DHDK, a Plant-Derived Natural Small Molecule, Protects Against Doxorubicin-Induced Cardiotoxicity via the PPARG-CPT1B-FAO Axis
Abstract
1. Introduction
2. Results
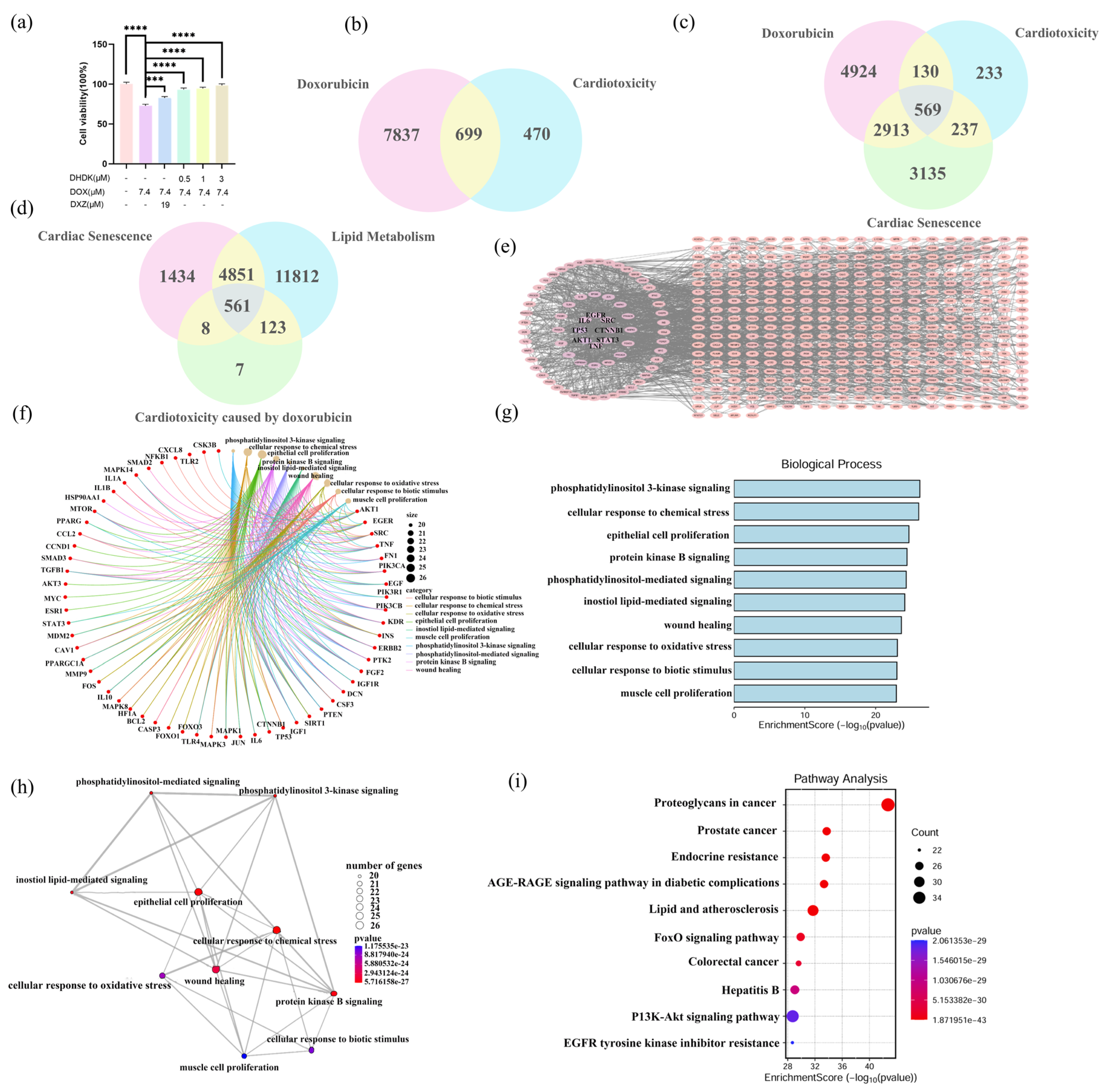
2.1. DHDK Attenuates DOX-Induced Cardiotoxicity In Vitro
2.2. Identification of DOX Exposure Induced Acute and Chronic (Lipotoxicity, Cardiac Senescence) Cardiotoxicity Targets with Functional Enrichment Analysis
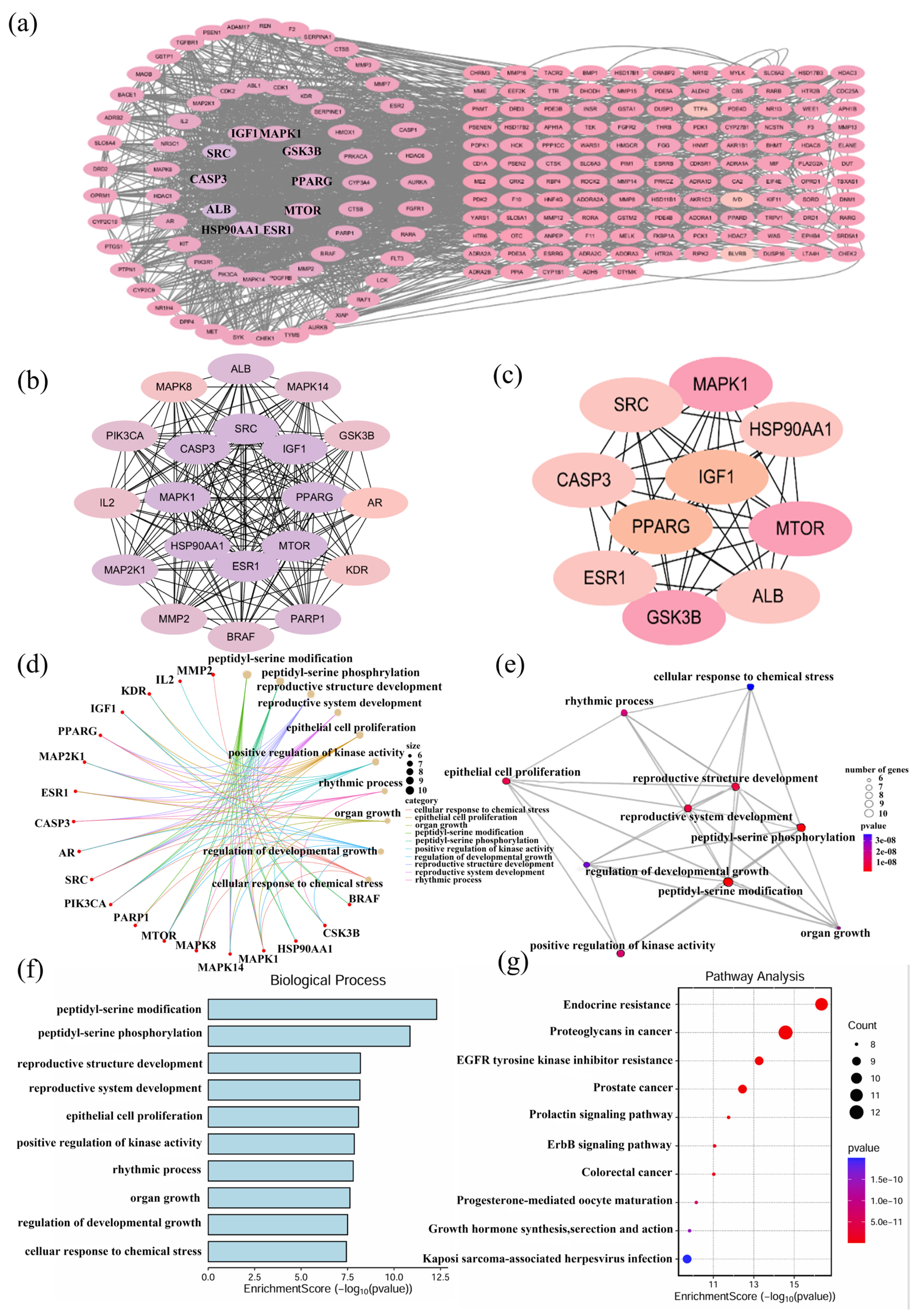
2.3. Identification of Potential Targets and Functional Enrichment Analysis of DHDK
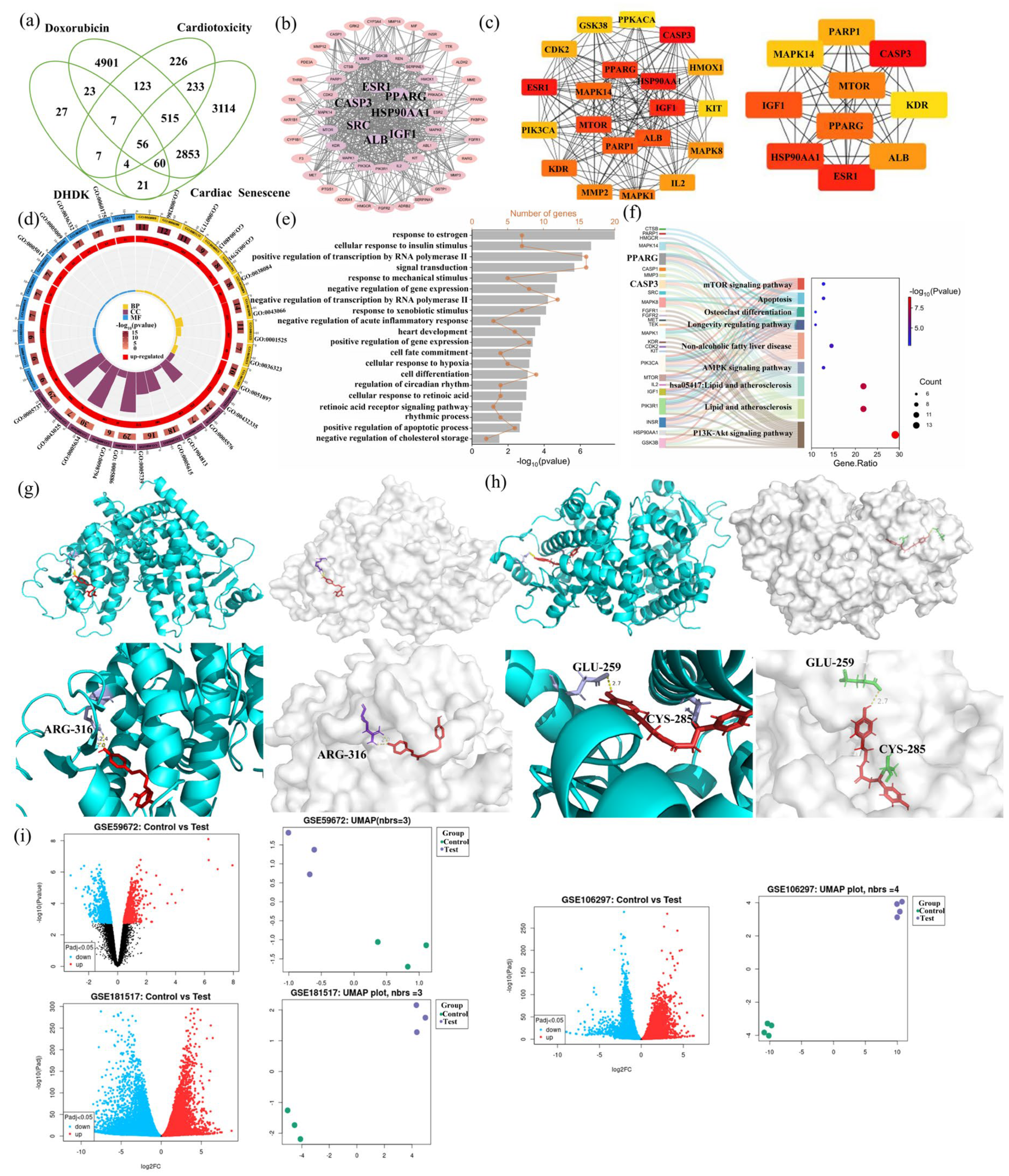
2.4. Hub Gene Identification and Enrichment Analysis of DOX Cardiotoxicity and DHDK-Mediated Cardio Protection
2.5. Molecular Docking Validation
2.6. The GEO Database Verified That the mRNA Expression Level of PPARG Was Significantly Affected by Doxorubicin
2.7. Lipidomic Alterations and Pathway Enrichment Analysis
2.8. Combined Analysis of Lipodomics with Hub Genes
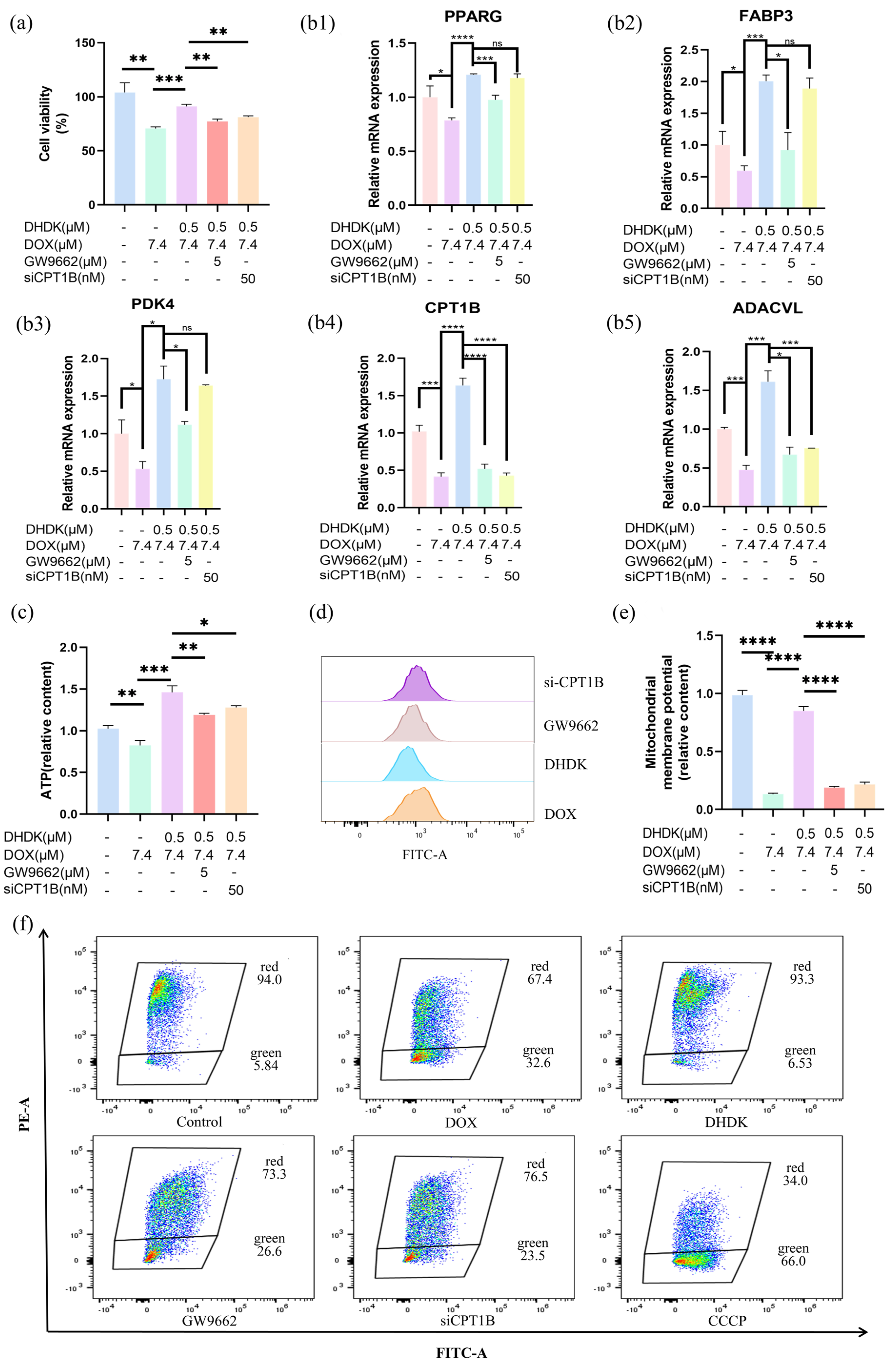
2.9. In Vitro Mechanism Validation of DHDK-Mediated Cardioprotection
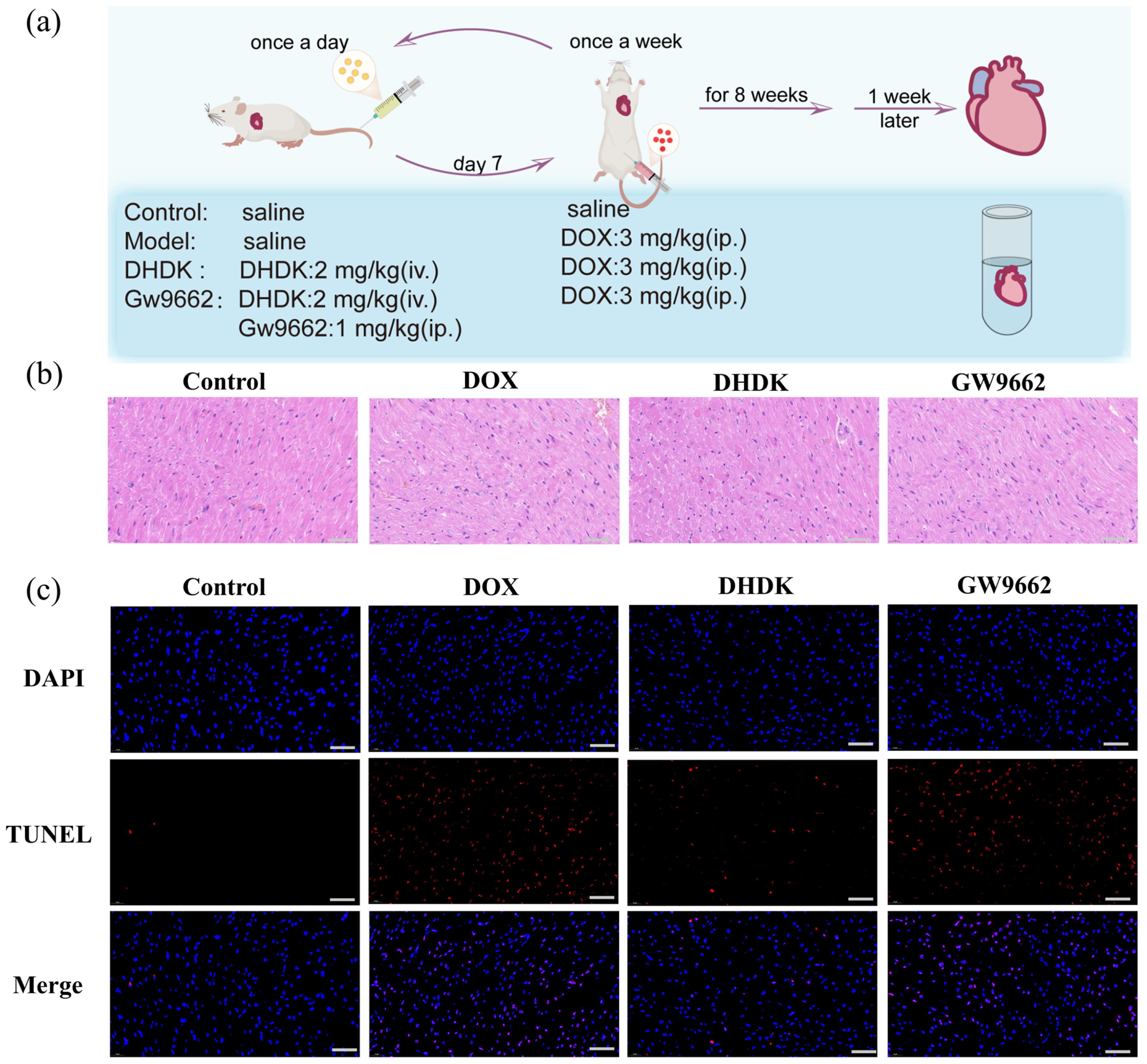
2.10. In Vivo Validation of DHDK-Mediated Cardioprotection via PPARG
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Availability
4.3. Data Collection and Target Extraction Involved Lipotoxicity, Cardiac Senescence and Cardiotoxicity of DOX
4.4. Network Construction and Core Target Identification Involved Lipotoxicity, Cardiac Senescence and Cardiotoxicity of DOX
4.5. DHDK Targets Prediction and Intersection with Cardiac Senescence and Cardiotoxicity
4.6. Network Construction and Hub Target Identification of the Protective Effect of DHDK on Doxorubicin-Induced Lipotoxicity, Cardiac Senescence and Cardiotoxicity
4.7. Targets Validation via Molecular Docking
4.8. Validate the Core Target from the GEO Database
4.9. Lipodomics
4.10. Combined Analysis of Lipodomics and Network Toxicology and Pharmacology
4.11. q-PCR
4.12. ROS Determination
4.13. Mitochondrial Membrane Potential Determination
4.14. ATP Assay
4.15. Animal Administration
4.16. HE and TUNEL Staining of Heart Tissues
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOX | Doxorubicin |
| DHDK | (1E, 4E)-1, 7-Bis(4-hydroxyphenyl) hepta-1, 4-dien-3-one |
| PPI | Protein–Protein Interaction |
| PPARG | Peroxisome Proliferator Activated Receptor Gamma |
| CPT1B | Carnitine Palmitoyltransferase 1B |
| DXZ | Dexrazoxane |
| GEO | Gene Expression Omnibus |
| GW9662 | PPARγ antagonist |
| VIP | Variable Importance in Projection |
| STRING | Search Tool for Recurring Instances of Neighbouring Genes |
| ATP | Adenosine triphosphate |
| FAO | Fatty Acid β-oxidation |
| AKT1 | AKT serine/threonine kinase 1 |
| MAPK3 | Mitogen-Activated Protein Kinase 3 |
| SIRT1 | Sirtuin1 |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein Kinase B |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| ESR1 | Estrogen receptor |
| IGF1 | Insulin-like growth factor 1 |
| MAPK1 | Mitogen-activated protein kinase 1 |
| HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| AMPK | Adenosine 5’-monophosphate (AMP)-activated protein kinase |
| HIF-1 | Hypoxia inducible factor-1 |
| ALB | Albumin |
| CASP3 | Caspase-3 |
| DG | Diacylglycerol |
| LPC | Lysophosphatidylcholine |
| CL | Cardiolipin |
| Cer | Ceramide |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| FABP3 | Fatty Acid Binding Protein 3 |
| PDK4 | Pyruvate Dehydrogenase Kinase 4 |
| ACADVL | Acyl-CoA Dehydrogenase Very Long Chain |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| PGC1α | Peroxisome proliferator-activated receptor-γcoactivator-1α |
| DMSO | Dimethyl Sulfoxide |
| OD | Optical Density |
| DCFH-DA | 2’,7’-Dichlorodihydrofluorescein diacetate |
References
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Gurova, K.; Sarthy, J.; Szabó, G.; Henikoff, S. Chromatin as an old and new anticancer target. Trend Cancer 2024, 10, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Damiani, R.M.; Moura, D.J.; Viau, C.M.; Caceres, R.A.; Henriques, J.A.P.; Saffi, J. Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch. Toxicol. 2016, 90, 2063–2076. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Bostany, G.; Chen, Y.; Francisco, L.; Dai, C.; Meng, Q.; Sparks, J.; Sessions, M.; Nabell, L.; Stringer-Reasor, E.; Khoury, K.; et al. Cardiac Dysfunction Among Breast Cancer Survivors: Role of Cardiotoxic Therapy and Cardiovascular Risk Factors. J. Clin. Oncol. 2025, 43, 32–45. [Google Scholar] [CrossRef]
- Vo, J.B.; Ramin, C.; Veiga, L.H.S.; Brandt, C.; Curtis, R.E.; Bodelon, C.; Barac, A.; Roger, V.L.; Feigelson, H.S.; Buist, D.S.M.; et al. Long-term cardiovascular disease risk after anthracycline and trastuzumab treatments in US breast cancer survivors. J. Natl. Cancer Inst. 2024, 116, 1384–1394. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, S.; Wang, H.; Zhang, P.; Liu, C.; Zhu, C.; Yang, C. Neoadjuvant chemotherapy and perioperative cardiotoxicity. J. Anesth. Transl. Med. 2024, 3, 171–180. [Google Scholar] [CrossRef]
- Palmieri, V.; Vietri, M.T.; Montalto, A.; Montisci, A.; Donatelli, F.; Coscioni, E.; Napoli, C. Cardiotoxicity, Cardioprotection, and Prognosis in Survivors of Anticancer Treatment Undergoing Cardiac Surgery: Unmet Needs. Cancers 2023, 15, 2224. [Google Scholar] [CrossRef]
- Rahimi, P.; Barootkoob, B.; ElHashash, A.; Nair, A. Efficacy of Dexrazoxane in Cardiac Protection in Pediatric Patients Treated with Anthracyclines. Cureus 2023, 15, e37308. [Google Scholar] [CrossRef]
- Langer, S.W. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag. Res. 2014, 6, 357–363. [Google Scholar] [CrossRef]
- Lee, S.R.; Mukae, M.; Jeong, K.J.; Park, S.H.; Shin, H.J.; Kim, S.W.; Won, Y.S.; Kwun, H.J.; Baek, I.J.; Hong, E.J. PGRMC1 Ablation Protects from Energy-Starved Heart Failure by Promoting Fatty Acid/Pyruvate Oxidation. Cells 2023, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, A.; Demaison, L. Fatty acid oxidation in the heart. J. Cardiovasc. Pharmacol. 1996, 28 (Suppl. 1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.P.; Ribeiro, A.P.D.; Monte, M.G.; Fujimori, A.S.S.; Tonon, C.R.; Ferreira, N.F.; Zanatti, S.G.; Minicucci, M.F.; Zornoff, L.A.M.; Paiva, S.A.R.; et al. Pera orange juice (Citrus sinensis L. Osbeck) alters lipid metabolism and attenuates oxidative stress in the heart and liver of rats treated with doxorubicin. Heliyon 2024, 10, e36834. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Vinayagam, S.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Suman, S.; Arunachalam, S.; Vellingiri, B.; Valsala Gopalakrishnan, A. Exploring the Pattern of Metabolic Alterations Causing Energy Imbalance via PPARα Dysregulation in Cardiac Muscle During Doxorubicin Treatment. Cardiovasc. Toxicol. 2022, 22, 436–461. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Hou, Y.; Zhang, Y.; Chen, J.; Gao, S.; Duan, H.; Gu, S.; Yu, S.; Cai, Y. SIRT6 activates PPARα to improve doxorubicin-induced myocardial cell aging and damage. Chem. Biol. Interact. 2024, 392, 110920. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Hu, J.; Wang, Y.; Lu, S.; Xiong, J.; Li, H.; Xiong, N.; Huang, Y.; Wang, Y.; et al. Overexpression of hnRNPK and inhibition of cytoplasmic translocation ameliorate lipid disorder in doxorubicin-induced cardiomyopathy via PINK1/Parkin-mediated mitophagy. Free Radic. Biol. Med. 2025, 231, 94–108. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, X.; Zhang, H.; Wu, Y.; Jing, J.; Huang, R.; Zhou, T.; Hu, J.; Wu, Y.; Li, Y.; et al. Doxorubicin downregulates autophagy to promote apoptosis-induced dilated cardiomyopathy via regulating the AMPK/mTOR pathway. Biomed. Pharmacother. 2023, 162, 114691. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Wang, X.; Zhang, Y.; Sun, Q.; Jiang, Y.; Yao, J.; Li, C.; Wang, Y.; Wang, W. Ferruginol Restores SIRT1-PGC-1α-Mediated Mitochondrial Biogenesis and Fatty Acid Oxidation for the Treatment of DOX-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 773834. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Wang, Y.; Gong, K.; Yan, T.; Wang, D.; Meng, X.; Yang, X.; Chen, Y.; Han, J.; et al. Daidzein alleviates doxorubicin-induced heart failure via the SIRT3/FOXO3a signaling pathway. Food Funct. 2022, 13, 9576–9588. [Google Scholar] [CrossRef]
- Abdellatif, M.; Rainer, P.P.; Sedej, S.; Kroemer, G. Hallmarks of cardiovascular ageing. Nat. Rev. Cardiol. 2023, 20, 754–777. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, X.Y.; Sun, L.X.; Fan, R.H.; Bi, K.S.; Yu, Z.G. Cytotoxic constituents of Viscum coloratum. Z. Naturforsch C J. Biosci. 2012, 67, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wei, X.Y.; Qiu, Z.D.; Gong, L.; Chen, Z.Y.; Ma, Y.; Shen, Y.; Zhao, Y.J.; Wang, W.H.; Lai, C.J.; et al. Exploring the resources of the genus Viscum for potential therapeutic applications. J. Ethnopharmacol. 2021, 277, 114233. [Google Scholar] [CrossRef] [PubMed]
- Anthracycline Global Market Report 2025—By Drugs (Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, Val-rubicin), By Dosage (Powder, Capsule, Solution, Injection, Suspension, Other Dosages), By Application (Acute Lymphocytic Leukemia, Acute Myelogenous Leukemia, Hodgkin’s Lymphoma, Non-Hodgkin’s Lymphoma, Bladder Cancer, Breast Cancer, Other Metastatic Cancers), By End User (Hospitals, Homecare, Specialty Clinics, Other End Users)—Market Size, Trends, And Global Forecast 2025–2034. Available online: https://www.thebusinessresearchcompany.com/report/anthracycline-global-market-report (accessed on 5 July 2025).
- Nebigil, C.G.; Désaubry, L. Updates in Anthracycline-Mediated Cardiotoxicity. Front. Pharmacol. 2018, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.; He, J.; Yu, L.; Li, X.; Zhang, J.; Li, S.; Zhang, C.; Kagan, J.C.; Karp, J.M.; et al. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity. Nat. Commun. 2023, 14, 3877. [Google Scholar] [CrossRef]
- Perry, J.M.; Tao, F.; Roy, A.; Lin, T.; He, X.C.; Chen, S.; Lu, X.; Nemechek, J.; Ruan, L.; Yu, X.; et al. Overcoming Wnt-β-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat. Cell Biol. 2020, 22, 689–700. [Google Scholar] [CrossRef]
- Tanwar, S.S.; Dwivedi, S.; Khan, S.; Sharma, S. Cardiomyopathies and a brief insight into DOX-induced cardiomyopathy. Egypt. Heart J. 2025, 77, 29. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARγ-NF-κB signaling pathway. Food Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Meng, Y.; Ding, P.; Wang, H.; Liu, J.; Xia, C.; Chen, Y.; Li, J. Pathological implication of CaMKII in NF-κB pathway and SASP during cardiomyocytes senescence. Mech. Ageing Dev. 2023, 209, 111758. [Google Scholar] [CrossRef] [PubMed]
- Renauld, S.; Tremblay, K.; Ait-Benichou, S.; Simoneau-Roy, M.; Garneau, H.; Staub, O.; Chraïbi, A. Stimulation of ENaC activity by rosiglitazone is PPARγ-dependent and correlates with SGK1 expression increase. J. Membr. Biol. 2010, 236, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Meng, L.; Yu, P.; Zhou, C.; Zhang, Z.; Yu, Z.; Qin, F.; Zhao, Y. Novel drug isolated from mistletoe (1E,4E)-1,7-bis(4-hydroxyphenyl)hepta-1,4-dien-3-one for potential treatment of various cancers: Synthesis, pharmacokinetics and pharmacodynamics. RSC Adv. 2020, 10, 27794–27804. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef]
- Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. Enhancing the Enrichment of Pharmacophore-Based Target Prediction for the Polypharmacological Profiles of Drugs. J. Chem. Inf. Model. 2016, 56, 1175–1183. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th anniversary: Update 2025. Nucleic Acids Res. 2025, 53, D1328–D1334. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30–1.30.33. [Google Scholar] [CrossRef]
- The GeneCards Suite. Practical Guide to Life Science Databases 27–56. 2022. Available online: https://www.genecards.org (accessed on 18 May 2025).
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome. Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.1–8.14.40. [Google Scholar] [CrossRef] [PubMed]
- PyMOL by Schrödinger. Available online: http://www.pymol.org/pymol (accessed on 7 June 2025).
- Sankar, K.; Trainor, K.; Blazer, L.L.; Adams, J.J.; Sidhu, S.S.; Day, T.; Meiering, E.; Maier, J.K.X. A Descriptor Set for Quantitative Structure-property Relationship Prediction in Biologics. Mol. Inform. 2022, 41, e2100240. [Google Scholar] [CrossRef] [PubMed]
- Tavella, D.; Ouellette, D.R.; Garofalo, R.; Zhu, K.; Xu, J.; Oloo, E.O.; Negron, C.; Ihnat, P.M. A novel method for in silico assessment of Methionine oxidation risk in monoclonal antibodies: Improvement over the 2-shell model. PLoS ONE 2022, 17, e0279689. [Google Scholar] [CrossRef]
- Sankar, K.; Krystek, S.R., Jr.; Carl, S.M.; Day, T.; Maier, J.K.X. AggScore: Prediction of aggregation-prone regions in proteins based on the distribution of surface patches. Proteins 2018, 86, 1147–1156. [Google Scholar] [CrossRef]
- Zhu, K.; Day, T.; Warshaviak, D.; Murrett, C.; Friesner, R.; Pearlman, D. Antibody structure determination using a combination of homology modeling, energy-based refinement, and loop prediction. Proteins 2014, 82, 1646–1655. [Google Scholar] [CrossRef]
- Salam, N.K.; Adzhigirey, M.; Sherman, W.; Pearlman, D.A. Structure-based approach to the prediction of disulfide bonds in proteins. Protein Eng. Des. Sel. 2014, 27, 365–374. [Google Scholar] [CrossRef]
- Beard, H.; Cholleti, A.; Pearlman, D.; Sherman, W.; Loving, K.A. Applying physics-based scoring to calculate free energies of binding for single amino acid mutations in protein-protein complexes. PLoS ONE 2013, 8, e82849. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.P.; Zhang, Y.; Bi, H.L.; Guan, X.M.; Wang, H.X.; Wang, X.; Du, J.; Xia, Y.L.; Li, H.H. Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein. Sci. Rep. 2016, 6, 28399. [Google Scholar] [CrossRef]
- Maillet, A.; Tan, K.; Chai, X.; Sadananda, S.N.; Mehta, A.; Ooi, J.; Hayden, M.R.; Pouladi, M.A.; Ghosh, S.; Shim, W.; et al. Modeling Doxorubicin-Induced Cardiotoxicity in Human Pluripotent Stem Cell Derived-Cardiomyocytes. Sci. Rep. 2016, 6, 25333. [Google Scholar] [CrossRef]
- Huang, H.; Christidi, E.; Shafaattalab, S.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. RARG S427L attenuates the DNA repair response to doxorubicin in induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rep. 2022, 17, 756–765. [Google Scholar] [CrossRef]
- Yixin, Z.; Fenglan, J.; Xin, W.; Xiao, W.; Bo, L.; Fuchun, W.; Tao, D.; Huibo, X. Study on the model of chronic heart failure induced by adriamycin in rats. Chin. J. Gerontol. 2021, 41, 2576–2581. [Google Scholar] [CrossRef]
- Liping, T.; Fengzhen, H.; Mingxia, X. Effects of portulaca polysaccharide on atheroscleroti plaque in rats by regulating PPARγ/NFκB pathway. Hebei Med. J. 2023, 45, 330–334. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef]

| Non-Covalent Molecule Docking (kcal/mol) | Covalent Molecule Docking (kcal/mol) | |
|---|---|---|
| ESR1 | −6.697 | - |
| HSP90AA1 | −7.058 | - |
| IGF1 | −6.081 | - |
| CASP3 | −7.543 | −4.323 |
| ALB | −8.398 | - |
| SRC | −6.219 | - |
| PPARG | −9.745 | −7.495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Zhang, F.; Zhang, R.; Fu, H.; Shen, D.; Wang, X.; Yang, Y.; Wu, J.; Meng, L.; Lü, H.; et al. DHDK, a Plant-Derived Natural Small Molecule, Protects Against Doxorubicin-Induced Cardiotoxicity via the PPARG-CPT1B-FAO Axis. Pharmaceuticals 2025, 18, 1759. https://doi.org/10.3390/ph18111759
Hong J, Zhang F, Zhang R, Fu H, Shen D, Wang X, Yang Y, Wu J, Meng L, Lü H, et al. DHDK, a Plant-Derived Natural Small Molecule, Protects Against Doxorubicin-Induced Cardiotoxicity via the PPARG-CPT1B-FAO Axis. Pharmaceuticals. 2025; 18(11):1759. https://doi.org/10.3390/ph18111759
Chicago/Turabian StyleHong, Jing, Fangyu Zhang, Ruizhen Zhang, Hongyang Fu, Dongang Shen, Xinyue Wang, Yuting Yang, Jiamei Wu, Lin Meng, Hongyang Lü, and et al. 2025. "DHDK, a Plant-Derived Natural Small Molecule, Protects Against Doxorubicin-Induced Cardiotoxicity via the PPARG-CPT1B-FAO Axis" Pharmaceuticals 18, no. 11: 1759. https://doi.org/10.3390/ph18111759
APA StyleHong, J., Zhang, F., Zhang, R., Fu, H., Shen, D., Wang, X., Yang, Y., Wu, J., Meng, L., Lü, H., Jiang, X., & Zhao, Y. (2025). DHDK, a Plant-Derived Natural Small Molecule, Protects Against Doxorubicin-Induced Cardiotoxicity via the PPARG-CPT1B-FAO Axis. Pharmaceuticals, 18(11), 1759. https://doi.org/10.3390/ph18111759






