Coleus aromaticus Benth.—A Plant with Strong Anticancer and Antioxidant Potential In Vitro
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Activity Assessment of C. aromaticus Extracts
2.2. Evaluation of Antioxidant Effect of C. aromaticus Extracts
2.3. Antimicrobial Effect of C. aromaticus Extracts
2.4. Phytochemical Analysis of C. aromaticus Extracts
2.4.1. Content of Phenolic Compounds
2.4.2. Compounds Identified in C. aromaticus Extracts by GC Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Evaluation of Cytotoxic Activity of C. aromaticus Extracts
4.2.1. Cell Lines
4.2.2. MTT Assay
4.3. Antioxidant Tests and Estimation of Metal Ion Complexation
4.3.1. Materials
4.3.2. Methods
ABTS Assay
DPPH Assay
Molybdenum Reduction Assay
4.3.3. Cu2+ Ion Complexation by C. aromaticus Extracts
4.4. Antimicrobial Activity
4.4.1. Microorganisms
4.4.2. Antibacterial Assay
4.5. Phytochemical Analysis of C. aromaticus Extracts
4.5.1. Total Phenolic Content
4.5.2. Total Flavonoid Content
4.5.3. Sample Preparation for GC Analysis
4.5.4. GC-FID Analysis
4.5.5. GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can Be Explained by Differences between Helicobacter Pylori Strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef]
- Nam, S.Y.; Park, B.J.; Ryu, K.H.; Nam, J.H. Effect of Helicobacter Pylori Eradication on the Regression of Gastric Polyps in National Cancer Screening Program. Korean J. Intern. Med. 2018, 33, 506–511. [Google Scholar] [CrossRef]
- Granados-Romero, J.J.; Valderrama-Treviño, A.I.; Contreras-Flores, E.H.; Barrera-Mera, B.; Enríquez, M.H.; Uriarte-Ruíz, K.; Ceballos-Villalba, J.C.; Estrada-Mata, A.G.; Rodríguez, C.A.; Arauz-Peña, G. Colorectal Cancer: A Review. Int. J. Res. Med. Sci. 2017, 5, 4667–4676. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A Review of Ethnobotanical Uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Arumugam, G.; Swamy, M.; Sinniah, U. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Jimmy, J.L. Coleus aromaticus Benth.: An Update on Its Bioactive Constituents and Medicinal Properties. All Life 2021, 14, 756–773. [Google Scholar] [CrossRef]
- Wadikar, D.D.; Patki, P.E. Coleus aromaticus: A Therapeutic Herb with Multiple Potentials. J. Food Sci. Technol. 2016, 53, 2895–2901. [Google Scholar] [CrossRef]
- Warrier, P.K. Indian Medicinal Plants; Orient Longman: Hyderabad, India, 1995; Volume 4. [Google Scholar]
- Damanik, R.; Damanik, N.; Daulay, Z.; Saragih, S.; Premier, R.; Wattanapenpaiboon, N.; Wahlqvist, M. Consumption of Bangun-Bangun Leaves (Coleus amboinicus Lour) to Increase Breast Milk Production among Batakneese Women in North Sumatra Island, Indonesia. Proc. Nutr. Soc. Aust. 2001, 25, S67. [Google Scholar]
- Gupta, S.; Jyothi Lakshmi, A.; Manjunath, M.N.; Prakash, J. Analysis of Nutrient and Antinutrient Content of Underutilized Green Leafy Vegetables. LWT-Food Sci. Technol. 2005, 38, 339–345. [Google Scholar] [CrossRef]
- Wadikar, D.D.; Premavalli, K.S. Development of a Hot Water Reconstitutable Appetizer Soup Mix from Coleus aromaticus Using Response Surface Methodology. Int. Food Res. J. 2013, 20, 3041–3046. [Google Scholar]
- Aguiar, J.J.S.; Sousa, C.P.B.; Araruna, M.K.A.; Silva, M.K.N.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.A.; Figueredo, F.G.; Bitu, V.C.N.; Coutinho, H.D.M.; et al. Antibacterial and Modifying-Antibiotic Activities of the Essential Oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Kusumoto, I.T.; Nakabayashi, T.; Kida, H.; Miyashiro, H.; Hattori, M.; Namba, T.; Shimotohno, K. Screening of Various Plant Extracts Used in Ayurvedic Medicine for Inhibitory Effects on Human Immunodeficiency Virus Type 1 (HIV-1) Protease. Phytother. Res. 1995, 9, 180–184. [Google Scholar] [CrossRef]
- Gurgel, A.P.A.D.; Da Silva, J.G.; Grangeiro, A.R.S.; Oliveira, D.C.; Lima, C.M.P.; Da Silva, A.C.P.; Oliveira, R.A.G.; Souza, I.A. In Vivo Study of the Anti-Inflammatory and Antitumor Activities of Leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J. Ethnopharmacol. 2009, 125, 361–363. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Majumder, P. Investigation of Phytochemicals and Anti-convulsant Activity of the Plant Coleus amboinicus (Lour.). Int. J. Green Pharm. 2013, 7, 211–215. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. Antioxidant and Free Radical Scavenging Activity of an Aqueous Extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kannappan, K.; Ramesh, K.; Hareendran, K.; Prabhu, S. Antimicrobial Properties of Aqueous Extract of Plectranthus amboinicus against Streptococcus mutans, Staphylococcus aureus and Candida albicans—An In-vitro Study. Int. J. Comm. Dent. 2024, 12, 78–85. [Google Scholar] [CrossRef]
- Ramalakshmi, P.; Subramanian, N.; Saravanan, R.; Mohanakrishnan, H.; Muthu, M. Anticancer effect of Coleus amboinicus (Karpooravalli) on human lung cancer cell line (A549). Int. J. Dev. Res. 2014, 4, 2442–2449. [Google Scholar]
- Subaiea, G.; Alafnan, A.; Alamri, A.; Hussain, T.; Hassoun, S.M.; Lila, A.S.A.; Khafagy, E.-S.; Katamesh, A.A. Coleus aromaticus Ethanolic Leaves Extract Mediates Inhibition of NF-κB Signaling Pathway in Lung Adenocarcinoma A549 Cells. Processes 2023, 11, 1332. [Google Scholar] [CrossRef]
- Sandra, P.; Safa, K.P.; Arippayil, F.; Arun Prakash, K.V.; Nandana, P.; Hiba Musthafa, K.P.R. Anticancer Activity of Ethanolic Extract of Coleus amboinicus. Int. J. Pharm. Sci. 2025, 3, 315–321. [Google Scholar] [CrossRef]
- Shekh, R.; Tiwari, R.K.; Ahmad, A.; Ahmad, I.; Alabdallah, N.M.; Saeed, M.; Ansari, I.A.; Mishra, A.; Ashfaque, M.; Bajpai, P. Ethanolic Extract of Coleus aromaticus Leaves Impedes the Proliferation and Instigates Apoptotic Cell Death in Liver Cancer HepG2 Cells through Repressing JAK/STAT Cascade. J. Food Biochem. 2022, 46, e14368. [Google Scholar] [CrossRef]
- Gulla, S.; Jabeen, S.; Thummala, C.; Reddy Lebaka, V.; Chinni, S.V.; Gopinath, S.C.B.; Lomada, D.; Reddy, M.C. Anti-Inflammatory, Anti-Bacterial, and Anti-Cancer Activities of Ag-Nanoparticles Generated by Plectranthus amboinicus. Inorg. Chem. Commun. 2024, 167, 112702. [Google Scholar] [CrossRef]
- Shubha, J.R.; Bhatt, P. Plectranthus amboinicus Leaves Stimulate Growth of Probiotic L. plantarum: Evidence for Ethnobotanical Use in Diarrhea. J. Ethnopharmacol. 2015, 166, 220–227. [Google Scholar] [CrossRef]
- Muniandy, K.; Hassan, Z.; Hafez Mohd Iza, M. Antimicrobial Activity of the Ethanolic Extract of Coleus aromaticus against Common Wound Pathogens. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1286–1296. [Google Scholar]
- Thenmozhi, S.; Bhuvana, M.; Ahmed John, S. Screening of Antimicrobial and Phytochemical Investigation of Coleus aromaticus (Benth) Leaf against Five Respiratory Pathogens. J. Pharm. Res. 2011, 4, 2261–2262. [Google Scholar]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic Activity of Indian Borage (Plectranthus amboinicus) Volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Pino, J.A.; Garcia, J.; Martinez, M.A. Comparative Chemical Composition of the Volatiles of Coleus aromaticus Produced by Steam Distillation, Solvent Extraction and Supercritical Carbon Dioxide Extraction. J. Essent. Oil Res. 1996, 8, 373–375. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Govindaraju, S.; Arulselvi, P.I. Characterization of Coleus aromaticus Essential Oil and Its Major Constituent Carvacrol for In Vitro Antidiabetic and Antiproliferative Activities. J. Herbs Spices Med. Plants 2018, 24, 37–51. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Kumar, N. Plectranthus amboinicus: A review on its pharmacological and, pharmacognostical studies. Am. J. Physiol. Biochem. Pharmacol. 2020, 10, 55–62. [Google Scholar] [CrossRef]
- Jain, A.; Dixit, A.; Mehta, S. Wound Healing Activity of Aqueous Extracts of Leaves and Roots of Coleus aromaticus in Rats. Acta Pol. Pharm. 2012, 69, 1119–1123. [Google Scholar] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Barreto, R.; Albuquerque-Júnior, R.; Araújo, A.; Almeida, J.; Santos, M.; Barreto, A.; DeSantana, J.; Siqueira-Lima, P.; Quintans, J.; Quintans-Júnior, L. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014, 19, 846–862. [Google Scholar] [CrossRef]
- El-hawary, S.S.; El-sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S. Phytochemical Screening, DNA Fingerprinting, and Nutritional Value of Plectranthus amboinicus (Lour.) Spreng. Pharmacogn. J. 2012, 4, 10–13. [Google Scholar] [CrossRef]
- Khanum, H.; Ramalakshmi, K.; Srinivas, P.; Borse, B.B. Synergistic Antioxidant Action of Oregano, Ajowan and Borage Extracts. Food Nutr. Sci. 2011, 2, 387–392. [Google Scholar] [CrossRef]
- Rao, B.S.S. Antioxidant, Anticlastogenic and Radioprotective Effect of Coleus aromaticus on Chinese Hamster Fibroblast Cells (V79) Exposed to Gamma Radiation. Mutagenesis 2006, 21, 237–242. [Google Scholar] [CrossRef]
- Kamal, B.S.; Kumar, M. In-Vitro Antioxidant and Anti-Inflammatory Studies of Coleus malabaricus Benth. and Its Morphotype on a Comparative Account with Coleus zeylanicus and Coleus amboinicus of Family Lamiaceae. Lett. Appl. NanoBioSci. 2025, 14, 1–13. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Hałasa, R.; Asztemborska, M.; Ochocka, J.R., III. Natural Cosmetics International Meeting Book of Abstracts; The University of Information Technology and Management in Rzeszów: Kielnarowa/Rzeszow, Poland, 17 September 2025. [Google Scholar]
- Borowska, S.; Tomczyk, M.; Strawa, J.W.; Brzóska, M.M. Estimation of the Chelating Ability of an Extract from Aronia melanocarpa L. Berries and Its Main Polyphenolic Ingredients Towards Ions of Zinc and Copper. Molecules 2020, 25, 1507. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; Oliveira, E.C.S.D.; Stasi, L.C.D.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Patwa, N.; Singh, G.; Sharma, V.; Chaudhary, P.; Sharma, B.; Haque, S.; Yadav, V.; Satapathy, S.R.; Tuli, H.S. Targeting Gastrointestinal Cancers with Carvacrol: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 777. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Bilgin, M.G.; Ergün, İ.S.; Dadak, A. Effects of Carvacrol on Human Fibroblast (WS-1) and Gastric Adenocarcinoma (AGS) Cells In Vitro and on Wistar Rats In Vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Mahwish; Imran, M.; Naeem, H.; Hussain, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Mujtaba, A.; Abdelgawad, M.A.; Ghoneim, M.M.; El-Ghorab, A.H.; et al. Antioxidative and Anticancer Potential of Luteolin: A Comprehensive Approach Against Wide Range of Human Malignancies. Food Sci. Nutr. 2025, 13, e4682. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Fan, L.; Wu, X.; Xin, A.; Ren, H.; Wang, X.J. Luteolin Inhibits Nrf2 Leading to Negative Regulation of the Nrf2/ARE Pathway and Sensitization of Human Lung Carcinoma A549 Cells to Therapeutic Drugs. Free Radic. Biol. Med. 2011, 50, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Mirzaei, S.; Hashemi, F.; Samarghandian, S.; Zabolian, A.; Hushmandi, K.; Ang, H.L.; Sethi, G.; Kumar, A.P.; et al. Gallic Acid for Cancer Therapy: Molecular Mechanisms and Boosting Efficacy by Nanoscopical Delivery. Food Chem. Toxicol. 2021, 157, 112576. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.; Villegas, C.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; González-Chavarría, I.; Nchiozem-Ngnitedem, V.-A.; Paz, C. Adjuvant Properties of Caffeic Acid in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 7631. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1–NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, L.; Ran, W.; Zhong, K.; Zhao, Y.; Gao, H. Antimicrobial Activities of Natural Flavonoids against Foodborne Pathogens and Their Application in Food Industry. Food Chem. 2024, 460, 140476. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Zhang, M.; Bai, B.; Yang, Y.; Fan, S. The Antibacterial Mechanism of Perilla Rosmarinic Acid. Biotechnol. Appl. Biochem. 2022, 69, 1757–1764. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.D.; Mahobia, N.K.; Singh, M.P.; Singh, A.; Sheikh, N.W.A.; Alam, G.; Singh, S.K. Antioxidant Potential of Leaves of Plectranthus amboinicus (Lour) Spreng. Pharm. Lett. 2010, 2, 240–245. [Google Scholar]
- Nguyen, N.Q.; Minh, L.V.; Trieu, L.H.; Bui, L.M.; Lam, T.D.; Hieu, V.Q.; Khang, T.V.; Trung, L.N.Y. Evaluation of Total Polyphenol Content, Total Flavonoid Content, and Antioxidant Activity of Plectranthus amboinicus Leaves. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062017. [Google Scholar] [CrossRef]
- Hernández-Barreto, E.; Ruz-Sanjuan, V.; Ribalta-Ribalta, V.; Torres-Gómez, L.A. Optimization of the Molybdenum Blue Method for Estimating the Antioxidant Activity of Natural Products. J. Pharm. Pharmacogn. Res. 2023, 11, 953–960. [Google Scholar] [CrossRef]
- Aydin, D.; Yalçin, E.; Çavuşoğlu, K. Metal Chelating and Anti-Radical Activity of Salvia Officinalis in the Ameliorative Effects against Uranium Toxicity. Sci. Rep. 2022, 12, 15845. [Google Scholar] [CrossRef]
- Jan, A.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef]
- Santos, C.C.D.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; De Almeida, A.A.C.; De Oliveira, G.A.L.; Costa, J.P.; De Sousa, D.P.; De Freitas, R.M.; De Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Costa, J.; Islam, T.; Santos, P.; Ferreira, P.; Oliveira, G.; Alencar, M.; Paz, M.; Ferreira, É.; Feitosa, C.; Citó, A.; et al. Evaluation of Antioxidant Activity of Phytol Using Non- and Pre-Clinical Models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef]
- Aristatile, B.; Al-Numair, K.S.; Al-Assaf, A.H.; Veeramani, C.; Pugalendi, K.V. Protective Effect of Carvacrol on Oxidative Stress and Cellular DNA Damage Induced by UVB Irradiation in Human Peripheral Lymphocytes: Protective effect of carvacrol. J. Biochem. Mol. Toxicol. 2015, 29, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.H.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.A.; Rocha, R.F.; Moreira, J.C.F.; et al. Bioassay-Guided Evaluation of Antioxidant and Antinociceptive Activities of Carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, S.H.A.; On, S.; Sanusi, S.N.A.; Hashim, A.A.; Addinna Zai, M.H. Antioxidant Activity of Camphor Leaves Extract Based on Variation Solvent. J. Phys. Conf. Ser. 2019, 1349, 012102. [Google Scholar] [CrossRef]
- Halliwell, B. Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Vijayanand, S.; Thiruvengadam, S.R.; Malath, R. Comparative Study on Free Radical Ameliorating Potential of Stem and the Leaf Extracts of Plectranthus amboinicus. J. Stress Physiol. Biochem. 2023, 19, 111–119. [Google Scholar]
- Bhatt, P.; Negi, P.S. Antioxidant and Antibacterial Activities in the Leaf Extracts of Indian Borage (Plectranthus amboinicus). Food Nutr. Sci. 2012, 3, 146–152. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, J.N. The Correlation between Antimutagenic Activity and Total Phenolic Content of Extracts of 31 Plant Species with High Antioxidant Activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-Inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Kwon, S.H.; Wang, Z.; Hwang, S.H.; Kang, Y.-H.; Lee, J.-Y.; Lim, S.S. Comprehensive Evaluation of the Antioxidant Capacity of Perilla frutescens Leaves Extract and Isolation of Free Radical Scavengers Using Step-Wise HSCCC Guided by DPPH-HPLC. Int. J. Food Prop. 2017, 20, 921–934. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Hering, A.; Cal, K.; Kowalczyk, M.; Kastsevich, A.; Ivashchanka, Y.; Ochocka, J.R.; Stefanowicz-Hajduk, J. Bufadienolide Penetration Through the Skin Membrane and Antiaging Properties of Kalanchoe spp. Juices in Dermal Applications. Molecules 2025, 30, 802. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/eucast_news/news_singleview/?tx_ttnews%5Btt_news%5D=464&cHash=ea8540c0fbdaa71b3bbcb3bf765239de (accessed on 20 June 2025).
- Lekogo, B.M.; Coroller, L.; Mathot, A.G.; Mafart, P.; Leguerinel, I. Modelling the Influence of Palmitic, Palmitoleic, Stearic and Oleic Acids on Apparent Heat Resistance of Spores of Bacillus cereus NTCC 11145 and Clostridium sporogenes Pasteur 79.3. Int. J. Food Microbiol. 2010, 141, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Båverud, V.; Gunnarsson, A.; Karlsson, M.; Franklin, A. Antimicrobial Susceptibility of Equine and Environmental Isolates of Clostridium difficile. Microb. Drug Resist. 2004, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, R.; Turecka, K.; Orlewska, C.; Werel, W. Comparison of Fluorescence Optical Respirometry and Microbroth Dilution Methods for Testing Antimicrobial Compounds. J. Microbiol. Methods 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Figueira, J.A.; Porto-Figueira, P.; Berenguer, C.; Pereira, J.A.M.; Câmara, J.S. Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules 2021, 26, 4202. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
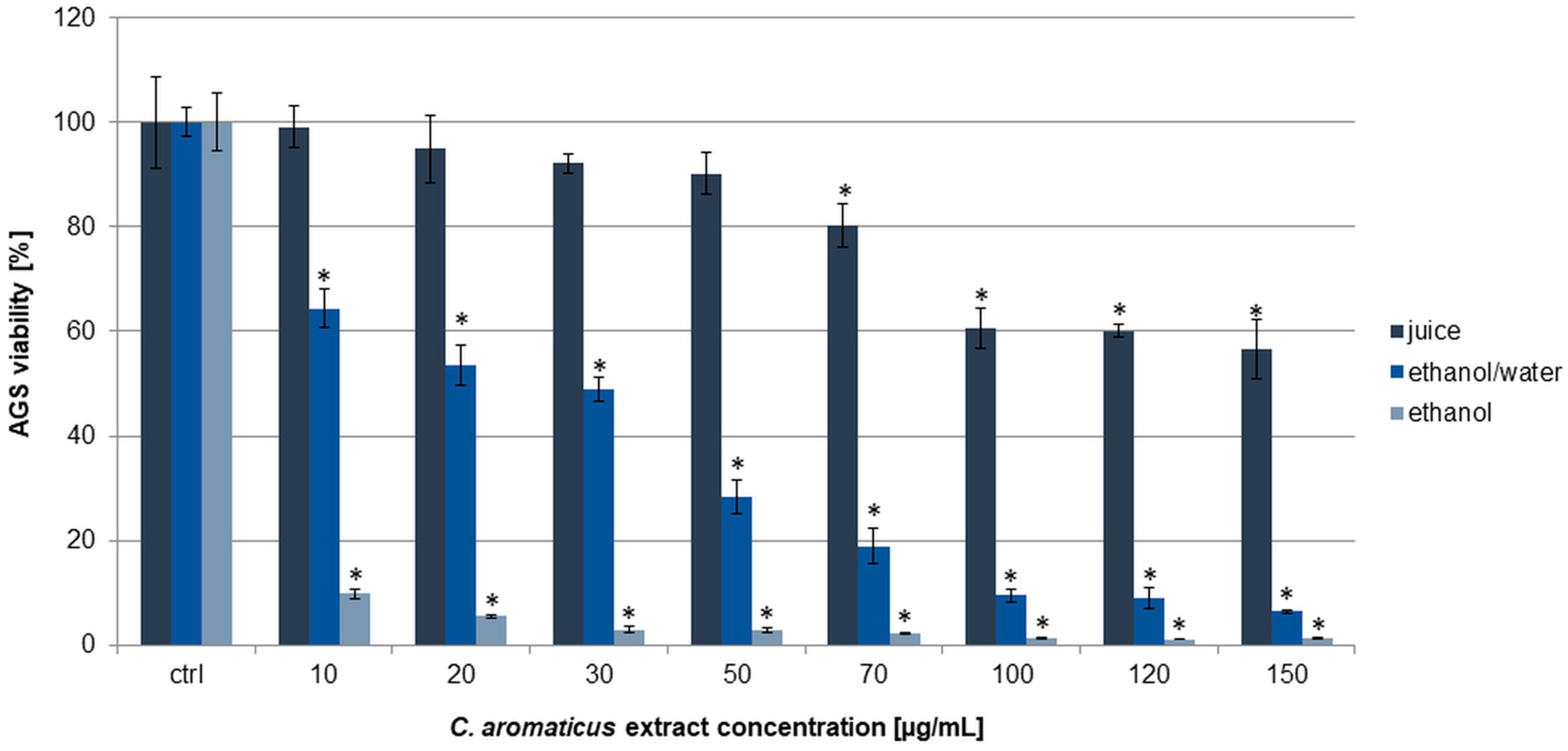

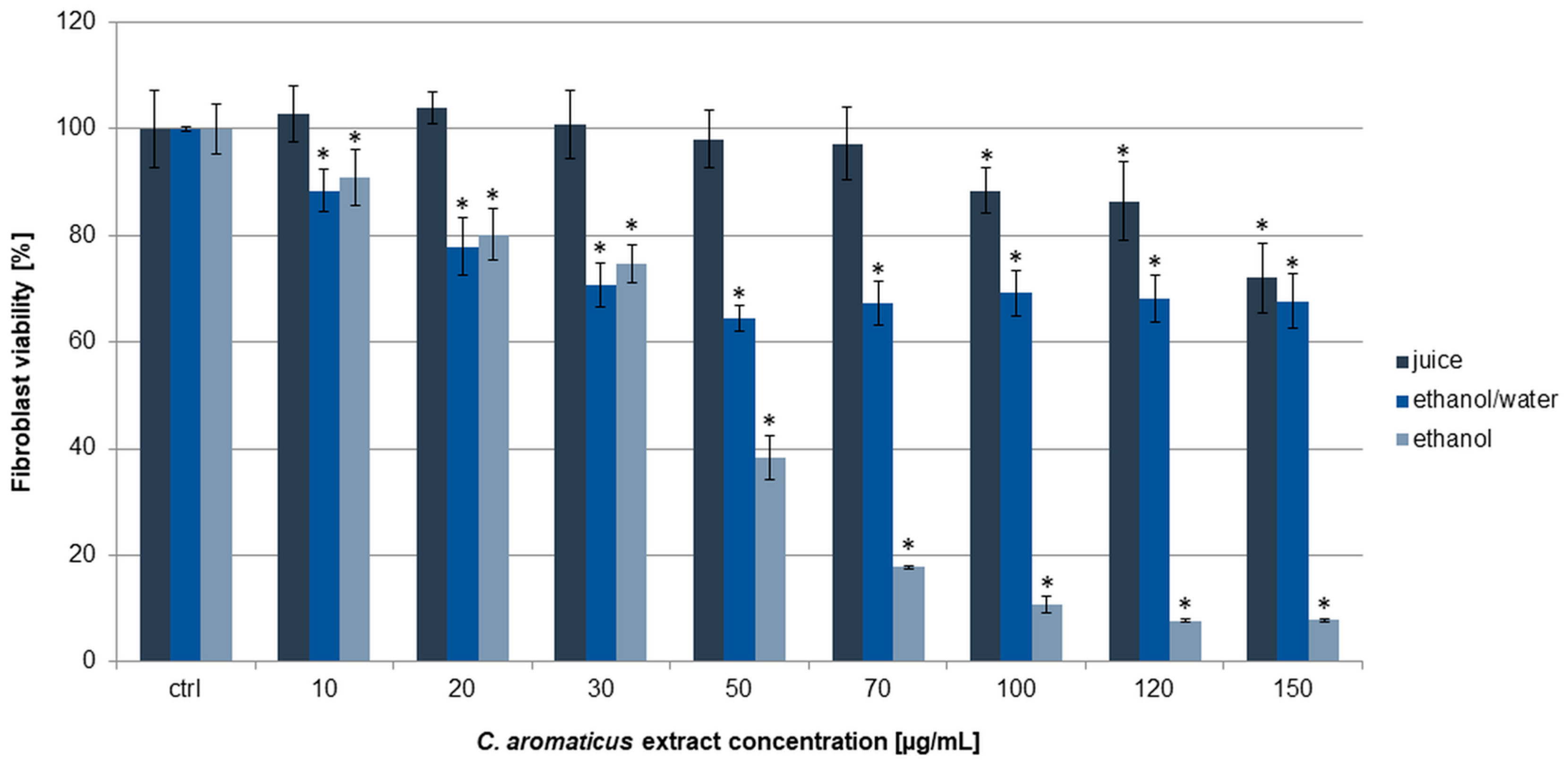
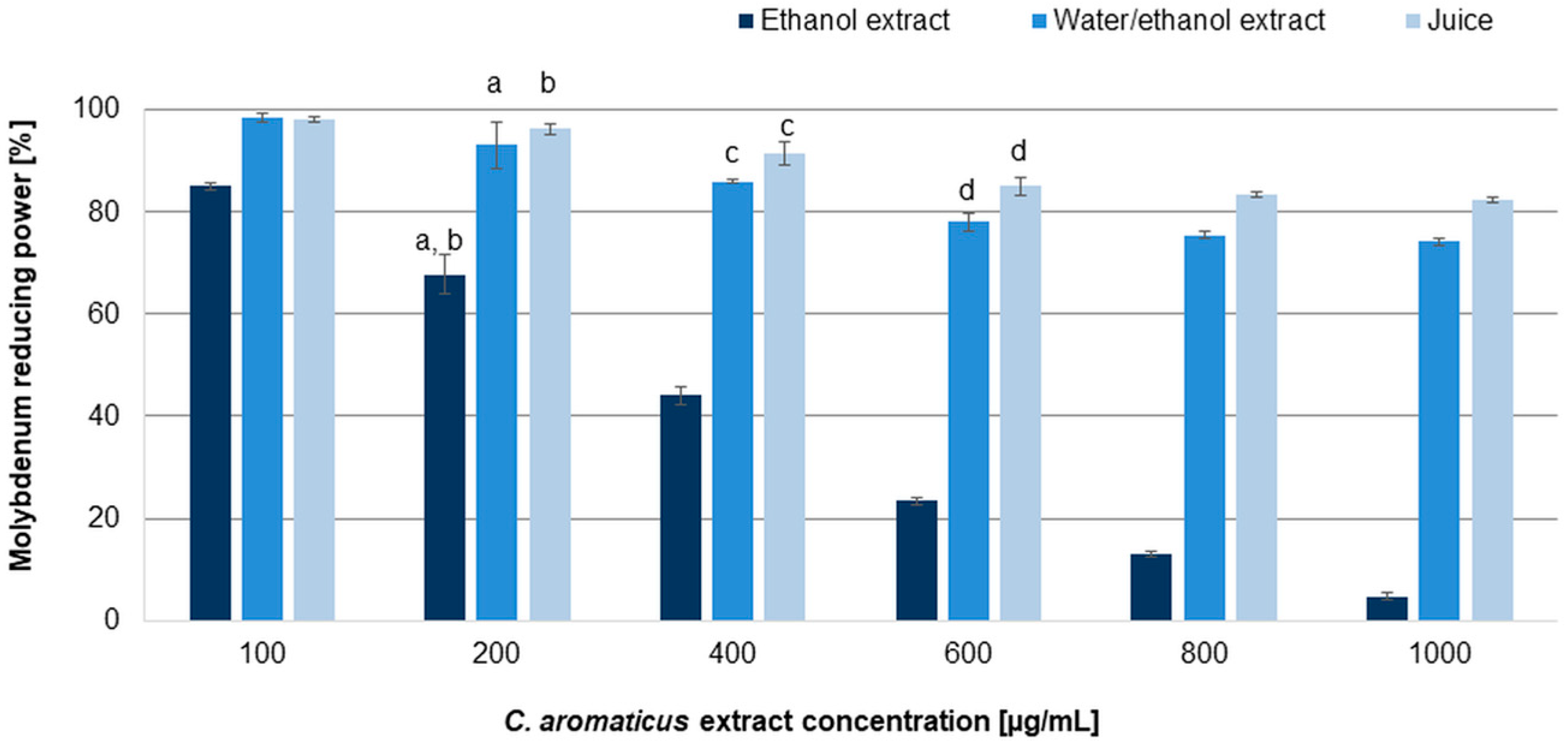
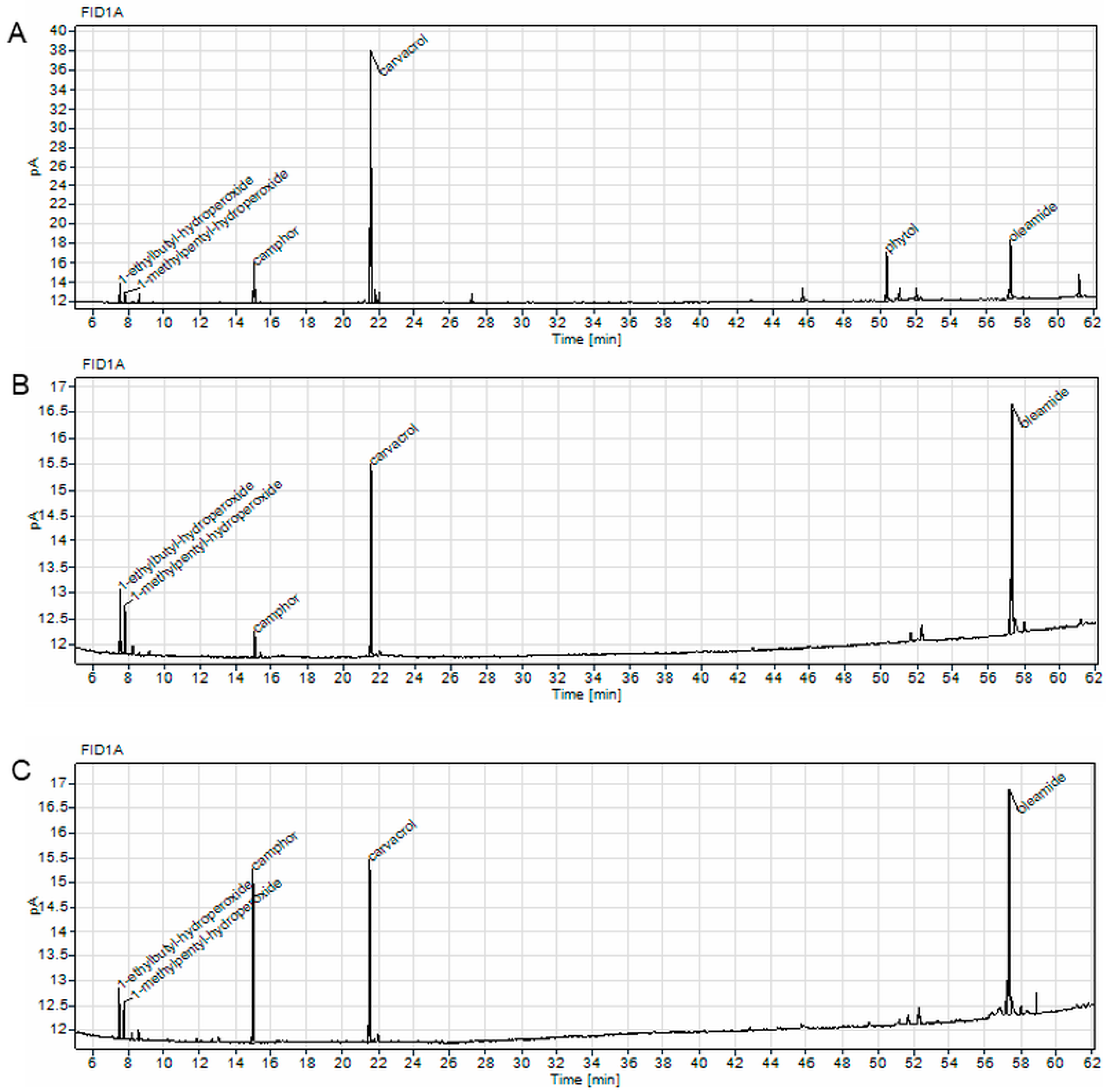
| Cell Line | IC50 [µg/mL] | |||
|---|---|---|---|---|
| C. aromaticus Ethanol Extract | C. aromaticus Ethanol/Water Extract | C. aromaticus Juice | Oxaliplatin | |
| AGS | 4.94 ± 0.48 | 22.82 ± 1.82 | n.r. | 17.90 ± 1.20 |
| HCT 116 | 24.99 ± 1.80 | n.r. | n.r. | n.r. |
| Fibroblasts | 45.31 ± 2.28 | n.r. | n.r. | 95.79 ± 3.24 |
| Assay | IC50 [µg/mL] | |||
|---|---|---|---|---|
| C. aromaticus Ethanol Extract | C. aromaticus Ethanol/Water Extract | C. aromaticus Juice | Ascorbic Acid | |
| ABTS | 13.34 ± 0.11 | 79.94 ± 0.55 | 148.90 ± 1.45 | 16.35 ± 0.97 |
| DPPH | 22.90 ± 1.30 | 418.40 ± 2.75 | 879.68 ± 2.55 | 20.15 ± 1.79 |
| Molybdenum reducing power | 290.17 ± 4.23 | n.r. | n.r. | 18.75 ± 1.54 |
| Microorganism | C. aromaticus Ethanol Extract | C. aromaticus Ethanol/Water Extract | C. aromaticus Juice | Ampicillin | |||
|---|---|---|---|---|---|---|---|
| [mg/mL] | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | |
| Streptococcus β-hemolytic group A PCM465 | 1 | 2 | 1 | 2 | >2 | >2 | 0.001 |
| Staphylococcus aureus ATCC6538 | 2 | 2 | >4 | >4 | >4 | >4 | 0.00008 |
| Staphylococcus aureus MRSA ATCC43300 | 1 | >2 | >2 | >2 | 2 | >2 | 0.01 |
| Enterococcus faecalis ATCC51299 | >4 | >4 | >4 | >4 | >4 | >4 | 0.000125 |
| Corynebacterium diphtheriae ZMF | 4 | 4 | >4 | >4 | >4 | >4 | 0.001 |
| Escherichia coli ATCC8739 | >4 | >4 | >4 | >4 | >4 | >4 | 0.0039 |
| Salmonella enterica ATCC13076 | >4 | >4 | >4 | >4 | >4 | >4 | 0.0005 |
| Shigella sonnei ATCC25931 | 2 | >4 | >4 | >4 | >4 | >4 | 0.002 |
| Yersinia enterocolitica ZMF4195 | 2 | 4 | >4 | >4 | >4 | >4 | >0.5 |
| Helicobacter pylori ATCC43504 | 2 | 4 | >4 | >4 | >4 | >4 | 0.0032 |
| Campylobacter jejuni ZMF | 4 | >4 | >4 | >4 | >4 | >4 | 0.032 |
| Clostridium sporogenes ATCC19404 | 1 | >2 | 2 | >2 | 2 | >2 | <0.0625 |
| Clostridium bifermentans ATCC638 | 1 | >2 | >2 | >4 | 1 | 2 | 0.016 |
| Clostridium perfringens ATCC13124 | <0.02 | <0.02 | <0.02 | <0.02 | >4 | >4 | <0.000016 |
| Listeria monocytogenes PCM2191 | >2 | >2 | >2 | >2 | >2 | >2 | 0.016 |
| Lactobacillus paracasei PCM2639 | 4 | >4 | >4 | >4 | >4 | >4 | >0.125 |
| Lactobacillus acidophilus PCM2499 | >4 | >4 | >4 | >4 | >4 | >4 | >0.125 |
| Candida albicans ATCC26790 | >4 | >4 | >4 | >4 | >4 | >4 | amphotericin B 0.001 |
| C. aromaticus Extract | Phenolic Content (mg GAE/g DE) | Flavonoid Content (mg QE/g DE) |
|---|---|---|
| Ethanol | 75.87 ± 0.96 | 176.01 ± 3.58 |
| Ethanol/water | 35.13 ± 1.18 | 26.59 ± 0.52 |
| Juice | 27.19 ± 0.15 | 12.90 ± 1.54 |
| Peak | RT [min] | Area% | Compound | Molecular Formula |
|---|---|---|---|---|
| C. aromaticus ethanol extract | ||||
| 1 | 7.441 | 2.77 | 1-ethylbutyl-hydroperoxide | C6H14O2 |
| 2 | 7.729 | 1.43 | 1-methylpentyl-hydroperoxide | C6H14O2 |
| 3 | 14.961 | 6.85 | camphor | C10H16O |
| 4 | 21.480 | 49.09 | carvacrol | C10H14O |
| 5 | 21.752 | 1.93 | n/i | - |
| 6 | 21.965 | 1.42 | n/i | - |
| 7 | 45.674 | 1.85 | n/i | - |
| 8 | 50.378 | 11.72 | phytol | C20H40O |
| 9 | 51.076 | 1.98 | n/i | - |
| 10 | 52.025 | 1.95 | n/i | - |
| 11 | 57.303 | 14.02 | oleamide | C18H35NO |
| 12 | 61.132 | 5 | n/i | - |
| C. aromaticus ethanol/water extract | ||||
| 1 | 7.470 | 6.49 | 1-ethylbutyl-hydroperoxide | C6H14O2 |
| 2 | 7.754 | 4.56 | 1-methylpentyl-hydroperoxide | C6H14O2 |
| 3 | 14.997 | 3.34 | camphor | C10H16O |
| 4 | 21.494 | 28.15 | carvacrol | C10H14O |
| 5 | 52.260 | 3.12 | n/i | - |
| 6 | 57.150 | 2.5 | n/i | - |
| 7 | 57.297 | 45.72 | oleamide | C18H35NO |
| 8 | 57.462 | 4.11 | n/i | - |
| 9 | 57.990 | 2.01 | n/i | - |
| C. aromaticus juice | ||||
| 1 | 7.433 | 4.34 | 1-ethylbutyl-hydroperoxide | C6H14O2 |
| 2 | 7.718 | 3.81 | 1-methylpentyl-hydroperoxide | C6H14O2 |
| 3 | 14.945 | 23.08 | camphor | C10H16O |
| 4 | 21.466 | 25.68 | carvacrol | C10H14O |
| 5 | 57.296 | 43.08 | oleamide | C18H35NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanowicz-Hajduk, J.; Hering, A.; Hałasa, R.; Masiak, S.; Turczyn, K.; Ochocka, J.R.; Asztemborska, M. Coleus aromaticus Benth.—A Plant with Strong Anticancer and Antioxidant Potential In Vitro. Pharmaceuticals 2025, 18, 1756. https://doi.org/10.3390/ph18111756
Stefanowicz-Hajduk J, Hering A, Hałasa R, Masiak S, Turczyn K, Ochocka JR, Asztemborska M. Coleus aromaticus Benth.—A Plant with Strong Anticancer and Antioxidant Potential In Vitro. Pharmaceuticals. 2025; 18(11):1756. https://doi.org/10.3390/ph18111756
Chicago/Turabian StyleStefanowicz-Hajduk, Justyna, Anna Hering, Rafał Hałasa, Szymon Masiak, Karolina Turczyn, J. Renata Ochocka, and Monika Asztemborska. 2025. "Coleus aromaticus Benth.—A Plant with Strong Anticancer and Antioxidant Potential In Vitro" Pharmaceuticals 18, no. 11: 1756. https://doi.org/10.3390/ph18111756
APA StyleStefanowicz-Hajduk, J., Hering, A., Hałasa, R., Masiak, S., Turczyn, K., Ochocka, J. R., & Asztemborska, M. (2025). Coleus aromaticus Benth.—A Plant with Strong Anticancer and Antioxidant Potential In Vitro. Pharmaceuticals, 18(11), 1756. https://doi.org/10.3390/ph18111756








