3.2. Synthesis
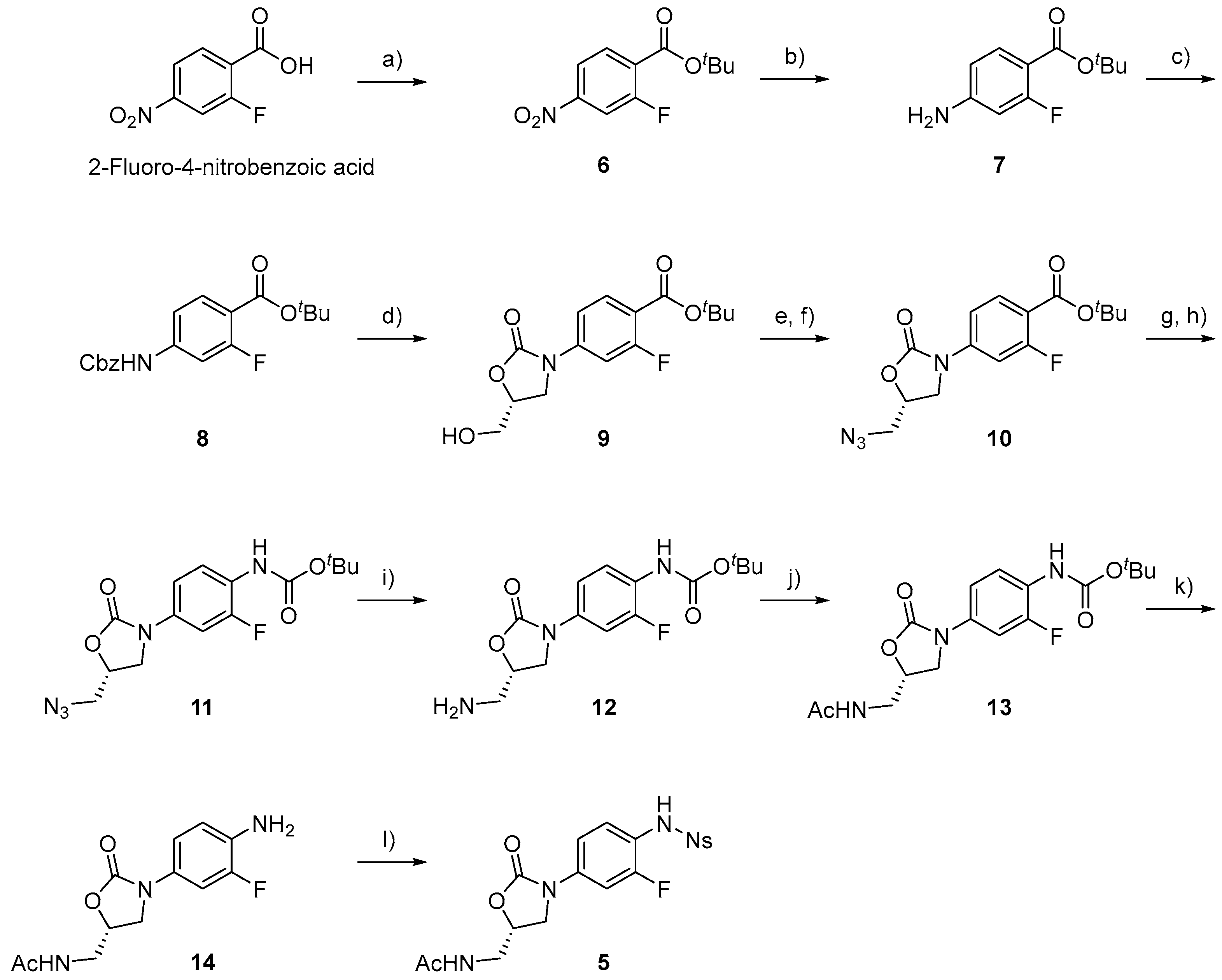
3.2.1. tert-Butyl 2-fluoro-4-nitrobenzoate (6) [20]
To a solution of 2-fluoro-4-nitrobenzoic acid (6.0 g, 32 mmol) in dichloromethane (DCM) (60 mL), triethylamine (TEA) (13 mL, 93 mmol), dimethylaminopyridine (DMAP) (1.2 g, 9.8 mmol), and di-tert-butyl dicarbonate (Boc2O) (11 mL, 49 mmol) were added. After stirring for 2 h at room temperature, the residue was treated with ethyl acetate (EtOAc) and 5% citric acid solution. The organic layer was washed with brine and dried over anhydrous Na2SO4. The filtrate was concentrated under reduced pressure to obtain a colorless solid 6 (6.6 g, 83%). M.P. 81–82 °C. 1H-NMR (600 MHz, CDCl3) δ 8.07–8.03 (m, 2H), 7.99–7.97 (m, 1H), 1.62 (s, 9H).
3.2.2. tert-Butyl 4-amino-2-fluorobenzoate (7) [20]
To a solution of 6 (1.1 g, 5.0 mmol) in methanol (MeOH) (30 mL), Pd/C (200 mg) was added. After stirring for 2 h under a H2 atmosphere at room temperature, the mixture was filtered through a Celite pad. The filtrate was concentrated under reduced pressure, and a colorless solid 7 was obtained (1.0 g, quant.). M.P. 94–96 °C. 1H-NMR (600 MHz, CDCl3) δ 7.68 (t, J = 8.6 Hz), 6.39 (dd, J = 2.4, 8.6 Hz), 6.31 (dd, J = 2.0, 12.7 Hz), 4.13 (bs, 2H), and 1.57 (s, 9H). HRMS (ESI): m/z calculated for C11H14FNNaO2: 234.0906, found [M + Na]+ 234.0914.
3.2.3. tert-Butyl 4-(((benzyloxy)carbonyl)amino)-2-fluorobenzoate (8) [21]
To a solution of 7 (3.8 g, 18 mmol) in pyridine (Py) (35 mL), benzyl chloroformate (13 g, 73 mmol) was added dropwise at 0 °C. The mixture was allowed to warm to room temperature. After stirring overnight at room temperature, the mixture was diluted with EtOAc. The residue was treated with EtOAc and 5% hydrochloric acid. The organic layer was washed with brine and dried over anhydrous Na2SO4. The filtrate was concentrated under reduced pressure. The resulting residue was separated by column chromatography (C.C.) (hexane/EtOAc = 6:1) to yield 8 as a white solid (6.2 g, 99%). M.P. 115–120 °C. 1H-NMR (600 MHz, CDCl3) δ 7.80 (t, J = 8.1 Hz, 1H), 7.41–7.35 (m, 6H), 7.04–6.97 (m, 2H), 5.21 (s, 2H), 1.58 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 163.2 (d, J = 4.3 Hz), 162.8 (d, J = 258.7 Hz), 152.7, 143.0 (d, J = 11.6 Hz), 135.5, 132.8, 128.7, 128.6, 128.4, 114.9 (d, J = 10.1 Hz), 113.0, 106.3 (d, J = 28.9 Hz), and 81.6, 67.5, 28.2. HRMS (ESI): m/z calculated for C19H20FNNaO4: 368.1274, found [M + Na]+ 368.1264.
3.2.4. tert-Butyl (R)-2-fluoro-4-(5-(hydroxymethyl)-2-oxooxazolidin-3-yl)benzoate (9) [21]
To a solution of 8 (2.8 g, 8.0 mmol) in THF (80 mL), n-butyllithium in hexane (6 mL, 9.6 mmol) was added dropwise at −78 °C. After stirring for 1 h at −78 °C, (R)-glycidyl butyrate (1.2 mL, 8.8 mmol) was added, and the mixture was allowed to warm to room temperature. After stirring overnight at room temperature, saturated NH4Cl was added, and the organic materials were extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by C.C. (hexane/EtOAc = 1:1) to yield 9 as a white solid (1.8 g, 71%). M.P. 143–145 °C. 1H-NMR (600 MHz, CDCl3) δ 7.87 (t, J = 8.4 Hz, 1H), 7.47 (dd, J = 13.1, 2.4 Hz, 1H), 7.26 (dd, J = 8.9, 2.4 Hz, 1H), 4.79–4.77 (m, 1H), 4.13–4.00 (m, 3H), 3.77 (d, J = 12.4 Hz, 1H), 1.59 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 163.0 (d, J = 4.3 Hz), 162.3 (d, J = 258.6 Hz), 154.3, 142.8 (d, J = 11.5 Hz), 132.8, 115.3 (d, J = 8.7 Hz), 112.4 (d, J = 2.9 Hz), 106.0 (d, J = 28.9 Hz), and 81.9, 62.5, 46.0, 28.2. HRMS (ESI): m/z calculated for C15H18FNNaO5: 334.1067, found [M + Na]+ 334.1076. = −118.5° (c 0.10, CHCl3).
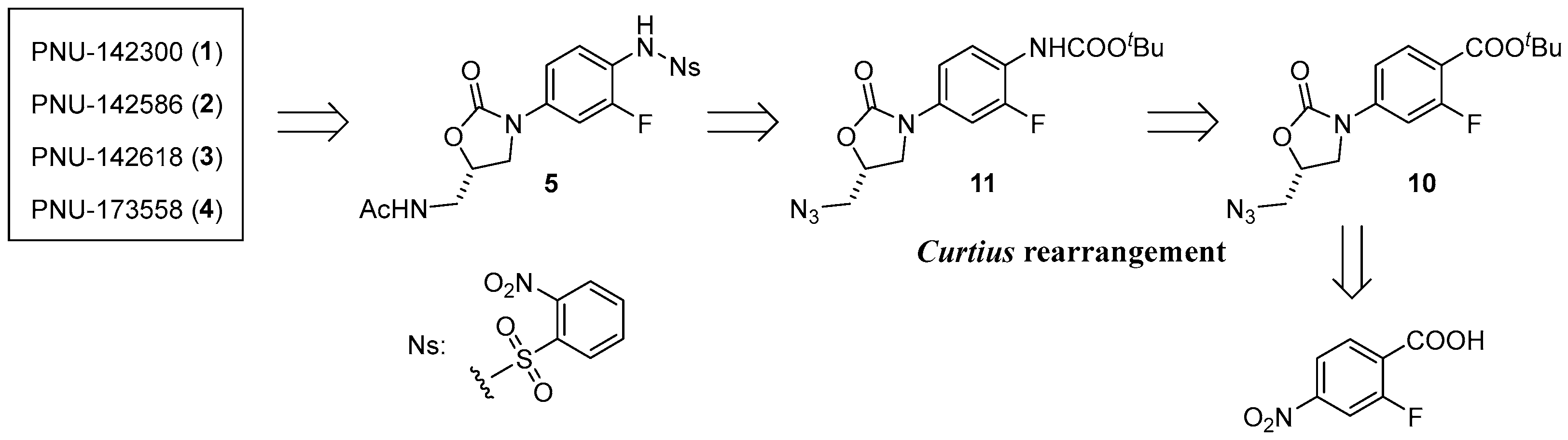
3.2.5. tert-Butyl (R)-4-(5-(azidomethyl)-2-oxooxazolidin-3-yl)-2-fluorobenzoate (10) [21]
To a solution of 9 (2.5 g, 8.0 mmol) in DCM (80 mL), TEA (7 mL) and methanesulphonyl chloride (1.9 mL, 24 mmol) were added dropwise at 0 °C, and the mixture was allowed to warm to room temperature. After stirring for 1 h at room temperature, the solvent was removed by vacuum drying. The reaction mixture was diluted with water, and the organic material was extracted using EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue (3.1 g, 8.0 mmol) was solved in DMF (35 mL), sodium azide (0.9 g, 15 mmol) was added. After stirring overnight at 60 °C, the mixture was diluted with hexane/EtOAc = 1:1. The organic layer was washed with brine, dried over anhydrous Na2SO4, and the filtrate was concentrated under reduced pressure. The resulting residue was separated by C.C. (hexane/EtOAc = 6:1) to yield 10 as a white solid (2.2 g, 82%). M.P. 93–95 °C. 1H-NMR (600 MHz, CDCl3) δ 7.87 (t, J = 8.4 Hz, 1H), 7.47 (dd, J = 13.1, 2.1 Hz, 1H), 7.26 (dd, J = 8.8, 2.2 Hz, 1H), 4.87–4.83 (m, 1H), 4.14–4.09 (m, 1H), 3.88 (dd, J = 8.9, 6.2 Hz, 1H), 3.76 (dd, J = 13.4, 4.1 Hz, 1H), 3.62 (dd, J = 13.4, 4.5 Hz, 1H), 1.59 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 162.9 (d, J = 2.9 Hz), 162.4 (d, J = 258.7 Hz), 153.5, 142.7 (d, J = 11.5 Hz), 132.8, 115.5 (d, J = 8.6 Hz), 112.4 (d, J = 2.9 Hz), 106.1 (d, J = 28.9 Hz), and 81.9, 70.9, 52.9, 47.0, 28.2. HRMS (ESI): m/z calculated for C15H17FN4NaO4: 359.1132, found [M + Na]+ 359.1133. = −320.5° (c 0.20, CHCl3).
3.2.6. tert-Butyl (R)-(4-(5-(azidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)carbamate (11) [22]
To a solution of 10 (672 mg, 2.0 mmol) in DCM (4 mL) was added TFA (4 mL, 52 mmol) dropwise at 0 °C, and the mixture was allowed to warm to room temperature. After stirring overnight at room temperature, the solvent was removed in vacuo. To a solution of the resulting residue (565 mg, 2.0 mmol) in THF (7.5 mL), tert-butyl alcohol (t-BuOH) (950 μL, 10 mmol), TEA (360 μL, 2.6 mmol), and diphenylphosphoryl azide (DPPA) (560 μL, 2.6 mmol) were added. After stirring for 2 h at room temperature and overnight at 70 °C, the solvent was concentrated under reduced pressure, and the mixture was diluted with EtOAc. Saturated NaHCO3 was added to the mixture, and the organic materials were extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by C.C. (hexane/EtOAc = 1:1) to yield 11 as a white solid (390 mg, 56%). M.P. 85–88 °C. 1H-NMR (600 MHz, CDCl3) δ 7.66–7.64 (m, 1H), 6.98 (d, J = 8.6 Hz, 1H), 6.67 (s, 1H), 4.79 (qd, J = 4.5, 1.7 Hz, 1H), 4.06 (t, J = 8.9 Hz, 1H), 3.83 (dd, J = 8.9, 6.2 Hz, 1H), 3.71 (dd, J = 13.4, 4.5 Hz, 1H), 3.59 (dd, J = 13.2, 4.6 Hz, 1H), 1.53 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 153.8, 152.4, 152.0 (d, J = 53.5 Hz), 133.1 (d, J = 10.1 Hz), 123.2 (d, J = 10.1 Hz), 113.3 (d, J = 2.8 Hz), 106.1 (d, J = 24.6 Hz),and 81.1, 70.6, 53.0, 47.4, 28.3. HRMS (ESI): m/z calculated for C15H18FN5NaO4: 374.1241, found [M + Na]+ 374.1241. [ = −178.0° (c 0.20, CHCl3).
3.2.7. tert-Butyl (S)-(4-(5-(aminomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)carbamate (12) [22]
To a solution of 11 (754 mg, 2.1 mmol) in methanol (MeOH) (50 mL), Pd/C (301 mg) was added. After stirring for 1 h under a H2 atmosphere at room temperature, the mixture was filtered through a Celite pad. The filtrate was concentrated under reduced pressure to obtain a colorless solid 12 (704 mg, quant.). M.P. 95–100 °C. 1H-NMR (600 MHz, CDCl3) δ 7.68 (d, J = 13.4 Hz, 1H), 6.99 (d, J = 8.9 Hz, 1H), 6.68 (bs, 1H), 4.68–4.66 (m, 1H), 4.02 (t, J = 8.8 Hz, 1H), 3.83 (t, J = 7.6 Hz, 1H), 3.11 (dd, J = 13.6, 4.0 Hz, 1H), 2.98 (dd, J = 13.6, 5.7 Hz, 1H), 1.53 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 154.5, 152.4, 152.0 (d, J = 243.0 Hz), 133.5 (d, J = 10.1 Hz), 129.7 (d, J = 24.6 Hz), 122.9 (d, J = 10.1 Hz), 113.2, 105.9 (d, J = 24.6 Hz), and 81.1, 73.8, 47.6, 44.9, 28.3. HRMS (ESI): m/z calculated for C15H20FN3NaO4: 348.1336, found [M + Na]+ 348.1329. = −119.0° (c 0.20, CHCl3).
3.2.8. tert-Butyl (S)-(4-(5-(acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)carbamate (13)
To a solution of 12 (978 mg, 3.0 mmol) in DCM (7 mL), TEA and Ac2O (473 μL, 4.3 mmol) were added dropwise at 0 °C, and the mixture was allowed to warm to room temperature. After stirring for 0.5 h at room temperature, the solvent was removed in vacuo, and a colorless solid 13 was obtained (814 mg, quant.). M.P. 105–108 °C. 1H-NMR (600 MHz, CDCl3) δ 7.90–8.17 (bs, 1H), 7.55 (dd, J = 13.1, 2.4 Hz, 1H), 6.99 (dd, J = 8.9, 1.4 Hz, 1H), 6.69 (bs, 1H), 6.59 (t, J = 6.2 Hz, 1H), 4.80–4.76 (m, 1H), 4.02 (t, J = 8.9 Hz, 1H), 3.76 (dd, J = 9.1, 6.7 Hz, 1H), 3.68–3.63 (m, 2H), 2.03 (s, 3H), 1.53 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ171.4, 154.4, 152.5, 152.0 (d, J = 242.8 Hz), 133.1 (d, J = 10.5 Hz), 123.2 (d, J = 11.5 Hz), 113.5 (d, J = 2.8 Hz), 106.0 (d, J = 2.6 Hz), and 81.2, 72.0, 47.5, 41.9, 28.3, 23.0. HRMS (ESI): m/z calculated for C17H22FN3NaO5: 390.1441, found [M + Na]+ 390.1432. = −102.5° (c 0.20, CHCl3).
3.2.9. (S)-N-((3-(4-Amino-3-fluorophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (14)
To a solution of 13 (332 mg, 0.9 mmol) in DCM (1 mL), TFA (1 mL) was added. After stirring for 1 h at room temperature, the solvent was removed in vacuo, and a colorless solid 14 was obtained (215 mg, 90%). M.P. 92–95 °C. 1H-NMR (600 MHz, CD3OD) δ 7.58 (dd, J = 12.7, 2.4 Hz, 1H), 7.49 (t, J = 8.6 Hz, 1H), 7.23–7.21 (m, 1H), 4.73–4.69 (m, 1H), 4.06 (t, J = 8.9 Hz, 1H), 3.73 (dd, J = 9.1, 6.4 Hz, 1H), 3.47–3.46 (m, 2H), 1.86 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ 172.8, 156.7, 156.1 (d, J = 37.5 Hz), 155.0 (d, J = 13.0 Hz), 138.5 (d, J = 10.1 Hz), 126.6, 118.3 (d, J = 13.0 Hz), 113.4 (d, J = 2.9 Hz), 106.0 (d, J = 24.6 Hz), 72.2, 41.8, 21.1.
3.2.10. (S)-N-((3-(3-Fluoro-4-((2-nitrophenyl)sulfonamido)phenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (5)
To a solution of 14 (59 mg, 0.2 mmol) in pyridine (Py) (1.5 mL), 2-nitrobenzenesulfonyl (Ns) chloride was added. After stirring for 1.5 h at room temperature, the solvent was concentrated under reduced pressure, and the mixture was diluted with CHCl3. Saturated NH4Cl was added to the mixture, and the organic materials were extracted with CHCl3. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was separated by C.C. (CHCl3/MeOH = 40:1) to yield 5 as a yellow solid (91 mg, 92%). M.P. 98–100 °C. 1H-NMR (600 MHz, CDCl3) δ 7.92 (dd, J = 7.9, 1.0 Hz, 1H), 7.81 (dd, J = 7.9, 1.4 Hz, 1H), 7.75 (td, J = 7.7, 1.4 Hz, 1H), 7.63 (td, J = 7.7, 1.1 Hz, 1H), 7.54 (t, J = 8.6 Hz, 1H), 7.46 (dd, J = 12.4, 2.4 Hz, 1H), 7.10 (dt, J = 8.9, 1.4 Hz, 1H), 6.42 (t, J = 6.2 Hz, 1H), 4.80–4.78 (m, 1H), 4.03 (t, J = 9.1 Hz, 1H), 3.77 (dd, J = 9.1, 6.7 Hz, 1H), 3.68–3.63 (m, 2H), 2.01 (s, 3H), 13C-NMR (151 MHz, CDCl3) δ171.4, 155.8 (d, J = 247.1 Hz), 154.2, 147.9, 137.7 (d, J = 10.1 Hz), 134.3, 132.8, 132.6, 131.2, 127.5, 125.7, 119.0 (d, J = 14.5 Hz), 113.6, 113.6, 106.1 (d, J = 26.0 Hz), and 72.1, 47.4, 41.8, 23.1. HRMS (ESI): m/z calculated for C18H17FN4NaO7S: 475.0700, found [M + Na]+ 475.0705. = −70.5° (c 0.20, CHCl3).
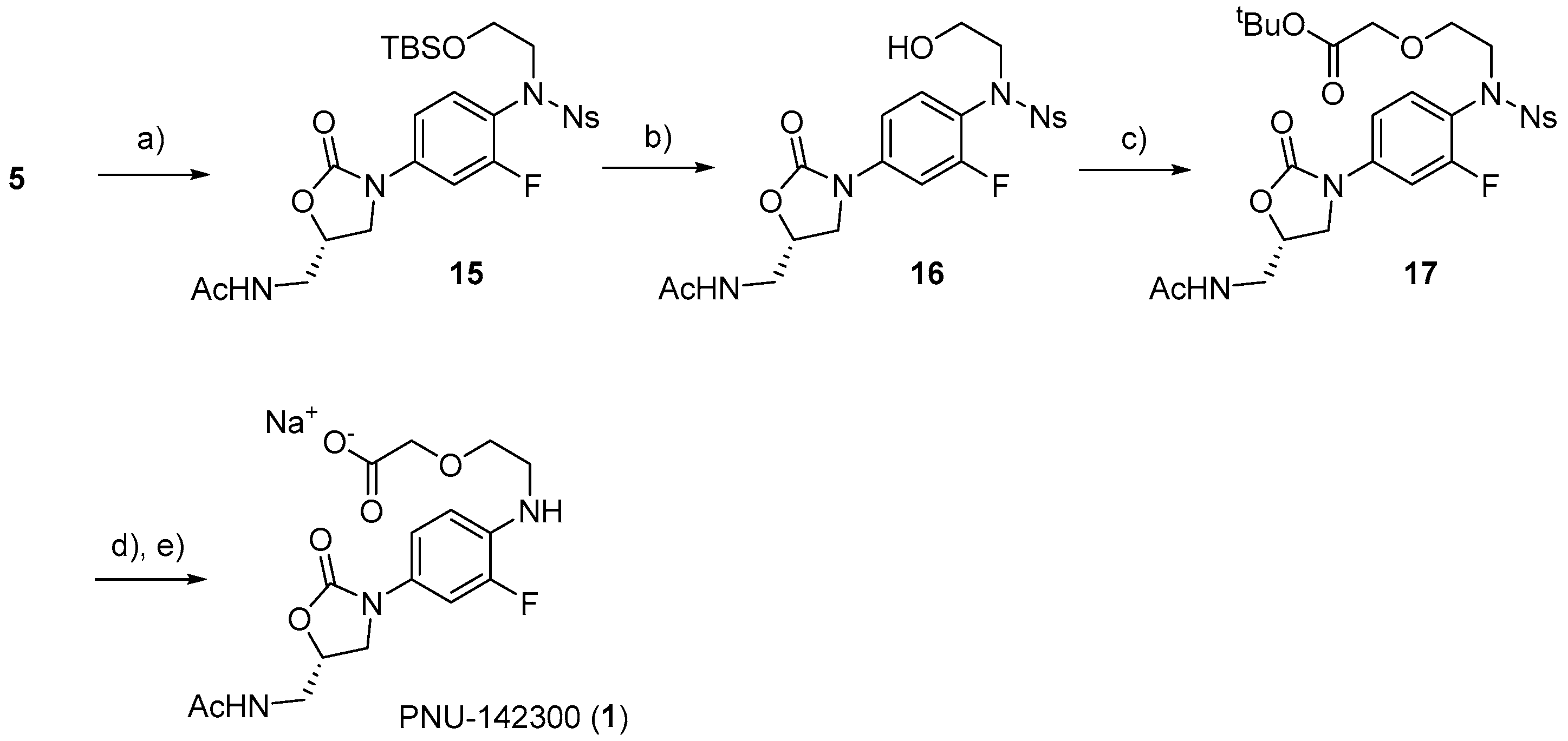
3.2.11. (S)-N-((3-(4-((N-(2-((tert-Butyldimethylsilyl)oxy)ethyl)-2-nitrophenyl)sulfonamido)-3-fluorophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (15)
To a solution of 5 (113 mg, 0.25 mmol) in DMF (0.3 mL), (2-bromoethoxy)-tert-butyldimethylsilane (107 μL, 0.5 mmol) and K2CO3 (52 mg, 0.75 mmol) were added. After stirring for 2 h at room temperature and 48 h at 50 °C, the reaction mixture was diluted with water, and the organic material was extracted with hexane/EtOAc (1:1). The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was separated by C.C. (CHCl3/MeOH = 40:1) to yield 15 as a yellow solid (137 mg, 86%). M.P. 88–90 °C. 1H-NMR (600 MHz, CDCl3) δ 6.80–6.77 (m, 2H), 6.70 (dd, J = 7.7, 0.9 Hz, 1H), 6.67–6.60 (m, 2H), 6.53 (t, J = 8.8 Hz, 1H), 6.22 (dd, J = 8.6, 2.1 Hz, 1H), 5.28 (t, J = 6.2 Hz, 1H), 3.90–3.88 (m, 1H), 3.15 (t, J = 9.1 Hz, 1H), 2.94–2.87 (m, 3H), 2.82–2.78 (m, 3H), 2.74–2.71 (m, 1H), 1.13 (s, 3H), -0.08 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 171.1,159.7 (d, J = 251.4 Hz), 154.0, 148.0, 139.7 (d, J = 10.2 Hz), 134.1, 133.7, 132.4, 131.3, 131.0, 124.0, 121.0 (d, J = 13.0 Hz), 113.1, 106.5 (d, J = 26.0 Hz), and 72.0, 61.1, 53.4, 47.4, 41.9, 25.8, 23.1, 18.1, -5.5. HRMS (ESI): m/z calculated for C26H35FN4NaO8SSi: 633.1827, found [M + Na]+ 633.1805. = −87.5° (c 0.20, CHCl3).
3.2.12. (S)-N-((3-(3-Fluoro-4-((N-(2-hydroxyethyl)-2-nitrophenyl)sulfonamido)phenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (16)
To a solution of 15 (137 mg, 0.23 mmol) in THF (0.5 mL), Tetrabutylammonium fluoride (TBAF) (350 μL, 0.35 mmol) was added dropwise at 0 °C, and the mixture was allowed to warm to room temperature. After stirring for 2 h at room temperature, the solvent was removed in vacuo. The reaction mixture was diluted with water, and the organic material was extracted with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was separated by C.C. (CHCl3/MeOH = 10:1) to yield 16 as a yellow solid (99 mg, 85%). M.P. 102–104 °C. 1H-NMR (600 MHz, CD3OD) δ 7.80 (ddd, J = 8.3, 7.0, 1.1 Hz, 1H), 7.74 (dd, J = 7.9, 1.4 Hz, 1H), 7.70 (dd, J = 7.9, 1.4 Hz, 1H), 7.66 (td, J = 7.6, 1.1 Hz, 1H), 7.56 (dd, J = 13.1, 2.4 Hz, 1H), 7.43 (t, J = 8.6 Hz, 1H), 7.31–7.29 (m, 1H), 4.81–4.80 (m, 1H), 4.15 (t, J = 8.9 Hz, 1H), 3.82 (dd, J = 9.3, 6.2 Hz, 3H), 3.59–3.56 (m, 4H), 1.96 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ 174.1, 161.2 (d, J = 250.0 Hz), 156.2, 149.6, 142.1 (d, J = 11.5 Hz), 135.6, 135.0, 133.0, 132.6, 132.2, 125.3, 121.6 (d, J = 13.0 Hz), 114.8, 107.5 (d, J = 27.4 Hz), and 73.6, 60.8, 54.5, 49.9, 49.5, 49.3, 49.2, 49.0, 48.9, 48.8, 48.6, 43.1, 22.5. HRMS (ESI): m/z calculated for C20H22FN4O8S: 497.1142, found [M + H]+ 497.1154. = −64.5° (c 0.20, CH3OH).
3.2.13. tert-Butyl (S)-2-(2-((N-(4-(5-(acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)-2-nitrophenyl)sulfonamido)ethoxy)acetate (17)
To a solution of 16 (30 mg, 0.06 mmol) in DCM (1 mL), tert-butyl bromoacetate (13 μL, 0.09 mmol), tetrabutylammonium hydrogen sulfate (20 mg, 0.06 mmol), and 1 M NaOH (90 μL, 0.09mmol) were added. After stirring vigorously for 3 h at room temperature, 1 M NaOH (90 μL, 0.09 mmol) was added to the reaction mixture. After stirring vigorously for 1 h at room temperature, the reaction mixture was diluted with water, and the organic material was extracted with DCM. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was separated by HPLC (CHCl3/MeOH = 40:1) to yield 17 as a yellow solid (16 mg, 75% based on conversion yield, 43% recovered 16). 1H-NMR (600 MHz, CDCl3) δ 7.73–7.68 (m, 2H), 7.61 (dd, J = 7.9, 1.4 Hz, 1H), 7.58–7.52 (m, 2H), 7.47 (t, J = 8.6 Hz, 1H), 7.13 (dd, J = 8.9, 2.4 Hz, 1H), 6.17 (t, J = 6.0 Hz, 1H), 4.79 (q, J = 3.0 Hz, 1H), 4.06 (t, J = 8.9 Hz, 1H), 3.92 (s, 2H), 3.78 (dd, J = 9.3, 6.9 Hz, 1H), 3.73–3.66 (m, 3H), 3.64–3.60 (m, 1H), 2.04 (s, 3H), 1.46 (s, 9H), 13C-NMR (151 MHz, CDCl3) δ 172.4, 160.8 (d, J = 248.7 Hz), 151.0 (d, J = 11.6 Hz), 147.9, 137.8, 133.7, 133.1, 132.3, 131.5, 131.4, 123.8, 123.2, 112.1 (d, J = 11.5 Hz), 109.9, 108.9, 99.5 (d, J = 24.6 Hz), and 69.1, 59.9, 59.0, 54.0, 46.5, 43.7, 29.7, 24.0, 23.1, 19.7, 13.7. HRMS (ESI): m/z calculated for C26H31FN4NaO10S: 633.1643, found [M + Na]+ 633.1651. = −77.0° (c 0.20, CHCl3).
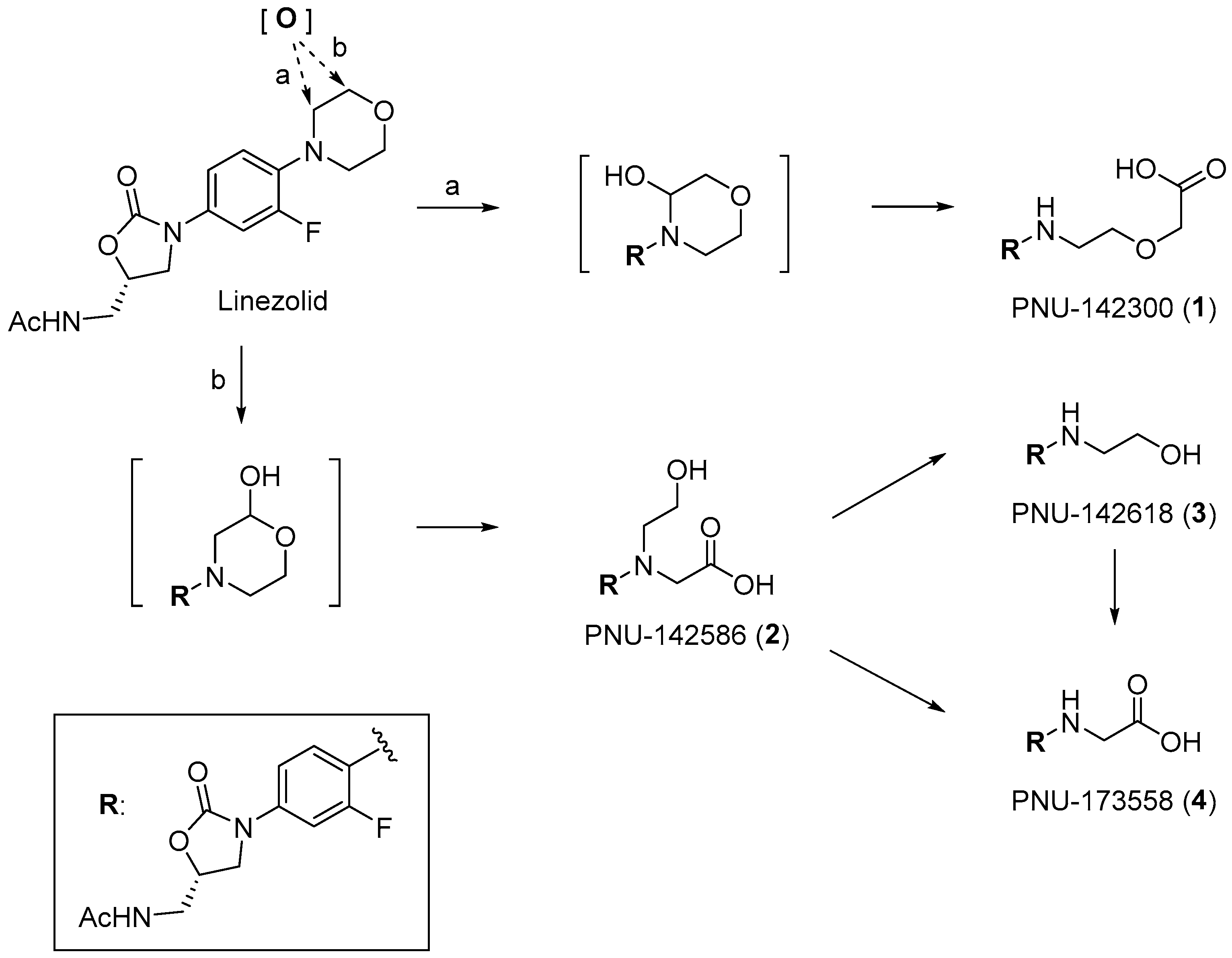
3.2.14. (S)-2-(2-((4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)amino)ethoxy)acetic acid (1)
To a solution of 17 (30 mg, 0.06 mmol) in DMF (300 μL), K2CO3 (30 mg, 0.22 mmol) and thiophenol (17 μL, 0.17 mmol) were added. After stirring for 6 h at room temperature, the reaction mixture was diluted with water, and the organic material was extracted with DCM. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by HPLC (CHCl3/MeOH = 40:1) to yield a colorless solid (16 mg, 86%). To a solution of the solid (15 mg, 0.04 mmol) in DCM (440 μL), TFA was added. After stirring for 1 h at room temperature and concentrating under reduced pressure, a colorless solid was obtained. To a solution of the solid in water, 1 M NaOH (60 μL) was added dropwise. The crude solution was purified using Sep Pak C18 (H2O-MeOH) to yield PNU-142300 (1) as a sodium salt (19 mg, 94%). M.P. 101–103 °C. 1H-NMR (600 MHz, CD3OD) δ 7.34 (dd, J = 13.4, 2.4 Hz, 1H), 7.03 (dt, J = 8.8, 1.3 Hz, 1H), 6.79 (t, J = 9.1 Hz, 1H), 4.75 (dd, J = 8.9, 6.2 Hz, 1H), 4.08 (t, J = 8.9 Hz, 1H), 3.89 (s, 2H), 3.75 (dd, J = 9.1, 6.4 Hz, 1H), 3.69 (t, J = 5.5 Hz, 2H), 3.54 (d, J = 4.8 Hz, 2H), 3.33 (t, J = 5.3 Hz, 2H), 1.97 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ 178.1, 174.1, 157.1, 153.4 (d, J = 238.4 Hz), 135.6 (d, J = 11.5 Hz), 129.0, 116.9, 113.5 (d, J = 4.3 Hz), and 108.5, 108.4, 73.4, 71.7, 70.3, 49.8, 44.5, 43.2, 22.5. HRMS (ESI): m/z calculated for C16H20FN3NaO6: 392.1234, found [M + H]+ 392.1219. = −63.0° (c 0.20, CH3OH).
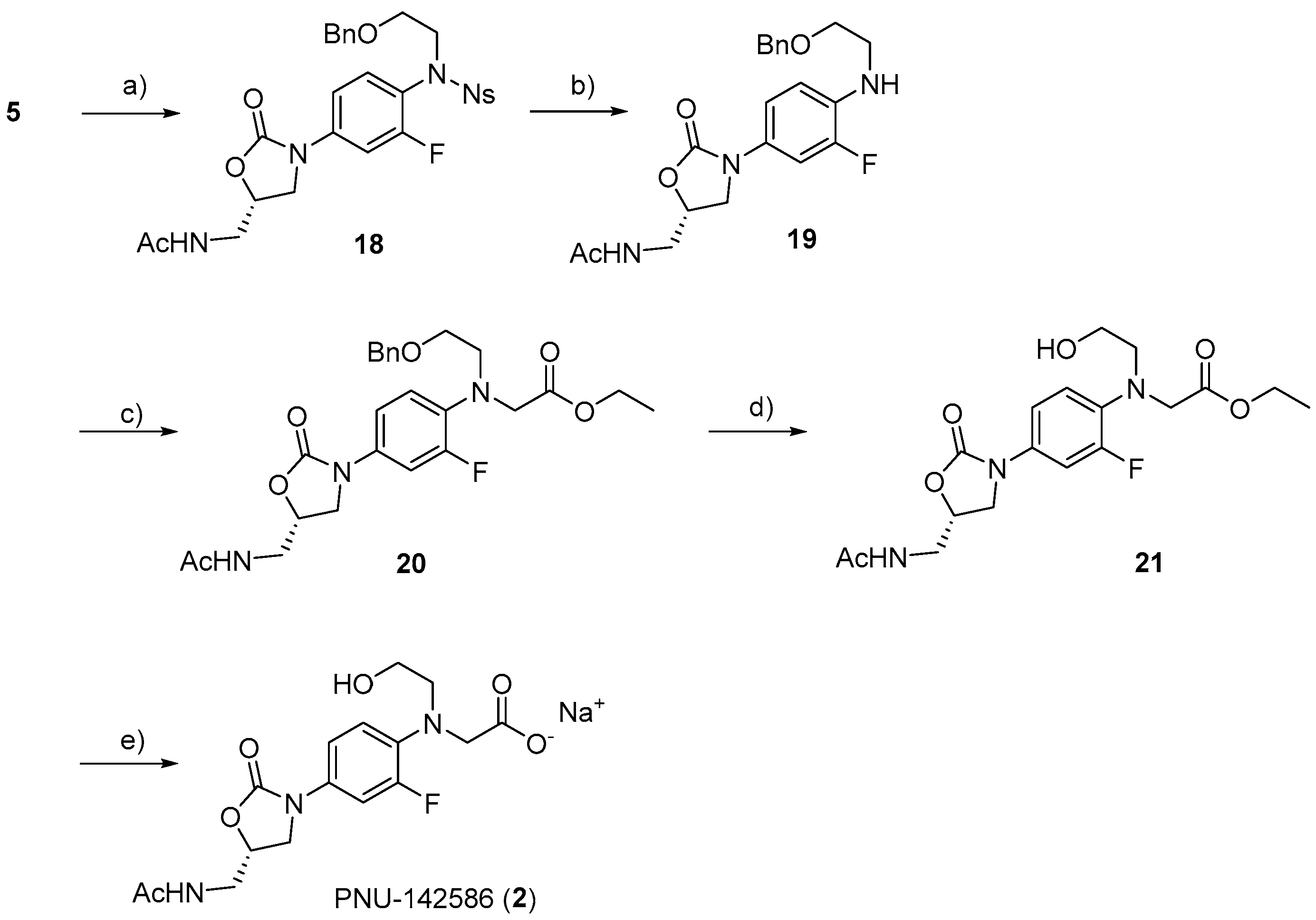
3.2.15. (S)-N-((3-(4-((N-(2-(Benzyloxy)ethyl)-2-nitrophenyl)sulfonamido)-3-fluorophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (18)
To a solution of 5 (45 mg, 0.1 mmol) in DMF (0.5 mL), K2CO3 (21 mg, 0.15 mmol) and benzyl 2-bromoethyl ether (32 μL, 0.2 mmol) were added. After stirring overnight at 50 °C, the reaction mixture was diluted with water, and the organic material was extracted with DCM. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by C.C. (CHCl3/MeOH = 30:1) to yield 18 as a colorless solid (137 mg, 93%). 1H-NMR (600 MHz, CDCl3) δ 7.72 (dd, J = 8.1, 1.2 Hz, 1H), 7.65 (td, J = 7.7, 1.4 Hz, 1H), 7.58 (dd, J = 8.1, 1.2 Hz, 1H), 7.51–7.48 (m, 2H), 7.35–7.25 (m, 4H), 7.22 (d, J = 6.5 Hz, 2H), 7.08 (dd, J = 8.9, 2.4 Hz, 1H), 6.42 (t, J = 6.2 Hz, 1H), 4.79–4.75 (m, 1H), 4.43 (s, 2H), 4.02–3.96 (m, 2H), 3.75 (dd, J = 9.1, 6.7 Hz, 1H), 3.67 (ddd, J = 14.6, 6.0, 3.6 Hz, 1H), 3.62–3.56 (m, 3H), 2.01 (s, 3H), 13C-NMR (151 MHz, CDCl3) δ 171.3, 159.8 (d, J = 252.9 Hz), 154.0, 148.0, 139.8 (d, J = 10.1 Hz), 137.8, 133.8 (d, J = 8.6 Hz), 132.3, 131.4, 131.1, 128.3, 127.7, 127.7, 123.9, 120.5 (d, J = 11.5 Hz), 113.3, 106.4 (d, J = 26.0 Hz), and 72.8, 72.0, 67.9, 50.9, 47.4, 41.8, 23.1. HRMS (ESI): m/z calculated for C27H27FN4NaO8S: 609.1431, found [M + Na]+ 609.1418. = −85.5° (c 0.20, CHCl3).
3.2.16. (S)-N-((3-(4-((2-(Benzyloxy)ethyl)amino)-3-fluorophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (19)
To a solution of 18 (79 mg, 0.14 mmol) in DMF (0.5 mL), K2CO3 (75 mg, 0.54 mmol), and thiophenol (41 μL, 0.4 mmol) were added. After stirring for 6 h at room temperature, the reaction mixture was diluted with water, and the organic material was extracted with DCM. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by HPLC (CHCl3/MeOH = 40:1) to yield a colorless solid 19 (15.6 mg, 86%). M.P. 80–83 °C. 1H-NMR (600 MHz, CDCl3) δ 7.37–7.30 (m, 6H), 6.97 (dq, J = 8.7, 1.2 Hz, 1H), 6.66 (t, J = 9.1 Hz, 1H), 6.23 (bs, 1H), 4.74 (t, J = 3.1 Hz, 1H), 4.56 (s, 2H), 4.19–4.31 (1H), 3.99 (t, J = 9.1 Hz, 1H), 3.72–3.68 (m, 4H), 3.61–3.57 (m, 1H), 3.33 (t, J = 4.6 Hz, 2H), 2.02 (s, 3H), 13C-NMR (151 MHz, CDCl3) δ13C-NMR (151 MHz, CHLOROFORM-D) δ 171.1, 154.6, 151.3 (d, J = 240.0 Hz), 137.9, 133.9 (d, J = 11.5 Hz), 128.5, 127.8, 127.8, 114.9, 112.2 (d, J = 4.4 Hz), 107.0 (d, J = 23.1 Hz), and 73.2, 71.8, 68.3, 48.0, 43.5, 42.0, 23.1. HRMS (ESI): m/z calculated for C21H24FN3NaO4: 424.1649, found [M + Na]+ 424.1671. = −89.5° (c 0.20, CHCl3).
3.2.17. Ethyl (S)-N-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)-N-(2-(benzyloxy)ethyl)glycinate (20)
To a solution of 19 (20 mg, 0.05 mmol) in acetonitrile (MeCN) (0.5 mL), K2CO3 (11 mg, 0.08 mmol) and ethyl bromoacetate (16 μL, 0.1 mmol) were added. After stirring for 2 days at 90 °C, the reaction mixture was diluted with water, and the organic material was extracted with DCM. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by HPLC (CHCl3/MeOH = 40:1) to yield 20 a colorless solid (16 mg, 68%). 1H-NMR (600 MHz, CDCl3) δ 7.42–7.22 (m, 6H), 7.03–6.99 (m, 1H), 6.93 (t, J = 9.3 Hz, 1H), 6.44–6.38 (m, 1H), 4.76–4.69 (m, 1H), 4.49 (s, 2H), 4.16–4.07 (m, 3H), 3.99 (t, J = 9.1 Hz, 1H), 3.75–3.66 (m, 4H), 3.61–3.54 (m, 3H), 3.48 (s, 2H), 2.06–1.96 (m, 3H), 1.22 (t, J = 7.2 Hz, 3H), 13C-NMR (151 MHz, CDCl3) δ 171.2, 171.1, 154.4, 154.3 (d, J = 244.2 Hz), 138.2, 134.3 (d, J = 8.6 Hz), 131.4 (d, J = 10.1 Hz), 128.5, 128.4, 127.8, 127.6, 127.5, 120.1 (d, J = 4,2 Hz), 114.0, 107.8 (d, J = 26.0 Hz), 73.2, 71.9, 69.1, 60.7, 54.4 (d, J = 4.4 Hz), 52.5, 50.8, 47.7, 41.9, 23.1, 14.2. HRMS (ESI): m/z calculated for C25H30FN3NaO6: 510.2016, found [M + Na]+ 510.2015. = −86.0° (c 0.20, CHCl3).
3.2.18. Ethyl (S)-N-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)-N-(2-hydroxyethyl)glycinate (21)
To a solution of 20 (16 mg, 0.04 mmol) in THF (1 mL), Pd/C (20 mg) was added. After stirring for 24 h under a H2 atmosphere at room temperature, the mixture was filtered through a Celite pad. The filtrate was concentrated under reduced pressure, and a colorless solid 21 was obtained (20 mg, quant.). M.P. 75–78 °C. 1H-NMR (600 MHz, CDCl3) δ 7.40–7.37 (m, 1H), 7.02–7.00 (m, 2H), 6.77–6.77 (m, 1H), 4.76–4.75 (m, 1H), 4.20 (q, J = 7.1 Hz, 1H), 4.01–3.97 (m, 3H), 3.75–3.72 (m, 2H), 3.67–3.61 (m, 4H), 3.47 (t, J = 4.6 Hz, 2H), 2.01 (s, 3H), 1.27 (q, J = 7.1 Hz, 3H), 13C-NMR (151 MHz, CDCl3) δ 172.6, 171.4, 155.0 (d, J = 244.2 Hz), 154.5, 133.6 (d, J = 10.1 Hz), 132.3 (d, J = 10.1 Hz), 120.9 (d, J = 2.9 Hz), 114.0, 107.8 (d, J = 26.0 Hz), and 72.0, 61.3, 59.5, 55.5, 55.5, 47.6, 41.9, 23.0, 14.2. = −55.0° (c 0.20, CHCl3).
3.2.19. (S)-N-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)-N-(2-hydroxyethyl)glycine (2)
To a solution of 21 (40 mg, 0.01 mmol) in THF (1 mL), 1 M NaOH (300 μL) was added. After stirring for 2 h at room temperature, the supernatant was removed to obtain a colorless solid. A solution of the solid in water was purified by Sep Pak C18 (water, MeOH) to yield PNU-142586 (2) as a sodium salt (38 mg, 80%). M.P. 80–82 °C.1H-NMR (600 MHz, CD3OD) δ 7.37 (dd, J = 15.8, 2.7 Hz, 1H), 7.06–7.04 (m, 1H), 6.93 (t, J = 9.5 Hz, 1H), 4.75 (dd, J = 8.8, 6.4 Hz, 1H), 4.09 (t, J = 9.1 Hz, 1H), 3.79 (d, J = 1.4 Hz, 2H), 3.75 (dd, J = 9.3, 6.5 Hz, 1H), 3.66 (t, J = 5.2 Hz, 2H), 3.54–3.50 (m, 4H), 1.96 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ180.2, 174.1, 156.9, 154.8 (d, J = 242.7 Hz), 136.1, 131.7 (d, J = 10.1 Hz), and 120.1, 115.9, 109.2 (d, J = 27.5 Hz), 73.4, 60.9, 59.1, 56.9, 43.2, 22.5. HRMS (ESI): m/z calculated for C16H20FN3NaO6: 392.1234, found [M + H]+ 392.1252. = −52.0° (c 0.20, CH3OH).
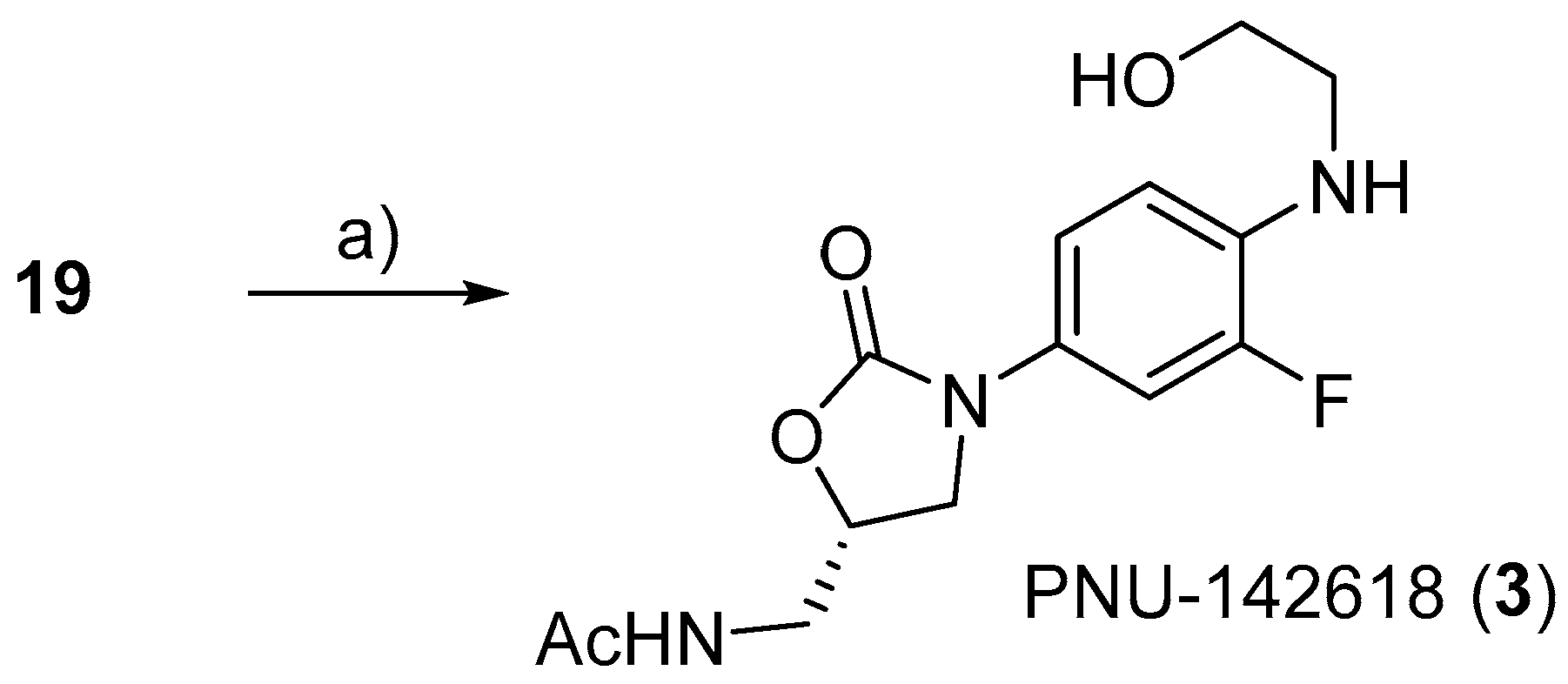
3.2.20. (S)-N-((3-(3-Fluoro-4-((2-hydroxyethyl)amino)phenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (3)
To a solution of 19 (15 mg, 0.04 mmol) in THF (1 mL), Pd/C (13 mg) was added. After stirring overnight under a H2 atmosphere at room temperature, the mixture was filtered through a Celite pad. The filtrate was concentrated under reduced pressure, and a colorless solid 3 was obtained (9 mg, 86%). M.P. 98–100 °C. 1H-NMR (600 MHz, CD3OD) δ 7.35 (dd, J = 13.6, 2.6 Hz, 1H), 7.03 (dq, J = 8.8, 1.2 Hz, 1H), 6.78 (t, J = 9.3 Hz, 1H), 4.76–4.74 (m, 1H), 4.07 (t, J = 8.9 Hz, 1H), 3.76–3.72 (m, 3H), 3.54 (d, J = 4.8 Hz, 2H), 3.26 (t, J = 5.7 Hz, 2H), 1.96 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ 174.1, 157.1, 152.5 (d, J = 238.4 Hz), 135.5 (d, J = 13.0 Hz), 129.0 (d, J = 10.1 Hz), 116.9, 113.3 (d, J = 4.4 Hz), 108.4 (d, J = 24.6 Hz), and 73.4, 61.4, 49.7, 49.4, 49.3, 49.1, 49.0, 48.9, 48.7, 48.6, 46.7, 43.2, 30.9, 22.4. HRMS (ESI): m/z calculated for C14H18FN3NaO4: 334.1179, found [M + Na]+ 334.1263. = −48.5° (c 0.20, CH3OH).
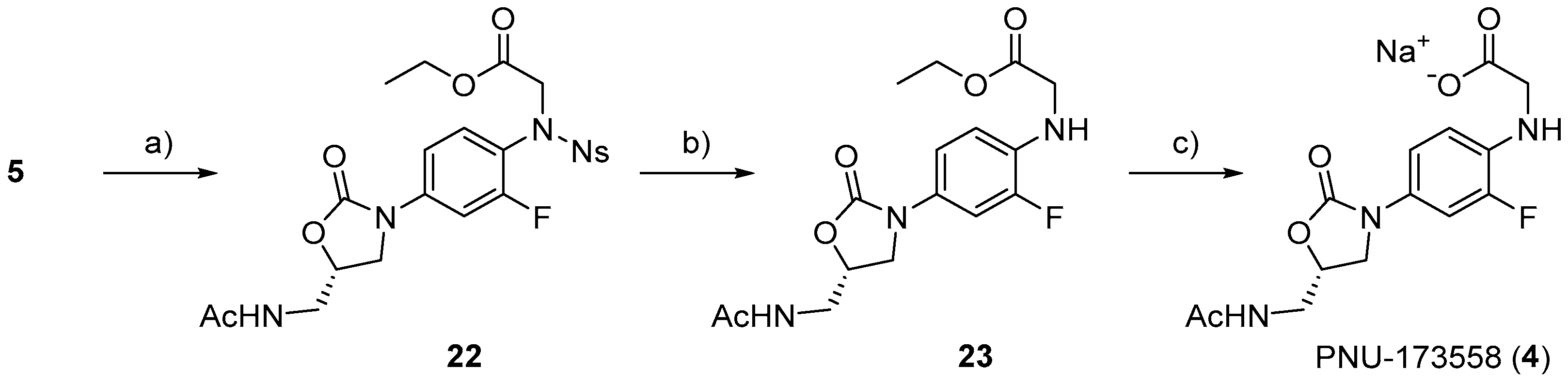
3.2.21. Ethyl (S)-N-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)-N-((4-nitrophenyl)sulfonyl)glycinate (22)
To a solution of 5 (100 mg, 0.22 mmol) in DMF (0.2 mL), K2CO3 (45 mg, 0.33 mmol), and ethyl bromoacetate (49 μL) were added. After stirring overnight at 50 °C, the reaction mixture was diluted with water, and the organic material was extracted with hexane/EtOAc (1:1). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by C.C. (CHCl3/MeOH = 20:1) to yield 22 as a colorless solid (112 mg, 95%). M.P. 78–80 °C. 1H-NMR (600 MHz, CDCl3) δ 7.73–7.66 (m, 4H), 7.60–7.53 (m, 2H), 7.12 (dd, J = 8.6, 2.1 Hz, 1H), 6.37 (s, 1H), 4.80–4.79 (m, 1H), 4.52 (s, 2H), 4.16 (q, J = 7.1 Hz, 2H), 4.06 (t, J = 8.9 Hz, 1H), 3.80 (dd, J = 9.1, 6.7 Hz, 1H), 3.68–3.61 (m, 2H), 2.02 (s, 3H), 1.25 (t, J = 7.0 Hz, 3H), 13C-NMR (151 MHz, CDCl3) δ 171.3, 168.7, 159.4 (d, J = 251.4 Hz), 154.0, 147.8, 140.2 (d, J = 10.1 Hz), 134.4, 134.1, 132.4, 131.7, 131.3, 124.3, 120.8 (d, J = 13.0 Hz), 113.3, 106.4 (d, J = 26.0 Hz), and 72.0, 61.7, 52.5, 47.4, 41.9, 23.1, 14.1. HRMS (ESI): m/z calculated for C22H23FN4NaO9S: 561.1067, found [M + Na]+ 561.1073. = −138.0° (c 0.20, CHCl3).
3.2.22. Ethyl (S)-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)glycinate (23)
To a solution of 22 (81 mg, 0.15 mmol) in DMF (0.2 mL), K2CO3 (83 mg, 0.6 mmol) and thiophenol (46 μL, 0.45 mmol) were added. After stirring overnight at room temperature, the reaction mixture was diluted with water, and the organic material was extracted with hexane/EtOAc (1:1). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was separated by HPLC (CHCl3/MeOH = 1:1) to yield 23 a colorless solid (50 mg, 95%). M.P. 93–95 °C. 1H-NMR (600 MHz, CDCl3) δ 7.36 (dd, J = 13.1, 2.4 Hz, 1H), 6.96 (dq, J = 8.7, 1.2 Hz, 1H), 6.88 (t, J = 6.0 Hz, 1H), 6.54 (t, J = 8.9 Hz, 1H), 4.76–4.73 (m, 1H), 4.24 (q, J = 7.1 Hz, 2H), 3.98 (t, J = 8.9 Hz, 1H), 3.92 (s, 2H), 3.73–3.69 (m, 2H), 3.65–3.58 (m, 2H), 2.01 (s, 3H), 1.30 (t, J = 7.2 Hz, 3H), 13C-NMR (151 MHz, CDCl3) δ 171.5, 170.7, 154.8, 151.1 (d, J = 239.8 Hz), 132.7 (d, J = 11.6 Hz), 128.5 (d, J = 8.6 Hz), 114.9 (d, J = 2.9 Hz), 112.1 (d, J = 4.4 Hz), 107.1 (d, J = 23.2 Hz), and 72.0, 61.5, 48.0, 45.5, 41.9, 23.0, 14.2. HRMS (ESI): m/z calculated for C16H20FN3NaO5: 376.1285, found [M + Na]+ 376.1277. = −36.0° (c 0.20, CHCl3).
3.2.23. (S)-(4-(5-(Acetamidomethyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)glycine (4)
To a solution of 23 (18 mg, 0.05 mmol) in THF (1 mL), 1M NaOH (79 μL) was added. After stirring for 2 h at room temperature, MeOH was added, and the reaction mixture was subjected to PTLC (CHCl3/MeOH = 1:1) to yield PNU-173558 (4) as a sodium salt (15 mg, 95%). M.P. 118–120 °C. 1H-NMR (600 MHz, CD3OD) δ 7.36 (dd, J = 13.4, 2.4 Hz, 1H), 7.01 (dt, J = 8.7, 1.3 Hz, 1H), 6.64 (t, J = 9.3 Hz, 1H), 4.76–4.74 (m, 1H), 4.08 (t, J = 9.1 Hz, 1H), 3.75 (dd, J = 9.1, 6.4 Hz, 1H), 3.65 (s, 2H), 3.54 (d, J = 5.2 Hz, 2H), 1.97 (s, 3H), 13C-NMR (151 MHz, CD3OD) δ 177.6, 174.0, 170.4, 157.1, 153.3 (d, J = 236.9 Hz), 135.5 (d, J = 13.0 Hz), 128.7 (d, J = 10.1 Hz), 116.9, 113.1 (d, J = 4.4 Hz), 108.3 (d, J = 24.4 Hz), and 73.4, 49.8, 48.4, 43.2, 30.8, 22.4. HRMS (ESI): m/z calculated for C14H16FN3NaO5: 348.0972, found [M + H]+ 348.0986. = −69.5° (c 0.20, CH3OH).