Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
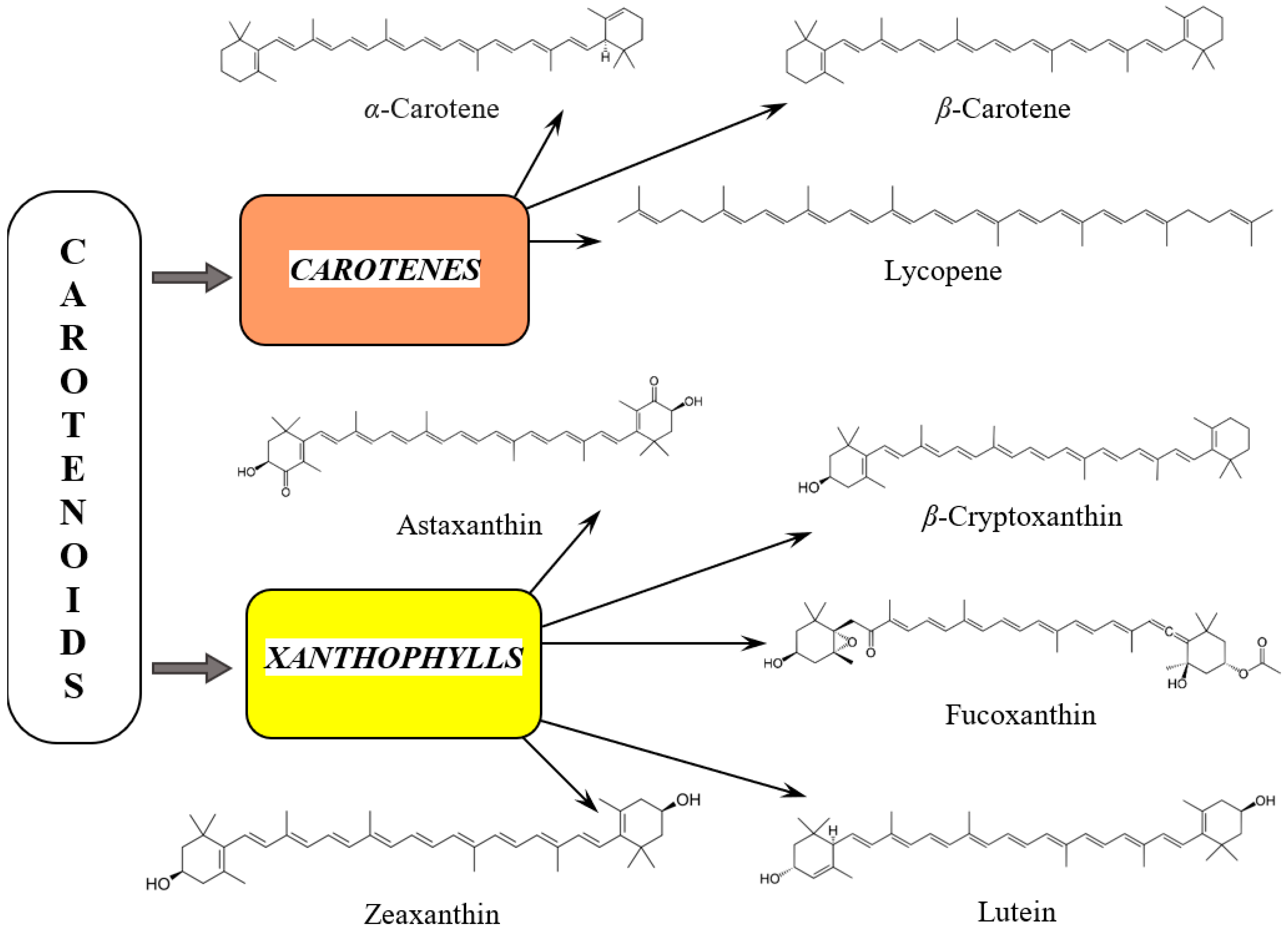
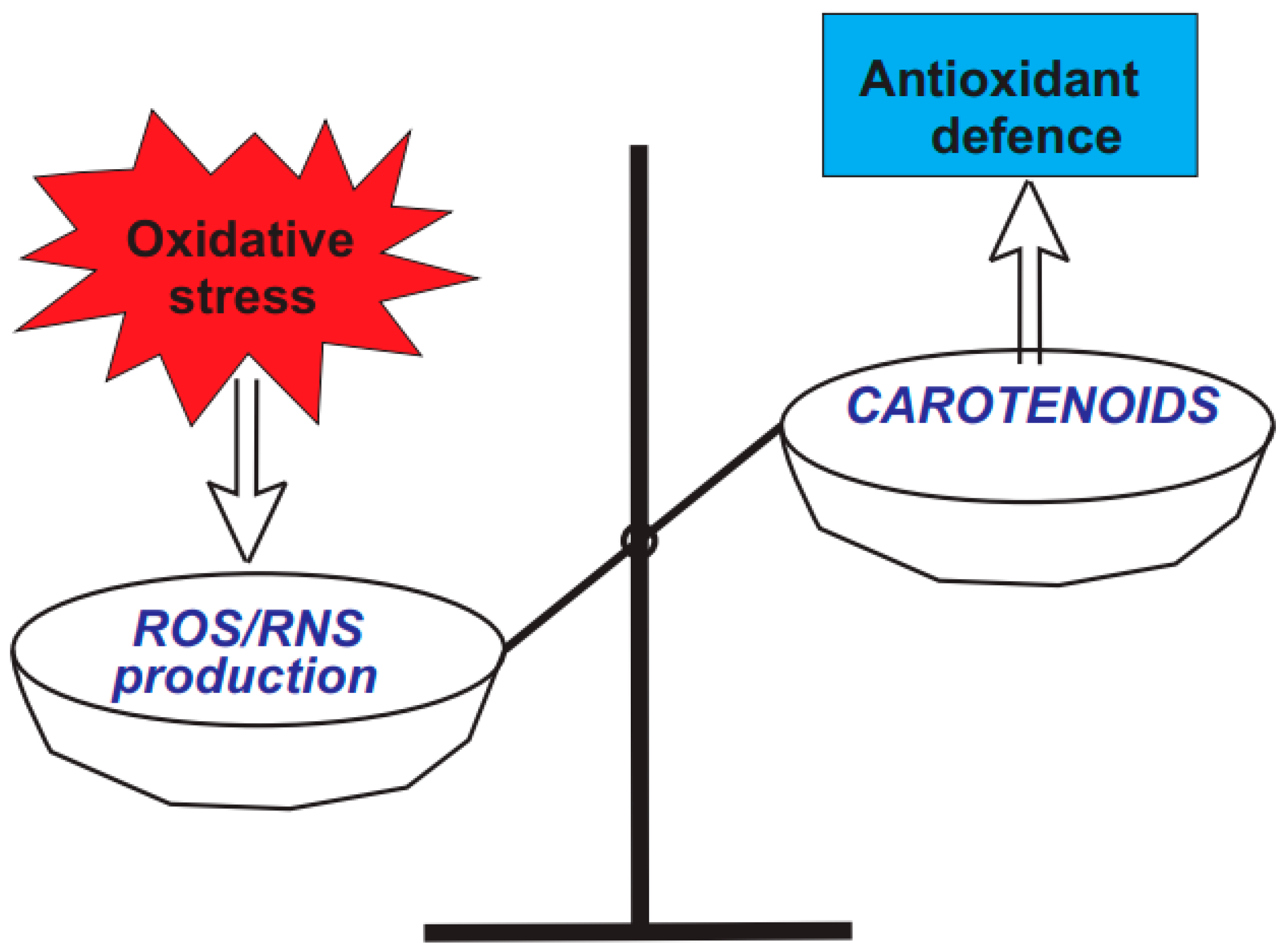
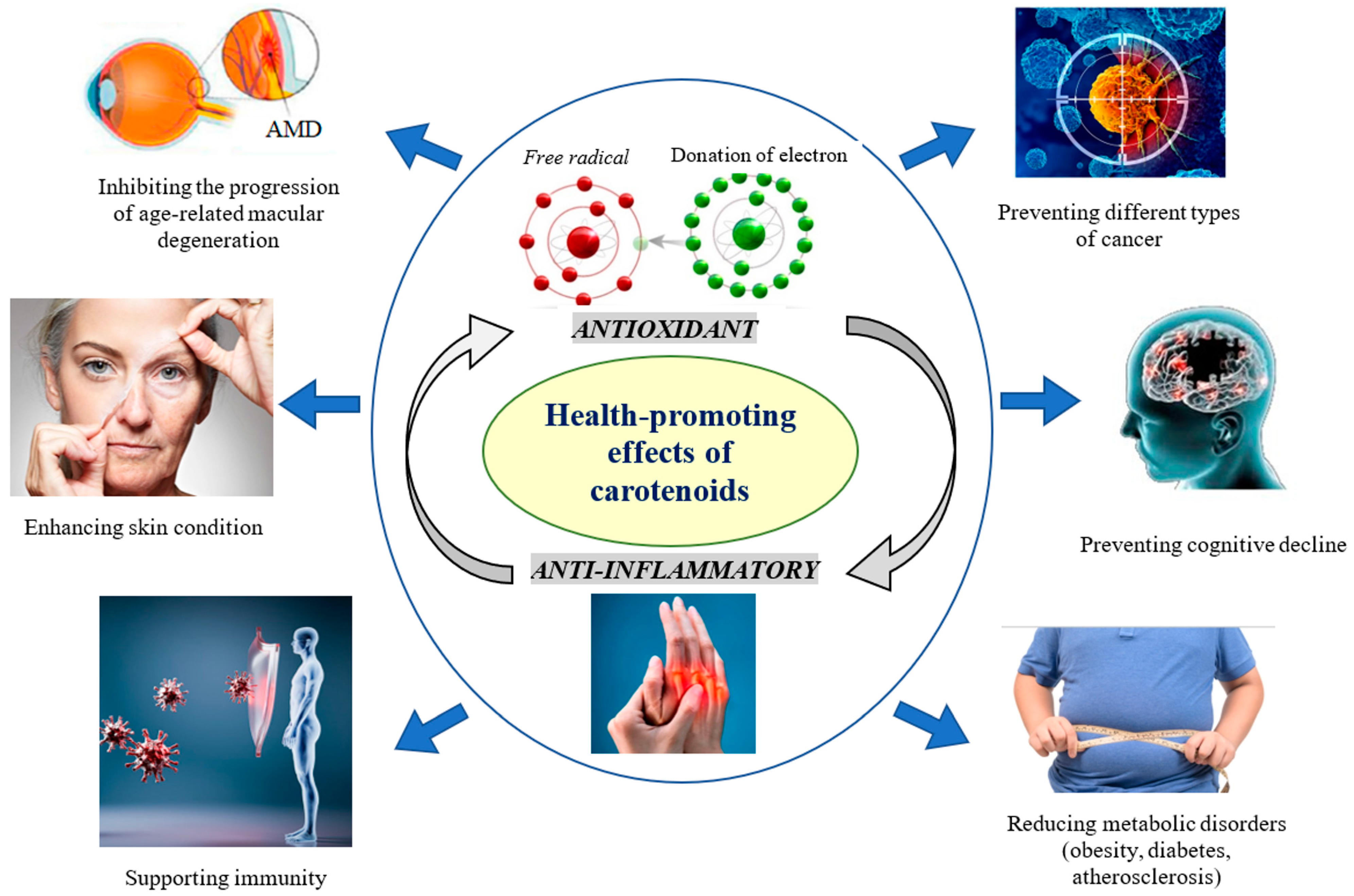
3.1. Diversity and Health-Promoting Effects of Carotenoids
3.2. Carotenes
3.2.1. α-Carotene
3.2.2. β-Carotene
3.2.3. Lycopene
3.3. Xanthophylls
3.3.1. Fucoxanthin
3.3.2. Lutein
3.3.3. Astaxanthin
3.3.4. β-Cryptoxanthin
3.3.5. Zeaxanthin
3.4. Clinical Trials on Carotenoids
3.5. Adverse Effects and Safety Concerns of Carotenoids
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Zepeda-Arzate, E.A.; Castillo-Vazquez, S.K.; Fleischmann-delaParra, P.; Hernandez-Pineda, J.; Flores-Soto, E.; Garcia-delaTorre, P.; Estrella-Parra, E.A.; Gomez-Verjan, J.C. Exploring the Geroprotective Potential of Nutraceuticals. Nutrients 2024, 16, 2835. [Google Scholar] [CrossRef]
- Mohammadi, E.; Mehri, S.; Badie Bostan, H.; Hosseinzadeh, H. Protective effect of crocin against d-galactose-induced aging in mice. Avicenna J. Phytomed. 2018, 8, 14–23. [Google Scholar]
- Stiefvatter, L.; Frick, K.; Lehnert, K.; Vetter, W.; Montoya-Arroyo, A.; Frank, J.; Schmid-Staiger, U.; Bischoff, S.C. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant-A Randomized Controlled Pilot Trial in Elderly Individuals. Mar. Drugs 2022, 20, 716. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Lin, Q.; Cai, J.; Liu, X.; Liang, Y. Nutrition Interventions of Herbal Compounds on Cellular Senescence. Oxidative Med. Cell. Longev. 2022, 2022, 1059257. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem.-Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef]
- Rudnicka, E.; Napierala, P.; Podfigurna, A.; Meczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 5 January 2025).
- Gansukh, E.; Nile, A.; Sivanesan, I.; Rengasamy, K.R.R.; Kim, D.H.; Keum, Y.S.; Saini, R.K. Chemopreventive Effect of beta-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis. Antioxidants 2019, 9, 28. [Google Scholar] [CrossRef]
- Brahma, D.; Dutta, D. Evaluating beta-cryptoxanthin antioxidant properties against ROS-induced macromolecular damages and determining its photo-stability and in-vitro SPF. World J. Microbiol. Biotechnol. 2023, 39, 310. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br. J. Nutr. 2015, 114, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Filipska, A.; Bohdan, B.; Wieczorek, P.; Hudz, N. Chronic kidney disease and dialysis therapy: Incidence and prevalence in the world. Pharmacia 2021, 68, 463–470. [Google Scholar] [CrossRef]
- WHO. Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 2 January 2025).
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Synergistic Effect of beta-Cryptoxanthin and Epigallocatechin Gallate on Obesity Reduction. Nutrients 2024, 16, 2344. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Antiaging Strategies and Remedies: A Landscape of Research Progress and Promise. ACS Chem. Neurosci. 2024, 15, 408–446. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Bjorklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Kahraman, C.; Kaya Bilecenoglu, D.; Sabuncuoglu, S.; Cankaya, I.T. Toxicology of pharmaceutical and nutritional longevity compounds. Expert Rev. Mol. Med. 2023, 25, e28. [Google Scholar] [CrossRef]
- Luy, M.; Di Giulio, P.; Di Lego, V.; Lazarevic, P.; Sauerberg, M. Life Expectancy: Frequently Used, but Hardly Understood. Gerontology 2020, 66, 95–104. [Google Scholar] [CrossRef]
- Shanaida, M.; Lysiuk, R.; Mykhailenko, O.; Hudz, N.; Abdulsalam, A.; Gontova, T.; Oleshchuk, O.; Ivankiv, Y.; Shanaida, V.; Lytkin, D.; et al. Alpha-lipoic Acid: An Antioxidant with Anti-Aging Properties for Disease Therapy. Curr. Med. Chem. 2024, 32, 23–54. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 381–406. [Google Scholar] [CrossRef]
- Batool, Z.; Chen, J.-H.; Gao, Y.; Lu, L.; Xu, H.; Liu, B.; Wang, m.; Chen, F. Natural Carotenoids as Neuroprotective Agents for Alzheimer’s Disease: An Evidence-Based Comprehensive Review. J. Agric. Food Chem. 2022, 70, 15631–15646. [Google Scholar] [CrossRef]
- Das, G.; Kameswaran, S.; Ramesh, B.; Bangeppagari, M.; Nath, R.; Das Talukdar, A.; Shin, H.S.; Patra, J.K. Anti-Aging Effect of Traditional Plant-Based Food: An Overview. Foods 2024, 13, 3785. [Google Scholar] [CrossRef]
- Bartali, B.; Semba, R. Carotenoids and healthy aging: The fascination continues. Am. J. Clin. Nutr. 2021, 113, 259–260. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Mandic, A.I.; Bantis, F.; Bohm, V.; Borge, G.I.A.; Brncic, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gomez-Villegas, P.; Gonda, M.L.; Leon-Vaz, A.; Leon, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, Seaweeds and Aquatic Bacteria, Archaea, and Yeasts: Sources of Carotenoids with Potential Antioxidant and Anti-Inflammatory Health-Promoting Actions in the Sustainability Era. Mar. Drugs 2023, 21, 340. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and Their Biosynthesis in Fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Bohm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Banati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Di Carlo, E.; Augustin, A.J. Prevention of the Onset of Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 3297. [Google Scholar] [CrossRef]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in human skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- Darawsha, A.; Trachtenberg, A.; Sharoni, Y. ARE/Nrf2 Transcription System Involved in Carotenoid, Polyphenol, and Estradiol Protection from Rotenone-Induced Mitochondrial Oxidative Stress in Dermal Fibroblasts. Antioxidants 2024, 13, 1019. [Google Scholar] [CrossRef]
- Chen, X.; He, C.; Yu, W.; Ma, L.; Gou, S.; Fu, P. Associations between dietary carotenoid and biological age acceleration: Insights from NHANES 2009–2018. Biogerontology 2024, 26, 24. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits-A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Bas, T.G. Bioactivity and Bioavailability of Carotenoids Applied in Human Health: Technological Advances and Innovation. Int. J. Mol. Sci. 2024, 25, 7603. [Google Scholar] [CrossRef]
- Mussagy, C.; Winterburn, J.; Santos-Ebinuma, V.; Pereira, J. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134 (Suppl. 12), 3479S–3485S. [Google Scholar] [CrossRef]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Significance of Genetic, Environmental, and Pre- and Postharvest Factors Affecting Carotenoid Contents in Crops: A Review. J. Agric. Food Chem. 2018, 66, 5310–5324. [Google Scholar] [CrossRef]
- Popa, C.; Calugar, R.E.; Varga, A.; Muntean, E.; Bacila, I.; Vana, C.D.; Racz, I.; Tritean, N.; Berindean, I.V.; Ona, A.D.; et al. Evaluating Maize Hybrids for Yield, Stress Tolerance, and Carotenoid Content: Insights into Breeding for Climate Resilience. Plants 2025, 14, 138. [Google Scholar] [CrossRef]
- Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. [Google Scholar] [CrossRef]
- Bohn, T.; Balbuena, E.; Ulus, H.; Iddir, M.; Wang, G.; Crook, N.; Eroglu, A. Carotenoids in Health as Studied by Omics-Related Endpoints. Adv. Nutr. 2023, 14, 1538–1578. [Google Scholar] [CrossRef]
- Meresse, S.; Fodil, M.; Fleury, F.; Chenais, B. Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Ortega-Regules, A.E.; Martinez-Thomas, J.A.; Schurenkamper-Carrillo, K.; de Parrodi, C.A.; Lopez-Mena, E.R.; Mejia-Mendez, J.L.; Lozada-Ramirez, J.D. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants 2024, 13, 1584. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef]
- Qiao, N.; Dumas, V.; Bergheau, A.; Ouillon, L.; Laroche, N.; Privet-Thieulin, C.; Perrot, J.-L.; Zahouani, H. Contactless mechanical stimulation of the skin using shear waves. J. Mech. Behav. Biomed. Mater. 2024, 156, 106597. [Google Scholar] [CrossRef]
- Darawsha, A.; Trachtenberg, A.; Levy, J.; Sharoni, Y. The Protective Effect of Carotenoids, Polyphenols, and Estradiol on Dermal Fibroblasts under Oxidative Stress. Antioxidants 2021, 10, 2023. [Google Scholar] [CrossRef]
- Argyropoulos, A.J.; Robichaud, P.; Balimunkwe, R.M.; Fisher, G.J.; Hammerberg, C.; Yan, Y.; Quan, T. Alterations of Dermal Connective Tissue Collagen in Diabetes: Molecular Basis of Aged-Appearing Skin. PLoS ONE 2016, 11, e0153806. [Google Scholar] [CrossRef]
- Birnbaum, J.; Le Moigne, A.; Dispensa, L.; Buchner, L. A Review of Clinical Trials Conducted With Oral, Multicomponent Dietary Supplements for Improving Photoaged Skin. J. Drugs Dermatol. 2015, 14, 1453–1461. [Google Scholar]
- Sadick, N.; Pannu, S.; Abidi, Z.; Arruda, S. Topical Treatments for Photoaged Skin. J. Drugs Dermatol. 2023, 22, 867–873. [Google Scholar] [CrossRef]
- Schupp, C.; Olano-Martin, E.; Gerth, C.; Morrissey, B.M.; Cross, C.E.; Werner, J.S. Lutein, zeaxanthin, macular pigment, and visual function in adult cystic fibrosis patients. Am. J. Clin. Nutr. 2004, 79, 1045–1052. [Google Scholar] [CrossRef]
- Kumar, P.; Banik, S.P.; Ohia, S.E.; Moriyama, H.; Chakraborty, S.; Wang, C.K.; Song, Y.S.; Goel, A.; Bagchi, M.; Bagchi, D. Current Insights on the Photoprotective Mechanism of the Macular Carotenoids, Lutein and Zeaxanthin: Safety, Efficacy and Bio-Delivery. J. Am. Nutr. Assoc. 2024, 43, 505–518. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary beta-carotene bioavailability in healthy men. J. Nutr. 2015, 145, 1740–1747. [Google Scholar] [CrossRef]
- Jiao, Y.; Reuss, L.; Wang, Y. β-cryptoxanthin: Chemistry, Occurrence, and Potential Health Benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Zhu, W.; Ye, D.; Xiao, Y.; Shi, M.; Zeng, R.; Bian, J.; Xu, X.; Chen, L.; et al. Relationship between serum carotenoids and telomere length in overweight or obese individuals. Front. Nutr. 2024, 11, 1479994. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Genc, Y.; Bardakci, H.; Yucel, C.; Karatoprak, G.S.; Kupeli Akkol, E.; Hakan Barak, T.; Sobarzo-Sanchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Su, W.; Xu, W.; Liu, E.; Su, W.; Polyakov, N.E. Improving the Treatment Effect of Carotenoids on Alzheimer’s Disease through Various Nano-Delivery Systems. Int. J. Mol. Sci. 2023, 24, 7652. [Google Scholar] [CrossRef]
- Guo, M.; Cui, W.; Li, Y.; Fei, S.; Sun, C.; Tan, M.; Su, W. Microfluidic fabrication of size-controlled nanocarriers with improved stability and biocompatibility for astaxanthin delivery. Food Res. Int. 2023, 170, 112958. [Google Scholar] [CrossRef]
- Medoro, A.; Scapagnini, G.; Brogi, S.; Jafar, T.H.; Trung, T.T.; Saso, L.; Davinelli, S. Carotenoid Interactions with PCSK9: Exploring Novel Cholesterol-Lowering Strategies. Pharmaceuticals 2024, 17, 1597. [Google Scholar] [CrossRef]
- Liu, S.; Yang, D.; Yu, L.; Aluo, Z.; Zhang, Z.; Qi, Y.; Li, Y.; Song, Z.; Xu, G.; Zhou, L. Effects of lycopene on skeletal muscle-fiber type and high-fat diet-induced oxidative stress. J. Nutr. Biochem. 2021, 87, 108523. [Google Scholar] [CrossRef]
- Gasmi, B.A.; Tippairote, T.; Gasmi, A.; Noor, S.; Avdeev, O.; Shanaida, Y.; Mojgani, N.; Emadali, A.; Dadar, M.; Bjørklund, G. Periodontitis Continuum: Antecedents, Triggers, Mediators, and Treatment Strategies. Curr. Med. Chem. 2024, 31, 6775–6800. [Google Scholar] [CrossRef]
- Naruishi, K. Carotenoids and Periodontal Infection. Nutrients 2020, 12, 269. [Google Scholar] [CrossRef]
- Li, F.; Wang, G.; Zhang, Y. Association between carotenoid intake and periodontitis in diabetic patients. J. Nutr. Sci. 2024, 13, e11. [Google Scholar] [CrossRef]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.; Naeem, M.; Corbo, F.; Clodoveo, M.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- He, X.; Yin, X.; Chen, X.; Chen, X. Aging and antioxidants: The impact of dietary carotenoid intakes on soluble klotho levels in aged adults. Front. Endocrinol. 2023, 14, 1283722. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Zampino, M.; Tanaka, T.; Bandinelli, S.; Moaddel, R.; Fantoni, G.; Candia, J.; Ferrucci, L.; Semba, R.D. The Plasma Proteome Fingerprint Associated with Circulating Carotenoids and Retinol in Older Adults. J. Nutr. 2022, 152, 40–48. [Google Scholar] [CrossRef]
- Gonzalez-Pena, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramirez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids-A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; De Nucci, S.; Sila, A.; Aresta, S.; Buscemi, C.; Randazzo, C.; Buscemi, S.; Triggiani, V.; De Pergola, G.; et al. Role of Dietary Carotenoids in Frailty Syndrome: A Systematic Review. Biomedicines 2022, 10, 632. [Google Scholar] [CrossRef]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef]
- Bruno, R.; Rosa, F.; Nahas, P.; Branco, F.; de Oliveira, E. Serum α-carotene, but Not Other Antioxidants, Is Positively Associated with Muscle Strength in Older Adults: NHANES 2001–2002. Antioxidants 2022, 11, 2386. [Google Scholar] [CrossRef]
- Flieger, J.; Forma, A.; Flieger, W.; Flieger, M.; Gawlik, P.J.; Dzierzynski, E.; Maciejewski, R.; Teresinski, G.; Baj, J. Carotenoid Supplementation for Alleviating the Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 8982. [Google Scholar] [CrossRef]
- Liu, X.; Dhana, K.; Furtado, J.; Agarwal, P.; Aggarwal, N.; Tangney, C.; Laranjo, N.; Carey, V.; Barnes, L.; Sacks, F. Higher circulating α-carotene was associated with better cognitive function: An evaluation among the MIND trial participants. J. Nutr. Sci. 2021, 10, e64. [Google Scholar] [CrossRef]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef]
- Gasmi, A.; Gasmi Benahmed, A.; Shanaida, M.; Chirumbolo, S.; Menzel, A.; Anzar, W.; Arshad, M.; Cruz-Martins, N.; Lysiuk, R.; Beley, N.; et al. Anticancer activity of broccoli, its organosulfur and polyphenolic compounds. Crit. Rev. Food Sci. Nutr. 2024, 64, 8054–8072. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.-F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic Aspects of Carotenoid Health Benefits—Where are we Now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Lademann, J.; Hagen, J.; Lohan, S.; Kolmar, H.; Meinke, M.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Lauretani, F.; Bandinelli, S.; Semba, R.; Bartali, B.; Cherubini, A.; Cappola, A.; Ceda, G.P.; Ferrucci, L. Relationship between Carotenoids, Retinol, and Estradiol Levels in Older Women. Nutrients 2015, 7, 6506–6519. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef]
- Darvin, M.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Vranesic-Bender, D. The role of nutraceuticals in anti-aging medicine. Acta Clin. Croat. 2010, 49, 537–544. [Google Scholar]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2022, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Beltran-de-Miguel, B.; Estevez-Santiago, R.; Olmedilla-Alonso, B. Assessment of dietary vitamin A intake (retinol, alpha-carotene, beta-carotene, beta-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010). Int. J. Food Sci. Nutr. 2015, 66, 706–712. [Google Scholar] [CrossRef]
- Ravi, M.; De, S.L.; Azharuddin, S.; Paul, S.F.D. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr. Diet. Suppl. 2010, 2, 73–83. [Google Scholar] [CrossRef]
- Miandoab, L.; Hejazi, M.; Bagherieh-Najjar, M.; Chaparzadeh, N. Optimization of the Four Most Effective Factors on Β-Carotene Production by Dunaliella Salina Using Response Surface Methodology Running title: Optimization of β-carotene production by Dunaliella salina. Iran. J. Pharm. Res. (IJPR) 2020, 18, 1566–1579. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, W.; Li, Y.; Qin, J.; Zhou, T.; Li, D.; Xu, Y.; Cheng, X.; Xiong, Y.; Chen, Z. Anti-aging effect of β-carotene through regulating the KAT7-P15 signaling axis, inflammation and oxidative stress process. Cell. Mol. Biol. Lett. 2022, 27, 86. [Google Scholar] [CrossRef]
- Henning, T.; Kochlik, B.; Ara, I.; Gonzalez-Gross, M.; Fiorillo, E.; Marongiu, M.; Cucca, F.; Rodriguez-Artalejo, F.; Carnicero Carreno, J.A.; Rodriguez-Manas, L.; et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients 2023, 15, 1142. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. beta-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical beta-carotene protects against infra-red-light-induced free radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leverett, J.; Krutmann, J. Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol. Photoimmunol. Photomed. 2021, 37, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, A.; Gramdorf, S.; Muller, R.H.; Kurz, T. Beta-carotene-loaded nanostructured lipid carriers. J. Food Sci. 2008, 73, N1–N6. [Google Scholar] [CrossRef]
- Lademann, J.; Vergou, T.; Darvin, M.E.; Patzelt, A.; Meinke, M.C.; Voit, C.; Papakostas, D.; Zastrow, L.; Sterry, W.; Doucet, O. Influence of Topical, Systemic and Combined Application of Antioxidants on the Barrier Properties of the Human Skin. Ski. Pharmacol. Physiol. 2016, 29, 41–46. [Google Scholar] [CrossRef]
- Trivedi, P.; Jena, G. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2015, 54, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Toti, E.; Chen, O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef] [PubMed]
- Abir, M.H.; Mahamud, A.G.M.S.U.; Tonny, S.H.; Anu, M.S.; Hossain, K.H.S.; Protic, I.A.; Khan, M.S.U.; Baroi, A.; Moni, A.; Uddin, M.J. Pharmacological potentials of lycopene against aging and aging-related disorders: A review. Food Sci. Nutr. 2023, 11, 5701–5735. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Petyaev, I.; Dovgalevsky, P.; Klochkov, V.; Chalyk, N.; Pristensky, D.; Chernyshova, M.; Udumyan, R.; Kocharyan, T.; Kyle, N.; Lozbiakova, M.; et al. Effect of lycopene supplementation on cardiovascular parameters and markers of inflammation and oxidation in patients with coronary vascular disease. Food Sci. Nutr. 2018, 6, 1770–1777. [Google Scholar] [CrossRef]
- Ozkan, G.; Gunal Köroglu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.; Al-Omari, B.; Calina, D.; Sharifi-Rad, J.; Cho, W. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023, 161, 114428. [Google Scholar] [CrossRef]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Kapala, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A narrative review on the potential of tomato and lycopene for the prevention of Alzheimer’s disease and other dementias. Crit. Rev. Food Sci. Nutr. 2022, 62, 4970–4981. [Google Scholar] [CrossRef]
- Moran, N.; Thomas-Ahner, J.; Wan, L.; Zuniga, K.; Erdman, J., Jr.; Clinton, S. Tomatoes, Lycopene, and Prostate Cancer: What Have We Learned from Experimental Models? J. Nutr. 2022, 152, 1381–1403. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef]
- Sengngam, K.; Hoc, T.H.; Hang, D.V.; Tran Ngoan, L. Trans-Lycopene and beta-Cryptoxanthin Intake and Stomach Cancer in Vietnamese Men: A Pilot Case-Control Study. Asian Pac. J. Cancer Prev. 2022, 23, 861–865. [Google Scholar] [CrossRef]
- Petyaev, I. Lycopene Deficiency in Ageing and Cardiovascular Disease. Oxidative Med. Cell. Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef] [PubMed]
- Bohm, V. Lycopene and heart health. Mol. Nutr. Food Res. 2012, 56, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Wu, Z.Z.; Zhang, Y.Q.; Wung, B.S. Lycopene inhibits ICAM-1 expression and NF-kappaB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016, 155, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Yang, X.; Ding, H. Lycopene ameliorates skin aging by regulating the insulin resistance pathway and activating SIRT1. Food Funct. 2022, 13, 11307–11320. [Google Scholar] [CrossRef]
- Rao, L.G.; Mackinnon, E.S.; Rao, A.V. Lycopene and Bone Tissue. In Lycopene: Nutritional, Medicinal and Therapeutic Properties; Preedy, V.R., Watson, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 309–328. [Google Scholar]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of Caffeine and Lycopene in Experimentally Induced Diabetes Mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef]
- Wang, J.; Suo, Y.; Zhang, J.; Zou, Q.; Tan, X.; Tian, Y.; Liu, Z.; Liu, X. Lycopene supplementation attenuates western diet-induced body weight gain through increasing the expressions of thermogenic/mitochondrial functional genes and improving insulin resistance in the adipose tissue of obese mice. J. Nutr. Biochem. 2019, 69, 63–72. [Google Scholar] [CrossRef]
- Lorenz, M.; Fechner, M.; Kalkowski, J.; Frohlich, K.; Trautmann, A.; Bohm, V.; Liebisch, G.; Lehneis, S.; Schmitz, G.; Ludwig, A.; et al. Effects of lycopene on the initial state of atherosclerosis in New Zealand White (NZW) rabbits. PLoS ONE 2012, 7, e30808. [Google Scholar] [CrossRef]
- Rakha, S.; Elmetwally, M.; El-Sheikh Ali, H.; Balboula, A.; Mahmoud, A.; Zaabel, S. Lycopene Reduces the In Vitro Aging Phenotypes of Mouse Oocytes by Improving Their Oxidative Status. Vet. Sci. 2022, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene Protects against Smoking-Induced Lung Cancer by Inducing Base Excision Repair. Antioxidants 2020, 9, 643. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araujo, G.R.; Martins, T.L.; Bandeira, A.C.B.; Costa, G.P.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene Improves Insulin Sensitivity through Inhibition of STAT3/Srebp-1c-Mediated Lipid Accumulation and Inflammation in Mice fed a High-Fat Diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610–617. [Google Scholar] [CrossRef]
- Song, X.; Sun, J.; Liu, H.; Mushtaq, A.; Huang, Z.; Li, D.; Zhang, L.; Chen, F. Lycopene Alleviates Endoplasmic Reticulum Stress in Steatohepatitis through Inhibition of the ASK1-JNK Signaling Pathway. J. Agric. Food Chem. 2024, 72, 7832–7844. [Google Scholar] [CrossRef]
- Kamel, S.; Zeidan, D.; Khaled, H.E.; Ali, Z.; Elrefaei, N.; El-Naggar, M. In Vivo Assessment of Lycopene Effect on Obesity-Induced Inflammation. Biomed. Pharmacol. J. 2022, 15, 1551–1560. [Google Scholar] [CrossRef]
- Vakili, S.; Samare-Najaf, M.; Karimi, A.; Jahromi, B.N.; Mohit, M.; Hashempur, M.H. Lycopene in male infertility. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, M.; Li, J.; Zhang, W.; Shang, X. Lycopene alleviates lipopolysaccharide-induced testicular injury in rats by activating the PPAR signaling pathway to integrate lipid metabolism and the inflammatory response. Transl. Androl. Urol. 2023, 12, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Shariatzadeh, N.; Gharavi, A.; Kalayi, A.; Khalaji, N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with Type 2 diabetes mellitus: A possible role in the prevention of long-term complications. J. Endocrinol. Investig. 2007, 30, 833–838. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Grande, S.; Guarnieri, S.; Gardana, C.; Simonetti, P.; Porrini, M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J. Agric. Food Chem. 2006, 54, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mendonca, P.; Messeha, S.; Soliman, K. Anticancer Effects of Fucoxanthin through Cell Cycle Arrest, Apoptosis Induction, and Angiogenesis Inhibition in Triple-Negative Breast Cancer Cells. Molecules 2023, 28, 6536. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jiménez-López, C.; Carpena, M.; González Pereira, A.; García Oliveira, P.; Prieto, M.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Bioactive significance of fucoxanthin and its effective extraction. Biocatal. Agric. Biotechnol. 2020, 26, 101639. [Google Scholar] [CrossRef]
- Quan, J.; Kim, S.-M.; Pan, C.-H.; Chung, D. Characterization of fucoxanthin-loaded microspheres composed of cetyl palmitate-based solid lipid core and fish gelatin–gum Arabic coacervate shell. Food Res. Int. 2013, 50, 31–37. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Yan, L.; Wu, Y.; Zhang, L.; Li, B.; Tong, H.; Lin, X. Molecular Mechanisms of Fucoxanthin in Alleviating Lipid Deposition in Metabolic Associated Fatty Liver Disease. J. Agric. Food Chem. 2024, 72, 10391–10405. [Google Scholar] [CrossRef]
- Satomi, Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Luan, H.; Yan, L.; Zhao, Y.; Ding, X.; Cao, L. Fucoxanthin induces apoptosis and reverses epithelial-mesenchymal transition via inhibiting Wnt/beta-catenin pathway in lung adenocarcinoma. Discov. Oncol. 2022, 13, 98. [Google Scholar] [CrossRef]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2021, 42, 210–219. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine Carotenoid Fucoxanthin Possesses Anti-Metastasis Activity: Molecular Evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Fang, C.; Cao, Q.; Xing, M.; Li, X.; Hou, J.; Ji, A.; Song, S. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar. Drugs 2020, 18, 634. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef]
- McClements, D.J.; Ozturk, B. Utilization of Nanotechnology to Improve the Handling, Storage and Biocompatibility of Bioactive Lipids in Food Applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhi, J.; Huang, S.; Zhang, X.; Kim, Y.R.; Xu, Y.; Wang, D.; Luo, K. Fabrication of starch/zein-based microcapsules for encapsulation and delivery of fucoxanthin. Food Chem. 2022, 392, 133282. [Google Scholar] [CrossRef]
- Spagolla Napoleao Tavares, R.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schafer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef]
- Smeriglio, A.; Lionti, J.; Ingegneri, M.; Burlando, B.; Cornara, L.; Grillo, F.; Mastracci, L.; Trombetta, D. Xanthophyll-Rich Extract of Phaeodactylum tricornutum Bohlin as New Photoprotective Cosmeceutical Agent: Safety and Efficacy Assessment on In Vitro Reconstructed Human Epidermis Model. Molecules 2023, 28, 4190. [Google Scholar] [CrossRef]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of Fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Natsume, C.; Aoki, N.; Aoyama, T.; Senda, K.; Matsui, M.; Ikegami, A.; Tanaka, K.; Azuma, Y.T.; Fujita, T. Fucoxanthin Ameliorates Atopic Dermatitis Symptoms by Regulating Keratinocytes and Regulatory Innate Lymphoid Cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef]
- Chen, S.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Lee, A.S.; Lin, C.H.; Tseng, K.W. Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Mar. Drugs 2021, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.; Ożarowski, M.; Alam, R.; Lochynska, M.; Stasiewicz, M. What Do We Know about Antimicrobial Activity of Astaxanthin and Fucoxanthin? Mar. Drugs 2021, 20, 36. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Ramesh, T.; Thayumanavan, P. Dual antidiabetic and antihypertensive activity of fucoxanthin isolated from Sargassum wightii Greville in in vivo rat model. Food Sci. Hum. Wellness 2023, 12, 1693–1700. [Google Scholar] [CrossRef]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2021, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ramos, A.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G. Effect of Fucoxanthin on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. J. Med. Food 2023, 26, 521–527. [Google Scholar] [CrossRef]
- Maury, J.; Delbrut, A.; Villard, V.; Pradelles, R. A Standardized Extract of Microalgae Phaeodactylum tricornutum (Mi136) Inhibit D-Gal Induced Cognitive Dysfunction in Mice. Mar. Drugs 2024, 22, 99. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.P.; Nishigaki, I. Cancer preventive efficacy of marine carotenoid fucoxanthin: Cell cycle arrest and apoptosis. Nutrients 2013, 5, 4978–4989. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Stability and biological activity enhancement of fucoxanthin through encapsulation in alginate/chitosan nanoparticles. Int. J. Biol. Macromol. 2024, 263, 130264. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukula-Koch, W.; Wawruszak, A.; Okoń, E.; Stępnik, K.; Gaweł-Bęben, K.; Setzer, W.; Dini, I.; Sharifi-Rad, J.; Calina, D. Fucoxanthin: From chemical properties and sources to novel anticancer mechanistic insights and synergistic therapeutic opportunities. Curr. Res. Biotechnol. 2024, 7, 100203. [Google Scholar] [CrossRef]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein production from microalgae: A review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef]
- Wang, M.; Tang, R.; Zhou, R.; Qian, Y.; Di, D. The protective effect of serum carotenoids on cardiovascular disease: A cross-sectional study from the general US adult population. Front. Nutr. 2023, 10, 1154239. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of beta-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Boribalnukul, P.; Morakul, B.; Junyaprasert, V.B. Influence of Vegetable Oils on In Vitro Performance of Lutein-Loaded Lipid Carriers for Skin Delivery: Nanostructured Lipid Carriers vs. Nanoemulsions. Pharmaceutics 2022, 14, 2160. [Google Scholar] [CrossRef] [PubMed]
- Pongcharoen, S.; Warnnissorn, P.; Lertkajornsin, O.; Limpeanchob, N.; Sutheerawattananonda, M. Protective effect of silk lutein on ultraviolet B-irradiated human keratinocytes. Biol. Res. 2013, 46, 39–45. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Salama, A.; Ali, S.I.; Elgohary, R. Lutein isolated from Scenedesmus obliquus microalga boosts immunity against cyclophosphamide-induced brain injury in rats. Sci. Rep. 2022, 12, 22601. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Tarighat-Esfanjani, A.; Ghoreishi, Z.; Sarreshtedari, M. Lutein supplementation combined with a low-calorie diet in middle-aged obese individuals: Effects on anthropometric indices, body composition and metabolic parameters. Br. J. Nutr. 2021, 126, 1028–1039. [Google Scholar] [CrossRef]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.; de Oliveira, B.M.M.; Cury, B.J.; Jeronimo, D.T.; Venzon, L.; Franca, T.C.S.; Mariott, M.; Silva-Nunes, R.; Santos, A.C.; Roman-Junior, W.A.; et al. Extract of Tagetes erecta L., a medicinal plant rich in lutein, promotes gastric healing and reduces ulcer recurrence in rodents. J. Ethnopharmacol. 2022, 293, 115258. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Effects of lutein on the growth and migration of bovine lens epithelial cells in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 360–363. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Ding, H.; Hu, S.; Wu, X.; Ma, A.; Ma, Y. Lutein Prevents Liver Injury and Intestinal Barrier Dysfunction in Rats Subjected to Chronic Alcohol Intake. Nutrients 2023, 15, 1229. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Jaggi, D.; Solberg, Y.; Dysli, C.; Lincke, J.; Habra, O.; Wyss, A.; Wolf, S.; Zinkernagel, M. Fluorescence lifetime imaging ophthalmoscopy and the influence of oral lutein/zeaxanthin supplementation on macular pigment (FLOS)—A pilot study. Clin. Nutr. ESPEN 2023, 56, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sakai, O.; Honda, T.; Kikuya, T.; Takeda, R.; Sawabe, A.; Inaba, M.; Koike, C. Effects of Astaxanthin, Lutein, and Zeaxanthin on Eye-Hand Coordination and Smooth-Pursuit Eye Movement after Visual Display Terminal Operation in Healthy Subjects: A Randomized, Double-Blind Placebo-Controlled Intergroup Trial. Nutrients 2023, 15, 1459. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, J.; Dobrzynska, M.M. Lutein and zeaxanthin—Radio- and chemoprotective properties. Mechanism and possible use. Rocz. Panstw. Zakl. Hig. 2023, 74, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini K, S.; Ramanna, M.K.; L, S.; Prasad, T.K. A Comparative study on Efficacy of Lutein and Atorvastatin on Lipid Profile and Lipoprotein(A) in Hypercholesterolemic male Wistar Rats. Biomed. Pharmacol. J. 2021, 14, 503–511. [Google Scholar] [CrossRef]
- Chew, E.; Clemons, T.; Agrón, E.; Domalpally, A.; Keenan, T.; Vitale, S.; Weber, C.; Smith, D.; Christen, W.; SanGiovanni, J.P.; et al. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Parekh, R.; Hammond, B.R., Jr.; Chandradhara, D. Lutein and Zeaxanthin Supplementation Improves Dynamic Visual and Cognitive Performance in Children: A Randomized, Double-Blind, Parallel, Placebo-Controlled Study. Adv. Ther. 2024, 41, 1496–1511. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawson, R.; Kong, L.; Tan, L. Lutein supplementation for early-life health and development: Current knowledge, challenges, and implications. Crit. Rev. Food Sci. Nutr. 2024, 1–16. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Zhang, D.; Li, S. The Mediation Role of the Risk of Non-Alcoholic Fatty Liver Disease in Relationship between Lutein and Zeaxanthin and Cognitive Functions among Older Adults in the United States. Nutrients 2022, 14, 578. [Google Scholar] [CrossRef]
- Shegokar, R.; Mitri, K. Carotenoid lutein: A promising candidate for pharmaceutical and nutraceutical applications. J. Diet. Suppl. 2012, 9, 183–210. [Google Scholar] [CrossRef]
- Rizzardi, N.; Pezzolesi, L.; Samorì, C.; Senese, F.; Zalambani, C.; Pitacco, W.; Calonghi, N.; Bergamini, C.; Prata, C.; Fato, R. Natural Astaxanthin Is a Green Antioxidant Able to Counteract Lipid Peroxidation and Ferroptotic Cell Death. Int. J. Mol. Sci. 2022, 23, 15137. [Google Scholar] [CrossRef]
- Ahmad, A.; Riaz, S.; Nadeem, M.; Mubeen, U.; Maham, K. Role of Carotenoids in Cardiovascular Disease. In Carotenoids-New Perspectives and Application; Martínez-Espinosa, R.M., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef]
- Abdelazim, K.; Ghit, A.; Assal, D.; Dorra, N.; Noby, N.; Khattab, S.N.; El Feky, S.E.; Hussein, A. Production and therapeutic use of astaxanthin in the nanotechnology era. Pharmacol. Rep. 2023, 75, 771–790. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Shanaida, M.; Ongenae, A.; Lysiuk, R.; Dosa, M.D.; Tsal, O.; Piscopo, S.; Chirumbolo, S.; Bjorklund, G. Calanus oil in the treatment of obesity-related low-grade inflammation, insulin resistance, and atherosclerosis. Appl. Microbiol. Biotechnol. 2020, 104, 967–979. [Google Scholar] [CrossRef]
- Nakano, M.; Onodera, A.; Saito, E.; Tanabe, M.; Yajima, K.; Takahashi, J.; Van Chuyen, N. Effect of astaxanthin in combination with alpha-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J. Nutr. Sci. Vitaminol. 2008, 54, 329–334. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, C.R.; Vasconcelo, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of Astaxanthin on Diabetes Pathogenesis and Chronic Complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Bjorklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef]
- Bahbah, E.; Ghozy, S.; Attia, M.; Negida, A.; Emran, T.; Mitra, S.; Albadrani, G.; Abdel Daim, M.; Uddin, M.; Simal-Gandara, J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs 2021, 19, 201. [Google Scholar] [CrossRef]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52, 382–392. [Google Scholar]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. A new alternative insight of nanoemulsion conjugated with kappa-carrageenan for wound healing study in diabetic mice: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 133, 236–250. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Zhang, S.; Xu, Y. Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3beta/Nrf2 signalling pathway in vitro. J. Cell. Mol. Med. 2020, 24, 8977–8985. [Google Scholar] [CrossRef]
- Fleischmann, C.; Bar-Ilan, N.; Horowitz, M.; Bruchim, Y.; Deuster, P.; Heled, Y. Astaxanthin supplementation impacts the cellular HSP expression profile during passive heating. Cell Stress. Chaperones 2020, 25, 549–558. [Google Scholar] [CrossRef]
- Gasmi, A.; Nasreen, A.; Lenchyk, L.; Lysiuk, R.; Peana, M.; Shapovalova, N.; Piscopo, S.; Komisarenko, M.; Shanaida, M.; Smetanina, K.; et al. An Update on Glutathione’s Biosynthesis, Metabolism, Functions, and Medicinal Purposes. Curr. Med. Chem. 2024, 31, 4579–4601. [Google Scholar] [CrossRef]
- Haung, H.Y.; Wang, Y.C.; Cheng, Y.C.; Kang, W.; Hu, S.H.; Liu, D.; Xiao, C.; Wang, H.D. A Novel Oral Astaxanthin Nanoemulsion from Haematococcus pluvialis Induces Apoptosis in Lung Metastatic Melanoma. Oxidative Med. Cell. Longev. 2020, 2020, 2647670. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef]
- Güdül Bacanlı, M.; Basaran, N.; Basaran, A. Lycopene: Is it Beneficial to Human Health as an Antioxidant? Turk. J. Pharm. Sci. 2017, 14, 311–318. [Google Scholar] [CrossRef]
- Linnewiel Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Claudi, I.; Palmeri, D.; Tonacci, A.; Allegra, A.; Gangemi, S. Alarmins in autoimmune diseases. Autoimmun. Rev. 2022, 21, 103142. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Boeder, S.C.; Mudaliar, S.R.; Giovannetti, E.R.; Henry, R.R.; Pettus, J.H. Astaxanthin, a natural antioxidant, lowers cholesterol and markers of cardiovascular risk in individuals with prediabetes and dyslipidaemia. Diabetes Obes. Metab. 2023, 25, 1985–1994. [Google Scholar] [CrossRef]
- Hudz, N.; Stepaniuk, N.; Stepaniuk, A. Gender-specific features of arterial hypertension development and treatment. Acta Pol. Pharm. Drug Res. 2020, 77, 667–671. [Google Scholar] [CrossRef]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Takara, T. Cognitive function improvement with astaxanthin and tocotrienol intake: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2020, 67, 307–316. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective Effects of Astaxanthin Supplementation against Ultraviolet-Induced Photoaging in Hairless Mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef]
- Liu, S.; Manabe, Y.; Sugawara, T. Oral administration of astaxanthin mitigates chronological skin aging in mice. Biosci. Biotechnol. Biochem. 2024, zbae205. [Google Scholar] [CrossRef]
- Zhen, A.X.; Kang, K.A.; Piao, M.J.; Madushan Fernando, P.D.S.; Lakmini Herath, H.M.U.; Hyun, J.W. Protective effects of astaxanthin on particulate matter 2.5 induced senescence in HaCaT keratinocytes via maintenance of redox homeostasis. Exp. Ther. Med. 2024, 28, 275. [Google Scholar] [CrossRef]
- Bakan, E.; Akbulut, Z.; İnanç, A. Carotenoids in Foods and their Effects on Human Health. Acad. Food J. 2014, 12, 61–68. [Google Scholar]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of beta-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Maeda, K.; Yamada, H.; Munetsuna, E.; Fujii, R.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Tsuboi, Y.; Ishikawa, H.; Ohashi, K.; et al. Serum carotenoid levels are positively associated with DNA methylation of thioredoxin-interacting protein. Int. J. Vitam. Nutr. Res. 2024, 94, 210–220. [Google Scholar] [CrossRef]
- Llopis, S.; Rodrigo, M.J.; Gonzalez, N.; Genoves, S.; Zacarias, L.; Ramon, D.; Martorell, P. beta-Cryptoxanthin Reduces Body Fat and Increases Oxidative Stress Response in Caenorhabditis elegans Model. Nutrients 2019, 11, 232. [Google Scholar] [CrossRef]
- Kobori, M.; Ni, Y.; Takahashi, Y.; Watanabe, N.; Sugiura, M.; Ogawa, K.; Nagashimada, M.; Kaneko, S.; Naito, S.; Ota, T. β-cryptoxanthin Alleviates Diet-Induced Nonalcoholic Steatohepatitis by Suppressing Inflammatory Gene Expression in Mice. PLoS ONE 2014, 9, e98294. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Mendiola, J.; Lopez-Espin, J.; Sarabia-Cos, L.; Vivero-Salmerón, G.; Vioque, J.; Navarrete-Muñoz, E.M.; Torres-cantero, A. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum. Reprod. 2012, 27, 2807–2814. [Google Scholar] [CrossRef]
- Yamanobe, H.; Yamamoto, K.; Kishimoto, S.; Nakai, K.; Oseko, F.; Yamamoto, T.; Mazda, O.; Kanamura, N. Anti-Inflammatory Effects of beta-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes. Molecules 2023, 28, 2935. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, H.; Li, Y.; Xiong, Y.; Liu, X.; Wang, L.; Chen, Z. beta-Cryptoxanthin Maintains Mitochondrial Function by Promoting NRF2 Nuclear Translocation to Inhibit Oxidative Stress-Induced Senescence in HK-2 Cells. Int. J. Mol. Sci. 2023, 24, 3851. [Google Scholar] [CrossRef]
- Lim, J.Y.; Liu, C.; Hu, K.Q.; Smith, D.E.; Wu, D.; Lamon-Fava, S.; Ausman, L.M.; Wang, X.D. Dietary beta-Cryptoxanthin Inhibits High-Refined Carbohydrate Diet-Induced Fatty Liver via Differential Protective Mechanisms Depending on Carotenoid Cleavage Enzymes in Male Mice. J. Nutr. 2019, 149, 1553–1564. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, D.; Wang, X.; Zhang, Y.; Duan, W.; Li, Y. beta-cryptoxanthin alleviates myocardial ischaemia/reperfusion injury by inhibiting NF-kappaB-mediated inflammatory signalling in rats. Arch. Physiol. Biochem. 2022, 128, 1128–1135. [Google Scholar] [CrossRef]
- Ke, J.; Zang, H.; Liu, Y.; Teng, Q.; Hua, J.; Peng, D.; Wang, P. beta-cryptoxanthin suppresses oxidative stress via activation of the Nrf2/HO-1 signaling pathway in diabetic kidney disease. Front. Pharmacol. 2024, 15, 1480629. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishita, Y.; Ando, F.; Shimokata, H.; Otsuka, R. Low Serum Total Carotenoids and beta-Cryptoxanthin Are Associated with Low Lean Body Mass in Older Community-Dwellers in the National Institute for Longevity Sciences-Longitudinal Study of Aging: A 4-Y Longitudinal Study. J. Nutr. 2024, 154, 3042–3047. [Google Scholar] [CrossRef]
- Iwamoto, M.; Imai, K.; Ohta, H.; Shirouchi, B.; Sato, M. Supplementation of highly concentrated beta-cryptoxanthin in a satsuma mandarin beverage improves adipocytokine profiles in obese Japanese women. Lipids Health Dis. 2012, 11, 52. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Transport and Delivery of Vitamin A and Carotenoids to the Retinal Pigment Epithelium. Mol. Nutr. Food Res. 2019, 63, e1801046. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Fanning, K.J.; Martin, I.F. Zeaxanthin biofortification of sweet-corn and factors affecting zeaxanthin accumulation and colour change. Arch. Biochem. Biophys. 2015, 572, 184–187. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; de Lourdes Bastos, M.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; et al. Safety and efficacy of lutein and lutein/zeaxanthin extracts from Tagetes erecta for poultry for fattening and laying (except turkeys). EFSA J. 2019, 17, e05698. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Abdel Jaleel, G.A.R. Zeaxanthin Isolated from Dunaliella salina Microalgae Ameliorates Age Associated Cardiac Dysfunction in Rats through Stimulation of Retinoid Receptors. Mar. Drugs 2019, 17, 290. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Jin, W.; Liu, Y.; Li, G.; Zhong, W.; Huang, J.; Wang, W. QTL Mapping of Zeaxanthin Content in Sweet Corn Using Recombinant Inbred Line Population across Different Environments. Plants 2023, 12, 3506. [Google Scholar] [CrossRef]
- Souza, A.S.N.; Schmidt, H.O.; Pagno, C.; Rodrigues, E.; Silva, M.; Flôres, S.H.; Rios, A.O. Influence of cultivar and season on carotenoids and phenolic compounds from red lettuce influence of cultivar and season on lettuce. Food Res. Int. 2022, 155, 111110. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimer’s Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Harju, N. Regulation of oxidative stress and inflammatory responses in human retinal pigment epithelial cells. Acta Ophthalmol. 2022, 100 (Suppl. S273), 3–59. [Google Scholar] [CrossRef]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef]
- Vrdoljak, N. Carotenoids and Carcinogenesis: Exploring the Antioxidant and Cell Signaling Roles of Carotenoids in the Prevention of Cancer. Crit. Rev. Oncog. 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Sheng, Y.N.; Luo, Y.H.; Liu, S.B.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Wang, C.Y.; et al. Zeaxanthin Induces Apoptosis via ROS-Regulated MAPK and AKT Signaling Pathway in Human Gastric Cancer Cells. Onco Targets Ther. 2020, 13, 10995–11006. [Google Scholar] [CrossRef]
- Bi, M.C.; Rosen, R.; Zha, R.Y.; McCormick, S.A.; Song, E.; Hu, D.N. Zeaxanthin Induces Apoptosis in Human Uveal Melanoma Cells through Bcl-2 Family Proteins and Intrinsic Apoptosis Pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 205082. [Google Scholar] [CrossRef]
- Huang, Z.; Li, G.; Zhang, Z.; Gu, R.; Wang, W.; Lai, X.; Cui, Z.-K.; Zeng, F.; Xu, S.; Deng, F. β2AR-HIF-1α-CXCL12 signaling of osteoblasts activated by isoproterenol promotes migration and invasion of prostate cancer cells. BMC Cancer 2019, 19, 1142. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Khachik, F.; de Moura, F.F.; Chew, E.Y.; Douglass, L.W.; Ferris, F.L., 3rd; Kim, J.; Thompson, D.J. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5234–5242. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation With Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 fatty acid, carotenoid and vitamin E supplementation improves working memory in older adults: A randomised clinical trial. Clin. Nutr. 2022, 41, 405–414. [Google Scholar] [CrossRef]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Li, H.; Yu, H.; Xi, Y. The Protective Effects of Zeaxanthin on Amyloid-β Peptide 1-42-Induced Impairment of Learning and Memory Ability in Rats. Front. Behav. Neurosci. 2022, 16, 912896. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Q.; You, Q.L.; Li, Z.L.; Hu, N.Y.; Wang, Y.; Jin, Z.L.; Li, S.J.; Li, X.W.; Yang, J.M.; et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020, 11, 5465. [Google Scholar] [CrossRef]
- La Russa, D.; Manni, G.; Di Santo, C.; Pieroni, B.; Pellegrino, D.; Barba, F.J.; Bagetta, G.; Fallarino, F.; Montesano, D.; Amantea, D. Zeaxanthin exerts anti-inflammatory effects in vitro and provides significant neuroprotection in mice subjected to transient middle cerebral artery occlusion. PharmaNutrition 2024, 27, 100368. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Gunal, M.Y.; Sakul, A.A.; Caglayan, A.B.; Erten, F.; Kursun, O.E.D.; Kilic, E.; Sahin, K. Protective Effect of Lutein/Zeaxanthin Isomers in Traumatic Brain Injury in Mice. Neurotox. Res. 2021, 39, 1543–1550. [Google Scholar] [CrossRef]
- Al-Thepyani, M.; Algarni, S.; Gashlan, H.; Elzubier, M.; Baz, L. Evaluation of the Anti-Obesity Effect of Zeaxanthin and Exercise in HFD-Induced Obese Rats. Nutrients 2022, 14, 4944. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Zhang, C.; Dai, Z.; Li, X.; Zhang, B. The Hypoglycemic, Hypolipidemic, and Anti-Diabetic Nephritic Activities of Zeaxanthin in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Appl. Biochem. Biotechnol. 2017, 182, 944–955. [Google Scholar] [CrossRef]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017, 4, 23. [Google Scholar] [CrossRef]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Kwon, O.; Shin, A.; Kim, J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2019, 9, 3406. [Google Scholar] [CrossRef]
- Van Hoang, D.; Pham, N.M.; Lee, A.H.; Tran, D.N.; Binns, C.W. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70. [Google Scholar] [CrossRef]
- Sahin, K.; Gencoglu, H.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Sahin, N.; Yilmaz, I.; Juturu, V. Lutein and zeaxanthin isomers may attenuate photo-oxidative retinal damage via modulation of G protein-coupled receptors and growth factors in rats. Biochem. Biophys. Res. Commun. 2019, 516, 163–170. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Li, B.; Blount, J.D.; Nwagbo, U.; Kim, H.J.; Sparrow, J.R.; Bernstein, P.S. Lutein and zeaxanthin reduce A2E and iso-A2E levels and improve visual performance in Abca4−/−/Bco2−/− double knockout mice. Exp. Eye Res. 2021, 209, 108680. [Google Scholar] [CrossRef]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The Effects of Lutein and Zeaxanthin Supplementation on Cognitive Function in Adults With Self-Reported Mild Cognitive Complaints: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef]
- NCT02147171; Carotenoid Supplementation and Normal Ocular Health. U.S. National Library of Medicine: Bethesda, MD, USA, 2014. Available online: https://clinicaltrials.gov/study/NCT02147171?cond=Aging&term=Lutein&rank=1 (accessed on 8 January 2025).
- NCT02200263; The Effects of Lutein and Zeaxanthin Supplementation on Vision in Patients With Albinism (LUVIA). U.S. National Library of Medicine: Bethesda, MD, USA, 2018. Available online: https://clinicaltrials.gov/study/NCT02200263?cond=skin&term=luteine&rank=5 (accessed on 8 January 2025).
- NCT00564902; The Zeaxanthin and Visual Function Study (ZVF). U.S. National Library of Medicine: Bethesda, MD, USA, 2012. Available online: https://clinicaltrials.gov/study/NCT00564902?term=zeaxanthin&rank=4 (accessed on 8 January 2025).
- NCT04832412; Effect of BrainPhyt, a Microalgae Based Ingredient on Cognitive Function in Healthy Older Subjects (PHAEOSOL-THREE). U.S. National Library of Medicine: Bethesda, MD, USA, 2023. Available online: https://clinicaltrials.gov/study/NCT04832412?cond=Ageing&term=Fucoxanthin&rank=2 (accessed on 8 January 2025).
- NCT00169845; Beta-Carotene and Alpha-tocopherol Chemoprevention of Second Primary Malignancies in Head and Neck Cancer Patients. U.S. National Library of Medicine: Bethesda, MD, USA, 2018. Available online: https://clinicaltrials.gov/study/NCT00169845?cond=cancer&term=carotene&rank=1 (accessed on 8 January 2025).
- NCT00836342; Correlation Between Skin Carotenoid Levels and Previous History of Skin Cancer. U.S. National Library of Medicine: Bethesda, MD, USA, 2012. Available online: https://clinicaltrials.gov/study/NCT00836342?cond=cancer&term=carotene&rank=10 (accessed on 8 January 2025).
- NCT00450749; Lycopene in Treating Patients Undergoing Radical Prostatectomy for Prostate Cancer. U.S. National Library of Medicine: Bethesda, MD, USA, 2019. Available online: https://clinicaltrials.gov/study/NCT00450749?cond=cancer&term=carotenoids&page=2&rank=17 (accessed on 8 January 2025).
- NCT03991286; The Effect of Astaxanthin on Oxidative Stress Indices in Patients with Polycystic Ovary Syndrome. U.S. National Library of Medicine: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT03991286?cond=cancer&term=astaxanthin&rank=1 (accessed on 8 January 2025).
- NCT03769779; Evaluation of the Benefits of FloraGLO™ Lutein on Skin Health. U.S. National Library of Medicine: Bethesda, MD, USA, 2021. Available online: https://clinicaltrials.gov/study/NCT03769779?cond=Aging&term=Lutein&rank=2 (accessed on 9 January 2025).
- NCT02373111; Effects of Isoflavone Combined With Astaxanthin on Skin Aging. U.S. National Library of Medicine: Bethesda, MD, USA, 2016. Available online: https://clinicaltrials.gov/study/NCT02373111?cond=Aging&term=astaxanthin&rank=1 (accessed on 9 January 2025).
- NCT03460860; Astaxanthin (2 mg) + Lycopene (1.8 mg) + D-Alpha-Tocopherol (10 IU) for The Treatment of Skin Aging. U.S. National Library of Medicine: Bethesda, MD, USA, 2019. Available online: https://clinicaltrials.gov/study/NCT03460860?cond=Aging&term=astaxanthin&rank=2 (accessed on 9 January 2025).
- NCT03811977; The Effect of 12-Week Dietary Intake of Lutein on Minimal Erythema Dose and Other Skin Parameters (VIST Lutein). U.S. National Library of Medicine: Bethesda, MD, USA, 2019. Available online: https://clinicaltrials.gov/study/NCT03811977?cond=skin&term=luteine&rank=2 (accessed on 9 January 2025).
- NCT05376501; A Study to Evaluate the Effect of Astaxanthin in Healthy Participants. U.S. National Library of Medicine: Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/study/NCT05376501?cond=aging&term=astaxanthin&rank=3 (accessed on 9 January 2025).
- Ma, L.; Dou, H.-L.; Huang, Y.; Lu, X.-R.; Xu, X.-R.; Qian, F.; Zou, Z.; Pang, H.-L.; Dong, P.-C.; Xiao, X.; et al. Improvement of Retinal Function in Early Age-Related Macular Degeneration After Lutein and Zeaxanthin Supplementation: A Randomized, Double-Masked, Placebo-Controlled Trial. Am. J. Ophthalmol. 2012, 154, 625–634.e1. [Google Scholar] [CrossRef]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.E.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef]
- Zielinska, M.A.; Wesolowska, A.; Pawlus, B.; Hamulka, J. Health Effects of Carotenoids during Pregnancy and Lactation. Nutrients 2017, 9, 838. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union 2006, 50, 26–38. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0026:0038:en:PDF (accessed on 20 February 2025).
- Devaraj, S.; Mathur, S.; Basu, A.; Aung, H.H.; Vasu, V.T.; Meyers, S.; Jialal, I. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J. Am. Coll. Nutr. 2008, 27, 267–273. [Google Scholar] [CrossRef]
- EFSA Panel On Dietetic Products, Nutrition and Allergies. Scientific Opinion on the safety of astaxanthin-rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. EFSA J. 2014, 12, 3757. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Bohn, T. Bioactivity of Carotenoids—Chasms of Knowledge. Int. J. Vitam. Nutr. Res. 2017, 87, 5–9. [Google Scholar] [CrossRef]
- de Oliveira, M.R. Vitamin A and Retinoids as Mitochondrial Toxicants. Oxidative Med. Cell. Longev. 2015, 2015, 140267. [Google Scholar] [CrossRef]
- Siems, W.; Salerno, C.; Crifò, C.; Sommerburg, O.; Wiswedel, I. β-Carotene Degradation Products—Formation, Toxicity and Prevention of Toxicity. In Food Factors for Health Promotion; Yoshikawa, T., Ed.; Karger Medical and Scientific Publishers: Basel, Switzerland, 2009; Volume 61, pp. 75–86. [Google Scholar]
- Qiu, Z.; Chen, X.; Geng, T.; Wan, Z.; Lu, Q.; Li, L.; Zhu, K.; Zhang, X.; Liu, Y.; Lin, X.; et al. Associations of Serum Carotenoids With Risk of Cardiovascular Mortality Among Individuals With Type 2 Diabetes: Results From NHANES. Diabetes Care 2022, 45, 1453–1461. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Effects of Carotenoids on Health: Are All the Same? Results from Clinical Trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef]
- Christian, M.S.; Schulte, S.; Hellwig, J. Developmental (embryo-fetal toxicity/teratogenicity) toxicity studies of synthetic crystalline lycopene in rats and rabbits. Food Chem. Toxicol. 2003, 41, 773–783. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
| Official Title | Clinical Trials.gov ID | Location | Condition | Protocol (Study Type) | Subjects (Ages Eligible for Study) | Duration | Intervention/Treatment | Reference |
|---|---|---|---|---|---|---|---|---|
| Carotenoid Supplementation and Normal Ocular Health | NCT02147171 | Manchester, UK | Older human eyes | Interventional (phases 2 and 3) | 88 (50–90 Years) | November 2011–April 2014 | Dietary supplement: VisionAce containing carotenoids. Lutein-containing ‘VisionAce’ daily for a period of 1 year | [285] |
| The Effects of Lutein and Zeaxanthin Supplementation on Vision in Patients With Albinism (LUVIA) | NCT02200263 | Baltimore, USA | Macular pigment and visual function in albinism | Interventional | 10 (12 Years and older) | November 2014–April 2018 | Lutein plus zeaxanthin. Participants received 20 mg of lutein plus 20 mg of zeaxanthin per day (for the duration of 12 months) | [286] |
| The Zeaxanthin and Visual Function Study (ZVF) | NCT00564902 | North Chicago VA Medical Center | Atrophic age-related macular degeneration | Interventional | 60 (45 Years to 90 Years) | October 2007–June2009 | Dietary supplement: 8 mg of lutein and 8 mg of zeaxanthin administered during 12 months | [287] |
| Effect of BrainPhyt, a Microalgae Based Ingredient on Cognitive Function in Healthy Older Subjects (PHAEOSOL-THREE) | NCT04832412 | Cork, Ireland | Cognitive function of healthy older subjects | Observational | 66 (healthy males and females, 55–75 Years) | April 2021–May 2023 | Dietary supplement: BrainPhyt (natural extract of Phaeodactylum tricornutum containing fucoxanhin) Doses: 2 capsules of 275 mg BrainPhyt for 24 weeks | [288] |
| Beta-carotene and Alpha-tocopherol Chemoprevention of Second Primary Malignancies in Head and Neck Cancer Patients | NCT00169845 | Quebec City, Canada | Malignancies in head and neck cancer patients | Interventional (phase 3) | 540 (18 Years and older) | October 2014–March 2018 | Dietary supplement: alpha-tocopherol and beta-carotene (daily supplementation of one capsule of 400 IU dl-alpha-tocopherol) and one capsule of 30 mg beta-carotene (for 3 years after the end of radiation therapy) | [289] |
| Correlation Between Skin Carotenoid Levels and Previous History of Skin Cancer | NCT00836342 | Boston, USA | Skin malignancies, including non- melanoma skin cancer | Observational | 81 (50–75 Years) | February 2009–January 2012 | Mean carotenoid levels in subjects with a history of squamous-cell carcinoma were compared to mean carotenoid levels in control subjects without a history of non-melanoma skin cancer | [290] |
| Lycopene in Treating Patients Undergoing Radical Prostatectomy for Prostate Cancer | NCT00450749 | USA | Prostate cancer | Interventional (phase 3) | 10 males (12 Years and older) | February 2008–May 2010 | Total tissue lycopene concentrations in radical prostatectomy specimens in participants receiving 4–7 weeks of preoperative supplementation with lycopene (60 or 30 mg/day) | [291] |
| The Effect of Astaxanthin on Oxidative Stress Indices in Patients With Polycystic Ovary Syndrome | NCT03991286 | Tehran, Iran | Polycystic ovary syndrome | Interventional | 48 Females (18–40 Years) | January 2020–April 2021 | Dietary supplement: Astaxanthin (8 mg), a 40-day course | [292] |
| Evaluation of the Benefits of FloraGLO™ Lutein on Skin Health | NCT03769779 | Port Chester, New York, USA | Healthy skin elasticity and hydration | Interventional | 60 (females, 30–65 Years) | June 2019–Marh 2020 | Dietary supplement: FloraGLO lutein (20 mg) (after 42 and 84 days of use) | [293] |
| Effects of Isoflavone Combined with Astaxanthin on Skin Aging | NCT02373111 | Seoul, Republic of Korea | Skin photoaging | Interventional | 90 (45 Years and older females) | March 2015–December 2015 | Dietary supplement: isoflavone Dietary supplement: astaxanthin. Each subject takes one tablet per day for 24 weeks. Each tablet contains isoflavone 27 mg and astaxanthin 4 mg | [294] |
| Astaxanthin (2 mg) + Lycopene (1.8 mg) + D-Alpha-Tocopherol (10 IU) For The Treatment Of Skin Aging | NCT03460860 | Manila, Philippines | Healthy skin | Interventional | 100 (Healthy individuals age 30 to 60 years old) | March 2018–July 2019 | Dietary supplement: Astaxanthin (2mg)+Lycopene (1.8mg)+D-Alpha-Tocopherol (10IU): once daily, for 12 weeks | [295] |
| The Effect of 12-Week Dietary Intake of Lutein on Minimal Erythema Dose and Other Skin Parameters (VIST Lutein) | NCT03811977 | Ljubljana, Slovenia | Skin erythema | Interventional | 30 Females (25–55 Years) | March 2019– August 2019 | Dietary supplement: lutein syrup: 12-week dietary supplementation (20 mg lutein/day) | [296] |
| A Study to Evaluate the Effect of Astaxanthin in Healthy Participants | NCT05376501 | Richardson, Texas, USA | Healthy females | Interventional | 60 females (18–50 Years) | June 2022–October 2022 | Dietary supplement: Astaxanthin (8 mg per capsule) Time frame: 4 and 12 weeks | [297] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. https://doi.org/10.3390/ph18030403
Shanaida M, Mykhailenko O, Lysiuk R, Hudz N, Balwierz R, Shulhai A, Shapovalova N, Shanaida V, Bjørklund G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals. 2025; 18(3):403. https://doi.org/10.3390/ph18030403
Chicago/Turabian StyleShanaida, Mariia, Olha Mykhailenko, Roman Lysiuk, Nataliia Hudz, Radosław Balwierz, Arkadii Shulhai, Nataliya Shapovalova, Volodymyr Shanaida, and Geir Bjørklund. 2025. "Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications" Pharmaceuticals 18, no. 3: 403. https://doi.org/10.3390/ph18030403
APA StyleShanaida, M., Mykhailenko, O., Lysiuk, R., Hudz, N., Balwierz, R., Shulhai, A., Shapovalova, N., Shanaida, V., & Bjørklund, G. (2025). Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals, 18(3), 403. https://doi.org/10.3390/ph18030403












