A Comprehensive Approach to the Antidepressant-like Effect and Toxicity of Thunbergia alata Bojer ex Sims (Acanthaceae): Involvement of the Serotoninergic System
Abstract
1. Introduction
2. Results
2.1. Aqueous Extract of T. alata (THA) Preparation
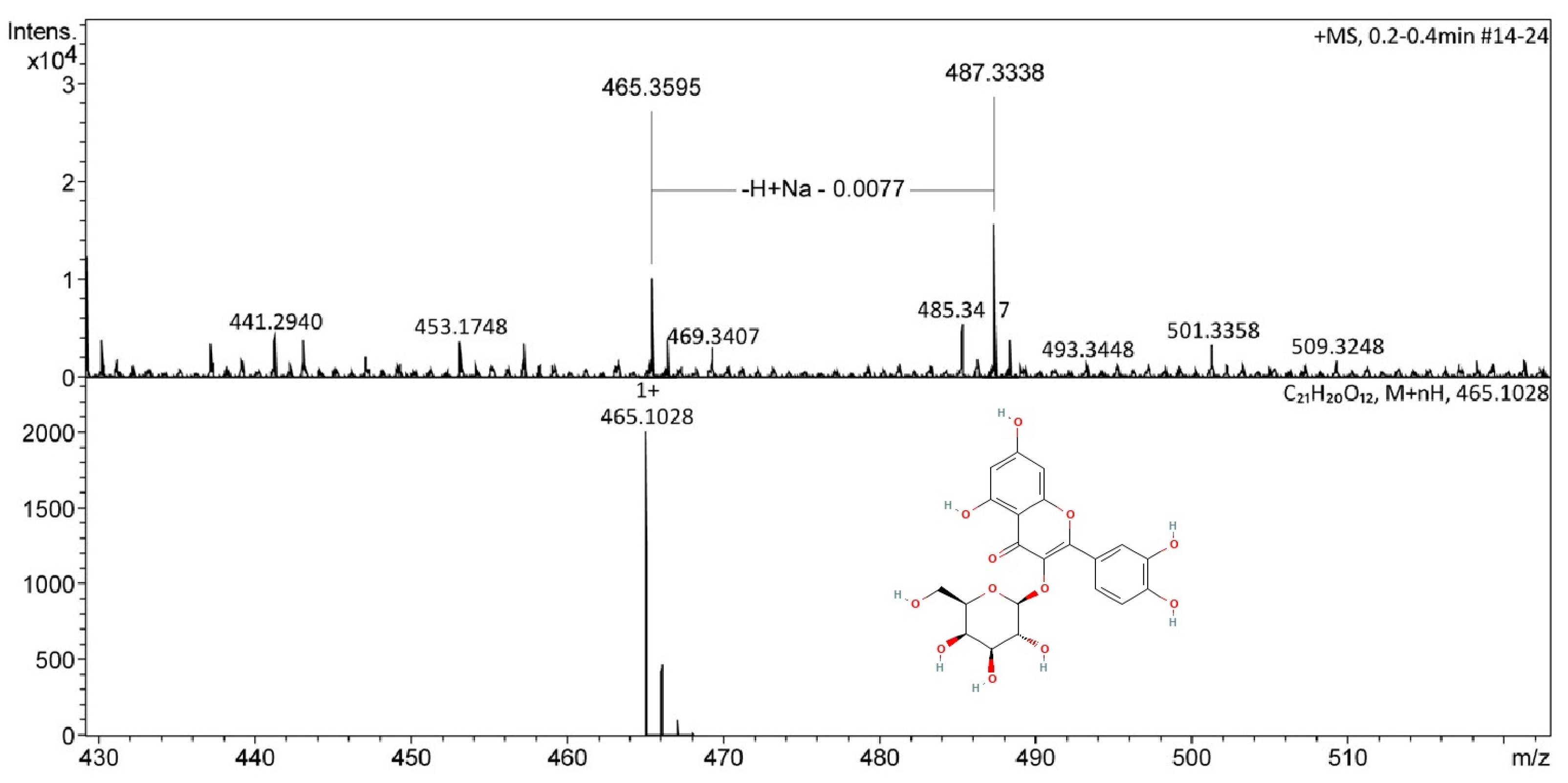
2.2. Chemical Analysis of Aerial Parts of T. alata
2.3. Neuropharmacological Evaluation
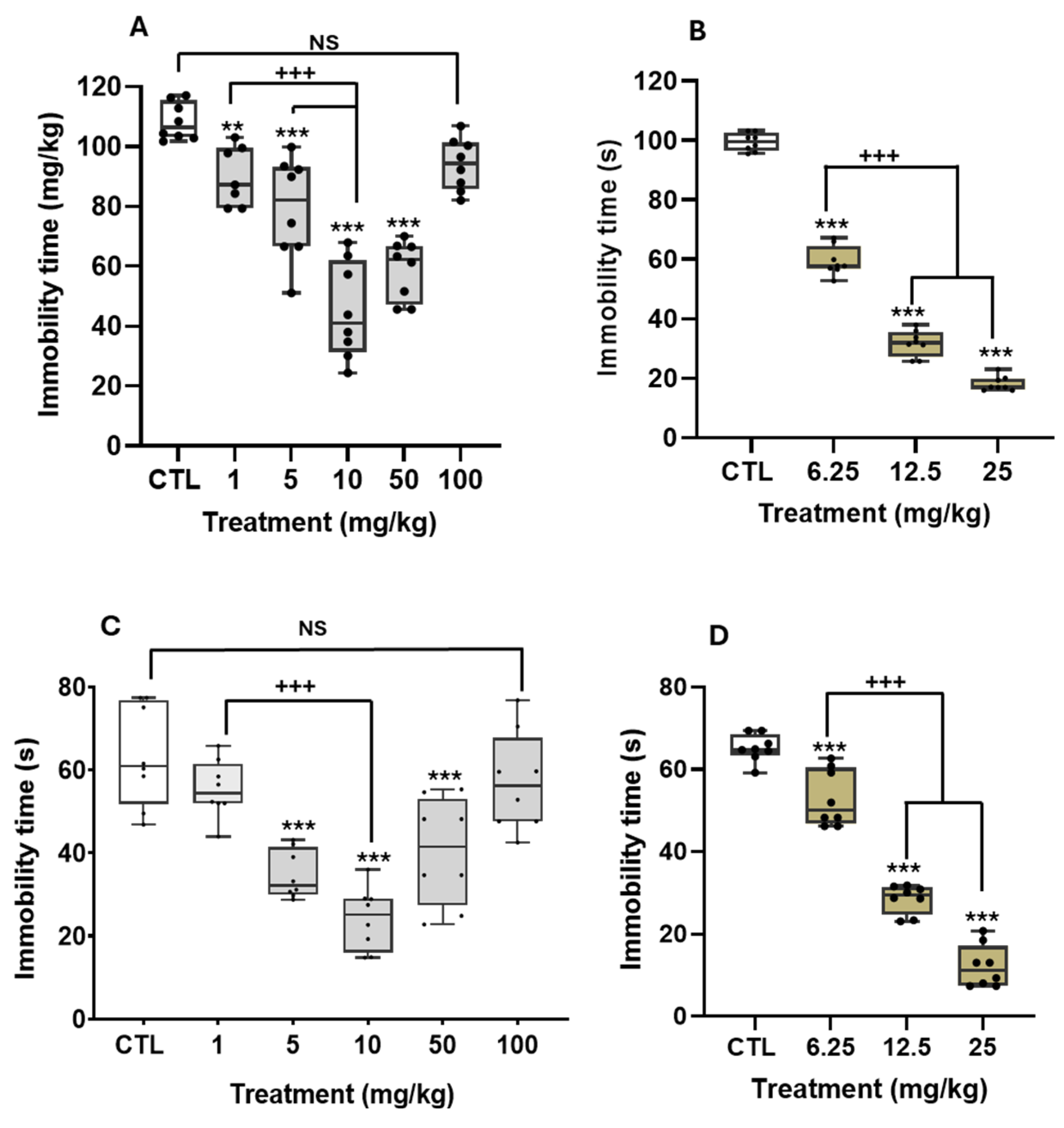
Antidepressant-like Effect of THA in Different Paradigms
2.4. Biochemical Mechanisms Associated with Antidepressant-like Actions of the THA in the FST
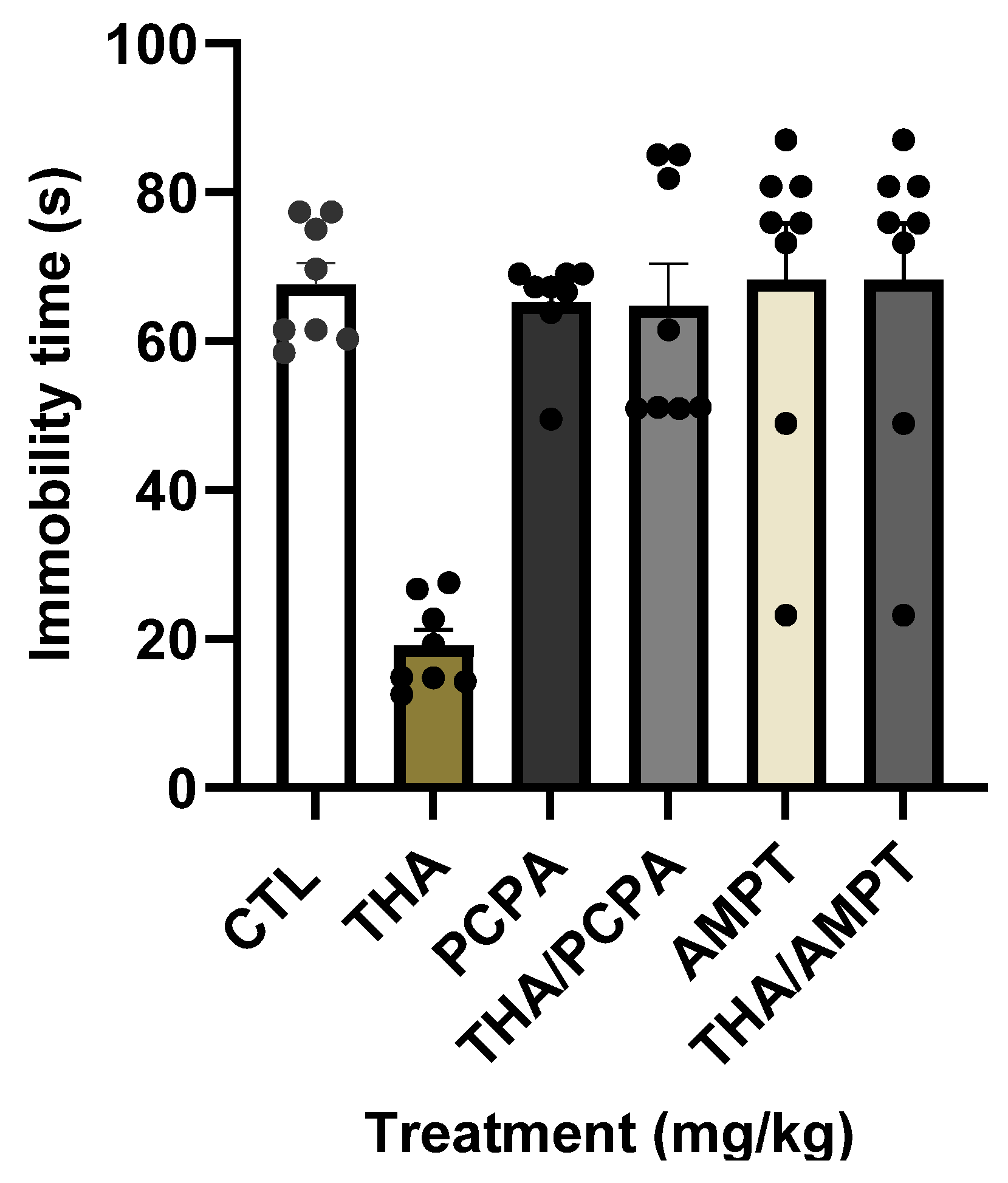
2.4.1. Role of the Monoamines in the Antidepressant Effects of the THA
2.4.2. Role of the Serotonergic Receptors in the Antidepressant Effects of the THA
2.4.3. Synergistic Actions of THA and Serotonergic Compounds on Despair
2.4.4. Effect of THA on Brain MAO Activity
2.5. Neurotoxicological Effects of THA in the Inverted Screen Test (IST) and Rota Rod Test (RRT)
2.6. Median Acute Lethal Dose (LD50) of the THA
2.7. Subacute Oral Toxicity of THA in Male and Female Mice
2.7.1. Determination of Apparent Signs of Toxicity
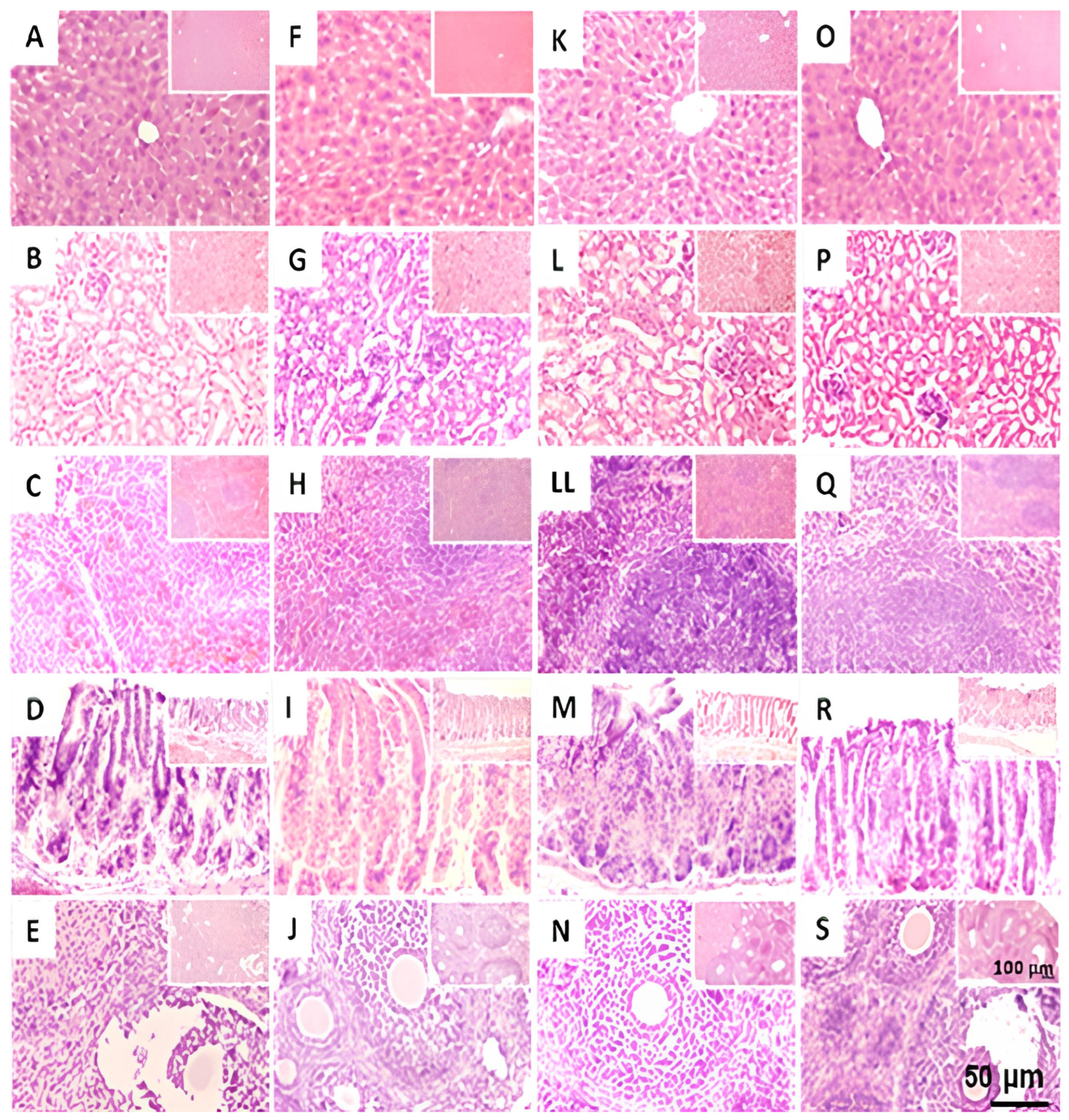
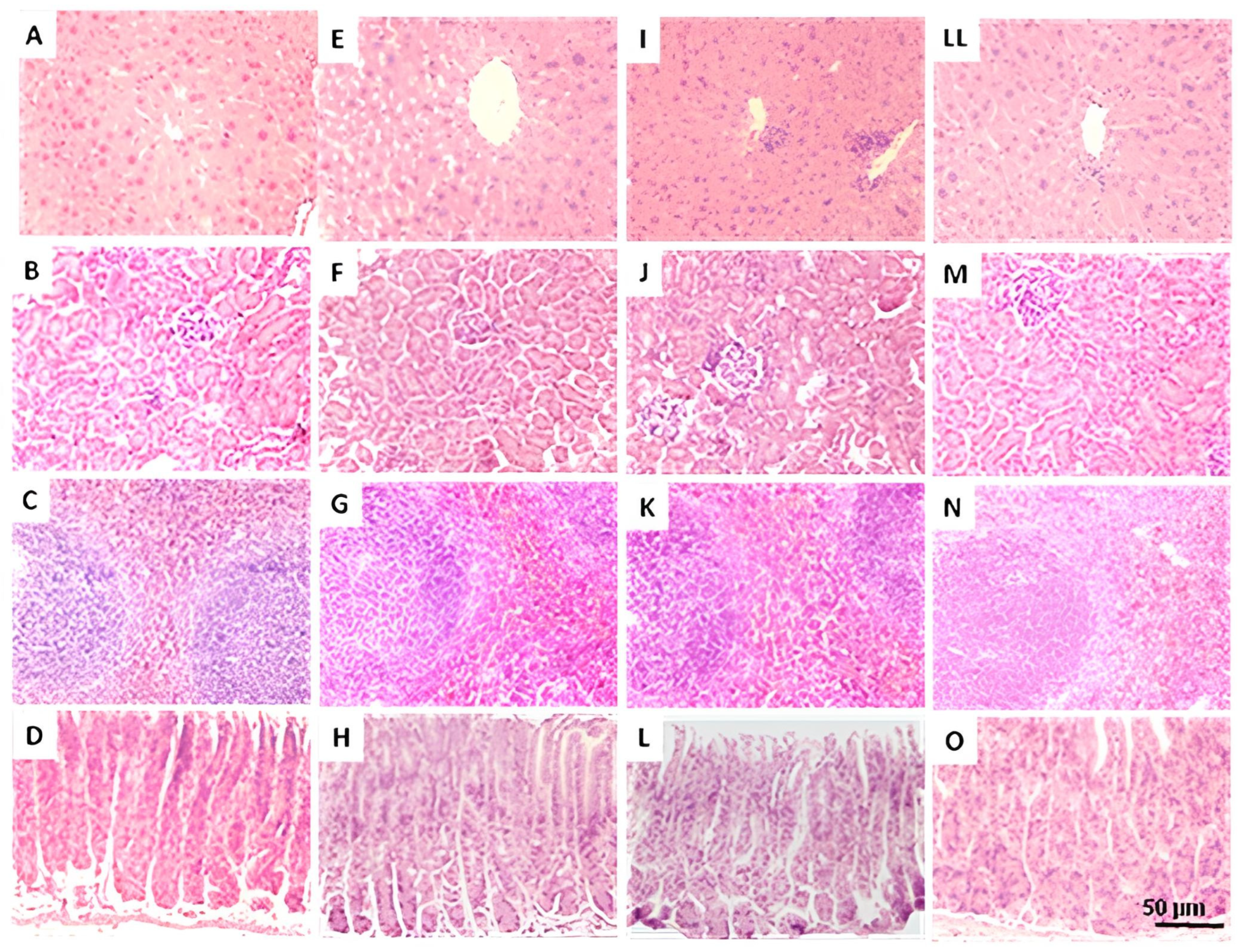
2.7.2. Gross Necropsy
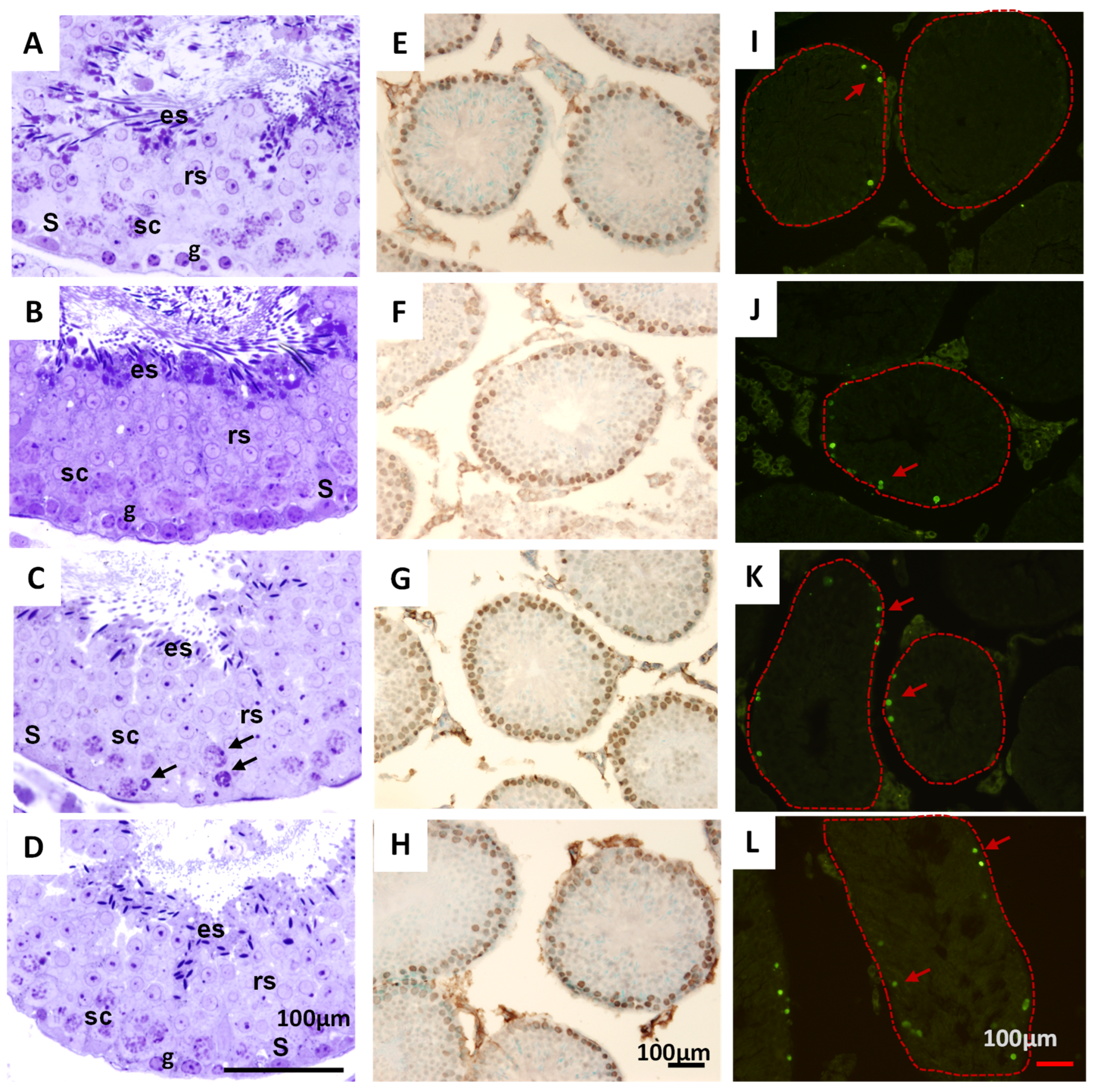
2.7.3. Histological Analysis of Testicles in Mice Treated with THA
3. Discussion
3.1. The THA Produces Antidepressant-like Effects, Apparently Without Adverse Side Effects
3.2. Monoamines and Serotonergic 5-HT1A Receptors Participate in the Antidepressant-like Effect of THA in the FST
3.3. The THA Does Not Produce Relevant Toxicological Effects in Mice
3.4. Chemical Composition of THA
4. Materials and Methods
4.1. Vegetal Material
4.2. NMR Data
4.3. DIESI-MS Analysis
4.4. Animals
4.5. Drugs
4.6. Behavioral Evaluation in Tests of Depressive Behavior
4.6.1. Effect of Different Doses of T. alata
4.6.2. Identification of Biochemical Pathways Underlying the Antidepressant-like Effects of THA
4.7. Neurotoxicological Evaluation of Mice Treated with the THA
4.8. Toxicological Evaluation of Animals Treated with THA
4.8.1. Acute Oral Toxicity of THA in Male Mice
4.8.2. Sub-Acute Oral Toxicity of THA
4.8.3. Histological Analyses of Peripheral Organs and Histopathological Analysis of Seminiferous Tubes
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, M. The World Mental Health Report: Transforming Mental Health for All. World Psychiatry 2022, 21, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO Prevalence Estimates of Mental Disorders in Conflict Settings: A Systematic Review and Meta-Analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef]
- Paniagua, F.A. Culture-Bound Syndromes, Cultural Variations, and Psychopathology. In Handbook of Multicultural Mental Health; Elsevier: Amsterdam, The Netherlands, 2013; pp. 25–47. [Google Scholar]
- Karunamoorthi, K.; Jegajeevanram, K.; Vijayalakshmi, J.; Mengistie, E. Traditional Medicinal Plants: A Source of Phytotherapeutic Modality in Resource-Constrained Health Care Settings. J. Evid.-Based Complement. Altern. Med. 2013, 18, 67–74. [Google Scholar] [CrossRef]
- Tabuti, J.R.S.; Lye, K.A.; Dhillion, S.S. Traditional Herbal Drugs of Bulamogi, Uganda: Plants, Use and Administration. J. Ethnopharmacol. 2003, 88, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Atlas de las Plantas de la Medicina Tradicional Mexicana Biblioteca Digital de la Medicina Tradicional Mexicana. 2009. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/introduccion.html (accessed on 30 January 2024).
- Housti, F.; Andary, C.; Gargadennec, A.; Amssa, M. Effects of wounding and salicylic acid on hydroxycinnamoylmalic acids in Thunbergia alata. Plant Physiol. Biochem. 2002, 40, 761–769. [Google Scholar] [CrossRef]
- Damtoft, S.; Frederiksen, L.B.; Jensen, S.R. Alatoside and thunaloside, two iridoid glucosides from Thunbergia alata. Phytochemistry 1994, 35, 1259–1261. [Google Scholar] [CrossRef]
- Charles, A.; Ramani, A.V. Chemosystematics of Genus Thunbergia (A-Mini review). Int. J. Sci. Res. Mod. Educ. 2016, 25, 5–11. [Google Scholar]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid glucosides from Thunbergia laurifolia. Phytochemistry 2002, 60, 769–771. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sultana, K.W.; Roy, A.; Chandra, I. An Overview of Ethnopharmacological and Phytochemical properties of Thunbergia sp. Med. Aromat. Plants 2015, 4, 1000217. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Cheng, C.; Bomser, J.; Ferruzzi, M.G.; Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 2007, 114, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.P.; Subramaneyaan, M. Phytochemical valuation and pharmacological activities of Thunbergia species—A comprehensive review. Int. J. Biol. Pharm. Allied Sci. 2021, 10, 233–243. [Google Scholar] [CrossRef]
- Parkinson, C.M.; O’Brien, A.; Albers, T.M.; Simon, M.A.; Clifford, C.B.; Pritchett-Corning, K.R. Diagnostic Necropsy and Selected Tissue and Sample Collection in Rats and Mice. J. Vis. Exp. 2011, 54, e2966. [Google Scholar] [CrossRef]
- Alarcón, R.D. Culture, Cultural Factors and Psychiatric Diagnosis: Review and Projections. World Psychiatry 2009, 8, 131–139. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Landy, D. The Culture-Bound Syndromes: Folk Illnesses of Psychiatric and Anthropological Interest. Ronald C. Simons and Charles C. Hughes. Med. Anthropol. Q. 1987, 1, 345–347. [Google Scholar] [CrossRef]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Pharmacol. 2010, 49, 5.8.1–5.8.14. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The Tail Suspension Test as a Model for Assessing Antidepressant Activity: Review of Pharmacological and Genetic Studies in Mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Lam, R.W.; Tam, E.M.; Grewl, A.; Yatham, L.N. Effects of Alpha-Methyl-Para-Tyrosine-Induced Catecholamine Depletion in Patients with Seasonal Affective Disorder in Summer Remission. Neuropsychopharmacology 2001, 25, S97–S101. [Google Scholar] [CrossRef]
- Waløen, K.; Kleppe, R.; Martinez, A.; Haavik, J. Tyrosine and Tryptophan Hydroxylases as Therapeutic Targets in Human Disease. Expert Opin. Ther. Targets 2017, 21, 167–180. [Google Scholar] [CrossRef]
- Berger, U.V.; Grzanna, R.; Molliver, M.E. Depletion of Serotonin Using P-Chlorophenylalanine (PCPA) and Reserpine Protects against the Neurotoxic Effects of p-Chloroamphetamine (PCA) in the Brain. Exp. Neurol. 1989, 103, 111–115. [Google Scholar] [CrossRef]
- Sauvé, Y.; Reader, T.A. Effects of Alpha-Methyl-p-Tyrosine on Monoamines and Catecholamine Receptors in Rat Cerebral Cortex and Neostriatum. Neurochem. Res. 1988, 13, 807–815. [Google Scholar] [CrossRef]
- Weng, R.; Shen, S.; Tian, Y.; Burton, C.; Xu, X.; Liu, Y.; Chang, C.; Bai, Y.; Liu, H. Metabolomics Approach Reveals Integrated Metabolic Network Associated with Serotonin Deficiency. Sci. Rep. 2015, 5, 11864. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, N.; Yang, D.; Oxenkrug, G.; Yang, Q. Regulating the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism. FEBS J. 2017, 284, 948–966. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.M. Update on the Pharmacology of Selective Inhibitors of MAO-A and MAO-B: Focus on Modulation of CNS Monoamine Neurotransmitter Release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef]
- Cho, H.-U.; Kim, S.; Sim, J.; Yang, S.; An, H.; Nam, M.-H.; Jang, D.-P.; Lee, C.J. Redefining Differential Roles of MAO-A in Dopamine Degradation and MAO-B in Tonic GABA Synthesis. Exp. Mol. Med. 2021, 53, 1148–1158. [Google Scholar] [CrossRef]
- Cassani, J.; Alberto Ferreyra-Cruz, O.; María Dorantes-Barrón, A.; Vigueras Villaseñor, R.M.; Arrieta-Baez, D.; Estrada-Reyes, R. Antidepressant-like and Toxicological Effects of a Standardized Aqueous Extract of Chrysactinia Mexicana A. Gray (Asteraceae) in Mice. J. Ethnopharmacol. 2015, 171, 295–306. [Google Scholar] [CrossRef]
- Detke, M.J.; Wieland, S.; Lucki, I. Blockade of the Antidepressant-like Effects of 8-OH-DPAT, Buspirone and Desipramine in the Rat Forced Swim Test by 5HT1A Receptor Antagonists. Psychopharmacology 1995, 119, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Assié, M.B.; Koek, W. [3H]-8-OH-DPAT Binding in the Rat Brain Raphe Area: Involvement of 5-HT1A and Non-5-HT1A Receptors. Br. J. Pharmacol. 2000, 130, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Melon, C.; De Vry, J. The Role of 5-HT Receptor Subtypes in the Anxiolytic Effects of Selective Serotonin Reuptake Inhibitors in the Rat Ultrasonic Vocalization Test. Psychopharmacology 1998, 135, 383–391. [Google Scholar] [CrossRef]
- Yaman, B.; Bal, R. Pindolol Potentiates the Antidepressant Effect of Venlafaxine by Inhibiting 5-HT1A Receptor in DRN Neurons of Mice. Int. J. Neurosci. 2022, 132, 23–30. [Google Scholar] [CrossRef]
- Arrieta-Báez, D.; Gómez-Patiño, M.B.; Jurado Hernández, N.; Mayagoitia-Novales, L.; Dorantes-Barrón, A.M.; Estrada-Reyes, R. Antidepressant-like Effects of a Methanol Extract of Leonotis nepetifolia in Mice. Nat. Prod. Res. 2022, 36, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Juergenliemk, G.; Boje, K.; Huewel, S.; Lohmann, C.; Galla, H.J.; Nahrstedt, A. In vitro studies indicate that miquelianin (quercetin 3-O-β-D-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 2003, 69, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Jürgenliemk, G.; Nahrstedt, A.; Winterhoff, H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000, 66, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Porras-Ramírez, J.; Estrada-Reyes, R.; Rodríguez-Zavala, J.S.; Dorantes-Barrón, A.M.; Jurado-Hernández, N.; Martínez-Vázquez, M. Antidepressant-like Effects of a New Dihydro Isoquinoline and Its Chemical Precursors in Mice: Involvement of Serotonin and Dopaminergic Systems. Can. J. Chem. 2021, 99, 455–464. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Measuring the Strength of Mice. J. Vis. Exp. 2013, 2, 2610. [Google Scholar] [CrossRef]
- Vigueras-Villaseñor, R.M.; Molina-Ortiz, D.; Reyes-Torres, G.; del Ángel, D.S.; Moreno-Mendoza, N.A.; Cruz, M.E.G.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Effect of Allopurinol on Damage Caused by Free Radicals to Cryptorchid Testes. Acta Histochem. 2009, 111, 127–137. [Google Scholar] [CrossRef]
| Treatment/(mg/kg) | Counts Number ± SEM | Rearing Number ± SEM |
|---|---|---|
| CTL | 53.8 ± 10.0 | 35.5 ± 7.0 |
| THA/1 | 54.1 ± 12.3 | 38.4 ± 10.5 |
| THA/5 | 50.2 ± 10.3 | 34.0 ± 7.7 |
| THA/10 | 47.8 ± 10.0 | 36.5 ± 6.7 |
| THA/50 | 47.4 ± 7.6 | 32.2 ± 3.7 |
| THA/100 | 50.5 ± 2.8 | 29.2 ± 1.7 |
| H = 3.62, df = 5, p = 0.60 | H = 7.9, df = 5, p = 0.1 | |
| CTL | 61.2 ± 18.0 | 34.3 ± 8.2 |
| DES/6.25 | 48.8 ± 13.8 * | 35.2 ± 10.4 |
| DES12.5 | 67.5 ± 21.7 | 30.7 ± 9.4 |
| DES/25 | 77.3 ± 18.8 ** | 34.8 ± 7.6 |
| H = 8.05, df = 3, p = 0.04 | H = 1.77, df = 3, p = 0.62 |
| Experiment of Antagonism | Experiment of Synergism | ||
|---|---|---|---|
| Treatment (mg/kg) | Immobility Time (s) | Treatment (mg/kg) | Immobility Time (s) |
| CTL | 67.6 ± 2.8 | CTL | 64.3 ± 1.7 |
| THA (10) | 19.0 ± 2.0 *** | THA (1) | 61.2 ± 2.3 |
| NAN-190 | 70.3 ± 8.2 | 8-OH-DPAT | 64.7± 3.8 |
| THA + NAN190 | 70.0 ± 6.2 +++ | THA + 8OHDPAT | 16.9 ± 2.5 *** |
| H = 18.3, df = 3, p ≤ 0.001 | H = 22.3, df = 3, p ≤ 0.001 | ||
| CTL | 67.6 ± 2.8 | CTL | 68.5 ± 3.0 |
| THA (10) | 19.0 ± 2.0 *** | THA (1) | 61.2 ± 2.3 |
| Ketanserin | 59.7 ± 4.8 | Pindolol (32) | 78.3 ± 3.7 |
| THA + ketanserin | 15.4 ± 2.4 *** | THA + pindolol | 14.73 ± 3.8 *** |
| H = 24.5, df = 3, p ≤ 0.001) | H = 22.9, df = 3, p ≤ 0.001 | ||
| CTL | 71.4 ± 2.3 | CTL | 68.5 ± 3.0 |
| THA (10) | 19.0 ± 2.0 *** | THA (1) | 61.2 ± 2.3 |
| MDL72222 | 73.2 ± 4.9 | FLX (10) | 74.6 ± 5.2 |
| THA + MDL72222 | 14.9 ± 2.7 *** | THA + FLX | 18.2 ± 2.2 *** |
| H = 23.6, df = 3, p ≤ 0.001 | H = 20.5, df = 3, p ≤ 0.001 | ||
| MAO-A | MAO-B | ||
|---|---|---|---|
| µg/mL | Activity µmol/min/mg Protein | µg/mL | Activity µmol/min/mg Protein |
| Basal total | 76.50 ± 2.2 | Basal total | 76.50 ± 2.2 |
| Clor | 33.20 ± 1.8 *** | Dep | 42.23 ± 4.60 *** |
| THA6.12 | 42.37 ± 2.0 *** | THA6.12 | 30.09 ± 4.39 *** |
| THA12.5 | 39.78 ± 0.31 *** | THA12.5 | 26.93 ± 3.82 *** |
| THA25 | 45.18 ± 2.00 *** | THA25 | 38.80 ± 6.3 |
| F(4, 10) = 82.7, p < 0.001 | F(4, 10) = 9.80, p = 0.002 | ||
| THA 10, 100, and 1000 mg/kg | Female Mice | Male Mice |
|---|---|---|
| Spleen | F(3, 8) = 3.22, p = 0.08 | F(3, 8) = 1.31, p = 0.33 |
| Liver | F(3, 8) = 0.87, p = 0.49 | F(3, 8) = 3.67, p = 0.06 |
| Stomach | F(3, 8) = 2.30, p = 0.15 | F(3, 8) = 1.93, p = 0.20 |
| Left kidney | F(3, 8) = 0.76, p = 0.54 | F(3, 8) = 0.70, p = 0.57 |
| Right kidney | F(3, 8) = 0.19, p = 0.89 | F(3, 8) = 0.27, p = 0.84 |
| Right ovarium | F(3, 8) = 0.33, p = 0.79 | |
| Left ovarium | F(3, 7) = 0.45, p = 0.72 | |
| Right teste | F(3, 8) = 0.87, p = 0.49 | |
| Left teste | F(3, 8) = 2.55, p = 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Patiño, M.B.; Dorantes-Barrón, A.M.; Arrieta-Báez, D.; Jurado-Hernández, N.; Cassani, J.; Vigueras-Villaseñor, R.M.; Martínez-Mota, L.; Ibarra Ocaña, J.A.; Estrada-Reyes, R. A Comprehensive Approach to the Antidepressant-like Effect and Toxicity of Thunbergia alata Bojer ex Sims (Acanthaceae): Involvement of the Serotoninergic System. Pharmaceuticals 2025, 18, 1812. https://doi.org/10.3390/ph18121812
Gómez-Patiño MB, Dorantes-Barrón AM, Arrieta-Báez D, Jurado-Hernández N, Cassani J, Vigueras-Villaseñor RM, Martínez-Mota L, Ibarra Ocaña JA, Estrada-Reyes R. A Comprehensive Approach to the Antidepressant-like Effect and Toxicity of Thunbergia alata Bojer ex Sims (Acanthaceae): Involvement of the Serotoninergic System. Pharmaceuticals. 2025; 18(12):1812. https://doi.org/10.3390/ph18121812
Chicago/Turabian StyleGómez-Patiño, Mayra Beatriz, Ana María Dorantes-Barrón, Daniel Arrieta-Báez, Noé Jurado-Hernández, Julia Cassani, Rosa María Vigueras-Villaseñor, Lucía Martínez-Mota, Jessica A. Ibarra Ocaña, and Rosa Estrada-Reyes. 2025. "A Comprehensive Approach to the Antidepressant-like Effect and Toxicity of Thunbergia alata Bojer ex Sims (Acanthaceae): Involvement of the Serotoninergic System" Pharmaceuticals 18, no. 12: 1812. https://doi.org/10.3390/ph18121812
APA StyleGómez-Patiño, M. B., Dorantes-Barrón, A. M., Arrieta-Báez, D., Jurado-Hernández, N., Cassani, J., Vigueras-Villaseñor, R. M., Martínez-Mota, L., Ibarra Ocaña, J. A., & Estrada-Reyes, R. (2025). A Comprehensive Approach to the Antidepressant-like Effect and Toxicity of Thunbergia alata Bojer ex Sims (Acanthaceae): Involvement of the Serotoninergic System. Pharmaceuticals, 18(12), 1812. https://doi.org/10.3390/ph18121812








