Regulation of Intestinal Barrier Function and Gut Microbiota by Hot Melt Extrusion-Drug Delivery System-Prepared Mulberry Anthocyanin in an Inflammatory Bowel Disease Model
Abstract
1. Introduction
2. Results and Discussion
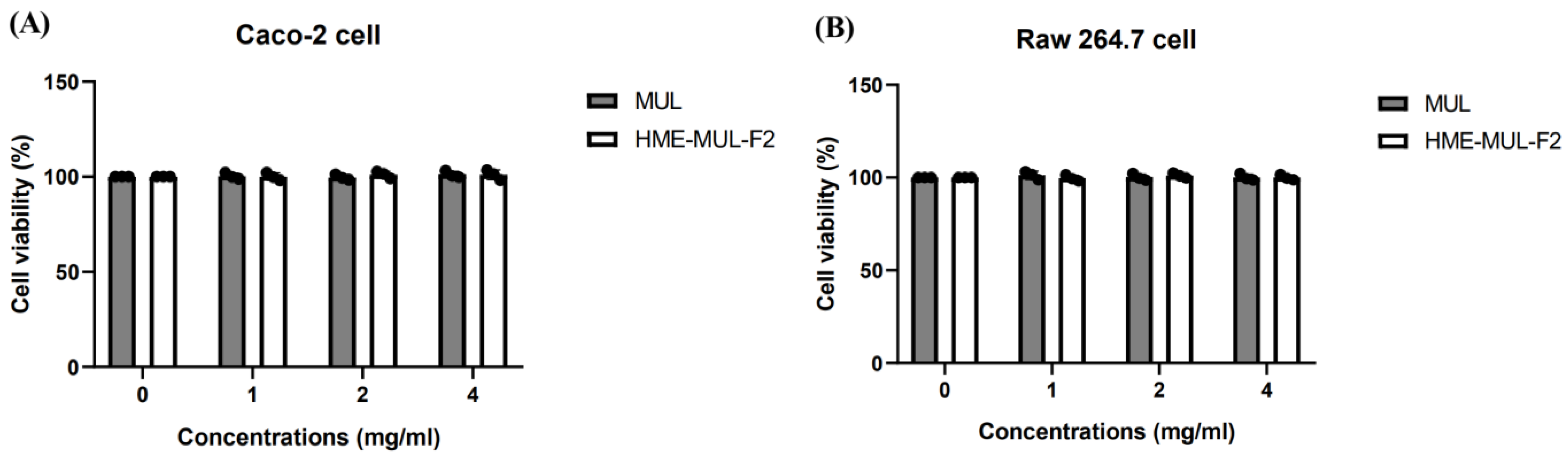
2.1. Toxicity of HME-MUL-F2 to Caco-2 and RAW 264.7 Cells
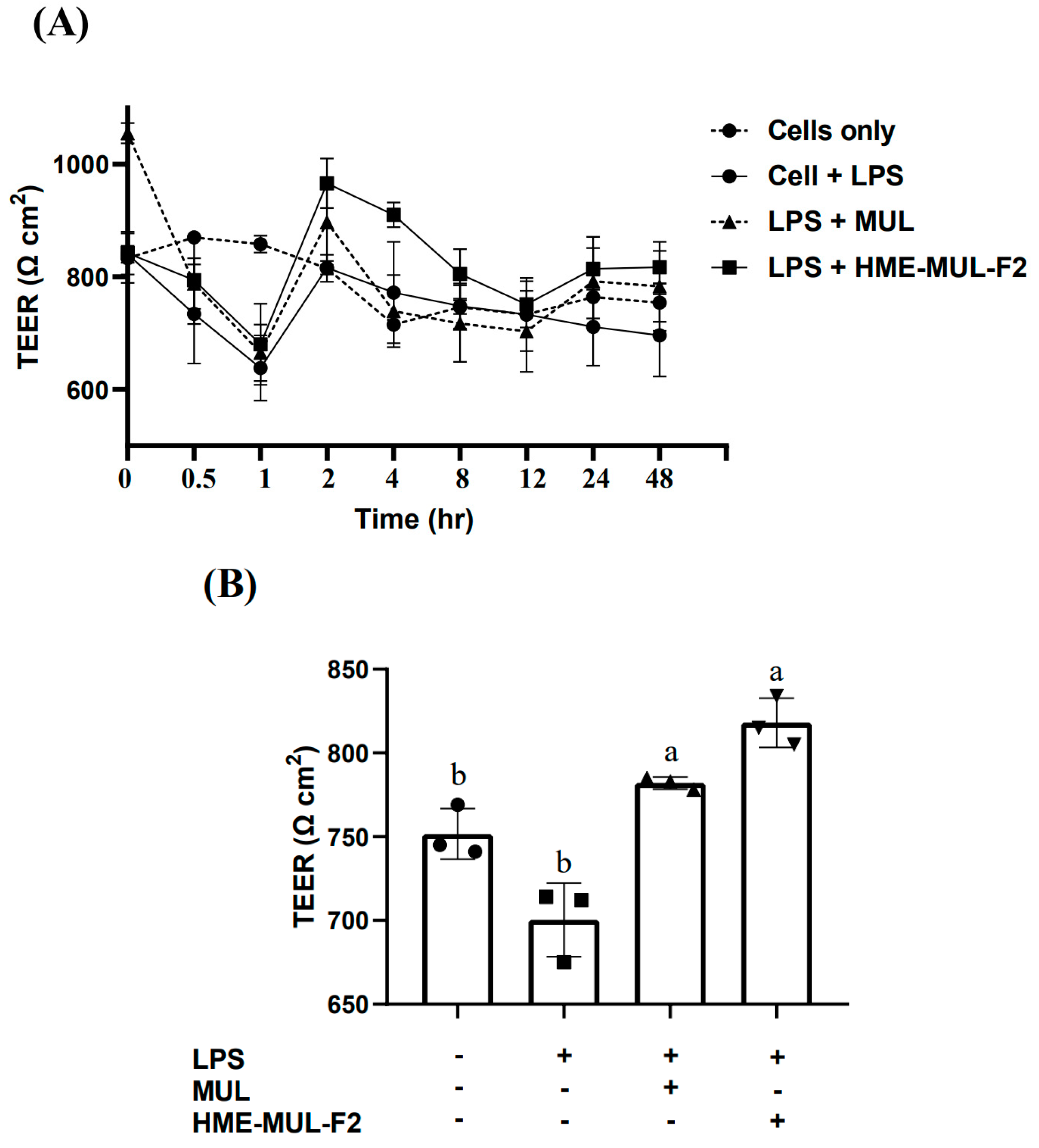
2.2. Effect of HME-MUL-F2 on Tight Junction Integrity of Caco-2 Cells
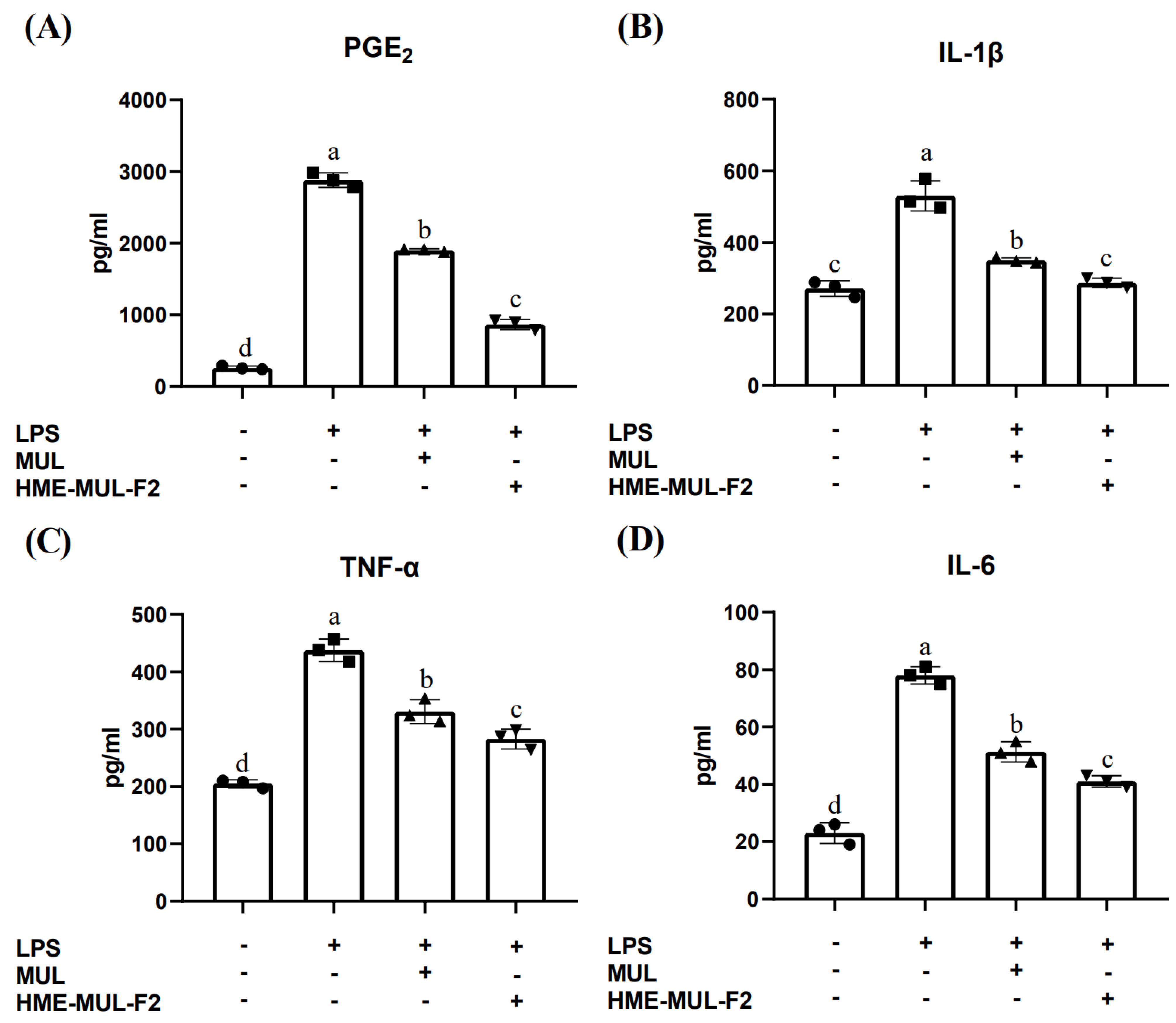
2.3. Effect of HME-MUL-F2 on Inhibition of Cytokine Production
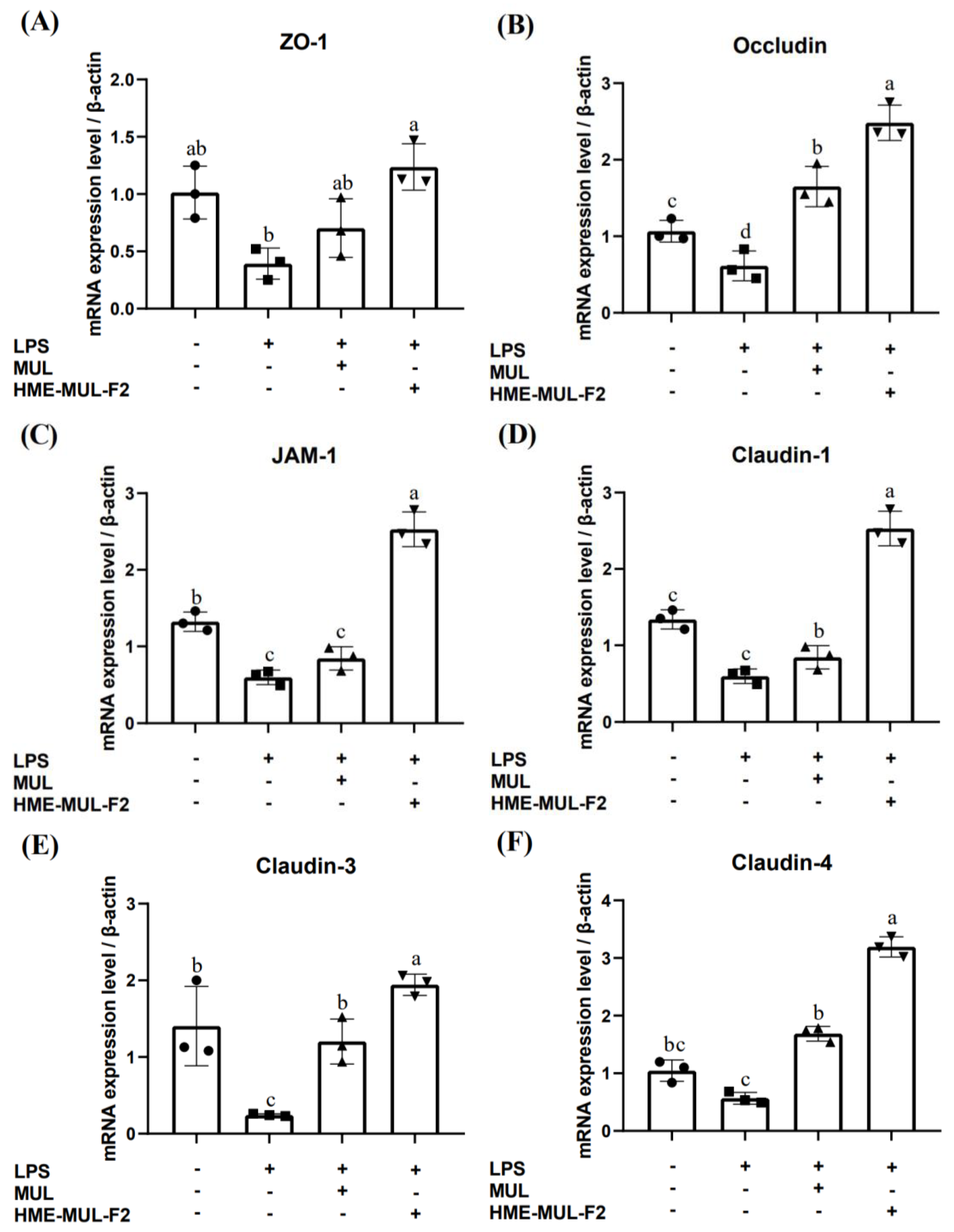
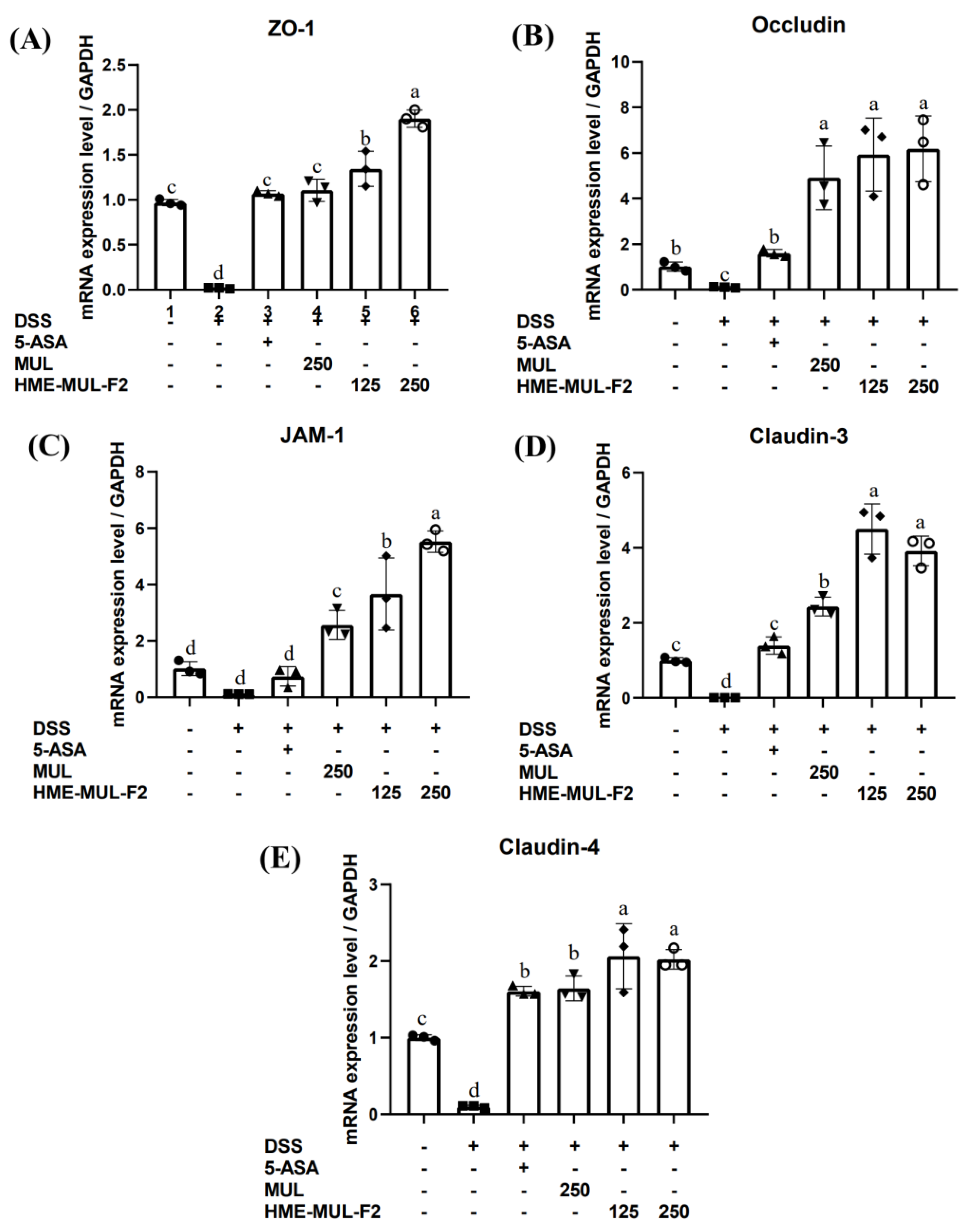
2.4. Effects of HME-MUL-F2 on Gene Expression Related to Tight Junction
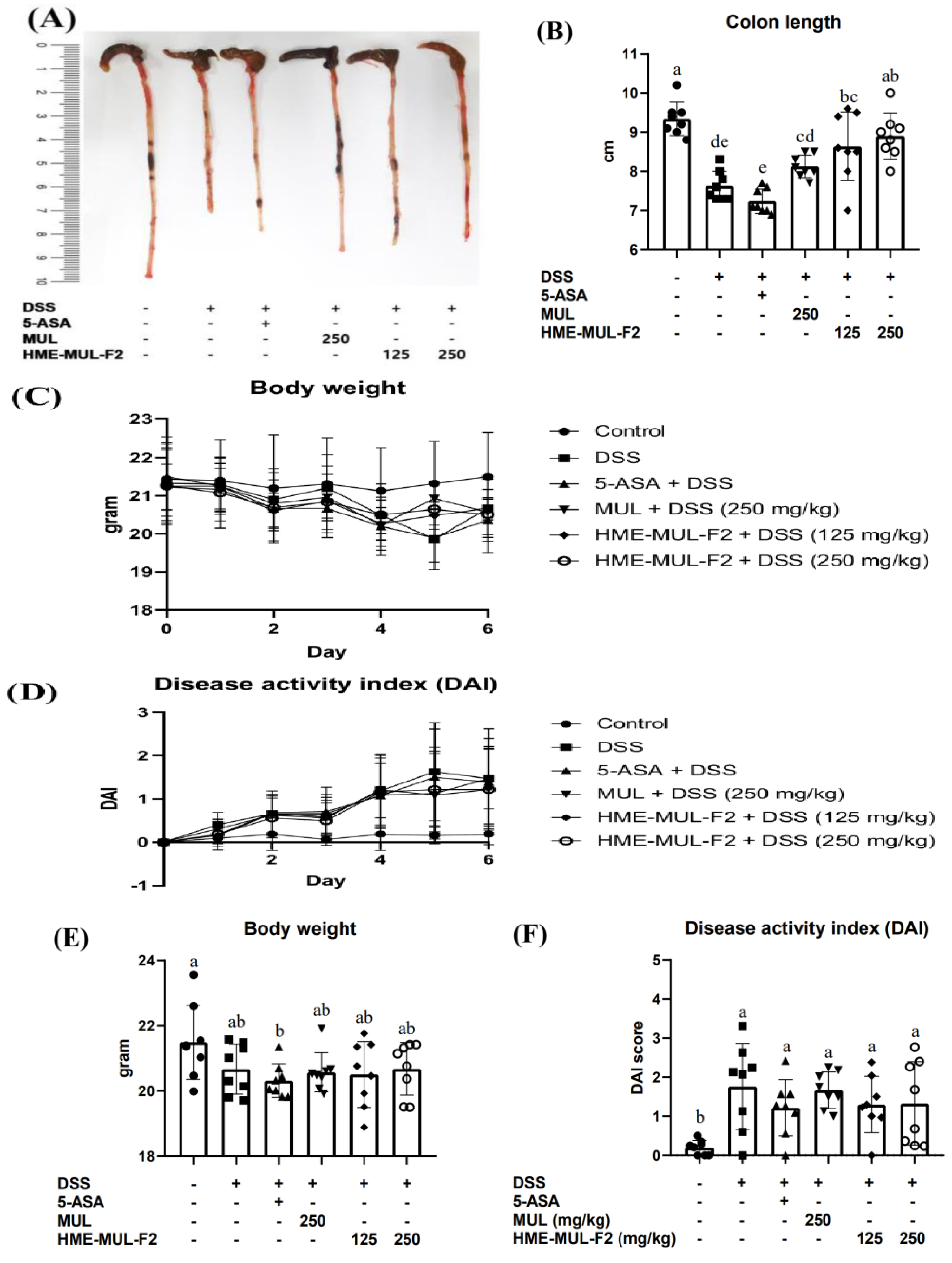
2.5. HME-MUL-F2 Ameliorates DSS-Induced Colitis
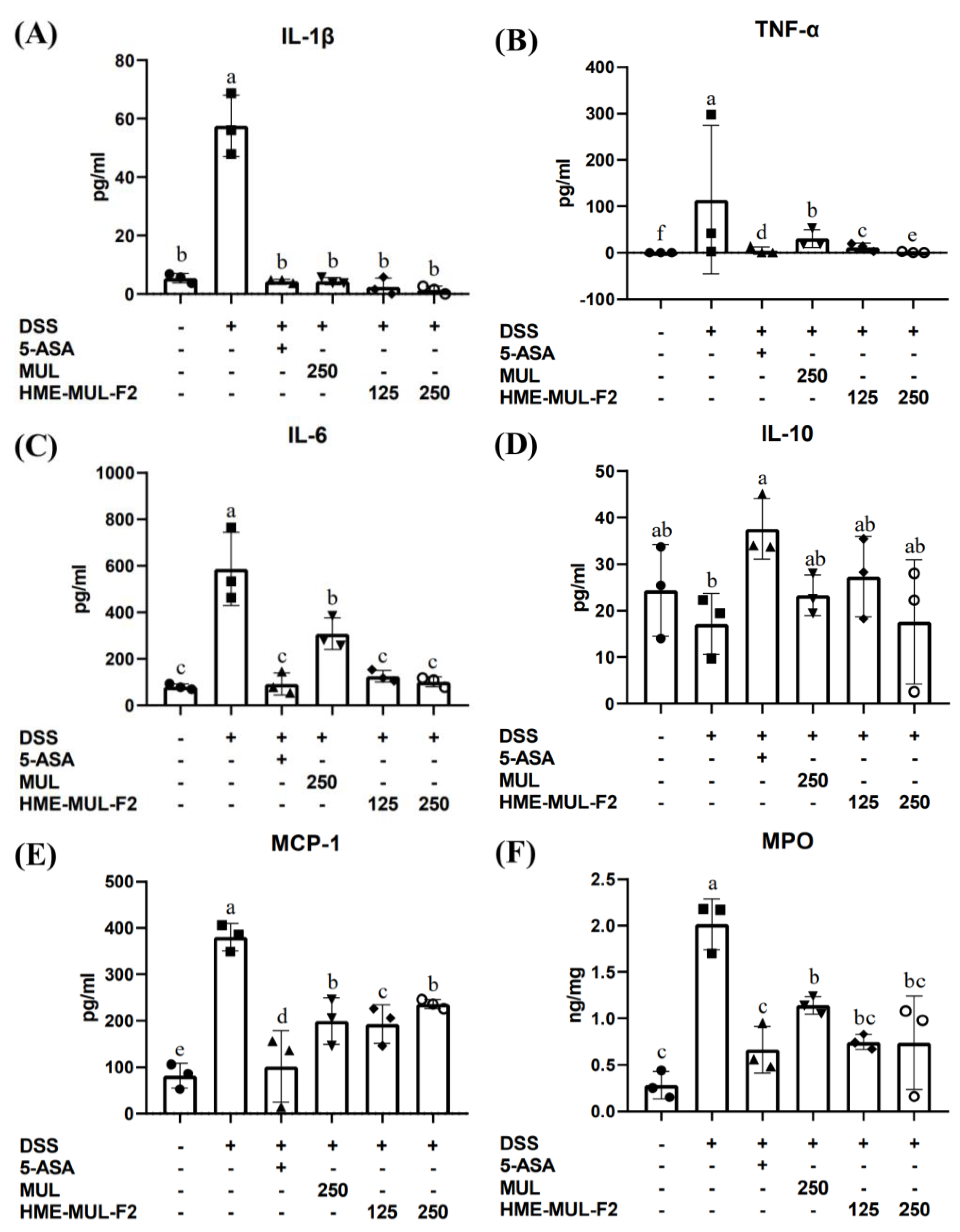
2.6. The Effects of HME-MUL-F2 on Serum Inflammatory Cytokines in DSS-Induced Colitis
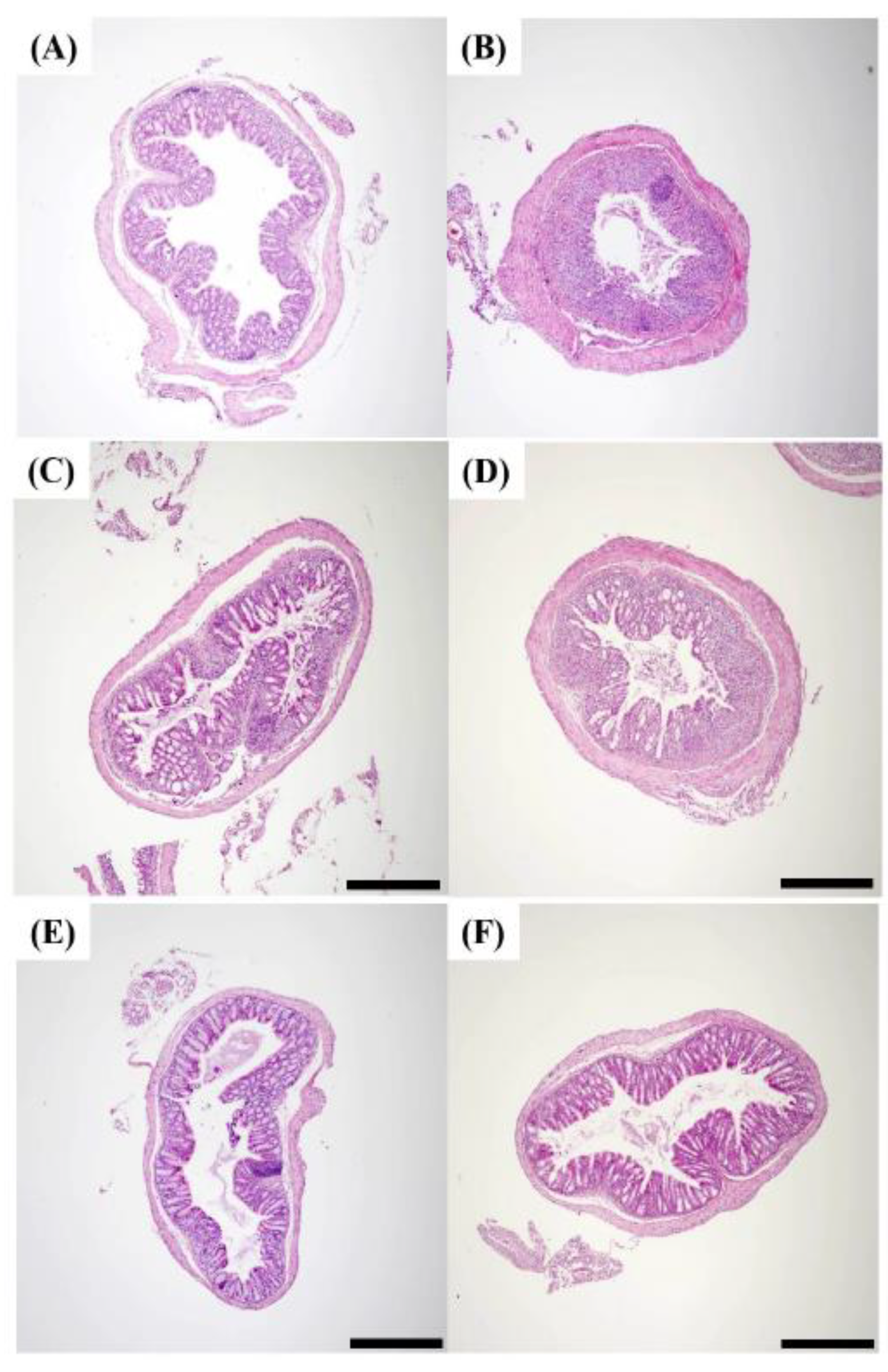
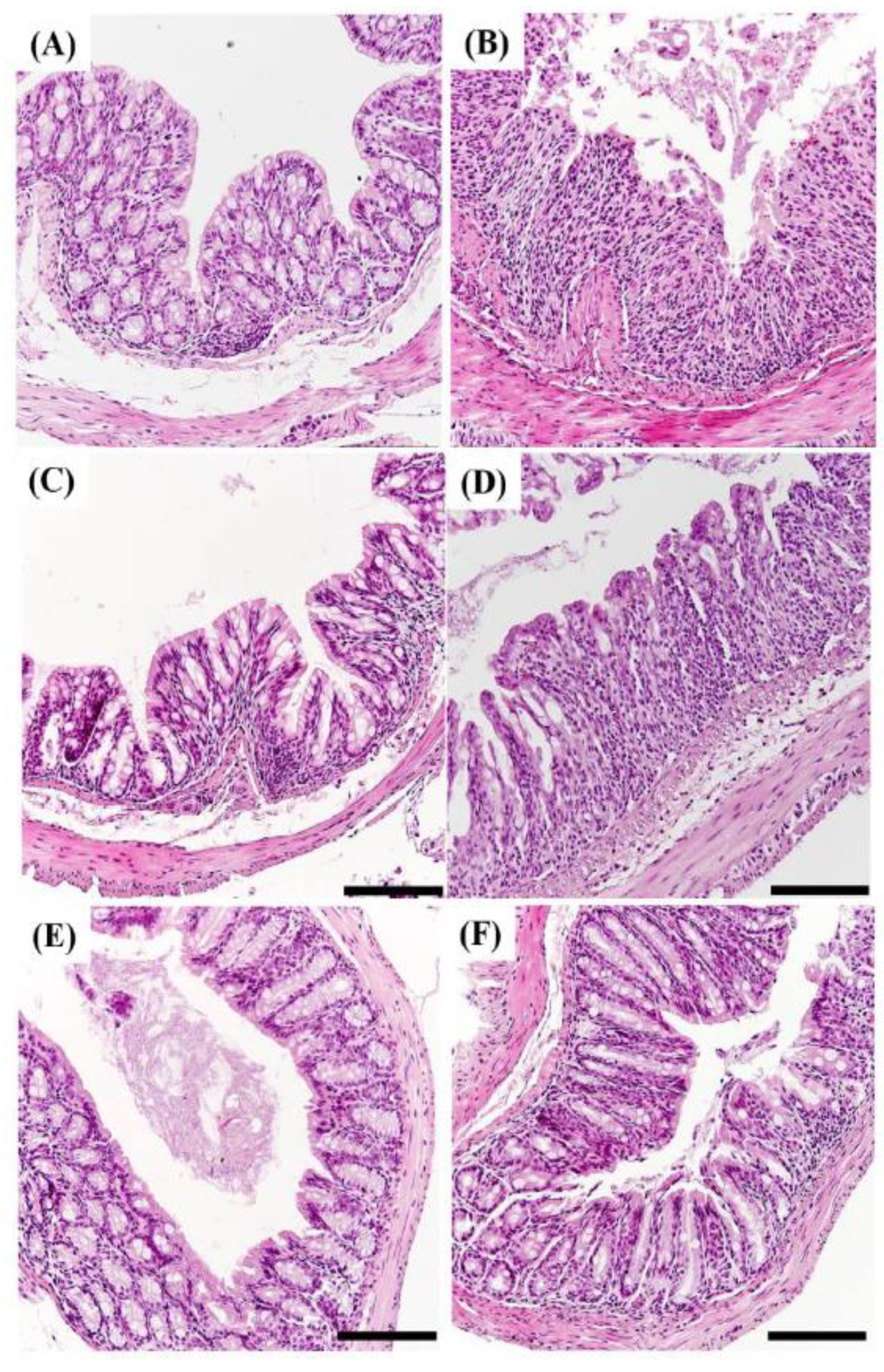
2.7. Histopathology of DSS Induced Colitis
2.8. HME-MUL-F2 Alleviated DSS-Induced Colonic Barrier Dysfunction
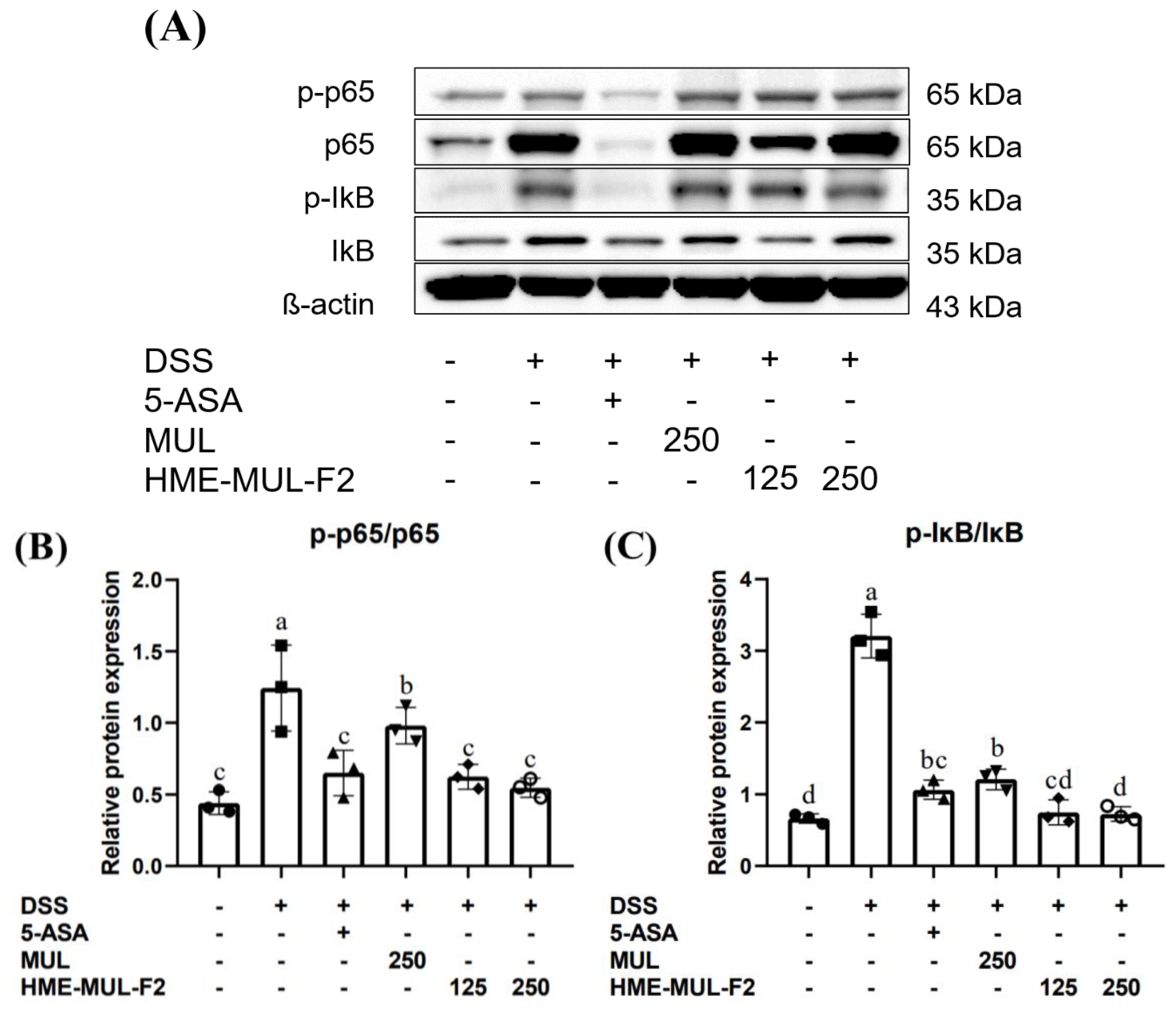
2.9. HME-MUL-F2 Suppressed the Expression of p-NF-κB p65 and IκB in DSS-Induced Colitis
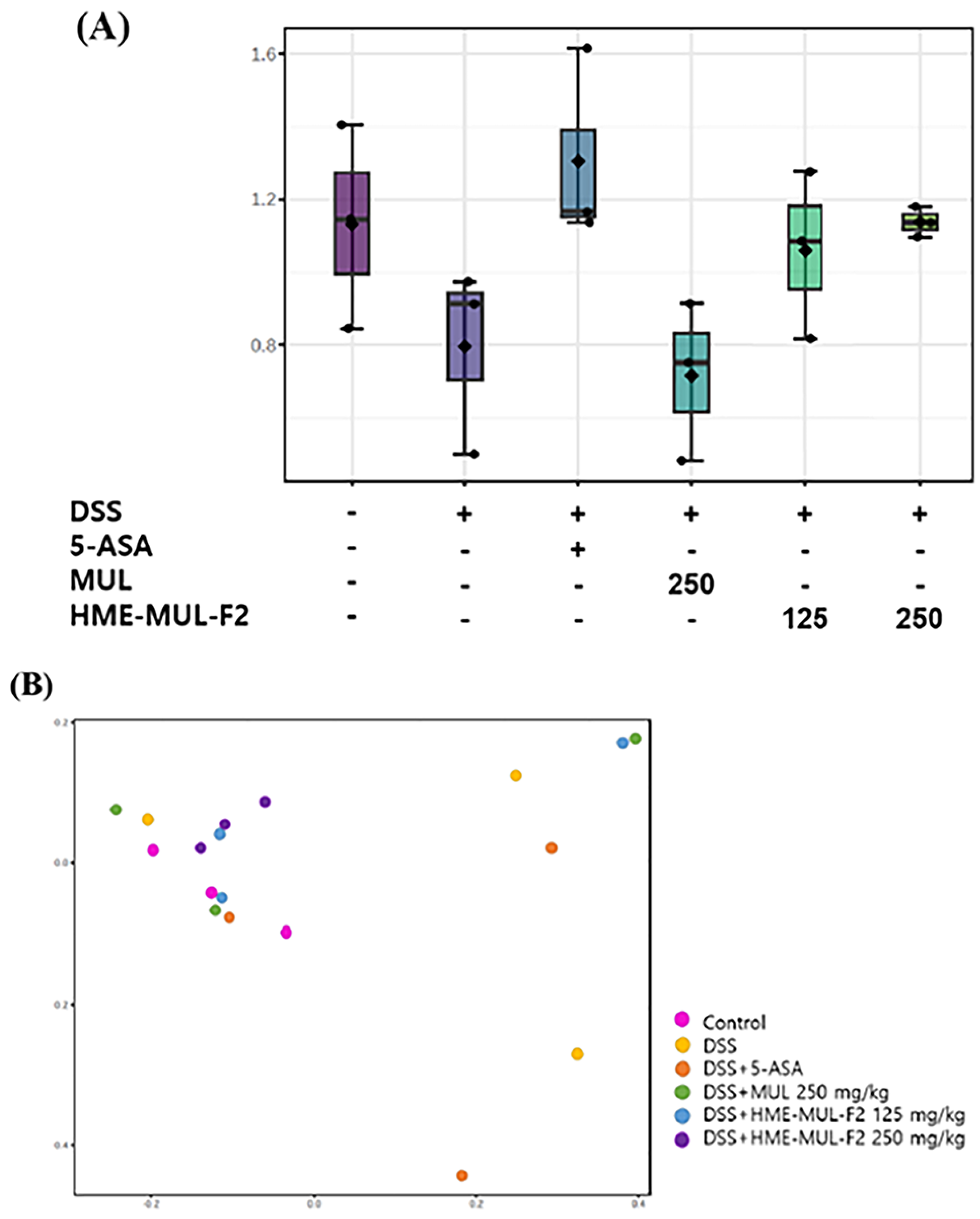
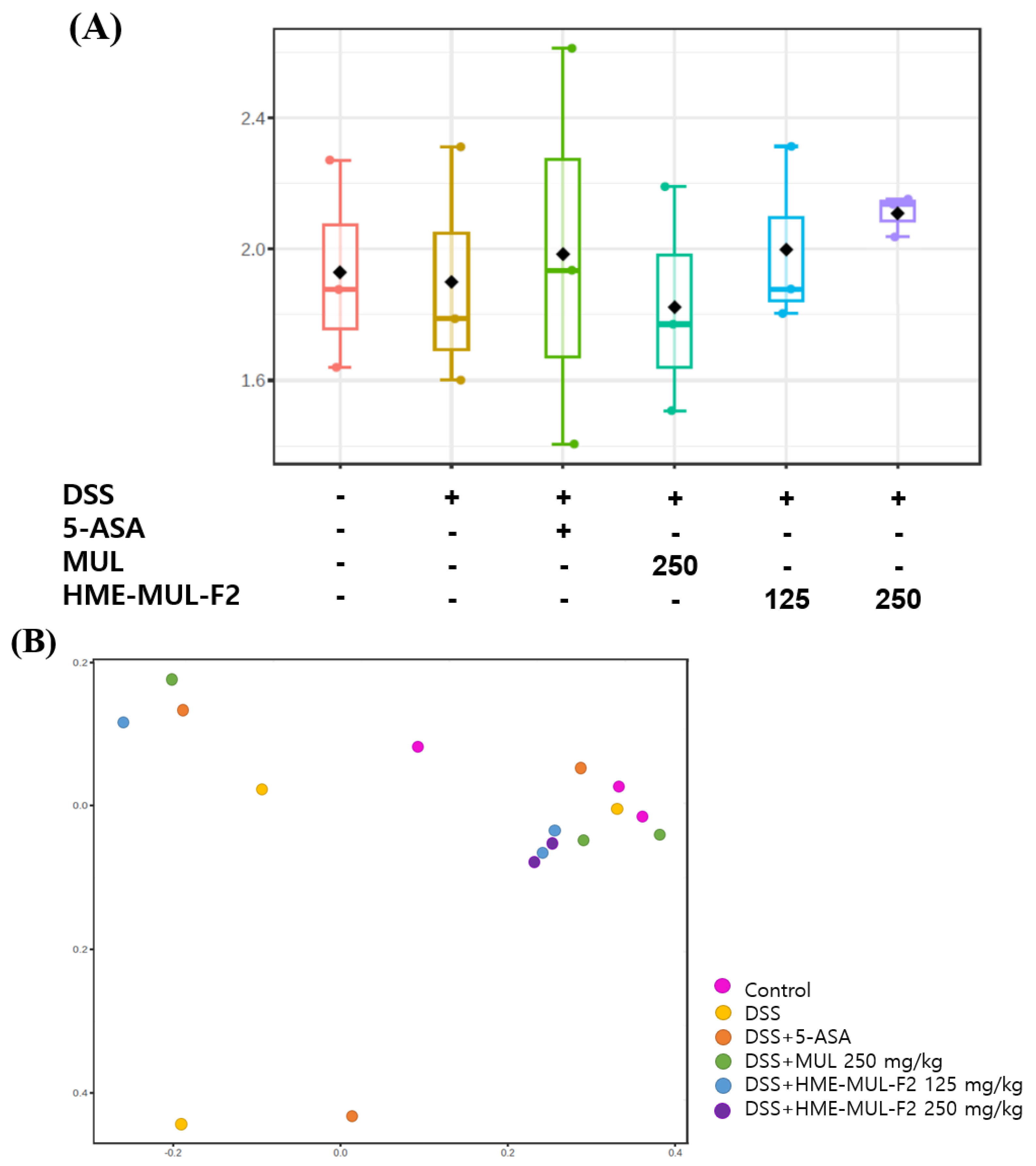
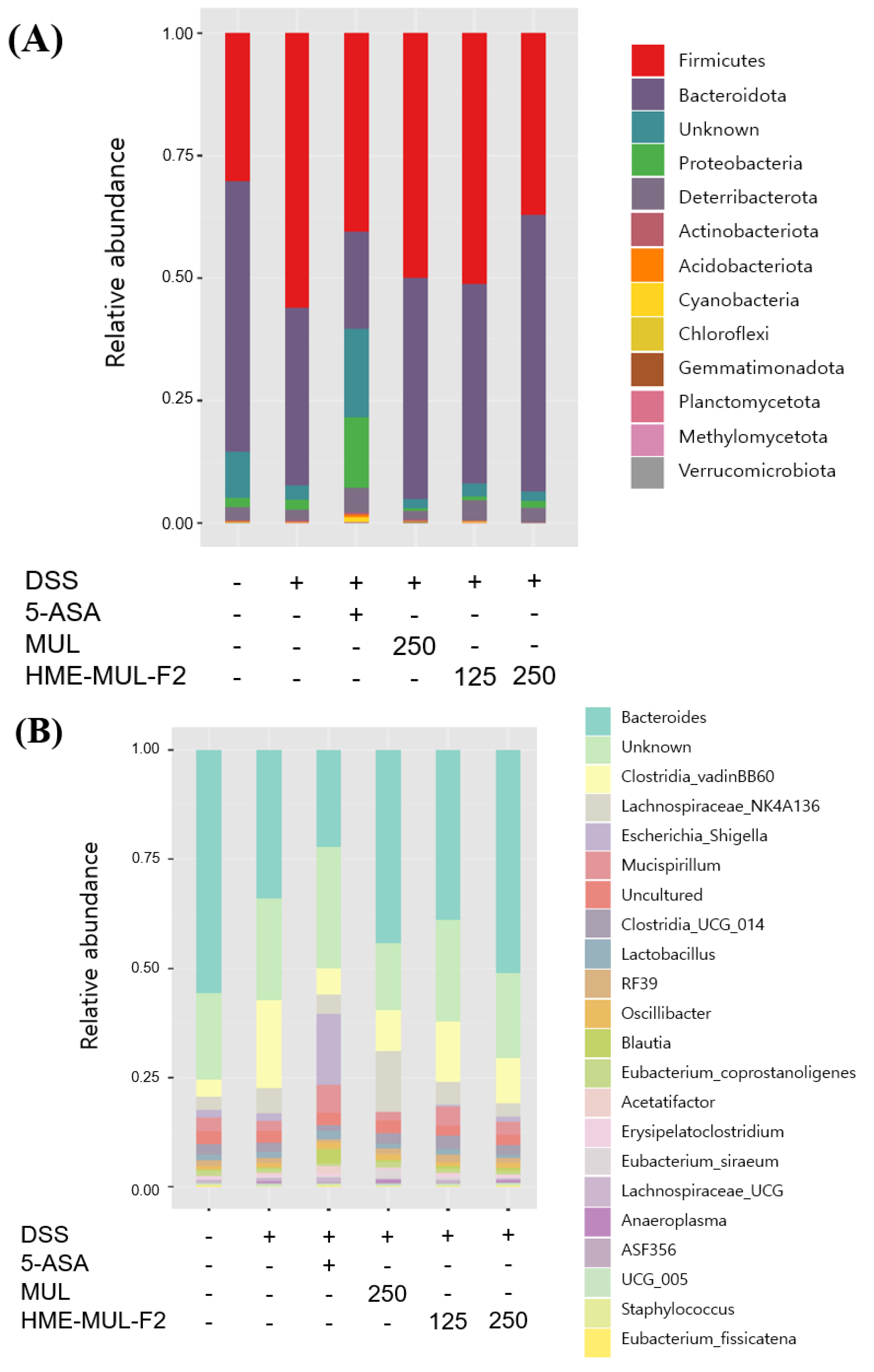
2.10. Effects of HME-MUL-F2 on Gut Microbiota
3. Materials and Methods
3.1. Materials
3.2. Cell Culture Conditions
3.3. Toxicity Evaluation
3.4. In Vitro Inflammation Model
3.5. TEER Measurement
3.6. Cytokine and RNA Analysis
3.7. Quantitative RT-PCR (qRT-PCR) Analysis
3.8. Animal Preparation
3.9. Clinical Indicators and Pathological Evaluation
3.10. Cytokine, Chemokine, and Protein Analysis
3.11. cDNA Synthesis and qPCR from Colon Tissue
3.12. Intestinal Microbial Community Metagenomic Analysis
3.13. Statistical Analysis
4. Conclusions and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Günther, J.; Koy, M.; Berthold, A.; Schuberth, H.-J.; Seyfert, H.-M. Comparison of the Pathogen Species-Specific Immune Response in Udder Derived Cell Types and Their Models. Vet. Res. 2016, 47, 22. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory Epithelial Cells Orchestrate Pulmonary Innate Immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A Tale of Two Diseases: The History of Inflammatory Bowel Disease. J. Crohns Colitis 2014, 8, 341–348. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-Rich Fractions from Red Raspberries Attenuate Inflammation in Both RAW264. 7 Macrophages and a Mouse Model of Colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar]
- Meng, M.; Klingensmith, N.J.; Coopersmith, C.M. New Insights into the Gut as the Driver of Critical Illness and Organ Failure. Curr. Opin. Crit. Care 2017, 23, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Iacob, D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front. Microbiol. 2019, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in Intestinal Permeability: The Role of the “Leaky Gut” in Health and Disease. J. Equine Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, S.J.; Kagnoff, M.F. Nod1 Is an Essential Signal Transducer in Intestinal Epithelial Cells Infected with Bacteria That Avoid Recognition by Toll-like Receptors. Infect. Immun. 2004, 72, 1487–1495. [Google Scholar] [CrossRef]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of Bacillus Subtilis Strains on Intestinal Barrier Function and Inflammatory Response. Front. Immunol. 2019, 10, 431111. [Google Scholar] [CrossRef]
- Vora, P.; Youdim, A.; Thomas, L.S.; Fukata, M.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Wada, A.; Hirayama, T.; Arditi, M. β-Defensin-2 Expression Is Regulated by TLR Signaling in Intestinal Epithelial Cells. J. Immunol. 2004, 173, 5398–5405. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [PubMed]
- McDonald, D.; Ackermann, G.; Khailova, L.; Baird, C.; Heyland, D.; Kozar, R.; Lemieux, M.; Derenski, K.; King, J.; Vis-Kampen, C. Extreme Dysbiosis of the Microbiome in Critical Illness. mSphere 2016, 1, 10-1128. [Google Scholar]
- Eun, C.S. Intestinal Microbiota and Inflammatory Bowel Diseases. J. Korean Med. Assoc. 2021, 64, 588–595. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Marian, E.; Tiţa, D. Compatibility Study between Ketoprofen and Pharmaceutical Excipients Used in Solid Dosage Forms. J. Pharm. Biomed. Anal. 2011, 56, 221–227. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Identificação Espectrométrica de Compostos Orgánicos (7a); Grupo Gen-LTC: Milano, Italy, 2010; ISBN 8521615213. [Google Scholar]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar]
- Hao, J.; Gao, Y.; Xue, J.; Yang, Y.; Yin, J.; Wu, T.; Zhang, M. Phytochemicals, Pharmacological Effects and Molecular Mechanisms of Mulberry. Foods 2022, 11, 1170. [Google Scholar] [CrossRef]
- Qin, S.; Lv, C.; Hou, D.-X. Biological Functions and Health Benefits of Food Polyphenols. Funct. Foods Biotechnol. 2020, 295–326. [Google Scholar]
- Augustin, M.A.; Hemar, Y. Nano-and Micro-Structured Assemblies for Encapsulation of Food Ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Reitz, C.; Kleinebudde, P. Solid Lipid Extrusion of Sustained Release Dosage Forms. Eur. J. Pharm. Biopharm. 2007, 67, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Sejal, S.; Jiannan, L.; Sindhuri, M.; Joe, M.; Ketaki, P.; Mohammed, N.N. Melt Extrusion: Process to Product. Expert. Opin. Drug Deliv. 2012, 9, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Eunsu, L.; Hyeongjeong, K.; Yu, J.; Cho, Y.; Kim, D.; Yuhyeon, S.; Kwon, O.; Bongjeon, A. Anti-Inflammatory Effect of Polygonum Multiflorum Extraction in Activated RAW 264.7 Cells with Lipopolysaccharide. Food Sci. Preserv. 2014, 21, 740–746. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jeon, H.; Lee, C.U.; Yoon, S.H.; Bae, S.K.; Chin, Y.-W.; Yoon, K.D. Isolation and Development of Quantification Method for Cyanidin-3-Glucoside and Cyanidin-3-Rutinoside in Mulberry Fruit by High-Performance Countercurrent Chromatography and High-Performance Liquid Chromatography. Nat. Product. Sci. 2015, 21, 20–24. [Google Scholar]
- Go, E.J.; Ryu, B.R.; Gim, G.J.; Lee, H.Y.; You, H.S.; Kim, H.B.; Lee, H.T.; Lee, J.Y.; Shim, M.S.; Baek, J.-S. Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms. Antioxidants 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Kura, A.U.; Fakurazi, S.; Hussein, M.Z.; Arulselvan, P. Nanotechnology in Drug Delivery: The Need for More Cell Culture Based Studies in Screening. Chem. Cent. J. 2014, 8, 46. [Google Scholar] [CrossRef]
- Marsousi, N.; Doffey-Lazeyras, F.; Rudaz, S.; Desmeules, J.A.; Daali, Y. Intestinal Permeability and P-glycoprotein-mediated Efflux Transport of Ticagrelor in Caco-2 Monolayer Cells. Fundam. Clin. Pharmacol. 2016, 30, 577–584. [Google Scholar] [CrossRef]
- Chantret, I.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Brot-Laroche, E.; Zweibaum, A.; Rousset, M. Differential Expression of Sucrase-Isomaltase in Clones Isolated from Early and Late Passages of the Cell Line Caco-2: Evidence for Glucose-Dependent Negative Regulation. J. Cell Sci. 1994, 107, 213–225. [Google Scholar] [CrossRef]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX Cocultures in an in Vitro Digestion/Cell Culture Model Used to Predict Iron Bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [PubMed]
- Yang, M.; Lu, X.; Xu, J.; Liu, X.; Zhang, W.; Guan, R.; Zhong, H. Cellular Uptake, Transport Mechanism and Anti-Inflammatory Effect of Cyanidin-3-Glucoside Nanoliposomes in Caco-2/RAW 264.7 Co-Culture Model. Front. Nutr. 2022, 9, 995391. [Google Scholar]
- Chan, E.P.; Lichtenstein, G.R. Chemoprevention: Risk Reduction with Medical Therapy of Inflammatory Bowel Disease. Gastroenterol. Clin. 2006, 35, 675–712. [Google Scholar]
- Sappington, P.L.; Han, X.; Yang, R.; Delude, R.L.; Fink, M.P. Ethyl Pyruvate Ameliorates Intestinal Epithelial Barrier Dysfunction in Endotoxemic Mice and Immunostimulated Caco-2 Enterocytic Monolayers. J. Pharmacol. Exp. Ther. 2003, 304, 464–476. [Google Scholar] [PubMed]
- Pinto, M.; Robine-Leon, S.; Appay, M.-D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J. Enterocyte-like Differentiation and Polarization of the Human Colon Carcinoma Cell Line Caco-2 in Culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Briske-Anderson, M.J.; Finley, J.W.; Newman, S.M. The Influence of Culture Time and Passage Number on the Morphological and Physiological Development of Caco-2 Cells. Proc. Soc. Exp. Biol. Med. 1997, 214, 248–257. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Li, X.; Yao, K.; Tan, B.; Yin, Y. 4-Phenylbutyric Acid Accelerates Rehabilitation of Barrier Function in IPEC-J2 Cell Monolayer Model. Anim. Nutr. 2021, 7, 1061–1069. [Google Scholar]
- Valdez, J.C.; Cho, J.; Bolling, B.W. Aronia Berry Inhibits Disruption of Caco-2 Intestinal Barrier Function. Arch. Biochem. Biophys. 2020, 688, 108409. [Google Scholar]
- Umare, V.; Pradhan, V.; Nadkar, M.; Rajadhyaksha, A.; Patwardhan, M.; Ghosh, K.K.; Nadkarni, A.H. Effect of Proinflammatory Cytokines (IL-6, TNF-α, and IL-1β) on Clinical Manifestations in Indian SLE Patients. Mediat. Inflamm. 2014, 2014, 385297. [Google Scholar]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-?B and Akt/MAPKs Signaling Pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef]
- Yang, D.; Jones, K.S. Effect of Alginate on Innate Immune Activation of Macrophages. J. Biomed. Mater. Res. A 2009, 90, 411–418. [Google Scholar] [CrossRef]
- Watari, A.; Sakamoto, Y.; Hisaie, K.; Iwamoto, K.; Fueta, M.; Yagi, K.; Kondoh, M. Rebeccamycin Attenuates TNF-α-Induced Intestinal Epithelial Barrier Dysfunction by Inhibiting Myosin Light Chain Kinase Production. Cell. Physiol. Biochem. 2017, 41, 1924–1934. [Google Scholar] [CrossRef]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar]
- Huang, Y.; Zhou, W. Microencapsulation of Anthocyanins through Two-Step Emulsification and Release Characteristics during in Vitro Digestion. Food Chem. 2019, 278, 357–363. [Google Scholar] [CrossRef]
- Ni, Y.; Teng, T.; Li, R.; Simonyi, A.; Sun, G.Y.; Lee, J.C. TNFα Alters Occludin and Cerebral Endothelial Permeability: Role of P38MAPK. PLoS ONE 2017, 12, e0170346. [Google Scholar]
- Ershad, M.; Shigenaga, M.K.; Bandy, B. Differential Protection by Anthocyanin-Rich Bilberry Extract and Resveratrol against Lipid Micelle-Induced Oxidative Stress and Monolayer Permeability in Caco-2 Intestinal Epithelial Cells. Food Funct. 2021, 12, 2950–2961. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins Protect the Gastrointestinal Tract from High Fat Diet-Induced Alterations in Redox Signaling, Barrier Integrity and Dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhu, L.; Yang, L.; Li, W.; Wei, S.; Tao, Y.; Du, G. The Soluble and Particulate Form of Alginates Positively Regulate Immune Response. Iran. J. Immunol. 2018, 15, 228–238. [Google Scholar]
- Wong, S.W.; Yao, Y.; Hong, Y.; Ma, Z.; Kok, S.H.L.; Sun, S.; Cho, M.; Lee, K.K.H.; Mak, A.F.T. Preventive Effects of Poloxamer 188 on Muscle Cell Damage Mechanics under Oxidative Stress. Ann. Biomed. Eng. 2017, 45, 1083–1092. [Google Scholar] [CrossRef]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.N.; De, A.; Kataria, V.; Mallick, S. Solvent-Free Hot Melt Extrusion Technique in Improving Mesalamine Release for Better Management of Inflammatory Bowel Disease. Indian. J. Pharm. Educ. Res. 2019, 53, S554–S562. [Google Scholar] [CrossRef]
- Jain, A.; Irizarry-Caro, R.A.; Chawla, A.S.; Philip, N.H.; Carroll, K.R.; Katz, J.D.; Oberst, A.; Chervonsky, A.V.; Pasare, C. T Cells Instruct Dendritic Cells to Produce Inflammasome Independent IL-1β Causing Systemic Inflammation. bioRxiv 2018, 475517. [Google Scholar]
- Evans, S.; Tzeng, H.-P.; Veis, D.J.; Matkovich, S.; Weinheimer, C.; Kovacs, A.; Barger, P.M.; Mann, D.L. TNF Receptor–Activated Factor 2 Mediates Cardiac Protection through Noncanonical NF-ΚB Signaling. JCI Insight 2018, 3, e98278. [Google Scholar]
- Allavena, P.; Bianchi, G.; Zhou, D.; Van Damme, J.; Jílek, P.; Sozzani, S.; Mantovani, A. Induction of Natural Killer Cell Migration by Monocyte Chemotactic Protein−1,−2 And−3. Eur. J. Immunol. 1994, 24, 3233–3236. [Google Scholar]
- Sutherland, A.D.; Gearry, R.B.; Frizelle, F.A. Review of Fecal Biomarkers in Inflammatory Bowel Disease. Dis. Colon. Rectum 2008, 51, 1283–1291. [Google Scholar]
- Smith, J.W.; Castro, G.A. Relation of Peroxidase Activity in Gut Mucosa to Inflammation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1978, 234, R72–R79. [Google Scholar]
- Sabra, R.; Billa, N.; Roberts, C.J. Cetuximab-Conjugated Chitosan-Pectinate (Modified) Composite Nanoparticles for Targeting Colon Cancer. Int. J. Pharm. 2019, 572, 118775. [Google Scholar]
- Zheng, S.; Zhuang, T.; Tang, Y.; Wu, R.; Xu, T.; Leng, T.; Wang, Y.; Lin, Z.; Ji, M. Leonurine Protects against Ulcerative Colitis by Alleviating Inflammation and Modulating Intestinal Microflora in Mouse Models. Exp. Ther. Med. 2021, 22, 1199. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-Induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight Junctions in Inflammatory Bowel Diseases and Inflammatory Bowel Disease Associated Colorectal Cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [PubMed]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-Regulation of MIR200C-3p, Which Degrades Occludin MRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar]
- Weber, C.R.; Turner, J.R. Inflammatory Bowel Disease: Is It Really Just Another Break in the Wall? Gut 2007, 56, 6–8. [Google Scholar] [PubMed]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar]
- Edelblum, K.L.; Turner, J.R. The Tight Junction in Inflammatory Disease: Communication Breakdown. Curr. Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in Expression and Distribution of Claudin 2, 5 and 8 Lead to Discontinuous Tight Junctions and Barrier Dysfunction in Active Crohn’s Disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Blair, S.A.; Kane, S.V.; Clayburgh, D.R.; Turner, J.R. Epithelial Myosin Light Chain Kinase Expression and Activity Are Upregulated in Inflammatory Bowel Disease. Lab. Investig. 2006, 86, 191–201. [Google Scholar]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-γ and TNF-α-Induced Intestinal Epithelial Barrier Dysfunction by Berberine via Suppression of MLCK-MLC Phosphorylation Signaling Pathway. PLoS ONE 2013, 8, e61944. [Google Scholar]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-ΚB and Rel Proteins: Evolutionarily Conserved Mediators of Immune Responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [PubMed]
- Beaulieu, L.M.; Freedman, J.E. NFκB Regulation of Platelet Function: No Nucleus, No Genes, No Problem? J. Thromb. Haemost. 2009, 7, 1329–1332. [Google Scholar] [PubMed]
- Karin, M.; Yamamoto, Y.; Wang, Q.M. The IKK NF-ΚB System: A Treasure Trove for Drug Development. Nat. Rev. Drug Discov. 2004, 3, 17–26. [Google Scholar] [CrossRef]
- Baldwin, A.S., Jr. The NF-ΚB and IκB Proteins: New Discoveries and Insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef]
- Tsang, S.W.; Ip, S.P.; Wu, J.C.-Y.; Ng, S.-C.; Yung, K.K.-L.; Bian, Z.-X. A Chinese Medicinal Formulation Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis by Suppressing the Activity of Nuclear Factor-KappaB Signaling. J. Ethnopharmacol. 2015, 162, 20–30. [Google Scholar] [CrossRef]
- Nam, N.-H. Naturally Occurring NF-ΚB Inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Jialing, L.; Yangyang, G.; Jing, Z.; Xiaoyi, T.; Ping, W.; Liwei, S.; Simin, C. Changes in Serum Inflammatory Cytokine Levels and Intestinal Flora in a Self-Healing Dextran Sodium Sulfate-Induced Ulcerative Colitis Murine Model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Species Divergence and the Measurement of Microbial Diversity. FEMS Microbiol. Rev. 2008, 32, 557–578. [Google Scholar]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria Expansion: A Microbial Signature of Epithelial Dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg White Peptides Ameliorate Dextran Sulfate Sodium-Induced Acute Colitis Symptoms by Inhibiting the Production of pro-Inflammatory Cytokines and Modulation of Gut Microbiota Composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-ΚB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral Honey from a Medical Plant, Prunella Vulgaris, Protected against Dextran Sulfate Sodium-Induced Ulcerative Colitis via Modulating Gut Microbial Populations in Rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Witten, J.; Samad, T.; Ribbeck, K. Selective Permeability of Mucus Barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar]
- Alosaimi, A.M.; Alorabi, R.O.; Katowah, D.F.; Al-Thagafi, Z.T.; Alsolami, E.S.; Hussein, M.A.; Qutob, M.; Rafatullah, M. Review on Biomedical Advances of Hybrid Nanocomposite Biopolymeric Materials. Bioengineering 2023, 10, 279. [Google Scholar] [CrossRef]
- Tang, H.Y.; Fang, Z.; Ng, K. Dietary Fiber-Based Colon-Targeted Delivery Systems for Polyphenols. Trends Food Sci. Technol. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Xie, J.; Cui, H.; Mozafari, M.R.; Chen, W. Potential Micro-/Nano-Encapsulation Systems for Improving Stability and Bioavailability of Anthocyanins: An Updated Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3362–3385. [Google Scholar] [CrossRef]
- Alkhader, E.; Billa, N.; Roberts, C.J. Mucoadhesive Chitosan-Pectinate Nanoparticles for the Delivery of Curcumin to the Colon. AAPS PharmSciTech 2017, 18, 1009–1018. [Google Scholar] [CrossRef]
- Sha, A.; Luo, Y.; Xiao, W.; He, J.; Chen, X.; Xiong, Z.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Plant-Derived Exosome-like Nanoparticles: A Comprehensive Overview of Their Composition, Biogenesis, Isolation, and Biological Applications. Int. J. Mol. Sci. 2024, 25, 12092. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Watanabe, C.; Ando, Y.; Kitaoka, S.; Egawa, Y.; Takashima, T.; Matsumoto, A.; Murakami, M. Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability. Pharmaceutics 2023, 15, 2338. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, M.; Zeng, H.; Zhao, H.; Zhang, M.; Yang, Z. Preparation, Characterization and Iron Absorption by Caco-2 Cells of the Casein Peptides-Iron Chelate. Int. J. Pept. Res. Ther. 2022, 28, 116. [Google Scholar] [CrossRef]
- Wongwanakul, R.; Aueviriyavit, S.; Furihata, T.; Gonil, P.; Sajomsang, W.; Maniratanachote, R.; Jianmongkol, S. Quaternization of High Molecular Weight Chitosan for Increasing Intestinal Drug Absorption Using Caco-2 Cells as an in Vitro Intestinal Model. Sci. Rep. 2023, 13, 7904. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed Polysaccharides as Prebiotics on Growth and Acidifying Activity of Probiotics in Vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Ishimoto, K.; Miki, S.; Ohno, A.; Nakamura, Y.; Otani, S.; Nakamura, M.; Nakagawa, S. β-Carotene Solid Dispersion Prepared by Hot-Melt Technology Improves Its Solubility in Water. J. Food Sci. Technol. 2019, 56, 3540–3546. [Google Scholar] [CrossRef]
- Cho, N.; Lee, S.G.; Kim, J.O.; Kim, Y.A.; Kim, E.M.; Park, C.; Ji, J.H.; Kim, K.K. Identification of Differentially Expressed Genes Associated with Extracellular Matrix Degradation and Inflammatory Regulation in Calcific Tendinopathy Using RNA Sequencing. Calcif. Tissue Int. 2020, 107, 489–498. [Google Scholar] [CrossRef]
- Kim, H.B.; Go, E.J.; Baek, J.S. Effect of Hot-Melt Extruded Morus Alba Leaves on Intestinal Microflora and Epithelial Cells. Heliyon 2024, 10, e23954. [Google Scholar] [CrossRef]
- Peach, M.; Marsh, N.; MacPhee, D.J. Protein Solubilization: Attend to the Choice of Lysis Buffer. Methods Mol. Biol. 2012, 869, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Láng, L.; McArthur, S.; Lazar, A.S.; Pourtau, L.; Gaudout, D.; Pontifex, M.G.; Müller, M.; Vauzour, D. Dietary (Poly)Phenols and the Gut–Brain Axis in Ageing. Nutrients 2024, 16, 1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, N.; Zhao, L.; Zhao, L. Black Rice Anthocyanins Nanoparticles Based on Bovine Serum Albumin and Hyaluronic Acid: Preparation, Characterization, Absorption and Intestinal Barrier Function Protection in Caco-2 Monolayers. Int. J. Biol. Macromol. 2024, 267, 131325. [Google Scholar] [CrossRef]
- Karpí Nski, M.; Doglio, A.; Zarowski, M.O.; Mosaddad, S.A.; Hussain, A.; Tebyaniyan, H. Green Alternatives as Antimicrobial Agents in Mitigating Periodontal Diseases: A Narrative Review. Microorganisms 2023, 11, 1269. [Google Scholar] [CrossRef]
| Anthocyanin Content (mg/g DW) | |||
|---|---|---|---|
| C3G | C3R | ||
| Raw materials | MUL | 43.13 ± 2.63 e | 2.99 ± 1.25 f |
| Extrusion materials | HME-MUL | 117.44 ± 1.44 d | 68.91 ± 1.35 e |
| HME-MUL-F2 | 325.02 ± 11.12 b | 154.73 ± 11.30 bc | |
| Gene | Orientation | Primers Sequence (5′→3′) |
|---|---|---|
| ZO-1 (NM_175610) | Forward | AAGGTTAGCTTACTGTCACACGCTT |
| Reverse | ACCTAGAAGACATTGAAGGCATC | |
| Occludin (NM_002538) | Forward | GTACCCACCAGTGACCAACA |
| Reverse | GTTGCTGGAGCTTAGCCTGT | |
| JAM-1 (NM_016946) | Forward | ACAGCCATGAGGTCAGAGGCT |
| Reverse | CGACTCTAGAAACACAAGAGCAAGA | |
| claudin-3 (NM_001306) | Forward | CACCACTACCAGCAGTCGATGAAC |
| Reverse | AGACTGTGTGTCGTCTGTCACCATC | |
| claudin-4 (NM_001305) | Forward | GGTAGCTCAGCTGTGACTTTGGACTC |
| Reverse | CTGGAGTAACGTGTAGGCTGAGTGAG | |
| GAPDH (NM_002046) | Forward | TCCCACTCTTCCACCTTCGA |
| Reverse | CAGGAAATGAGCTTGACAAAGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, E.-J.; Ryu, B.R.; Gim, G.J.; Shin, Y.R.; Kang, M.J.; Kim, M.J.; Baek, J.-S.; Lim, J.D. Regulation of Intestinal Barrier Function and Gut Microbiota by Hot Melt Extrusion-Drug Delivery System-Prepared Mulberry Anthocyanin in an Inflammatory Bowel Disease Model. Pharmaceuticals 2025, 18, 475. https://doi.org/10.3390/ph18040475
Go E-J, Ryu BR, Gim GJ, Shin YR, Kang MJ, Kim MJ, Baek J-S, Lim JD. Regulation of Intestinal Barrier Function and Gut Microbiota by Hot Melt Extrusion-Drug Delivery System-Prepared Mulberry Anthocyanin in an Inflammatory Bowel Disease Model. Pharmaceuticals. 2025; 18(4):475. https://doi.org/10.3390/ph18040475
Chicago/Turabian StyleGo, Eun-Ji, Byeong Ryeol Ryu, Gyeong Ju Gim, Ye Rim Shin, Min Ji Kang, Min Jun Kim, Jong-Suep Baek, and Jung Dae Lim. 2025. "Regulation of Intestinal Barrier Function and Gut Microbiota by Hot Melt Extrusion-Drug Delivery System-Prepared Mulberry Anthocyanin in an Inflammatory Bowel Disease Model" Pharmaceuticals 18, no. 4: 475. https://doi.org/10.3390/ph18040475
APA StyleGo, E.-J., Ryu, B. R., Gim, G. J., Shin, Y. R., Kang, M. J., Kim, M. J., Baek, J.-S., & Lim, J. D. (2025). Regulation of Intestinal Barrier Function and Gut Microbiota by Hot Melt Extrusion-Drug Delivery System-Prepared Mulberry Anthocyanin in an Inflammatory Bowel Disease Model. Pharmaceuticals, 18(4), 475. https://doi.org/10.3390/ph18040475









