Synergistic Effects of Low-Frequency Ultrasound and Therapeutic Agents on Endothelial and Renal Cells: Emphasis on Cell Functionality, Oxidative Stress, and Inflammatory Markers
Abstract
1. Introduction
2. Results
2.1. Assessment of Cell Function Following Treatment with Captopril, Losartan, and Dexamethasone, Combined with LFU
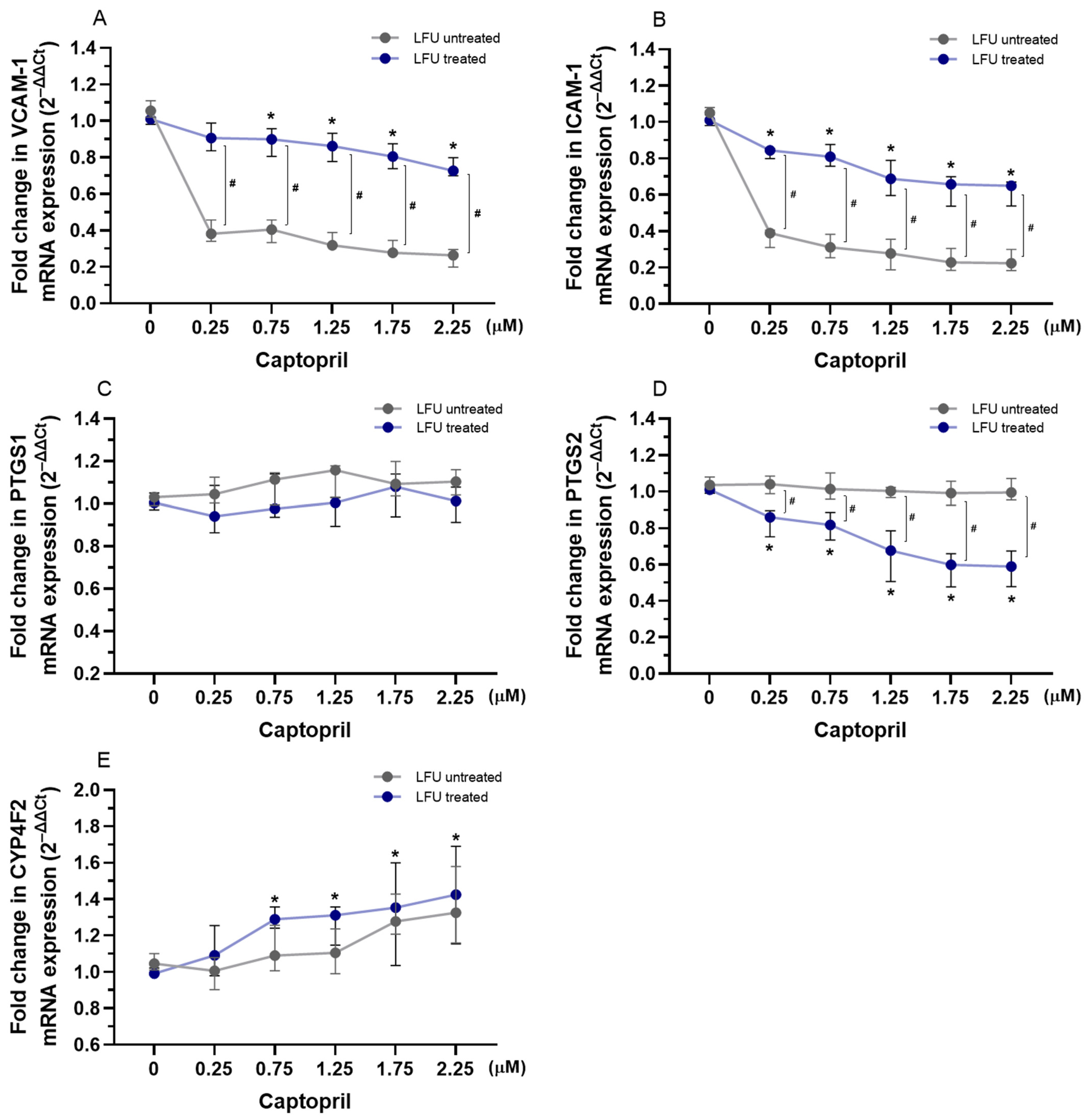
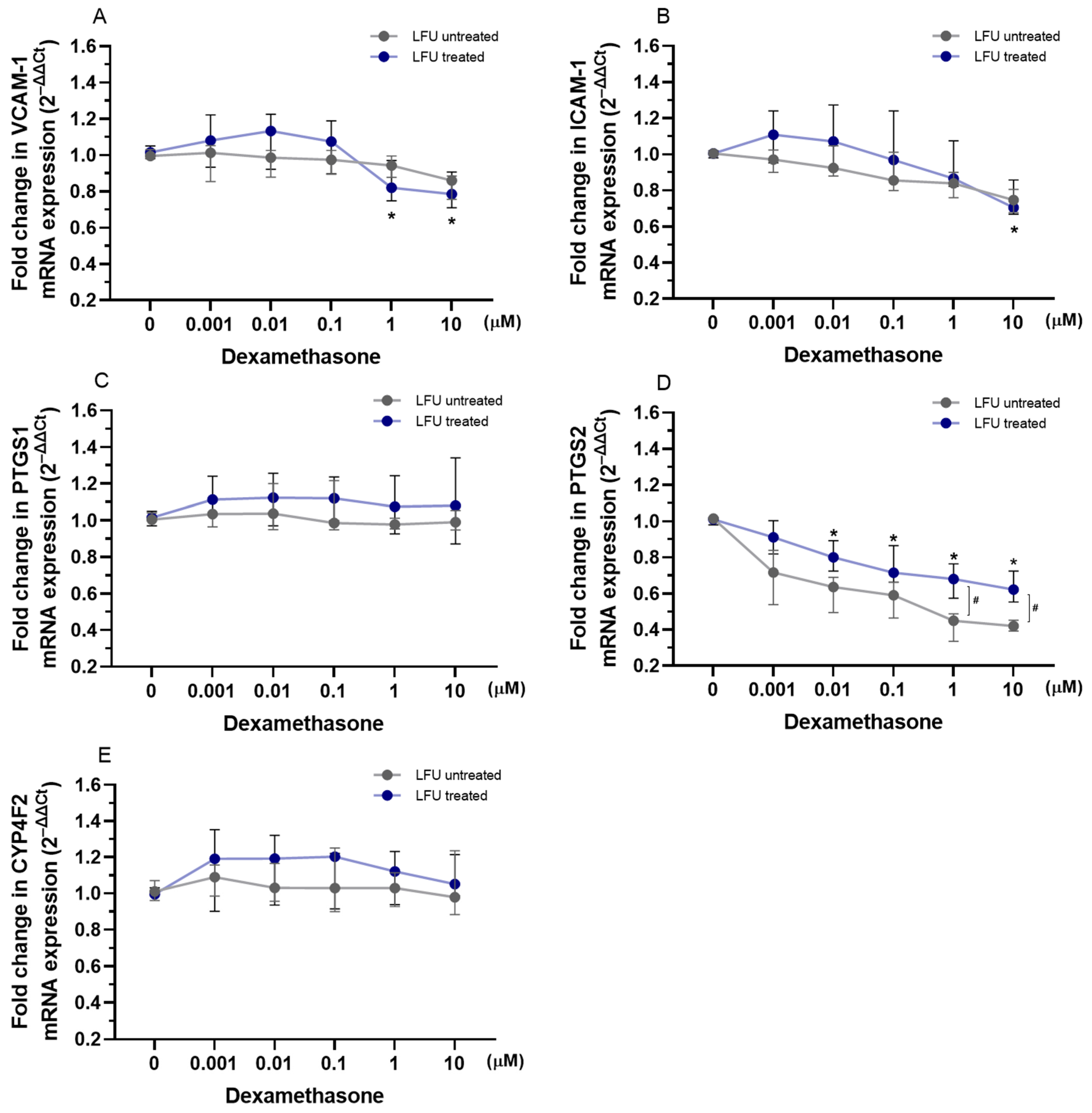
2.2. Effects of Captopril, Losartan, and Dexamethasone Combined with LFU on the mRNA Expression of Genes Related to Inflammation in HUVECs
2.3. Effects of Captopril, Losartan, and Dexamethasone Combined with LFU on the mRNA Expression of Genes Related to Kidney Proximal Tubule Function in RPTEC/TERT1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture Cultivation
4.2. Treatment of Cell Cultures with Drugs Combined with Low-Frequency Ultrasound (LFU)

4.3. LFU Device Design
4.4. Metabolic Activity of the Cells
4.5. Wound-Healing Assay
4.6. Nitric Oxide Assay
4.7. Reactive Oxygen Species Detection Assay
4.8. Gene Expression Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Karamzad, N.; Singh, K.; Carson-Chahhoud, K.; Adams, C.; Nejadghaderi, S.A.; Almasi-Hashiani, A.; Sullman, M.J.M.; Mansournia, M.A.; Bragazzi, N.L.; et al. Burden of Ischemic Heart Disease and Its Attributable Risk Factors in 204 Countries and Territories, 1990-2019. Eur. J. Prev. Cardiol. 2022, 29, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Aboyans, V.; Vardas, P.; Townsend, N.; Torbica, A.; Kavousi, M.; Boriani, G.; Huculeci, R.; Kazakiewicz, D.; Scherr, D.; et al. European Society of Cardiology: The 2023 Atlas of Cardiovascular Disease Statistics. Eur. Heart. J. 2024, 45, 4019–4062. [Google Scholar] [CrossRef]
- Welén Schef, K.; Tornvall, P.; Alfredsson, J.; Hagström, E.; Ravn-Fischer, A.; Soderberg, S.; Yndigegn, T.; Jernberg, T. Prevalence of Angina Pectoris and Association with Coronary Atherosclerosis in a General Population. Heart 2023, 109, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Blöndal, M.; Ainla, T.; Eha, J.; Lõiveke, P.; Marandi, T.; Saar, A.; Veldre, G.; Edfors, R.; Lewinter, C.; Jernberg, T.; et al. Comparison of Management and Outcomes of ST-Segment Elevation Myocardial Infarction Patients in Estonia, Hungary, Norway, and Sweden According to National Ongoing Registries. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 307–314. [Google Scholar] [CrossRef]
- Aldujeli, A.; Tsai, T.Y.; Haq, A.; Tatarunas, V.; Knokneris, A.; Briedis, K.; Unikas, R.; Onuma, Y.; Brilakis, E.S.; Serruys, P.W. Impact of Coronary Microvascular Dysfunction on Functional Left Ventricular Remodeling and Diastolic Dysfunction. J. Am. Heart Assoc. 2024, 13, e033596. [Google Scholar] [CrossRef]
- Patel, S.; Shokr, H.; Greenstein, A.; Gherghel, D. Macro- and Microvascular Function in Middle-Aged Individuals with Low Cardiovascular Disease Risk. J. Clin. Med. 2022, 11, 6962. [Google Scholar] [CrossRef]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial Dysfunction as a Target for Prevention of Cardiovascular Disease. Diabetes Care 2009, 32, S314–S321. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of Hydrogen Sulfide in Endothelial Dysfunction: Pathophysiology and Therapeutic Approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Ruggieri, S.; Annese, T.; Crivellato, E. Surface Markers: An Identity Card of Endothelial Cells. Microcirculation 2020, 27, e12587. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular Consequences of Endothelial Nitric Oxide Synthase Uncoupling for the Activity and Expression of the Soluble Guanylyl Cyclase and the CGMP-Dependent Protein Kinase. Arter. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef]
- Ray, A.; Ch. Maharana, K.; Meenakshi, S.; Singh, S. Endothelial Dysfunction and Its Relation in Different Disorders: Recent Update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Aldujeli, A.; Patel, R.; Grabauskyte, I.; Hamadeh, A.; Lieponyte, A.; Tatarunas, V.; Khalifeh, H.; Briedis, K.; Skipskis, V.; Aldujeili, M.; et al. The Impact of Trimethylamine N-Oxide and Coronary Microcirculatory Dysfunction on Outcomes Following ST-Elevation Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2023, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef]
- Cepoi, M.R.; Duca, S.T.; Chetran, A.; Costache, A.D.; Spiridon, M.R.; Afrăsânie, I.; Leancă, S.A.; Dmour, B.A.; Matei, I.T.; Miftode, R.S.; et al. Chronic Kidney Disease Associated with Ischemic Heart Disease: To What Extent Do Biomarkers Help? Life 2024, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Meariman, J.K.; Zulli, H.; Perez, A.; Bajracharya, S.D.; Mohandas, R. Small Vessel Disease: Connections between the Kidney and the Heart. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 26, 100257. [Google Scholar] [CrossRef]
- Baaten, C.C.F.M.J.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients with Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Maki-Petaja, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular Consequences of Inflammation: A Position Statement Fromthe Eshworking Group Onvascular Structure and Function and Thearterysociety. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Rios, F.J.; de Ciuceis, C.; Georgiopoulos, G.; Lazaridis, A.; Nosalski, R.; Pavlidis, G.; Tual-Chalot, S.; Agabiti-Rosei, C.; Camargo, L.L.; Dąbrowska, E.; et al. Mechanisms of Vascular Inflammation and Potential Therapeutic Targets: A Position Paper from the ESH Working Group on Small Arteries. Hypertension 2024, 81, 1218–1232. [Google Scholar] [CrossRef]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-Aldosterone System in Vascular Inflammation and Remodeling. Int. J. Inflamm. 2014, 2014, 689360. [Google Scholar] [CrossRef]
- Félétou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-Mediated Control of Vascular Tone: COX-1 and COX-2 Products. Br. J. Pharmacol. 2011, 164, 894–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, D.; Zhang, W.; Xing, Y.; Guo, Y.; Wang, F.; Jia, J.; Yan, T.; Liu, Y.; Lin, S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3–5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs 2020, 80, 797–811. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.F.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin–Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Liu, B.; Kanasaki, K.; Koya, D.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Loss of Endothelial Glucocorticoid Receptor Accelerates Diabetic Nephropathy. Nat. Commun. 2021, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef] [PubMed]
- Altland, O.D.; Dalecki, D.; Suchkova, V.N.; Francis, C.W. Low-Intensity Ultrasound Increases Endothelial Cell Nitric Oxide Synthase Activity and Nitric Oxide Synthesis. J. Thromb. Haemost. 2004, 2, 637–643. [Google Scholar] [CrossRef]
- Yang, Q.; Nanayakkara, G.K.; Drummer, C.; Sun, Y.; Johnson, C.; Cueto, R.; Fu, H.; Shao, Y.; Wang, L.; Yang, W.Y.; et al. Low-Intensity Ultrasound-Induced Anti-Inflammatory Effects Are Mediated by Several New Mechanisms Including Gene Induction, Immunosuppressor Cell Promotion, and Enhancement of Exosome Biogenesis and Docking. Front. Physiol. 2017, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Lin, J.; Sun, X.; Ding, Q. Exosomes Derived from Low-Intensity Pulsed Ultrasound-Treated Dendritic Cells Suppress Tumor Necrosis Factor–Induced Endothelial Inflammation. J. Ultrasound Med. 2019, 38, 2081–2091. [Google Scholar] [CrossRef]
- Yuan, X.; Zhao, X.; Wang, W.; Li, C. Mechanosensing by Piezo1 and Its Implications in the Kidney. Acta Physiol. 2024, 240, e14152. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Hong, N.; Garvin, J.L. Effects of Reactive Oxygen Species on Renal Tubular Transport. Am. J. Physiol. Ren. Physiol. 2019, 317, F444–F455. [Google Scholar] [CrossRef] [PubMed]
- Stellato, C. Post-Transcriptional and Nongenomic Effects of Glucocorticoids. Proc. Am. Thorac. Soc. 2004, 1, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing Sound: The Use of Ultrasound in Drug Delivery and Other Therapeutic Applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Liang, H.D.; Tang, J.; Halliwell, M. Sonoporation, Drug Delivery, and Gene Therapy. Proc. Inst. Mech. Eng. Part H 2010, 224, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Thum, T. Critical Role of the Nitric Oxide/Reactive Oxygen Species Balance in Endothelial Progenitor Dysfunction. Antioxid. Redox Signal 2011, 15, 933–948. [Google Scholar] [CrossRef]
- Iida, K.; Luo, H.; Hagisawa, K.; Akima, T.; Shah, P.K.; Naqvi, T.Z.; Siegel, R.J. Noninvasive Low-Frequency Ultrasound Energy Causes Vasodilation in Humans. J. Am. Coll. Cardiol. 2006, 48, 532–537. [Google Scholar] [CrossRef]
- Hamdi, H.K.; Castellon, R. ACE Inhibition Actively Promotes Cell Survival by Altering Gene Expression. Biochem. Biophys. Res. Commun. 2003, 310, 1227–1235. [Google Scholar] [CrossRef]
- Yu, W.; Akishita, M.; Xi, H.; Nagai, K.; Sudoh, N.; Hasegawa, H.; Kozaki, K.; Toba, K. Angiotensin Converting Enzyme Inhibitor Attenuates Oxidative Stress-Induced Endothelial Cell Apoptosis via P38 MAP Kinase Inhibition. Clin. Chim. Acta 2006, 364, 328–334. [Google Scholar] [CrossRef]
- Watanabe, T.; Suzuki, J.; Yamawaki, H.; Sharma, V.K.; Sheu, S.S.; Berk, B.C. Losartan Metabolite EXP3179 Activates Akt and Endothelial Nitric Oxide Synthase via Vascular Endothelial Growth Factor Receptor-2 in Endothelial Cells: Angiotensin II Type 1 Receptor-Independent Effects of EXP3179. Circulation 2005, 112, 1798–1805. [Google Scholar] [CrossRef]
- Elijovich, F.; Laffer, C.L. Detrimental Effects of Dual ACEI-ARB Therapy: Is the (Pro)Renin Receptor the Culprit. Kidney Int. 2011, 80, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, J.; Kaneko, Y.; Okumura, K.; Yoshida, O.; Nakayama, H.; Fujita, J. Inhibitory effect of dexamethasone and progesterone in vitro on proliferation of human renal cell carcinomas and effects on expression of interleukin-6 or interleukin-6 receptor. J. Urol. 1995, 153, 858–862. [Google Scholar] [CrossRef] [PubMed]
- De Haij, S.; Adcock, I.M.; Bakker, A.C.; Gobin, S.J.P.; Daha, M.R.; Van Kooten, C. Steroid Responsiveness of Renal Epithelial Cells: Dissociation of Transrepression and Transactivation. J. Biol. Chem. 2003, 278, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, R.; Nyström, A. Balance and Circumstance: The Renin Angiotensin System in Wound Healing and Fibrosis. Cell. Signal. 2018, 51, 34–46. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, Q.; Chen, X.; Wang, J.; Wang, L. Low-frequency Ultrasound Enhances Vascular Endothelial Growth Factor Expression, Thereby Promoting the Wound Healing in Diabetic Rats. Exp. Ther. Med. 2019, 18, 4040–4048. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhou, Q. Different Effects of a Perioperative Single Dose of Dexamethasone on Wound Healing in Mice with or without Sepsis. Front. Surg. 2023, 10, 927168. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Zhang, D.; Barksdale, A.N.; Wadman, M.C.; Muelleman, R.L.; Li, Y.L. Dexamethasone Improves Wound Healing by Decreased Inflammation and Increased Vasculogenesis in Mouse Skin Frostbite Model. Wilderness Environ. Med. 2020, 31, 407–417. [Google Scholar] [CrossRef]
- Momose, N.; Fukuo, K.; Morimoto, S.; Ogihara, T. Captopril Inhibits Endothelin-1 Secretion from Endothelial Cells through Bradykinin. Hypertension 1993, 21, 921–924. [Google Scholar] [CrossRef]
- Wenzel, P.; Schulz, E.; Oelze, M.; Müller, J.; Schuhmacher, S.; Alhamdani, M.S.S.; Debrezion, J.; Hortmann, M.; Reifenberg, K.; Fleming, I.; et al. AT1-Receptor Blockade by Telmisartan Upregulates GTP-Cyclohydrolase I and Protects ENOS in Diabetic Rats. Free Radic. Biol. Med. 2008, 45, 619–626. [Google Scholar] [CrossRef]
- Liu, H.; Kitazato, K.T.; Uno, M.; Yagi, K.; Kanematsu, Y.; Tamura, T.; Tada, Y.; Kinouchi, T.; Nagahiro, S. Protective Mechanisms of the Angiotensin II Type 1 Receptor Blocker Candesartan against Cerebral Ischemia: In-Vivo and In-Vitro Studies. J. Hypertens. 2008, 26, 1435–1445. [Google Scholar] [CrossRef]
- Miguel-Carrasco, J.L.; Zambrano, S.; Blanca, A.J.; Mate, A.; Vázquez, C.M. Captopril Reduces Cardiac Inflammatory Markers in Spontaneously Hypertensive Rats by Inactivation of NF-KB. J. Inflamm. 2010, 7, 21. [Google Scholar] [CrossRef]
- Van Der Giet, M.; Erinola, M.; Zidek, W.; Tepel, M. Captopril and Quinapril Reduce Reactive Oxygen Species. Eur. J. Clin. Investig. 2002, 32, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, X.; Marfavi, Z.; Chen, Y.; Zhang, L.; Chu, J.; Sun, K.; Li, M.; Tao, K. A Perspective on Ultrasound-Triggered Production of Reactive Oxygen Species by Inorganic Nano/Microparticles. Adv. Nanobiomed Res. 2024, 4, 2400060. [Google Scholar] [CrossRef]
- Pialoux, V.; Foster, G.E.; Ahmed, S.B.; Beaudin, A.E.; Hanly, P.J.; Poulin, M.J. Losartan Abolishes Oxidative Stress Induced by Intermittent Hypoxia in Humans. J. Physiol. 2011, 589, 5529–5537. [Google Scholar] [CrossRef]
- Raei, M.; Ahmadi, M.; Abrotan, S.; Razavi, A.; Hedayatizadeh-Omran, A.; Shamshirian, A.; Heydari, K.; Saeedi, M.; Alizadeh-Navaei, R. Effect of Losartan on Cell Proliferation and Reactive Oxygen Species Scavenging in Gastric Cancer Cell Lines. Eurasian J. Med. Oncol. 2024, 8, 135–140. [Google Scholar] [CrossRef]
- Fürst, R.; Zahler, S.; Vollmar, A.M. Dexamethasone-Induced Expression of Endothelial Mitogen-Activated Protein Kinase Phosphatase-1 Involves Activation of the Transcription Factors Activator Protein-1 and 3′,5′-Cyclic Adenosine 5′-Monophosphate Response Element-Binding Protein and the Genera. Endocrinology 2008, 149, 3635–3642. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huo, Y.; Rangarajan, P.; Ling, E.A.; Dheen, S.T. Dexamethasone Inhibits the Nox-Dependent ROS Production via Suppression of MKP-1-Dependent MAPK Pathways in Activated Microglia. BMC Neurosci. 2011, 12, 49. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Ni, K.-D.; Liu, J.-Y. The Functions of Cytochrome P450 ω-Hydroxylases and the Associated Eicosanoids in Inflammation-Related Diseases. Front. Pharmacol. 2021, 12, 716801. [Google Scholar] [CrossRef]
- Mullins, G.E.; Sunden-Cullberg, J.; Johansson, A.S.; Rouhiainen, A.; Erlandsson-Harris, H.; Yang, H.; Tracey, K.J.; Rauvala, H.; Palmblad, J.; Andersson, J.; et al. Activation of Human Umbilical Vein Endothelial Cells Leads to Relocation and Release of High-Mobility Group Box Chromosomal Protein 1. Scand. J. Immunol. 2004, 60, 566–573. [Google Scholar] [CrossRef]
- Ahwin, P.; Martinez, D. The Relationship between SGLT2 and Systemic Blood Pressure Regulation. Hypertens. Res. 2024, 47, 2094–2103. [Google Scholar] [CrossRef]
- Mistry, D.; Stockley, R.A. Gamma-Glutamyl Transferase: The Silent Partner? COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 285–290. [Google Scholar] [CrossRef]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 Inhibitor Empagliflozin Reduces Renal Growth and Albuminuria in Proportion to Hyperglycemia and Prevents Glomerular Hyperfiltration in Diabetic Akita Mice. Am. J. Physiol. Ren. Physiol. 2014, 306, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Huang, D.; Jiang, J.; Li, Y.; Li, H.; Ke, Y. Captopril Alleviates Hypertension-Induced Renal Damage, Inflammation, and NF-κB Activation. Braz. J. Med. Biol. Res. 2018, 51, e7338. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 Protein Inhibition Decreases Renal Lipid Accumulation, Inflammation, and the Development of Nephropathy in Diabetic Mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef]
- Hunter, R.W.; Ivy, J.R.; Bailey, M.A. Glucocorticoids and Renal Na+ Transport: Implications for Hypertension and Salt Sensitivity. J. Physiol. 2014, 592, 1731–1744. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and Microbubble Mediated Therapeutic Delivery: Underlying Mechanisms and Future Outlook. J. Control. Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.; Worzella, T.; Minor, L. Cell Viability Assays. In The Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration In Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 622, 611–622. [Google Scholar] [CrossRef]







| Frequency | ~69 kHz |
| Power | 40 W |
| Capacity | 0.5 L |
| Adjust power | 40–100% |
| Pan size (mm) | 120 × 80 × 10 |
| Dimensions (mm) | 130 × 90 × 60 |
| Power supply | 220 V/50 Hz |
| Timing | 1–99 min |
| Accessories | bracket |
| Gene Symbol | Accession | Name | Primer | Primer Sequence |
|---|---|---|---|---|
| VCAM-1 | NM_001078.4 | Vascular Cell Adhesion Molecule 1 | Forward | 5′-AGGTGGAGATCTACTCTTTTCCT-3′ |
| Reverse | 5′-ACACTTGACTGTGATCGGCT-3′ | |||
| ICAM-1 | NM_000201.3 | Intercellular Adhesion Molecule 1 | Forward | 5′-GGTGCTGGTGAGGAGAGATCA-3′ |
| Reverse | 5′-AGTCGCTGGCAGGACAAAGG-3′ | |||
| PTGS1 | NM_000962.4 | Prostaglandin-Endoperoxide Synthase 1 | Forward | 5′-CTGGTGGATGCCTTCTCTCG-3′ |
| Reverse | 5′-CATCTCCCGAGACTCCCTGA-3′ | |||
| PTGS2 | NM_000963.4 | Prostaglandin-Endoperoxide Synthase 2 | Forward | 5′-CGGTGAAACTCTGGCTAGACAG-3′ |
| Reverse | 5′-GCAAACCGTAGATGCTCAGGGA-3′ | |||
| CYP4F2 | NM_001082.5 | Cytochrome P450 Family 4 Subfamily F Member 2 | Forward | 5′-GACAGCCATTGTCAGGAGAAACC-3′ |
| Reverse | 5′-TGCAGGAGGATCTCATGGTGTC-3′ | |||
| SGLT2/ SLC5A2 | NM_003041.4 | Solute Carrier Family 5 (Sodium/ Glucose Cotransporter), Member 2 | Forward | 5′-AGTGCCTGCTCTGGTTTTGT-3′ |
| Reverse | 5′-TTAGGCATAGAAGCCCCAGA-3′ | |||
| GGT1 | NM_013421.3 | Gamma-Glutamyltransferase 1 | Forward | 5′-TGCTCGAAGATTGGGAGGGATG-3′ |
| Reverse | 5′-ACACAACAGGGCTGCAATGG-3′ | |||
| TFRC | NM_003234.4 | Transferrin Receptor | Forward | 5′-ACTTGCCCAGATGTTCTCAGAT-3′ |
| Reverse | 5′-CGAAAGGTATCCCTCTAGCCAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čiapienė, I.; Vėžys, J.; Lesauskaitė, V.; Matulevičiūtė, I.; Meškauskaitė, U.; Skipskis, V.; Strazdauskas, A.; Trumbeckaitė, S.; Bubulis, A.; Jūrėnas, V.; et al. Synergistic Effects of Low-Frequency Ultrasound and Therapeutic Agents on Endothelial and Renal Cells: Emphasis on Cell Functionality, Oxidative Stress, and Inflammatory Markers. Pharmaceuticals 2025, 18, 404. https://doi.org/10.3390/ph18030404
Čiapienė I, Vėžys J, Lesauskaitė V, Matulevičiūtė I, Meškauskaitė U, Skipskis V, Strazdauskas A, Trumbeckaitė S, Bubulis A, Jūrėnas V, et al. Synergistic Effects of Low-Frequency Ultrasound and Therapeutic Agents on Endothelial and Renal Cells: Emphasis on Cell Functionality, Oxidative Stress, and Inflammatory Markers. Pharmaceuticals. 2025; 18(3):404. https://doi.org/10.3390/ph18030404
Chicago/Turabian StyleČiapienė, Ieva, Joris Vėžys, Vaiva Lesauskaitė, Indrė Matulevičiūtė, Ugnė Meškauskaitė, Vilius Skipskis, Arvydas Strazdauskas, Sonata Trumbeckaitė, Algimantas Bubulis, Vytautas Jūrėnas, and et al. 2025. "Synergistic Effects of Low-Frequency Ultrasound and Therapeutic Agents on Endothelial and Renal Cells: Emphasis on Cell Functionality, Oxidative Stress, and Inflammatory Markers" Pharmaceuticals 18, no. 3: 404. https://doi.org/10.3390/ph18030404
APA StyleČiapienė, I., Vėžys, J., Lesauskaitė, V., Matulevičiūtė, I., Meškauskaitė, U., Skipskis, V., Strazdauskas, A., Trumbeckaitė, S., Bubulis, A., Jūrėnas, V., Ostaševičius, V., Tamakauskas, V., & Tatarūnas, V. (2025). Synergistic Effects of Low-Frequency Ultrasound and Therapeutic Agents on Endothelial and Renal Cells: Emphasis on Cell Functionality, Oxidative Stress, and Inflammatory Markers. Pharmaceuticals, 18(3), 404. https://doi.org/10.3390/ph18030404








