Morin Alleviates Fructose-Driven Disturbance of Podocyte Mitochondrial Energy Metabolism by Inhibiting Adenosine 5′-Monophosphate Deaminase Activity to Improve Glomerular Injury
Abstract
1. Introduction
2. Results
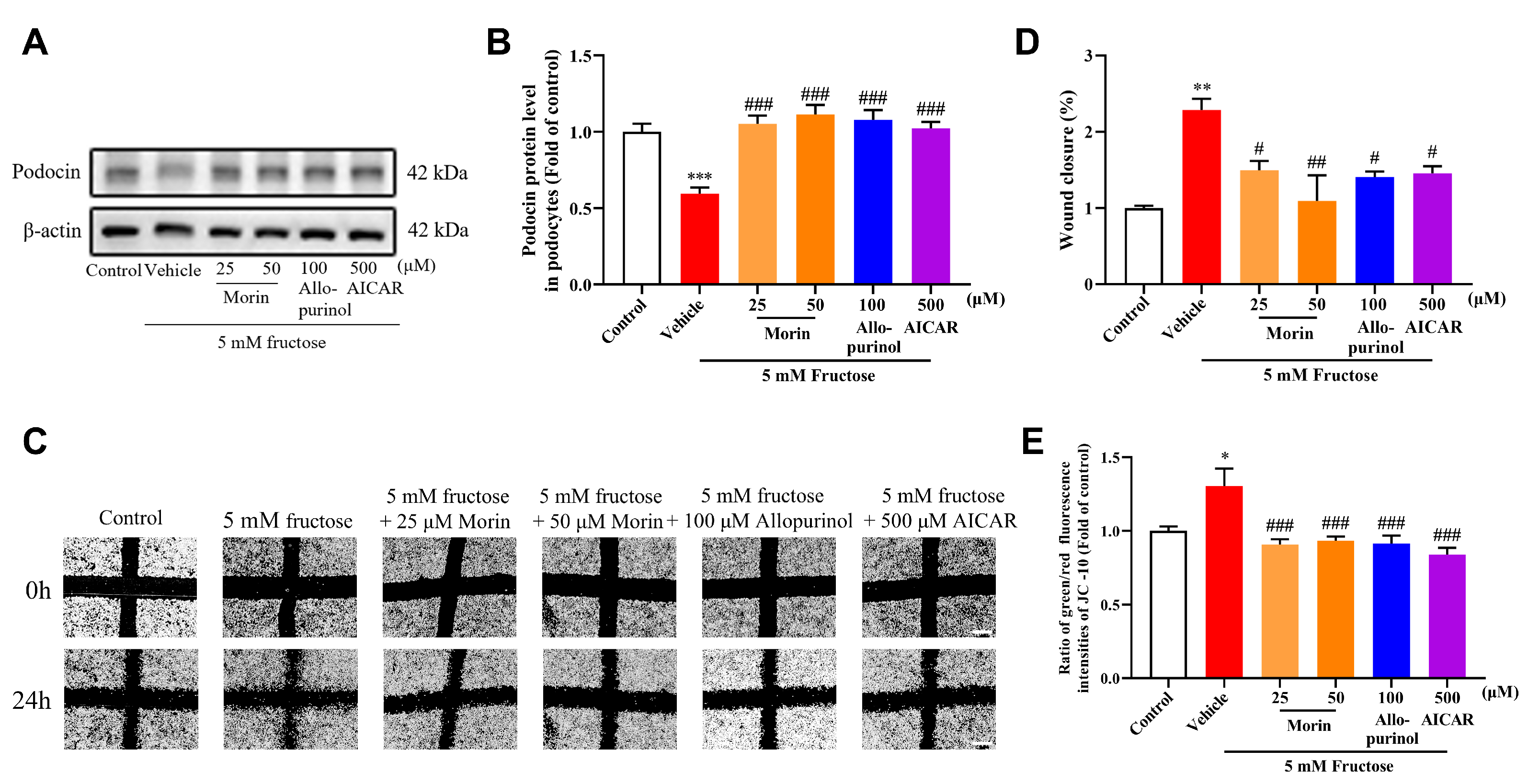
2.1. Morin Ameliorates High-Fructose-Induced Podocyte Injury and Mitochondrial Dysfunction in Mouse Podocyte Clone-5 (MPC5)
2.2. Morin Inhibits the Fructose-Induced Enhancement of AMPD Activity in MPC5
2.3. Morin Improves Mitochondrial Energetic Disturbance Through AMPD2 Suppression in MPC5
2.4. Morin Ameliorates Podocyte Injury of High-Fructose-Fed Rats by Inhibiting AMPD Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Treatments
4.3. OGTT and ITT
4.4. Biochemical Analysis
4.5. Transmission Electron Microscopy Analysis
4.6. Immunohistofluorescence Assay
4.7. Cell Culture and Treatment
4.8. Wound-Healing Assay
4.9. Western Blot Assay
4.10. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assay
4.11. JC-10 Assay
4.12. Seahorse Measurement
4.13. Determination of AMPD Activity
4.14. Determination of ADSS and ADSL Activity
4.15. Inhibitory Effect of AMPD Activity by Morin
4.16. Data Mining in HPA Database
4.17. Molecular Docking
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UACR | urinary albumin to creatinine ratio |
| AMPD | adenosine 5′-monophosphate deaminase |
| MPC5 | mouse podocyte clone-5 |
| PNC | purine nucleotide cycle |
| OCR | oxygen consumption rate |
| 2-DG | 2-deoxyglucose |
| ADSS | adenylosuccinate synthetase |
| ADSL | adenylosuccinate lyase |
| ECAR | extracellular acidification rate |
| AICAR | 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside |
| OGTT | oral glucose tolerance test |
| ITT | insulin tolerance test |
| CYP2E1 | cytochrome P450 family 2 subfamily e polypeptide 1 |
| MAPK | mitogen-activated protein kinase |
| PMSF | phenylmethanesulfonyl fluoride |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
References
- Fang, L.; Li, T.S.; Zhang, J.Z.; Liu, Z.H.; Yang, J.; Wang, B.H.; Wang, Y.M.; Zhou, J.; Kong, L.D. Fructose drives mitochondrial metabolic reprogramming in podocytes via Hmgcs2-stimulated fatty acid degradation. Signal Transduct. Tar. 2021, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Flisinski, M.; Brymora, A.; Skoczylas-Makowska, N.; Stefanska, A.; Manitius, J. Fructose-Rich Diet Is a Risk Factor for Metabolic Syndrome, Proximal Tubule Injury and Urolithiasis in Rats. Int. J. Mol. Sci. 2022, 23, 203. [Google Scholar] [CrossRef]
- Muller-Deile, J.; Schiffer, M. The podocyte power-plant disaster and its contribution to glomerulopathy. Front. Endocrinol. 2014, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Ueda, S.; Imamura, H.; Mori, K.; Asanuma, K.; Yanagita, M.; Nakagawa, T. Glycolysis, but not Mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci. Rep. 2015, 5, 18575. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Liang, W.; Chen, Z.W.; Hu, J.J.; Feng, J.; Cao, Y.; Ma, Y.Q.; Ding, G.H. Mitoquinone Protects Podocytes from Angiotensin II-Induced Mitochondrial Dysfunction and Injury via the Keap1-Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2021, 2021, 1394486. [Google Scholar] [CrossRef]
- Chen, Z.W.; Zhu, Z.J.; Liang, W.; Luo, Z.L.; Hu, J.J.; Feng, J.; Zhang, Z.W.; Luo, Q.; Yang, H.X.; Ding, G.H. Reduction of anaerobic glycolysis contributes to angiotensin II-induced podocyte injury with foot process effacement. Kidney Int. 2023, 103, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, J.P.; Holmes, E.W. Control of the Purine Nucleotide Cycle in Extracts of Rat Skeletal-Muscle—Effects of Energy-State and Concentrations of Cycle Intermediates. Arch. Biochem. Biophys. 1984, 233, 515–529. [Google Scholar] [CrossRef]
- Swain, J.L.; Hines, J.J.; Sabina, R.L.; Harbury, O.L.; Holmes, E.W. Disruption of the Purine Nucleotide Cycle by Inhibition of Adenylosuccinate Lyase Produces Skeletal-Muscle Dysfunction. J. Clin. Investig. 1984, 74, 1422–1427. [Google Scholar] [CrossRef]
- Flanagan, W.F.; Holmes, E.W.; Sabina, R.L.; Swain, J.L. Importance of purine nucleotide cycle to energy production in skeletal muscle. Am. J. Physiol. 1986, 251, C795–C802. [Google Scholar] [CrossRef]
- Lowenstein, J.M. Ammonia production in muscle and other tissues: The purine nucleotide cycle. Physiol. Rev. 1972, 52, 382–414. [Google Scholar] [CrossRef]
- van Waarde, A. Operation of the purine nucleotide cycle in animal tissues. Biol. Rev. Camb. Philos. Soc. 1988, 63, 259–298. [Google Scholar] [CrossRef] [PubMed]
- Cader, M.Z.; Rodrigues, R.P.D.; West, J.A.; Sewell, G.W.; Md-Ibrahim, M.N.; Reikine, S.; Sirago, G.; Unger, L.W.; Inglesias-Romero, A.B.; Ramshorn, K.; et al. FAMIN Is a Multifunctional Purine Enzyme Enabling the Purine Nucleotide Cycle. Cell 2020, 180, 278–295. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, Q.; Xi, Y.M.; Xie, D.; Morisaki, H.; Morisaki, T.; Cheng, J.D. AMPD2 plays important roles in regulating hepatic glucose and lipid metabolism. Mol. Cell Endocrinol. 2023, 577, 112039. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Bontemps, F.; Vincent, M.F.; Van den Bergh, F. The purine nucleotide cycle and its molecular defects. Prog. Neurobiol. 1992, 39, 547–561. [Google Scholar] [CrossRef]
- Stepinski, J.; Pawelczyk, T.; Bizon, D.; Angielski, S. Purine nucleotide cycle in rat renal cortex and medulla under conditions that mimic normal and low oxygen supply. Kidney Int. 1996, 50, 1195–1201. [Google Scholar] [CrossRef]
- Bogusky, R.T.; Steele, K.A.; Lowenstein, L.M. The purine nucleotide cycle in the regulation of ammoniagenesis during induction and cessation of chronic acidosis in the rat kidney. Biochem. J. 1981, 196, 323–326. [Google Scholar] [CrossRef]
- Bogusky, R.T.; Lowenstein, L.M.; Lowenstein, J.M. The purine nucleotide cycle. A pathway for ammonia production in the rat kidney. J. Clin. Investig. 1976, 58, 326–335. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. The anticarcinogenic and anticancer effects of the dietary flavonoid, morin: Current status, challenges, and future perspectives. Phytother. Res. 2021, 35, 6843–6861. [Google Scholar] [CrossRef]
- Carmona Mata, V.; Goldberg, J. Morin and isoquercitrin protect against ischemic neuronal injury by modulating signaling pathways and stimulating mitochondrial biogenesis. Nutr. Neurosci. 2023, 26, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.T.; Song, L.; Chen, T.Y.; Wang, X.; Zhao, X.J.; Ding, X.Q.; Yang, Y.Z.; Pan, Y.; Zhang, D.M.; Kong, L.D. Fructose downregulates miR-330 to induce renal inflammatory response and insulin signaling impairment: Attenuation by morin. Mol. Nutr. Food Res. 2017, 61, 1600760. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ding, X.Q.; Gu, T.T.; Song, L.; Li, J.M.; Xue, Q.C.; Kong, L.D. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic. Biol. Med. 2015, 83, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Lucove, J.; Vupputuri, S.; Heiss, G.; North, K.; Russell, M. Metabolic syndrome and the development of CKD in American Indians: The Strong Heart Study. Am. J. Kidney Dis. 2008, 51, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Akase, T.; Ninomiya, D.; Kumagi, T.; Kikuchi, A. Metabolic syndrome is a predictor of decreased renal function among community-dwelling middle-aged and elderly Japanese. Int. Urol. Nephrol. 2019, 51, 2285–2294. [Google Scholar] [CrossRef]
- Li, T.S.; Chen, L.; Wang, S.C.; Yang, Y.Z.; Xu, H.J.; Gu, H.M.; Zhao, X.J.; Dong, P.; Pan, Y.; Shang, Z.Q.; et al. Magnesium isoglycyrrhizinate ameliorates fructose-induced podocyte apoptosis through downregulation of miR-193a to increase WT1. Biochem. Pharmacol. 2019, 166, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tang, C.; Wang, W.; Pan, Y.; Jiao, R.; Kong, L. Polydatin Ameliorates High Fructose-Induced Podocyte Oxidative Stress via Suppressing HIF-1alpha/NOX4 Pathway. Pharmaceutics 2022, 14, 2202. [Google Scholar] [CrossRef]
- Gu, T.T.; Zhang, D.M.; Wan, Z.Y.; Li, T.S.; Jiao, R.Q.; Chen, T.Y.; Zhao, X.J.; Kong, L.D. Polydatin enhances glomerular podocyte autophagy homeostasis by improving Nrf2-dependent antioxidant capacity in fructose-fed rats. Mol. Cell Endocrinol. 2021, 520, 111079. [Google Scholar] [CrossRef]
- Goodman, M.N.; Lowenstein, J.M. The purine nucleotide cycle. Studies of ammonia production by skeletal muscle in situ and in perfused preparations. J. Biol. Chem. 1977, 252, 5054–5060. [Google Scholar] [CrossRef]
- Lowenstein, J.; Tornheim, K. Ammonia production in muscle: The purine nucleotide cycle. Science 1971, 171, 397–400. [Google Scholar] [CrossRef]
- Strzelecki, T.; Rogulski, J.; Angielski, S. The purine nucleotide cycle and ammonia formation from glutamine by rat kidney slices. Biochem. J. 1983, 212, 705–711. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Cicerchi, C.; Kuwabara, M.; Orlicky, D.J.; Sanchez-Lozada, L.G.; Nakagawa, T.; Johnson, R.J.; Lanaspa, M.A. Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation. Nat. Metab. 2021, 3, 1189–1201. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Jiang, L.; Liu, W.; Hong, H.; Qian, Y.; Li, S.; Huang, W.; Zhao, H.; Yang, Z.; et al. Morin alleviates aflatoxin B1-induced liver and kidney injury by inhibiting heterophil extracellular traps release, oxidative stress and inflammatory responses in chicks. Poult. Sci. 2021, 100, 101513. [Google Scholar] [CrossRef]
- Wei, Z.; He, X.; Kou, J.; Wang, J.; Chen, L.; Yao, M.; Zhou, E.; Fu, Y.; Guo, C.; Yang, Z. Renoprotective mechanisms of morin in cisplatin-induced kidney injury. Int. Immunopharmacol. 2015, 28, 500–506. [Google Scholar] [CrossRef]
- Ni, Z.; Tao, L.; Xiaohui, X.; Zelin, Z.; Jiangang, L.; Zhao, S.; Weikang, H.; Hongchao, X.; Qiujing, W.; Xin, L. Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. J. Cell Physiol. 2017, 232, 2776–2787. [Google Scholar] [CrossRef]
- Kong, Z.L.; Che, K.; Hu, J.X.; Chen, Y.; Wang, Y.Y.; Wang, X.; Lü, W.S.; Wang, Y.G.; Chi, J.W. Orientin Protects Podocytes from High Glucose Induced Apoptosis through Mitophagy. Chem. Biodivers. 2020, 17, e1900647. [Google Scholar] [CrossRef] [PubMed]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Audzeyenka, I.; Bierzynska, A.; Lay, A.C. Podocyte Bioenergetics in the Development of Diabetic Nephropathy: The Role of Mitochondria. Endocrinology 2022, 163, bqab234. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Kovari, I.; Soofi, A.; Nihalani, D.; Patrie, K.; Holzman, L.B. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Investig. 2006, 116, 1346–1359. [Google Scholar] [CrossRef]
- Abe, Y.; Sakairi, T.; Kajiyama, H.; Shrivastav, S.; Beeson, C.; Kopp, J.B. Bioenergetic characterization of mouse podocytes. Am. J. Physiol. Cell Physiol. 2010, 299, C464–C476. [Google Scholar] [CrossRef]
- Yuan, Q.; Miao, J.; Yang, Q.Q.; Fang, L.; Fang, Y.; Ding, H.; Zhou, Y.; Jiang, L.; Dai, C.S.; Zen, K.; et al. Role of pyruvate kinase M2-mediated metabolic reprogramming during podocyte differentiation. Cell Death Dis. 2020, 11, 355. [Google Scholar] [CrossRef]
- Aragon, J.J.; Tornheim, K.; Goodman, M.N.; Lowenstein, J.M. Replenishment of citric acid cycle intermediates by the purine nucleotide cycle in rat skeletal muscle. Curr. Top. Cell Regul. 1981, 18, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef]
- Hu, G.; Xu, L.; Ito, O. Impacts of High Fructose Diet and Chronic Exercise on Nitric Oxide Synthase and Oxidative Stress in Rat Kidney. Nutrients 2023, 15, 2322. [Google Scholar] [CrossRef]
- Tavazzi, B.; Amorini, A.M.; Fazzina, G.; Di Pierro, D.; Tuttobene, M.; Giardina, B.; Lazzarino, G. Oxidative stress induces impairment of human erythrocyte energy metabolism through the oxygen radical-mediated direct activation of AMP-deaminase. J. Biol. Chem. 2001, 276, 48083–48092. [Google Scholar] [CrossRef]
- Igaki, Y.; Osanami, A.; Tanno, M.; Sato, T.; Ogawa, T.; Yano, T.; Kouzu, H.; Miura, T. Inhibition of xanthine oxidase ameliorates functional and metabolic impairment in type 2 diabetic hearts under pressure overload. Eur. Heart J. 2020, 41, ehaa946.3618. [Google Scholar] [CrossRef]
- Fernanda Arias-Santé, M.; Fuentes, J.; Ojeda, C.; Aranda, M.; Pastene, E.; Speisky, H. Amplification of the antioxidant properties of myricetin, fisetin, and morin following their oxidation. Food Chem. 2024, 435, 137487. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, L.; Yu, Q.; Lin, G.; Ma, X.; Li, M.; Guan, F.; Liu, Y.; Huang, X.; Xie, J.; et al. Metformin alleviates long-term high-fructose diet-induced skeletal muscle insulin resistance in rats by regulating purine nucleotide cycle. Eur. J. Pharmacol. 2022, 933, 175234. [Google Scholar] [CrossRef]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Siddaraju, N.; Khan, K.M.; Oommen, A.M.; Jagannath, M.R.; Rao, R.P. Modulation of de novo purine biosynthesis leads to activation of AMPK and results in improved glucose handling and insulin sensitivity. J. Diabetes Metab. Disord. 2014, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Visnjic, D.; Lalic, H.; Dembitz, V.; Tomic, B.; Smoljo, T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, J.H.; Qin, M.T.; Yi, W.J.; Yu, S.; Chen, Y.; Guan, J.; Zhang, R. Amelioration of streptozotocin-induced pancreatic beta cell damage by morin: Involvement of the AMPK-FOXO3-catalase signaling pathway. Int. J. Mol. Med. 2018, 41, 1409–1418. [Google Scholar] [CrossRef]
- Zhang, X.D.; Han, X.P.; Zhang, P.; Zhou, T.T.; Chen, Y.; Jin, J.; Ma, X. Morin attenuates oxidized low-density lipoprotein-mediated injury by inducing autophagy via activating AMPK signalling in HUVECs. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1053–1060. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.X.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.F.; Fong, W.P.; Cheng, C.H.K. The dual actions of morin (3,5,7,2′,4′-pentahydroxyflavone) as a hypouricemic agent: Uricosuric effect and xanthine oxidase inhibitory activity. J. Pharmacol. Exp. Ther. 2006, 316, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Banek, C.T.; Bauer, A.J.; Needham, K.M.; Dreyer, H.C.; Gilbert, J.S. AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1159–H1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, T.; Wang, Z.; Wang, C.; Fang, L.; Kong, L. Loss of Nup155 promotes high fructose-driven podocyte senescence by inhibiting INO80 mRNA nuclear export. J. Adv. Res. 2025, 73, 535–548. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, C.N.; Tain, Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients 2020, 12, 448. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.M.; Gu, T.T.; Ding, X.Q.; Fan, C.Y.; Zhu, Q.; Shi, Y.W.; Hong, Y.; Kong, L.D. Morin reduces hepatic inflammation-associated lipid accumulation in high fructose-fed rats via inhibiting sphingosine kinase 1/sphingosine 1-phosphate signaling pathway. Biochem. Pharmacol. 2013, 86, 1791–1804. [Google Scholar] [CrossRef]
- Nissim, I.; Yudkoff, M.; Segal, S. Effect of 5-amino-4-imidazolecarboxamide riboside on renal ammoniagenesis. Study with [15N]aspartate. J. Biol. Chem. 1986, 261, 6509–6514. [Google Scholar] [CrossRef]
- Ding, X.Q.; Gu, T.T.; Wang, W.; Song, L.; Chen, T.Y.; Xue, Q.C.; Zhou, F.; Li, J.M.; Kong, L.D. Curcumin protects against fructose-induced podocyte insulin signaling impairment through upregulation of miR-206. Mol. Nutr. Food Res. 2015, 59, 2355–2370. [Google Scholar] [CrossRef]
- Koop, K.; Eikmans, M.; Baelde, H.J.; Kawachi, H.; De Heer, E.; Paul, L.C.; Bruijn, J.A. Expression of podocyte-associated molecules in acquired human kidney diseases. J. Am. Soc. Nephrol. 2003, 14, 2063–2071. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.L.; Liu, Z.H.; Pan, Y.; Jiao, R.Q.; Kong, L.D. Atractylodin inhibits fructose-induced human podocyte hypermotility via anti-oxidant to down-regulate TRPC6/p-CaMK4 signaling. Eur. J. Pharmacol. 2021, 913, 174616. [Google Scholar] [CrossRef]
- Guan, T.J.; Qin, F.J.; Du, J.H.; Geng, L.; Zhang, Y.Y.; Li, M. AICAR inhibits proliferation and induced S-phase arrest, and promotes apoptosis in CaSki cells. Acta Pharmacol. Sin. 2007, 28, 1984–1990. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.X.; Fang, L.; Li, T.S.; Liu, Z.H.; Pan, Y.; Kong, L.D. Atractylodes lancea and Magnolia officinalis combination protects against high fructose-impaired insulin signaling in glomerular podocytes through upregulating Sirt1 to inhibit p53-driven miR-221. J. Ethnopharmacol. 2023, 300, 115688. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Wang, Y.M.; Ding, H.; Li, T.S.; Liu, Z.H.; Chen, L.; Jiao, R.Q.; Zhang, D.M.; Kong, L.D. IL-6/STAT3 signaling activation exacerbates high fructose-induced podocyte hypertrophy by ketohexokinase-A-mediated tristetraprolin down-regulation. Cell. Signal. 2021, 86, 110082. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Powers, R.E.; Sabina, R.L.; Theibert, A.B. Elevated adenosine monophosphate deaminase activity in Alzheimer’s disease brain. Neurobiol. Aging 1998, 19, 385–391. [Google Scholar] [CrossRef]
- Akizu, N.; Cantagrel, V.; Schroth, J.; Cai, N.; Vaux, K.; McCloskey, D.; Naviaux, R.K.; Van Vleet, J.; Fenstermaker, A.G.; Silhavy, J.L.; et al. AMPD2 regulates GTP synthesis and is mutated in a potentially treatable neurodegenerative brainstem disorder. Cell 2013, 154, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.S.; Steinberg, J.R.; Svingen, B.A.; Luecke, R.W. Increased purine nucleotide cycle activity associated with dietary zinc deficiency. Biochem. Biophys. Res. Commun. 1977, 78, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Momose, H.; Shio, I. Regulation of purine nucleotide synthesis in Bacillus subtilis. II. Specificity of purine derivatives for enzyme repression. J. Biochem. 1967, 62, 92–98. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Marti-Renom, M.A.; Stuart, A.C.; Fiser, A.; Sanchez, R.; Melo, F.; Sali, A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 291–325. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5–6. [Google Scholar] [CrossRef]
- Fiser, A.; Do, R.K.; Sali, A. Modeling of loops in protein structures. Protein Sci. 2000, 9, 1753–1773. [Google Scholar] [CrossRef]
- Han, B.W.; Bingman, C.A.; Mahnke, D.K.; Bannen, R.M.; Bednarek, S.Y.; Sabina, R.L.; Phillips, G.N., Jr. Membrane association, mechanism of action, and structure of Arabidopsis embryonic factor 1 (FAC1). J. Biol. Chem. 2006, 281, 14939–14947. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; Pintro, V.O.; de Azevedo, W.F., Jr. Docking with AutoDock4. Methods Mol. Biol. 2019, 2053, 125–148. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Ampd2 | TTGATAGCGTGGATGATGAG | CCCGTGGGAGATGTTCTCGG |
| Ampd3 | GTTGGCGGAGAAGGTGTTTG | CTGCGACCGGATCATCTTGAA |
| Adss | TGCAAACGCAGCATTGTTAGA | GGAAAGGCACCAATACCAACTC |
| Adsl | TACTTCAGCCCCATCCACTC | TCACTGTAACCGGGTTCTCC |
| β-actin | GGGAAATCGTGCGTGAC | AGGCTGGAAAAGAGCCT |
| Ampd2 siRNA | GUGCAUGCGGACAGGAAUATT | UAUUCCUGUCCGCAUGCACTT |
| Ampd3 siRNA | GGAAGAUGCUGGAGAACAUTT | AUGUUCUCCAGCAUCUUCCTT |
| Negative control siRNA | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wan, Z.; Huang, L.; Zhou, Z.; Wang, W.; Xing, Y.; Li, S.; Du, Y.; Huang, J.; Wu, Y.; et al. Morin Alleviates Fructose-Driven Disturbance of Podocyte Mitochondrial Energy Metabolism by Inhibiting Adenosine 5′-Monophosphate Deaminase Activity to Improve Glomerular Injury. Pharmaceuticals 2025, 18, 1883. https://doi.org/10.3390/ph18121883
Yang Y, Wan Z, Huang L, Zhou Z, Wang W, Xing Y, Li S, Du Y, Huang J, Wu Y, et al. Morin Alleviates Fructose-Driven Disturbance of Podocyte Mitochondrial Energy Metabolism by Inhibiting Adenosine 5′-Monophosphate Deaminase Activity to Improve Glomerular Injury. Pharmaceuticals. 2025; 18(12):1883. https://doi.org/10.3390/ph18121883
Chicago/Turabian StyleYang, Yingzhi, Ziyan Wan, Luyi Huang, Ziang Zhou, Wanru Wang, Yu Xing, Shijie Li, Yufan Du, Jiufang Huang, Yanqing Wu, and et al. 2025. "Morin Alleviates Fructose-Driven Disturbance of Podocyte Mitochondrial Energy Metabolism by Inhibiting Adenosine 5′-Monophosphate Deaminase Activity to Improve Glomerular Injury" Pharmaceuticals 18, no. 12: 1883. https://doi.org/10.3390/ph18121883
APA StyleYang, Y., Wan, Z., Huang, L., Zhou, Z., Wang, W., Xing, Y., Li, S., Du, Y., Huang, J., Wu, Y., Fan, M., Li, J., Kong, L., & Zhang, D. (2025). Morin Alleviates Fructose-Driven Disturbance of Podocyte Mitochondrial Energy Metabolism by Inhibiting Adenosine 5′-Monophosphate Deaminase Activity to Improve Glomerular Injury. Pharmaceuticals, 18(12), 1883. https://doi.org/10.3390/ph18121883





