Antioxidant and Anticancer Activities of Water Extracts from Flowers, Leaves and Stems of In Vitro Cultivated and Wild-Growing Marrubium vulgare Plants
Abstract
1. Introduction
2. Results
2.1. In Vitro Culture
2.1.1. Shoot Multiplication
2.1.2. In Vitro Rooting, Adaptation, and Acclimatization Ex Vitro
2.2. Content of Total Polyphenols, Total Flavonoids, and Antioxidant Activity
2.3. Assessment of Anticancer Activity
2.3.1. Antiproliferative Activity
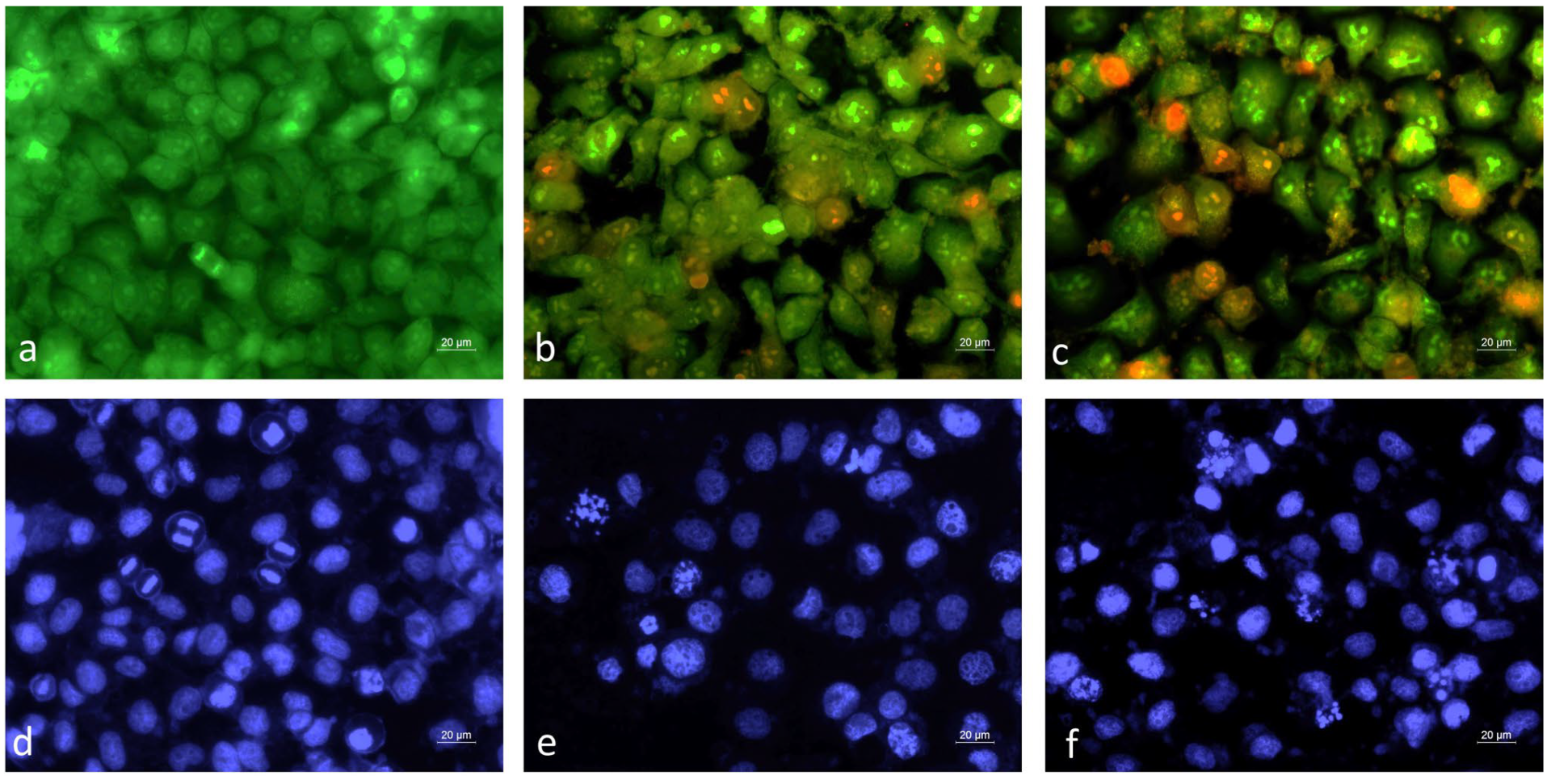
2.3.2. Fluorescent Microscopy
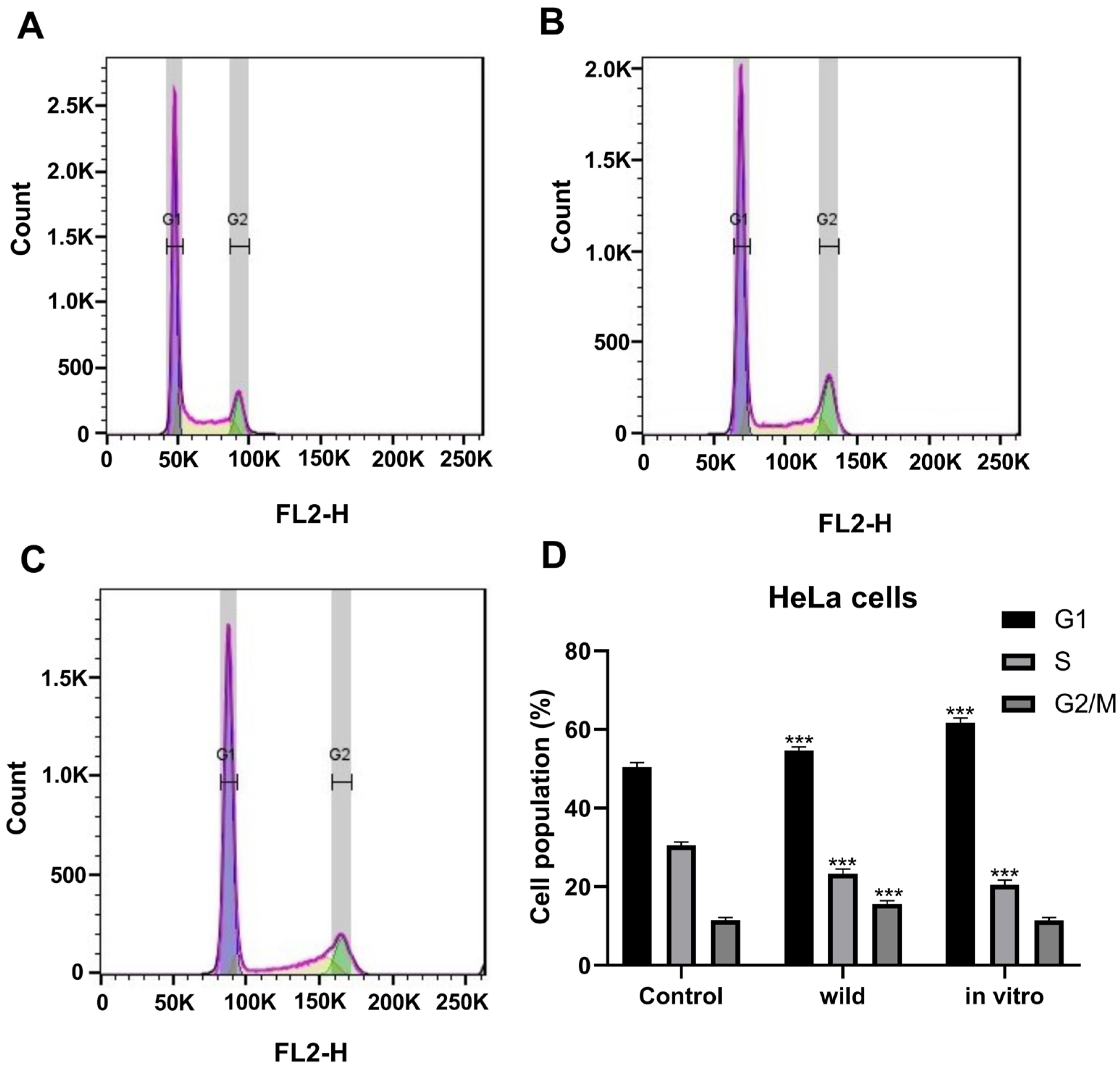
2.3.3. Cell Cycle Analysis
3. Discussion
4. Materials and Methods
4.1. In Vitro Culture
4.1.1. Initial Plant Material
4.1.2. Sterilization of Plant Material
4.1.3. Media Composition for in Vitro Micropropagation
4.1.4. In Vitro Rooting and Acclimatization of Obtained Plants
4.1.5. Conditions for In Vitro Cultures
4.2. Plant Material Extraction and Analysis
4.2.1. Plant Material
4.2.2. Extraction of Polyphenols and Flavonoids
4.2.3. Determination of Total Polyphenol and Total Flavonoid Content
4.2.4. Determination of Marrubiin Content
4.2.5. Antioxidant Activity Assays
4.2.6. Preparation of Freeze-Dried Extract for Anti-Tumor Activity Analysis
4.3. Anti-Tumor Activity
4.3.1. Cell Lines
4.3.2. Assessment of Cell Viability
4.3.3. Analysis of Cell Death
4.3.4. Nuclear Morphology Analysis
4.3.5. Cell Cycle Flow Cytometry Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutabia, L.; Telailia, S.; Mena, M. Traditional therapeutic uses of Marrubium vulgare L. by local populations in the Haddada region (Souk Ahras, Algeria). Ethnobot. Res. Appl. 2020, 19, 44. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.; Sharma, V.; Usman, M. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429. [Google Scholar] [CrossRef]
- Nawal, H.M.; Atta, E.M. Cytotoxic and antioxidant activity of Marrubium vulgare and flavonoid constituents. In Proceedings of the 2nd International Conference on Chemical, Environmental and Biological Sciences, Dubai, United Arab Emirates, 17–18 March 2013. [Google Scholar]
- Belayachi, L.; Aceves-Luquero, C.; Merghoub, N.; Bakri, Y.; Fernández de Mattos, S.; Amzazi, S.; Villalonga, P. Screening of North African medicinal plant extracts for cytotoxic activity against tumor cell lines. Eur. J. Med. Plants 2013, 3, 310–332. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Liggett, J.L.; Kim, N.C.; Baek, S.J. Anti-proliferative effect of horehound leaf and wild cherry bark extracts on human colorectal cancer cells. Oncol. Rep. 2006, 15, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Joha, S.; Cheok, M.; Roumy, V.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F.; Hennebelle, T. Activity of ladanein on leukemia cell lines and its occurrence in Marrubium vulgare. Planta Med. 2010, 76, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Zarai, Z.; Kadri, A.; Chobba, I.B.; Mansour, R.B.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 161. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Prashant, S.P.; Bhawana, M. An update on biotechnological intervention mediated by plant tissue culture to boost secondary metabolite production in medicinal and aromatic plants. Physiol. Plant 2024, 176, e14400. [Google Scholar] [CrossRef]
- Knöss, W. Marrubium vulgare (White Horehound): In vitro culture, and the production of diterpene marrubiin and other secondary me-tabolites. In Medicinal and Aromatic Plants XI; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 43, pp. 274–289. [Google Scholar]
- Abdallah, S.A.S.; Hassan, H.M.S.; El-Mekawy, M.A.; Ali, N.A.M. Application of tissue culture technique on Marrubium vulgare L. plant. Hortscience J. Suez Canal Uni 2018, 7, 1–10. [Google Scholar]
- Mehalaine, S.; Chenchouni, H. New insights for the production of medicinal plant materials: Ex vitro and in vitro propagation of valuable Lamiaceae species from northern Africa. Curr. Plant Biol. 2021, 27, 100216. [Google Scholar] [CrossRef]
- Aeineh, M.; Najafian, S.; Hosseinifarahi, M. Evaluation of pharmaceutical compounds at vegetative and reproductive growth stages of Marrubium vulgare, a medicinal plant to cleanse the body. Nat. Prod. Res. 2024, 38, 3433–3437. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Stryjecka, M.; Zagórska-Dziok, M.; Żarnowiec, P. Biological activity of horehound (Marrubium vulgare L.) herb grown in Poland and its phytochemical composition. Pharmaceuticals 2024, 17, 780. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mehalaine, S.; Menasria, T.; Chenchouni, H. Screening of seed germination requirements of some native plants of the Mediterranean region. Sci. Hortic. 2023, 312, 111838. [Google Scholar] [CrossRef]
- Mehalaine, S.; Menasria, T.; Bouguessa, S.; Yahia, A. In vitro seed germination of some Algerian medicinal plants and the effect of gibberellic acid (GA3) on breaking dormancy. J. Mater. Environ. Sci. 2017, 8, 2034–2039. [Google Scholar]
- Gaspar, T.H.; Kevers, C.; Faivre-Rampant, O.; Crevecoeur, M.; Penel, C.; Greppin, H.; Dommes, J. Changing concepts in plant hormone action. In Vitro Cell. Dev. Biol. Plant 2003, 39, 85–106. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Guedri Mkaddem, M.; Zrig, A.; Ben Abdallah, M.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Hegab, M.Y.; Madany, M.M.Y.; Hassan, A.H.A.; et al. Variation of the chemical composition of essential oils and total phenols content in natural populations of Marrubium vulgare L. Plants 2022, 11, 612. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef]
- Gavaric, A.; Vladić, J.; Ambrus, R.; Jokić, S.; Szabo-Revesz, P.; Tomić, M.; Blažić, M.; Vidović, S. Spray drying of a subcritical extract using Marrubium vulgare as a method of choice for obtaining high quality powder. Pharmaceutics 2019, 11, 523. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. leave extract: Phytochemical composition, antioxidant and wound healing properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A Phytochemical and pharmacological overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Radulović, N.S.; Đorđević Zlatković, M.R.; Stojanović, N.M.; Nešić, M.S.; Zlatković, D.B.; Potić Floranović, M.S.; Tričković Vukić, D.S.; Randjelovic, P.J. Marrubiin inhibits peritoneal inflammatory response induced by carrageenan application in C57 Mice. Int. J. Mol. Sci. 2024, 25, 4496. [Google Scholar] [CrossRef]
- Acquaviva, R.; Malfa, G.A.; Loizzo, M.R.; Xiao, J.; Bianchi, S.; Tundis, R. Advances on natural abietane, labdane and clerodane diterpenes as anti-cancer agents: Sources and mechanisms of action. Molecules 2022, 27, 4791. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Sabarwal, A.; Yadav, U.C.S.; Singh, R.P. Lupeol induces S-phase arrest and mitochondria-mediated apoptosis in cervical cancer cells. J. Biosci. 2018, 43, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Suryati Santoni, A.; Ulia, R.V. Imelda cytotoxic and molecular docking potential of β-sitosterol isolated from Lantana camara leaves against breast (T47D) and cervical cancer (HeLa) cell lines. TJNPR 2024, 8, 6911–6917. [Google Scholar]
- Song, X.; Liu, C.C.; Hong, Y.R.; Zhu, X.C. Anticancer activity of novel oleanolic acid methyl ester derivative in HeLa cervical cancer cells is mediated through apoptosis induction and reactive oxygen species production. Bangladesh J. Pharmacol. 2015, 10, 896–902. [Google Scholar] [CrossRef]
- Xiaofei, J.; Mingqing, S.; Miao, S.; Yizhen, Y.; Shuang, Z.; Qinhua, X.; Kai, Z. Oleanolic acid inhibits cervical cancer Hela cell proliferation through modulation of the ACSL4 ferroptosis signaling pathway. Biochem. Biophys. Res. Commun. 2021, 545, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Indap, M.A.; Susarla, R.; Motiwale, L.; Rao, K.V.K. Anticancer activity of phenolic antioxidants against breast cancer cells and a spontaneous mammary tumor. Indian. J. Pharm. Sci. 2006, 68, 470–474. [Google Scholar]
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 Cells. J. Biochem. Mol. Toxicol. 2014, 28, 387–393. [Google Scholar] [CrossRef]
- Kolahi, M.; Tabandeh, M.; Saremy, S.; Hosseini, S.; Hashemitabar, M. The study of apoptotic effect of p-coumaric acid on breast cancer cells MCF-7. JSSU 2016, 24, 211–221. [Google Scholar]
- Kozyra, M.; Korga, A.; Ostrowska, M.; Humeniuk, E.; Adamczuk, G.; Gieroba, R.; Makuch-Kocka, A.; Dudka, J. Cytotoxic activity of methanolic fractions of different Marrubium spp. against melanoma cells is independent of antioxidant activity and total phenolic content. FEBS Open Bio 2020, 10, 86–95. [Google Scholar] [CrossRef]
- Paunović, V.; Košić, M.; Djordjević, S.; Žugić, A.; Djalinać, N.; Gašić, U.; Trajković, V.; Harhaji-Trajković, J. Marrubium vulgare ethanolic extract induces proliferation block, apoptosis, and cytoprotective autophagy in cancer cells in vitro. Cell Mol. Biol. 2016, 62, 108–114. [Google Scholar]
- Mannoubi, I.E.; Alghamdi, N.M.; Bashir, S.H.; Mohamed, S.A.; Chaabane, H.; Abdalla, A.N.; Abid, M.; Kadri, A.; de Oliveira, M.S. UPLC-ESI-QTOF-MS/MS profiling, antioxidant, and cytotoxicity potentials of Marrubium vulgare L. extracts: Experimental analysis and computational validation. Chem. Biodivers. 2025, 22, e00400. [Google Scholar] [CrossRef]
- Ullah, R.; Alqahtani, A.S.; Shahat, A.A.; Nasr, F.; Wadaan, M.A.; Farooq, M. The antiproliferative effects of Marrubium vulgare, and toxicity screening in zebrafish embryos. J. King Saud. Univ.-Sci. 2024, 36, 103241. [Google Scholar] [CrossRef]
- Okur, M.E.; Karakaş, N.; Karadağ, A.E.; Öztürk, R.Y.; Demirci, F. In vitro cytotoxicity evaluation of Marrubium vulgare L. methanol extract. J. Res. Pharm. 2019, 23, 711–718. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- White Horehound. Ph. Eur. Monograph 1835, European Pharmacopoeia, 7th ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2013. [Google Scholar]
- Lazarova, M.; Stefanova, M.; Denev, P.; Taseva, T.; Vassileva, V.; Tasheva, K. Neuroprotective effect of Marrubium vulgare extract in scopolamine-induced cognitive impairment in rats: Behavioral and biochemical approaches. Biology 2024, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]




| Plant Growth Regulators [mg/L] | New Shoot Formation [%] | Number of Shoot per Explant | Shoot Height [cm] |
|---|---|---|---|
| K1 | 60 | 3.08 ± 0.33 c | 2.00 ± 0.20 cd |
| K1.5 | 80 | 2.75 ± 0.26 c | 1.60 ± 0.16 b |
| K2 | 95 | 3.00 ± 0.24 c | 1.80 ± 0.16 bc |
| K1IAA0.1 | 90 | 4.00 ± 0.22 d | 3.70 ± 0.24 f |
| Z1IAA0.1 | 70 | 2.35 ± 0.13 b | 3.5 ± 0.22 f |
| BAP1IAA0.1 | 80 | 1.93 ± 0.19 a | 2.70 ± 0.23 e |
| K1NAA0.1 | 80 | 2.81 ± 0.20 c | 2.21 ± 0.19 d |
| K2IBA0.2 | 40 | 2.25 ± 0.16 ab | 1.01 ± 0.10 a |
| LSD | - | 0.37 | 0.33 |
| Nutrient Medium | Root Induction [%] | Number of Roots Per Explants | Root Length [cm] |
|---|---|---|---|
| IBA0.1 | 50 | 2.8 ± 0.24 a | 1.5 ± 0.15 b |
| IBA1.0 | 70 | 6.0 ± 0.23 b | 1.0 ± 0.09 a |
| IBA2.0 IAA0.2 | 90 | 6.4 ± 0.22 b | 1.3 ± 0.13 b |
| LSD | 0.45 | 0.25 |
| Sample | Total Polyphenols, mg GAE/100 g DW | Total Flavonoids, mg RE/100 g DW | ORAC, µmol TE/g DW | HORAC, µmol GAE/g DW |
|---|---|---|---|---|
| FCP | 1205.9 ± 10.0 a | 215.2 ± 9.0 b | 297.8 ± 12.4 a | 118.0 ± 5.2 a |
| FWP | 1274.5 ± 5.0 b | n.d. | 67.8 ± 1.2 b | 28.8 ± 1.9 b |
| LCP | 2100.5 ± 3.3 c | 325.4 ± 15.9 d | 518.6 ± 20.8 c | 194.5 ± 11.3 c |
| LWP | 1996.1 ± 7.7 d | 236.5 ± 10.0 c | 492.9 ± 2.4 d | 193.6 ± 15.7 d |
| SCP | 1030.1 ± 3.4 e | 68.2 ± 3.9 a | 254.4 ± 20.6 e | 108.1 ± 6.4 e |
| SWP | 481.6 ± 5.6 f | n.d | 118.9 ± 5.8 f | 50.1 ± 3.6 e |
| LSD | 6.50 | 19.95 | 13.61 | 8.99 |
| Sample | Marrubiin, mg/100 g DW |
|---|---|
| FCP | 279.3 ± 17.0 a |
| FWP | 350.0 ± 14.1 b |
| LCP | 120.7 ± 11.0 bc |
| LWP | 138.5 ± 8.8 c |
| SCP | 101.5 ± 14.1 d |
| SWP | 128.5 ± 6.9 e |
| LSD | 13.14 |
| Cell Line | LWP | LCP | FWP | FCP | SWP | SCP |
|---|---|---|---|---|---|---|
| HeLa 24 h | >1000 | >1000 | 548.0 ± 16.1 a | 692.3 ± 20.1 b | >1000 | >1000 |
| HeLa 72 h | 235.2 ± 7.2 c | 307.0 ± 4.6 d | 42.0 ± 1.0 a | 168.0 ± 7.0 b | 484.4 ± 12.5 e | 306.0 ± 7.9 d |
| HT-29 24 h | >1000 | >1000 | 486.2 ± 11.6 a | 757.1 ± 16.7 b | >1000 | >1000 |
| HT-29 72 h | 377.1 ± 12.1 b | 307.5 ± 7.0 a | 439.2 ± 8.2 c | 330.1 ± 6.1 a | 703.3 ± 21.7 d | 776.8 ± 19.3 e |
| MCF-7 24 h | >1000 | >1000 | 732.7 ± 18.0 a | 906.8 ± 24.4 b | >1000 | >1000 |
| MCF-7 72 h | 234.0 ± 7.6 a | 375.6 ± 11.7 c | 342.8 ± 10.4 b | 361.4 ± 10.2 c | >1000 | 448.9 ± 14.5 d |
| BALB/3T3 24 h | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| BALB/3T3 72 h | 535.2 ± 17.0 a | 669.3 ± 15.5 c | 596.0 ± 13.9 b | 694.2 ± 17.5 c | >1000 | 631.4 ± 16.0 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasheva, K.; Georgieva, A.; Sulikovska, I.; Petrova, M.; Dimitrova, M.; Dimitrova, L.; Georgieva, E.; Denev, P.; Lazarova, M.; Petkova-Kirova, P. Antioxidant and Anticancer Activities of Water Extracts from Flowers, Leaves and Stems of In Vitro Cultivated and Wild-Growing Marrubium vulgare Plants. Pharmaceuticals 2025, 18, 1806. https://doi.org/10.3390/ph18121806
Tasheva K, Georgieva A, Sulikovska I, Petrova M, Dimitrova M, Dimitrova L, Georgieva E, Denev P, Lazarova M, Petkova-Kirova P. Antioxidant and Anticancer Activities of Water Extracts from Flowers, Leaves and Stems of In Vitro Cultivated and Wild-Growing Marrubium vulgare Plants. Pharmaceuticals. 2025; 18(12):1806. https://doi.org/10.3390/ph18121806
Chicago/Turabian StyleTasheva, Krasimira, Ani Georgieva, Inna Sulikovska, Maria Petrova, Margarita Dimitrova, Lyudmila Dimitrova, Elena Georgieva, Petko Denev, Maria Lazarova, and Polina Petkova-Kirova. 2025. "Antioxidant and Anticancer Activities of Water Extracts from Flowers, Leaves and Stems of In Vitro Cultivated and Wild-Growing Marrubium vulgare Plants" Pharmaceuticals 18, no. 12: 1806. https://doi.org/10.3390/ph18121806
APA StyleTasheva, K., Georgieva, A., Sulikovska, I., Petrova, M., Dimitrova, M., Dimitrova, L., Georgieva, E., Denev, P., Lazarova, M., & Petkova-Kirova, P. (2025). Antioxidant and Anticancer Activities of Water Extracts from Flowers, Leaves and Stems of In Vitro Cultivated and Wild-Growing Marrubium vulgare Plants. Pharmaceuticals, 18(12), 1806. https://doi.org/10.3390/ph18121806











