Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications
Abstract
1. Introduction
2. Pharmacotherapeutic Mechanisms of Polyphenols in Main Skin Diseases
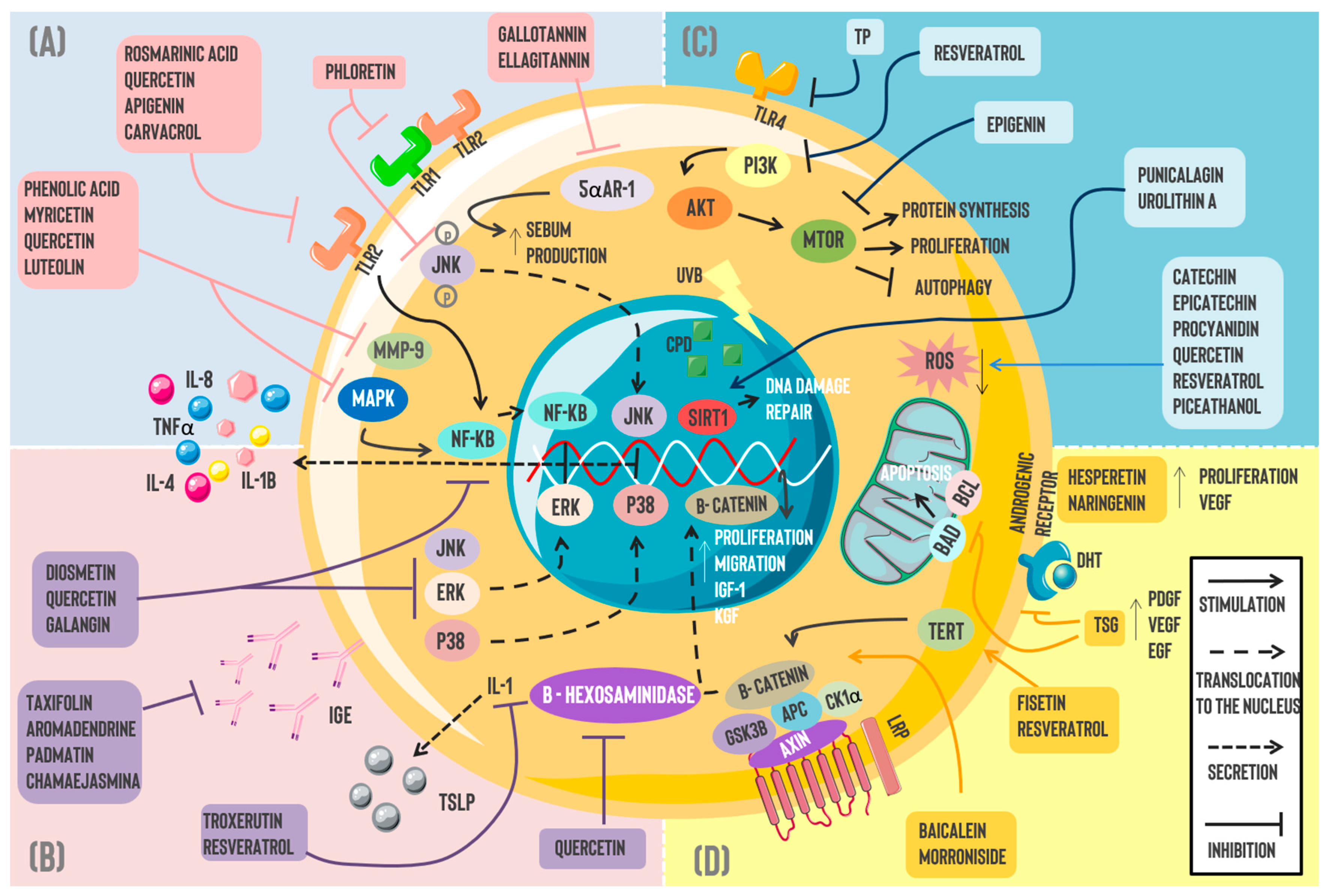
2.1. Acne Vulgaris (AV)
2.2. Dermatitis
2.3. Skin Fungal Infections
2.4. Alopecia
2.5. Skin Cancer
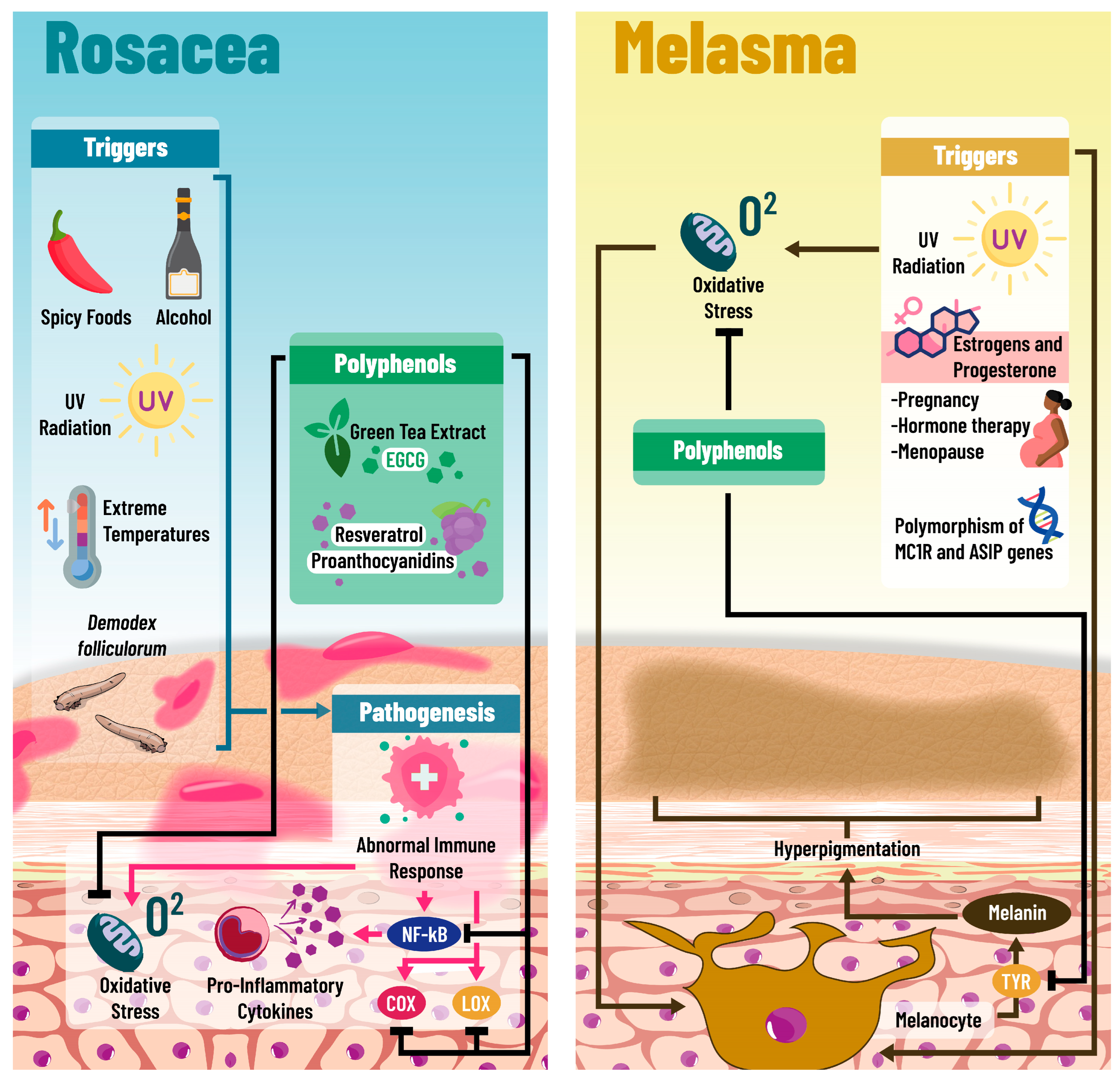
2.6. Rosacea
2.7. Melasma
3. Clinical Evidence of Polyphenols in Dermatological Diseases
4. Bioavailability of Polyphenols and Routes of Administration
5. Natural Components Other than Polyphenols Being Used in Skin Conditions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBD | Global Burden Disease |
| SD | Skin disorders |
| AV | Acne vulgaris |
| ROS | Reactive oxygen species |
| TPCLE | Total phenolic content leaf extract |
| IL-8 | Interleukin-8 |
| IL-1β | Interleukin-1β |
| TNFα | Tumor Necrosis Factor Alpha |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of activated B cells |
| MPP-9 | Matrix Metalloproteinase 9 |
| TLR2 | Toll-like receptor 2 |
| mRNA | Messenger RNA |
| NOS | Nitrous Oxide |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| COX-2 | Cyclooxygenase-2 |
| COX-1 | Cyclooxygenase-1 |
| JNK | c-Jun N-terminal kinase |
| KAS-III | 3-Ketoacyl ACP synthase |
| QML | Quercus mongolica leaf extract |
| PD | Pedunculagin |
| IL-6 | Interleukin-6 |
| PP | Pomegranate peels |
| 5αAR-1 | 5α reductase type 1 |
| BK | Bokusoku |
| AD | Atopic dermatitis |
| TCS | Topical corticosteroids |
| CI | Calcineurin inhibitors |
| IL-4 | Interleukin-4 |
| ERK | Extracellular Signal-Regulated Kinase |
| IgE | Immunoglobulin E |
| TSLP | Thymic Stromal Lymphopoietin |
| TTLE | Tambourissa trichophylla leaf extract |
| AP | Acacia polyphenol |
| STB | Schizonepeta tenuifolia Briquet |
| AOM | Alpinia oxyphylla Miquel |
| CD | Contact dermatitis |
| mTOR | Mammalian Target of Rapamycin |
| TLR4 | Toll-like receptor 4 |
| SIRT1 | Sirtuin 1 |
| IGF-1 | Insulin-like Growth Factor 1 |
| KGF | Keratinocyte growth factor |
| TERT | Telomerase reverse transcriptase |
| VEGF | Vascular Endothelial Growth Factor |
| DPC | Dermal papilla cell |
| PDGF | Platelet-derived Growth Factor |
| EGF | Epidermal Growth Factor |
| DHT | Dihydrotestosterone |
| GA | Gallic acid |
| EA | Ellagic acid |
| CYP51 | Sterol 14α-demethylase P450 |
| SE | Squalane Epoxidase |
| CA | Caffeic Acid |
| LicoA | Licochalcone A |
| FAS | Fatty acid synthase |
| CDK | Cyclin-Dependent Kinase |
| ATP | Adenosine triphosphate |
| MMP | Mitochondrial membrane potential |
| ISO | Isoquercitrin |
| SOD | Superoxide Dismutase |
| 5-LOX | 5-Lipooxygenase |
| HL | Hair loss |
| AGA | Androgenic alopecia |
| HF | Hair follicle |
| TE | Telogen effluvium |
| H2O2 | Hydrogen peroxide |
| ALP | Alkaline phosphatase |
| ORSC | Outer root sheath cells |
| PM | Polygonum multiflorum |
| TSG | 2,3,5,4-Tetrahydroxystilbene-2-O-β-d-glucoside |
| AR | Androgenic receptor |
| UV | Ultraviolet |
| NMSC | Non-Melanoma Skin Cancer |
| BCC | Basal Cell Carcinoma |
| SCC | Squamous cell carcinoma |
| MSC | Melanoma Skin Cancer |
| CPD | Cyclobutane pyrimidine dimer |
| NER | Nucleotide Excision Repair |
| PI3K | Phosphatidylinositol 3 Kinase |
| AKT | Kinase protein B |
| TP | Tea polyphenol |
| ETR | Erythematotelangiectatic Rosacea |
| PPR | Papulopustular Rosacea |
| EGCG | Epigallocatechin gallate |
| RD | Radiation dermatitis |
| INFγ | Interferon-Gamma |
| IL-17 | Interleukin-17 |
| IL-10 | Interleukin-10 |
References
- Ortiz, A.; Herrera, T.; del Molino, C.P.; Piñeiro, F.; Perales, M.L.; Muñoz, P. Epidemiología de las enfermedades dermatologicas en atencion primaria. Rev. Española Salud Pública 1992, 66, 71–82. [Google Scholar]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, M.E.; Delost, G.R. Burden of skin disease and associated socioeconomic status in Europe: An ecologic study from the Global Burden of Disease Study 2017. JAAD Int. 2020, 1, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Cano, C.; Pérez, J.L.; Castro, A.; Díaz, M.P.; Garrido, B.; Carrasquero, R.; Chacín, M.; Velasco, M.; D’marco, L.; et al. Role of Dietary Polyphenols in Adipose Tissue Browning: A Narrative Review. Curr. Pharm. Des. 2020, 26, 4444–4460. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Kosina, P.; Paloncýová, M.; Rajnochová Svobodová, A.; Zálešák, B.; Biedermann, D.; Ulrichová, J.; Vostálová, J. Dermal Delivery of Selected Polyphenols from Silybum marianum. Theoretical and Experimental Study. Molecules 2018, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Chilicka, K.; Gold, M.H.; Nowicka, D. Acne vulgaris and the most popular and new cosmetological treatments. J. Cosmet. Dermatol. 2023, 22, 1946–1950. [Google Scholar] [CrossRef]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef] [PubMed]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.-C.; Kim, Y. Target Proteins of Phloretin for Its Anti-Inflammatory and Antibacterial Activities Against Propionibacterium acnes-Induced Skin Infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, T.-H.; Huang, C.-J.; Li, Y.-Y.; Chyuan, J.-H.; Chuang, L.-T.; Tsai, P.-J. Inhibitory Effects of Wild Bitter Melon Leaf Extract on Propionibacterium acnes-Induced Skin Inflammation in Mice and Cytokine Production In Vitro. Food Funct. 2015, 6, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-T.; Tsai, T.-H.; Lien, T.-J.; Huang, W.-C.; Liu, J.-J.; Chang, H.; Chang, M.-L.; Tsai, P.-J. Ethanolic Extract of Origanum vulgare Suppresses Propionibacterium acnes-Induced Inflammatory Responses in Human Monocyte and Mouse Ear Edema Models. Molecules 2018, 23, 1987. [Google Scholar] [CrossRef] [PubMed]
- Chrząszcz, M.; Miazga-Karska, M.; Klimek, K.; Granica, S.; Tchórzewska, D.; Ginalska, G.; Szewczyk, K. Extracts from Cephalaria Uralensis (Murray) Roem. & Schult. and Cephalaria Gigantea (Ledeb.) Bobrov as Potential Agents for Treatment of Acne Vulgaris: Chemical Characterization and In Vitro Biological Evaluation. Antioxidants 2020, 9, 796. [Google Scholar] [CrossRef]
- Albouchi, F.; Avola, R.; Dico, G.M.L.; Calabrese, V.; Graziano, A.C.E.; Abderrabba, M.; Cardile, V. Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes. Molecules 2018, 23, 2526. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yin, J.; Hwang, I.H.; Park, D.H.; Lee, E.K.; Kim, M.J.; Lee, M.W. Anti-Acne Vulgaris Effects of Pedunculagin from the Leaves of Quercus mongolica by Anti-Inflammatory Activity and 5α-Reductase Inhibition. Molecules 2020, 25, 2154. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63, 3392. [Google Scholar] [CrossRef]
- Koseki, J.; Matsumoto, T.; Matsubara, Y.; Tsuchiya, K.; Mizuhara, Y.; Sekiguchi, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Hattori, T.; et al. Inhibition of Rat 5α-Reductase Activity and Testosterone-Induced Sebum Synthesis in Hamster Sebocytes by an Extract of Quercus acutissima Cortex. Evid.-Based Complement. Alternat. Med. 2015, 2015, 853846. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, H.J.; Ryu, J.-H. Prenylated Polyphenols from Broussonetia kazinoki as Inhibitors of Nitric Oxide Production. Molecules 2018, 23, 639. [Google Scholar] [CrossRef]
- Tanei, R. Atopic Dermatitis in Older Adults: A Review of Treatment Options. Drugs Aging 2020, 37, 149–160. [Google Scholar] [CrossRef]
- Bridgman, A.C.; Qureshi, A.; Li, T.; Tabung, F.K.; Cho, E.; Drucker, A.M. Inflammatory dietary pattern and incident psoriasis, psoriatic arthritis, and atopic dermatitis in women: A cohort study. J. Am. Acad. Dermatol. 2019, 80, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Kingkaw, A.; Nakphaichit, M.; Suratannon, N.; Nitisinprasert, S.; Wongoutong, C.; Chatchatee, P.; Krobthong, S.; Charoenlappanit, S.; Roytrakul, S.; Vongsangnak, W. Analysis of the infant gut microbiome reveals metabolic functional roles associated with healthy infants and infants with atopic dermatitis using metaproteomics. PeerJ 2020, 8, e9988. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Kang, Y.-M.; Eom, Y.-J.; Hong, C.-H.; An, H.-J. Anti-Atopic Dermatitis Effect of Seaweed Fulvescens Extract via Inhibiting the STAT1 Pathway. Mediat. Inflamm. 2019, 2019, 3760934. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.K.; Choi, J.; Jang, H.; Seol, J.W. Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int. Immunopharmacol. 2020, 89, 107046. [Google Scholar] [CrossRef]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Jegal, J.; Park, N.-J.; Kim, T.-Y.; Choi, S.; Lee, S.W.; Hang, J.; Kim, S.-N.; Yang, M.H. Effect of Topically Applied Wikstroemia dolichantha Diels on the Development of Atopic Dermatitis-Like Skin Symptoms in Mice. Nutrients 2019, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef] [PubMed]
- Fitoussi, J.; Virassamynaïk, S.; Callejon, S.; Weber, S.; Collet, E.; Scalia, J.; Chavagnac-Bonneville, M.; Trompezinski, S.; Sayag, M. Inhibition of thymic stromal lymphopoietin production to improve pruritus and quality of life in infants and children with atopic dermatitis. J. Cosmet. Dermatol. 2020, 19, 2061–2069. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesions through effects on the epithelium. PeerJ 2016, 4, e1889. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Fujitate, N.; Togashi, T.; Takayama, N.; Fukuda, N.; Kon, R.; Sakai, H.; Kamei, J.; Sugiyama, K. Acacia Polyphenol Ameliorates Atopic Dermatitis in Trimellitic Anhydride-Induced Model Mice via Changes in the Gut Microbiota. Foods 2020, 9, 773. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, J.; Wu, X.; Huang, S.; Yuan, H.; Park, S. Schizonepeta tenuifolia with Alpinia oxyphylla Alleviates Atopic Dermatitis and Improves the Gut Microbiome in Nc/Nga Mice. Pharmaceutics 2020, 12, 722. [Google Scholar] [CrossRef]
- Jegal, J.; Park, N.-J.; Lee, S.-Y.; Jo, B.-G.; Bong, S.-K.; Kim, S.-N.; Yang, M.H. Quercitrin, the Main Compound in Wikstroemia indica, Mitigates Skin Lesions in a Mouse Model of 2,4-Dinitrochlorobenzene-Induced Contact Hypersensitivity. Evid.-Based Complement. Altern. Med. 2020, 2020, 4307161. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Cho, S.-H.; Youn, S.-K.; Park, J.-S.; Choi, J.T.; Bak, Y.-S.; Yu, Y.-B.; Kim, Y.K. Epidemiological Characterization of Skin Fungal Infections Between the Years 2006 and 2010 in Korea. Osong Public Health Res. Perspect. 2015, 6, 341–345. [Google Scholar] [CrossRef] [PubMed]
- White, T.C.; Findley, K.; Dawson, T.L.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef]
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Med. 2015, 25, e44–e58. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Rossi, A. Pathogenesis of Dermatophytosis: Sensing the Host Tissue. Mycopathologia 2017, 182, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal Activity of Ellagic Acid In Vitro and In Vivo. Phytother. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef]
- Cantelli, B.A.M.; Bitencourt, T.A.; Komoto, T.T.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Caffeic acid and licochalcone A interfere with the glyoxylate cycle of Trichophyton rubrum. Biomed. Pharmacother. 2017, 96, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Leibundgut, M.; Boehringer, D.; Ban, N. Structure and function of eukaryotic fatty acid synthases. Q. Rev. Biophys. 2010, 43, 373–422. [Google Scholar] [CrossRef]
- Da Silva, C.R.; De Andrade-Neto, J.B.; Campos, R.D.S.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; De Andrade, G.M.; Lima, I.S.P.; et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complement. Altern. Med. 2013, 13, 229. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; TakahasiKomoto, T.; Marins, M.; Fachin, A.L. Antifungal activity of flavonoids and modulation of expression of genes of fatty acid synthesis in the dermatophyte Trichophyton rubrum. BMC Proc. 2014, 8 (Suppl. S4), 53. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Macedo, C.; Franco, M.E.; Rocha, M.C.; Moreli, I.S.; Cantelli, B.A.M.; Sanches, P.R.; Beleboni, R.O.; Malavazi, I.; Passos, G.A.; et al. Trans-chalcone activity against Trichophyton rubrum relies on an interplay between signaling pathways related to cell wall integrity and fatty acid metabolism. BMC Genom. 2019, 20, 411. [Google Scholar] [CrossRef]
- Gaziano, R.; Campione, E.; Iacovelli, F.; Marino, D.; Pica, F.; Di Francesco, P.; Aquaro, S.; Menichini, F.; Falconi, M.; Bianchi, L. Antifungal activity of Cardiospermum halicacabum L. (Sapindaceae) against Trichophyton rubrum occurs through molecular interaction with fungal Hsp90. Drug Des. Dev. Ther. 2018, 12, 2185–2193. [Google Scholar] [CrossRef]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal Activity and Mechanism of Action of Ou-gon (Scutellaria Root Extract) Components Against Pathogenic Fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Oliveira, A.P.; Cunha, D.; Pereira, D.M.; Valentão, P.; Pinto, E.; Araújo, L.; Andrade, P.B. Flavonoid Composition of Salacia senegalensis (Lam.) DC. Leaves, Evaluation of Antidermatophytic Effects, and Potential Amelioration of the Associated Inflammatory Response. Molecules 2019, 24, 2530. [Google Scholar] [CrossRef]
- Tosti, A.; Gray, J. Assessment of Hair and Scalp Disorders. J. Investig. Dermatol. Symp. Proc. 2007, 12, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.; Parthasaradhi, A.; Kharkar, R.; Ghoshal, A.; Ganjoo, A.; Ravichandran, G.; Saraswat, A.; Shah, Y.; Singh, M.; Remadevi, T.; et al. Expert consensus on the management of androgenetic alopecia in India. Int. J. Trichology 2019, 11, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef]
- Malkud, S. Telogen Effluvium: A Review. J. Clin. Diagn. Res. 2015, 9, WE01–WE03. [Google Scholar] [CrossRef] [PubMed]
- Santos, Z.; Avci, P.; Hamblin, M.R. Drug discovery for alopecia: Gone today, hair tomorrow. Expert Opin. Drug Discov. 2015, 10, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 2007, 14, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Perez, C.I.; Schachner, L.A.; Jimenez, J.J. Prevention and treatment of alopecia areata with quercetin in the C3H/HeJ mouse model. Cell. Stress. Chaperones 2012, 17, 267–274. [Google Scholar] [CrossRef]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124 Pt 8, 1179–1182. [Google Scholar] [CrossRef]
- Madaan, A.; Joshi, V.; Kishore, A.; Verma, R.; Singh, A.; Jaggi, M.; Sung, Y.K. In vitro Hair Growth Promoting Effects of Naringenin and Hesperetin on Human Dermal Papilla Cells and Keratinocytes. Am. J. Dermatol. Venereol. 2017, 6, 51–57. [Google Scholar]
- Xing, F.; Yi, W.J.; Miao, F.; Su, M.Y.; Lei, T.C. Baicalin increases hair follicle development by increasing canonical Wnt/β-catenin signaling and activating dermal papillar cells in mice. Int. J. Mol. Med. 2018, 41, 2079–2085. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Morroniside regulates hair growth and cycle transition via activation of the Wnt/β-catenin signaling pathway. Sci. Rep. 2018, 8, 13785. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.-H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef]
- Kubo, C.; Ogawa, M.; Uehara, N.; Katakura, Y. Fisetin Promotes Hair Growth by Augmenting TERT Expression. Front. Cell Dev. Biol. 2020, 8, 566617. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global incidence and mortality of skin cancer by histological subtype and its relationship with the human development index (HDI); an ecology study in 2018. World Cancer Res. J. 2019, 6, e1265. [Google Scholar] [CrossRef]
- Fahradyan, A.; Howell, A.C.; Wolfswinkel, E.M.; Tsuha, M.; Sheth, P.; Wong, A.K. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Mohammadbeigi, A.; Khazaei, S.; Veisani, Y.; Delpisheh, A.; Jenabi, E. Global inequality in the incidence and mortality rate of melanoma skin cancer according to human development index: A country-level analysis. Egypt. J. Dermatol. Venerol. 2021, 41, 26. [Google Scholar] [CrossRef]
- Chen, J.T.; Kempton, S.J.; Rao, V.K.M. The Economics of Skin Cancer: An Analysis of Medicare Payment Data. Plast. Reconstr. Surg. Glob. Open 2016, 4, e868. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Chen, X.; Chang, L.; Qu, Y.; Liang, J.; Jin, W.; Xia, X. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int. J. Immunopathol. Pharmacol. 2018, 32, 394632017739531. [Google Scholar] [CrossRef] [PubMed]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef]
- Ikehata, H.; Mori, T.; Kamei, Y.; Douki, T.; Cadet, J.; Yamamoto, M. Wavelength- and Tissue-dependent Variations in the Mutagenicity of Cyclobutane Pyrimidine Dimers in Mouse Skin. Photochem. Photobiol. 2020, 96, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.; Matsuo, H.; Onoue, S.; Yamamoto, H.; Ito, H.; Katakura, Y. Identification of polyphenols that repair the ultraviolet-B-induced DNA damage via SIRT1-dependent XPC/XPA activation. J. Funct. Foods 2019, 54, 119–127. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef]
- Rossi, Y.E.; Bohl, L.P.; Braber, N.L.V.; Ballatore, M.B.; Escobar, F.M.; Bodoira, R.; Maestri, D.M.; Porporatto, C.; Cavaglieri, L.R.; Montenegro, M.A. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interaction with ROS. J. Funct. Foods 2020, 67, 103862. [Google Scholar] [CrossRef]
- Eskandari, M.; Rembiesa, J.; Startaitė, L.; Holefors, A.; Valančiūtė, A.; Faridbod, F.; Ganjali, M.R.; Engblom, J.; Ruzgas, T. Polyphenol-hydrogen peroxide reactions in skin: In vitro model relevant to study ROS reactions at inflammation. Anal. Chim. Acta 2019, 1075, 91–97. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention. Cell. Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Yadav, S.A. Synergistic inhibitory actions of resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: A combinational drug approach. Med. Oncol. 2024, 41, 64. [Google Scholar] [CrossRef]
- Zujko-Kowalska, K.; Masłowska, J.; Knaś-Dawidziuk, M.; Hamulka, J.; Zujko, M.E. Dietary Antioxidants May Support Cosmetic Treatment in Patients with Rosacea. Antioxidants 2024, 13, 381. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and Diet: What is New in 2021? J. Clin. Aesthet. Dermatol. 2021, 14, 49–54. [Google Scholar]
- Paiva-Santos, A.C.; Gonçalves, T.; Peixoto, D.; Pires, P.C.; Velsankar, K.; Jha, N.K.; Chavda, V.P.; Mohammad, I.S.; Cefali, L.C.; Mazzola, P.G.; et al. Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems. Mol. Pharm. 2023, 20, 3804–3828. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Wang, Y.; Fang, R.; Sun, Q. Rosacea Treatment: Review and Update. Dermatol. Ther. 2021, 11, 13–24. [Google Scholar] [CrossRef]
- Maden, S. Epidermal Skin Barrier and Skin Care in Rosacea: A Narrative Review. Dermis 2024, 4, 15. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Nasca, M.R.; Gerbino, C.; Micali, G. Advances in pharmacotherapy for rosacea: What is the current state of the art? Expert Opin. Pharmacother. 2022, 23, 1845–1854. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef] [PubMed]
- Artzi, O.; Horovitz, T.; Bar-Ilan, E.; Shehadeh, W.; Koren, A.; Zusmanovitch, L.; Mehrabi, J.N.; Salameh, F.; Nelkenbaum, G.I.; Zur, E.; et al. The pathogenesis of melasma and implications for treatment. J. Cosmet. Dermatol. 2021, 20, 3432–3445. [Google Scholar] [CrossRef]
- Doolan, B.J.; Gupta, M. Melasma. Aust. J. Gen. Pract. 2021, 50, 880–885. [Google Scholar] [CrossRef]
- Cassiano, D.P.; Espósito, A.C.C.; Da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Miot, L.D.B.; Miot, H.A.; Bagatin, E. Update on Melasma—Part II: Treatment. Dermatol. Ther. 2022, 12, 1989–2012. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Bs, K.M.B.; Babbush, R.A.; Khachemoune, A. Treatment of melasma: A review of less commonly used antioxidants. Int. J. Dermatol. 2021, 60, 166–173. [Google Scholar] [CrossRef]
- AlSalem, S.; Alexis, A. Melasma hyperpigmentation: An overview of current topical therapeutics. Dermatol. Rev. 2022, 4, 38–52. [Google Scholar] [CrossRef]
- Kim, S.; Park, T.H.; Kim, W.I.; Park, S.; Kim, J.H.; Cho, M.K. The effects of green tea on acne vulgaris: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 35, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Waranuch, N.; Phimnuan, P.; Yakaew, S.; Nakyai, W.; Grandmottet, F.; Onlom, C.; Srivilai, J.; Viyoch, J. Antiacne and antiblotch activities of a formulated combination of Aloe barbadensis leaf powder, Garcinia mangostana peel extract, and Camellia sinensis leaf extract. Clin. Cosmet. Investig. Dermatol. 2019, 12, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kanoya, Y.; Nagata, S. Effects of a foot bath containing green tea polyphenols on interdigital tinea pedis. Foot 2013, 23, 58–62. [Google Scholar] [CrossRef]
- Loing, E.; Lachance, R.; Ollier, V.; Hocquaux, M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J. Cosmet. Sci. 2013, 64, 45–58. [Google Scholar]
- Takahashi, T.; Kamimura, A.; Kagoura, M.; Toyoda, M.; Morohashi, M. Investigation of the topical application of procyanidin oligomers from apples to identify their potential use as a hair-growing agent. J. Cosmet. Dermatol. 2005, 4, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Mehrbani, M.; Choopani, R.; Fekri, A.; Mehrabani, M.; Mosaddegh, M.; Mehrabani, M. The efficacy of whey associated with dodder seed extract on moderate-to-severe atopic dermatitis in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2015, 172, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, W.; Zhao, X.; Li, X.; Zhou, Z.; Zheng, M.; Meng, X.; Kong, L.; Zhang, S.; He, D.; et al. Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients with Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 779. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Noaimi, A.A.; Al-Salih, M.M. Topical therapy of acne vulgaris using 2% tea lotion in comparison with 5% zinc sulphate solution. Saudi Med. J. 2008, 29, 1757–1761. [Google Scholar] [PubMed]
- Sharquie, K.E.; Al-Turfi, I.A.; Al-Shimary, W.M. Treatment of acne vulgaris with 2% topical tea lotion. Saudi Med. J. 2006, 27, 83–85. [Google Scholar] [PubMed]
- Elsaie, M.L.; Abdelhamid, M.F.; Elsaaiee, L.T.; Emam, H.M. The efficacy of topical 2% green tea lotion in mild-to-moderate acne vulgaris. J. Drugs Dermatol. 2009, 8, 358–364. [Google Scholar] [PubMed]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Khan, H.M.S.; Saeed, T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn. J. Basic Med. Sci. 2010, 10, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Ha, S.; Son, J.-A.; Song, J.H.; Houh, Y.; Cho, E.; Chun, J.H.; Yoon, S.R.; Yang, Y.; Ban, S.I.; et al. Polyphenon-60 displays a therapeutic effect on acne by suppression of TLR2 and IL-8 expression via down-regulating the ERK1/2 pathway. Arch. Dermatol. Res. 2012, 304, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Lu, P.; Hsu, C. Does supplementation with green tea extract improve acne in post-adolescent women? A randomized, double-blind, and placebo-controlled clinical trial. Complement. Ther. Med. 2016, 25, 159–163. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Moldovan, C. A comparison of the effects of topical green tea and lotus on facial sebum control in healthy humans. Hippokratia 2013, 17, 64–67. [Google Scholar]
- Seok, J.; Kim, T.S.; Kwon, H.J.; Lee, S.P.; Kang, M.H.; Kim, B.J.; Kim, M.N. Efficacy of Cistanche Tubulosa and Laminaria Japonica Extracts (MK-R7) Supplement in Preventing Patterned Hair Loss and Promoting Scalp Health. Clin. Nutr. Res. 2015, 4, 124–131. [Google Scholar] [CrossRef]
- Kamimura, A.; Takahashi, T.; Watanabe, Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine 2000, 7, 529–536. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, P.; Joshi, S.C. Topical Treatment of Dermatophytic Lesion on Mice (Mus musculus) Model. Indian J. Microbiol. 2011, 51, 217–222. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Rojas, G.; Navarro, V.; Herrera-Arellano, A.; Zamilpa-Álvarez, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: An explorative pilot study controlled with ketoconazole. Planta Medica 2006, 72, 1257–1261. [Google Scholar] [CrossRef]
- Herrera-Arellano, A.; Rodríguez-Soberanes, A.; de los Angeles Martínez-Rivera, M.; Martínez-Cruz, E.; Zamilpa, A.; Alvarez, L.; Tortoriello, J. Effectiveness and tolerability of a standardized phytodrug derived from Solanum chrysotrichum on Tinea pedis: A controlled and randomized clinical trial. Planta Med. 2003, 69, 390–395. [Google Scholar] [PubMed]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of an encecalin standardized extract of Ageratina pichinchensis on the treatment of onychomycosis in patients with diabetes mellitus. Phytother. Res. 2020, 34, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; Jiménez-Ferrer, E.; Vega-Pimentel, A.M.; de los Angeles Martínez-Rivera, M.; Hernández-Hernández, M.; Zamilpa, A.; Tortoriello, J. Clinical and mycological evaluation of therapeutic effectiveness of Solanum chrysotrichum standardized extract on patients with Pityriasis capitis (dandruff). A double blind and randomized clinical trial controlled with ketoconazole. Planta Med. 2004, 70, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ngatu, N.R.; Saruta, T.; Hirota, R.; Eitoku, M.; Luzitu, N.S.; Muzembo, B.A.; Matsui, T.; Suganuma, N. Brazilian green propolis extracts improve Tinea pedis interdigitalis and Tinea corporis. J. Altern. Complement. Med. 2012, 18, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Ngatu, N.R.; Saruta, T.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Matsui, T.; Nangana, L.S.; Mbenza, M.A.; Kumagai, N.; Suganuma, N. Antifungal efficacy of Brazilian green propolis extracts and honey on Tinea capitis and Tinea versicolor. Eur. J. Integr. Med. 2011, 3, e281–e287. [Google Scholar] [CrossRef]
- Obot, M.J.; Aluyi, H.S. Treatment of superficial mycoses with Ocimum gratissimum. Int. J. Infect. Dis. 2002, 6, 151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oyelami, O.A.; Onayemi, O.; Oladimeji, F.A.; Ogundaini, A.O.; Olugbade, T.A.; Onawunmi, G.O. Clinical evaluation of Acalypha ointment in the treatment of superficial fungal skin diseases. Phytother. Res. 2003, 17, 555–557. [Google Scholar] [CrossRef]
- Carmo, E.S.; Pereira, F.d.O.; Cavalcante, N.M.; Gayoso, C.W.; Lima, E.d.O. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf—Therapeutic pilot study. An. Bras. Dermatol. 2013, 88, 381–385. [Google Scholar] [CrossRef]
- Damodaran, S.; Venkataraman, S. A study on the therapeutic efficacy of Cassia alata, Linn. leaf extract against Pityriasis versicolor. J. Ethnopharmacol. 1994, 42, 19–23. [Google Scholar] [CrossRef]
- Buck, D.S.; Nidorf, D.M.; Addino, J.G. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J. Fam. Pract. 1994, 38, 601–605. [Google Scholar]
- Patrizi, A.; Raone, B.; Neri, I.; Gurioli, C.; Carbonara, M.; Cassano, N.; Vena, G.A. Randomized, controlled, double-blind clinical study evaluating the safety and efficacy of MD2011001 cream in mild-to-moderate atopic dermatitis of the face and neck in children, adolescents and adults. J. Dermatol. Treat. 2016, 27, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Brasiel, P.G.d.A.; Guimarães, F.V.; Rodrigues, P.M.; Bou-Habib, D.C.; Carvalho, V.d.F. Therapeutic Efficacy of Flavonoids in Allergies: A Systematic Review of Randomized Controlled Trials. J. Immunol. Res. 2022, 2022, 8191253. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef]
- Xie, J.; Jia, L.; Xie, P.; Yin, X.; Zhu, W.; Zhao, H.; Wang, X.; Meng, X.; Xing, L.; Zhao, H.; et al. Efficacy and safety of epigallocatechin-3-gallate in treatment acute severe dermatitis in patients with cancer receiving radiotherapy: A phase I clinical trial. Sci. Rep. 2023, 13, 13865. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Hosseini, S.; Elyasi, S.; Pakdel, A.F.; Karimi, G. Topical silymarin administration for prevention of acute radiodermatitis in breast cancer patients: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 379–386. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E.; Magrone, M.; Russo, M.A.; Romita, P.; Massari, F.; Foti, C. Red Grape Polyphenol Oral Administration Improves Immune Response in Women Affected by Nickel-Mediated Allergic Contact Dermatitis. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 374–384. [Google Scholar] [CrossRef]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.R.; da Silva, D.V.T.; Baião, D.d.S.; Paschoalin, V.M.F. Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases. Molecules 2021, 26, 4621. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial lipid-nanosystem for dermal delivery of 5-fluorouracil and resveratrol against skin cancer: Delineation of improved dermatokinetics and epidermal drug deposition enhancement analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Menaa, B. Polyphenols Nano-Formulations for Topical Delivery and Skin Tissue Engineering. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 839–848. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured lipidic carriers for dual drug delivery in the management of psoriasis: Systematic optimization, dermatokinetic and preclinical evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Hasan, N.; Imran, M.; Nadeem, M.; Jain, D.; Haider, K.; Alam Rizvi, M.M.; Sheikh, A.; Kesharwani, P.; Jain, G.K.; Ahmad, F.J. Formulation and development of novel lipid-based combinatorial advanced nanoformulation for effective treatment of non-melanoma skin cancer. Int. J. Pharm. 2023, 632, 122580. [Google Scholar] [CrossRef]
- Gupta, N.K.; Dixit, V.K. Development and evaluation of vesicular system for curcumin delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Trepa, M.; Sułkowska-Ziaja, K.; Kała, K.; Muszyńska, B. Therapeutic Potential of Fungal Terpenes and Terpenoids: Application in Skin Diseases. Molecules 2024, 29, 1183. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, Z.D.; Aligiannis, N.; Graikou, K.; Bilia, A.R.; Kolašinac, S.; Kilibarda, S. Bioactivity of carotenoids and their role in skin disorders: From tradition to application. Maced. Pharm. Bull. 2022, 68, 149–150. [Google Scholar] [CrossRef]
- Maurya, S.K.; Divakar, S.; Patil, U.K. Potentials of plant derived products for the treatment of skin disorders. Ger. J. Pharm. Biomater. 2023, 2, 9–31. [Google Scholar] [CrossRef]
- Park, J.; Nguyen, T.M.N.; Park, H.-A.; Nguyen, M.T.T.; Lee, N.-Y.; Ban, S.-Y.; Park, K.-B.; Lee, C.-K.; Kim, J.; Park, J.-T. Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. IJMS 2023, 24, 12803. [Google Scholar] [CrossRef]
- Yan, Z.F.; Yang, Y.; Tian, F.H.; Mao, X.X.; Li, Y.; Li, C.T. Inhibitory and Acceleratory Effects of Inonotus obliquus on Tyrosinase Activity and Melanin Formation in B16 Melanoma Cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 259836. [Google Scholar] [CrossRef]
- Kreuzenbeck, N.B.; Dhiman, S.; Roman, D.; Burkhardt, I.; Conlon, B.H.; Fricke, J.; Guo, H.; Blume, J.; Görls, H.; Poulsen, M.; et al. Isolation, (bio)synthetic studies and evaluation of antimicrobial properties of drimenol-type sesquiterpenes of Termitomyces fungi. Commun. Chem. 2023, 6, 79. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef]
- Ren, Q.; Lu, X.-Y.; Han, J.-X.; Aisa, H.A.; Yuan, T. Triterpenoids and phenolics from the fruiting bodies of Inonotus hispidus and their activations of melanogenesis and tyrosinase. Chin. Chem. Lett. 2017, 28, 1052–1056. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- I Ibiebele, T.; van der Pols, J.C.; Hughes, M.C.; Marks, G.C.; Williams, G.M.; Green, A.C. Dietary pattern in association with squamous cell carcinoma of the skin: A prospective study. Am. J. Clin. Nutr. 2007, 85, 1401–1408. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Melchi, F.; A Pilla, M.; Antonelli, G.; Camaioni, D.; Alotto, M.; Pasquini, P. A protective effect of the Mediterranean diet for cutaneous melanoma. Leuk. Res. 2008, 37, 1018–1029. [Google Scholar] [CrossRef]
- Heinen, M.M.; Hughes, M.C.; Ibiebele, T.I.; Marks, G.C.; Green, A.C.; van der Pols, J.C. Intake of antioxidant nutrients and the risk of skin cancer. Eur. J. Cancer 2007, 43, 2707–2716. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study. Ski. Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Heinrich, U.; Wiebusch, M.; Tronnier, H.; Gärtner, C.; Eichler, O.; Sies, H.; Stahl, W. Supplementation with β-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Biskanaki, F.; Kalofiri, P.; Tertipi, N.; Sfyri, E.; Andreou, E.; Kefala, V.; Rallis, E. Carotenoids and Dermoaesthetic Benefits: Public Health Implications. Cosmetics 2023, 10, 120. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Żmudzki, P.; Podolak, I. Anti-melanoma potential of two benzoquinone homologues embelin and rapanone—A comparative in vitro study. Toxicol. In Vitro 2020, 65, 104826. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Laila, S.P.; Fernandez, A. A Fluoro Derivative of Embelin, as Potent B-RAF Inhibitor in Melanoma. Anti-Cancer Agents Med. Chem. 2021, 21, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.J.; Chandy, A.S.; Joseph, A.A.; Gorantla, J.N.; Donadkar, A.D.; Nath, L.R.; Sharifi-Rad, J.; Calina, D. Embelin: A multifaceted anticancer agent with translational potential in targeting tumor progression and metastasis. EXCLI J. 2023, 22, 1311–1329. [Google Scholar] [CrossRef]
| Author | Skin Disorder | Methodology | Results |
|---|---|---|---|
| Kim et al. [93] | Acne vulgaris | Meta-analysis with five randomized controlled clinical trials evaluated the efficacy and safety of green tea extract for treating acne. | Green tea extract significantly reduced the number of inflammatory lesions (−9.38; 95% CI: −14.13 to −4.63), inflammatory lesion counts (−11.39; 95% CI: −15.91 to −6.86), and non-inflammatory acne lesions (−32.44; 95% CI: −39.27 to −25.62). |
| Waranuch et al. [94] | Acne vulgaris | A single-center, parallel, randomized controlled trial assessed the anti-acne and anti-blotch activity of a hydrogel formulated with a combination of Aloe barbadensis leaf extract and Garcinia mangostana peel extract. | There was a reduction in total acne lesions (p < 0.0001) and mean acne severity index. Also, a decrease in skin redness (p < 0.05) and in mean melanin value (p = 0.037) was found. |
| Ikeda et al. [95] | Skin fungal infection | A double-blind, randomized, placebo-controlled trial evaluating the effects of a foot bath containing green tea polyphenols in patients with interdigital tinea pedis. | The use of a foot bath containing green tea polyphenols for 12 weeks produced a significant reduction in the size of the affected area (p < 0.001). |
| Loing et al. [96] | Alopecia | A randomized, placebo-controlled study assessed the efficacy of Trifolium pratense flower extract and a biomimetic peptide in alopecia. | The anagen/telogen (A/T) ratio increased by +46%, anagen hair increased at an average of +13%, and telogen hair density decreased by −29% after 4 months of treatment. |
| Takahashi et al. [97] | Alopecia | A double-blind, randomized, placebo-controlled trial evaluated the efficacy of the external application of 0.7% apple procyanidin oligomers in patients with pattern baldness. | There was a significant increase in total number of hair (procyanidin, 3.3 ± 13.0 (mean ± SD)/0.50 cm2; placebo, −3.6 ± 8.1/0.50 cm2; p < 0.001, two-sample t-test). |
| Mehrbani et al. [98] | Atopic Dermatitis | A double-blind, randomized, placebo, controlled trial assessed the effect of powdered lyophilized milk serum with cuscuta extract to treat AD. | A marked increase in skin humidity and elasticity was observed (p < 0.001), along with diminished pruritus (p < 0.05) and sleep disturbances (p < 0.05). |
| Zhao et al. [99] | Radiation Dermatitis | A phase II, double-blind, randomized, controlled clinical trial was performed to determine the therapeutic and preventive properties of EGCG on RD patients with breast cancer treated with post-operatory radiotherapy. | EGCG significantly reduced the incidence, severity, and clinical manifestations of RD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, J.; Ortega, Á.; Pérez, J.L.; Garrido, B.; Santeliz, R.; Galbán, N.; Díaz, M.P.; Cano, R.; Cano, G.; Contreras-Velasquez, J.C.; et al. Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications. Pharmaceuticals 2025, 18, 247. https://doi.org/10.3390/ph18020247
Salazar J, Ortega Á, Pérez JL, Garrido B, Santeliz R, Galbán N, Díaz MP, Cano R, Cano G, Contreras-Velasquez JC, et al. Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications. Pharmaceuticals. 2025; 18(2):247. https://doi.org/10.3390/ph18020247
Chicago/Turabian StyleSalazar, Juan, Ángel Ortega, José Luis Pérez, Bermary Garrido, Raquel Santeliz, Néstor Galbán, Maria Paula Díaz, Raquel Cano, Gabriel Cano, Julio Cesar Contreras-Velasquez, and et al. 2025. "Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications" Pharmaceuticals 18, no. 2: 247. https://doi.org/10.3390/ph18020247
APA StyleSalazar, J., Ortega, Á., Pérez, J. L., Garrido, B., Santeliz, R., Galbán, N., Díaz, M. P., Cano, R., Cano, G., Contreras-Velasquez, J. C., & Chacín, M. (2025). Role of Polyphenols in Dermatological Diseases: Exploring Pharmacotherapeutic Mechanisms and Clinical Implications. Pharmaceuticals, 18(2), 247. https://doi.org/10.3390/ph18020247









