Coating Doyle Nasal Silicone Splints with a Sustained Release Varnish Containing Antibiotics Provides Long-Term Protection from Staphylococcus aureus: An In Vitro Study
Abstract
1. Introduction
2. Results
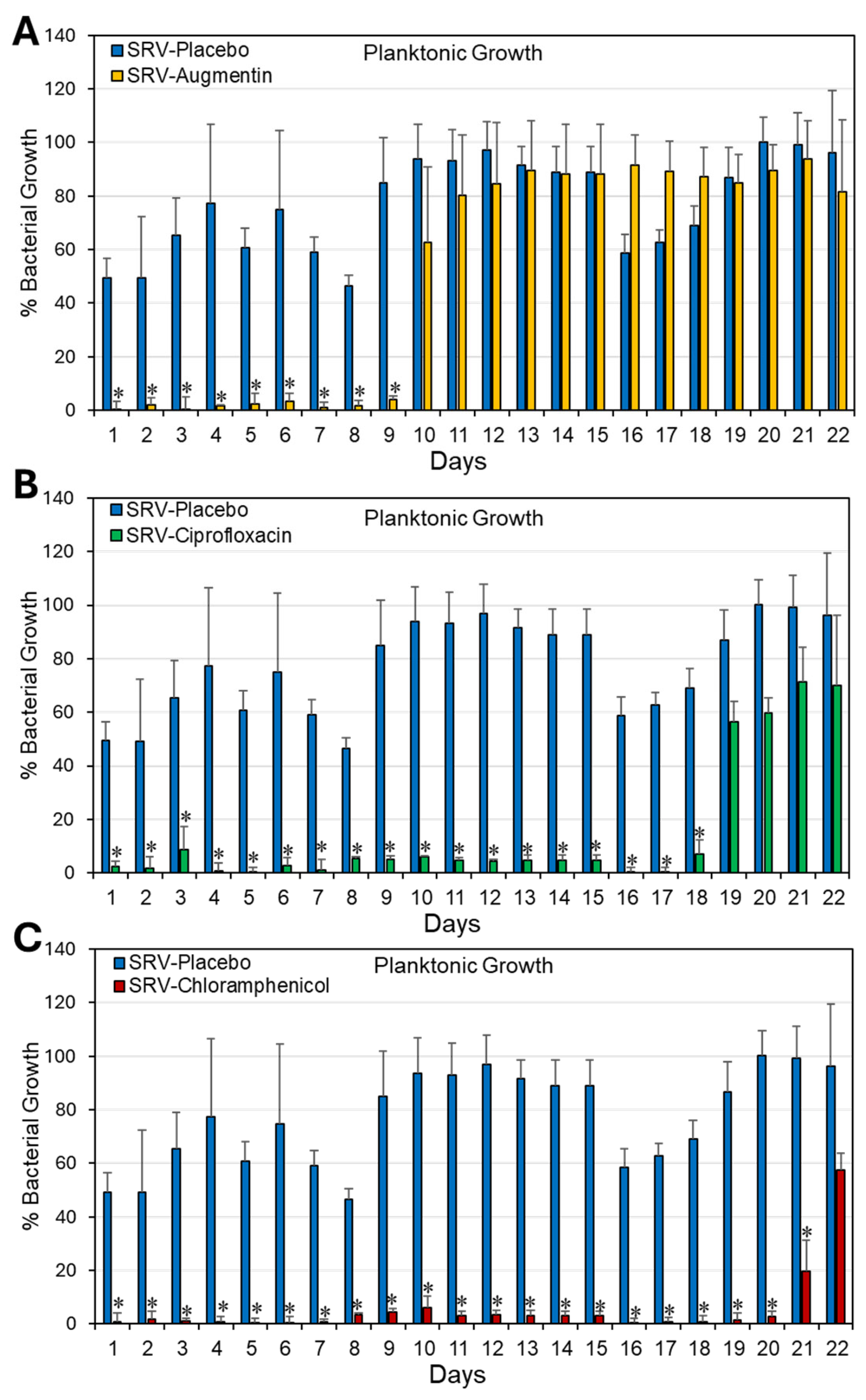
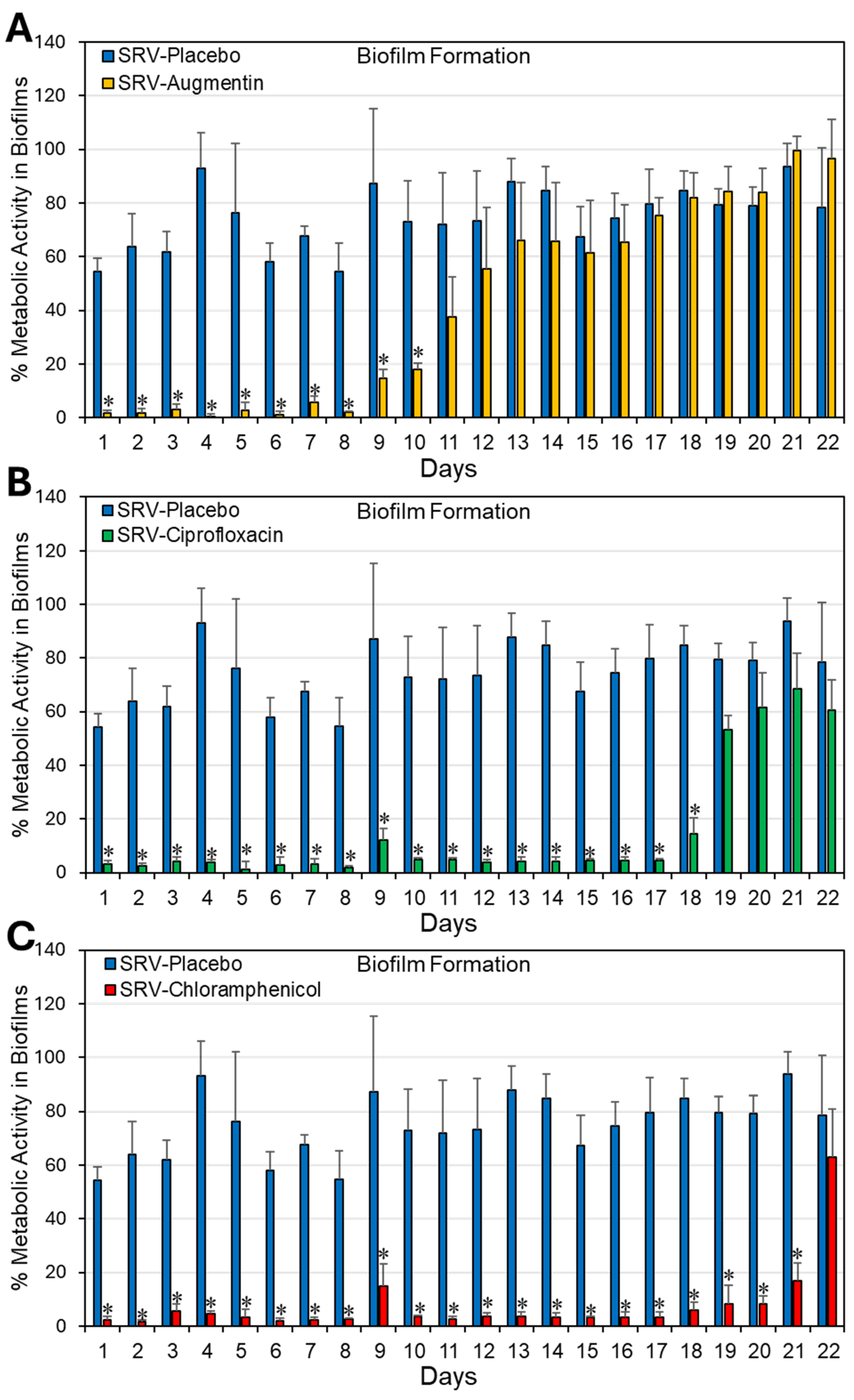
2.1. Coating Doyle Nasal Silicone Splints with SRV Containing Antibiotics Caused Long-Term Inhibition of S. aureus Planktonic Growth and Biofilm Formation
2.2. Coating Doyle Nasal Silicone Splints with SRV Containing Antibiotics Resulted in Long-Term Inhibition of S. aureus Growth in Disk Diffusion Assay
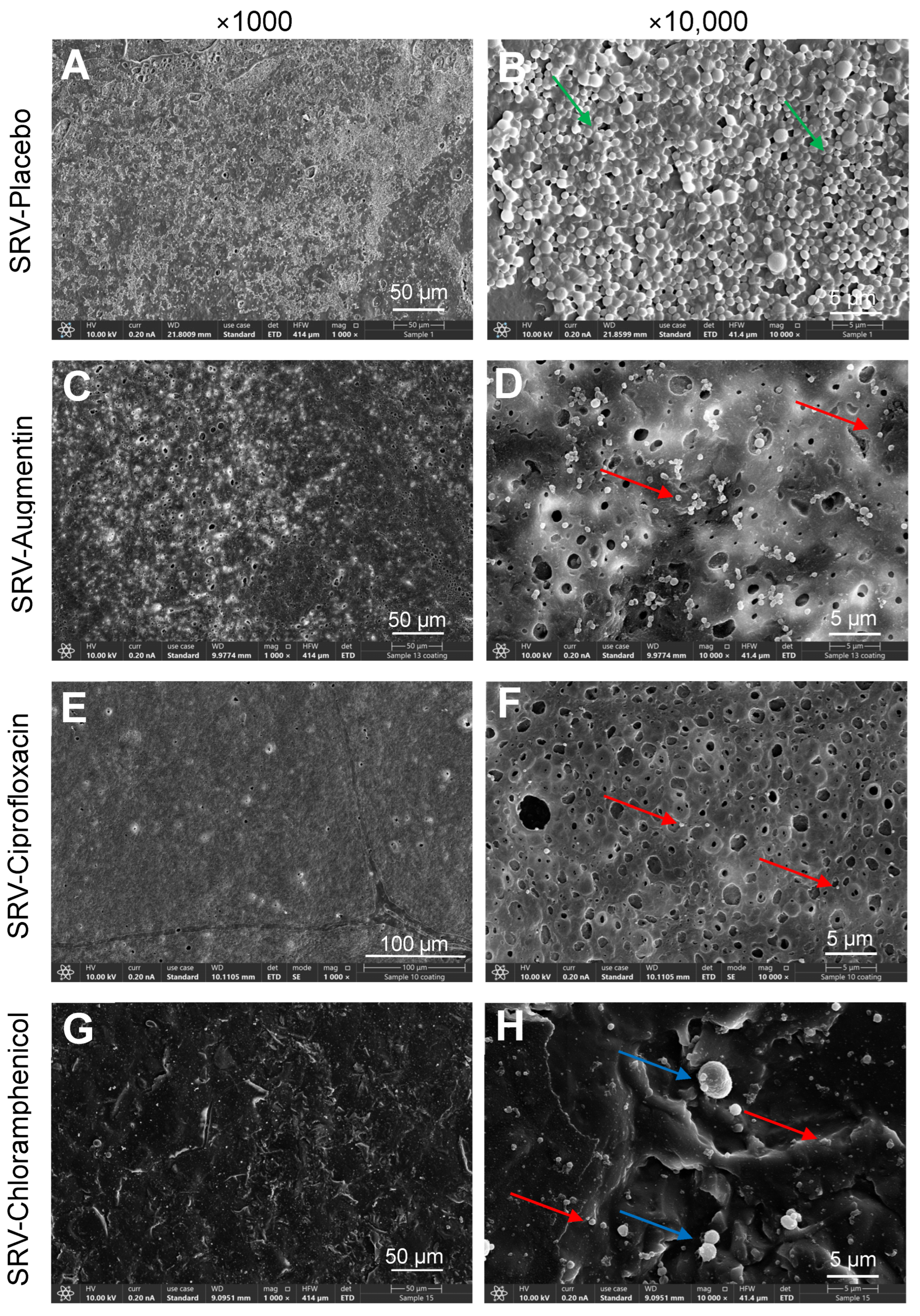
2.3. HR-SEM Images Showed Significant Reduction of Biofilm Formation on Doyle Nasal Silicone Splints Coated with SRV–Antibiotics
3. Discussion
4. Materials and Methods
4.1. Preparation of Sustained Release Varnish (SRV)
4.2. Doyle Nasal Silicone Splints
4.3. Coating of Doyle Nasal Silicone Splints with SRV
4.4. Cultivation of Staphylococcus aureus
4.5. In Vitro Model for Studying the Long-Term Antibacterial and Antibiofilm Properties of SRV-Coated Splint Segments
4.5.1. Measuring the Effect of SRV-Coated Splint Segments on Planktonic Bacterial Growth
4.5.2. Biofilm Metabolic Assay
4.6. Kinetic Disk Diffusion Assay to Determine the Long-Term Anti-Bacterial Activity of SRV-Coated Splints
4.7. High-Resolution-Scanning Electron Microscope (HR-SEM)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDW | Double-distilled water |
| HR-SEM | High-Resolution Scanning Electron Microscopy |
| OD | Optical Density |
| SRV | Sustained-release varnish |
References
- Kar, M.; Bayar Muluk, N.; Alqunaee, M.; Manole, F.; Cingi, C. Functional Endoscopic Sinus Surgery: Key Points for Safer Surgery. Ear Nose Throat J. 2024, 103, 5S–14S. [Google Scholar] [CrossRef]
- Yim, M.T.; Smith, K.A.; Alt, J.A.; Orlandi, R.R. The value of endoscopic sinus surgery in chronic rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2021, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.R.; Jafari, A.; DeConde, A.S. Revision rates and time to revision following endoscopic sinus surgery: A large database analysis. Laryngoscope 2018, 128, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.A.; Soler, Z.M.; Koochakzadeh, S.; Desiato, V.M.; Yoo, F.; Nguyen, S.A.; Schlosser, R.J. Revision surgery rates in chronic rhinosinusitis with nasal polyps: Meta-analysis of risk factors. Int. Forum Allergy Rhinol. 2020, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Alaryani, R.A.; Alhedaithy, R.A. Preventive Measures of Middle Turbinate Lateralization After Endoscopic Sinus Surgery: An Updated Review. Cureus 2021, 13, e15763. [Google Scholar] [CrossRef]
- Stępiński, M.J.; Banaszewski, J. Risk factors leading to the formation of intranasal synechiae (adhesions) in patients who underwent septo- and septoturbinoplasty (a retrospective cohort Study). Ann. Med. Surg. 2025, 87, 3082–3096. [Google Scholar] [CrossRef]
- Stępiński, M.J.; Banaszewski, J. Intranasal Synechiae as Complications of Rhinosurgical Treatment-A Review of Current Knowledge. J. Clin. Med. 2023, 12, 6831. [Google Scholar] [CrossRef]
- Welch, K.C.; Stankiewicz, J.A. A contemporary review of endoscopic sinus surgery: Techniques, tools, and outcomes. Laryngoscope 2009, 119, 2258–2268. [Google Scholar] [CrossRef]
- Shrime, M.G.; Tabaee, A.; Hsu, A.K.; Rickert, S.; Close, L.G. Synechia formation after endoscopic sinus surgery and middle turbinate medialization with and without FloSeal. Am. J. Rhinol. 2007, 21, 174–179. [Google Scholar] [CrossRef]
- Bernardo, M.T.; Alves, S.; Lima, N.B.; Helena, D.; Condé, A. Septoplasty with or without postoperative nasal packing? Prospective study. Braz. J. Otorhinolaryngol. 2013, 79, 471–474. [Google Scholar] [CrossRef]
- Alghanem, B.; Almotairi, N.; Alrasheedi, A. Septoplasty and Nasal Packs From Otolaryngologists’ Perspectives: A Cross-Sectional Survey. Cureus 2024, 16, e54691. [Google Scholar] [CrossRef]
- Tripathi, P.B.; Majd, P.; Ngo, T.; Gu, J.T.; Sharma, G.K.; Badger, C.; Bhandarkar, N.D.; Wong, B.J.F. Evaluation of Safety and Efficacy for an Intranasal Airway Device in Nasal Surgery. JAMA Facial Plast. Surg. 2019, 21, 38–43. [Google Scholar] [CrossRef]
- Dal, S.B. Doyle silicone splint insertion: Endoscopy-assisted versus nasal speculum assisted. Braz. J. Otorhinolaryngol. 2021, 87, 578–582. [Google Scholar] [CrossRef]
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bomar, L.; Brugger, S.D.; Lemon, K.P. Bacterial microbiota of the nasal passages across the span of human life. Curr. Opin. Microbiol. 2018, 41, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qin, F.; Li, S.; Yin, J.; Hu, L.; Zheng, S.; He, L.; Xia, H.; Liu, J.; Hu, W. The mechanisms of biofilm antibiotic resistance in chronic rhinosinusitis: A review. Medicine 2022, 101, e32168. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Daffinee, K.E.; Piehl, E.C.; Bleick, C.; LaPlante, K.L. Eradication of Staphylococcus epidermidis within Biofilms: Comparison of Systemic versus Supratherapeutic Concentrations of Antibiotics. Antimicrob. Agents Chemother. 2023, 67, e0010823. [Google Scholar] [CrossRef]
- Bari, A.K.; Belalekar, T.S.; Poojary, A.; Rohra, S. Combination drug strategies for biofilm eradication using synthetic and natural agents in KAPE pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1155699. [Google Scholar] [CrossRef]
- Da Silva, R.A.G.; Afonina, I.; Kline, K.A. Eradicating biofilm infections: An update on current and prospective approaches. Curr. Opin. Microbiol. 2021, 63, 117–125. [Google Scholar] [CrossRef]
- Ma, R.; Hu, X.; Zhang, X.; Wang, W.; Sun, J.; Su, Z.; Zhu, C. Strategies to prevent, curb and eliminate biofilm formation based on the characteristics of various periods in one biofilm life cycle. Front. Cell. Infect. Microbiol. 2022, 12, 1003033. [Google Scholar] [CrossRef] [PubMed]
- Karayamparambil, B.J.; Vadakkan, K.; Thomas, S. The Impact of Antibiofilm Strategies in Controlling Microbial Colonization. Curr. Microbiol. 2025, 82, 351. [Google Scholar] [CrossRef] [PubMed]
- Fontanot, A.; Ellinger, I.; Unger, W.W.J.; Hays, J.P. A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms-January 2020 to September 2023. Antibiotics 2024, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. Bacteriophage-mediated approaches for biofilm control. Front. Cell. Infect. Microbiol. 2024, 14, 1428637. [Google Scholar] [CrossRef]
- Head, K.; Chong, L.Y.; Piromchai, P.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 4, CD011994. [Google Scholar] [CrossRef]
- Whitehead, R.A.; Talati, V.; Baird, A.M.; Owen, G.S.; Hansen, R.; Filip, P.; Tajudeen, B.A.; Batra, P.S.; Papagiannopoulos, P. The Efficacy of Mupirocin Nasal Irrigations for Treatment of Refractory Chronic Rhinosinusitis After Endoscopic Sinus Surgery. Am. J. Rhinol. Allergy 2025, 39, 364–370. [Google Scholar] [CrossRef]
- Ha, K.R.; Psaltis, A.J.; Butcher, A.R.; Wormald, P.J.; Tan, L.W. In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope 2008, 118, 535–540. [Google Scholar] [CrossRef]
- Gregory, J.; Huynh, B.; Tayler, B.; Korgaonkar-Cherala, C.; Garrison, G.; Ata, A.; Sorum, P. High-Dose vs Standard-Dose Amoxicillin Plus Clavulanate for Adults with Acute Sinusitis: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e212713. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.R.; Miller, J.E.; Suh, J.D. Safety of Antibiotic Irrigations for Acute Exacerbations of Chronic Rhinosinusitis in Patients with Identical Drug Allergies or Adverse Reactions: A Pilot Study. Ear Nose Throat J. 2023, 1455613231189057. [Google Scholar] [CrossRef] [PubMed]
- Olwoch, I.P. Microbiology of acute complicated bacterial sinusitis at the University of the Witwatersrand. S. Afr. Med. J. 2010, 100, 529–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feldman, M.; Moustafa Elsayed, W.S.; Friedman, M.; Gati, I.; Steinberg, D.; Marei, H. Prolonged Inhibition of Streptococcus mutans Growth and Biofilm Formation by Sustained Release of Chlorhexidine from Varnish Coated Dental Abutments: An In Vitro Study. Int. J. Dent. 2022, 2022, 7246155. [Google Scholar] [CrossRef]
- Cataldo Russomando, A.; Vogt Sionov, R.; Friedman, M.; Gati, I.; Eliashar, R.; Steinberg, D.; Gross, M. Sinonasal Stent Coated with Slow-Release Varnish of Chlorhexidine Has Sustained Protection against Bacterial Biofilm Growth in the Sinonasal Cavity: An In Vitro Study. Pharmaceutics 2021, 13, 1783. [Google Scholar] [CrossRef]
- Feldman, M.; Gati, I.; Sionov, R.V.; Sahar-Helft, S.; Friedman, M.; Steinberg, D. Potential Combinatory Effect of Cannabidiol and Triclosan Incorporated into Sustained Release Delivery System against Oral Candidiasis. Pharmaceutics 2022, 14, 1624. [Google Scholar] [CrossRef]
- Risheq, S.; Sancho, A.; Friedman, M.; Gati, I.; Eliashar, R.; Steinberg, D.; Gross, M. Ciprofloxacin-Coated Tympanostomy Tubes with Sustained-Release Varnish: A Novel Strategy to Combat Biofilm Formation by Pseudomonas aeruginosa. Microorganisms 2025, 13, 2039. [Google Scholar] [CrossRef]
- Sionov, R.V.; Gati, I.; Kirmayer, D.; Friedman, M.; Steinberg, D.; Gross, M. Voice Prosthesis Coated with Sustained Release Varnish Containing Clotrimazole Shows Long-Term Protection Against Candida albicans: An In Vitro Study. Molecules 2021, 26, 5395. [Google Scholar] [CrossRef]
- Segev, G.; Bankirer, T.; Steinberg, D.; Duvdevani, M.; Shapur, N.K.; Friedman, M.; Lavy, E. Evaluation of urinary catheters coated with sustained-release varnish of chlorhexidine in mitigating biofilm formation on urinary catheters in dogs. J. Vet. Intern. Med. 2013, 27, 39–46. [Google Scholar] [CrossRef]
- Czerninski, R.; Pikovsky, A.; Gati, I.; Friedman, M.; Steinberg, D. Comparison of the efficacy of a novel sustained release clotrimazole varnish and clotrimazole troches for the treatment of oral candidiasis. Clin. Oral Investig. 2015, 19, 467–473. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-release drug delivery of antimicrobials in controlling of supragingival oral biofilms. Expert Opin. Drug Deliv. 2017, 14, 571–581. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Sustained-release delivery of antimicrobial drugs for the treatment of periodontal diseases: Fantasy or already reality? Periodontol. 2000 2020, 84, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Elhassan, H.A.; Singh, N. History of intranasal splints. J. Laryngol. Otol. 2018, 132, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.S.; Guven, M.; Buyukarslan, D.G.; Kaymaz, R.; Erkorkmaz, U. Do silicone nasal septal splints with integral airway reduce postoperative eustachian tube dysfunction? Otolaryngol. Head Neck Surg. 2012, 146, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, Z.; Kurc, M.A.; Kaya, A.D.; Saracoglu, G.V.; Deniz, M.; Gultekin, E. Do we really need to coat the novel silicone intranasal splints with antibiotics? Am. J. Otolaryngol. 2016, 37, 447–451. [Google Scholar] [CrossRef]
- Abi Zeid Daou, C.; Zaytoun, G.; Hadi, U.; Fakhri, S.; Korban, Z. The use of different intranasal splints Doyle splints versus Reuter bivalve splints versus no splints in primary septal surgeries: A randomised clinical trial looking at morbidity. Clin. Otolaryngol. 2024, 49, 87–93. [Google Scholar] [CrossRef]
- Law, R.H.; Ko, A.B.; Jones, L.R.; Peterson, E.L.; Craig, J.R.; Deeb, R.H. Postoperative pain with or without nasal splints after septoplasty and inferior turbinate reduction. Am. J. Otolaryngol. 2020, 41, 102667. [Google Scholar] [CrossRef]
- Jung, Y.G.; Hong, J.W.; Eun, Y.G.; Kim, M.G. Objective usefulness of thin silastic septal splints after septal surgery. Am. J. Rhinol. Allergy 2011, 25, 182–185. [Google Scholar] [CrossRef]
- Koçak, İ.; Şentürk, E. A new modification of Doyle splint (Hemi-split Doyle) in rhinoplasty with alar base reduction. Eur. Arch. Otorhinolaryngol. 2017, 274, 3667–3672. [Google Scholar] [CrossRef]
- Kasapgil, E.; Badv, M.; Cantú, C.A.; Rahmani, S.; Erbil, H.Y.; Anac Sakir, I.; Weitz, J.I.; Hosseini-Doust, Z.; Didar, T.F. Polysiloxane Nanofilaments Infused with Silicone Oil Prevent Bacterial Adhesion and Suppress Thrombosis on Intranasal Splints. ACS Biomater. Sci. Eng. 2021, 7, 541–552. [Google Scholar] [CrossRef]
- Yildirim, G.; Kumral, T.L.; Altindağ, C.; Özdemir, E.; Uyar, Y. The Effect of Dexpanthenol-Vitamin A (Nazalnem) on Silastic Splints After Nasal Septal Surgery. J. Craniofac Surg. 2017, 28, 2139–2142. [Google Scholar] [CrossRef]
- Acar, M.; Catli, T.; Dag, I.; San, T.; Cingi, C. Microbial biofilm formation on silicone nasal splints: Optimal time for splint removal. Rhinology 2014, 52, 371–375. [Google Scholar] [CrossRef][Green Version]
- Ozdogan, F.; Ozel, H.E.; Esen, E.; Yuce, T.; Eyisarac, S.; Genc, S.; Selcuk, A. Optimal time for intranasal splint removal after septoplasty: A prospective clinical study. Eur. Arch. Otorhinolaryngol. 2016, 273, 3203–3206. [Google Scholar] [CrossRef]
- Sionov, R.V.; Siag, A.; Mersini, E.T.; Kogan, N.M.; Alkhazov, T.; Koman, I.; Rowlo, P.; Gutkin, V.; Gross, M.; Steinberg, D. The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus. Pharmaceutics 2025, 17, 463. [Google Scholar] [CrossRef]

| Component | Placebo | Augmentin | Ciprofloxacin | Chloramphenicol |
|---|---|---|---|---|
| Ethylcellulose | 1000 mg | 1000 mg | 1000 mg | 1000 mg |
| Hydroxypropylcellulose | 200 mg | 200 mg | 200 mg | 200 mg |
| PEG-400 | 120 mg | 120 mg | 120 mg | 120 mg |
| Active ingredient | None | 600 mg Augmentin | 600 mg Ciprofloxacin | 600 mg Chloramphenicol |
| Ethanol | 12 mL | 12 mL | 12 mL | 12 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siag, A.; Sionov, R.V.; Gati, I.; Friedman, M.; Steinberg, D.; Gross, M. Coating Doyle Nasal Silicone Splints with a Sustained Release Varnish Containing Antibiotics Provides Long-Term Protection from Staphylococcus aureus: An In Vitro Study. Pharmaceuticals 2025, 18, 1746. https://doi.org/10.3390/ph18111746
Siag A, Sionov RV, Gati I, Friedman M, Steinberg D, Gross M. Coating Doyle Nasal Silicone Splints with a Sustained Release Varnish Containing Antibiotics Provides Long-Term Protection from Staphylococcus aureus: An In Vitro Study. Pharmaceuticals. 2025; 18(11):1746. https://doi.org/10.3390/ph18111746
Chicago/Turabian StyleSiag, Ahmad, Ronit Vogt Sionov, Irith Gati, Michael Friedman, Doron Steinberg, and Menachem Gross. 2025. "Coating Doyle Nasal Silicone Splints with a Sustained Release Varnish Containing Antibiotics Provides Long-Term Protection from Staphylococcus aureus: An In Vitro Study" Pharmaceuticals 18, no. 11: 1746. https://doi.org/10.3390/ph18111746
APA StyleSiag, A., Sionov, R. V., Gati, I., Friedman, M., Steinberg, D., & Gross, M. (2025). Coating Doyle Nasal Silicone Splints with a Sustained Release Varnish Containing Antibiotics Provides Long-Term Protection from Staphylococcus aureus: An In Vitro Study. Pharmaceuticals, 18(11), 1746. https://doi.org/10.3390/ph18111746







