Comparison of Three Consecutive Monthly Administrations Between Aflibercept 8 mg and Brolucizumab 6 mg in Polypoidal Choroidal Vasculopathy
Abstract
1. Introduction
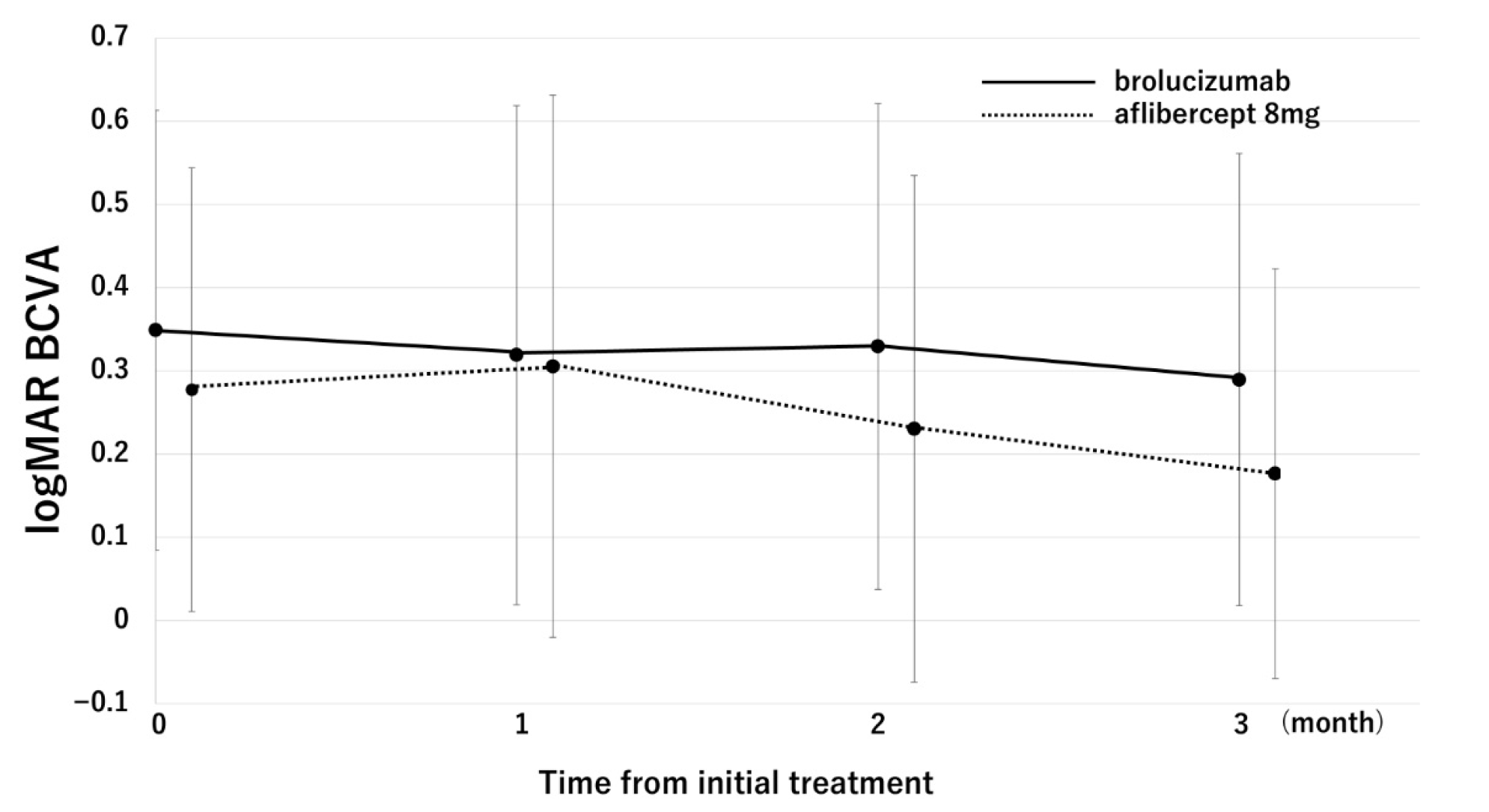
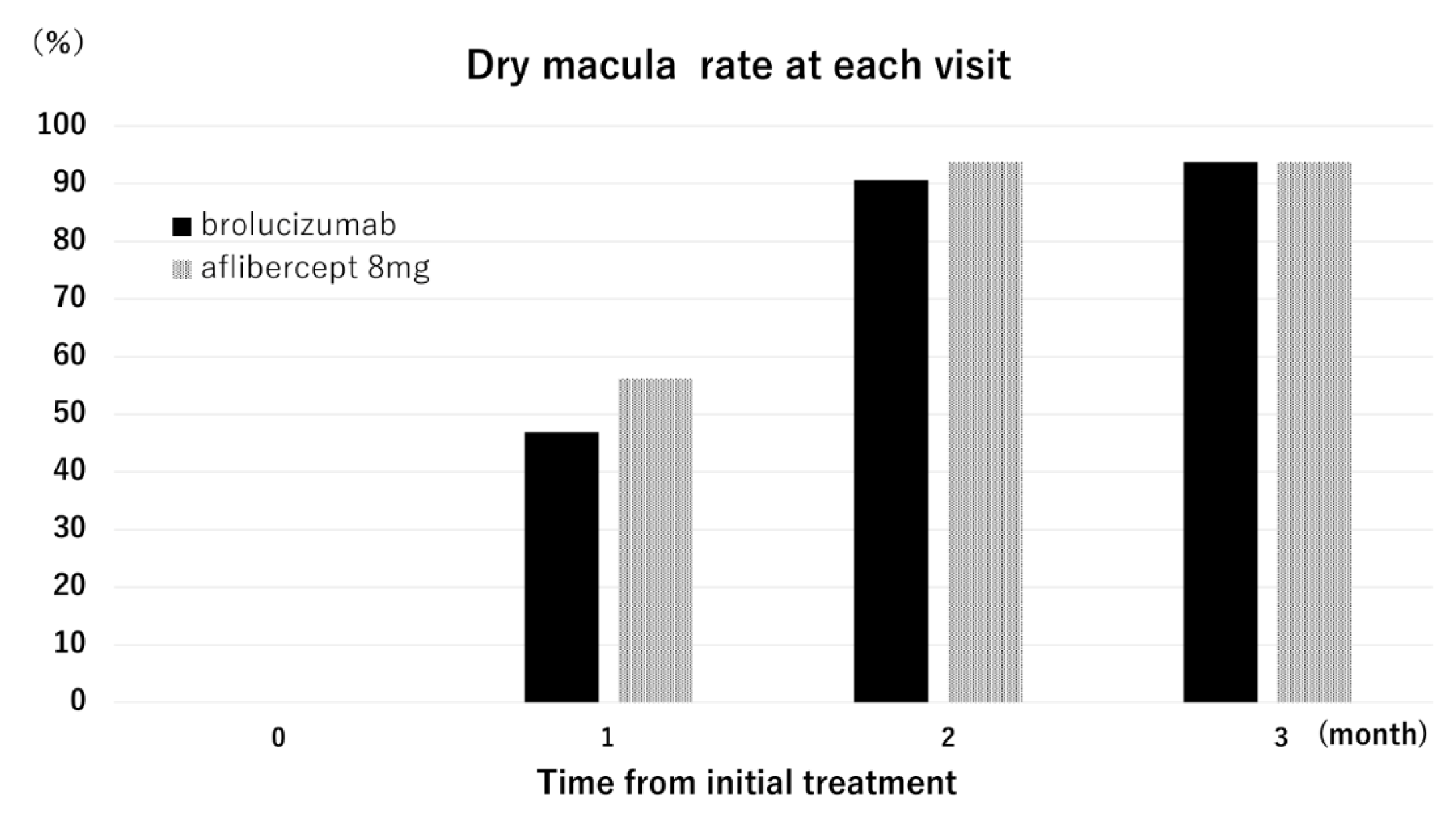
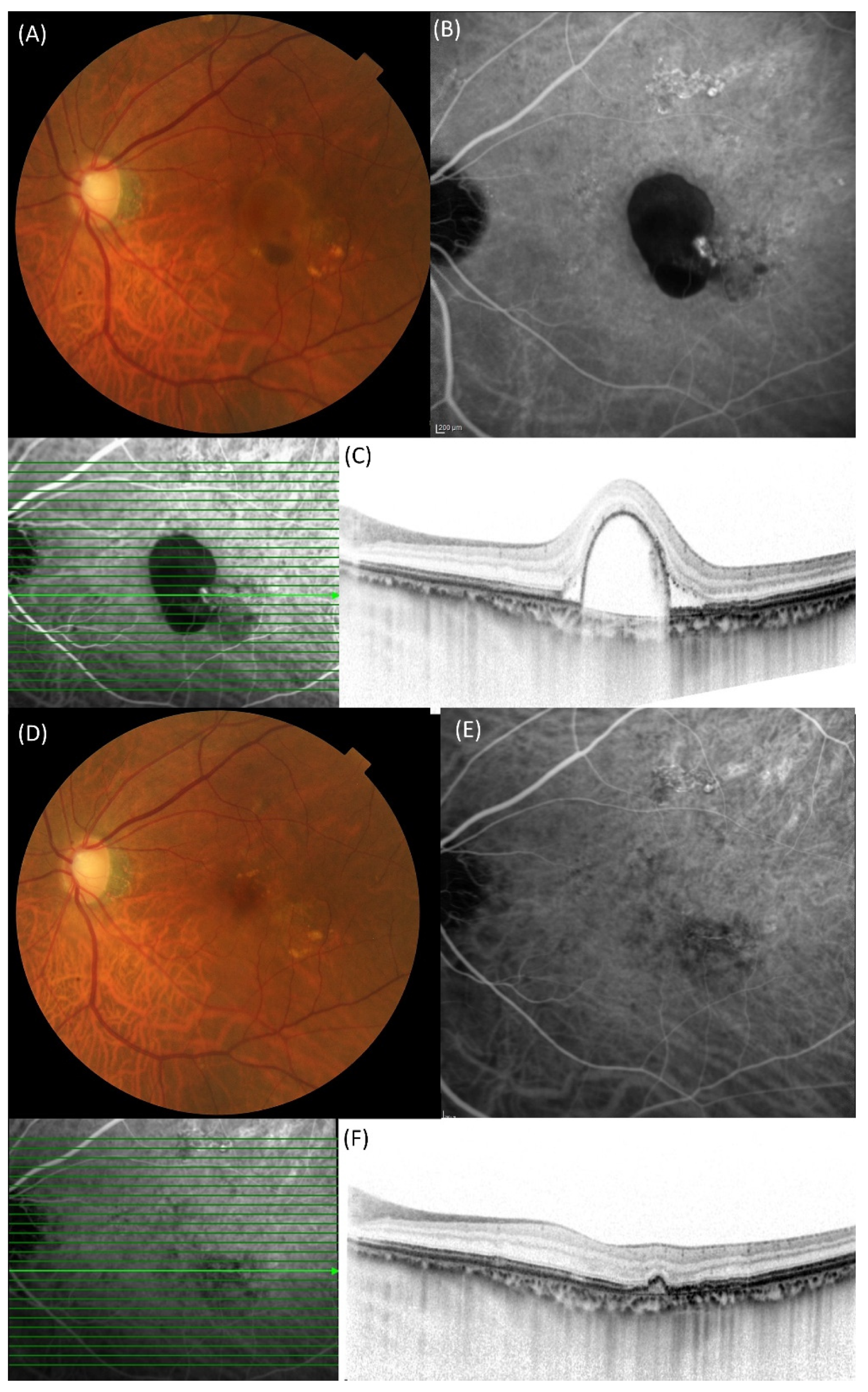
2. Results
3. Discussion
4. Materials and Methods
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| BCVA | best-corrected visual acuity |
| CRT | central retinal thickness |
| ICGA | indocyanine green angiography |
| IOI | intraocular inflammation |
| MNV | macular neovascularization |
| PCV | polypoidal choroidal vasculopathy |
| PDT | photodynamic therapy |
| SCT | subfoveal choroidal thickness |
| VEGF | vascular endothelial growth factor |
References
- Spaide, R.F.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.; Orlach, D.A. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995, 15, 100–110. [Google Scholar] [CrossRef]
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Tanaka, K.; Fragiotta, S. Differentiating drusen and drusenoid deposits subtypes on multimodal imaging and risk of advanced age-related macular degeneration. Jpn. J. Ophthalmol. 2023, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Dansingani, K.K.; Koizumi, H.; Lai, T.Y.Y.; Sivaprasad, S.; Boon, C.J.F.; Van Dijk, E.H.C.; Chhablani, J.; Lee, W.K.; Freund, K.B. Pachychoroid disease: Review and update. Eye 2025, 39, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Sakurada, Y.; Yoneyama, S.; Kikushima, W.; Sugiyama, A.; Matsubara, M.; Tanabe, N.; Iijima, H. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci. Rep. 2019, 9, 11906. [Google Scholar] [CrossRef]
- Saito, M.; Iida, T.; Saito, K.; Kano, M.; Itagaki, K.; Maruko, I.; Sekiryu, T. Long-term characteristics of exudative age-related macular degeneration in Japanese patients. PLoS ONE 2021, 16, e0261320. [Google Scholar] [CrossRef]
- Maruko, I.; Iida, T.; Saito, M.; Nagayama, D.; Saito, K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am. J. Ophthalmol. 2007, 144, 15–22. [Google Scholar] [CrossRef]
- Lafaut, B.A.; Leys, A.M.; Snyers, B.; Rasquin, F.; De Laey, J.J. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 752–759. [Google Scholar] [CrossRef]
- Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch. Ophthalmol. 1999, 117, 1329–1345.
- Gomi, F.; Ohji, M.; Sayanagi, K.; Sawa, M.; Sakaguchi, H.; Oshima, Y.; Ikuno, Y.; Tano, Y. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2008, 115, 141–146. [Google Scholar] [CrossRef]
- Hang, A.; Feldman, S.; Amin, A.P.; Ochoa, J.A.R.; Park, S.S. Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Retinal Disorders. Pharmaceuticals 2023, 16, 1140. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; Group, A.S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Rich, R.M.; Lalwani, G.A. Ranibizumab: Phase III clinical trial results. Ophthalmol. Clin. N. Am. 2006, 19, 361–372. [Google Scholar] [CrossRef]
- Oishi, A.; Kojima, H.; Mandai, M.; Honda, S.; Matsuoka, T.; Oh, H.; Kita, M.; Nagai, T.; Fujihara, M.; Bessho, N.; et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am. J. Ophthalmol. 2013, 156, 644–651. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Dugel, P.U.; Singh, R.P.; Koh, A.; Ogura, Y.; Weissgerber, G.; Gedif, K.; Jaffe, G.J.; Tadayoni, R.; Schmidt-Erfurth, U.; Holz, F.G. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 89–99. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Matsubara, M.; Kotoda, Y.; Kasai, Y.; Sugiyama, A.; Kashiwagi, K. Comparison of one-year outcomes between as-needed brolucizumab and aflibercept for polypoidal choroidal vasculopathy. Jpn. J. Ophthalmol. 2023, 67, 402–409. [Google Scholar] [CrossRef]
- Allehyani, M.H.; Alsaeedi, A.K.; Alqthmi, R.O.; Saleh, R.E.; Alsamli, R.S.; Almalki, H.A.; Alshehri, A.F.; Felimban, S.A.; Kambiji, G.J.; Almatrafi, M.I.; et al. Comparative Efficacy of Brolucizumab and Aflibercept in Polypoidal Choroidal Vasculopathy: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e77073. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Mukai, R.; Nakamura, K.; Akiyama, H. Short-term outcomes of intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci. Rep. 2021, 11, 6759. [Google Scholar] [CrossRef]
- Kikushima, W.; Sakurada, Y.; Fukuda, Y.; Matsubara, M.; Kotoda, Y.; Sugiyama, A.; Kashiwagi, K. A Treat-and-Extend Regimen of Intravitreal Brolucizumab for Exudative Age-Related Macular Degeneration Refractory to Aflibercept: A 12-Month Result. Pharmaceuticals 2023, 16, 562. [Google Scholar] [CrossRef]
- Ueda-Consolvo, T.; Tanigichi, A.; Numata, A.; Oiwake, T.; Nakamura, T.; Ishida, M.; Yanagisawa, S.; Hayashi, A. Switching to brolucizumab from aflibercept in age-related macular degeneration with type 1 macular neovascularization and polypoidal choroidal vasculopathy: An 18-month follow-up study. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Lanzetta, P.; Korobelnik, J.F.; Heier, J.S.; Leal, S.; Holz, F.G.; Clark, W.L.; Eichenbaum, D.; Iida, T.; Xiaodong, S.; Berliner, A.J.; et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sakurada, Y.; Matsubara, M.; Hasebe, Y.; Sugiyama, A.; Kikushima, W.; Kashiwagi, K. Comparison of Outcomes between 3 Monthly Brolucizumab and Aflibercept Injections for Polypoidal Choroidal Vasculopathy. Biomedicines 2021, 9, 1164. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef]
- Moorthy, R.S.; Lyon, A.T.; Rabb, M.F.; Spaide, R.F.; Yannuzzi, L.A.; Jampol, L.M. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology 1998, 105, 1380–1385. [Google Scholar] [CrossRef]
- Spaide, R.F.; Donsoff, I.; Lam, D.L.; Yannuzzi, L.A.; Jampol, L.M.; Slakter, J.; Sorenson, J.; Freund, K.B. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina 2002, 22, 529–535. [Google Scholar] [CrossRef]
- Eandi, C.M.; Ober, M.D.; Freund, K.B.; Slakter, J.S.; Yannuzzi, L.A. Selective photodynamic therapy for neovascular age-related macular degeneration with polypoidal choroidal neovascularization. Retina 2007, 27, 825–831. [Google Scholar] [CrossRef]
- Chan, W.M.; Lam, D.S.; Lai, T.Y.; Liu, D.T.; Li, K.K.; Yao, Y.; Wong, T.H. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: One-year results of a prospective case series. Ophthalmology 2004, 111, 1576–1584. [Google Scholar] [CrossRef]
- Lee, S.C.; Seong, Y.S.; Kim, S.S.; Koh, H.J.; Kwon, O.W. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica 2004, 218, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ciardella, A.P.; Donsoff, I.M.; Yannuzzi, L.A. Polypoidal choroidal vasculopathy. Ophthalmol. Clin. N. Am. 2002, 15, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Hussain, A.; Natarajan, S. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Indian J. Ophthalmol. 2005, 53, 101–104. [Google Scholar] [CrossRef]
- Silva, R.M.; Figueira, J.; Cachulo, M.L.; Duarte, L.; Faria de Abreu, J.R.; Cunha-Vaz, J.G. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 973–979. [Google Scholar] [CrossRef]
- Song, M.H.; Ryu, H.W.; Roh, Y.J. One-year results of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy. Ophthalmologica 2011, 226, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, K.; Kim, D.G.; Yu, S.Y.; Kwak, H.W. Two-year results of photodynamic therapy combined with intravitreal anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy. Ophthalmologica 2011, 226, 205–213. [Google Scholar] [CrossRef]
- Koh, A.; Lee, W.K.; Chen, L.J.; Chen, S.J.; Hashad, Y.; Kim, H.; Lai, T.Y.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef]
- Lee, W.K.; Iida, T.; Ogura, Y.; Chen, S.J.; Wong, T.Y.; Mitchell, P.; Cheung, G.C.M.; Zhang, Z.; Leal, S.; Ishibashi, T.; et al. Efficacy and Safety of Intravitreal Aflibercept for Polypoidal Choroidal Vasculopathy in the PLANET Study: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 786–793. [Google Scholar] [CrossRef]
- Baumal, C.R.; Bodaghi, B.; Singer, M.; Tanzer, D.J.; Seres, A.; Joshi, M.R.; Feltgen, N.; Gale, R. Expert Opinion on Management of Intraocular Inflammation, Retinal Vasculitis, and Vascular Occlusion after Brolucizumab Treatment. Ophthalmol. Retina 2021, 5, 519–527. [Google Scholar] [CrossRef]
- Sayanagi, K.; Fujimoto, S.; Hara, C.; Fukushima, Y.; Maruyama, K.; Kawasaki, R.; Sato, S.; Nishida, K. Effect of polyp regression and reduction on treatment efficacy in polypoidal choroidal vasculopathy treated with aflibercept. Sci. Rep. 2024, 14, 1833. [Google Scholar] [CrossRef]
- Kimura, M.; Sakurada, Y.; Fukuda, Y.; Matsubara, M.; Kotoda, Y.; Kasai, Y.; Sugiyama, A.; Kikushima, W.; Tsuru, D.V.; Kashiwagi, K. Association of Polyp Regression after Loading Phase with 12-Month Outcomes of Eyes with Polypoidal Choroidal Vasculopathy. Pharmaceuticals 2024, 17, 687. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Bhatt, V.; Das, S.; Lakhlan, P.; Luthra, G.; Luthra, S. Intraocular Inflammation Following Intravitreal Faricimab Injection in Neovascular Age-Related Macular Degeneration. Cureus 2024, 16, e75937. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, A.; Cohen, S.Y.; Nghiem-Buffet, S.; Smadja, J.; Paques, M.; Fajnkuchen, F.; Mrejen, S. Vitritis following intravitreal faricimab: A retrospective monocentric analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 965–972. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Numaga, S.; Mimura, K.; Asatori, Y.; Akiyama, H. Retinal vasculitis after intravitreal aflibercept 8 mg for neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2024, 68, 531–537. [Google Scholar] [CrossRef]
- Mukai, R.; Matsumoto, H.; Akiyama, H. Risk factors for emerging intraocular inflammation after intravitreal brolucizumab injection for age-related macular degeneration. PLoS ONE 2021, 16, e0259879. [Google Scholar] [CrossRef]
- Yoneyama, S.; Sakurada, Y.; Shijo, T.; Fukuda, Y.; Kotoda, Y.; Kikushima, W.; Mabuchi, F.; Kashiwagi, K. Genetic Factors Associated with Intraocular Inflammation After Brolucizumab Administration in Patients with Exudative Age-Related Macular Degeneration. Genes 2025, 16, 797. [Google Scholar] [CrossRef]
- Holz, F.G.; Iida, T.; Maruko, I.; Sadda, S.R. A Consensus on Risk Mitigation for Brolucizumab in Neovascular Age-Related Macular Degeneration: Patient Selection, Evaluation, and Treatment. Retina 2022, 42, 1629–1637. [Google Scholar] [CrossRef]
- Binder, K.E.; Bleidissel, N.; Charbel Issa, P.; Maier, M.; Coulibaly, L.M. Noninfectious Intraocular Inflammation After Intravitreal Aflibercept. JAMA Ophthalmol. 2025, 143, 499–506. [Google Scholar] [CrossRef]
- Hoffmann, L.; Michels, S.; Eandi, C.; Karam, M.A.; Figueiredo, E.C.O.; Hatz, K. Aflibercept high-dose (8 mg) related intraocular inflammation (IOI)—A case series. BMC Ophthalmol. 2024, 24, 520. [Google Scholar] [CrossRef]
| Brolucizumab (n = 32) | Aflibercept 8 mg (n = 16) | p-Value | |
|---|---|---|---|
| Age (years) | 75.40 ± 8.0 | 73.9 ± 9.2 | 0.61 |
| Male | 24 (75%) | 11 (68.8%) | 0.91 |
| Mean baseline log MAR BCVA | 0.35 ± 0.26 | 0.28 ± 0.27 | 0.40 |
| Baseline central retinal thickness (μm) | 382.0 ± 157.1 | 358.2 ± 152.2 | 0.55 |
| Baseline subfoveal choroidal thickness (μm) | 201.5 ± 77.9 | 185.9 ± 76.1 | 0.55 |
| Number of polyps | 2 ± 1.22 | 1.4 ± 0.6 | 0.083 |
| Maximum polyp size (μm) | 378.1 ± 236.3 | 434.4 ± 154.1 | 0.082 |
| Variables | β-Coefficient | p-Value |
|---|---|---|
| Age (years) | −0.03 | 0.74 |
| Type of treatment (0: aflibercept 8 mg, 1: brolucizumab) | 0.099 | 0.27 |
| Baseline log MAR BCVA | 0.81 | 2.1 × 10−10 |
| Baseline subfoveal choroidal thickness (μm) | −0.027 | 0.77 |
| Baseline central retinal thickness (μm) | 0.24 | 0.013 |
| Number of polyps | −0.09 | 0.35 |
| Maximum polyp size (μm) | −0.06 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, Y.; Sakurada, Y.; Kotoda, Y.; Kimura, M.; Kashiwagi, K. Comparison of Three Consecutive Monthly Administrations Between Aflibercept 8 mg and Brolucizumab 6 mg in Polypoidal Choroidal Vasculopathy. Pharmaceuticals 2025, 18, 1811. https://doi.org/10.3390/ph18121811
Fukuda Y, Sakurada Y, Kotoda Y, Kimura M, Kashiwagi K. Comparison of Three Consecutive Monthly Administrations Between Aflibercept 8 mg and Brolucizumab 6 mg in Polypoidal Choroidal Vasculopathy. Pharmaceuticals. 2025; 18(12):1811. https://doi.org/10.3390/ph18121811
Chicago/Turabian StyleFukuda, Yoshiko, Yoichi Sakurada, Yumi Kotoda, Misa Kimura, and Kenji Kashiwagi. 2025. "Comparison of Three Consecutive Monthly Administrations Between Aflibercept 8 mg and Brolucizumab 6 mg in Polypoidal Choroidal Vasculopathy" Pharmaceuticals 18, no. 12: 1811. https://doi.org/10.3390/ph18121811
APA StyleFukuda, Y., Sakurada, Y., Kotoda, Y., Kimura, M., & Kashiwagi, K. (2025). Comparison of Three Consecutive Monthly Administrations Between Aflibercept 8 mg and Brolucizumab 6 mg in Polypoidal Choroidal Vasculopathy. Pharmaceuticals, 18(12), 1811. https://doi.org/10.3390/ph18121811








