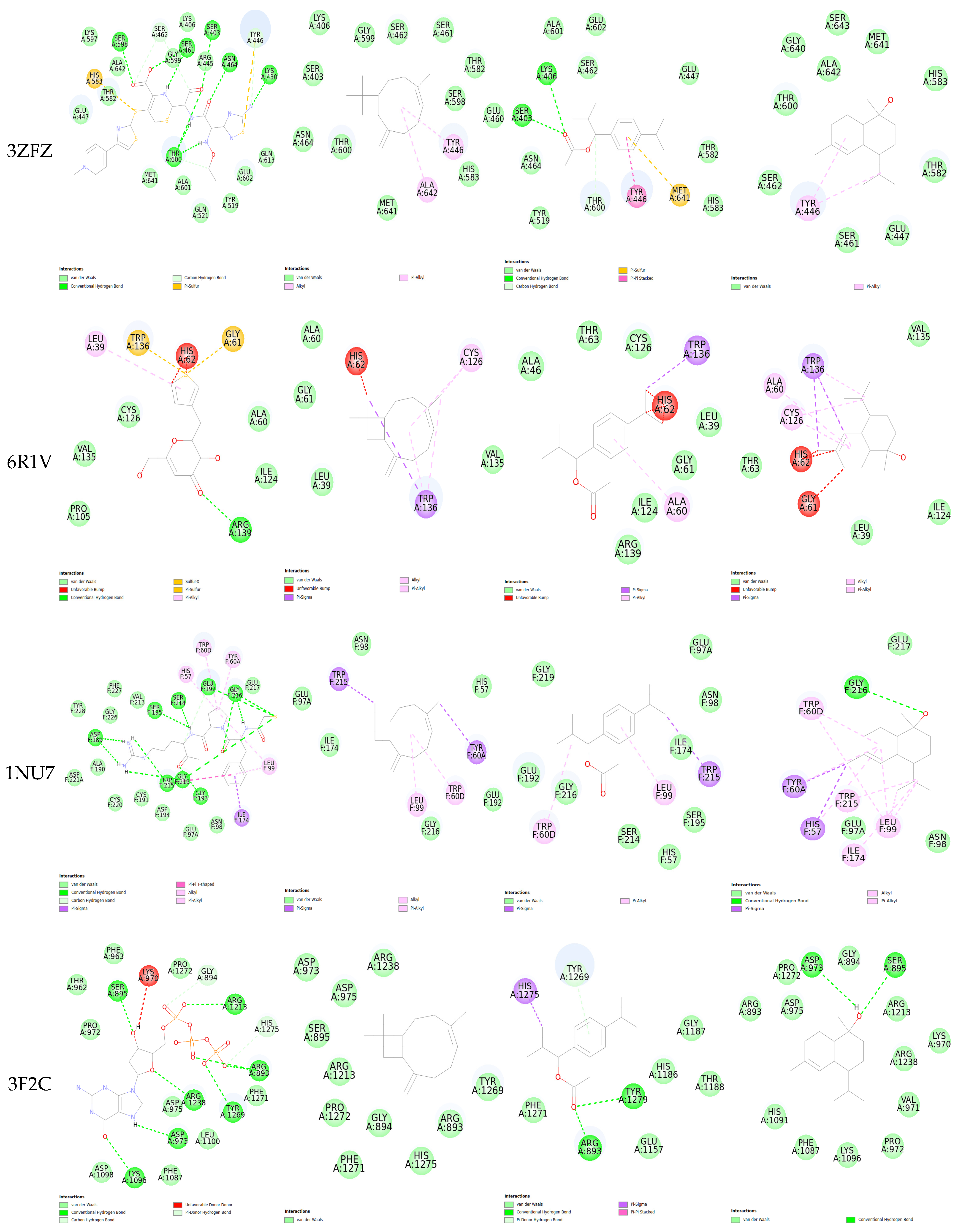
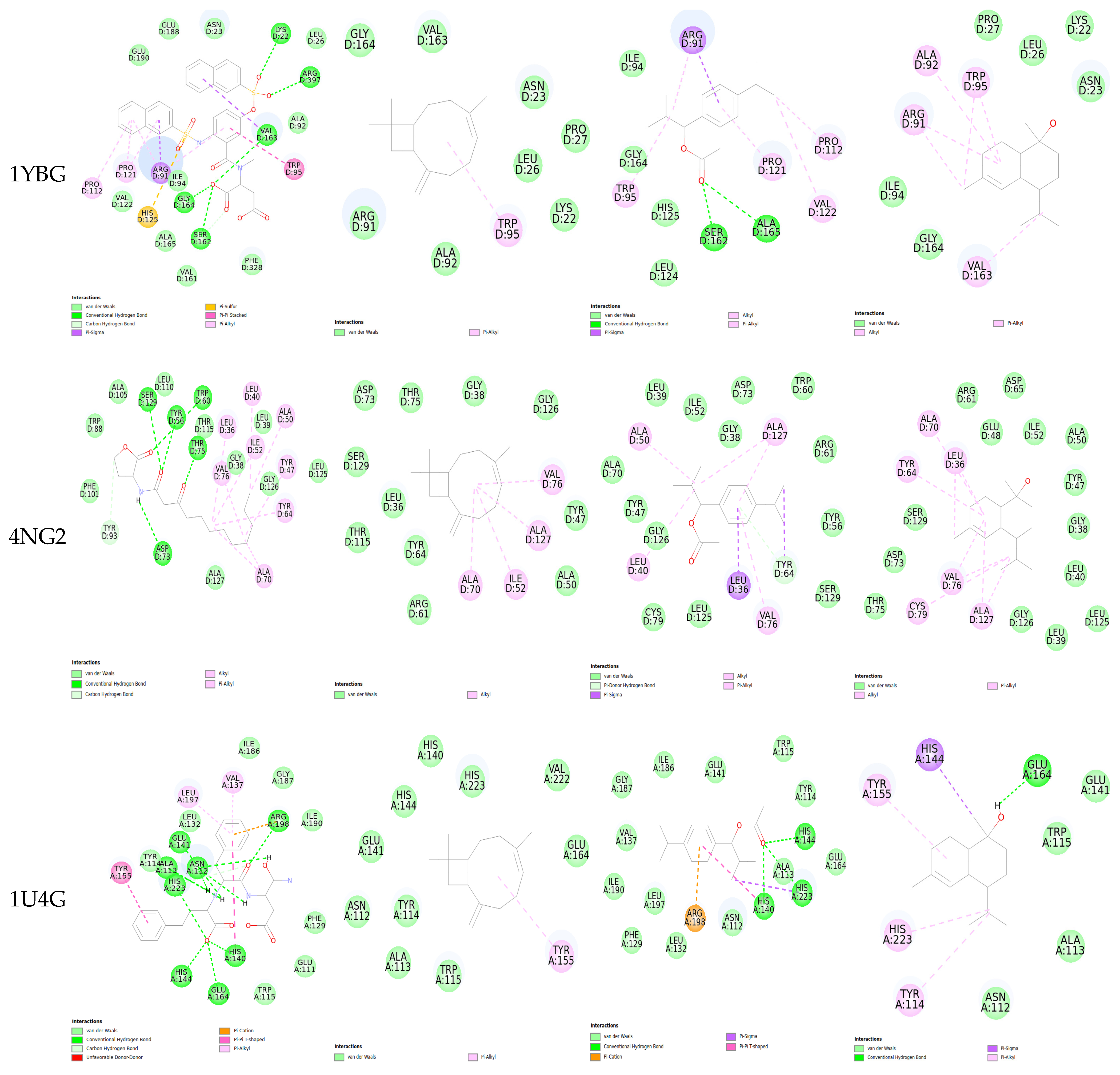
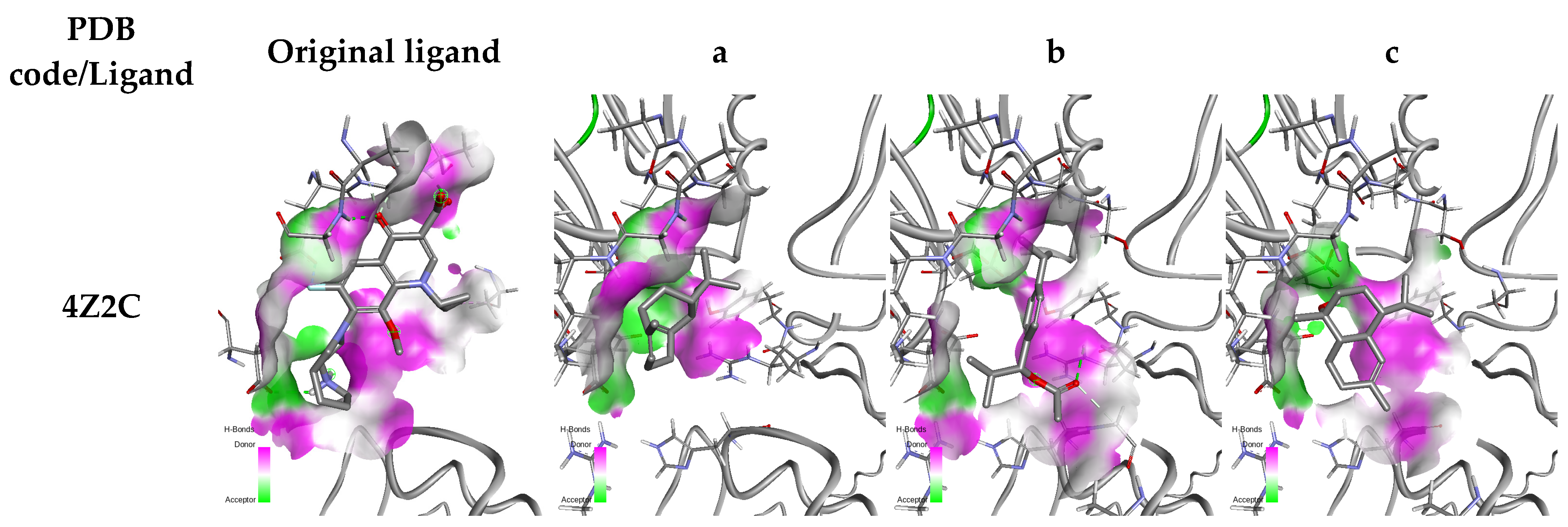
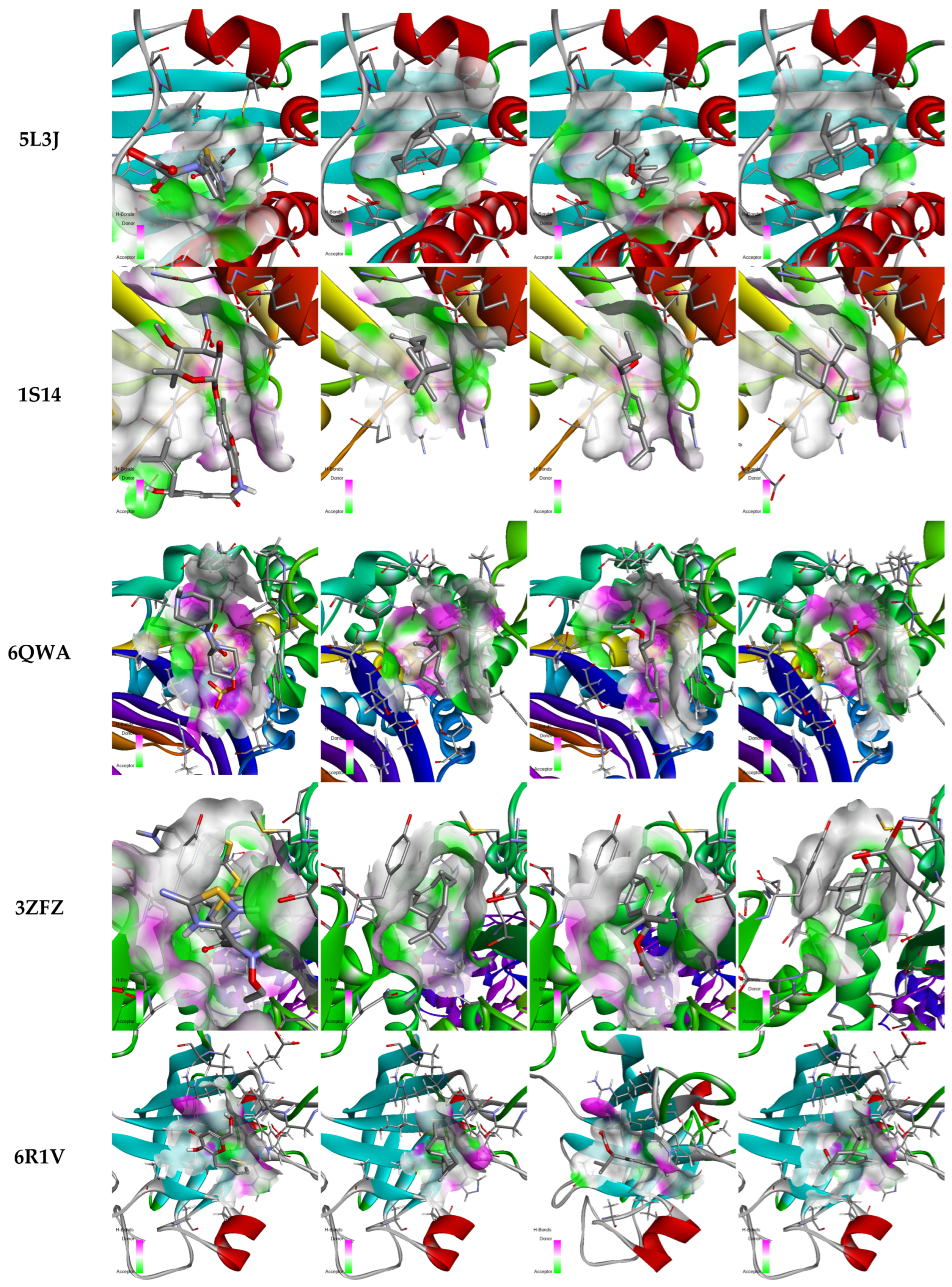
2.5. Molecular Docking
3. Material and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Phytochemical Analysis
3.4. Antioxidant Activity
3.4.1. 2,2-Diphenylpicrylhydrazyl Method (DPPH)
3.4.2. Ferric Reducing Antioxidant Power (FRAP)
3.4.3. Total Antioxidant Capacity Test (TAC)
3.4.4. Beta-Carotene Bleaching Inhibition Assay
3.5. Antibacterial Activity
3.6. ADMET Investigation
3.7. Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laccourreye, O.; Maisonneuve, H. French scientific medical journals confronted by developments in medical writing and the transformation of the medical press. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 475–480. [Google Scholar] [CrossRef]
- Cox, P.B.; Gupta, R. Contemporary computational applications and tools in drug discovery. ACS Med. Chem. Lett. 2022, 13, 1016–1029. [Google Scholar] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Küpeli Akkol, E.; Sobarzo-Sánchez, E. Multifunctional therapeutic potential of phytocomplexes and natural extracts for antimicrobial properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [PubMed]
- Halaska, M.; Beles, P.; Gorkow, C.; Sieder, C. Treatment of cyclical mastalgia with a solution containing a Vitex agnus castus extract: Results of a placebo-controlled double-blind study. Breast 1999, 8, 175–181. [Google Scholar] [CrossRef]
- Wuttke, W.; Jarry, H.; Christoffel, V.; Spengler, B.; Seidlova-Wuttke, D. Chaste tree (Vitex agnus-castus)—Pharmacology and clinical indications. Phytomedicine 2003, 10, 348–357. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar]
- Drioua, S.; Azalmad, H.; El-Guourrami, O.; Ameggouz, M.; Benkhouili, F.Z.; Assouguem, A.; Kara, M.; Ullah, R.; Ali, E.A.; Ercisli, S.; et al. Phytochemical screening and antioxidant activity of Vitex agnus-castus L. Open Chem. 2024, 22, 20230190. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Özbilgin, A.; Çavuş, İ.; Tunalı, V.; Özel, Y.; Alagöz, Ç.Y.; Kayalar, H. In vitro efficacy of Vitex agnus-castus extracts against trichomonas vaginalis: A potential therapeutic approach. Univers. J. Pharm. Res. 2024, 9, 14–20. [Google Scholar]
- Sucharitha, P.; Reddy, K.R.; Satyanarayana, S.V.; Garg, T. Absorption, distribution, metabolism, excretion, and toxicity assessment of drugs using computational tools. In Computational Approaches for Novel Therapeutic and Diagnostic Designing to Mitigate SARS-CoV-2 Infection; Academic Press: Cambridge, MA, USA, 2022; pp. 335–355. [Google Scholar]
- Mousavi, H.; Zeynizadeh, B.; Rimaz, M. Green and efficient one-pot three-component synthesis of novel drug-like furo [2, 3-d] pyrimidines as potential active site inhibitors and putative allosteric hotspots modulators of both SARS-CoV-2 MPro and PLPro. Bioorg. Chem. 2023, 135, 106390. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, H.; Rimaz, M.; Zeynizadeh, B. Practical Three-Component Regioselective Synthesis of Drug-Like 3-Aryl (or heteroaryl)-5, 6-dihydrobenzo [h] cinnolines as Potential Non-Covalent Multi-Targeting Inhibitors to Combat Neurodegenerative Diseases. ACS Chem. Neurosci. 2024, 15, 1828–1881. [Google Scholar] [CrossRef]
- Siddique, F.; Daoui, O.; Ayoub, M.; Elkhattabi, S.; Chtita, S.; Afzal, S.; Mohyuddin, A.; Kaukab, I.; Ejaz, S.; Salamatullah, A.; et al. Identification and in silico screening of natural phloroglucinols as potential PI3Kα inhibitors: A computational approach for drug discovery. Open Chem. 2024, 22, 20240064. [Google Scholar]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Golkar, P.; Moattar, F. Essential oil composition, bioactive compounds, and antioxidant activities in Iberis amara L. Nat. Prod. Commun. 2019, 14, 1934578X19846355. [Google Scholar]
- Zhelev, I.; Petkova, Z.; Kostova, I.; Damyanova, S.; Stoyanova, A.; Dimitrova-Dyulgerova, I.; Antova, G.; Ercisli, S.; Assouguem, A.; Kara, M.; et al. Chemical Composition and Antimicrobial Activity of Essential Oil of Fruits from Vitex agnus-castus L., Growing in Two Regions in Bulgaria. Plants 2022, 11, 896. [Google Scholar] [CrossRef]
- Arora Vimal, A.V.; Lohar Vikram, L.V.; Sandeep Singhal, S.S.; Bhandari Anil, B.A. Vitex negundo: A Chinese Chaste tree. Int. J. Pharmaceut. Innov. 2011, 1, 9–20. [Google Scholar]
- Meier, B.; Berger, D.; Hoberg, E.; Sticher, O.; Schaffner, W. Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine 2000, 7, 373–381. [Google Scholar] [CrossRef]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar]
- ELMoussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Majid Mahmood, H.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- Ghannadi, A.; Bagherinejad, M.R.; Abedi, D.; Jalali, M.; Absalan, B.; Sadeghi, N. Antibacterial activity and composition of essential oils from Pelargonium graveolens L’Her and Vitex agnus-castus L. Iran. J. Microbiol. 2012, 4, 171. [Google Scholar]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [PubMed]
- Parida, K.K.; Lahiri, M.; Ghosh, M.; Dalal, A.; Kalia, N.P. P-glycoprotein inhibitors as an adjunct therapy for TB. Drug Discov. Today 2024, 29, 104108. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Omidi, Y.; Kianinejad, N.; Kwon, Y.; Omidian, H. Drug delivery and targeting to brain tumors: Considerations for crossing the blood-brain barrier. Expert Rev. Clin. Pharmacol. 2021, 14, 357–381. [Google Scholar] [PubMed]
- Keitel, S. Pharmacopoeial Standards: European Pharmacopoeia. Encycl. Pharm. Sci. Technol. 2013, 6, 2691–2703. [Google Scholar]
- Azzouni, D.; Mrani, S.A.; Bertani, R.; Alanazi, M.M.; En-Nabety, G.; Taleb, M. Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium. Molecules 2024, 29, 3323. [Google Scholar] [CrossRef]
- Mrani, S.A.; Zejli, H.; Azzouni, D.; Fadili, D.; Alanazi, M.M.; Hassane, S.O.S.; Sabbahi, R.; Kabra, A.; Moussaoui, A.E.; Hammouti, B.; et al. Chemical Composition, Antioxidant, Antibacterial, and Hemolytic Properties of Ylang-Ylang (Cananga odorata) Essential Oil: Potential Therapeutic Applications in Dermatology. Pharmaceuticals 2024, 17, 1376. [Google Scholar] [CrossRef]
- Gani, I.H.; Al-Obaidi, Z. MgO NPs catalyzed the synthesis of novel pyridin-3-yl-pyrimidin-2-yl-aminophenyl-amide derivatives and evaluation of pharmacokinetic profiles and biological activity. Front. Mater. 2023, 10, 1057677. [Google Scholar]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids–ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Asomadu, R.O.; Ezeorba, T.P.C.; Ezike, T.C.; Uzoechina, J.O. Exploring the antioxidant potential of endophytic fungi: A review on methods for extraction and quantification of total antioxidant capacity (TAC). 3 Biotech 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Esmaeilzadeh Kenari, R.; Asnaashari, M.; Shahrampour, D.; Bakhshandeh, T. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr. 2019, 7, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Usman, L.A.; Adebayo, S.A.; Zubair, M.F.; Elaigwu, S.E. Chemical constituents of leaf essential oil of north-central Nigerian grown Vitex agnus-castus L. Adv. Environ. Biol. 2010, 4, 250–253. [Google Scholar]
- Ouedrhiri, W.; Mounyr, B.; Harki, E.H.; Moja, S.; Greche, H. Synergistic antimicrobial activity of two binary combinations of marjoram, lavender, and wild thyme essential oils. Int. J. Food Prop. 2017, 20, 3149–3158. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar]
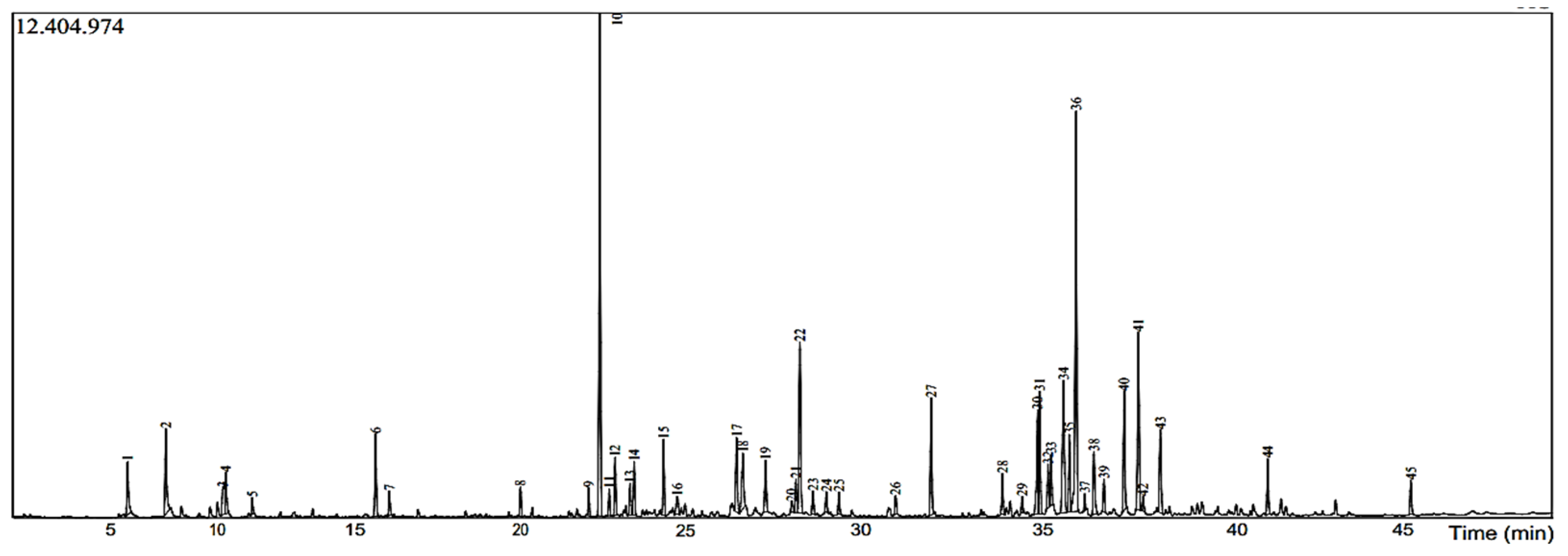


| Peak | Retention Time | Phytochemical Compounds | Area (%) |
|---|---|---|---|
| 1 | 7.899 | 2-Pinene | 1.61 |
| 2 | 9.028 | Bicyclo [3.1.0]hexane, 4-methylene-1-(1-methylethyl) | 2.67 |
| 3 | 10.710 | D-Limonene | 1.36 |
| 4 | 10.799 | Eucalyptol | 1.24 |
| 5 | 11.583 | Gamma.-Terpinene | 0.42 |
| 6 | 15.228 | Terpinen-4-ol | 2.37 |
| 7 | 15.634 | (1S)-1,3,3-trimethylnorbornan-2-ol | 0.67 |
| 8 | 19.513 | Bicycloelemene | 0.72 |
| 9 | 21.531 | Alpha.-Gurjunene | 0.69 |
| 10 | 21.860 | Caryophyllene | 13.87 |
| 11 | 22.136 | Alpha.-Bergamotene | 0.63 |
| 12 | 22.310 | Cyclohexene, 3-(1,5-dimethyl-4-hexenyl)-6 | 1.41 |
| 13 | 22.752 | alpha.-Humulene | 0.83 |
| 14 | 22.872 | Alloaromadendrene | 1.43 |
| 15 | 23.743 | (1S,2E,6E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | 2.01 |
| 16 | 24.150 | 4-Isopropyl-1,6-dimethyl-1,2,3,4,4a,7,8,8a-octahydro-1-naphthalenol | 0.63 |
| 17 | 25.903 | 1H-Cycloprop[e]azulen-7-ol, decahydro-1,1,7-trimethyl-4-methylene-, [1ar (1a.alpha.,4a.alpha.,7.beta.,7a.beta.,7b.alpha.)] | 2.39 |
| 18 | 26.086 | 5-Oxatricyclo[8.2.0.04,6]dodecane, 4,12,12-trimethyl-9-methylene-, (1R,4R,6R,10S) | 2.17 |
| 19 | 26.757 | Ledol | 1.63 |
| 20 | 27.525 | Isospathulenol | 0.42 |
| 21 | 27.657 | 10,10-Dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5.beta.-ol | 0.99 |
| 22 | 27.773 | Tau.-Cadinol | 5.41 |
| 23 | 28.160 | Viridifloro | 0.69 |
| 24 | 28.553 | Isoaromadendrene epoxide | 0.60 |
| 25 | 28.928 | alpha.-Bisabolol | 0.60 |
| 26 | 30.602 | 2,5-Dimethylbicyclo[3.3.0]oct-6-en-8-one | 0.52 |
| 27 | 31.653 | Androsta-4,6-dien-3-one, 17-.beta.-hydroxy | 3.38 |
| 28 | 33.761 | (E)-4-(1,3,3-trimethylnorcaran-2-yl)but-3-en-2-one | 1.09 |
| 29 | 34.342 | Kolavelool | 0.46 |
| 30 | 34.802 | cis-Valerenyl acetate | 3.47 |
| 31 | 34.853 | 1,1,2,3,3,5-hexamethyl-2H-indene | 3.74 |
| 32 | 35.107 | 3-Buten-2-one, 3-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 1.13 |
| 33 | 35.199 | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, acetate, (3.beta.,4.alpha.,5.alpha.) | 1.22 |
| 34 | 35.566 | (3E,6E)-5-isopropylidene-6-methyl-deca-3,6,9-trien-2-one | 5.23 |
| 35 | 35.735 | Humulane-1,6-dien-3-ol | 2.22 |
| 36 | 35.934 | 1-(4-Isopropylphenyl)-2-methylpropyl acetate | 12.20 |
| 37 | 36.190 | 1H-Naphtho[2,1-b]pyran, 3-ethenyldodecahydro-3,4a,7,7,10a-pentamethyl-, [3R-(3.alpha.,4a.beta.,6a.alpha.,10a.beta.,10b.alpha.)] | 0.40 |
| 38 | 36.462 | Seychellene | 2.11 |
| 39 | 36.761 | Kaur-15-ene | 0.86 |
| 40 | 37.358 | Podocarpa-8,11,13-triene, 13-isopropyl | 3.58 |
| 41 | 37.777 | cis-3,14-Clerodadien-13-ol | 5.36 |
| 42 | 37.915 | Androsta-1,4-dien-3-one, 6.beta.,17.beta.-dihydroxy-, 17-acetate | 0.45 |
| 43 | 38.427 | Kolavelool | 2.65 |
| 44 | 41.603 | (1R,2R,4aS,8aS)-1-(2-(Furan-3-yl)ethyl)-2,5 | 1.56 |
| 45 | 45.832 | Rotundifuran | 0.90 |
| Total (%) | 99.98 |
| Name | Chemical Formula | Structure | Molar Mass (g/mol) | |
|---|---|---|---|---|
| a | Caryophyllene | C15H24 |  | 204.35 |
| b | 1-(4-Isopropylphenyl)-2-methylpropyl acetate | C15H22O2 | 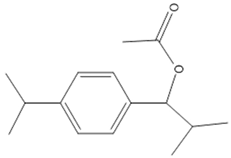 | 234.34 |
| c | τ-Cadinol | C15H26O | 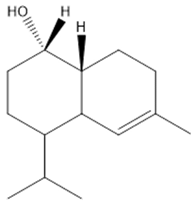 | 222.36 |
| VACEO | BHT | Acid Ascorbic | Quercetin | |
|---|---|---|---|---|
| DPPH (IC 50 mg/mL) | 0.93 ± 0.03 | 0.11 ± 0.001 | - | - |
| FRAP (EC50 mg/mL) | 0.146 ± 0.004 | - | - | 0.03 ± 0.004 |
| Relative antioxidant activity in % | 72.69 ± 0.3% | - | 100% | - |
| TAC in mg eqv BHT/g BHT/g EO | 0.794 ± 0.02 | - | - | - |
| Escherichia coli | Staphylococcus aureus | Bacillus subtilis | Pseudomonas aeruginosa | |||||
|---|---|---|---|---|---|---|---|---|
| ID (mm) | MIC (mg/mL) | ID (mm) | MIC (mg/mL) | ID (mm) | MIC (mg/mL) | ID (mm) | MIC (mg/mL) | |
| VACEO | 18.25 ± 0.75 | 0.02 | 21.11 ± 0.25 | 0.02 | 13.25 ± 1.00 | 0.04 | 17.35 ± 1.00 | 0.02 |
| Kanamycin | 19.3 ± 1.56 | 0.002 | 21.4 ± 1.2 | 0.016 | 19.3 ± 1.5 | 0.004 | 17.00 ± 0.00 | 0.004 |
| Mol | MW | RB | HA | HB | MR | MLOGP | GI | BBB | Pgp | C19 | C9 | L | G | V | E | M | BS | PA | BA | LV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 204.35 | 0 | 0 | 0 | 68.78 | 4.63 | Low | No | No | Yes | Yes | 1 | 0 | 0 | 0 | 1 | 0.55 | 0 | 1 | 2 |
| b | 234.33 | 5 | 2 | 0 | 71.31 | 3.65 | High | Yes | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 | 0 | 2 |
| c | 222.37 | 1 | 1 | 1 | 70.72 | 3.67 | High | Yes | No | Yes | No | 0 | 0 | 0 | 0 | 1 | 0.55 | 0 | 1 | 1 |
| Predicted LD50: mg/kg | Class | Average Similarity | Prediction Accuracy | |
|---|---|---|---|---|
| a | 5300 | 5 | 86.96% | 70.97% |
| b | 5000 | 5 | 82.83% | 70.97% |
| c | 2830 | 5 | 96.55% | 72.90% |
| Protein/Ligand | PDB Code | Original Ligand | a | b | c |
|---|---|---|---|---|---|
| GyrA | 4Z2C | −5.79 | −4.59 | −4.424 | −5.007 |
| GyrB | 5L3J | −6.589 | −5.923 | −6.915 | −5.812 |
| Topoisomérase IV | 1S14 | −7.367 | −5.967 | −5.572 | −5.483 |
| Beta-lactamase | 6QWA | −7.618 | −6.279 | −7.083 | −6.436 |
| Penicillin-binding protein 2a (PBP2a) | 3ZFZ | −8.985 | −6.96 | −7.069 | −6.656 |
| Sortase A | 6R1V | −4.613 | −5.053 | −4.815 | −5.366 |
| Staphylocoagulase | 1NU7 | −8.955 | −8.014 | −6.296 | −7.074 |
| DNA polymerase III | 3F2C | −8.311 | −5.267 | −5.446 | −6.063 |
| MurA | 1YBG | −10.27 | −6.56 | −6.667 | −6.282 |
| LasR | 4NG2 | −9.611 | −7.405 | −8.762 | −8.622 |
| Elastase | 1U4G | −7.752 | −5.949 | −5.620 | −5.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzouni, D.; Alaoui Mrani, S.; Bahij, F.; Zejli, H.; Alanazi, M.M.; Fadili, D.; El Moussaoui, A.; Mahmoud, A.M.; Taleb, M. Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies. Pharmaceuticals 2025, 18, 462. https://doi.org/10.3390/ph18040462
Azzouni D, Alaoui Mrani S, Bahij F, Zejli H, Alanazi MM, Fadili D, El Moussaoui A, Mahmoud AM, Taleb M. Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies. Pharmaceuticals. 2025; 18(4):462. https://doi.org/10.3390/ph18040462
Chicago/Turabian StyleAzzouni, Dounia, Soukaina Alaoui Mrani, Fadoua Bahij, Hind Zejli, Mohammed M. Alanazi, Driss Fadili, Abdelfattah El Moussaoui, Ayman M. Mahmoud, and Mustapha Taleb. 2025. "Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies" Pharmaceuticals 18, no. 4: 462. https://doi.org/10.3390/ph18040462
APA StyleAzzouni, D., Alaoui Mrani, S., Bahij, F., Zejli, H., Alanazi, M. M., Fadili, D., El Moussaoui, A., Mahmoud, A. M., & Taleb, M. (2025). Comprehensive Phytochemical, Antioxidant, and Antibacterial Analysis of Vitex agnus-castus L. Essential Oil (VACEO): Insights from ADMET and Molecular Docking Studies. Pharmaceuticals, 18(4), 462. https://doi.org/10.3390/ph18040462







