Abstract
Background/Objectives: In the current medical era, Topoisomerase II is recognized as an essential enzyme that regulates DNA topology during critical biological processes such as DNA replication, transcription, and repair. This study aimed to design, synthesize, and biologically evaluate a new series of pyrazolo[3,4-b]pyridines (8a–g, 10a–g, and 12) as potential anticancer agents and Topoisomerase II inhibitors. Methods: The synthesized compounds were subjected to in vitro anticancer screening at the National Cancer Institute (NCI, USA). Active derivatives were further evaluated through a five-dose screening to determine their antiproliferative potency. Selected compounds were examined for their effects on leukemia cell lines (K562 and MV4-11), and mechanistic studies were performed to assess DNA damage, cell cycle distribution, and apoptosis-related protein modulation. Additionally, enzyme inhibition assays were conducted to determine Topoisomerase IIα (TOPIIα) inhibition. Results: Initial single-dose screening identified several active compounds, notably 8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f. Among these, compound 8c exhibited potent and broad-spectrum antiproliferative activity across the NCI cancer cell line panel, with a GI50 MG-MID value of 1.33 µM (range: 0.54–2.08 µM). The synthesized molecules showed moderate to good anti-leukemic efficacy against K562 and MV4-11 cells. Mechanistic investigations revealed that compound 8c induced DNA damage and S-phase cell cycle arrest, leading to apoptosis as evidenced by the modulation of PARP-1, Bax, XIAP, and Caspases. Furthermore, target-based assays confirmed that compound 8c significantly inhibited the DNA relaxation activity of TOPIIα in a dose-dependent manner, comparable to etoposide. Conclusions: The study highlights compound 8c as a promising pyrazolo[3,4-b]pyridine derivative with potent antiproliferative activity and effective inhibition of Topoisomerase IIα. These findings suggest its potential as a lead scaffold for further optimization in anticancer drug development..
1. Introduction
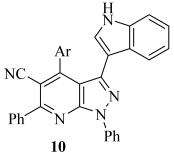
Cancer is still one of the leading causes of death worldwide and remains a serious public health concern [1,2]. By 2050, the global burden of cancer is anticipated to reach 19 million cancer diagnoses worldwide, with an estimated 10.5 million cancer-related deaths [3]. Among the diverse types of cancer, leukemia stands out as one of the most aggressive and potentially fatal hematological malignancies [4]. Acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), acute lymphocytic leukemia (ALL), and chronic lymphocytic leukemia (CLL) are the four primary subtypes of leukemia that are clinically distinguished based on cell lineage and the rate of disease progression [5]. Each subtype exhibits distinct pathological, genetic, and clinical features, which require individualized treatment approaches. Although leukemia often responds well to chemotherapy, the range of safe and durable treatment options remains limited [6]. As a result, the development of novel, more effective therapies for leukemia remains a pressing and continuous task. Pyrazolo [3,4-b]pyridine is a privileged fused heterocyclic scaffold commonly found in pharmaceuticals and drug-like substances [7]. Research has shown that derivatives of pyrazolo [3,4-b]pyridine (Figure 1) exhibit anticancer properties through various mechanisms, including inhibition of several targets such as cyclin-dependent kinases (I–IV) [8,9,10], tubulin polymerization (V) [11], glycogen synthase kinase-3 (VI) [12], hematopoietic cell kinase (VII) [13], fibroblast growth factor receptor (VIII) [14], tropomyosin receptor kinases (IX) [15] and TOPII (X–XI) [16,17].
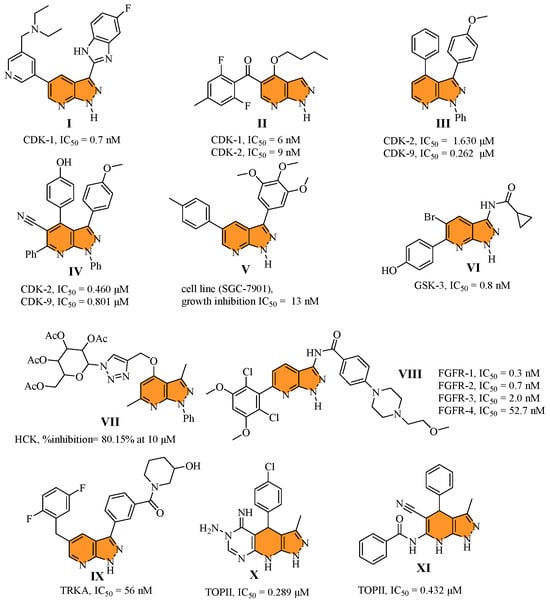
Figure 1.
Structure of some reported pyrazolo [3,4-b]pyridine (I–XI) derivatives alongside their biological activity.
Indole is a simple chemical scaffold with a bicyclic planar structure found in many natural products [18]. The biological actions of indole derivatives include antibacterial [19], antiinflammatory [20], anticancer [21,22], anticonvulsant [23], antiviral [24], antidiabetic [25], and antioxidant [26] properties. Regarding cancer proliferation inhibition, indole derivatives are highly effective because they strongly target multiple pathways (Figure 2), including PIM (XII) [27], CDK (XIII) [28], EGFR (XIV) [29], PI3Kα (XV) [30], Bcl-2/Mcl-1 (XVI) [31], TOPII (XV–XVI) [32], etc. [33].
Figure 2.
Structure of some reported indole (XII–XVI) derivatives alongside their biological activity.
The endonuclease class enzymes known as DNA topoisomerases (TOPs) are widely distributed throughout all life spheres and play essential roles in fundamental cellular processes [34,35]. TOP enzymes carry out a variety of tasks linked to the maintenance of DNA metabolism and regulate DNA topology during transcription and replication [36,37]. Based on their structure, amino acid sequence, catalytic activity, and reaction mechanism, TOPs can be divided into type I and type II [38,39]. Numerous investigations have demonstrated that TOPs are overexpressed in tumor cells, and their inhibition suppressed tumor cell growth, establishing TOPs as valuable targets for the development of anticancer therapeutics [40,41]. Furthermore, leukemia’s higher topoisomerase IIα expression levels have been associated with increased sensitivity to agents targeting this enzyme [42]. TOPII inhibitors, such as doxorubicin and etoposide, have been extensively employed in cancer therapy. Although these compounds demonstrate clinical efficacy, their application is often limited by toxicity and adverse side effects [43,44]. Doxorubicin, for instance, is known to induce dose-dependent cardiotoxicity primarily through the generation of reactive oxygen species (ROS) and mitochondrial dysfunction, leading to cardiomyocyte apoptosis [45,46]. Etoposide, on the other hand, is associated with myelosuppression due to its interference with DNA synthesis and repair in rapidly dividing hematopoietic cells [47]. Moreover, resistance to TOPII inhibitors in leukemia patients remains a significant clinical challenge. Mechanisms contributing to drug resistance include the deletion or downregulation of the TOPIIα gene, upregulation of DNA repair enzymes such as PARP and RAD51, and enhanced drug efflux via ATP-binding cassette (ABC) transporters [48]. These adaptations reduce drug efficacy and complicate treatment outcomes, thus, the development of novel and more efficient TOPII inhibitors is the subject of extensive research [41,49].
TOPII inhibitors are known for their inflexible, coplanar aromatic structures, which serve as templates for additional structural optimization to facilitate the intercalation of stable ternary complexes with DNA. The molecular scope of inhibitor design tactics has been expanded beyond “poison inhibition” to encompass “catalytic inhibition”, “dual-targeting”, and “structure-induced blockade” [50]. As a result, new TOPII inhibitors with non-classical structures are appearing in preclinical research. A tendency towards structural and mechanistic diversity is reflected in these structures, which extend beyond conventional scaffolds and incorporate a variety of chemotypes [50]. Furthermore, the creation of dual-target compounds that integrate TOPII inhibitors with other target inhibitors, such as PARP [51], HDAC [52], and tubulin [53], results in long-lasting lethal stress and reduces resistance.
Previous studies have highlighted planar structures as a key pharmacophoric feature of TOPII inhibitors such as the scaffolds found in compounds X [16] and XI [17], both of which contain pyrazolo [3,4-b]pyridine in their structure. Additionally, the indole ring, due to its planar structure, can act as an intercalation site for DNA interaction, as demonstrated by compounds XV and XVI, which showed promising TOPII inhibition activities. All things considered, the compounds in this study were based on a hybridization strategy involving pyrazolo [3,4-b]pyridine as the main scaffold bearing the indole moiety at three varying positions to achieve promising activity against TOPII. As a part of the structure, phenyl rings were also grafted to afford binding interaction with the lipophilic DNA groove binding regions (Figure 3). The obtained compounds were tested in a preliminary anticancer screening at the National Cancer Institute (NCI), and the most promising were subjected to biological mechanistic studies and molecular docking to explore their potential anticancer mechanisms.
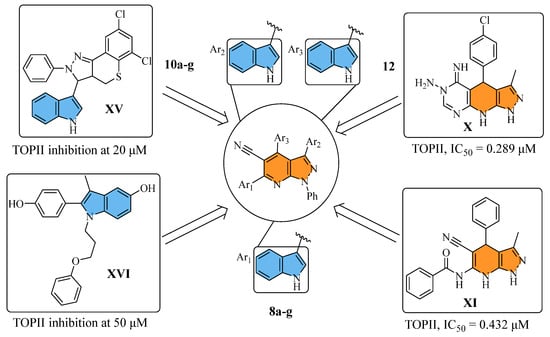
Figure 3.
The adopted hybridization strategy for the design of small molecules 8a–g, 10a–g, and 12.
2. Results and Discussion
2.1. Chemistry
Scheme 1 and Scheme 2 show the synthetic routes taken to produce the target pyrazolo [3,4-b]pyridine derivatives (8a–g, 10a–g, and 12). To obtain 3-(1H-indol-3-yl)-3-oxopropanenitrile (2), indole (1) was heated with cyanoacetic acid, and acetic anhydride was involved [54].
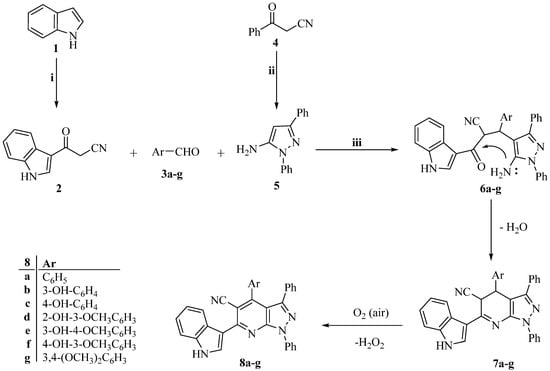
Scheme 1.
The synthetic route of target pyrazolo [3,4-b]pyridine compounds (8a–g). Reagents and conditions: i. Cyanoacetic acid, acetic anhydride, 85 °C 30 min; ii. Abs. EtOH, C6H5NHNH2, reflux 1 h; iii. Abs. EtOH, reflux 12 h.
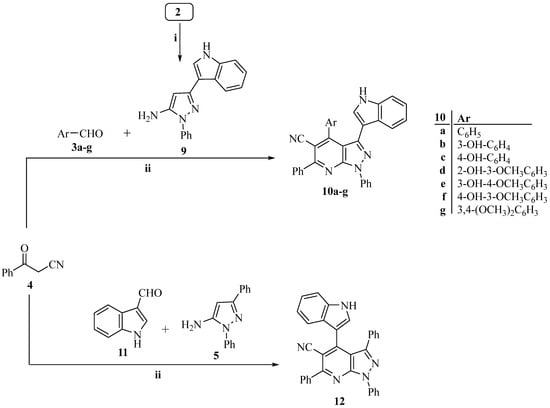
Scheme 2.
The synthetic route of target pyrazolo [3,4-b]pyridine compounds (10a–g and 12). Reagents and conditions: i. Abs. EtOH, C6H5NHNH2, reflux 1 h; ii. Abs. EtOH, reflux 12 h.
Additionally, 3-oxo-3-phenylpropanenitrile (4) and 3-(1H-indol-3-yl)-3-oxopropanenitrile (2) were condensed with phenylhydrazine in refluxing absolute ethanol to create the 3-substituted-1-phenyl-1H-pyrazol-5-amines (5 and 9) [55,56,57,58,59,60]. As shown in Scheme 1 and Scheme 2, the target pyrazolo [3,4-b]pyridine derivatives (8a–g, 10a–g, and 12) were then prepared using a one-pot, three-component reaction] that included the relevant 3-substituted-1-phenyl-1H-pyrazol-5-amine (5 or 9), an equimolar amount of the corresponding 3-oxo-3-arylpropanenitrile (2 or 4), and the appropriate aldehyde (3a-g and 11).
Intermediates 6a–g are produced as part of the reaction mechanism, and they are cyclized and then water-eliminated to produce intermediates 7a–g. The required compounds 8a–g are subsequently obtained through a final oxidative dehydrogenation process, in which atmospheric oxygen acts as the oxidant. Nuclear magnetic resonance (NMR) spectroscopy (1H and 13C) (Figures S1–S37) and high-resolution mass spectrometry (HRMS) (Figures S38–S51) validated the structures of the synthesized compounds, which were in agreement with the proposed structures.
2.2. Structure Elucidation of the Target Compounds
The 1H NMR spectra of target compounds 8a–g, 10a–g, and 12 confirmed their structures. In particular, the absence of the aldehydic proton (CH=O) signals from aldehydes (3a–g and 11) and the active methylene protons (CH2) from nitriles (2 and 4) around δ 3.5 ppm, as well as the disappearance of characteristic signals around δ 5.0 ppm, which correspond to the two NH2 protons of the precursor 3-substituted-1-phenyl-1H-pyrazol-5-amines (5 and 9). The addition of aromatic moieties throughout the process was further supported by the spectra, which showed an increase in aromatic proton signals.
The suggested structures were further supported by the 13C NMR spectra, which revealed the elimination of carbon signals associated with nitrile and aldehyde carbonyl groups, typically detected at δ 190 ppm. These modifications verified that the initial materials had completely changed into the final pyrazolo [3,4-b]pyridine derivatives.
With variances falling within the permissible range of ±0.4%, the elemental analysis findings showed good agreement with the estimated values for the target compounds’ molecular formulae. The structures were further validated by molecular ion peaks obtained from high-resolution mass spectrometry (HRMS) that matched the predicted molecular weights. The effectiveness and selectivity of the synthetic processes were further demonstrated by high-performance liquid chromatography (HPLC) analysis, which verified that all synthesized compounds had a purity higher than 95.00% (Figures S52–S66).
2.3. Biological Evaluation
The NCI 60 human cancer cell line panel was used for preliminary in vitro anticancer screening at a single dose of 10 μM.
The United States’ National Cancer Institute (NCI) conducted the first anticancer assessment of the recently synthesized indole-conjugated pyrazolo [3,4-b]pyridine derivatives (8a–g, 10a–g, and 12). The comprehensive NCI 60 human tumor cell line panel, which comprises leukemia, non-small cell lung, melanoma, central nervous system (CNS), ovarian, renal, prostate, and breast cancer cell lines, was first screened in vitro at a fixed concentration of 10 µM. The findings, presented as the mean percentage growth (G%) of treated cells compared to untreated controls, shed light on the cytotoxic effects (G% less than 0) as well as the cytostatic activity (G% between 0 and 100).
The single-dose activity profiles of compounds 8a–g, 10a–g, and 12 were examined using the COMPARE algorithm on 60 distinct cancer cell lines. The pyrazolo [3,4-b]pyridines assessed exhibited a broad range of cytotoxic effects on various cancer types, with anticancer activities ranging from weak to very potent [61]. Table 1 summarizes the growth inhibition percentages (GI%) for each target molecule, which are computed as (100–G%).

Table 1.
Percentage cell growth inhibition (GI%) of an in vitro panel of 60 human tumor cell lines at 10 µM concentration of compounds 8a–g, 10a–g, and 12.
According to the NCI criteria, derivatives 8d, 8g, and 10d (mean GI% values below 20) did not exhibit significant cytotoxicity against the examined cell lines (except for 8d towards NCI-H226 and 10d towards CCRF-CEM, HL-60(TB), and MOLT-4) [62]. Compounds 8a (mean GI% = 24), 10a (mean GI% = 43), 10g (mean GI% = 29), and 12 (mean GI% = 23) exposed promising sensitivity towards limited representative cell lines (leukemia; K-562 and renal cancer; RXF 393) (Table 1).
The remaining eight compounds; 3-hydroxyphenyl derivatives (8b and its counterpart 10b), 4-hydroxyphenyl derivatives (8c and its counterpart 10c), 4-hydroxy-3-methoxyphenyl derivatives (8e and its counterpart 10e) and 3-hydroxy-4-methoxyphenyl derivatives (8f and its counterpart 10f) showed auspicious results in NCI preliminary screening with mean GI% = 120, 154, 138, 125, 126, 118, 138 and 122 (Table 1).
The antiproliferative activity of compounds 8a (mean GI% = 24) and 10a (mean GI% = 43) was considerably increased by adding a hydroxyl group to the meta position of one of the phenyl rings. This resulted in 8b and 10b derivatives, with mean growth inhibition (GI%) values of 120% and 126%, respectively. Among the 60 cell lines examined, both compounds showed the most potent anticancer effects. According to Table 1, each demonstrated fatal activity against 42 cell lines, 33 of which were shared by both, and inhibitory effects on the other lines. The GI% values for these lines ranged from 46 to 99.
When the hydroxyl group in compound 8b was shifted from the meta to the para position, obtaining compound 8c, the antiproliferative activity increased. Both derivatives exerted a lethal effect on 47 cell lines. The same structure modification of 10b, yielding compound 10c, led to a modest decrease in activity, and 10c exerted a lethal effect only on 37 cell lines. The mean GI% values for 8c and 10c were 154% and 118%, respectively (Table 1).
Introducing a methoxy group at the para position adjacent to the hydroxyl group in compounds 8b and 10b resulted in enhanced antiproliferative activity, as observed in the obtained derivatives 8e and 10e, both of which showed a mean GI% of 138. These compounds exerted lethal effects on 39 common cancer cell lines. Lastly, compounds 8e and 10e showed enhanced anticancer activity with mean GI% values of 125 and 122, respectively, when the hydroxyl and methoxy groups were repositioned, with the hydroxyl in the para position and the methoxy in the meta position, as in compounds 8f and 10f with 34 and 35 cancer cell lines were fatally affected by these compounds, respectively.
Based on their in vitro cytotoxic characteristics against the NCI-60 human cancer cell line panel, the structure–activity relationship (SAR) of the synthesized compounds is depicted in Figure 4. The mean growth inhibition percentages (GI%) presented in Table 1 serve as the basis for this analysis. Important pharmacophoric characteristics that contribute to potency and selectivity across several cancer cell types were identified by establishing a correlation between the observed anticancer activity and the structural alterations within the pyrazolo [3,4-b]pyridine scaffold. This SAR analysis lays the groundwork for future structural optimization in the development of more potent anticancer drugs, providing insightful information on how specific substituents affect biological activity.
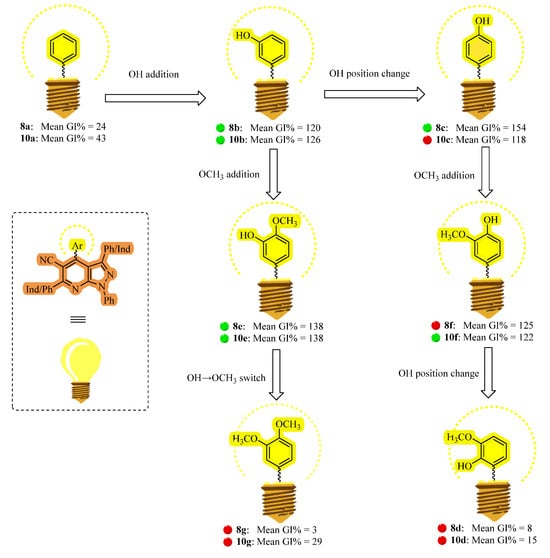
Figure 4.
The target compounds’ structure–activity relationship (SAR) as in vitro anticancer agents based on their mean GI% values across 60 human cancer cell lines.
In the next step, the most potent derivatives, including 3-hydroxyphenyl derivatives (8b and its counterpart 10b), 4-hydroxyphenyl derivatives (8c and its counterpart 10c), 4-hydroxy-3-methoxyphenyl derivatives (8e and its counterpart 10e) and 3-hydroxy-4-methoxyphenyl derivatives (8f and its counterpart 10f), were screened against the 60 cancer cell lines at 10-fold dilutions and five different concentrations (0.01, 0.1, 1, 10 and 100 μM) [61]. Following established experimental procedures, the sulforhodamine B (SRB) colorimetric assay was used to evaluate cell viability [63]. Cell density and vitality after chemical treatment are indirectly assessed by measuring cellular protein content in this experiment. The molar concentrations of the substances needed to inhibit 50% of cell proliferation (GI50) and induce 50% cell death (LC50) are represented by the GI50 and LC50 values for each cancer cell line that is tested. Table 2 summarizes these important pharmacological parameters, providing information on the cytotoxic and antiproliferative efficacy of the investigated agents [64,65]. The NCI 60 cancer cell line panel demonstrated strong antiproliferative activity across all chosen compounds (8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f), with efficacy ranging from sub-micromolar to low micromolar concentrations, according to the results. These results demonstrate compounds’ strong growth inhibition against a wide range of cancer cell types, underscoring their considerable potential as potent anticancer agents.

Table 2.
Five dose in vitro anticancer activity results expressed as GI50 (μM) for compounds 8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f against all sixty cancer cell lines.
The results showed that most compounds had significant inhibitory effects, with GI50 values ranging from 0.15 µM to 5 µM. Only a few cell lines showed reduced sensitivity, with GI50 values not exceeding 15 µM. Among the tested compounds, 8c (a 4-hydroxyphenyl derivative) and 8e (a 4-hydroxy-3-methoxyphenyl derivative) demonstrated remarkable potency, particularly against leukemia, followed by CNS, renal, and breast cancer cell lines.
Compound 8c displayed outstanding activity with GI50 values ranging from 0.15 to 4.43 μM among the tested NCI panel, including 15 cell lines with GI50 below 1.00 μM. Interestingly, it showed sub-micromolar inhibitory activity towards all leukemia, all CNS (except SF-268 and SNB-19), renal (786-0, RXF 393, and UO-31), and breast cancer cell lines (MCF7 and HS 578T). Regarding compound 8e, it displayed remarkable activity with GI50 values ranging from 0.23 to 4.68 μM (with 10 cancer cell lines with GI50 below 1.00 μM). Compound 8e showed sub-micromolar inhibitory activity towards all leukemia (except K-562 and MOLT-4), all CNS (except SF-268 and SNB-19), and renal cancer cell lines (786-0 and RXF 393) (Table 2).
Additionally, an average activity metric for all cell lines is provided by the mean graph midpoint (MG-MID). The compounds 8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f showed MID (average sensitivity of all cell lines in µM) values of 2.43, 1.33, 1.93, 3.04, 3.37, 4.23, 2.65, and 2.49 µM, respectively (Table 3).

Table 3.
Selectivity ratio of the compounds 8b, 8c, 8e, 8f, 10b, 10c, 10e and 10f towards NCI nine tumor subpanels.
The ratio of the entire panel mean graph midpoint (MID) to the individual subpanel MID was used to calculate the selectivity of these substances. The subpanel MID shows the average sensitivity of cell lines within a particular tumor subpanel, whereas the whole panel MID shows the average sensitivity of all cell lines to the test agent. Compounds with selectivity ratios below this cutoff are regarded as nonselective, whilst those with ratios above 2 may suggest a preference for specific tumor types. With selectivity ratios ranging from 0.54 to 2.46, the drugs in this investigation showed broad-spectrum anticancer efficacy across the nine tumor subpanels examined. With an average MID of 1.33 μM, compound 8c demonstrated the highest potency overall and the most effective and selective treatment of leukemia (MID = 0.54 μM, selectivity = 2.46). Likewise, compound 8e had significant potency and selectivity against leukemia, attaining a MID of 0.97 μM and a selectivity ratio of 1.98, with an average MID of 1.93 μM.
2.4. Antiproliferative Activity Against Leukemia Cells
Following the NCI 60 screening, the synthesized derivatives (8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f) were further evaluated for their antiproliferative activity against leukemia cancer cell lines, including chronic myeloid leukemia (K562) and acute monocytic leukemia (MV4-11), as shown in Table 4.

Table 4.
Antiproliferative activity of pyrazolo [3,4-b]pyridines 8b, 8c, 8e, 8f, 10b, 10c, 10e and 10f against leukemia MV4-11 and K562 cell lines.
With the exception of compound 8e, the chosen pyrazolo [3,4-b]pyridine derivatives efficiently suppressed the proliferation of MV4-11 cells, with GI50 values ranging from 0.72 to 9.03 µM. With a GI50 value of 0.72 µM, pyrazolo [3,4-b]pyridine 8c exhibited the strongest sub-micromolar cytotoxic effect. Furthermore, with GI50 values of 3.55 and 3.70 µM, respectively, compounds 8b and 8f demonstrated low single-digit micromolar cytotoxicity against the MV4-11 cell line. The remaining compounds (10b, 10c, 10e, and 10f), on the other hand, demonstrated substantial single-digit micromolar cytotoxic activity, as indicated by Table 4, with GI50 values ranging from 8.03 to 9.03 µM.
The pyrazolo [3,4-b]pyridine derivatives showed GI50 values ranging from 0.72 to 6.52 µM for the K562 leukemia cell line. Compound 8c once more exhibited exceptional sub-micromolar cytotoxicity (GI50 = 0.72 µM). With GI50 values ranging from 2.50 to 6.52 µM, compounds 8b, 8f, 10b, 10c, 10e, and 10f demonstrated single-digit micromolar cytotoxic action. However, as Table 4 summarizes, compound 8e exhibited noticeably less activity, with a GI50 value exceeding 10 µM.
Overall, SAR analysis indicates that subtle structural modifications have a marked influence on biological activity, notably enhancing potency against leukemia cancer cell lines. Among the tested compounds, 8c exhibited the highest potency and was therefore selected for further experiments to elucidate the molecular mechanism of action of the presented derivatives.
2.5. Immunodetection and Cell Cycle Analysis of MV4-11
Based on the promising antiproliferative activity observed in the initial screening and SAR analysis, further experiments were conducted to characterize the molecular mechanisms underlying the cytotoxic effects of the most potent compound 8c. Asynchronously growing MV4-11 cells were incubated with increasing concentrations of 8c (1.25, 2.5, 5.0, and 10 µM) for 24 h and subsequently analyzed by immunodetection (Figure 5) and cell cycle flow cytometry (Figure 6).
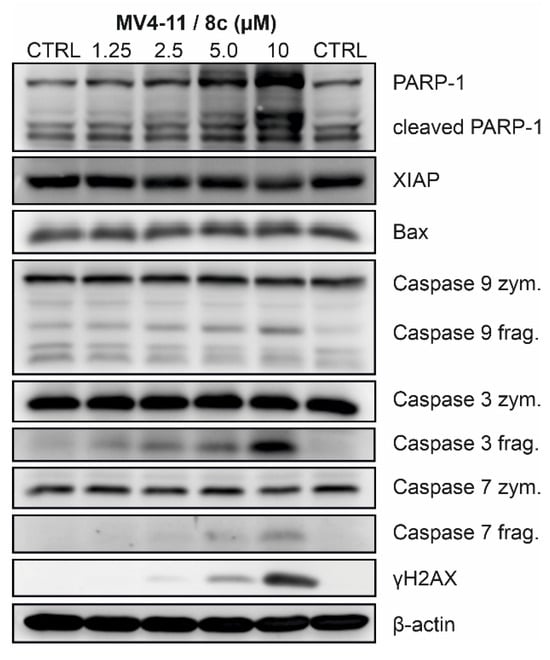
Figure 5.
Effect of 8c on the induction of cell death and DNA damage in MV4-11 cells treated for 24 h with indicated concentrations (µM). Representative results from three independent experiments are presented; quantitative data are provided in the Supplementary Materials Information.
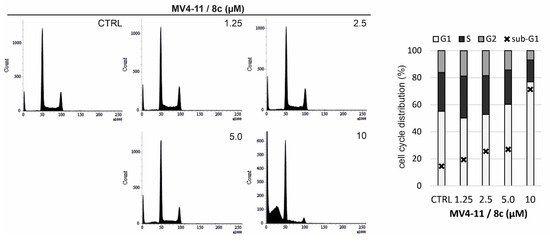
Figure 6.
The cell cycle phases (%) in MV4-11 cells treated with the indicated concentrations (µM) of compound 8c for 24 h.
Treatment with 8c efficiently induced the cleavage of initiator caspase-9, which was accompanied by cleavage of executioner caspases −3 and −7. Once activated, they cleave a variety of cellular substrates, including poly(ADP-ribose) polymerase 1 (PARP-1), thereby promoting commitment to cell death [66]. The presence of the cleaved 89 kDa fragment of PARP-1 clearly indicates a concentration-dependent progression of cell death in MV4-11 cells following 8c treatment. This was further associated with a slight increase in the proapoptotic protein Bax and, conversely, a decrease in the antiapoptotic protein XIAP. In addition, 8c induced phosphorylation of histone H2AX at serine 139 (γ-H2AX), indicative of DNA damage, which is likely the trigger responsible for the initial activation of caspases [67,68] (Figure 5).
To further support these findings, flow cytometry analysis of cell cycle distribution was performed in MV4-11 cells treated with 8c for 24 h, using propidium iodide staining. Treatment with 8c resulted in a dose-dependent decrease in the S-phase cell population, accompanied by a reduction in the number of cells progressing to the G2/M phase and a significant increase in the sub-G1 population. These changes are indicative of replication stress during the S-phase and corroborate the strong induction of cell death previously observed by immunoblotting (Figure 6).
2.6. Topoisomerase Relaxation Assay
Finally, we sought to investigate the underlying mechanism responsible for the replication stress induced by compound 8c, leading to cell death. Replication stress is often associated with inhibiting TOPs, enzymes essential for maintaining DNA topology during replication. Notably, several established TOP inhibitors, such as camptothecin (a TOPI inhibitor) and etoposide (a TOPII inhibitor), possess planar aromatic structures that facilitate DNA intercalation [44]. Given the planar aromatic character of the synthesized compound series, we hypothesized that 8c might interfere with the activity of TOPI or TOPII.
To test this hypothesis, we performed topoisomerase relaxation assays to evaluate the inhibitory potential of 8c on the ability of TOPI and TOPIIα to relax supercoiled plasmid DNA. Reaction products were separated by agarose gel electrophoresis and visualized using GelRed nucleic acid stain. Camptothecin (CPT) and etoposide were employed as positive controls for TOPI and TOPIIα inhibition, respectively. 8c did not inhibit TOPI-mediated DNA relaxation; however, it impaired the relaxation activity of TOPIIα in a concentration-dependent manner (Figure 7).
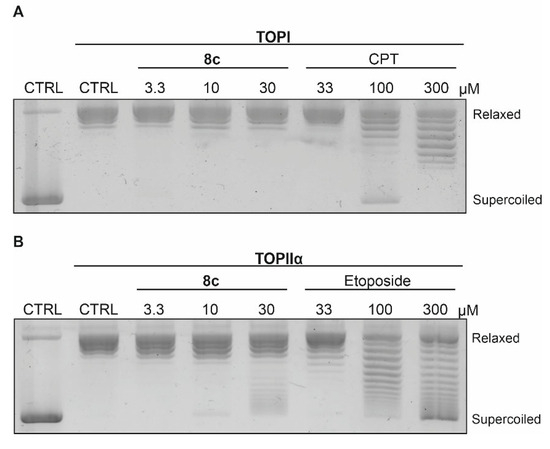
Figure 7.
Effect of 8c on the ability of (A) TOPI and (B) TOPIIα to relax supercoiled plasmid DNA at the indicated concentrations using camptothecin (CPT) and etoposide as references.
2.7. Kinase Assays
The newly prepared pyrazolo [3,4-b]pyridines (8b, 8c, 8e, 8f, 10b, 10c, 10e, and 10f) underwent further evaluation against a focused kinase panel (Abl, FLT3-ITD, and PDGFR) to assess off-target effects and confirm TOPIIα selectivity. At concentrations up to 10 µM, these molecules exhibited minimal inhibitory activity (Table S1) across the tested kinases, demonstrating no clinically relevant off-target interactions. Furthermore, we intend to extend the profiling to include a broader kinase panel in our future work, which will allow for a thorough assessment of kinome-wide selectivity.
2.8. Docking
The structure of 8c was subjected to molecular docking modelling in order to assess the potential mode of binding inside TOPII. As anticipated from the best docking pose, 8c fits well into the major groove region of the DNA double helix (Figure 8). The intercalation was observed for the cocrystal with the DNA bases DC8 and DC13, while 8c was observed with DC8, DC9, DC12, and DC13. The π-interactions were observed between these bases and the aromatic systems of 8c, including the main scaffold, one of the phenyl rings, the indole ring, and the phenolic ring. Additionally, the cyano group formed a hydrogen bond with Gln778, and a similar interaction occurred with one of the oxygen atoms of the cocrystal ligand. In addition, lipophilic interactions with Met782 and Arg503 were also observed with 8c. Furthermore, the OH group in the para position enabled sulfur-x interaction with Met782 and improved the π-stacking interaction with DT9. The docking score of 8c (−10.4 kcal/mol) was lower than that of the cocrystal ligand (−14.7 kcal/mol) while maintaining the important interaction patterns.
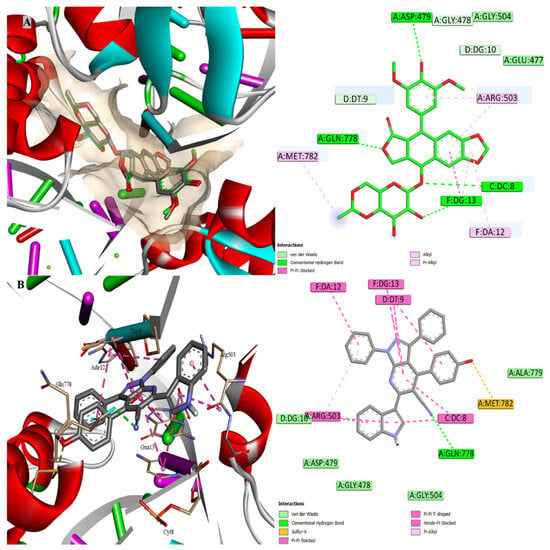
Figure 8.
Molecular docking inside the active site of TOPII (PDB: 3QX3); (A) overlay of the docked and cocrystal ligand “green” with RMSD 0.3503 and (B) docking of 8c.
3. Materials and Methods
3.1. Chemistry
3.1.1. General
Commercial suppliers provided all of the materials, which were used exactly as supplied. Silica gel plates (Merck KGaA, Darmstadt, Germany) were subjected to TLC in order to track the purity and development of the reaction. Uncorrected melting points were measured using a Stuart SMP30 apparatus (Cole-Parmer Ltd., Stone, Staffordshire, UK), and standard equipment was used for elemental analysis using a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany), FTIR using a PerkinElmer Spectrum Two FTIR spectrometer (Waltham, MA, USA), and NMR (1H, 13C, and DEPT-135) were obtained on a Bruker Avance III HD 400 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). A Bruker MicroTOF spectrometer was used to record high-resolution mass spectra (Bruker Daltonics, Billerica, MA, USA). As stated in the literature, starting ingredients 2 [54], 5 [55], and 9 [55] were synthesized. A ZORBAX Eclipse Plus C18 column (4.6 × 150 mm, 5 µm; Agilent Technologies, Santa Clara, CA, USA) was used for HPLC analysis, which verified that the produced chemicals were more than 95% pure.
3.1.2. General Procedure for Synthesis of Pyrazolo [3,4-b]Pyridines (8a–g, 10a–g, and 12)
TLC was used to track the reaction progress of a combination of different aldehydes (3a–g and 11) (0.0015 mole), equimolar quantities of 3-oxo-3-arylpropanenitriles (2 and 4) (0.0015 mole), and 3-substituted-1-phenyl-1H-pyrazol-5-amines (5 and 9) (0.0015 mole) that were refluxed in 20 mL of absolute ethanol for 12 h. The reaction mixture was finished and then left to cool to room temperature. Filtration, air drying, and recrystallization of the resultant precipitate from ethanol produced the required very pure pyrazolo [3,4-b]pyridine derivatives (8a–g, 10a–g, and 12).
3.1.3. 6-(1H-Indol-3-yl)-1,3,4-Triphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8a)
Yield (85%) as a white powder, with Mp: 245 °C. HPLC: RT 7.098 min (purity: 96.00%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.68 (d, J = 2.9 Hz, 1H, NH), 7.81–7.78 (m, 3H, Arom-H), 7.63–7.57 (m, 4H, Arom-H), 7.49–7.39 (m, 3H, Arom-H), 7.33–7.29 (m, 6H, Arom-H), 7.20–7.16 (m, 2H, Arom-H), 7.13 (tt, J = 7.2, 2.3 Hz, 2H, Arom-H). 13C NMR (101 MHz, DMSOd6) δ (ppm): 149.7, 145.6, 145.3, 139.1, 138.2, 136.3, 134.5, 130.7, 129.8, 129.3, 129.1, 128.9, 128.3, 127.4, 127.2, 125.7, 125.2, 123.7, 122.4, 122.2, 121.4, 120.0, 111.8, 108.1, 99.4, 85.7. HRMS (ESI): m/z: [M + H]+ calcd. 488.1870 and found 488.1862. Anal. Calcd. (Found) For C33H21N5: C, 81.29 (81.15); H, 4.34 (4.35); N, 14.36 (14.29)%.
3.1.4. 4-(3-Hydroxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8b)
Yield (83%) as a white powder, with Mp: 232 °C. HPLC: RT 5.770 min (purity: 99.40%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.68 (d, J = 2.9 Hz, 1H, NH), 9.80 (s, 1H, OH), 7.81–7.78 (m, 3H, Arom-H), 7.72–7.54 (m, 7H, Arom-H), 7.21–7.07 (m, 5H, Arom-H), 6.83–6.74 (m, 2H, Arom-H), 6.71 (t, J = 2.1 Hz, 1H, Arom-H), 6.59 (dd, J = 8.1, 2.4 Hz, 1H, Arom-H). 13C NMR (101 MHz, DMSOd6) δ (ppm): 158.5, 150.5, 144.8, 143.8, 139.3, 136.0, 134.1, 130.8, 129.7, 128.6, 128.1, 128.0, 127.3, 124.0, 121.5, 119.0, 115.2, 114.6, 112.9, 102.1. HRMS (ESI): m/z: [M + H]+ calcd. 504.1819 and found 504.1810. Anal. Calcd. (Found) For C33H21N5O: C, 78.71 (78.87); H, 4.20 (4.18); N, 13.91 (13.86)%.
3.1.5. 4-(4-Hydroxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8c)
Yield (89%) as a white powder, with Mp: 295 °C. HPLC: RT 5.165 min (purity: 98.53%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.92 (s, 1H, NH), 9.85 (s, 1H, OH), 8.53–8.44 (m, 2H, Arom-H), 8.39–8.29 (m, 2H, Arom-H), 7.69–7.61 (m, 2H, Arom-H), 7.57 (dd, J = 8.3, 3.3 Hz, 1H, Arom-H), 7.49–7.43 (m, 1H, Arom-H), 7.31–7.25 (m, 2H, Arom-H), 7.23–7.11 (m, 7H, Arom-H), 6.71–6.56 (m, 2H, Arom-H). 13C NMR (126 MHz, DMSOd6) δ (ppm): 159.2, 156.3, 153.7, 151.0, 147.5, 138.9, 136.9, 132.1, 131.5, 130.1, 129.7, 129.4, 128.5, 128.0, 127.4, 126.4, 124.8, 123.1, 122.3, 122.0, 121.4, 119.7, 115.2, 113.3, 112.7, 110.8, 100.3. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 131.5 (↑), 130.1 (↑), 129.7 (↑), 129.4 (↑), 128.5 (↑), 128.0 (↑), 127.4 (↑), 123.1 (↑), 122.3 (↑), 122.0 (↑), 121.4 (↑), 115.2 (↑), 112.7 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 504.1819 and found 504.1809. Anal. Calcd. (Found) For C33H21N5O: C, 78.71 (78.59); H, 4.20 (4.23); N, 13.91 (13.97)%.
3.1.6. 4-(2-Hydroxy-3-Methoxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8d)
Yield (85%) as a white powder, with Mp: 271 °C. HPLC: RT 7.888 min (purity: 99.41%). 1H NMR (400 MHz, DMSOd6) δ (ppm): 11.33 (s, 1H, NH), 9.41 (s, 1H, OH), 8.45–6.76 (m, 18H, Arom-H), 3.88 (s, 3H, OCH3). 13C NMR (101 MHz, DMSOd6) δ (ppm): 160.3, 153.2, 150.0, 149.7, 147.1, 143.0, 139.1, 138.3, 136.2, 130.5, 129.9, 129.8, 128.9, 127.8, 127.5, 126.9, 126.2, 122.4, 121.7, 120.5, 118.3, 116.9, 112.5, 112.0, 106.4, 102.5, 56.2. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.38); H, 4.34 (4.30); N, 13.13 (13.09)%.
3.1.7. 4-(3-Hydroxy-4-Methoxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8e)
Yield (91%) as a white powder, with Mp: 266 °C. HPLC: RT 7.413 min (purity: 96.44%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.92 (s, 1H, NH), 9.13 (s, 1H, OH), 8.48 (d, J = 7.9 Hz, 2H, Arom-H), 8.36–8.33 (m, 2H, Arom-H), 7.67–7.63 (m, 2H, Arom-H), 7.57 (d, J = 8.0 Hz, 1H, Arom-H), 7.48–7.45 (m, 1H, Arom-H), 7.28 (ddt, J = 8.1, 5.6, 1.9 Hz, 2H, Arom-H), 7.22 (td, J = 7.5, 1.2 Hz, 1H, Arom-H), 7.18–7.11 (m, 4H, Arom-H), 6.82 (d, J = 2.1 Hz, 1H, Arom-H), 6.78 (d, J = 8.3 Hz, 1H, Arom-H), 6.73 (dd, J = 8.2, 2.1 Hz, 1H, Arom-H), 3.77 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 156.2, 153.4, 150.9, 149.3, 147.4, 146.8, 138.9, 136.9, 132.1, 130.1, 129.7, 129.2, 128.6, 127.9, 127.4, 126.9, 126.4, 123.1, 122.4, 122.0, 121.4, 119.5, 117.0, 113.3, 112.7, 112.2, 110.8, 100.3, 56.3. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.1 (↑), 129.7 (↑), 129.2 (↑), 128.6 (↑), 127.9 (↑), 127.4 (↑), 123.1 (↑), 122.4 (↑), 122.0 (↑), 121.4 (↑), 117.0 (↑), 112.7 (↑), 112.2 (↑), 56.3 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 534.1925 and found 534.1922. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.65); H, 4.34 (4.32); N, 13.13 (13.16)%.
3.1.8. 4-(4-Hydroxy-3-Methoxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8f)
Yield (87%) as a white powder, with Mp: 239 °C. HPLC: RT 7.355 min (purity: 98.55%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.92 (d, J = 2.9 Hz, 1H, NH), 9.40 (s, 1H, OH), 8.49 (d, J = 2.7 Hz, 1H, Arom-H), 8.47 (d, J = 7.9 Hz, 1H, Arom-H), 8.36–8.31 (m, 2H, Arom-H), 7.68–7.63 (m, 2H, Arom-H), 7.57 (d, J = 8.0 Hz, 1H, Arom-H), 7.49–7.45 (m, 1H, Arom-H), 7.31–7.25 (m, 2H, Arom-H), 7.22 (td, J = 7.4, 1.2 Hz, 1H, Arom-H), 7.17 (d, J = 4.4 Hz, 4H, Arom-H), 7.05 (dd, J = 8.1, 2.1 Hz, 1H, Arom-H), 6.82 (d, J = 8.1 Hz, 1H, Arom-H), 6.74 (d, J = 2.0 Hz, 1H, Arom-H), 3.34 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 156.4, 153.5, 151.0, 148.6, 147.5, 147.4, 138.9, 136.9, 132.4, 130.2, 129.7, 129.4, 128.6, 127.9, 127.4, 126.4, 125.0, 123.1, 123.0, 122.4, 122.0, 121.4, 119.8, 115.5, 114.9, 113.3, 112.7, 110.7, 100.2, 55.7. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.2 (↑), 129.7 (↑), 129.4 (↑), 128.6 (↑), 127.9 (↑), 127.4 (↑), 123.1 (↑), 123.0 (↑), 122.4 (↑), 122.0 (↑), 121.4 (↑), 115.5 (↑), 114.9 (↑), 112.7 (↑), 55.7 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 534.1925 and found 534.1912. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.67); H, 4.34 (4.38); N, 13.13 (13.18)%.
3.1.9. 4-(3,4-Dimethoxyphenyl)-6-(1H-Indol-3-yl)-1,3-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (8g)
Yield (88%) as a white powder, with Mp: 228 °C. HPLC: RT 10.632 min (purity: 99.55%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 12.26 (s, 1H, NH), 8.44 (s, 2H, Arom-H), 8.22–8.18 (m, 4H, Arom-H), 7.81 (d, J = 2.2 Hz, 2H, Arom-H), 7.73 (dd, J = 8.5, 2.1 Hz, 2H, Arom-H), 7.56 (d, J = 7.1 Hz, 2H), 7.28 (td, J = 7.6, 1.5 Hz, 4H, Arom-H), 7.19 (d, J = 8.5 Hz, 2H, Arom-H), 3.88 (s, 3H, OCH3), 3.84 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 181.9, 153.1, 152.9, 149.1, 137.1, 135.8, 126.7, 126.3, 125.4, 123.9, 122.8, 121.9, 119.1, 114.2, 113.3, 112.9, 112.3, 108.4, 56.3, 56.0. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 152.9 (↑), 135.8 (↑), 126.3 (↑), 123.9 (↑), 122.8 (↑), 121.9 (↑), 113.3 (↑), 112.9 (↑), 112.3 (↑), 56.3 (↑), 56.0 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 548.2081 and found 548.2080. Anal. Calcd. (Found) For C35H25N5O2: C, 76.77 (76.91); H, 4.60 (4.57); N, 12.79 (12.89)%.
3.1.10. 3-(1H-Indol-3-yl)-1,4,6-Triphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10a)
Yield (86%) as a white powder, with Mp: 280 °C. HPLC: RT 7.001 min (purity: 98.27%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.22–11.19 (m, 1H, NH), 8.38 (d, J = 8.0 Hz, 2H, Arom-H), 8.20 (d, J = 7.7 Hz, 1H, Arom-H), 8.02 (dd, J = 6.6, 2.9 Hz, 2H, Arom-H), 7.68–7.61 (m, 6H, Arom-H), 7.59 (s, 1H, Arom-H), 7.56–7.53 (m, 2H, Arom-H), 7.48 (t, J = 7.6 Hz, 2H, Arom-H), 7.42 (t, J = 7.4 Hz, 1H, Arom-H), 7.35 (d, J = 7.8 Hz, 1H, Arom-H), 7.17–7.12 (m, 2H, Arom-H). 13C NMR (126 MHz, DMSOd6) δ (ppm): 160.4, 153.1, 150.0, 142.8, 139.0, 138.2, 136.1, 135.0, 130.6, 130.5, 129.9, 129.9, 129.9, 129.0, 128.9, 127.3, 127.1, 126.2, 122.4, 121.8, 121.4, 120.5, 118.1, 112.4, 112.0, 106.3, 102.4. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.6 (↑), 130.5 (↑), 129.9 (↑), 129.9 (↑), 129.9 (↑), 129.0 (↑), 128.9 (↑), 127.3 (↑), 127.1 (↑), 122.4 (↑), 121.8 (↑), 121.4 (↑), 120.5 (↑), 112.0 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 488.1870 and found 488.1868. Anal. Calcd. (Found) For C33H21N5: C, 81.29 (81.42); H, 4.34 (4.37); N, 14.36 (14.41)%.
3.1.11. 4-(3-Hydroxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10b)
Yield (83%) as a white powder, with Mp: 247 °C. HPLC: RT 5.736 min (purity: 99.79%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.32 (d, J = 2.9 Hz, 1H, NH), 9.88 (s, 1H, OH), 8.40 (d, J = 8.0 Hz, 2H, Arom-H), 8.03–8.01 (m, 2H, Arom-H), 7.68–7.62 (m, 6H, Arom-H), 7.39–7.32 (m, 3H, Arom-H), 7.20–7.13 (m, 3H, Arom-H), 7.04–7.02 (m, 1H, Arom-H), 6.98 (d, J = 7.6 Hz, 1H, Arom-H), 6.96 (d, J = 2.1 Hz, 1H, Arom-H). HRMS (ESI): m/z: [M+H]+ calcd. 504.1819 and found 504.1824. Anal. Calcd. (Found) For C33H21N5O: C, 78.71 (78.90); H, 4.20 (4.19); N, 13.91 (13.94)%.
3.1.12. 4-(4-Hydroxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10c)
Yield (85%) as a white powder, with Mp: 259 °C. HPLC: RT 5.054 min (purity: 99.32%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.33 (d, J = 2.8 Hz, 1H, NH), 10.07 (s, 1H, OH), 8.38 (d, J = 8.1 Hz, 2H, Arom-H), 8.24–8.22 (m, 1H, Arom-H), 8.02–8.00 (m, 2H, Arom-H), 7.65–7.61 (m, 5H, Arom-H), 7.42 (s, 1H, Arom-H), 7.37 (dd, J = 8.6, 6.9 Hz, 3H, Arom-H), 7.20–7.09 (m, 3H, Arom-H), 6.86–6.83 (m, 2H, Arom-H). HRMS (ESI): m/z: [M + H]+ calcd. 504.1819 and found 504.1817. Anal. Calcd. (Found) For C33H21N5O: C, 78.71 (78.86); H, 4.20 (4.16); N, 13.91 (13.95)%.
3.1.13. 4-(2-Hydroxy-3-Methoxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10d)
Yield (90%) as a white powder, with Mp: 277 °C. HPLC: RT 7.845 min (purity: 99.81%). 1H NMR (400 MHz, DMSOd6) δ (ppm): 11.67 (s, 1H, NH), 9.21 (s, 1H, OH), 8.27 (d, J = 7.6 Hz, 1H, Arom-H), 7.88 (d, J = 2.5 Hz, 1H, Arom-H), 7.81–7.71 (m, 3H, Arom-H), 7.60 (t, J = 7.9 Hz, 3H, Arom-H), 7.51–7.42 (m, 3H, Arom-H), 7.32–7.30 (m, 1H, Arom-H), 7.21–7.14 (m, 5H, Arom-H), 6.95 (s, 1H, Arom-H), 3.82 (s, 3H, OCH3). 13C NMR (101 MHz, DMSOd6) δ (ppm): 163.3, 150.1, 148.9, 148.8, 148.4, 139.4, 137.0, 129.6, 127.6, 125.4, 124.6, 124.5, 123.4, 122.1, 121.4, 120.2, 120.1, 119.7, 116.3, 112.2, 109.3, 91.9, 56.3. HRMS (ESI): m/z: [M + H]+ calcd. 534.1925 and found 534.1918. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.66); H, 4.34 (4.32); N, 13.13 (13.16)%.
3.1.14. 4-(3-Hydroxy-4-Methoxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10e)
Yield (93%) as a white powder, with Mp: 249 °C. HPLC: RT 7.380 min (purity: 99.95%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.35 (d, J = 2.9 Hz, 1H, NH), 9.45 (s, 1H, OH), 8.39 (d, J = 8.0 Hz, 2H, Arom-H), 8.33–8.30 (m, 1H, Arom-H), 8.03–7.99 (m, 2H, Arom-H), 7.67–7.62 (m, 5H, Arom-H), 7.55–7.35 (m, 3H, Arom-H), 7.17 (tt, J = 7.1, 5.4 Hz, 2H, Arom-H), 7.05 (d, J = 8.3 Hz, 1H, Arom-H), 6.98 (d, J = 2.2 Hz, 1H, Arom-H), 6.95 (dd, J = 8.2, 2.2 Hz, 1H, Arom-H), 3.89 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 160.4, 153.3, 150.0, 149.7, 147.1, 143.0, 139.1, 138.3, 136.2, 130.6, 129.9, 129.9, 129.6, 129.0, 128.6, 127.8, 127.5, 127.0, 126.2, 122.4, 121.8, 121.7, 121.2, 120.6, 118.3, 116.9, 112.5, 112.4, 112.0, 106.4, 102.6, 56.2. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.6 (↑), 129.9 (↑), 129.9 (↑), 129.0 (↑), 127.8 (↑), 127.0 (↑), 122.4 (↑), 121.8 (↑), 121.7 (↑), 121.2 (↑), 120.6 (↑), 116.9 (↑), 112.5 (↑), 112.0 (↑), 56.2 (OCH3 ↑). HRMS (ESI): m/z: [M + H]+ calcd. 534.1925 and found 534.1929. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.38); H, 4.34 (4.28); N, 13.13 (13.08)%.
3.1.15. 4-(4-Hydroxy-3-Methoxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10f)
Yield (88%) as a white powder, with Mp: 276 °C. HPLC: RT 7.283 min (purity: 99.42%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.34 (d, J = 2.8 Hz, 1H, NH), 9.61 (s, 1H, OH), 8.38 (d, J = 8.3 Hz, 2H, Arom-H), 8.17 (d, J = 7.8 Hz, 1H, Arom-H), 8.04–7.99 (m, 2H, Arom-H), 7.67–7.59 (m, 5H, Arom-H), 7.45–7.36 (m, 2H, Arom-H), 7.15 (dt, J = 21.7, 7.2 Hz, 2H, Arom-H), 7.05 (dt, J = 8.1, 1.8 Hz, 1H, Arom-H), 7.01 (d, J = 1.7 Hz, 1H, Arom-H), 6.88 (dd, J = 8.0, 1.4 Hz, 1H, Arom-H), 6.16 (t, J = 2.0 Hz, 1H, Arom-H), 3.38 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 160.6, 153.4, 150.1, 148.9, 147.7, 142.9, 139.0, 138.3, 136.1, 130.6, 130.0, 129.8, 128.9, 127.7, 127.0, 126.2, 125.1, 123.2, 122.3, 121.8, 121.2, 120.5, 118.6, 115.7, 114.9, 112.4, 111.9, 106.5, 102.5, 55.9. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.6 (↑), 130.0 (↑), 129.8 (↑), 128.9 (↑), 127.7 (↑), 127.0 (↑), 123.3 (↑), 122.3 (↑), 121.8 (↑), 121.2 (↑), 120.5 (↑), 115.7 (↑), 114.9 (↑), 111.9 (↑), 55.9 (OCH3 ↑). HRMS (ESI): m/z: [M + H]+ calcd. 534.1925 and found 534.1920. Anal. Calcd. (Found) For C34H23N5O2: C, 76.53 (76.37); H, 4.34 (4.33); N, 13.13 (13.11)%.
3.1.16. 4-(3,4-Dimethoxyphenyl)-3-(1H-Indol-3-yl)-1,6-Diphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (10g)
Yield (91%) as a white powder, with Mp: 284 °C. HPLC: RT 9.795 min (purity: 99.32%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.30 (d, J = 2.8 Hz, 1H, NH), 8.38 (d, J = 8.0 Hz, 2H, Arom-H), 8.12 (d, J = 7.9 Hz, 1H, Arom-H), 8.05–8.00 (m, 2H, Arom-H), 7.69–7.61 (m, 5H, Arom-H), 7.41 (dd, J = 20.8, 7.7 Hz, 2H, Arom-H), 7.19–7.01 (m, 5H, Arom-H), 6.12 (d, J = 2.7 Hz, 1H, Arom-H), 3.85 (s, 3H, OCH3), 3.36 (s, 3H, OCH3). 13C NMR (126 MHz, DMSOd6) δ (ppm): 160.6, 153.1, 150.7, 150.1, 148.7, 142.9, 139.0, 138.3, 136.1, 130.6, 130.0, 129.9, 129.0, 127.6, 127.1, 126.6, 126.2, 122.9, 122.3, 121.8, 121.1, 120.4, 118.5, 114.3, 112.5, 112.0, 111.9, 106.4, 102.4, 56.2, 55.7. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.6 (↑), 130.0 (↑), 129.9 (↑), 129.8 (↑), 129.0 (↑), 127.6 (↑), 127.1 (↑), 122.9 (↑), 122.3 (↑), 121.8 (↑), 121.1 (↑), 120.4 (↑), 114.3 (↑), 112.0 (↑), 111.9 (↑), 56.2 (OCH3 ↑), 55.7 (OCH3 ↑). HRMS (ESI): m/z: [M + H]+ calcd. 548.2081 and found 548.2074 Anal. Calcd. (Found) For C35H25N5O2: C, 76.77 (76.59); H, 4.60 (4.62); N, 12.79 (12.85)%.
3.1.17. 4-(1H-Indol-3-yl)-1,3,6-Triphenyl-1H-Pyrazolo [3,4-b]Pyridine-5-Carbonitrile (12)
Yield (89%) as a white powder, with Mp: 257 °C. HPLC: RT 7.166 min (purity: 99.25%). 1H NMR (500 MHz, DMSOd6) δ (ppm): 11.62 (d, J = 2.8 Hz, 1H, NH), 8.35–8.29 (m, 2H, Arom-H), 8.09–8.03 (m, 2H, Arom-H), 7.66–7.61 (m, 5H, Arom-H), 7.46–7.39 (m, 4H, Arom-H), 7.18–7.09 (m, 4H, Arom-H), 7.02–6.94 (m, 3H, Arom-H). 13C NMR (126 MHz, DMSOd6) δ (ppm): 161.4, 150.7, 147.7, 147.6, 138.8, 138.4, 136.2, 132.2, 130.6, 130.0, 129.8, 129.0, 128.9, 128.7, 127.7, 127.4, 126.2, 122.4, 122.3, 120.4, 120.0, 118.9, 112.7, 112.2, 109.0, 102.9. 13C NMR-DEPT-135 (126 MHz, DMSOd6) δ: 130.6 (↑), 130.0 (↑), 129.8 (↑), 129.0 (↑), 128.9 (↑), 128.9 (↑), 128.7 (↑), 127.7 (↑), 127.4 (↑), 122.4 (↑), 122.3 (↑), 120.4 (↑), 120.0 (↑), 112.2 (↑). HRMS (ESI): m/z: [M + H]+ calcd. 488.18697 and found 488.18698. Anal. Calcd. (Found) For C33H21N5: C, 81.29 (81.44); H, 4.34 (4.32); N, 14.36 (14.41)%.
3.2. Biological Evaluation
3.2.1. In Vitro Antitumor Screening Against 60 Cancer Cell Lines
In accordance with the NCI’s usual technique, the synthetic compounds’ in vitro anticancer activity was assessed against a panel of 60 human tumor cell lines from nine different tissue types (Figures S67–S105). To evaluate each compound’s potency and cytotoxic profile, key dose–response parameters, including GI50, TGI, and LC50, were computed [69,70,71,72,73,74,75,76,77], and detailed data are provided in the Supplementary Information.
3.2.2. Cell Lines
Recognized cell banks provided the MV4-11 and K562 cancer cell lines, which were then cultivated at 37 °C in a humidified CO2 incubator under standard conditions in RPMI-1640 or DMEM medium supplemented with fetal bovine serum and antibiotics, and detailed data are provided in the Supplementary Materials Information.
3.2.3. Cell Viability Assay
In order to calculate GI50 values from dose–response curves using fluorescence measurements, cells were seeded in 96-well plates and treated with different doses of the test substances for 72 h. This was followed by a viability assessment based on resazurin, and detailed data are provided in the Supplementary Materials Information.
3.2.4. Flow Cytometry
The test substance was applied at different concentrations to asynchronously developing cells, which were then harvested after 24 h, fixed in ice-cold 70% ethanol, and incubated on ice for 30 min. Cell cycle distribution was examined by flow cytometry with a 488 nm laser after PBS washing and propidium iodide staining, and ModFit LT software (version 5.0.9) was used to quantify the results, and detailed data are provided in the Supplementary Materials Information.
3.2.5. Immunoblotting
RIPA buffer was used to generate cell lysates, and proteins were separated using SDS-PAGE before being transferred to nitrocellulose membranes. Following blocking, membranes were incubated with certain primary antibodies overnight at 4 °C. Peroxidase-conjugated secondary antibodies were then added, and SuperSignal West Pico reagents and a LAS-4000 CCD camera were used for detection, and detailed data are provided in the Supplementary Materials Information.
3.2.6. Topoisomerase Relaxation Assay
Supercoiled pBR322 plasmid and the corresponding assay buffers were used for the Topoisomerase I and IIα assays. The assays were then incubated for 30 min at 37 °C at 350 rpm. Complete experimental details are included in the Supplementary Materials Information. Reactions were halted, products were separated using 5% agarose gel electrophoresis, and they were then seen using GelRed staining and a FLA-7000 digital image analyzer.
3.2.7. Molecular Docking
The RCSB-PDB site was used to obtain the coordinates of TOPII (PDB: 3QX3 [78]). The three-dimensional structures of 8c and the cocrystal ligand were prepared and optimized using Marvin Sketch [79] as part of the docking investigation. AutoDock Vina [80] was used to carry out docking operations. The active site’s coordinates (x, y, z) were 32.9/95.4/50.8 with a size 24.5/20.2/17.5. The 2D schematic presentation and 3D visualization were created using the Discovery Studio 2021 client [81].
4. Conclusions
The synthesized pyrazolo [3,4-b]pyridine derivatives demonstrated antiproliferative activity, particularly against leukemia-derived cancer cell lines, with 8c emerging as the most potent candidate. Detailed mechanistic studies revealed that 8c induces DNA damage leading to cell death in MV4-11 cells, as evidenced by caspase activation, PARP-1 cleavage, and modulation of pro- and antiapoptotic proteins. Additionally, 8c caused S-phase cell cycle perturbations indicative of replication stress and confirmed strong induction of cell death. Topoisomerase relaxation assays identified TOPIIα as a potential molecular target, with 8c inhibiting its activity in a concentration-dependent manner. These findings highlight 8c as a promising lead compound for the further development of anticancer agents targeting TOPIIα-associated pathways.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18111770/s1, Figures S1–S37. NMR spectra of compounds 8a-g, 10a-g and 12, Figures S38–S51. HRMS spectra of compounds 8a-c, 8e-g, 10a-g and 12, Figures S52–S66. HPLC spectrum of compounds 8a-g, 10a-g and 12: title, Figures S67–S105. NCI charts of compounds 8a-g, 10a-g and 12; Table S1. Kinases assays.
Author Contributions
Conceptualization, W.M.E., V.K. and H.A.A.-A.; Formal analysis, W.M.E., H.O.T., A.T.N., T.A.M., M.M.E., P.K. and V.K.; Investigation, D.V., V.V., A.T.N., Z.M.E., M.A.S., P.K. and G.H.A.-A.; Resources, W.M.E. and V.K.; Writing—original draft preparation, W.M.E., H.O.T., Z.M.E., D.V. and V.K.; Writing—review and editing, H.O.T., D.V., A.T.N., M.A.S. and V.K.; Visualization, D.V., M.M.E., G.H.A.-A., V.K. and H.A.A.-A.; Supervision, W.M.E., and V.K.; Project administration, W.M.E., and V.K.; Funding acquisition, W.M.E., V.K. and H.A.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (RGP2/500/46). The study was also supported by The European Union—Next Generation EU (The project National Institute for Cancer Research, Programme EXCELES, ID No. LX22NPO5102) and Palacky University (IGA_PrF_2025_011).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Bizuayehu, H.M.; Dadi, A.F.; Ahmed, K.Y.; Tegegne, T.K.; Hassen, T.A.; Kibret, G.D.; Ketema, D.B.; Bore, M.G.; Thapa, S.; Odo, D.B.; et al. Burden of 30 cancers among men: Global statistics in 2022 and projections for 2050 using population-based estimates. Cancer 2024, 130, 3708–3723. [Google Scholar] [CrossRef]
- Spring, J.; Munshi, L. Hematology Emergencies in Adults With Critical Illness: Malignant Hematology. Chest 2022, 162, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Daltveit, D.S.; Morgan, E.; Colombet, M.; Steliarova-Foucher, E.; Bendahhou, K.; Marcos-Gragera, R.; Rongshou, Z.; Smith, A.; Wei, H.; Soerjomataram, I. Global patterns of leukemia by subtype, age, and sex in 185 countries in 2022. Leukemia 2025, 39, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Leukaemia ‘firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Donaire-Arias, A.; Montagut, A.M.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo [3,4-b]pyridines: Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef]
- Lin, R.; Connolly, P.J.; Lu, Y.; Chiu, G.; Li, S.; Yu, Y.; Huang, S.; Li, X.; Emanuel, S.L.; Middleton, S.A.; et al. Synthesis and evaluation of pyrazolo[3,4-b]pyridine CDK1 inhibitors as anti-tumor agents. Bioorganic Med. Chem. Lett. 2007, 17, 4297–4302. [Google Scholar] [CrossRef]
- Almansour, B.S.; Binjubair, F.A.; Abdel-Aziz, A.A.-M.; Al-Rashood, S.T. Synthesis and In Vitro Anticancer Activity of Novel 4-Aryl-3-(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridines Arrest Cell Cycle and Induce Cell Apoptosis by Inhibiting CDK2 and/or CDK9. Molecules 2023, 28, 6428. [Google Scholar] [CrossRef]
- Misra, R.N.; Xiao, H.-Y.; Rawlins, D.B.; Shan, W.; Kellar, K.A.; Mulheron, J.G.; Sack, J.S.; Tokarski, J.S.; Kimball, S.D.; Webster, K.R. 1H-Pyrazolo[3,4-b]pyridine inhibitors of cyclin-dependent kinases: Highly potent 2,6-Difluorophenacyl analogues. Bioorganic Med. Chem. Lett. 2003, 13, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.A.; Liu, S.; Gao, M.; Wang, L.; Sun, J.; Du, J.; Guan, Q.; Bao, K.; Zuo, D.; Wu, Y.; et al. 3,5-Diaryl-1H-pyrazolo[3,4-b]pyridines as potent tubulin polymerization inhibitors: Rational design, synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 168, 426–435. [Google Scholar] [CrossRef]
- Witherington, J.; Bordas, V.; Gaiba, A.; Garton, N.S.; Naylor, A.; Rawlings, A.D.; Slingsby, B.P.; Smith, D.G.; Takle, A.K.; Ward, R.W. 6-Aryl-pyrazolo[3,4-b]pyridines: Potent inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorganic Med. Chem. Lett. 2003, 13, 3055–3057. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Tiwari, G.; Khanna, A.; Mishra, V.K.; Yadav, Y.; Malviya, M.; Sagar, R. Molecular Design, Synthesis and Anticancer Activity of Novel Pyrazolo[3,4-b]pyridine-based Glycohybrid Molecules. Bioorganic Chem. 2025, 156, 108161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, Y.; Xu, P.; Dai, Y.; Luo, C.; Sun, Y.; Ai, J.; Geng, M.; Duan, W. Discovery of Substituted 1H-Pyrazolo[3,4-b]pyridine Derivatives as Potent and Selective FGFR Kinase Inhibitors. ACS Med. Chem. Lett. 2016, 7, 629–634. [Google Scholar] [CrossRef]
- Liu, N.; Wang, X.; Fu, Q.; Qin, Q.; Wu, T.; Lv, R.; Zhao, D.; Cheng, M. Design, synthesis and biological evaluation of pyrazolo[3,4-b]pyridine derivatives as TRK inhibitors. RSC Med. Chem. 2023, 14, 85–102. [Google Scholar] [CrossRef]
- El-Gohary, N.S.; Hawas, S.S.; Gabr, M.T.; Shaaban, M.I.; El-Ashmawy, M.B. New series of fused pyrazolopyridines: Synthesis, molecular modeling, antimicrobial, antiquorum-sensing and antitumor activities. Bioorganic Chem. 2019, 92, 103109. [Google Scholar] [CrossRef] [PubMed]
- Hawas, S.S.; El-Gohary, N.S.; Gabr, M.T.; Shaaban, M.I.; El-Ashmawy, M.B. Synthesis, molecular docking, antimicrobial, antiquorum-sensing and antiproliferative activities of new series of pyrazolo[3,4-b]pyridine analogs. Synth. Commun. 2019, 49, 2466–2487. [Google Scholar] [CrossRef]
- Kazemi, Z.; Rudbari, H.A.; Moini, N.; Momenbeik, F.; Carnamucio, F.; Micale, N. Indole-Containing Metal Complexes and Their Medicinal Applications. Molecules 2024, 29, 484. [Google Scholar] [CrossRef]
- Qin, H.-L.; Liu, J.; Fang, W.-Y.; Ravindar, L.; Rakesh, K.P. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 194, 112245. [Google Scholar] [CrossRef]
- Siddique, S.; Ahmad, K.R.; Nawaz, S.K.; Raza, A.R.; Ahmad, S.N.; Ali, R.; Inayat, I.; Suleman, S.; Kanwal, M.A.; Usman, M. Evaluation of the antiinflammatory, analgesic, anti-pyretic and anti-ulcerogenic potentials of synthetic indole derivatives. Sci. Rep. 2023, 13, 8639. [Google Scholar] [CrossRef]
- Mehra, A.; Sharma, V.; Verma, A.; Venugopal, S.; Mittal, A.; Singh, G.; Kaur, B. Indole Derived Anticancer Agents. ChemistrySelect 2022, 7, e202202361. [Google Scholar] [CrossRef]
- Hassan, S.M.; Farid, A.; Panda, S.S.; Bekheit, M.S.; Dinkins, H.; Fayad, W.; Girgis, A.S. Indole Compounds in Oncology: Therapeutic Potential and Mechanistic Insights. Pharmaceuticals 2024, 17, 922. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Siddiqui, N. Anticonvulsant evaluation of clubbed indole-1,2,4-triazine derivatives: A synthetic approach. Eur. J. Med. Chem. 2014, 80, 509–522. [Google Scholar] [CrossRef]
- Singh, A.; Bhutani, C.; Khanna, P.; Talwar, S.; Singh, S.K.; Khanna, L. Recent report on indoles as a privileged antiviral scaffold in drug discovery. Eur. J. Med. Chem. 2025, 281, 117017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef] [PubMed]
- Süzen, S. Antioxidant Activities of Synthetic Indole Derivatives and Possible Activity Mechanisms. In Bioactive Heterocycles V; Khan, M.T.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 145–178. [Google Scholar]
- Nishiguchi, G.A.; Atallah, G.; Bellamacina, C.; Burger, M.T.; Ding, Y.; Feucht, P.H.; Garcia, P.D.; Han, W.; Klivansky, L.; Lindvall, M. Discovery of novel 3,5-disubstituted indole derivatives as potent inhibitors of Pim-1, Pim-2, and Pim-3 protein kinases. Bioorganic Med. Chem. Lett. 2011, 21, 6366–6369. [Google Scholar] [CrossRef]
- Al-Warhi, T.; El Kerdawy, A.M.; Aljaeed, N.; Ismael, O.E.; Ayyad, R.R.; Eldehna, W.M.; Abdel-Aziz, H.A.; Al-Ansary, G.H. Synthesis, Biological Evaluation and In Silico Studies of Certain Oxindole-Indole Conjugates as Anticancer CDK Inhibitors. Molecules 2020, 25, 2031. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Pansare, D.N.; Lokwani, D.K.; Bhandari, S.V.; Kanode, V.V.; Tandale, O.V.; Kadam, A.R. Appraisal and synthesis of novel indole-thiazole derivatives as epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors against resistance mutation for lung cancer treatment. Results Chem. 2024, 11, 101760. [Google Scholar] [CrossRef]
- Bruel, A.; Logé, C.; de Tauzia, M.-L.; Ravache, M.; Le Guevel, R.; Guillouzo, C.; Lohier, J.-F.; Oliveira Santos, J.S.-d.; Lozach, O.; Meijer, L.; et al. Synthesis and biological evaluation of new 5-benzylated 4-oxo-3,4-dihydro-5H-pyridazino[4,5-b]indoles as PI3Kα inhibitors. Eur. J. Med. Chem. 2012, 57, 225–233. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhou, G.; Zhang, J.; Teng, Y.; Bai, Z.; Liu, T. Design, synthesis and anticancer activity studies of novel indole derivatives as Bcl-2/Mcl-1 dual inhibitors. Med. Chem. Res. 2023, 32, 99–108. [Google Scholar] [CrossRef]
- Zidar, N.; Secci, D.; Tomašič, T.; Mašič, L.P.; Kikelj, D.; Passarella, D.; Argaez, A.N.G.; Hyeraci, M.; Dalla Via, L. Synthesis, Antiproliferative Effect, and Topoisomerase II Inhibitory Activity of 3-Methyl-2-phenyl-1H-indoles. ACS Med. Chem. Lett. 2020, 11, 691–697. [Google Scholar] [CrossRef]
- Asati, V.; Bhupal, R.; Bhattacharya, S.; Kaur, K.; Gupta, G.D.; Pathak, A.; Mahapatra, D.K. Recent Updates on Indole Derivatives as Kinase Inhibitors in the Treatment of Cancer. Anticancer Agents Med. Chem. 2023, 23, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Swedan, H.K.; Kassab, A.E.; Gedawy, E.M.; Elmeligie, S.E. Topoisomerase II inhibitors design: Early studies and new perspectives. Bioorganic Chem. 2023, 136, 106548. [Google Scholar] [CrossRef] [PubMed]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. BioEssays News Rev. Mol. Cell. Dev. Biol. 2021, 43, e2000286. [Google Scholar] [CrossRef]
- Pommier, Y.; Nussenzweig, A.; Takeda, S.; Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 2022, 23, 407–427. [Google Scholar] [CrossRef]
- Baranello, L.; Kouzine, F.; Levens, D. Topoisomerase Regulation of Cancer Gene Expression. Annu. Rev. Biochem. 2025, 94, 333–359. [Google Scholar] [CrossRef]
- Buzun, K.; Bielawska, A.; Bielawski, K.; Gornowicz, A. DNA topoisomerases as molecular targets for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2020, 35, 1781–1799. [Google Scholar] [CrossRef]
- Matias-Barrios, V.M.; Dong, X. The Implication of Topoisomerase II Inhibitors in Synthetic Lethality for Cancer Therapy. Pharmaceuticals 2023, 16, 94. [Google Scholar] [CrossRef]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, A.D.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Lagunas-Rangel, F.A.; Schiöth, H.B. Recent developments of topoisomerase inhibitors: Clinical trials, emerging indications, novel molecules and global sales. Pharmacol. Res. 2024, 209, 107431. [Google Scholar] [CrossRef]
- Pentheroudakis, G.; Goussia, A.; Voulgaris, E.; Nikolaidis, K.; Ioannidou, E.; Papoudou-Bai, A.; Grepi, K.; Kanavaros, P.; Pavlidis, N.; Bai, M. High levels of topoisomerase IIα protein expression in diffuse large B-cell lymphoma are associated with high proliferation, germinal center immunophenotype, and response to treatment. Leuk. Lymphoma 2010, 51, 1260–1268. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.; Im, E.; Kim, N.D. Etoposide as a Key Therapeutic Agent in Lung Cancer: Mechanisms, Efficacy, and Emerging Strategies. Int. J. Mol. Sci. 2025, 26, 796. [Google Scholar] [CrossRef]
- Skok, Ž.; Zidar, N.; Kikelj, D.; Ilaš, J. Dual Inhibitors of Human DNA Topoisomerase II and Other Cancer-Related Targets. J. Med. Chem. 2019, 63, 884–904. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update. J. Cardiovasc. Dev. Dis. 2025, 12, 207. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, M. The Synthesis of Nano-Doxorubicin and its Anticancer Effect. Anticancer Agents Med. Chem. 2021, 21, 2466–2477. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ratain, M.J. Pharmacodynamics and long-term toxicity of etoposide. Cancer Chemother. Pharmacol. 1994, 34, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Bahot, A.; Sekar, G.; Bansode, M.; Khunteta, K.; Sonar, P.V.; Hebale, A.; Salokhe, V.; Sinha, B.K. Understanding Cancer’s Defense against Topoisomerase-Active Drugs: A Comprehensive Review. Cancers 2024, 16, 680. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, X.-S.; Wu, J.-F.; Yang, L.; Zheng, Y.-T.; Shen, Y.-M.; Li, Z.-Y.; Li, X. Discovery of Novel Topoisomerase II Inhibitors by Medicinal Chemistry Approaches. J. Med. Chem. 2018, 61, 8947–8980. [Google Scholar] [CrossRef]
- Shen, G.; Li, S.; Zhu, Y.; Xu, Z.; Liu, X.; Lv, C.; Xing, Z.; Cui, L.; Li, W. Recent progress in topoisomerase inhibitors as anticancer agents: Research and design strategies for Topo I and II inhibitors via structural optimization. Bioorganic Chem. 2025, 165, 109040. [Google Scholar] [CrossRef]
- Qiu, G.; Xie, J.; Li, F.; Han, K.; Long, Q.; Kowah, J.A.H.; Gao, R.; Wang, L.; Liu, X. Design, synthesis and biological evaluation of matrine contains benzimidazole derivatives as dual TOPOI and PARP inhibitors for cancer therapy. Eur. J. Med. Chem. 2024, 270, 116348. [Google Scholar] [CrossRef]
- Wang, B.; Shi, T.; Jia, S.; Wang, E.; Ruan, X.; Sheng, C.; Wu, S.; Zhou, Q. Indolo[3,2-c]isoquinoline Hydroxamic Acid Derivatives as Novel Orally Topoisomerase-Histone Deacetylase Dual Inhibitors for NSCLC Therapy. J. Med. Chem. 2025, 68, 1300–1315. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; Abd El-Hafeez, A.A.; Ebeid, K.; Mekkawy, A.I.; Abourehab, M.A.S.; Wafa, E.I.; Alhaj-Suliman, S.O.; Salem, A.K.; Ghosh, P.; Abuo-Rahma, G.E.-D.A.; et al. New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization. J. Enzym. Inhib. Med. Chem. 2022, 37, 1346–1363. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Tawfik, H.O.; Abdulla, M.-H.; Nafie, M.S.; Aref, H.; Shaldam, M.A.; Alhassan, N.S.; Al Obeed, O.; Elsayed, Z.M.; Abdel-Aziz, H.A. Identification of indole-grafted pyrazolopyrimidine and pyrazolopyridine derivatives as new anticancer agents: Synthesis, biological assessments, and molecular modeling insights. Bioorganic Chem. 2024, 153, 107804. [Google Scholar] [CrossRef]
- Barghash, R.F.; Eldehna, W.M.; Kovalová, M.; Vojáčková, V.; Kryštof, V.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel pyrazolo[3,4-b]pyridines as potent antileukemic agents. Eur. J. Med. Chem. 2022, 227, 113952. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Abdulla, M.-H.; Nafie, M.S.; Elsawi, A.E.; Ayman, S.; Shahin, M.I.; Alhassan, N.S.; Zubaidi, A.M.; Ghabbour, H.A.; Elaasser, M.; et al. Unveiling the anticancer potential of novel spirooxindole-tethered pyrazolopyridine derivatives. Bioorganic Chem. 2024, 153, 107778. [Google Scholar] [CrossRef] [PubMed]
- Syamala, M. Recent progress in three-component reactions. An update. Org. Prep. Proced. Int. 2009, 41, 1–68. [Google Scholar] [CrossRef]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Zhang, Z.; Chen, W.; Liu, Y.; Xia, R. Recent Advances in Three-Component Synthesis of Difluorinated Compounds. Org. Biomol. Chem. 2025, 62, 848–879. [Google Scholar] [CrossRef]
- Tripolitsiotis, N.P.; Thomaidi, M.; Neochoritis, C.G. The Ugi three-component reaction; a valuable tool in modern organic synthesis. Eur. J. Org. Chem. 2020, 2020, 6525–6554. [Google Scholar] [CrossRef]
- Katariya, K.D.; Shah, S.R.; Reddy, D. Anticancer, antimicrobial activities of quinoline based hydrazone analogues: Synthesis, characterization and molecular docking. Bioorganic Chem. 2020, 94, 103406. [Google Scholar] [CrossRef]
- Tawfik, H.O.; Petreni, A.; Supuran, C.T.; El-Hamamsy, M.H. Discovery of new carbonic anhydrase IX inhibitors as anticancer agents by toning the hydrophobic and hydrophilic rims of the active site to encounter the dual-tail approach. Eur. J. Med. Chem. 2022, 232, 114190. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Rashid, M.; Husain, A.; Mishra, R.; Karim, S.; Khan, S.; Ahmad, M.; Al-wabel, N.; Husain, A.; Ahmad, A.; Khan, S.A. Design and synthesis of benzimidazoles containing substituted oxadiazole, thiadiazole and triazolo-thiadiazines as a source of new anticancer agents. Arab. J. Chem. 2019, 12, 3202–3224. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Synthesis and in vitro evaluation of novel triazine analogues as anticancer agents and their interaction studies with bovine serum albumin. Eur. J. Med. Chem. 2016, 117, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, F.; Cho, Y.-Y.; Tang, F.; Zykova, T.; Ma, W.-y.; Bode, A.M.; Dong, Z. Cell apoptosis: Requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol. Cell 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Zhu, P.; Qian, J.; Xu, Z.; Meng, C.; Liu, J.; Shan, W.; Zhu, W.; Wang, Y.; Yang, Y.; Zhang, W.; et al. Piperlonguminine and Piperine Analogues as TrxR Inhibitors that Promote ROS and Autophagy and Regulate p38 and Akt/mTOR Signaling. J. Nat. Prod. 2020, 83, 3041–3049. [Google Scholar] [CrossRef]
- Elsebaie, H.A.; El-Bastawissy, E.A.; Elberembally, K.M.; Khaleel, E.F.; Badi, R.M.; Shaldam, M.A.; Eldehna, W.M.; Tawfik, H.O.; El-Moselhy, T.F. Novel 4-(2-arylidenehydrazineyl)thienopyrimidine derivatives as anticancer EGFR inhibitors: Design, synthesis, biological evaluation, kinome selectivity and in silico insights. Bioorganic Chem. 2023, 140, 106799. [Google Scholar] [CrossRef]
- Tawfik, H.O.; Mousa, M.H.A.; Zaky, M.Y.; El-Dessouki, A.M.; Sharaky, M.; Abdullah, O.; El-Hamamsy, M.H.; Al-Karmalawy, A.A. Rationale design of novel substituted 1,3,5-triazine candidates as dual IDH1(R132H)/IDH2(R140Q) inhibitors with high selectivity against acute myeloid leukemia: In vitro and in vivo preclinical investigations. Bioorganic Chem. 2024, 149, 107483. [Google Scholar] [CrossRef]
- Abo Al-Hamd, M.G.; Tawfik, H.O.; Abdullah, O.; Yamaguchi, K.; Sugiura, M.; Mehany, A.B.M.; El-Hamamsy, M.H.; El-Moselhy, T.F. Recruitment of hexahydroquinoline as anticancer scaffold targeting inhibition of wild and mutants EGFR (EGFRWT, EGFRT790M, and EGFRL858R). J. Enzym. Inhib. Med. Chem. 2023, 38, 2241674. [Google Scholar] [CrossRef]
- Aboukhatwa, S.M.; Sidhom, P.A.; Angeli, A.; Supuran, C.T.; Tawfik, H.O. Terminators or Guardians? Design, Synthesis, and Cytotoxicity Profiling of Chalcone-Sulfonamide Hybrids. ACS Omega 2023, 8, 7666–7683. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.O.; Shaldam, M.A.; Nocentini, A.; Salem, R.; Almahli, H.; Al-Rashood, S.T.; Supuran, C.T.; Eldehna, W.M. Novel 3-(6-methylpyridin-2-yl)coumarin-based chalcones as selective inhibitors of cancer-related carbonic anhydrases IX and XII endowed with antiproliferative activity. J. Enzym. Inhib. Med. Chem. 2022, 37, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Salem, R.; Elsayed, Z.M.; Al-Warhi, T.; Knany, H.R.; Ayyad, R.R.; Traiki, T.B.; Abdulla, M.-H.; Ahmad, R.; Abdel-Aziz, H.A.; et al. Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with apoptosis inducing mechanism in Colon cancer. J. Enzym. Inhib. Med. Chem. 2021, 36, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Bonardi, A.; Avgenikos, M.; Baselious, F.; Schmidt, M.; Al-Warhi, T.; Abdel-Aziz, H.A.; Rennert, R.; Peat, T.S.; et al. 4-(Pyrazolyl)benzenesulfonamide Ureas as Carbonic Anhydrases Inhibitors and Hypoxia-Mediated Chemo-Sensitizing Agents in Colorectal Cancer Cells. J. Med. Chem. 2024, 67, 20438–20454. [Google Scholar] [CrossRef]
- Elsawi, A.E.; Elbadawi, M.M.; Nocentini, A.; Almahli, H.; Giovannuzzi, S.; Shaldam, M.; Salem, R.; Ibrahim, T.M.; Abdel-Aziz, H.A.; Supuran, C.T.; et al. 1,5-Diaryl-1,2,4-triazole Ureas as New SLC-0111 Analogues Endowed with Dual Carbonic Anhydrase and VEGFR-2 Inhibitory Activities. J. Med. Chem. 2023, 66, 10558–10578. [Google Scholar] [CrossRef]
- Elbadawi, M.M.; Eldehna, W.M.; Wang, W.; Agama, K.K.; Pommier, Y.; Abe, M. Discovery of 4-alkoxy-2-aryl-6,7-dimethoxyquinolines as a new class of topoisomerase I inhibitors endowed with potent in vitro anticancer activity. Eur. J. Med. Chem. 2021, 215, 113261. [Google Scholar] [CrossRef]
- Wu, C.-C.; Li, T.-K.; Farh, L.; Lin, L.-Y.; Lin, T.-S.; Yu, Y.-J.; Yen, T.-J.; Chiang, C.-W.; Chan, N.-L. Structural Basis of Type II Topoisomerase Inhibition by the Anticancer Drug Etoposide. Science 2011, 333, 459–462. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Mohamed, R.; Elnagar, R.M.; Giovannuzzi, S.; Tayel, A.; Abdulla, M.-H.; Alhassan, N.S.; Shaldam, M.A.; Nocentini, A.; Supuran, C.T.; et al. Identification of isatin-triazole-benzenesulfonamide hybrids as dual hCA IX/XII and c-met inhibitors with hypoxia-mediated chemo-sensitizing activity. Bioorganic Chem. 2025, 166, 109071. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abo-Kamar, A.M.; Abdelaziz, A.A.; Ashour, A.E.; Shaldam, M.A.; Elekhnawy, E. Employing diclofenac sodium as a novel therapeutic frontier for Staphylococcus epidermidis infections. Sci. Rep. 2025, 15, 31377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).