Borylated 5-Membered Ring Iminosugars: Synthesis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 2
Abstract
1. Introduction
2. Results and Discussion
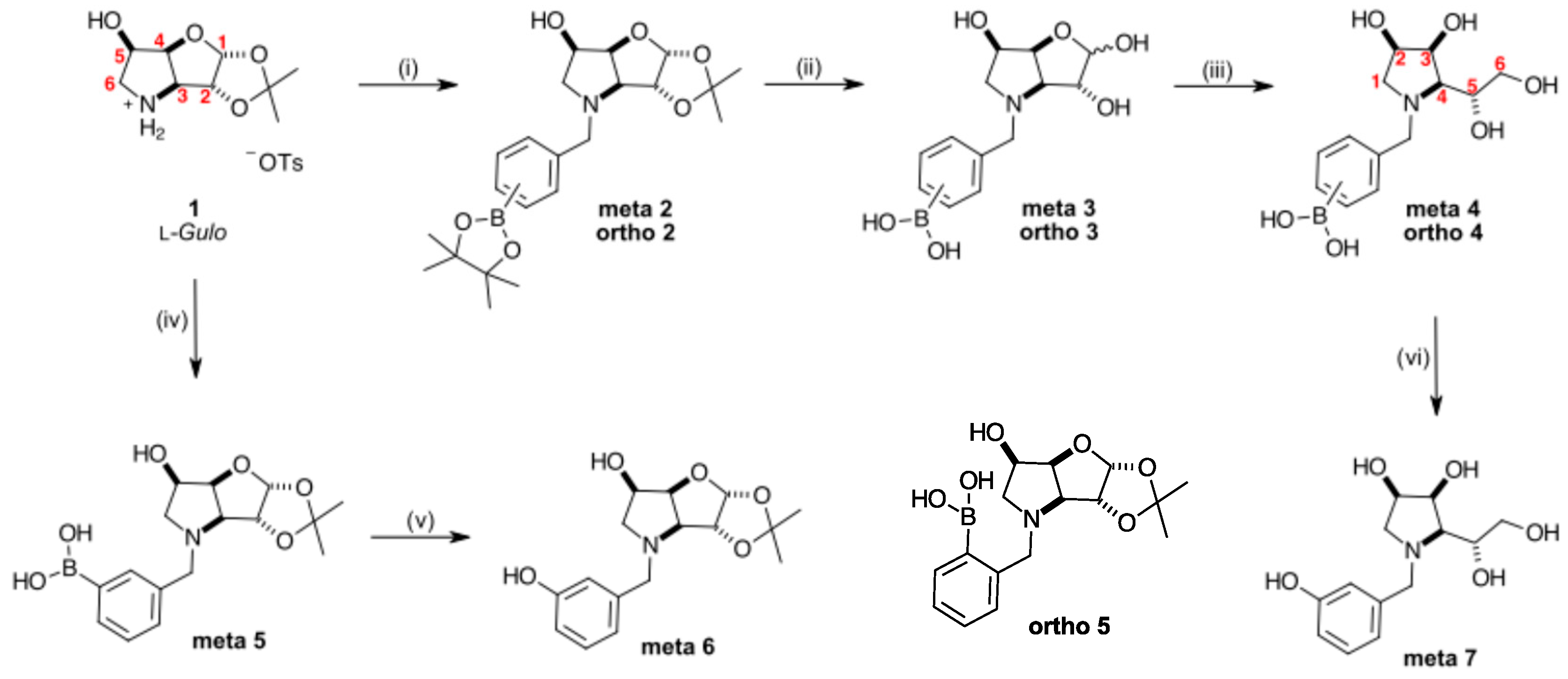
2.1. Synthesis
2.2. Biological Assays
2.2.1. Glycosidase Inhibition—Background
2.2.2. Glycosidase Inhibitions (Table 2, Scheme 1, Figure 1)
| Drug | α-d-Glucosidase | β-d-Glucosidase | α-d- Galactosidase | β-d-Galactosidase | ||||||
| Rice | Yeast | Rat Intestinal Maltase | Human Lysosome | Almond | Bovine Liver | Human Lysosome | Coffee Beans | Bovine Liver | E. coli | |
| BSH | aNI b(0%) | aNI b(6.9%) | aNI b(0%) | NA | aNI b(0%) | aNI b(15%) | NA | aNI b(12.3%) | aNI b(0%) | NA |
| 10B-BSH | aNI b(0%) | aNI b(5.6%) | aNI b(0%) | NA | aNI b(0%) | aNI b(11.9%) | NA | aNI b(3.9%) | aNI b(22.3%) | NA |
| BPA | cNI d(0%) | cNI d(0%) | cNI d(0%) | NA | cNI d(0%) | cNI d(0%) | NA | cNI d(4.3%) | cNI d(10.9%) | NA |
| 10B-BPA | cNI d(0%) | cNI d(0%) | cNI d(0%) | NA | cNI d(0%) | cNI d(14.2%) | NA | cNI d(1.2%) | cNI d(0%) | NA |
| ortho 2 | cNI d(0%) | cNI d(1%) | cNI d(15.5%) | cNI d(3.3%) | cNI d(35%) | cNI d(4.9%) | cNI d(5.6%) | cNI d(0%) | 362 | cNI d(8.8%) |
| ortho 2 | aNI b(0%) | aNI b(0%) | aNI b(0%) | NA | aNI b(7.2%) | aNI b(19.7%) | NA | aNI b(2.9%) | aNI b(21.8%) | NA |
| ortho 3 | cNI d(0%) | cNI d(2.9%) | cNI d(0%) | cNI d(0.3%) | cNI d(13.7%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | 617 | cNI d(4.4%) |
| ortho 3 | aNI b(0%) | aNI b(1%) | aNI b(0.7%) | NA | aNI b(38.8%) | 710 | NA | aNI b(0%) | 141 | NA |
| ortho 4 | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(2.2%) | cNI d(2.6%) | cNI d(3.3%) | cNI d(0%) | cNI d(0%) | cNI d(3.3%) | cNI d(14%) |
| ortho 4 | 622 | 784 | aNI b(29.4%) | NA | aNI b(40.1%) | aNI b(25.7%) | NA | aNI b(2.7%) | 533 | NA |
| ortho 5 | cNI d(6.7%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(2.7%) | cNI d(13.4%) | cNI d(3.5%) | cNI d(8.5%) | cNI d(6.1%) | cNI d(0%) |
| meta 2 | cNI d(0%) | cNI d(8.8%) | cNI d(5.8%) | cNI d(3.9%) | cNI d(6.8%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(2.2%) | cNI d(0%) |
| meta 2 | aNI b(2.9%) | aNI b(0%) | aNI b(12.4%) | NA | aNI b(12.6%) | aNI b(32.7%) | NA | aNI b(0%) | aNI b(41.8%) | NA |
| meta 3 | cNI d(0%) | cNI (1.4%) | cNI d(12.3%) | cNI d(3.5%) | cNI d(24.8%) | cNI d(8.2%) | cNI d(6.6%) | cNI d(0%) | 546 | cNI d(0.2%) |
| meta 3 | aNI b(5.9%) | aNI b(37%) | aNI b(2.0%) | NA | aNI b(45.8%) | 633 | NA | aNI b(0.7%) | 116 | NA |
| meta 4 | cNI d(8%) | cNI d(8.1%) | cNI d(22.8%) | cNI d(7.2%) | cNI d(0.6%) | cNI d(0%) | cNI d(13.6%) | cNI d(1.1%) | cNI d(24.6%) | cNI d(4.9%) |
| meta 4 | 106 | aNI b(4.9%) | 588 | NA | aNI b(43%) | aNI b(37.9%) | NA | aNI b(3.7%) | 322 | NA |
| meta 5 | cNI d(0%) | cNI d(7.8%) | cNI d(0%) | cNI d(0.3%) | cNI d(0%) | cNI d(23%) | cNI d(0%) | cNI d(1.5%) | cNI d(0%) | cNI d(0%) |
| meta 6 | cNI d(0%) | cNI d(0%) | cNI d(4.7%) | cNI d(3.3%) | cNI d(6.3%) | cNI d(15.8%) | cNI d(0%) | cNI d(0%) | cNI d(15.5%) | cNI d(0.8%) |
| meta 7 | cNI d(0%) | cNI d(3.4%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(9.9%) | cNI d(1.8%) | cNI d(17.5%) | cNI d(0%) |
| meta 7 | aNI b(5.0%) | aNI b(4.3%) | aNI b(7.2%) | NA | aNI b(9.6%) | aNI b(13.6%) | NA | aNI b(1.7%) | aNI b(8.1%) | NA |
| Drug | α-d- Mannosidase | β-d- Mannosidase | α-l- Rhamnosidase | α-l- Fucosidase | β-d-Glucuronidase | Trehalase | β-d- Glucanase | Amyloglucosidase | ||
| Jack Bean | Snail | P. decumbens | Bovine Kidney | E. coli | Bovine Liver | Porcine Kidney | T. Longibrachiatum | A. niger | ||
| BSH | aNI b(7.9%) | aNI b(19.2%) | aNI b(0.2%) | aNI b(0%) | aNI b(6%) | aNI b(19.6%) | aNI b(4.2%) | NA | aNI b(0%) | |
| 10B-BSH | aNI b(7.7%) | aNI b(15%) | aNI b(0%) | aNI b(6.5%) | aNI b(3.2%) | aNI b(12.5%) | aNI b(2.3%) | NA | aNI b(0%) | |
| BPA | cNI d(1.1%) | cNI d(2.1%) | cNI d(0%) | cNI d(0%) | cNI d(0.5%) | cNI d(7.4%) | cNI d(0%) | NA | cNI d(0%) | |
| 10B-BPA | cNI d(0%) | cNI d(0.3%) | cNI d(0%) | cNI d(0%) | cNI d(2.4%) | cNI d(0%) | cNI d(0%) | NA | cNI d(0%) | |
| ortho 2 | cNI d(9.4%) | cNI d(0%) | cNI d(1.5%) | NA | cNI d(4.1%) | cNI d(2.2%) | cNI d(5.6%) | cNI d(21%) | cNI d(0%) | |
| ortho 2 | aNI b(1.3%) | aNI b(7.8%) | aNI b(2.0%) | aNI b(7.8%) | aNI b(5.2%) | aNI b(8.4%) | aNI b(1.8%) | NA | aNI b(3.2%) | |
| ortho 3 | cNI d(3.8%) | cNI d(0%) | cNI d(0%) | NA | cNI d(0%) | cNI d(2.9%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | |
| ortho 3 | aNI b(0%) | aNI b(0%) | aNI b(3.2%) | aNI b(2.1%) | aNI b(8.4%) | aNI b(6.6%) | aNI b(0%) | NA | aNI b(2.1%) | |
| ortho 4 | cNI d(5.6%) | cNI d(5.3%) | cNI d(4.9%) | NA | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | |
| ortho 4 | aNI b(0.4%) | aNI b(2.3%) | aNI b(9.5%) | aNI b(1.3%) | aNI b(31.8%) | aNI b(0.9%) | aNI b(0%) | NA | aNI b(0%) | |
| ortho 5 | cNI d(12.9%) | cNI d(7.0%) | cNI d(0%) | NA | cNI d(1.2%) | cNI d(0%) | cNI d(4.9%) | cNI d(5.2%) | cNI d(4.9%) | |
| meta 2 | cNI d(7.5%) | cNI d(8.4%) | cNI d(1.5%) | NA | cNI d(13.7%) | cNI d(1.4%) | cNI d(0%) | cNI d(3.9%) | cNI d(1.4%) | |
| meta 2 | aNI b(0%) | aNI b(3.7%) | aNI b(0%) | aNI b(3.8%) | aNI b(5.6%) | aNI b(10.7%) | aNI b(5.8%) | NA | aNI b(0%) | |
| meta 3 | cNI d(0%) | cNI d(0%) | cNI d(1.2%) | NA | cNI d(16.2%) | cNI d(1.2%) | cNI d(20.9%) | cNI d(0%) | cNI d(24.6%) | |
| meta 3 | aNI b(0%) | aNI b(0%) | aNI b(3.2%) | aNI b(3.8%) | aNI b(36.9%) | aNI b(7.2%) | aNI b(0%) | NA | aNI b(1.5%) | |
| meta 4 | cNI d(0%) | cNI d(0%) | cNI d(8.3%) | NA | cNI d(3.5%) | cNI d(0%) | cNI d(0.8%) | cNI d(1.2%) | cNI d(6.6%) | |
| meta 4 | aNI b(0%) | aNI b(0%) | aNI b(14.7%) | aNI b(3.0%) | aNI b(20.6%) | aNI b(14.7%) | aNI b(3.4%) | NA | aNI b(3.6%) | |
| meta 5 | cNI d(6.3%) | cNI d(2.3%) | cNI d(0.6%) | NA | cNI d(4.1%) | cNI d(2.9%) | cNI d(0%) | cNI d(0%) | cNI d(0%) | |
| meta 6 | cNI d(9.5%) | cNI d(0.6%) | cNI d(6.5%) | NA | cNI d(0%) | cNI d(1.6%) | cNI d(0%) | cNI d(3.1%) | cNI d(4.8%) | |
| meta 7 | cNI d(0%) | cNI d(0%) | cNI d(3.8%) | NA | cNI d(0.5%) | cNI d(1.2%) | cNI d(0%) | cNI d(0%) | cNI d(5.1%) | |
| meta 7 | aNI b(0%) | aNI b(0%) | aNI b(2.1%) | aNI b(2.1%) | aNI b(7.5%) | aNI b(0%) | aNI b(1.9%) | NA | aNI b(2.1%) | |
2.2.3. Glycosidase Inhibitions (Table 3, Scheme 1, Figure 1)
| Drug | Sample Appearance | α-d-Glucosidase | β-d-Glucosidase | α-d-Mannosidase | N-Acetyl-β-d-glucosaminidase | N-Acetyl-β-d-Hexosaminidase | β-d-Glucuronidase | ||
|---|---|---|---|---|---|---|---|---|---|
| Yeast | Bacillus | Rat Intestine | Almond | Jack Bean | Bovine Kidney | Rat Intestine | Bovine Liver | ||
| BSH | In solution | 59 | 48.1 | NA | 47.3 | −18.7 | 37.1 | NA | 31.6 |
| 10B-BSH | In solution | 65.9 | 53 | NA | 49.9 | −16.8 | 40.9 | NA | 44.1 |
| BPA | Some in solution with undissolved sediment | 2.9 | 19.9 | NA | 3.9 | 0.6 | 6.7 | NA | −0.7 |
| 10B-BPA | 3.4 | 19.5 | NA | 3 | −0.7 | 6.3 | NA | −1 | |
| ortho 2 | In solution | −2 | NA | −6.7 | 73.3 | NA | NA | 13.8 | −1.8 |
| ortho 3 | In solution | 4.1 | NA | −1.4 | 68.6 | NA | NA | 19.2 | 1.3 |
| ortho 4 | In solution | −6.7 | NA | 17.4 | 49.8 | NA | NA | −0.2 | 2.2 |
| ortho 5 | In solution | 8.7/11 | NA | 4.2 | 8.7 | NA | NA | 5.2 | 1.4 |
| meta 2 | In solution | 9.3/7.9 | NA | 16.8 | 13.2 | NA | NA | 11.5 | 9.9 |
| meta 3 | In solution | −16.7 | NA | 12.1/8.9 | 61.7 | NA | NA | 8.7 | 13.3 |
| meta 4 | In solution | 23.5 | NA | 41.1 | 47.9 | NA | NA | −2.2 | −2.7 |
| meta 5 | In solution | 14.8/13.7 | NA | 11.6 | 7.7 | NA | NA | 5 | −0.9 |
| meta 6 | In solution | 6.6 | NA | 4.8 | 0.7 | NA | NA | −0.7 | 3.6 |
| meta 7 | In solution | 19.5 | NA | −10.7 | 2.9 | NA | NA | −2.5 | −3 |
2.2.4. Cancer Screening (Table 4, Scheme 1, Figure 1)
| Carcinomas | Normal | |||||
|---|---|---|---|---|---|---|
| Drug | HT29 Colon | A2780 Ovarian | H460 Lung | A431 Skin | MIA-Pa-Ca2 Pancreatic | MCF10A Breast |
| BSH | * 3 ± 2 | * 2 ± 5 | * 8 ± 2 | * <0 | * 2 ± 6 | * 8 ± 3 |
| 10B-BSH | * 5 ± 1 | * 5 ± 4 | * 4 ± 2 | * <0 | * 2 ± 4 | * 13 ± 4 |
| BPA | * 14 ± 0 | * 4 ± 1 | * 7 ± 8 | * 4 ± 6 | * 3 ± 3 | * 4 ± 1 |
| 10B-BPA | * 15 ± 4 | * 8 ± 4 | * 8 ± 5 | * 4 ± 4 | * 11 ± 3 | * <0 |
| ortho 2 | 7 ± 0 | 7 ± 2 | 11 ± 4 | 10 ± 5 | 9 ± 7 | 9 ± 4 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| ortho 3 | <0 | 17 ± 2 | 2 ± 1 | 3 ± 1 | 5 ± 5 | 9 ± 7 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| ortho 4 | <0 | 16 ± 8 | 8 ± 2 | 17 ± 2 | 7 ± 2 | 8 ± 4 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| ortho 5 | 4 ± 4 | 19 ± 2 | 2 ± 2 | 9 ± 4 | 12 ± 3 | 7 ± 6 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 2 | 1 ± 10 | 25 ± 2 | 5 ± 2 | 8 ± 6 | 19 ± 8 | 6 ± 4 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 3 | <0 | 18 ± 1 | 2 ± 2 | 3 ± 0 | 17 ± 4 | 6 ± 4 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 4 | <0 | 18 ± 5 | 7 ± 1 | 6 ± 1 | 16 ± 5 | 14 ± 13 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 5 | <0 | 32 ± 7 | 10 ± 2 | 8 ± 8 | 16 ± 1 | 7 ± 4 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 6 | 4 ± 3 | 23 ± 6 | 6 ± 3 | 4 ± 9 | 12 ± 4 | 4 ± 2 |
| >50 | >50 | >50 | >50 | >50 | >50 | |
| meta 7 | 29 ± 1 | 48 ± 7 | 37 ± 3 | 56 ± 6 | 39 ± 2 | 31 ± 16 |
| 50 ± 0.0 | 30 ± 20 | 47 ± 7.0 | 17 ± 8.5 | 51 ± 1.5 | 43 ± 17 | |
3. Materials and Methods
3.1. Glycosidase Inhibition for Table 2
3.2. Glycosidase Inhibition for Table 3
3.3. Cancer Screening
3.4. Numbering System
3.5. General Chemical Characterisation Methods
3.6. Reagents and Solvents
3.7. Chemistry Experimental Method
3.7.1. N-(3-Methylphenyl boronic acid pinacol ester)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose meta 2 (Scheme 1, Step (i))

3.7.2. N-(3-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-d-gulofuranose meta 3 (Scheme 1, Step (ii))

3.7.3. N-(3-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-l-gulitol meta 4 (Scheme 1, Step (iii))
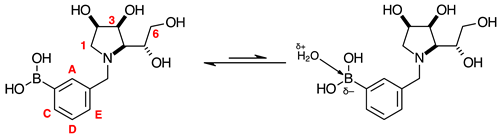
3.7.4. N-(2-Methylphenyl boronic acid pinacol ester)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose ortho 2 (Scheme 1, Step (i))
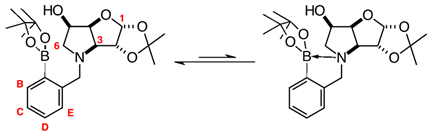
3.7.5. N-(2-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-d-gulofuranose ortho 3 (Scheme 1, Step (ii))

3.7.6. N-(2-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-l-gulitol ortho 4 (Scheme 1, Step (iii))
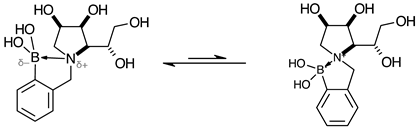
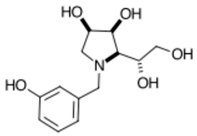
3.7.7. N-(3-Hydroxyphenyl)-1,4-dideoxy-1,4-imino-l-gulitol meta 7 (Scheme 1, step (vi))
3.7.8. N-(3-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-glucofuranose meta 5 (Scheme 1, step (iv)) and N-(3-hydroxyphenyl)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose meta 6 (Scheme 1, step (v))

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, H.C. Organic Syntheses via Boranes; John Wiley & Sons, Inc.: New York, NY, USA, 1975. [Google Scholar]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Brown, H.C.; Kulkarni, S.U. Organoboranes: XXV. Hydridation of dialkylhaloboranes. New practical syntheses of dialkylboranes under mild conditions. J. Organomet. Chem. 1981, 2018, 299. [Google Scholar] [CrossRef]
- Hall, D.G. Chapter 1: Structure, properties, and preparation of boronic acid derivatives. Overview of their reactions and applications. In Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley—VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Martin, J.E. Physics for Radiation Protection: A Handbook; WILEY—VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Hampshire, S. 2.01 Fundamental Aspects of Hard Ceramics. In Comprehensive Hard Materials; Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2. [Google Scholar]
- Gupta, S.; Chunduri, A.; Spreitzer, M.; Kržmanc, M.M.; Patel, N. 3—Synthesis and catalytic applications of metal boride ceramics. In Ceramic Catalysts, Materials, Synthesis, and Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Hong, J.; Mutalik, S.; Pescarmona, P.P.; Protesescu, L. Metal Borides: From Industrial Classics to Versatile Colloidal Nanocrystals for Energy, Catalysis, and Hard Coatings Applications. Chem. Mater. 2024, 36, 2147–2164. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Cheng, Z.; Han, Y.; Zhang, Y.; Dong, Y. Applications, prospects and challenges of metal borides in lithium sulfur batteries. J. Colloid Interface Sci. 2024, 657, 511–528. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, H.; Dudley, J.; Coca, A. Chapter 1: Boron Chemistry: An Overview. In Boron Reagents in Synthesis; ACS Symposium Series eBooks; ACS Publications: Washington, DC, USA, 2016; Volume 1236. [Google Scholar]
- Matsuda, I.; Wu, K. 2D Boron: Boraphene, Borophene, Boronene; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Reyes, H.; García, M.C.; Flores, B.M.; López-Rebolledo, H.; Santillán, R.; Farfán, N. Synthesis, NMR and X-Ray Diffraction Analysis of Boron Complexes Derived from Hydroxychalcones. J. Mex. Chem. Soc. 2006, 50, 106–113. [Google Scholar]
- Yamashita, M.; Yamamoto, Y.; Akiba, K.-Y.; Hashizume, D.; Iwasaki, F.; Takagi, N.; Nagase, S. Syntheses and Structures of Hypervalent Pentacoordinate Carbon and Boron Compounds Bearing an Anthracene Skeleton- Elucidation of Hypervalent Interaction Based on X-ray Analysis and DFT Calculation. J. Am. Chem. Soc. 2005, 127, 4354–4371. [Google Scholar] [CrossRef]
- Woods, W.G. An Introduction to Boron: History, Sources, Uses, and Chemistry. Environ. Health Perspect 1994, 102, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, J.; Hosmane, N.S.; Zhang, Y. Chapter 1: Introduction: Basic concept of boron and its physical and chemical properties. In Fundamentals and Applications of Boron Chemistry; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chatterjee, S.; Tripathi, N.M.; Bandyopadhyay, A. The modern role of boron as a ‘magic element’ in biomedical science: Chemistry perspective. Chem. Commun. 2021, 100, 13629–13640. [Google Scholar] [CrossRef]
- Légareé, M.-A.; Pranckevicius, C.; Braunschweig, H. Metallomimetic Chemistry of Boron. Chem. Rev. 2019, 119, 8231–8261. [Google Scholar] [CrossRef]
- Duret, G.; Quinlan, R.; Bisseret, P.; Blanchard, N. Boron chemistry in a new light. Chem. Sci. 2015, 10, 5366–5382. [Google Scholar] [CrossRef]
- Rubanov, Z.M.; Supranovich, V.I.; Levin, V.V.; Dilman, A.D. BF2-Chelates of N-Acylhydrazones as Versatile Coupling Partners in Photoredox Promoted Reactions. Eur. J. Org. Chem. 2023, 26, e202300247. [Google Scholar] [CrossRef]
- Yan, Q.-Q.; Zhao, X.Y.; Zhang, T.; Li, S.-D. Perfect Core-Shell Octahedral B@B38+, Be@B38, and Zn@B38 with an Octa-Coordinate Center as Superatoms Following the Octet Rule. ChemPhysChem 2023, 24, e202200947. [Google Scholar] [CrossRef]
- Selvaraj, C.; Rudhra, O.; Alothaim, A.S.; Alkhanani, M.; Singh, S.K. Structure and chemistry of enzymatic active sites that play a role in the switch and conformation mechanism. In Advances in Protein Chemistry and Structural Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 130. [Google Scholar]
- Goh, C.-S.; Milburn, D.; Gerstein, M. Conformational changes associated with protein–protein interactions. Curr. Opin. Struct. Biol. 2004, 14, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Belorkar, S.A.; Jogaiah, S. Chapter 1—Enzymes—Past, present, and future. In Protocols and Applications in Enzymology; Academic Press: London, UK, 2022. [Google Scholar]
- Lowering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. Med. Chem. Commun. 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Thompson, A.L.; Simone, M. Methyl 2-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)-4-nitrobenzoate. Acta Cryst. 2012, E68, o2699–o2700. [Google Scholar] [CrossRef]
- Simone, M. Diastereoselective Synthesis of the Borylated D-Galactose Monosaccharide 3-Boronic-3-Deoxy-D-Galactose and Biological Evaluation in Glycosidase Inhibition and in Cancer for Boron Neutron Capture Therapy (BNCT). Molecules 2023, 28, 4321. [Google Scholar] [CrossRef]
- Legge, W.J.; Shimadate, Y.; Sakoff, J.; Houston, T.A.; Kato, A.; Bernhardt, P.V.; Simone, M. Borylated methyl cinnamates: Green synthesis, characterization, crystallographic analysis and biological activities—In glycosidase inhibition and in cancer cells lines. Beilstein Arch. 2021, 2021, 20214. [Google Scholar] [CrossRef]
- Campkin, D.M.; Shimadate, Y.; Bartholomew, B.; Bernhardt, P.V.; Nash, R.J.; Sakoff, J.A.; Kato, A.; Simone, M. Borylated 2,3,4,5-Tetrachlorophthalimide and Their 2,3,4,5-Tetrachlorobenzamide Analogues: Synthesis, Their Glycosidase Inhibition and Anticancer Properties in View to Boron Neutron Capture Therapy. Molecules 2022, 27, 3447. [Google Scholar] [CrossRef]
- Prichard, K.; Yamamoto, S.; Shimadate, Y.; Yoshimura, K.; Bartholomew, B.; Gilbert, J.; Sakoff, J.; Nash, R.; Kato, A.; Simone, M. Borylated 5-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1. Pharmaceuticals 2025, 18, 1302. [Google Scholar] [CrossRef]
- Simone, M.; Soengas, R.G.; Jenkinson, S.F.; Evinson, E.L.; Nash, R.J.; Fleet, G.W.J. Synthesis of three branched iminosugars [(3R,4R,5S)-3-(hydroxymethyl)piperidine-3,4,5-triol, (3R,4R,5R)-3-(hydroxymethyl)piperidine-3,4,5-triol and (3S,4R,5R)-3-(hydroxymethyl)piperidine-3,4,5-triol] and a branched trihydroxynipecotic acid [(3R,4R,5R)-3,4,5-trihydroxypiperidine-3-carboxylic acid] from sugar lactones with a carbon substituent at C-2. Tetrahedron: Asymm. 2012, 23, 401–408. [Google Scholar] [CrossRef]
- Simone, M. Borylated Monosaccharide 3-Boronic-3-deoxy-D-galactose: Detailed NMR Spectroscopic Characterisation, and Method for Spectroscopic Analysis of Anomeric and Boron Equilibria. Int. J. Mol. Sci. 2024, 25, 12396. [Google Scholar] [CrossRef]
- Simone, M. Borylated 5-Membered Ring Iminosugars: Detailed Nuclear Magnetic Resonance Spectroscopic Characterisation, and Method for Analysis of Anomeric and Boron Equilibria. Molecules 2025, 30, 1402. [Google Scholar] [CrossRef] [PubMed]
- Glenister, A.; Simone, M.; Hambley, T.W. A Warburg effect targeting vector designed to increase the uptake of compounds by cancer cells demonstrates glucose and hypoxia dependent uptake. PLoS ONE 2019, 14, e0217712. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Peach, J.M.; Smith, P.W.; Austin, G.N.; Baird, P.D.; Watkin, D.J. 3,6-Dideoxy-3,6-imino-1,2-O-isopropylidene-α-D-glucofuranose as a divergent intermediate for the synthesis of hydroxylated pyrrolidines: Synthesis of 1,4-dideoxy-1,4-imino-L-gulitol, 1,4-dideoxy-1,4-imino-D-lyxitol, (2S,3S,4R)-3,4-dihydroxyproline and (1S,2R,8S,8aR)-1,2,8-trihydroxyoctahydroindolizine [8-episwainsonine]. X-ray crystal structure of (1S,2R,8S,8aR)-1,2,8-trihydroxy-5-oxooctahydroindolizine. Tetrahedron 1987, 43, 3095–3108. [Google Scholar] [CrossRef]
- Boshagen, H.; Heiker, F.-R.; Schuller, M. The chemistry of the 1-deoxynojirimycin system. Synthesis of 2-acetamido-1,2-dideoxynojirimycin from 1-deoxynojirimycin. Carbohydr. Res. 1987, 164, 141–148. [Google Scholar] [CrossRef]
- Zinner, H.; Wulf, G.; Heinatz, R. Derivate der Zucker-mercaptale, XXXV. Darstellung und Mercaptalbildung der 2-Desoxy-D-xylose. Chem. Ber. 1964, 97, 3536–3540. [Google Scholar] [CrossRef]
- Moore, J.L.; Taylor, S.M.; Soloshonok, V.A. An efficient and operationally convenient general synthesis of tertiary amines by direct alkylation of secondary amines with alkyl halides in the presence of Huenig’s base. Arkivoc 2005, vi, 287–292. [Google Scholar] [CrossRef]
- Wang, G.-N.; Twigg, G.; Butters, T.D.; Zhang, S.; Zhang, L.; Zhang, L.-H.; Ye, X.-S. Synthesis of N-substituted ε-hexonolactams as pharmacological chaperones for the treatment of N370S mutant Gaucher disease. Org. Biomol. Chem. 2012, 10, 2923–2927. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Chen, C.; Xiong, Y.; Zhang, L.-H.; Ye, J.; Ye, X.-S. Synthesis of N-substituted iminosugar derivatives and their immunosuppressive activities. Carbohydr. Res. 2010, 345, 780–786. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Zhou, X.; Zhou, J.; Zhang, L.-H.; Yea, X.-S.; Zhang, X.-L. An expeditious one-pot synthesis of 1,6-dideoxy-N-alkylated nojirimycin derivatives and their inhibitory effects on the secretion of IFN-g and IL-4. Bioorg. Med. Chem. 2008, 16, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, Y.; Boyle, K.M.O.; Murphy, P.V. Hybrid angiogenesis inhibitors: Synthesis and biological evaluation of bifunctional compounds based on 1-deoxynojirimycin and aryl-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2008, 18, 954–958. [Google Scholar] [CrossRef]
- Daher, S.A.; Fleet, G.; Namgoong, S.K.; Winchester, B. Change in specificity of glycosidase inhibition by N-alkylation of amino sugars. Biochem. J. 1989, 258, 613–615. [Google Scholar] [CrossRef]
- Guazzelli, L.; D’Andrea, F.; Sartini, S.; Giorgelli, F.; Confini, G.; Quattrini, L.; Piano, I.; Gargini, C.; Motta, C.L. Synthesis and investigation of polyhydroxylated pyrrolidine derivatives as novel chemotypes showing dual activity as glucosidase and aldose reductase inhibitors. Bioorg. Chem. 2019, 92, 103298. [Google Scholar] [CrossRef]
- Yang, L.-F.; Shimadate, Y.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and glycosidase inhibition of N-substituted derivatives of 1,4-dideoxy-1,4-imino-D-mannitol (DIM). Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef]
- Winchester, B.; Daher, S.A.; Carpenter, N.C.; Bello, I.C.D.; Choi, S.S.; Fairbanks, A.J.; Fleet, G.W.J. The structural basis of the inhibition of human α-mannosidases by azafuranose analogues of mannose. Biochem. J. 1993, 290, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Šesták, S.; Bella, M.; Klunda, T.; Gurská, S.; Dzubáck, P.; Wöls, F.; Wilson, I.B.H.; Sladek, V.; Hajdúch, M.; Poláková, M.; et al. N-Benzyl Substitution of Polyhydroxypyrrolidines: The Way to Selective Inhibitors of Golgi α-Mannosidase II. ChemMedChem 2018, 13, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L.; Houston, T.A. A drug targeting motif for glycosidase inhibitors: An iminosugar–boronate shows unexpectedly selective β-galactosidase inhibition. Tetrahedron Lett. 2002, 43, 8905–8908. [Google Scholar] [CrossRef]
- Cox, T.M. Gaucher’s Disease—An exemplary monogenic disorder. QJM Int. J. Med. 2001, 94, 399–402. [Google Scholar] [CrossRef][Green Version]
- Mena-Barragán, T.; García-Moreno, M.I.; Sevšek, A.; Okazaki, T.; Nanba, E.; Higaki, K.; Martin, N.I.; Pieters, R.J.; Fernández, J.M.G.; Mellet, C.O. Probing the Inhibitor versus Chaperone Properties of sp2-Iminosugars towards Human β-Glucocerebrosidase: A Picomolar Chaperone for Gaucher Disease. Molecules 2018, 23, 927. [Google Scholar] [CrossRef]
- Martín-Banderas, L.; Holgado, M.A.; Durán-Lobato, M.; Infante, J.J.; Álvarez-Fuentes, J.; Fernández-Arévalo, M. Role of Nanotechnology for Enzyme Replacement Therapy in Lysosomal Diseases. A Focus on Gaucher’s Disease. Curr. Med. Chem. 2016, 23, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Zhang, S.; Smith, A.C.; Callahan, J.W. Impaired Elastic-Fiber Assembly by Fibroblasts from Patients with Either Morquio B Disease or Infantile GM1-Gangliosidosis Is Linked to Deficiency in the 67-kD Spliced Variant of β-Galactosidase. Am. J. Hum. Genet. 2000, 67, 23–26. [Google Scholar] [CrossRef]
- Prichard, K.; Campkin, D.; O’Brien, N.; Kato, A.; Fleet, G.W.J.; Simone, M. Biological Activities of 3,4,5-Trihydroxypiperidines and their O- and N-Alkylated Derivatives. Chem. Biol. Drug Des. 2018, 92, 1171–1197. [Google Scholar] [CrossRef] [PubMed]
- Nicoll-Griffith, D.A.; Falgueyret, J.P.; Silva, J.M.; Morin, P.E.; Trimble, L.; Chan, C.C.; Clas, S.; Leger, S.; Wang, Z.; Yergey, J.A.; et al. Oxidative bioactivation of the lactol prodrug of a lactone cyclooxygenase-2 inhibitor. Drug. Metab. Dispos. 1999, 27, 403–409. [Google Scholar] [CrossRef]
- Gajula, S.N.R.; Nadimpalli, N.; Sonti, R. Drug metabolic stability in early drug discovery to develop potential lead compounds. Drug Metabolism Rev. 2021, 53, 459–477. [Google Scholar] [CrossRef]
- Li, L.; Chenna, B.C.; Yang, K.S.; Cole, T.R.; Goodall, Z.T.; Giardini, M.; Moghadamchargari, Z.; Hernandez, E.A.; Gomez, J.; Calvet, C.M.; et al. Self-Masked Aldehyde Inhibitors: A Novel Strategy for Inhibiting Cysteine Proteases. J. Med. Chem. 2021, 64, 11267–11287. [Google Scholar] [CrossRef]
- Volle, J.-N.; Filippini, D.; Krawczy, B.; Kaloyanov, N.; Lee, A.V.d.; Maurice, T.; Pirat, J.-L.; Virieux, D. Drug discovery: Phosphinolactone, in vivo bioisostere of the lactol group. Org. Biomol. Chem. 2010, 8, 1438–1444. [Google Scholar] [CrossRef]
- Ahuja-Casarín, A.I.; Merino-Montiel, P.; Vega-Baez, J.L.; Montiel-Smith, S.; Fernandes, M.X.; Lagunes, I.; Maya, I.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Tuning the activity of iminosugars: Novel N-alkylated deoxynojirimycin derivatives as strong BuChE inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 36, 138–146. [Google Scholar] [CrossRef]
- Iftikhar, M.; Lu, Y.; Zhou, M. An overview of therapeutic potential of N-alkylated 1-deoxynojirimycin congeners. Carbohydr. Res. 2021, 504, 108317. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Tanaka, Y.; Oki, H.; Sato, S.; Shibata, S.; Maru, T.; Tanaka, Y.; Tanaka, M.; Onishi, T. A new brain-penetrant glucosylceramide synthase inhibitor as potential Therapeutics for Gaucher disease. J. Neurochem. 2021, 159, 543–553. [Google Scholar] [CrossRef]
- Rawlings, A.J.; Lomas, H.; Pilling, A.W.; Lee, M.J.-R.; Alonzi, D.S.; Rountree, J.S.S.; Jenkinson, S.F.; Fleet, G.W.J.; Dwek, R.A.; Jones, J.H.; et al. Synthesis and Biological Characterisation of Novel N-Alkyl-Deoxynojirimycin a-Glucosidase Inhibitors. ChemBioChem 2009, 10, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Glenister, A.; Chen, C.K.J.; Paterson, D.J.; Renfrew, A.K.; Simone, M.; Hambley, T.W. Warburg Effect Targeting Co(III) Cytotoxin Chaperone Complexes. J. Med. Chem. 2021, 64, 2678–2690. [Google Scholar] [CrossRef]
- Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Müller, M.; Semenza, G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim. Biophys. Acta 1978, 506, 136–154. [Google Scholar] [CrossRef] [PubMed]
- IUPAC and IUBMB Joint Commission on Biochemical Nomenclature; McNaught, A.D. Nomenclature of Carbohydrates. Pure Appl. Chem. 1996, 68, 1919–2008. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Bruker. NMR Software TopSpin. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html?utm_source=Advertising&utm_medium=GoogleAd&utm_campaign=BBIO-Software-Cross-All-TopSpin-H2-2025&utm_source=Advertising&utm_medium=GoogleAd&utm_campaign=BBIO-Software-Cross-All-H2-2022&gad_source=1&gad_campaignid=15149563659&gbraid=0AAAAADsq6H1Plj_hwRuSXc2BkFGqPxpxq&gclid=EAIaIQobChMI6qmQ4oXojgMVLaNmAh3L9Q0jEAAYASABEgJxrfD_BwE (accessed on 20 September 2025).
- Peak, D.; Luther, G.W., III; Sparks, D.L. ATR-FTIR spectroscopic studies of boric acid adsorption on hydrous ferric oxide. Geochim. Cosmochim. Acta 2003, 67, 2551–2560. [Google Scholar] [CrossRef]
- Faniran, J.A.; Shurvell, H.F. Infrared spectra of phenylboronic acid (normal and deuterated) and diphenyl phenylboronate. Can. J. Chem. 1968, 46, 2089–2095. [Google Scholar] [CrossRef]
- Balachander, L.; Ramadevudu, G.; Shareefuddina, M.; Sayanna, R.; Venudhar, Y.C. IR analysis of borate glasses containing three alkali oxides. ScienceAsia 2013, 39, 278–283. [Google Scholar] [CrossRef]
- Chaplin, M. Structure and Properties of Water in its Various States Fundamentals of Water, Chemistry, Particles, and Ecology. In Encyclopedia of Water: Science, Technology, and Society; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Finch, A.; Pearn, E.J. Boron-oxygen stretching modes in cyclic systems. Spectrochim. Acta 1963, 19, 1621–1623. [Google Scholar] [CrossRef]
- Stuart, B.H. Chapter 4: Organic Molecules. In Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 71–93. [Google Scholar]
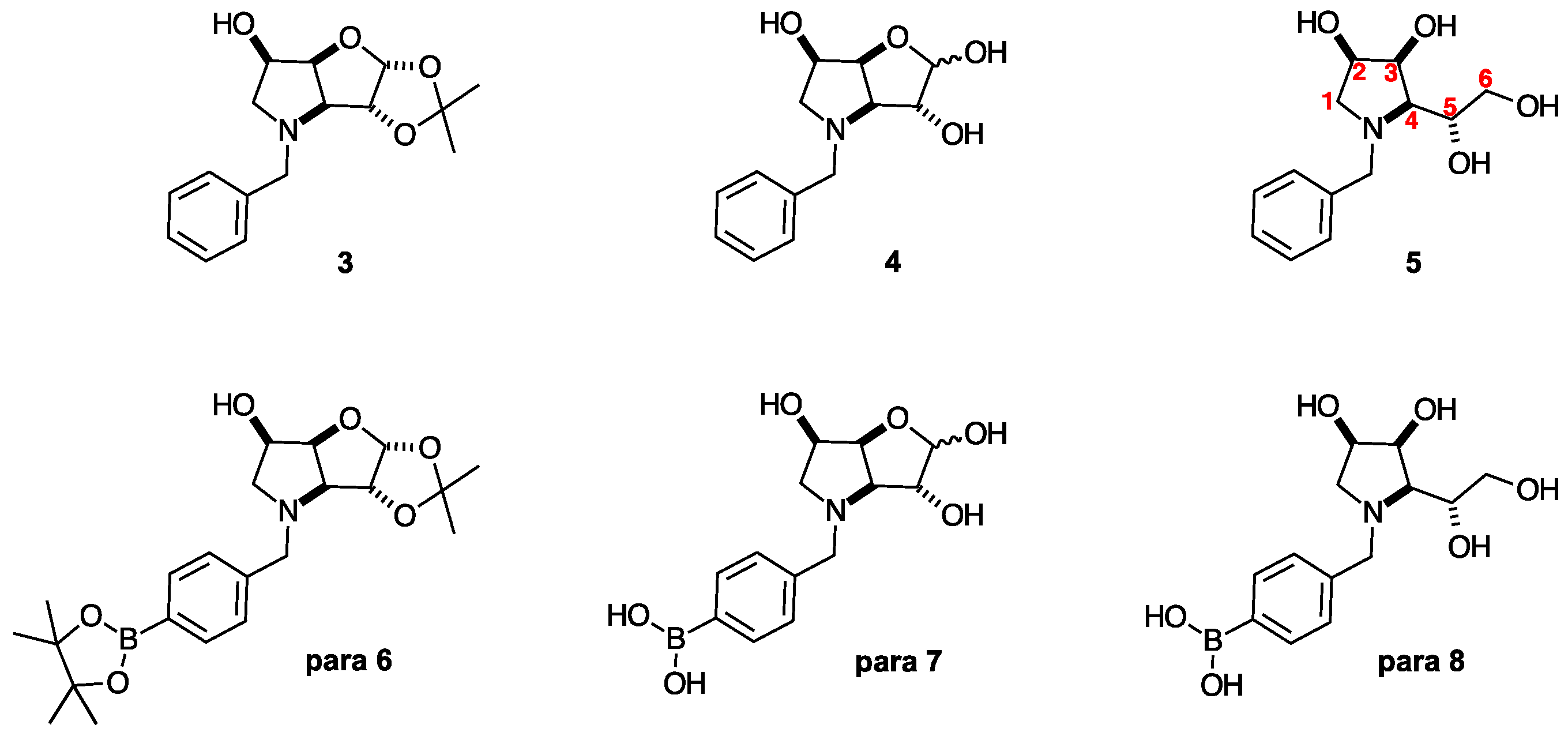
| Iminosugar | Most Inhibited Glycosidase/s |
|---|---|
| N-Benzyl-1,4-dideoxy-1,4-imino- | |
| d-allitol [43] | α-l-Fucosidase (76%) |
| d-galactitol [44] | α-d-Glucosidase (93.2%), IC50 = 40.6 μM |
| l-gulitol 5 [30] | NI |
| d-mannitol.HCl [45,46,47] | α-d-Mannosidases (lysosomal acidic, 34%), (neutral, 44%) and (Golgi II, 72%) |
| d-talitol.HCl [48] | NI |
| N-(2-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-d-talitol.HCl [48] | β-d-Galactosidase (E. coli) (44–55%) |
| N-Benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 [30] | β-d-Galactosidase (bovine liver), IC50 = 133 μM |
| N-(4-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-d-gulofuranose para 7 [30] | β-d-Galactosidase (bovine liver), IC50 = 218 μM |
| N-(4-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-l-gulitol para 8 [30] | β-d-Galactosidase (bovine liver), IC50 = 501 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prichard, K.; Yoshimura, K.; Yamamoto, S.; Taguchi, A.; Bartholomew, B.; Gilbert, J.; Sakoff, J.; Nash, R.; Kato, A.; Simone, M. Borylated 5-Membered Ring Iminosugars: Synthesis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 2. Pharmaceuticals 2025, 18, 1739. https://doi.org/10.3390/ph18111739
Prichard K, Yoshimura K, Yamamoto S, Taguchi A, Bartholomew B, Gilbert J, Sakoff J, Nash R, Kato A, Simone M. Borylated 5-Membered Ring Iminosugars: Synthesis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 2. Pharmaceuticals. 2025; 18(11):1739. https://doi.org/10.3390/ph18111739
Chicago/Turabian StylePrichard, Kate, Kosuke Yoshimura, Suzuka Yamamoto, Atsumi Taguchi, Barbara Bartholomew, Jayne Gilbert, Jennette Sakoff, Robert Nash, Atsushi Kato, and Michela Simone. 2025. "Borylated 5-Membered Ring Iminosugars: Synthesis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 2" Pharmaceuticals 18, no. 11: 1739. https://doi.org/10.3390/ph18111739
APA StylePrichard, K., Yoshimura, K., Yamamoto, S., Taguchi, A., Bartholomew, B., Gilbert, J., Sakoff, J., Nash, R., Kato, A., & Simone, M. (2025). Borylated 5-Membered Ring Iminosugars: Synthesis and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 2. Pharmaceuticals, 18(11), 1739. https://doi.org/10.3390/ph18111739







