Bacteriophages and Their Enzymes: Allies Against Microbial Biofilms
Abstract
1. Introduction
2. Characterization of Bacterial Biofilms
2.1. Biofilm Formation
2.2. Antibiotic Resistance in Biofilms
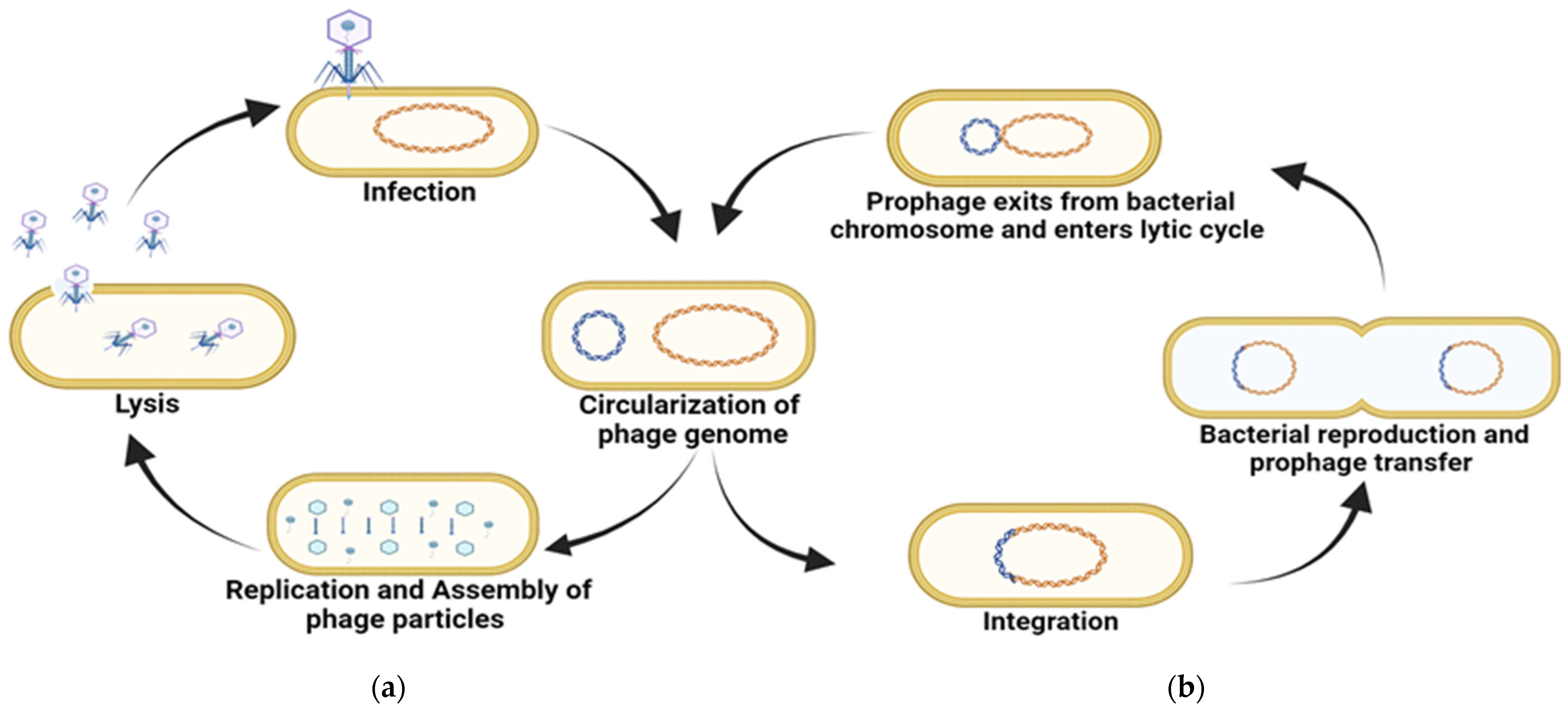
3. Bacteriophages as Inhibitors of Bacterial Biofilm
3.1. Use of Phages Against Biofilm-Forming Bacteria
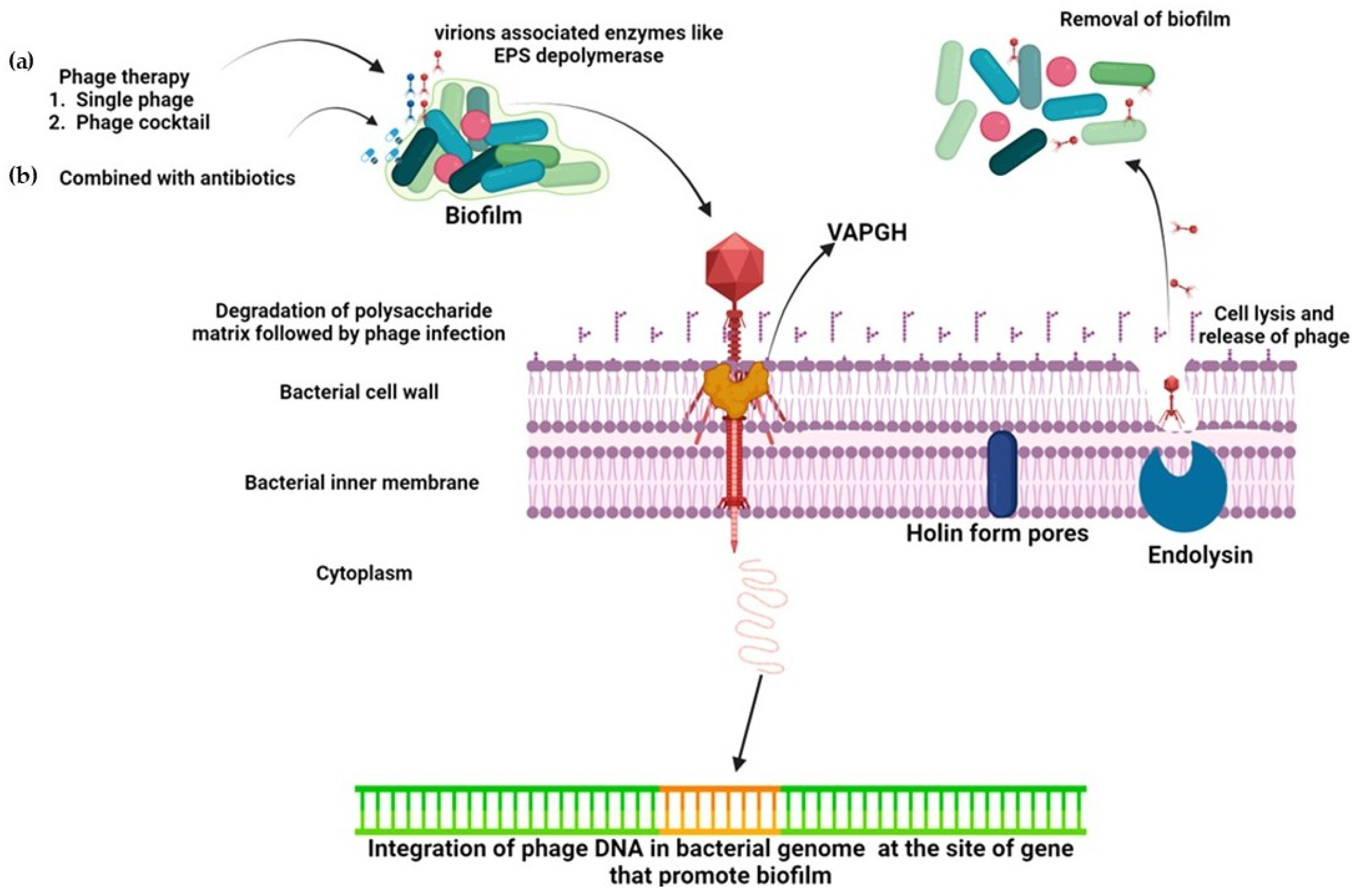
3.2. Phage Cocktail Therapy
4. Phage-Derived Enzymes
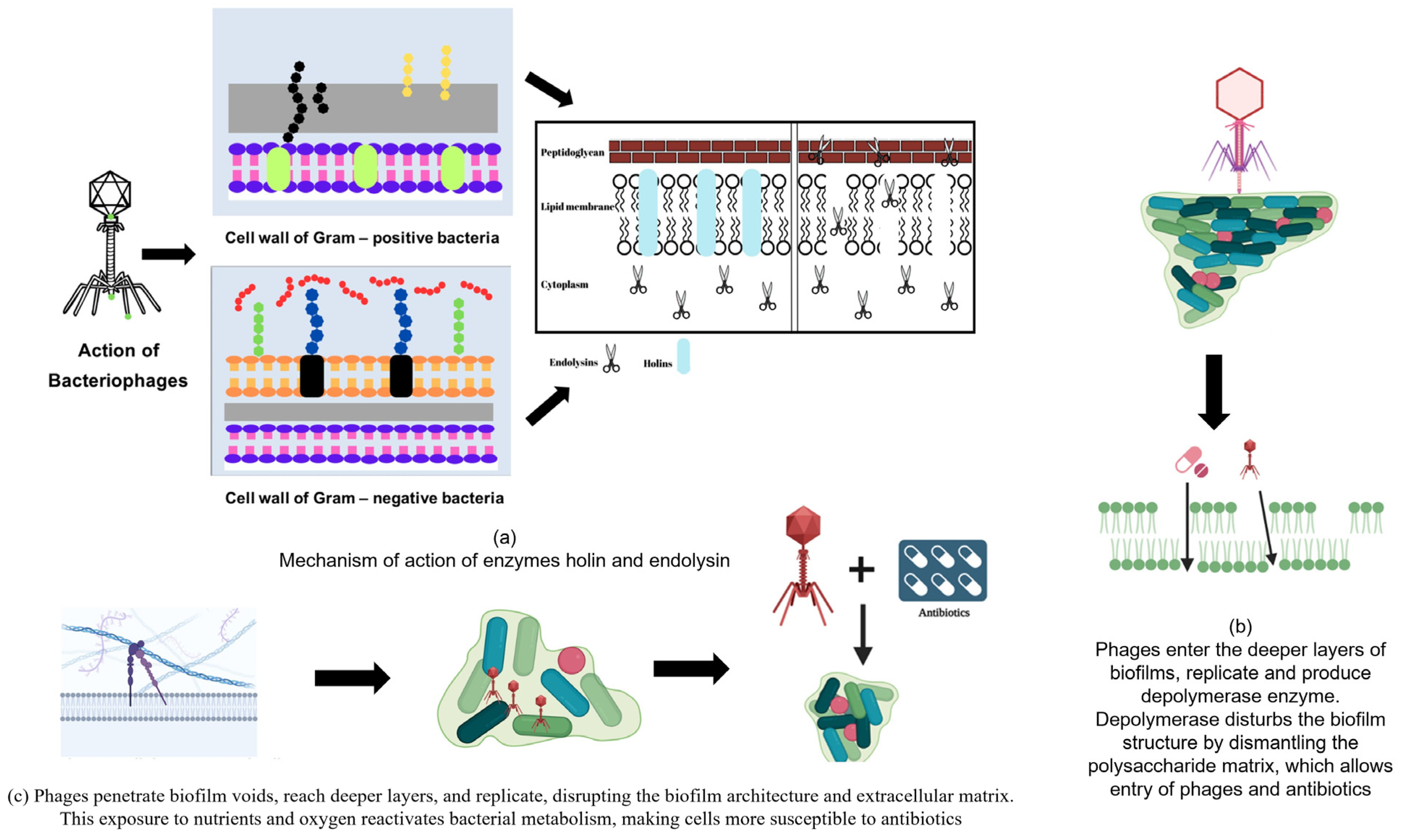
4.1. Holin-Endolysin System
4.1.1. Holins
4.1.2. Role of Lysin in Phage-Mediated Biofilm Control
5. Engineered Endolysins
5.1. Innolysins
5.2. Lysocin (Lysin-Bacteriocin)
5.3. Pinholins–Signal Arrest Release (SAR) System
5.4. Spanins
5.5. Virion-Associated Lysins (VAL)/Virion-Associated Peptidoglycan Hydrolase (VAPGH)
5.6. Amurins
6. Role of Phage-Encoded Depolymerases in Biofilm Control
7. Combination Therapy with Phage and Antibiotics
8. Mechanism of Action
9. Resistance of Bacterial Biofilms Against Phage Infections
10. Limitations of Phage Therapy
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upadhayay, A.; Ling, J.; Pal, D.; Xie, Y.; Ping, F.F.; Kumar, A. Resistance-Proof Antimicrobial Drug Discovery to Combat Global Antimicrobial Resistance Threat. Drug Resist. Updates 2023, 66, 100890. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277. [Google Scholar] [PubMed]
- Gould, I.M.; Bal, A.M. New Antibiotic Agents in the Pipeline and How They Can Help Overcome Microbial Resistance. Virulence 2013, 4, 185–191. [Google Scholar] [CrossRef]
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.P. The Multifaceted Roles of Antibiotics and Antibiotic Resistance in Nature. Front. Microbiol. 2013, 4, 38490. [Google Scholar] [CrossRef]
- Piddock, L.J.V. The Crisis of No New Antibiotics—What Is the Way Forward? Lancet Infect. Dis. 2012, 12, 249–253. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Read, A.F.; Woods, R.J. Antibiotic Resistance Management. Evol. Med. Public Health 2014, 2014, 147. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. Antibiotic-Resistant Bugs in the 21st Century—A Clinical Super-Challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, R.; Bielejewska, A.; Bociek, A.; Bociek, M. Resistance to ceftaroline—2018 review. Eur. J. Biol. Res. 2018, 8, 112–120. [Google Scholar] [CrossRef]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for Antibiotic Discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef]
- Laxminarayan, R. Antibiotic Effectiveness: Balancing Conservation against Innovation. Science 2014, 345, 1299–1301. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A Review of the Scientific Evidence for Biofilms in Wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in Food Processing Environments: Challenges and Opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, M.; Melander, C.; Kjellerup, B.V. Dispersal and Inhibition of Biofilms Associated with Infections. J. Appl. Microbiol. 2020, 128, 1279–1288. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.M.; Donlan, R.M. Bacteriophage-Mediated Control of a Two-Species Biofilm Formed by Microorganisms Causing Catheter-Associated Urinary Tract Infections in an in Vitro Urinary Catheter Model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Vandenheuvel, D.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Two Phages, PhiIPLA-RODI and PhiIPLA-C1C, Lyse Mono-and Dual-Species Staphylococcal Biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef]
- Abedon, S.T. Ecology and Evolutionary Biology of Hindering Phage Therapy: The Phage Tolerance vs. Phage Resistance of Bacterial Biofilms. Antibiotics 2023, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms 2023, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Mizan, M.F.R.; Jahid, I.K.; Ha, S. Do Microbial Biofilms in Seafood: A Food-Hygiene Challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 315815. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The Role of van Der Waals Forces and Hydrogen Bonds in “Hydrophobic Interactions” between Biopolymers and Low Energy Surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum Sensing in Bacteria: The LuxR-LuxI Family of Cell Density- Responsive Transcriptional Regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef]
- Lappin-Scott, H.M.; Costerton, J.W. Bacterial Biofilms and Surface Fouling. Biofouling 1989, 1, 323–342. [Google Scholar] [CrossRef]
- Jackson, K.D.; Starkey, M.; Kremer, S.; Parsek, M.R.; Wozniak, D.J. Identification of Psl, a Locus Encoding a Potential Exopolysaccharide That Is Essential for Pseudomonas aeruginosa PAO1 Biofilm Formation. J. Bacteriol. 2004, 186, 4466–4475. [Google Scholar] [CrossRef]
- Matsukawa, M.; Greenberg, E.P. Putative Exopolysaccharide Synthesis Genes Influence Pseudomonas aeruginosa Biofilm Development. J. Bacteriol. 2004, 186, 4449–4456. [Google Scholar] [CrossRef]
- Gjermansen, M.; Nilsson, M.; Yang, L.; Tolker-Nielsen, T. Characterization of Starvation-Induced Dispersion in Pseudomonas Putida Biofilms: Genetic Elements and Molecular Mechanisms. Mol. Microbiol. 2010, 75, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Purevdorj-Gage, B.; Costerton, W.J.; Stoodley, P. Phenotypic Differentiation and Seeding Dispersal in Non-Mucoid and Mucoid Pseudomonas aeruginosa Biofilms. Microbiology 2005, 151, 1569–1576. [Google Scholar] [CrossRef]
- Wilksch, J.J.; Yang, J.; Clements, A.; Gabbe, J.L.; Short, K.R.; Cao, H.; Cavaliere, R.; James, C.E.; Whitchurch, C.B.; Schembri, M.A.; et al. MrkH, a Novel c-Di-GMP-Dependent Transcriptional Activator, Controls Klebsiella pneumoniae Biofilm Formation by Regulating Type 3 Fimbriae Expression. PLoS Pathog. 2011, 7, e1002204. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, Y.; Guo, J.H.; Losick, R. Evidence for Cyclic Di-GMP-Mediated Signaling in Bacillus subtilis. J. Bacteriol. 2012, 194, 5080–5090. [Google Scholar] [CrossRef]
- Ryan, R.P.; Tolker-Nielsen, T.; Dow, J.M. When the PilZ Don’t Work: Effectors for Cyclic Di-GMP Action in Bacteria. Trends. Microbiol. 2012, 20, 235–242. [Google Scholar] [CrossRef]
- Monds, R.D.; Newell, P.D.; Gross, R.H.; O’Toole, G.A. Phosphate-Dependent Modulation of c-Di-GMP Levels Regulates Pseudomonas Fluorescens Pf0-1 Biofilm Formation by Controlling Secretion of the Adhesin LapA. Mol. Microbiol. 2007, 63, 656–679. [Google Scholar] [CrossRef]
- O’Connor, J.R.; Kuwada, N.J.; Huangyutitham, V.; Wiggins, P.A.; Harwood, C.S. Surface Sensing and Lateral Subcellular Localization of WspA, the Receptor in a Chemosensory-like System Leading to c-Di-GMP Production. Mol. Microbiol. 2012, 86, 720–729. [Google Scholar] [CrossRef]
- Hickman, J.W.; Tifrea, D.F.; Harwood, C.S. A Chemosensory System That Regulates Biofilm Formation through Modulation of Cyclic Diguanylate Levels. Proc. Natl. Acad. Sci. USA 2005, 102, 14422–14427. [Google Scholar] [CrossRef]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa Uses a Cyclic-Di-GMP-Regulated Adhesin to Reinforce the Biofilm Extracellular Matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.R.; Sauer, K. Small RNAs and Their Role in Biofilm Formation. Trends Microbiol. 2013, 21, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; De La Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A Broad-Spectrum Antibiofilm Peptide Enhances Antibiotic Action against Bacterial Biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm Formation and Control Strategies of Foodborne Pathogens: Food Safety Perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic Treatment of Biofilm Infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Husain, F.M.; Al-Shabib, N.A.; Noor, S.; Khan, R.A.; Khan, M.S.; Ansari, F.A.; Khan, M.S.; Khan, A.; Ahmad, I. Current Strategy to Target Bacterial Quorum Sensing and Virulence by Phytocompounds. New Look Phytomedicine Adv. Herb. Prod. Nov. Drug Leads 2018, 301–329. [Google Scholar] [CrossRef]
- Chua, S.L.; Yam, J.K.H.; Hao, P.; Adav, S.S.; Salido, M.M.; Liu, Y.; Givskov, M.; Sze, S.K.; Tolker-Nielsen, T.; Yang, L. Selective Labelling and Eradication of Antibiotic-Tolerant Bacterial Populations in Pseudomonas aeruginosa Biofilms. Nat. Commun. 2016, 7, 10750. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.L.; Cagnazzo, J.; Phan, C.Q.; Barnes, A.M.T.; Dunny, G.M. Multiple Roles for Enterococcus faecalis Glycosyltransferases in Biofilm-Associated Antibiotic Resistance, Cell Envelope Integrity, and Conjugative Transfer. Antimicrob. Agents Chemother. 2015, 59, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Breyne, K.; De Rycke, R.; Vermote, A.; Van Nieuwerburgh, F.; Meyer, E.; Van Calenbergh, S.; Coenye, T. The Quorum Sensing Inhibitor Hamamelitannin Increases Antibiotic Susceptibility of Staphylococcus aureus Biofilms by Affecting Peptidoglycan Biosynthesis and EDNA Release. Sci. Rep. 2016, 6, 20321. [Google Scholar] [CrossRef]
- Rafii, F.; Hart, M.E. Antimicrobial Resistance in Clinically Important Biofilms. World J. Pharmacol. 2015, 4, 31–46. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Wang, L.; Zhu, M.; Zhang, P.; Zhu, X. Extracellular Polymeric Substances Affect the Responses of Multi-Species Biofilms in the Presence of Sulfamethizole. Environ. Pollut. 2018, 235, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material Properties of Biofilms—A Review of Methods for Understanding Permeability and Mechanics. Rep. Prog. Phys. 2015, 78, 036601. [Google Scholar] [CrossRef]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef]
- Miyaue, S.; Suzuki, E.; Komiyama, Y.; Kondo, Y.; Morikawa, M.; Maeda, S. Bacterial Memory of Persisters: Bacterial Persister Cells Can Retain Their Phenotype for Days or Weeks after Withdrawal from Colony-Biofilm Culture. Front. Microbiol. 2018, 9, 379354. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial Persister Cell Formation and Dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Bowler, L.L.; Zhanel, G.G.; Ball, T.B.; Saward, L.L. Mature Pseudomonas aeruginosa Biofilms Prevail Compared to Young Biofilms in the Presence of Ceftazidime. Antimicrob. Agents Chemother. 2012, 56, 4976–4979. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas Aeruginosa biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 453354. [Google Scholar] [CrossRef]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 531825. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Wang, S.; Guan, L.; Li, X.; Hu, D.; Gao, D.; Song, J.; Chen, H.; Qiana, P. A Novel Tail-Associated O91-Specific Polysaccharide Depolymerase from a Podophage Reveals Lytic Efficacy of Shiga Toxin-Producing Escherichia coli. Appl. Environ. Microbiol. 2020, 86, e00145-20. [Google Scholar] [CrossRef] [PubMed]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.J.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in Nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage Resistance Mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as Weapons against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 189912. [Google Scholar] [CrossRef]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage—A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205–217. [Google Scholar] [CrossRef]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 502215. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Yu, P.; Zuo, P.; Da Silva, M.L.B.; Alvarez, P.J.J. Going Viral: Emerging Opportunities for Phage-Based Bacterial Control in Water Treatment and Reuse. Acc. Chem. Res. 2019, 52, 849–857. [Google Scholar] [CrossRef]
- Pires, D.P.; Melo, L.D.R.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage Therapy as an Alternative or Complementary Strategy to Prevent and Control Biofilm-Related Infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Alemayehu, D.; Casey, P.G.; Mcauliffe, O.; Guinane, C.M.; Martin, J.G.; Shanahan, F.; Coffey, A.; Ross, R.P.; Hill, C. Bacteriophages ΦMR299-2 and ΦNH-4 Can Eliminate Pseudomonas aeruginosa in the Murine Lung and on Cystic Fibrosis Lung Airway Cells. mBio 2012, 3, e00029-12. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Nannapaneni, R. Removal of Listeria monocytogenes Biofilms with Bacteriophage P100. J. Food Prot. 2010, 73, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Ganegama Arachchi, G.J.; Cridge, A.G.; Dias-Wanigasekera, B.M.; Cruz, C.D.; McIntyre, L.; Liu, R.; Flint, S.H.; Mutukumira, A.N. Effectiveness of Phages in the Decontamination of Listeria monocytogenes Adhered to Clean Stainless Steel, Stainless Steel Coated with Fish Protein, and as a Biofilm. J. Ind. Microbiol. Biotechnol. 2013, 40, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, L.; Brosh, Y.; Gelman, D.; Coppenhagen-Glazer, S.; Beyth, S.; Poradosu-Cohen, R.; Que, Y.A.; Beyth, N.; Hazan, R. Targeting Enterococcus faecalis Biofilms with Phage Therapy. Appl. Environ. Microbiol. 2015, 81, 2696–2705. [Google Scholar] [CrossRef]
- Nzakizwanayo, J.; Hanin, A.; Alves, D.R.; McCutcheon, B.; Dedi, C.; Salvage, J.; Knox, K.; Stewart, B.; Metcalfe, A.; Clark, J.; et al. Bacteriophage Can Prevent Encrustation and Blockage of Urinary Catheters by Proteus mirabilis. Antimicrob. Agents Chemother. 2016, 60, 1530–1536. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-Threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-Biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Patel, J.; Sharma, M.; Millner, P.; Calaway, T.; Singh, M. Inactivation of Escherichia coli O157:H7 Attached to Spinach Harvester Blade Using Bacteriophage. Foodborne Pathog. Dis. 2011, 8, 541–546. [Google Scholar] [CrossRef]
- Kelly, D.; Mcauliffe, O.; Ross, R.P.; Coffey, A. Prevention of Staphylococcus aureus Biofilm Formation and Reduction in Established Biofilm Density Using a Combination of Phage K and Modified Derivatives. Lett. Appl. Microbiol. 2012, 54, 286–291. [Google Scholar] [CrossRef]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T.A. Combined Use of Bacteriophage K and a Novel Bacteriophage to Reduce Staphylococcus aureus Biofilm Formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef]
- Alves, D.R.; Perez-Esteban, P.; Kot, W.; Bean, J.E.; Arnot, T.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T.A. A Novel Bacteriophage Cocktail Reduces and Disperses Pseudomonas aeruginosa Biofilms under Static and Flow Conditions. Microb. Biotechnol. 2016, 9, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.P. Differential Effect of Newly Isolated Phages Belonging to PB1-Like, PhiKZ-Like and LUZ24-Like Viruses against Multi-Drug Resistant Pseudomonas aeruginosa under Varying Growth Conditions. Viruses 2017, 9, 315. [Google Scholar] [CrossRef]
- Forti, F.; Roach, D.R.; Cafora, M.; Pasini, M.E.; Horner, D.S.; Fiscarelli, E.V.; Rossitto, M.; Cariani, L.; Briani, F.; Debarbieux, L.; et al. Design of a Broad-Range Bacteriophage Cocktail That Reduces Pseudomonas aeruginosa Biofilms and Treats Acute Infections in Two Animal Models. Antimicrob. Agents Chemother. 2018, 62, e02573-17. [Google Scholar] [CrossRef] [PubMed]
- Racenis, K.; Kroica, J.; Rezevska, D.; Avotins, L.; Skuditis, E.; Popova, A.; Puide, I.; Kuzema, V.; Petersons, A.S. Aureus Colonization, Biofilm Production, and Phage Susceptibility in Peritoneal Dialysis Patients. Antibiotics 2020, 9, 582. [Google Scholar] [CrossRef]
- Mangieri, N.; Foschino, R.; Picozzi, C. Application of Bacteriophages on Shiga Toxin-Producing Escherichia coli (STEC) Biofilm. Antibiotics 2021, 10, 1423. [Google Scholar] [CrossRef]
- Zurabov, F.; Glazunov, E.; Kochetova, T.; Uskevich, V.; Popova, V. Bacteriophages with Depolymerase Activity in the Control of Antibiotic Resistant Klebsiella pneumoniae Biofilms. Sci. Rep. 2023, 13, 15188. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.G.; Wang, I.N.; Struck, D.K.; Young, R. Breaking Free: “Protein Antibiotics” and Phage Lysis. Res. Microbiol. 2002, 153, 493–501. [Google Scholar] [CrossRef]
- Abeysekera, G.S.; Love, M.J.; Manners, S.H.; Billington, C.; Dobson, R.C.J. Bacteriophage-Encoded Lethal Membrane Disruptors: Advances in Understanding and Potential Applications. Front. Microbiol. 2022, 13, 1044143. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Łepek, K.; Stasiłojć, M.; Kosznik-Kwaśnicka, K.; Zdrojewska, K.; Maciąg-Dorszyńska, M.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Encoded Enzymes Destroying Bacterial Cell Membranes and Walls, and Their Potential Use as Antimicrobial Agents. Microbiol. Res. 2021, 248, 126746. [Google Scholar] [CrossRef]
- Cahill, J.; Holt, A.; Theodore, M.; Moreland, R.; O’Leary, C.; Martin, C.; Bettridge, K.; Xiao, J.; Young, R. Spatial and Temporal Control of Lysis by the Lambda Holin. mBio 2023, 15, e0129023. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage Lysins as Effective Antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Chen, J.H.; Zhang, R.; Liu, B. A Comprehensive Review of the Applications of Bacteriophage-Derived Endolysins for Foodborne Bacterial Pathogens and Food Safety: Recent Advances, Challenges, and Future Perspective. Front. Microbiol. 2023, 14, 1259210. [Google Scholar] [CrossRef]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell Wall Hydrolases in Bacteria: Insight on the Diversity of Cell Wall Amidases, Glycosidases and Peptidases toward Peptidoglycan. Front. Microbiol. 2019, 10, 418687. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Lee, S.J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.J. Antibacterial and Biofilm Removal Activity of a Podoviridae Staphylococcus aureus Bacteriophage SAP-2 and a Derived Recombinant Cell-Wall-Degrading Enzyme. Appl. Microbiol. Biotechnol. 2010, 86, 1439–1449. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective Removal of Staphylococcal Biofilms by the Endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Keary, R.; McAuliffe, O.; Ross, R.P.; O’Mahony, J.; Coffey, A. Bacteriophage-Derived Peptidase CHAPK Eliminates and Prevents Staphylococcal Biofilms. Int. J. Microbiol. 2013, 2013, 625341. [Google Scholar] [CrossRef]
- Yang, H.; Bi, Y.; Shang, X.; Wang, M.; Linden, S.B.; Li, Y.; Li, Y.; Nelson, D.C.; Wei, H. Antibiofilm Activities of a Novel Chimeolysin against Streptococcus Mutans under Physiological and Cariogenic Conditions. Antimicrob. Agents Chemother. 2016, 60, 7436–7443. [Google Scholar] [CrossRef]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, e02666-16. [Google Scholar] [CrossRef]
- Vázquez, R.; García, P. Synergy between Two Chimeric Lysins to Kill Streptococcus pneumoniae. Front. Microbiol. 2019, 10, 464211. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered Endolysin-Based “Artilysins” to Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 238301. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, Y.; Dai, J.; Zhou, J.; Yu, R.; Zhang, C. Design SMAP29-LysPA26 as a Highly Efficient Artilysin against Pseudomonas Aeruginosa with Bactericidal and Antibiofilm Activity. Microbiol. Spectr. 2021, 9, e0054621. [Google Scholar] [CrossRef]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Grimon, D.; Antenucci, F.; Vitt, A.R.; Bortolaia, V.; Briers, Y.; Brøndsted, L. Exploiting Phage Receptor Binding Proteins to Enable Endolysins to Kill Gram-Negative Bacteria. Sci. Rep. 2020, 10, 12087. [Google Scholar] [CrossRef]
- Heselpoth, R.D.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Lysocins: Bioengineered Antimicrobials That Deliver Lysins across the Outer Membrane of Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2019, 63, 00342-19. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, T.; Khan, R.H.; Sahu, I.D.; Drew, D.L.; Faul, E.; Li, T.; McCarrick, R.M.; Lorigan, G.A. Pinholin S21 Mutations Induce Structural Topology and Conformational Changes. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183771. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Cahill, J.; Ramsey, J.; Martin, C.; O’Leary, C.; Moreland, R.; Maddox, L.T.; Galbadage, T.; Sharan, R.; Sule, P.; et al. Phage-Encoded Cationic Antimicrobial Peptide Required for Lysis. J. Bacteriol. 2022, 204, JB0021421. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.H.; Rotich, N.C.; Morris, A.; Ahammad, T.; Baral, B.; Sahu, I.D.; Lorigan, G.A. Probing the Structural Topology and Dynamic Properties of Gp28 Using Continuous Wave Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. B 2023, 127, 9236–9247. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Martínez, B.; Donovan, D.M.; Rodríguez, A.; García, P. Bacteriophage Virion-Associated Peptidoglycan Hydrolases: Potential New Enzybiotics. Crit. Rev. Microbiol. 2013, 39, 427–434. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H. Phage Products for Fighting Antimicrobial Resistance. Microorganisms 2022, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Chamakura, K.R.; Edwards, G.B.; Young, R. Mutational Analysis of the MS2 Lysis protein L. Microbiology 2017, 163, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Antillon, S.F.; Bernhardt, T.G.; Chamakura, K.; Young, R. Physiological Characterization of Single Gene Lysis proteins. bioRxiv 2023, 562596. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Zhang, P.; Xu, M.; Yuan, W.; Bian, L.; Liu, Y.; Xia, J.; Leung, S.S.Y. Phage-Derived Depolymerase as an Antibiotic Adjuvant Against Multidrug-Resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 845500. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-Neck Appendage Protein (Dpo7) from Phage VB_SepiS-PhiIPLA7 as an Anti-Biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 167086. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Yan, G.; Lei, L.; Wang, S.; Yu, L.; Zhou, L.; Gao, A.; Feng, X.; Han, W.; et al. Identification and Characterization of Dpo42, a Novel Depolymerase Derived from the Escherichia coli Phage VB_EcoM_ECOO78. Front. Microbiol. 2017, 8, 273039. [Google Scholar] [CrossRef]
- Hernandez-Morales, A.C.; Lessor, L.L.; Wood, T.L.; Migl, D.; Mijalis, E.M.; Cahill, J.; Russell, W.K.; Young, R.F.; Gill, J.J. Genomic and Biochemical Characterization of Acinetobacter Podophage Petty Reveals a Novel Lysis Mechanism and Tail-Associated Depolymerase Activity. J. Virol. 2018, 92, e01064-17. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Liu, Y.; Wang, C.; He, T.; Gao, S.; Xing, S.; Huang, Y.; Fan, H.; Zhang, X.; Yu, W.; et al. Identification of a Lytic Pseudomonas Aeruginosa Phage Depolymerase and Its Anti-Biofilm Effect and Bactericidal Contribution to Serum. Virus Genes 2019, 55, 394–405. [Google Scholar] [CrossRef]
- Kamal, F.; Dennis, J.J. Burkholderia cepacia Complex Phage-Antibiotic Synergy (PAS): Antibiotics Stimulate Lytic Phage Activity. Appl. Environ. Microbiol. 2015, 81, 1132–1138. [Google Scholar] [CrossRef]
- Lu, T.K.; Collins, J.J. Dispersing Biofilms with Engineered Enzymatic Bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef]
- Bedi, M.S.; Verma, V.; Chhibber, S. Amoxicillin and Specific Bacteriophage Can Be Used Together for Eradication of Biofilm of Klebsiella pneumoniae B5055. World J. Microbiol. Biotechnol. 2009, 25, 1145–1151. [Google Scholar] [CrossRef]
- Rahman, M.; Kim, S.; Kim, S.M.; Seol, S.Y.; Kim, J. Characterization of Induced Staphylococcus aureus Bacteriophage SAP-26 and Its Anti-Biofilm Activity with Rifampicin. Biofouling 2011, 27, 1087–1093. [Google Scholar] [CrossRef]
- Yilmaz, C.; Colak, M.; Yilmaz, B.C.; Ersoz, G.; Kutateladze, M.; Gozlugol, M. Bacteriophage Therapy in Implant-Related Infections: An Experimental Study. J. Bone Jt. Surg. 2013, 95, 117–125. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Das, T.; Manos, J.; Kutter, E.; Morales, S.; Chan, H.K. Bacteriophage PEV20 and Ciprofloxacin Combination Treatment Enhances Removal of Pseudomonas aeruginosa Biofilm Isolated from Cystic Fibrosis and Wound Patients. AAPS J. 2019, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Latka, A.; Drulis-Kawa, Z. Advantages and Limitations of Microtiter Biofilm Assays in the Model of Antibiofilm Activity of Klebsiella Phage KP34 and Its Depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef]
- Fan, X.; Li, W.; Zheng, F.; Xie, J. Bacteriophage Inspired Antibiotics Discovery against Infection Involved Biofilm. Crit. Rev. Trade Eukaryot. Gene Expr. 2013, 23, 317–326. [Google Scholar] [CrossRef]
- Ojha, A.; Anand, M.; Bhatt, A.; Kremer, L.; Jacobs, W.R.; Hatfull, G.F. GroEL1: A Dedicated Chaperone Involved in Mycolic Acid Biosynthesis during Biofilm Formation in Mycobacteria. Cell 2005, 123, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Moak, M.; Molineux, I.J. Peptidoglycan Hydrolytic Activities Associated with Bacteriophage Virions. Mol. Microbiol. 2004, 51, 1169–1183. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.M.R.T.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Yazdi, M.; Bouzari, M.; Ghaemi, E.A. Isolation and Characterization of a Lytic Bacteriophage (VB_PmiS-TH) and Its Application in Combination with Ampicillin against Planktonic and Biofilm Forms of Proteus mirabilis Isolated from Urinary Tract Infection. J. Mol. Microbiol. Biotechnol. 2018, 28, 37–46. [Google Scholar] [CrossRef]
- Akturk, E.; Oliveira, H.; Santos, S.B.; Costa, S.; Kuyumcu, S.; Melo, L.D.R.; Azeredo, J. Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms. Antibiotics 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM Is a Widespread Bacterial Defence System with Broad Anti-Phage Activities. Nat. Microbiol. 2017, 3, 90–98. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting Phage Therapy: New Applications for Old Resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A Thermostable Salmonella Phage Endolysin, Lys68, with Broad Bactericidal Properties against Gram-Negative Pathogens in Presence of Weak Acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef]
- Plotka, M.; Kaczorowska, A.K.; Stefanska, A.; Morzywolek, A.; Fridjonsson, O.H.; Dunin-Horkawicz, S.; Kozlowski, L.; Hreggvidsson, G.O.; Kristjansson, J.K.; Dabrowski, S.; et al. Novel Highly Thermostable Endolysin from Thermus Scotoductus MAT2119 Bacteriophage Ph2119 with Amino Acid Sequence Similarity to Eukaryotic Peptidoglycan Recognition Proteins. Appl. Environ. Microbiol. 2014, 80, 886–895. [Google Scholar] [CrossRef]
- Müller, M.; Calvert, M.; Hottmann, I.; Kluj, R.M.; Teufel, T.; Balbuchta, K.; Engelbrecht, A.; Selim, K.A.; Xu, Q.; Borisova, M.; et al. The Exo-β-N-Acetylmuramidase NamZ from Bacillus subtilis Is the Founding Member of a Family of Exo-Lytic Peptidoglycan Hexosaminidases. J. Biol. Chem. 2021, 296, 100519. [Google Scholar] [CrossRef]
- Dik, D.A.; Marous, D.R.; Fisher, J.F.; Mobashery, S. Lytic Transglycosylases: Concinnity in Concision of the Bacterial Cell Wall. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 503. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Henry, X.; El Ghachi, M.; Auger, G.; Blanot, D.; Parquet, C.; Mengin-Lecreulx, D.; Bouhss, A. Active Site Mapping of MraY, a Member of the Polyprenyl-Phosphate N-Acetylhexosamine 1-Phosphate Transferase Superfamily, Catalyzing the First Membrane Step of Peptidoglycan Biosynthesis. Biochemistry 2008, 47, 8919–8928. [Google Scholar] [CrossRef]
- van Heijenoort, J. Lipid Intermediates in the Biosynthesis of Bacterial Peptidoglycan. Microbiol. Mol. Biol. Rev. 2007, 71, 620. [Google Scholar] [CrossRef] [PubMed]
- Orta, A.K.; Riera, N.; Li, Y.E.; Tanaka, S.; Yun, H.G.; Klaic, L.; Clemons, W.M. The Mechanism of the Phage-Encoded Protein Antibiotic from FX174. Science 2023, 381, eadg9091. [Google Scholar] [CrossRef]
- Chamakura, K.; Young, R. Phage Single-Gene Lysis: Finding the Weak Spot in the Bacterial Cell Wall. J. Biol. Chem. 2019, 294, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Chamakura, K.R.; Tran, J.S.; Young, R. MS2 Lysis of Escherichia Coli Depends on Host Chaperone DnaJ. J. Bacteriol. 2017, 199, e00058-17. [Google Scholar] [CrossRef]
- Montañez-Izquierdo, V.Y.; Salas-Vázquez, D.I.; Rodríguez-Jerez, J.J. Use of Epifluorescence Microscopy to Assess the Effectiveness of Phage P100 in Controlling Listeria monocytogenes Biofilms on Stainless Steel Surfaces. Food Control 2012, 23, 470–477. [Google Scholar] [CrossRef]
- Sharma, M.; Ryu, J.H.; Beuchat, L.R. Inactivation of Escherichia coli O157:H7 in Biofilm on Stainless Steel by Treatment with an Alkaline Cleaner and a Bacteriophage. J. Appl. Microbiol. 2005, 99, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.; Ayariga, J.A.; Robertson, B.K. Bacteriophages as Biocontrol Agents in Livestock Food Production. Microorganisms 2022, 10, 2126. [Google Scholar] [CrossRef]
- Vandersteegen, K.; Kropinski, A.M.; Nash, J.H.E.; Noben, J.P.; Hermans, K.; Lavigne, R. Romulus and Remus, Two Phage Isolates Representing a Distinct Clade within the Twortlikevirus Genus, Display Suitable Properties for Phage Therapy Applications. J. Virol. 2013, 87, 3237–3247. [Google Scholar] [CrossRef]
- Maszewska, A.; Zygmunt, M.; Grzejdziak, I.; Różalski, A. Use of Polyvalent Bacteriophages to Combat Biofilm of Proteus Mirabilis Causing Catheter-Associated Urinary Tract Infections. J. Appl. Microbiol. 2018, 125, 1253–1265. [Google Scholar] [CrossRef]
- Siringan, P.; Connerton, P.L.; Payne, R.J.H.; Connerton, I.F. Bacteriophage-Mediated Dispersal of Campylobacter Jejuni Biofilms. Appl. Environ. Microbiol. 2011, 77, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Gungor, C.; Onmaz, N.E.; Gundog, D.A.; Yavas, G.T.; Koskeroglu, K.; Gungor, G. Four Novel Bacteriophages from Slaughterhouse: Their Potency on Control of Biofilm-Forming MDR S. Aureus in Beef Model. Food Control 2024, 156, 110146. [Google Scholar] [CrossRef]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Brabban, A.D.; Diez-Gonzalez, F. Isolation and Characterization of Lytic Bacteriophages against Enterohaemorrhagic Escherichia coli. J. Appl. Microbiol. 2011, 110, 1323–1331. [Google Scholar] [CrossRef]
- Kazi, M.; Annapure, U.S. Bacteriophage Biocontrol of Foodborne Pathogens. J. Food Sci. Technol. 1996, 53, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.; Tobar-Calfucoy, E.; Rojas-Martínez, V.; Norambuena, R.; Serrano, M.J.; Cifuentes, O.; Zamudio, M.S.; San Martín, D.; Lara, P.; Sabag, A.; et al. Development and Characterization of a Bacteriophage Cocktail with High Lytic Efficacy against Field-Isolated Salmonella enterica. Poult. Sci. 2023, 102, 103125. [Google Scholar] [CrossRef]
- Sass, P.; Bierbaum, G. Lytic Activity of Recombinant Bacteriophage Φ11 and Φ12 Endolysins on Whole Cells and Biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Yu, J.; Huang, Y.; Zhang, X.E.; Wei, H. Novel Chimeric Lysin with High-Level Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus in Vitro and in Vivo. Antimicrob. Agents Chemother. 2014, 58, 536–542. [Google Scholar] [CrossRef]
- Sun, X.; Pu, B.; Qin, J.; Xiang, J. Effect of a Depolymerase Encoded by Phage168 on a Carbapenem-Resistant Klebsiella pneumoniae and Its Biofilm. Pathogens 2023, 12, 1396. [Google Scholar] [CrossRef]
- Verma, V.; Harjai, K.; Chhibber, S. Structural Changes Induced by a Lytic Bacteriophage Make Ciprofloxacin Effective against Older Biofilm of Klebsiella pneumoniae. Biofouling 2010, 26, 729–737. [Google Scholar] [CrossRef]
- Verma, V.; Harjai, K.; Chhibber, S. Characterization of a T7-Like Lytic Bacteriophage of Klebsiella pneumoniae B5055: A Potential Therapeutic Agent. Curr. Microbiol. 2009, 59, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.A. Synergistic Interaction Between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Molecular Sciences Review Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- De Soir, S.; Parée, H.; Kamarudin, N.H.N.; Wagemans, J.; Lavigne, R.; Braem, A.; Merabishvili, M.; De Vos, D.; Pirnay, J.P.; Van Bambeke, F. Exploiting Phage-Antibiotic Synergies to Disrupt Pseudomonas aeruginosa PAO1 Biofilms in the Context of Orthopedic Infections. Microbiol. Spectr. 2023, 12, e0321923. [Google Scholar] [CrossRef] [PubMed]
- Coulter, L.B.; McLean, R.J.C.; Rohde, R.E.; Aron, G.M. Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms. Viruses 2014, 6, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Harjai, K.; Chhibber, S. Aeromonas punctata Derived Depolymerase Improves Susceptibility of Klebsiella pneumoniae Biofilm to Gentamicin Microbial Biochemistry, Physiology and Metabolism. BMC Microbiol. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Issa, R.; Chanishvili, N.; Caplin, J.; Kakabadze, E.; Bakuradze, N.; Makalatia, K.; Cooper, I. Antibiofilm Potential of Purified Environmental Bacteriophage Preparations against Early Stage Pseudomonas aeruginosa Biofilms. J. Appl. Microbiol. 2019, 126, 1657–1667. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, R.; Xu, M.; Liu, Y.; Zhu, X.; Qiu, J.; Liu, Q.; He, P.; Li, Q. A Novel Polysaccharide Depolymerase Encoded by the Phage SH-KP152226 Confers Specific Activity Against Multidrug-Resistant Klebsiella pneumoniae via Biofilm Degradation. Front. Microbiol. 2019, 10, 476285. [Google Scholar] [CrossRef]
| Biofilm Type | Efficacy | Reference | |
|---|---|---|---|
| Phage | |||
| Phage P100 | L. monocytogenes | Reduced cell counts from 3.5 to 5.4 log units/cm2 | [84] |
| Decreased biofilm cells to undetectable levels after 48 h | [152] | ||
| Phage KH1 | E. coli O157:H7 | 1.2 log units per coupon reduction after 4 days application | [153,154] |
| Phage SAP-26 | S. aureus | 28% reduction in bacterial biomass | [141] |
| Phages ISP, Romulus and Remus | S. aureus | 37.8%, 34.4%, and 60% reduction in biofilm by Phages ISP, Romulus, and Remus, respectively | [155] |
| Cocktail of phages | |||
| Phage cocktail containing 39APmC32, 65APm2833 and 72APm5211 | P. mirabilis | The phage cocktail showed antibiofilm activity against 2–3 strains more than the activity of single phages without hindering the activity of each other | [156] |
| Phage K and phage derivatives | S. aureus | Complete inhibition of biofilm formation | [91] |
| Phage K and DRA88 | S. aureus | Complete biomass inhibition after 48 h of phage application | [92] |
| Phages LiMN4L, LiMN4p and LiMN17 | L. monocytogenes | Within 75 min biofilm cells reduced to undetectable levels | [80,85] |
| Phage CP8 and CP30 | C. jejuni | 1–3 logs unit/cm2 decrease in biofilm cell counts | [157] |
| Phages of Herelleviridae family (B2-102, O1-102, T2-102, and O2-92) | S. aureus | Log reduction in viable cell counts ranged from 3.1 to 4.2 cfu/g | [158] |
| 01 BEC8 (Phage cocktail) | E. coli O157:H7 | Significant biofilm reduction after 1 h of phage treatment | [159,160] |
| Cocktail of 3 phages (L8, SAEN098P01, and SAEN098P03) | Salmonella | It was highly effective against several serovars of the bacteria | [161] |
| Phage protein | |||
| Endolysin (Phage phi11) | S. aureus | Complete of inhibition of S. aureus biomass | [162,163] |
| Endolysin SAL-2 | S. aureus | Reduced biomass after 2 h of application | [80,108] |
| Endolysin LysH5 | S. aureus | Biofilm cell counts reduced by 1–3 log units | [109] |
| Domain CHAPk derived from endolysin LysK | S. aureus | Complete biofilm inhibition was recorded | [110] |
| Chimeric lysin ClyH | S. aureus | More than 60% reduction in biomass | [164] |
| Endolysin Lys68 | S. typhimurium | 1 log unit reduction in biofilm biomass | [165] |
| Depolymerases | |||
| Exoplysaccharide depolymerase Dpo7 | S. aureus | Inhibition of biofilm polysaccharide matrix | [134] |
| Depolymerase Dpo42 | E. coli | Reduction in capsular exopolysaccharides and biofilm in a dose dependent manner | [135] |
| Depolymerase Dpo1 | A. nosocomialis, A. baumanii | Inhibition of capsular exopolysaccharides and biofilm | [136] |
| phage IME180 depolymerase | P. aeruginosa | Inhibition of pre-formed biofilm at 30 µg/ml | [137] |
| Dep6 | Shiga toxin producing E. coli (STEC) | Reduced 24 h and 48 h biofilm by 29% and 54% | [72] |
| Combined therapy with antibiotics | |||
| Depolymerase encoded by phage 168 along with polymyxin B | Carbapenem-Resistant K. pneumoniae | Disruption of biofilm was done by depolymerase and the polymyxin exerted its bactericidal effects. They showed symbiotic action and bacterial load reduced | [166] |
| Depolymerase from phage KPO1K2 and ciprofloxacin | K. pneumoniae strain B5055 | Increased biofilm inhibition and removal | [67,167,168] |
| Phage cocktail with ciproflaxin or meropenem (2.5 X MIC) | P. aeruginosa | It inhibited the regrowth of phage-resistant mutants | [169] |
| Phage SAP-26 and rifampicin/azithromycin/vancomycin | S. aureus | Disruption of biofilm biomass matrix and 4-log reduction | [141,170] |
| Phage PSPS (Pbunavirus) with ciproflaxin | P. aeruginosa PAO1 biofilms | The combination resulted in decrease in biomass reduction by 24.7%. Up to 29.7% decrease in biomass of biofilm | [171] |
| T4 phage and cefotaxime | E. coli | Synergistic action resulted in reduced MBEC of cefotaxime by 2–8 folds against E. coli | [172] |
| T4 phage and tobramycin | E. coli | Synergistic action resulted in approx. 99.99% reduction | [173] |
| Depolymerase from phage KPO1K2 and gentamicin | K. pneumoniae B5055 | Reduced biofilm biomass counts of young biofilms (up to 4 days) | [174] |
| Phage vB_PmiS_TH and ampicillin | P. mirabilis | Highest biofilm removal at 24 h | [147] |
| PT-bacteriophages and ciprofloxacin | P. aeruginosa PS573 | Reduced bacterial load by ≥50% | [175] |
| Depolymerase Dep42 from phage SH-KP15226 and polymyxin | K. pneumoniae 2226 | Decreased bacterial counts | [176] |
| PEV20 phage and ciprofloxacin | P. aeruginosa | Increased biofilm removal | [144] |
| Depolymerase KP34p57 from phage KP34 and ciprofloxacin | K. pneumoniae 77 | Diminished bacterial colony counts | [145] |
| Phage M1 and ceftazidime and avibactam | K. pneumoniae | Decreased bacterial counts in mature biofilms | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, F.M.; Zahra, A.; Ali, A.; Kamthan, M.; Al-Shabib, N.A.; Farooqui, Z.; Ahmad, N.; Albalawi, T.; Alam, P.; Munawar, N. Bacteriophages and Their Enzymes: Allies Against Microbial Biofilms. Pharmaceuticals 2025, 18, 1771. https://doi.org/10.3390/ph18121771
Husain FM, Zahra A, Ali A, Kamthan M, Al-Shabib NA, Farooqui Z, Ahmad N, Albalawi T, Alam P, Munawar N. Bacteriophages and Their Enzymes: Allies Against Microbial Biofilms. Pharmaceuticals. 2025; 18(12):1771. https://doi.org/10.3390/ph18121771
Chicago/Turabian StyleHusain, Fohad Mabood, Andaleeb Zahra, Asghar Ali, Mohan Kamthan, Nasser A. Al-Shabib, Zeba Farooqui, Naved Ahmad, Thamer Albalawi, Pravej Alam, and Nayla Munawar. 2025. "Bacteriophages and Their Enzymes: Allies Against Microbial Biofilms" Pharmaceuticals 18, no. 12: 1771. https://doi.org/10.3390/ph18121771
APA StyleHusain, F. M., Zahra, A., Ali, A., Kamthan, M., Al-Shabib, N. A., Farooqui, Z., Ahmad, N., Albalawi, T., Alam, P., & Munawar, N. (2025). Bacteriophages and Their Enzymes: Allies Against Microbial Biofilms. Pharmaceuticals, 18(12), 1771. https://doi.org/10.3390/ph18121771








