Thymoquinone Overcomes Hypoxia-Induced Carboplatin Resistance Through ROS-Independent Apoptosis but Promotes Cancer Stem Cell Enrichment: Implications on Oral Cancer Adaptation and Recurrence
Abstract
1. Introduction
2. Results
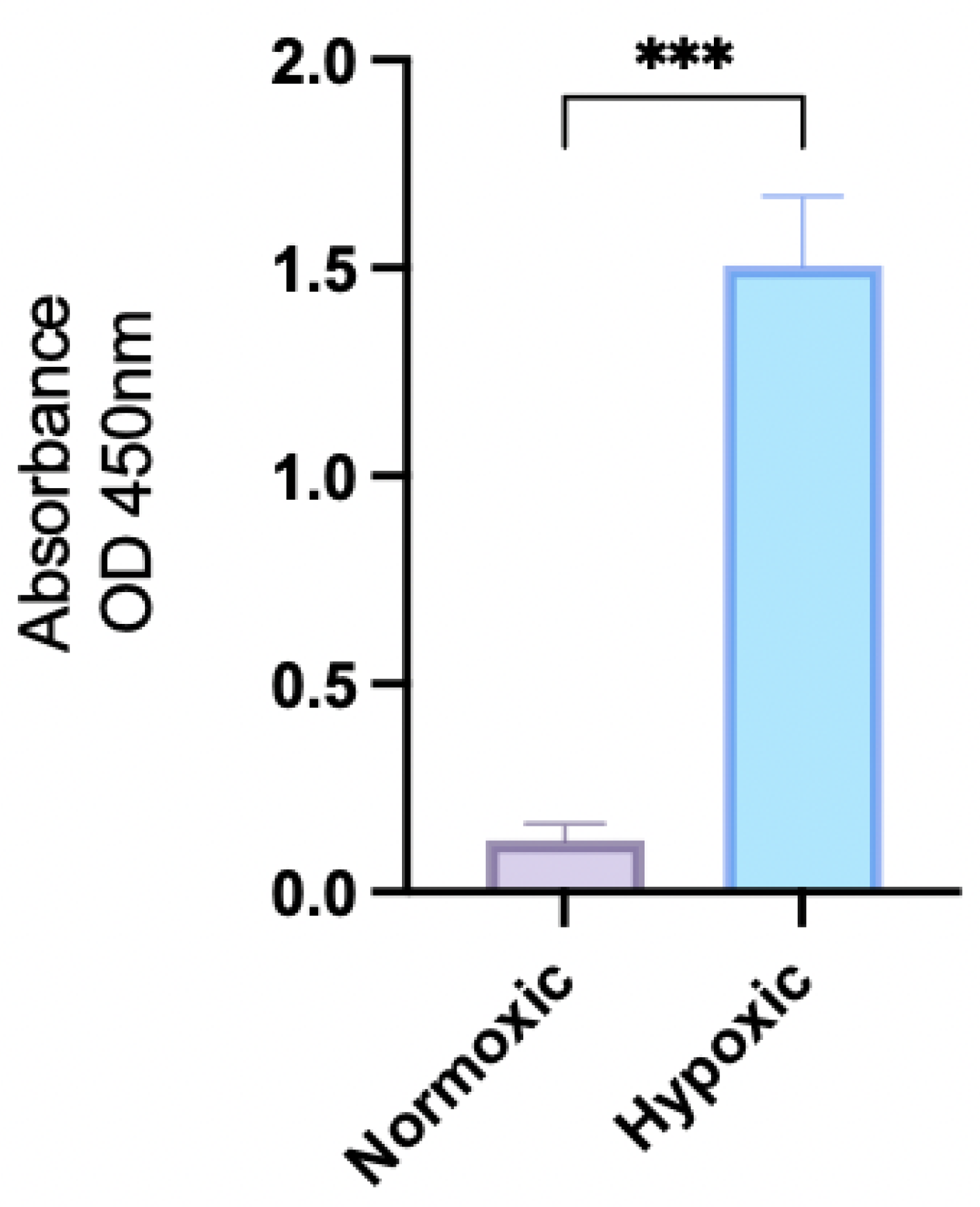
2.1. HIF-1a Expression
2.2. MTT Cytotoxicity Results
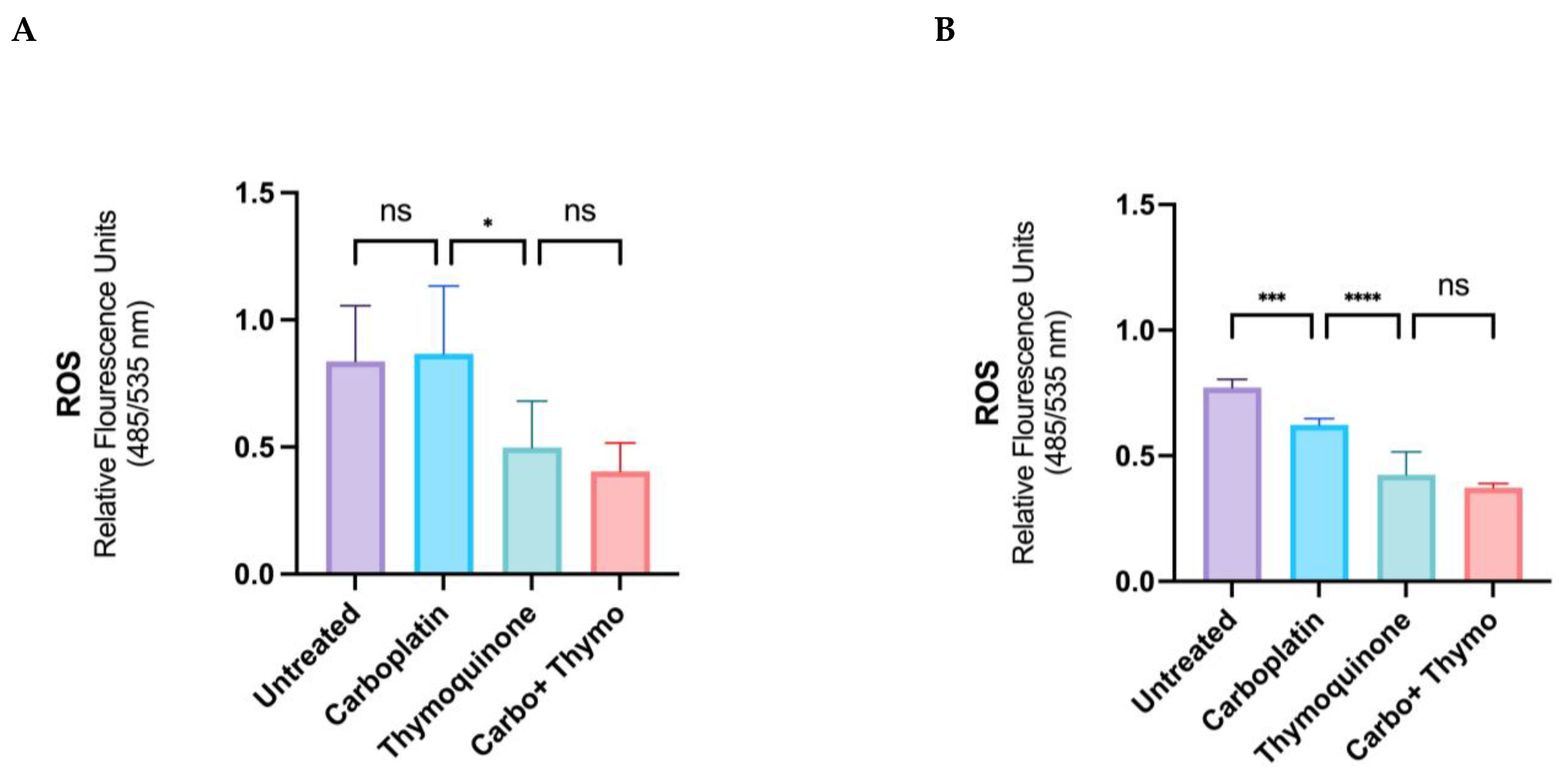
2.3. ROS
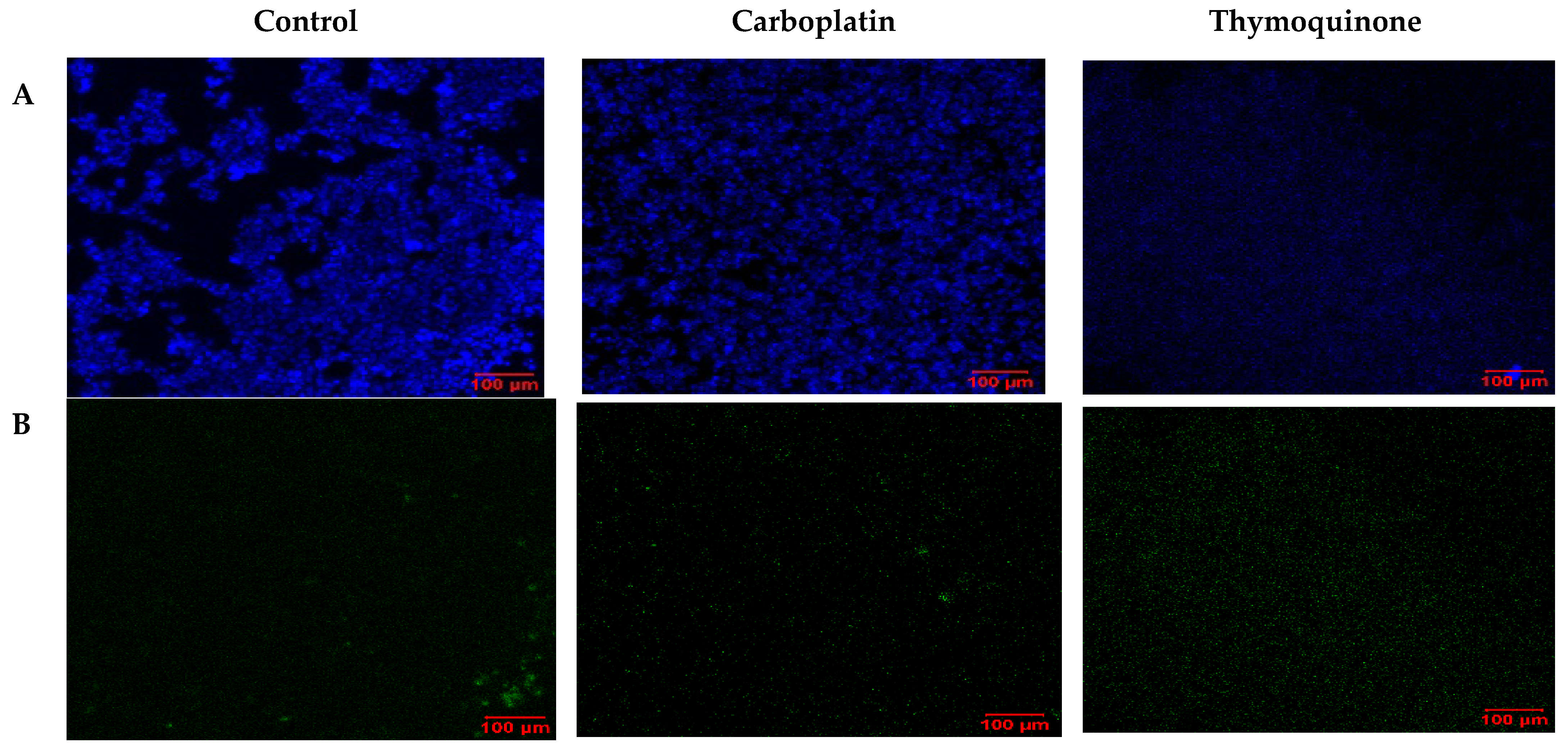
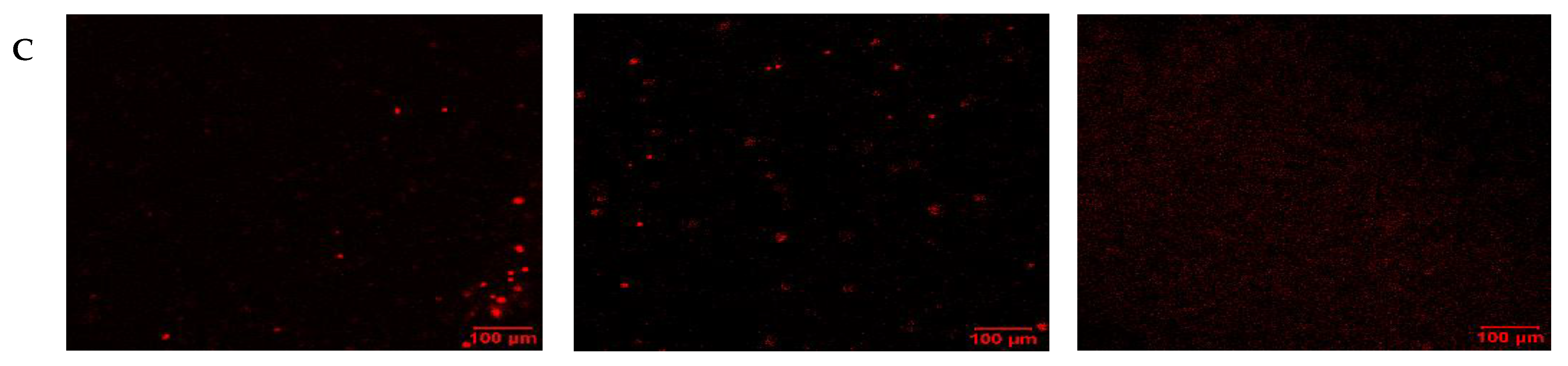
2.4. Cell Death and Repair Mechanisms
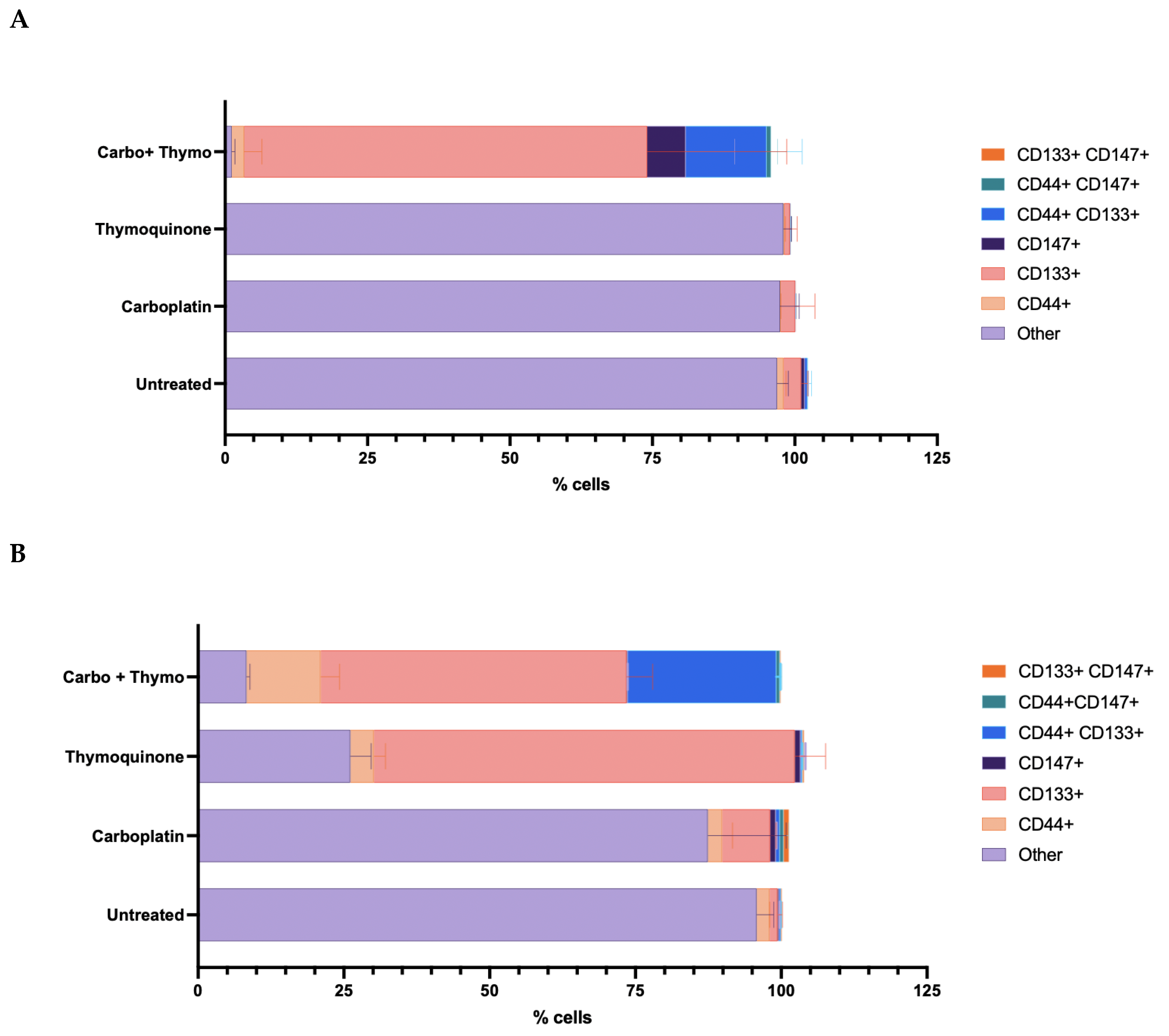
2.5. Phenotyping with Stem Cell Surface Marker Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment with Test and Control Drugs
4.2. Cell Lysis and Protein Determination
4.3. HIF-1a ELISA
4.4. MTT Cytotoxicity Assay
4.5. Oxidative Stress and Reactive Oxygen Species
4.6. Apoptosis
4.7. Confocal Imaging LSM
4.8. Autophagy
4.9. Stem Cell Surface Marker Detection
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Elaiwy, O.; El Ansari, W.; AlKhalil, M.; Ammar, A. Epidemiology and Pathology of Oral Squamous Cell Carcinoma in a Multi-Ethnic Population: Retrospective Study of 154 Cases over 7 Years in Qatar. Ann. Med. Surg. 2020, 60, 195–200. [Google Scholar] [CrossRef]
- Rahman, I.; Athar, M.T.; Islam, M. Type 2 Diabetes, Obesity, and Cancer Share Some Common and Critical Pathways. Front. Oncol. 2021, 10, 2995. [Google Scholar] [CrossRef]
- Motz, K.M.; Tan, M. Patient Populations at Increased Risk for Oral Cavity Cancer. In Oral Cancer: Evaluation, Therapy, and Rehabilitation; Fakhry, C., Pitman, K.T., Kiess, A.P., Eds.; Thieme Verlag: New York, NY, USA, 2020; p. 14. ISBN 9781626239685. [Google Scholar]
- Basha, S.; Mohamed, R.; Al-Thomali, Y.; Shamrani, A. The Prevalence of Oral Cancer in Saudi Arabia â A Systematic Review. Ann. Med. Health Sci. Res. 2019, 9, 553–557. [Google Scholar]
- Farooq, I.; Bugshan, A. Oral Squamous Cell Carcinoma: Metastasis, Potentially Associated Malignant Disorders, Etiology and Recent Advancements in Diagnosis. F1000Research 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Panarese, I.; Aquino, G.; Ronchi, A.; Longo, F.; Montella, M.; Cozzolino, I.; Roccuzzo, G.; Colella, G.; Caraglia, M.; Franco, R. Oral and Oropharyngeal Squamous Cell Carcinoma: Prognostic and Predictive Parameters in the Etiopathogenetic Route. Expert. Rev. Anticancer. Ther. 2019, 19, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Young, C.K.; Chien, H.T.; Chin, S.C.; Landelli, A.; Liao, C.T.; Tsao, C.K.; Kang, C.J.; Huang, S.F. Characteristics and Outcome Differences in Male and Female Oral Cavity Cancer Patients in Taiwan. Medicine 2021, 100, e27674. [Google Scholar] [CrossRef]
- Alharbi, F. Incidence of Head and Neck Cancers in Jazan Province, Saudi Arabia. Saudi J. Otorhinolaryngol. Head. Neck Surg. 2017, 19, 47. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global Epidemiology of Oral and Oropharyngeal Cancer. Oral. Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, S.; Gao, L.; Zhi, K.; Ren, W. The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 761379. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Aminuddin, A.; Ng, P.Y.; Leong, C.O.; Chua, E.W. Mitochondrial DNA Alterations May Influence the Cisplatin Responsiveness of Oral Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 7885. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Paderno, A.; Bossi, P.; Piazza, C. Editorial: Advances in the Multidisciplinary Management of Oral Cancer. Front. Oncol. 2021, 11, 817756. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin Based Therapy: The Role of the Mitogen Activated Protein Kinase Signaling Pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.L.; Zulli, A. Cisplatin for Cancer Therapy and Overcoming Chemoresistance. Heliyon 2022, 8, e10608. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Gillies, R.J. The Role of Carbonic Anhydrase IX in Cancer Development: Links to Hypoxia, Acidosis, and Beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and Cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83. [Google Scholar] [CrossRef]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains. Mol. Cell Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell. Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and Beneficial Role of ROS. Oxid. Med. Cell. Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Saito, S.; Lin, Y.C.; Tsai, M.H.; Lin, C.S.; Murayama, Y.; Sato, R.; Yokoyama, K.K. Emerging Roles of Hypoxia-Inducible Factors and Reactive Oxygen Species in Cancer and Pluripotent Stem Cells. Kaohsiung J. Med. Sci. 2015, 31, 279–286. [Google Scholar] [CrossRef]
- Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.; Aljammaz, N.A.; Huwaizi, S.U.; Suliman, R.; Kahtani, K.M.; Albadrani, G.M.; Barhoumi, T.; et al. The Role of Myrrh Metabolites in Cancer, Inflammation, and Wound Healing: Prospects for a Multi-Targeted Drug Therapy. Pharmaceuticals 2022, 15, 944. [Google Scholar] [CrossRef]
- Sinha, N.; Mukhopadhyay, S.; Das, D.N.; Panda, P.K.; Bhutia, S.K. Relevance of Cancer Initiating/Stem Cells in Carcinogenesis and Therapy Resistance in Oral Cancer. Oral. Oncol. 2013, 49, 854–862. [Google Scholar] [CrossRef]
- Wang, S.S.; Jiang, J.; Liang, X.H.; Tang, Y.L. Links between Cancer Stem Cells and Epithelial–Mesenchymal Transition. OncoTargets Ther. 2015, 8, 2973. [Google Scholar] [CrossRef]
- Patel, S.; Shah, K.; Mirza, S.; Daga, A.; Rawal, R. Epigenetic Regulators Governing Cancer Stem Cells and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. Curr. Stem Cell Res. Ther. 2015, 10, 140–152. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Lu, R.; Zhang, J.; Chen, X.; Zhou, G. CD47 as a Potential Prognostic Marker for Oral Leukoplakia and Oral Squamous Cell Carcinoma. Oncol. Lett. 2018, 15, 9075–9080. [Google Scholar] [CrossRef]
- Adnan, Y.; Ali, S.M.A.; Farooqui, H.A.; Kayani, H.A.; Idrees, R.; Awan, M.S. High CD44 Immunoexpression Correlates with Poor Overall Survival: Assessing the Role of Cancer Stem Cell Markers in Oral Squamous Cell Carcinoma Patients from the High-Risk Population of Pakistan. Int. J. Surg. Oncol. 2022, 2022, 9990489. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, S.N.; Choudhari, S.K.; Patankar, S.; Ghule, S.S.; Jadhav, Y.B.; Masne, S. Cancer Stem Cell Markers, CD44 and ALDH1, for Assessment of Cancer Risk in OPMDs and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Head. Neck Pathol. 2022, 16, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Raju, K.L.; Augustine, D.; Patil, S. Prognostic Significance of ALDH1, Bmi1, and OCT4 Expression in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Cancer Control 2020, 27, 1073274820904959. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, H.; Esmail, A.D.; Zahani, A.M.N.; Elmahdi, A.H.; Ibrahiem, A. Clinicopathological Correlation of Stem Cell Markers Expression in Oral Squamous Cell Carcinoma; Relation to Patients` Outcome. J. Immunoass. Immunochem. 2021, 42, 571–595. [Google Scholar] [CrossRef]
- Yu, C.C.; Hu, F.W.; Yu, C.H.; Chou, M.Y. Targeting CD133 in the Enhancement of Chemosensitivity in Oral Squamous Cell Carcinoma-Derived Side Population Cancer Stem Cells. Head. Neck 2016, 38 (Suppl. S1), E231–E238. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231. [Google Scholar] [CrossRef]
- Krause, W. Resistance to Anti-Tubulin Agents: From Vinca Alkaloids to Epothilones. Cancer Drug Resist. 2019, 2, 82–106. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Cas, M.D.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Sharrif Moghaddasi, M. Nigella Sativa Treditional Usages (Black Seed). Adv. Environ. Biol. 2011, 5, 5–16. [Google Scholar]
- Al-Jassir, M.S. Chemical Composition and Microflora of Black Cumin (Nigella Sativa L.) Seeds Growing in Saudi Arabia. Food Chem. 1992, 45, 239–242. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An Emerging Natural Drug with a Wide Range of Medical Applications. Iran. J. Basic. Med. Sci. 2014, 17, 950. [Google Scholar]
- Rahman, I.; Mohammed, A.; AlSheddi, M.A.; Algazlan, A.; Alwably, A.; Hebbal, M.; Omar, M.G. Nigella Sativa Oil as a Treatment for Gingivitis: A Randomized Active-Control Trial. Asian Pac. J. Trop. 2023, 17, 138. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone Antioxidant/pro-Oxidant Effect as Potential Anticancer Remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive Oxygen Species Mediate Thymoquinone-Induced Apoptosis and Activate ERK and JNK Signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, M.E.; Khowailed, A.A.; Aboulhoda, B.E.; Rashed, L.A.; Gaber, S.S.; Ashour, H. Thymoquinone Potentiated the Anticancer Effect of Cisplatin on Hepatic Tumorigenesis by Modulating Tissue Oxidative Stress and Endoplasmic GRP78/CHOP Signaling. Nutr. Cancer 2022, 74, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral Squamous Cell Carcinomas: State of the Field and Emerging Directions. Int. J. Oral. Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; von Delwig, A.A. Oxygen Therapy in Traditional and Immunotherapeutic Treatment Protocols of Cancer Patients: Current Reality and Future Prospects. Expert. Rev. Anticancer. Ther. 2022, 22, 575–581. [Google Scholar] [CrossRef]
- Khorshidi, S.; Younesi, S.; Karkhaneh, A. Peroxide Mediated Oxygen Delivery in Cancer Therapy. Colloids Surf. B Biointerfaces 2022, 219, 112832. [Google Scholar] [CrossRef]
- El-Far, A.H.; Tantawy, M.A.; Al Jaouni, S.K.; Mousa, S.A. Thymoquinone-Chemotherapeutic Combinations: New Regimen to Combat Cancer and Cancer Stem Cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1581–1598. [Google Scholar] [CrossRef]
- Liou, Y.F.; Chen, P.N.; Chu, S.C.; Kao, S.H.; Chang, Y.Z.; Hsieh, Y.S.; Chang, H.R. Thymoquinone Suppresses the Proliferation of Renal Cell Carcinoma Cells via Reactive Oxygen Species-Induced Apoptosis and Reduces Cell Stemness. Environ. Toxicol. 2019, 34, 1208–1220. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Ge, X.; Huang, L.; Pan, P.Y.; Shen, H.; Pettigrew, R.I.; Chen, S.H.; Mai, J. Novel Platinum Therapeutics Induce Rapid Cancer Cell Death through Triggering Intracellular ROS Storm. Biomaterials 2025, 314, 122835. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Dong, L.; Luo, L.; Wang, K. Redox Regulation of Autophagy in Cancer: Mechanism, Prevention and Therapy. Life 2022, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.; Sleem, R.; Abd-Elaziz, A.; Khalil, H. Regulation of NF-ΚB Expression by Thymoquinone; A Role in Regulating Pro-Inflammatory Cytokines and Programmed Cell Death in Hepatic Cancer Cells. Asian Pac. J. Cancer Prev. 2023, 24, 3739. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; S, S.; Martin, T.M.; Kumar, M.S.K. Combined Therapeutic Potential of Thymoquinone and Theaflavin Through P13k Pathway Suppression. Int. J. Environ. Sci. 2025, 1201–1210. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Alghamdi, S.S.; Shami, A.; Suliman, R.S.; Aabed, K.; Alotaibi, M.O.; Rahman, I. In Silico Prediction of Malvaviscus Arboreus Metabolites and Green Synthesis of Silver Nanoparticles—Opportunities for Safer Anti-Bacterial and Anti-Cancer Precision Medicine. Int. J. Nanomedicine 2023, 18, 2141–2162. [Google Scholar] [CrossRef]
- Sakagami, H. Apoptosis-Inducing Activity and Tumor-Specificity of Antitumor Agents against Oral Squamous Cell Carcinoma. Jpn. Dent. Sci. Rev. 2010, 46, 173–187. [Google Scholar] [CrossRef]
- Gonzalez, V.M.; Fuertes, M.A.; Alonso, C.; Perez, J.M. Is Cisplatin-Induced Cell Death Always Produced by Apoptosis? Mol. Pharmacol. 2001, 59, 657–663. [Google Scholar] [CrossRef]
- Liu, K.; Huang, J.; Liu, J.; Klionsky, D.J.; Kang, R.; Tang, D. Induction of Autophagy-Dependent Ferroptosis to Eliminate Drug-Tolerant Human Retinoblastoma Cells. Cell Death Disease 2022 13:6 2022, 13, 521. [Google Scholar] [CrossRef]
- Parkinson, E.I.; Hergenrother, P.J. Runaway ROS as a Selective Anticancer Strategy. ChemMedChem 2011, 6, 1957–1959. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Alghashem, S.A.; Ali, R.; Alsubait, A.; Suliman, R.S.; Mohammed, A.E.; Alehaideb, Z.; Alshafi, R.A.; Alturki, A.Y.; Rahman, I. Exploring the Potential of Ziziphus Nummularia and Luteolin-7-O-Glucoside as Tubulin Inhibitors in Cancer Therapy and Survival. Sci. Rep. 2024, 14, 7202. [Google Scholar] [CrossRef] [PubMed]
- Gremke, N.; Polo, P.; Dort, A.; Schneikert, J.; Elmshäuser, S.; Brehm, C.; Klingmüller, U.; Schmitt, A.; Reinhardt, H.C.; Timofeev, O.; et al. MTOR-Mediated Cancer Drug Resistance Suppresses Autophagy and Generates a Druggable Metabolic Vulnerability. Nat. Commun. 2020, 11, 4684. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Song, Y.; Kim, S.H.; Kim, J.; Seo, H.R. Potential Mechanisms of CD133 in Cancer Stem Cells. Life Sci. 2017, 184, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Jyosthsna, K.; Rani, G.; Purnachandra Nagaraju, G. CD44/PD-L1-Mediated Networks in Drug Resistance and Immune Evasion of Breast Cancer Stem Cells: Promising Targets of Natural Compounds. Int. Immunopharmacol. 2024, 138, 112613. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Wang, X.; Zhao, C.; Wang, H. CD44, a Marker of Cancer Stem Cells, Is Positively Correlated with PD-L1 Expression and Immune Cells Infiltration in Lung Adenocarcinoma. Cancer Cell Int. 2020, 20, 583. [Google Scholar] [CrossRef]
- Sipos, F.; Műzes, G. Interconnection of CD133 Stem Cell Marker with Autophagy and Apoptosis in Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 11201. [Google Scholar] [CrossRef]
- Mohammed, E.A.; Aabed, K.; Benabdelkamel, H.; Shami, A.; Alotaibi, M.O.; Alanazi, M.; Alfadda, A.A.; Rahman, I. Proteomic Profiling Reveals Cytotoxic Mechanisms of Action and Adaptive Mechanisms of Resistance in Porphyromonas Gingivalis: Treatment with Juglans Regia and Melaleuca Alternifolia. ACS Omega 2023, 8, 12980–12991. [Google Scholar] [CrossRef]
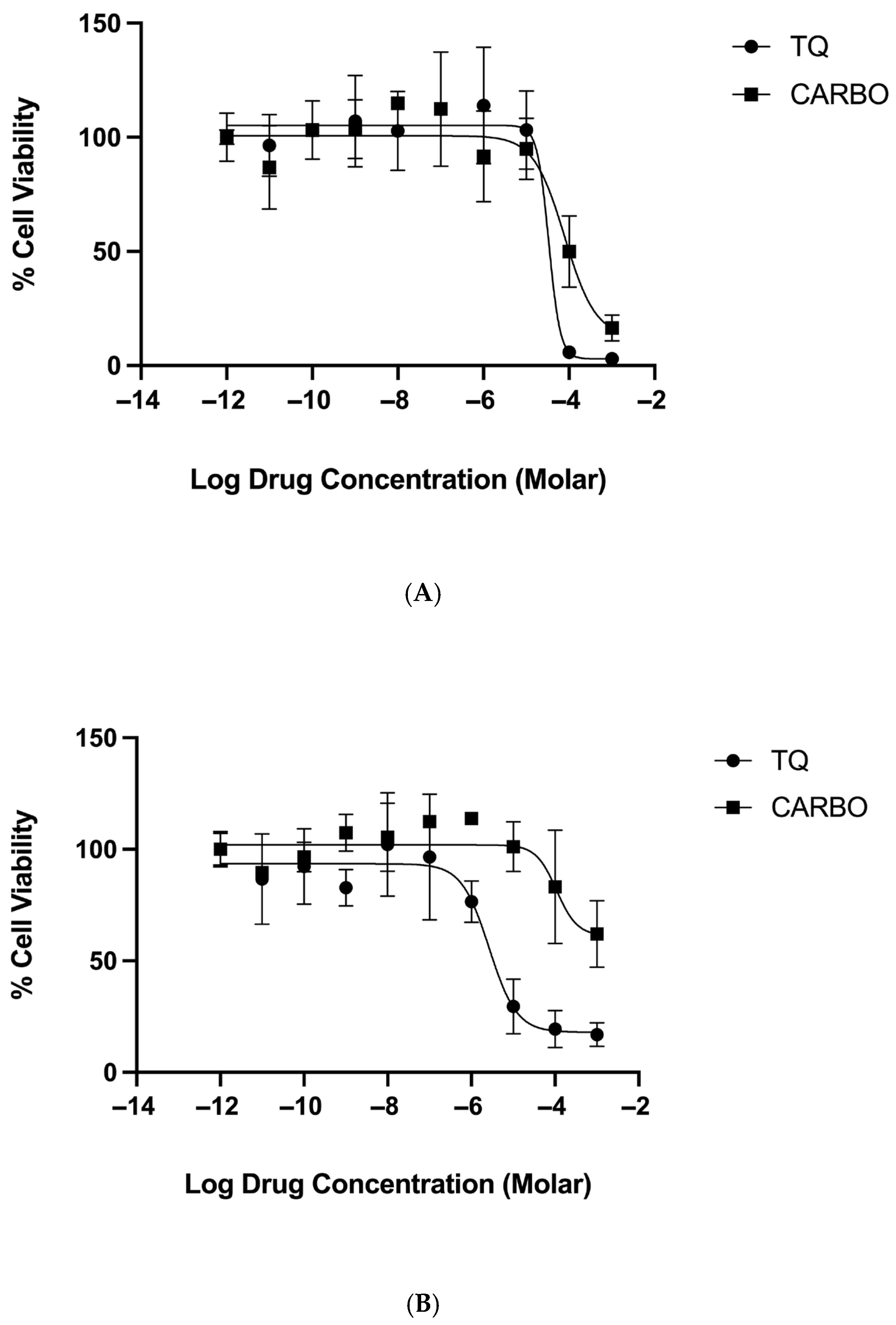





| IC50 (µM) ± SD | Percentage Span | |||
|---|---|---|---|---|
| Normoxic | Hypoxic | Normoxic | Hypoxic | |
| Thymoquinone | 27.9 ± 9.4 | 12.0 ± 14 | 99.9 ± 6.1 | 79.5 ± 5.7 |
| Carboplatin | 81.9 ± 5.1 | 110 ± 15 | 88.0 ± 13 | 39.7 ± 1.4 |
| Thymoquinone + Carboplatin | 49.1 ± 33 | 24 ± 12 | 94.0 ± 9.5 | 91.4 ± 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, I.; Henidi, H.; Alkahtani, M.M.; Makhlafi, Z.A.; ElRefai, S.; AlSheddi, M.A.; Ali, R.; Albassam, S.K.; Alharbi, H.S.; Omar, M.G.; et al. Thymoquinone Overcomes Hypoxia-Induced Carboplatin Resistance Through ROS-Independent Apoptosis but Promotes Cancer Stem Cell Enrichment: Implications on Oral Cancer Adaptation and Recurrence. Pharmaceuticals 2025, 18, 1758. https://doi.org/10.3390/ph18111758
Rahman I, Henidi H, Alkahtani MM, Makhlafi ZA, ElRefai S, AlSheddi MA, Ali R, Albassam SK, Alharbi HS, Omar MG, et al. Thymoquinone Overcomes Hypoxia-Induced Carboplatin Resistance Through ROS-Independent Apoptosis but Promotes Cancer Stem Cell Enrichment: Implications on Oral Cancer Adaptation and Recurrence. Pharmaceuticals. 2025; 18(11):1758. https://doi.org/10.3390/ph18111758
Chicago/Turabian StyleRahman, Ishrat, Hanan Henidi, Manal M. Alkahtani, Zaha Al Makhlafi, Sahar ElRefai, Manal A. AlSheddi, Rizwan Ali, Sara K. Albassam, Hazar S. Alharbi, Maha G. Omar, and et al. 2025. "Thymoquinone Overcomes Hypoxia-Induced Carboplatin Resistance Through ROS-Independent Apoptosis but Promotes Cancer Stem Cell Enrichment: Implications on Oral Cancer Adaptation and Recurrence" Pharmaceuticals 18, no. 11: 1758. https://doi.org/10.3390/ph18111758
APA StyleRahman, I., Henidi, H., Alkahtani, M. M., Makhlafi, Z. A., ElRefai, S., AlSheddi, M. A., Ali, R., Albassam, S. K., Alharbi, H. S., Omar, M. G., Salem, H. M., Alturki, A., Alnofaie, H., Alharbi, A., Aloraini, N., Alswied, R., Almutairi, S., Alshahrani, J., Alsuwaidan, R. F., ... Altwaim, S. A. (2025). Thymoquinone Overcomes Hypoxia-Induced Carboplatin Resistance Through ROS-Independent Apoptosis but Promotes Cancer Stem Cell Enrichment: Implications on Oral Cancer Adaptation and Recurrence. Pharmaceuticals, 18(11), 1758. https://doi.org/10.3390/ph18111758






