Effect of Nano-Gefitinib on Solid Ehrlich Carcinoma via Targeting EGFR, RIPK2 Pathways, and Macrophage Reprogramming
Abstract
1. Introduction
2. Results
2.1. Bilosomes Characterization
Particle size, Zeta Potential, Poly Dispersity Index (PDI), and Drug Content
2.2. Transmission Electron Microscope (TEM) Results
2.3. FTIR Analysis Results
2.4. Effect of Nano-Gefitinib on Tumor Volume and Tumor Weight in the Ehrlich Carcinoma Mice Model
2.5. Effect of Nano-Gefitinib Against Ehrlich Carcinoma-Induced Alterations of Hepatic Function in Mice
2.6. Effect of Nano-Gefitinib Against Ehrlich Carcinoma-Induced Hepatic Oxidative Stress in Mice
2.7. Effect of Nano-Gefitinib Against Ehrlich Carcinoma-Induced Alterations in Serum Tumor Marker in Mice
2.8. Effect of Nano-Gefitinib Against Ehrlich Carcinoma-Induced Hepatic Apoptosis in Mice
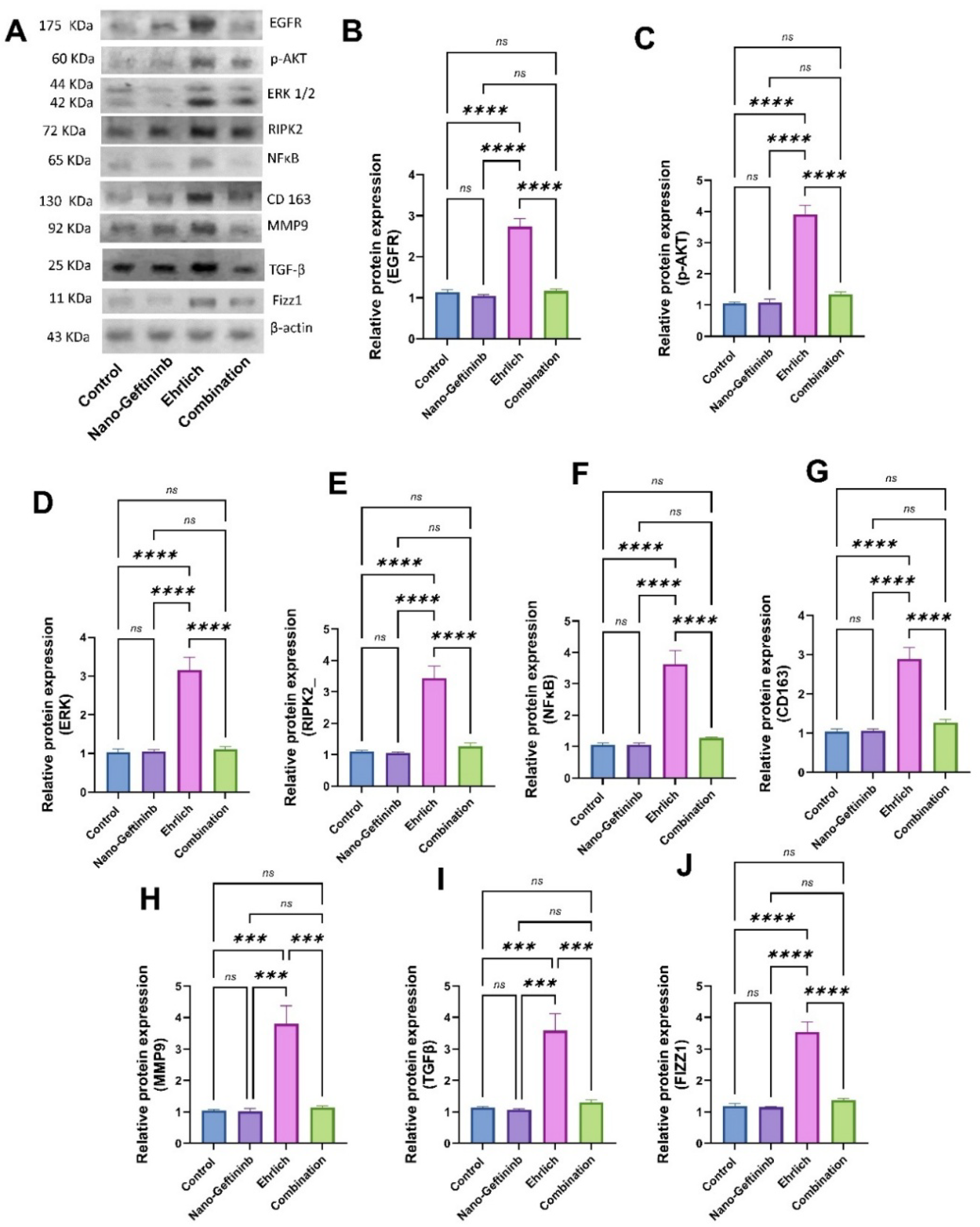
2.8.1. Effect of Nano-Gefitinib on Protein Expression of EGFR/p-AKT/ERK/RIPK2/NFκB in Ehrlich Mice Model
2.8.2. Effect of Nano-Gefitinib on Protein Expression of CD163/MMP9/TGF-β/Fizz1 in Ehrlich Mice Model
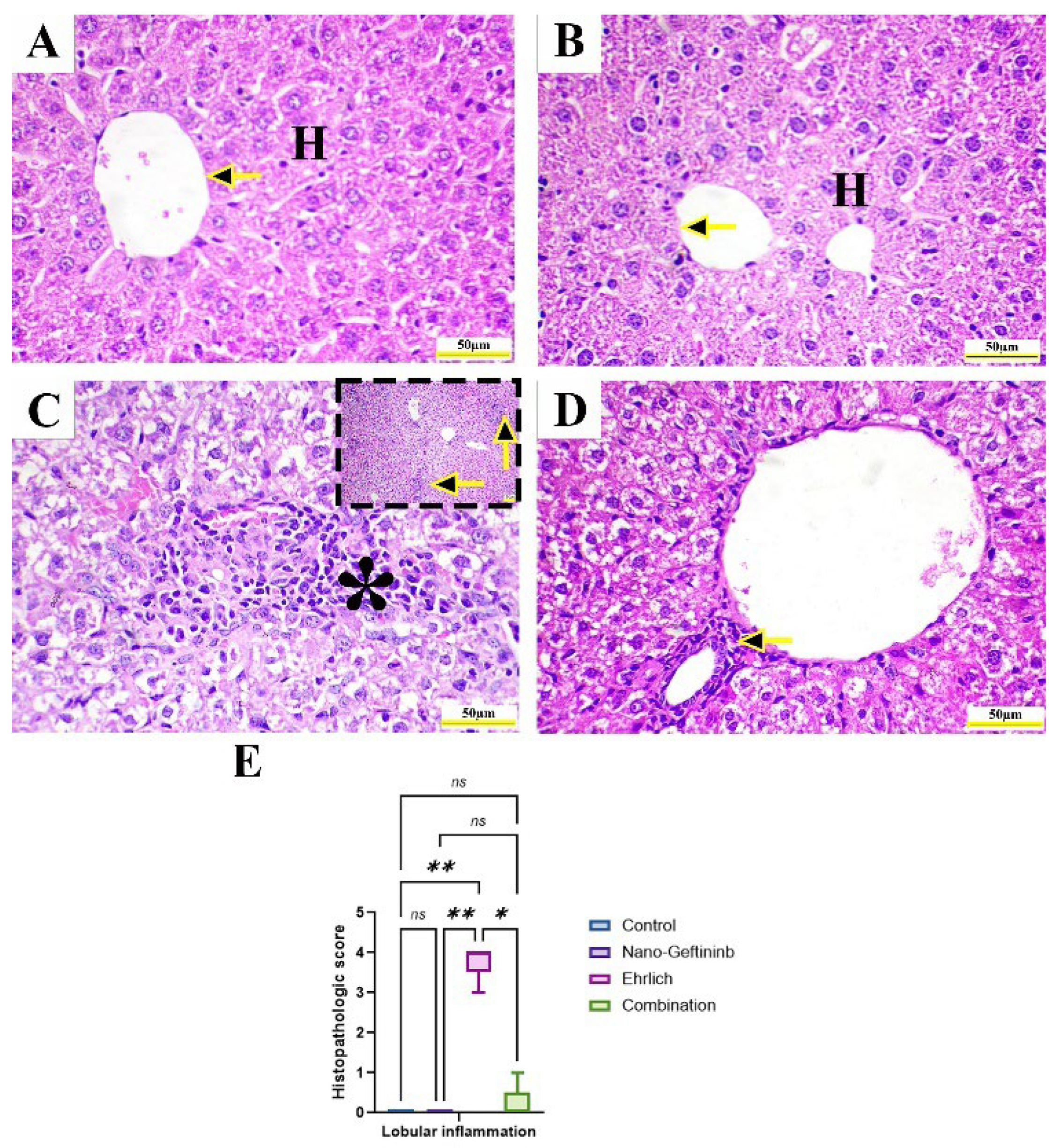
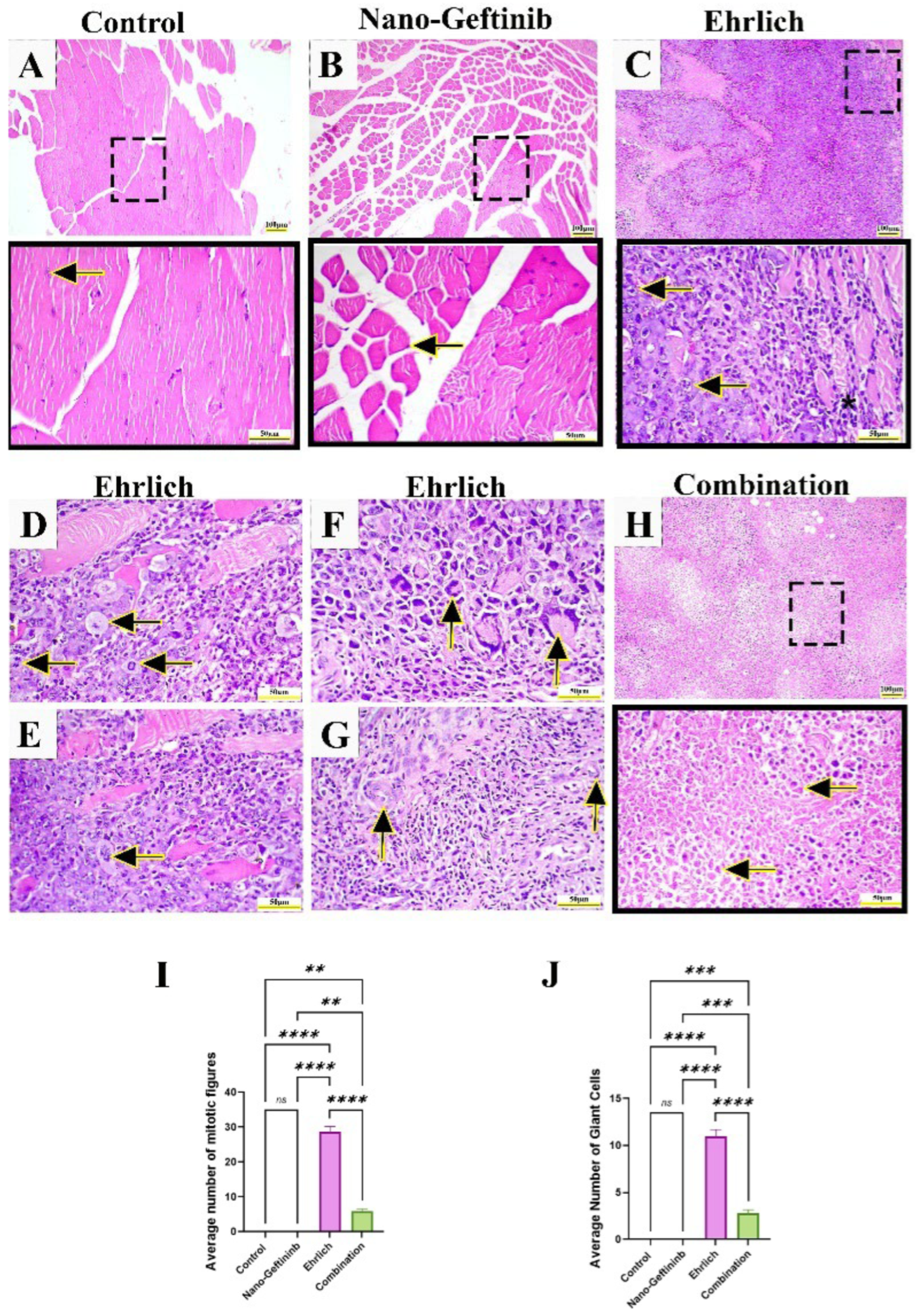
2.8.3. Effect of Nano-Gefitinib on Histopathological Alterations in Ehrlich Mice Model
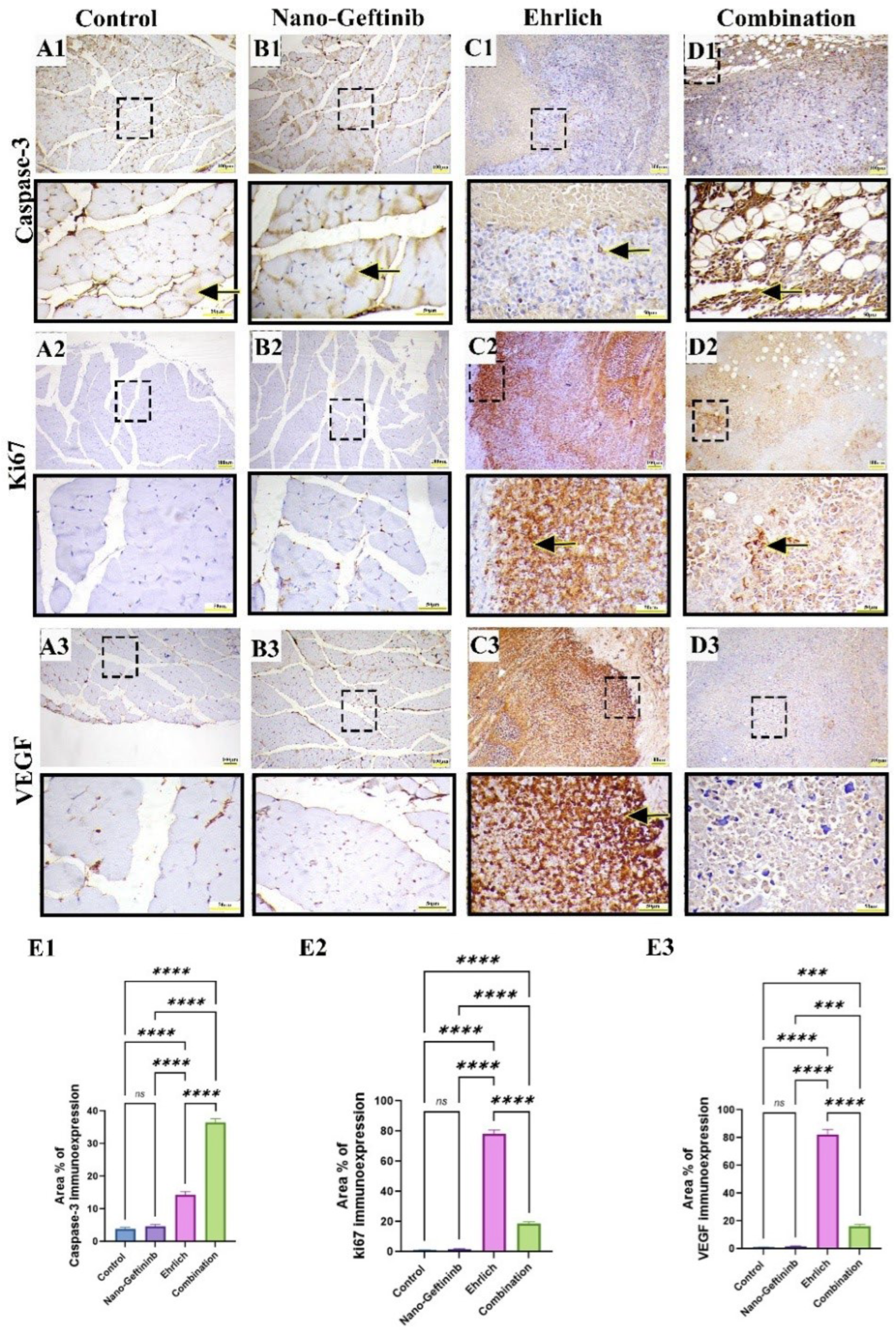
2.8.4. Effect of Nano-Gefitinib on Immunohistochemical Expression of Caspase-3, Ki67, and VEGF1 in Ehrlich Mice Model
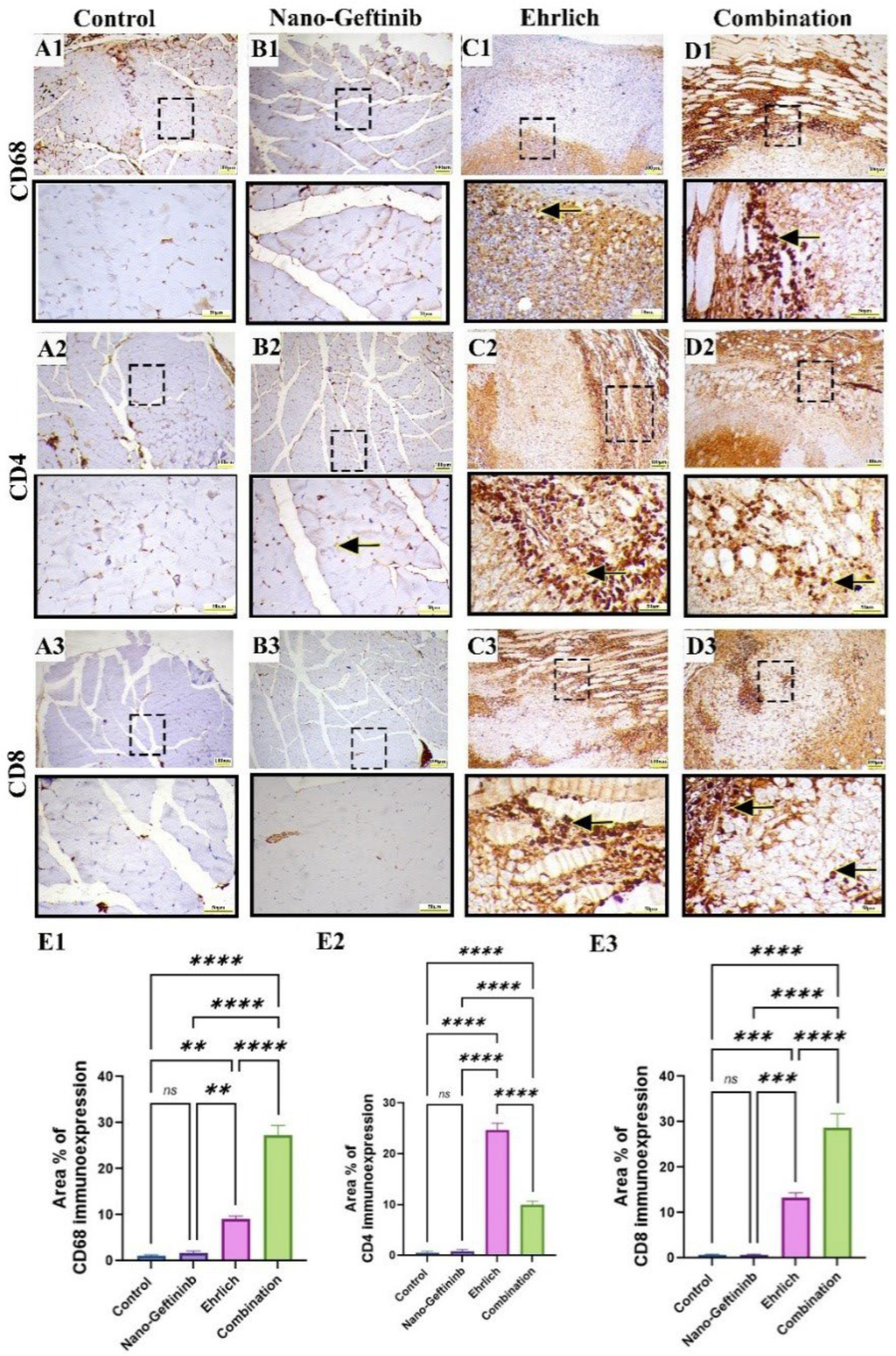
2.9. Effect of Nano-Gefitinib on Immunohistochemical Expression of CD68 Macrophage, CD4 and CD8 Lymphocytes in Ehrlich Mice Model
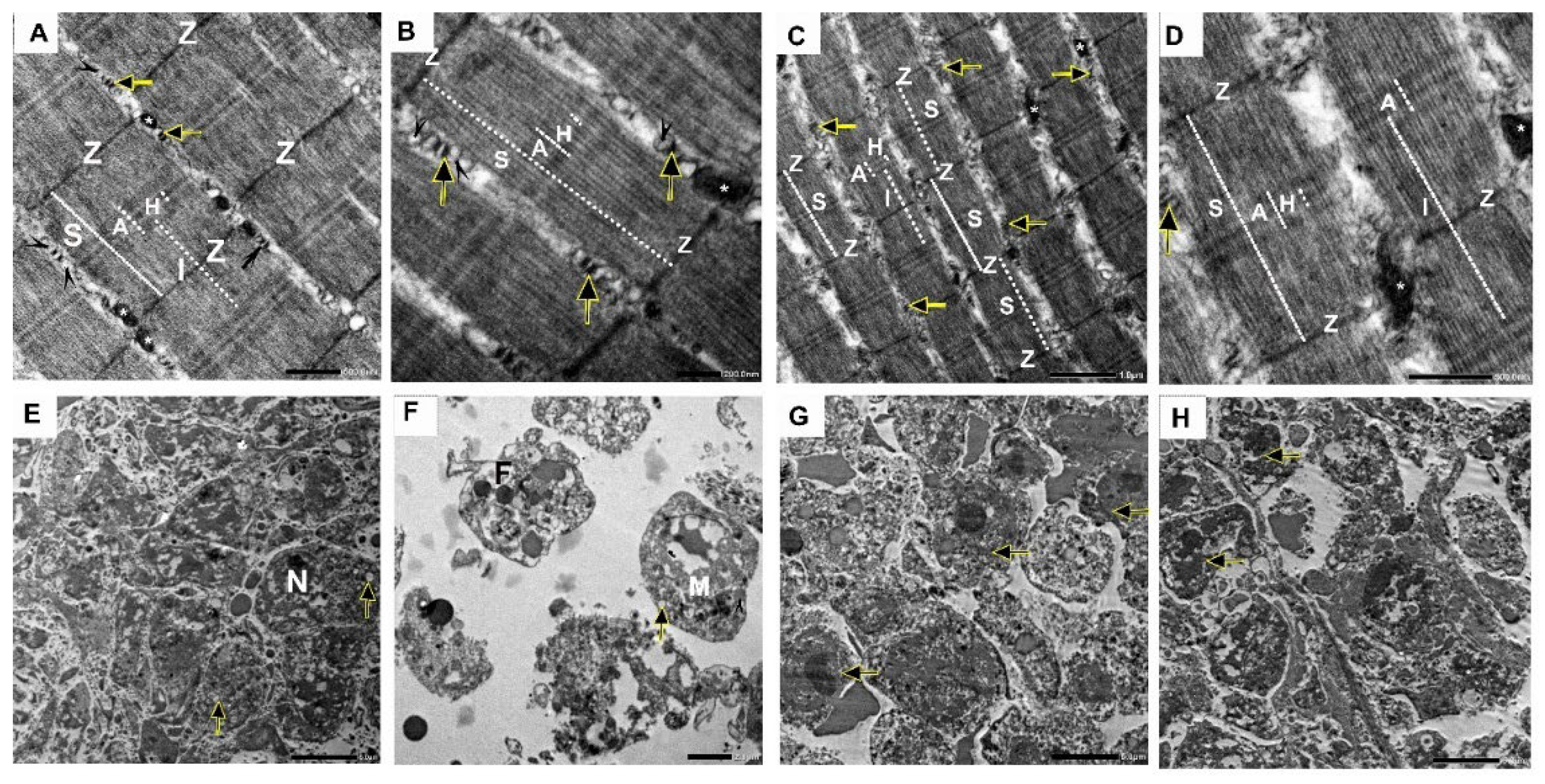
2.10. Effect of Nano-Gefitinib on Ultrastructural Changes Induced by Ehrlich Inoculation in Mice Model
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Preparation of Bilosomes
4.3. Bilosomes Characterization
4.3.1. Particle Size, Zeta Potential, Poly Disperisty Index (PDI)
4.3.2. Drug Content and % Entrapment Efficiency (EE)
4.3.3. Transmission Electron Microscope (TEM)
4.3.4. FTIR Analysis
4.4. Experimental Animals
4.5. Tumor Induction and Experimental Design
4.6. Evaluation of Tumor Volume and Weight
4.7. Blood and Tissue Sampling
4.8. Evaluation of Hepatic Performance Using Serum Biochemical Markers
4.9. Evaluation of Serum Tumor Markers
4.10. Evaluation of Hepatic Oxidative Stress Biomarkers
4.11. Hepatic Apoptotic Markers Assessment
4.12. Western Blot Analysis
4.13. Histopathological Assessment
4.14. Immunohistochemical Protein Assay
4.15. Electron Microscope Assessment
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFR-TKIs | Epidermal growth factor receptor-tyrosine kinase inhibitors |
| TG | Triglyceride |
| LDL | Low density lipoprotein |
| HDL | High density lipoprotein |
| TGF | Transforming growth factor |
| NF-κB | Nuclear factor kappa B |
| VEGF | Vascular endothelial growth factor |
| MMP | Matrix metalloproteinase |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Ehrlich, P. Experimentelle carcinomstudien an Mäusen; G. Fischer: Frankfurt am Main, Germany, 1906; Available online: https://cir.nii.ac.jp/crid/1970867909782048153 (accessed on 8 October 2025).
- Queiroz, M.L.; Valadares, M.; Bincoletto, C.; Dieamant, G. Ehrlich ascites tumor as a tool in the development of compounds with immunomodulatory properties. Immunopharmacol. Immunotoxicol. 2004, 26, 511–525. [Google Scholar] [CrossRef]
- Bahr, H.I.; Toraih, E.A.; Mohammed, E.A.; Mohammad, H.M.F.; Ali, E.A.I.; Zaitone, S.A. Chemopreventive effect of leflunomide against Ehrlich’s solid tumor grown in mice: Effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015, 141, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.P.; Panigrahy, U.P.; Panda, S.; Jena, B.R. Stem extract of Tabebuia chrysantha induces apoptosis by targeting sEGFR in Ehrlich Ascites Carcinoma. J. Ethnopharmacol. 2019, 235, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGFR family and its ligands in human cancer: Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37, 3–8. [Google Scholar] [CrossRef]
- El-Rayes, B.; LoRusso, P. Targeting the epidermal growth factor receptor. Br. J. Cancer 2004, 91, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Corvaja, C.; Passaro, A.; Attili, I.; Aliaga, P.T.; Spitaleri, G.; Del Signore, E.; de Marinis, F. Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging biological insights and therapeutic development. Cancer Treat. Rev. 2024, 130, 102824. [Google Scholar] [CrossRef]
- Ahmad, I.; Patel, H.M. From challenges to solutions: A review of fourth-generation EGFR tyrosine kinase inhibitors to overcome the C797S triple mutation in non-small cell lung cancer. Eur. J. Med. Chem. 2025, 284, 117178. [Google Scholar] [CrossRef]
- Averbuch, S.D. The impact of gefitinib on epidermal growth factor receptor signaling pathways in cancer. Clin. Lung Cancer 2003, 5 (Suppl. 1), S5–S10. [Google Scholar] [CrossRef]
- Han, W.; Shi, L.; Ren, L.; Zhou, L.; Li, T.; Qiao, Y.; Wang, H. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal Transduct. Target. Ther. 2018, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Gao, G.; Habaz, I.A.; Wang, Y. Mechanisms of resistance to tyrosine kinase inhibitor-targeted therapy and overcoming strategies. MedComm 2024, 5, e694. [Google Scholar] [CrossRef]
- Alawi, M.; Hilles, A.R.; Kumar, M.; Ansari, M.D.; Mahmood, S. Lipid-polymer hybrid nanoparticles: A cutting-edge frontier in breast cancer treatment strategies. Nanomedicine 2025, 20, 1775–1798. [Google Scholar] [CrossRef]
- Ni, X.L.; Chen, L.X.; Zhang, H.; Yang, B.; Xu, S.; Wu, M.; Liu, J.; Yang, L.L.; Chen, Y.; Fu, S.Z. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017, 24, 1501–1512. [Google Scholar] [CrossRef]
- Mukherjee, S.; Joshi, V.; Reddy, K.P.; Singh, N.; Das, P.; Datta, P. Biopharmaceutical and pharmacokinetic attributes to drive nanoformulations of small molecule tyrosine kinase inhibitors. Asian J. Pharm. Sci. 2024, 19, 100980. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bouchnita, A.; Volpert, V.; Koury, M.J.; Hellander, A. A multiscale model to design therapeutic strategies that overcome drug resistance to tyrosine kinase inhibitors in multiple myeloma. Math. Biosci. 2020, 319, 108293. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Doddapaneni, R.; Patel, A.R.; Singh, R.; Mercer, R.; Singh, M. Novel Gefitinib Formulation with Improved Oral Bioavailability in Treatment of A431 Skin Carcinoma. Pharm. Res. 2016, 33, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Chougule, M.B.; Patel, A.R.; Patlolla, R.; Jackson, T.; Singh, M. Epithelial transport of noscapine across cell monolayer and influence of absorption enhancers on in vitro permeation and bioavailability: Implications for intestinal absorption. J. Drug Target. 2014, 22, 498–508. [Google Scholar] [CrossRef]
- Phillip Lee, Y.-H.; Sathigari, S.; Jean Lin, Y.-J.; Ravis, W.R.; Chadha, G.; Parsons, D.L.; Rangari, V.K.; Wright, N.; Babu, R.J. Gefitinib–cyclodextrin inclusion complexes: Physico-chemical characterization and dissolution studies. Drug Dev. Ind. Pharm. 2009, 35, 1113–1120. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile salts-containing vesicles: Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2016, 23, 1847–1867. [Google Scholar] [CrossRef]
- Said, A.R.; Arafa, M.F.; El-Dakroury, W.A.; Alshehri, S.; El Maghraby, G.M. Bilosomes and Niosomes for Enhanced Intestinal Absorption and In Vivo Efficacy of Cytarabine in Treatment of Acute Myeloid Leukemia. Pharmaceuticals 2024, 17, 1572. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M.; Abdulabbas, H.T.; Jasim, S.A.; Kouhpayeh, S.A.; Barbaresi, S.; Ahmadi, S.; Ghasemian, A. Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges. J. Funct. Biomater. 2023, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Kaurav, H.; Tripathi, M.; Kaur, S.D.; Bansal, A.; Kapoor, D.N.; Sheth, S. Emerging Trends in Bilosomes as Therapeutic Drug Delivery Systems. Pharmaceutics 2024, 16, 697. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Khafaga, D.S.R.; El-Morsy, M.T.; Farhan, M.; Aatif, M.; Hosney, M. Targeting tumor-associated macrophages with nanocarrier-based treatment for breast cancer: A step toward developing innovative anti-cancer therapeutics. Heliyon 2024, 10, e37217. [Google Scholar] [CrossRef]
- D’Angelo, J. FT-IR determination of aliphatic and aromatic C-H contents of fossil leaf compressions. Part II: Applications. Anu. Latinoam. Educ. Química (ALDEQ) 2004, 18, 34–38. [Google Scholar]
- Alshehri, S.; Alanazi, A.; Elzayat, E.M.; Altamimi, M.A.; Imam, S.S.; Hussain, A.; Alqahtani, F.; Shakeel, F. Formulation, in vitro and in vivo evaluation of gefitinib solid dispersions prepared using different techniques. Processes 2021, 9, 1210. [Google Scholar] [CrossRef]
- Kumar, D.; Tiwari, S. Bile Salts Trigger Deformability in Liposomal Vesicles through Edge-Activating Action. Mol. Pharm. 2025, 22, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, T.M.; Adel, Y.A.; Teaima, M.; Nassar, N.N.; Nemr, A.A.; Al-Samadi, I.; El-Nabarawi, M.A.; Elhabal, S.F. Intranasal bilosomes in thermosensitive hydrogel: Advancing desvenlafaxine succinate delivery for depression management. Pharm. Dev. Technol. 2024, 29, 663–674. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Xie, S.; Chen, T.; Xie, D.; Cai, Y.; Cui, D.; Wang, L.; Chen, W.; Wang, X. Gefitinib enhances the anti-tumor immune response against EGFR-mutated NSCLC by upregulating B7H5 expression and activating T cells via CD28H. Int. J. Oncol. 2022, 61, 146. [Google Scholar] [CrossRef]
- Kuhlmann-Hogan, A.; Cordes, T.; Xu, Z.; Kuna, R.S.; Traina, K.A.; Robles-Oteiza, C.; Ayeni, D.; Kwong, E.M.; Levy, S.; Globig, A.M.; et al. EGFR-driven lung adenocarcinomas coopt alveolar macrophage metabolism and function to support EGFR signaling and growth. Cancer Discov. 2024, 14, 524–545. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.-Q.; Liang, G.-K.; He, Q.-J.; Ding, L.; Yang, B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1501–1511. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of metformin hydrochloride tailoring bilosomes as an effective transdermal nanocarrier. Int. J. Nanomed. 2022, 17, 1185–1201. [Google Scholar] [CrossRef]
- Souza, T.G.; Ciminelli, V.S.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; Soliman, M.M.; El Sayed, I.E.T.; Abdel Aleem, A.A.H.; Elsharkawy, H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Pollut. Res. 2021, 28, 44226–44238. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; Badr, O.M.; Abd-Eltawab, H.M. Ameliorative effect of saffron extract on mice bearing solid tumors. ISESCO J. Sci. Technol. 2011, 12, 60–70. [Google Scholar]
- Salem, F.S.; Badr, M.O.; Neamat-Allah, A.N.F. Biochemical and pathological studies on the effects of levamisole and chlorambucil on Ehrlich ascites carcinoma-bearing mice. Vet. Ital. 2011, 47, 89–95. [Google Scholar] [PubMed]
- Alamoudi, J.A.; El-Masry, T.A.; Nasr, M.; Ibrahim, I.T.; Ibrahim, H.A.; Saad, H.M.; El-Nagar, M.M.; Alshawwa, S.Z.; Alrashidi, A.; El Zahaby, E.I. Fabrication of nanocrystals for enhanced distribution of a fatty acid synthase inhibitor (Orlistat) as a promising method to relieve solid Ehrlich Carcinoma-Induced hepatic damage in mice. Pharmaceuticals 2024, 17, 96. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; El Sayed, I.E.T.; Abd Elmaksoud, A.Z.; Ahmed, A.A. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma–induced hepatorenal injury in mice. Environ. Sci. Pollut. Res. 2021, 28, 21835–21850. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, M.; El Atrash, A.; Tousson, E. Ehrlich Solid Tumor Induced Injury and Toxicity in Liver and Kidney in Female Mice. Asian Oncol. Res. J. 2022, 5, 113–119. [Google Scholar]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic activity and curative role of avenanthramides against the growth of Ehrlich solid tumors in mice. Oxidative Med. Cell. Longev. 2019, 2019, 5162687. [Google Scholar] [CrossRef]
- El-Masry, T.; Al-Shaalan, N.; Tousson, E.; Buabeid, M.; Al-Ghadeer, A. Potential therapy of vitamin B17 against Ehrlich solid tumor induced changes in Interferon gamma, Nuclear factor kappa B, DNA fragmentation, p53, Bcl2, survivin, VEGF and TNF-α Expressions in mice. Pak. J. Pharm. Sci. 2020, 33, 393–401. [Google Scholar] [PubMed]
- Li, L.; Cai, L.; Zheng, L.; Hu, Y.; Yuan, W.; Guo, Z.; Li, W. Gefitinib Inhibits Bleomycin-Induced Pulmonary Fibrosis via Alleviating the Oxidative Damage in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 8249693. [Google Scholar] [CrossRef]
- Gönenç, A.; Özkan, Y.; Torun, M.; Şimşek, B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001, 26, 141–144. [Google Scholar] [CrossRef]
- El-Din, N.K.B. Protective role of sanumgerman against γ-irradiation–induced oxidative stress in Ehrlich carcinoma-bearing mice. Nutr. Res. 2004, 24, 271–291. [Google Scholar] [CrossRef]
- Noaman, E.; El-Din, N.K.B.; Bibars, M.A.; Abou Mossallam, A.A.; Ghoneum, M. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 2008, 268, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.; Wakasugi, H. Cancer and oxidative stress. Jpn. Med. Assoc. J. 2001, 44, 535–539. [Google Scholar]
- Ali, D.A.; El-Din, N.K.B.; Abou-El-magd, R.F. Antioxidant and hepatoprotective activities of grape seeds and skin against Ehrlich solid tumor induced oxidative stress in mice. Egypt. J. Basic Appl. Sci. 2015, 2, 98–109. [Google Scholar] [CrossRef]
- El-Shorbagy, H.M.; Eissa, S.M.; Sabet, S.; El-Ghor, A.A. Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice. Int. J. Nanomed. 2019, 14, 3911–3928. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kaneko, F.; Niwa, Y. Changes in lipid peroxide levels and activity of reactive oxygen scavenging enzymes in skin, serum and liver following UVB irradiation in mice. Life Sci. 1992, 50, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- K P, S.H.K.; Babu, T.D.; M, P.C.; Joshy, G.; Mathew, D.; Thayyil, M.S. Antioxidant activity of erlotinib and gefitinib: Theoretical and experimental insights. Free Radic. Res. 2022, 56, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.-S.; Chang, J.-H.; Hung, W.-Y.; Yang, Y.-C.; Chien, M.-H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Lazarevich, N. Molecular mechanisms of alpha-fetoprotein gene expression. Biochemistry (Mosc.) 2000, 65, 117–133. [Google Scholar] [PubMed]
- Hussein, J.; El-khayat, Z.; Farouk, H. Antitumor activity of selenium in Ehrlich ascites carcinoma bearing mice. Biomed. Pharmacol. J. 2023, 16, 1353–1364. [Google Scholar] [CrossRef]
- Farnebo, M.; Bykov, V.J.; Wiman, K.G. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2010, 396, 85–89. [Google Scholar] [CrossRef]
- Wang, P.; Tian, Q.; Liang, Z.X.; Yang, Z.; Xu, S.F.; Sun, J.P.; Chen, L.A. Gefitinib attenuates murine pulmonary fibrosis induced by bleomycin. Chin. Med. J. 2010, 123, 2259–2264. [Google Scholar] [PubMed]
- El-Masry, T.A.; El-Nagar, M.M.F.; Oriquat, G.A.; Alotaibi, B.S.; Saad, H.M.; El Zahaby, E.I.; Ibrahim, H.A. Therapeutic efficiency of Tamoxifen/Orlistat nanocrystals against solid Ehrlich carcinoma via targeting TXNIP/HIF1-α/MMP-9/P27 and BAX/Bcl2/P53 signaling pathways. Biomed. Pharmacother. 2024, 180, 117429. [Google Scholar] [CrossRef]
- Areida, S.K.; El-Azim, A.; Amira, O.; Amer, M.E. Protective and curative effect of Thymoquinone on Ehrlich solid carcinoma inoculated mice. Egypt. J. Hosp. Med. 2015, 58, 129–142. [Google Scholar] [CrossRef]
- Chen, J.; Niu, N.; Zhang, J.; Qi, L.; Shen, W.; Donkena, K.V.; Feng, Z.; Liu, J. Polyploid giant cancer cells (PGCCs): The evil roots of cancer. Curr. Cancer Drug Targets 2019, 19, 360–367. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Mansour, S.M.; Ibrahim, R.Y.M. Zofenopril antitumor activity in mice bearing Ehrlich solid carcinoma: Modulation of PI3K/AKT signaling pathway. Braz. J. Pharm. Sci. 2022, 58, e19922. [Google Scholar] [CrossRef]
- Humphries, F.; Yang, S.; Wang, B.; Moynagh, P.N. RIP kinases: Key decision makers in cell death and innate immunity. Cell Death Differ. 2015, 22, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Toledo, B.; Zhu Chen, L.; Paniagua-Sancho, M.; Marchal, J.A.; Perán, M.; Giovannetti, E. Deciphering the performance of macrophages in tumour microenvironment: A call for precision immunotherapy. J. Hematol. Oncol. 2024, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z. Applications of Matrix Metalloproteinase-9-Related Nanomedicines in Tumors and Vascular Diseases. Pharmaceutics 2025, 17, 479. [Google Scholar] [CrossRef]
- Kalish, S.V.; Lyamina, S.V.; Usanova, E.A.; Manukhina, E.B.; Larionov, N.P.; Malyshev, I.Y. Macrophages Reprogrammed In Vitro Towards the M1 Phenotype and Activated with LPS Extend Lifespan of Mice with Ehrlich Ascites Carcinoma. Med. Sci. Monit. Basic Res. 2015, 21, 226–234. [Google Scholar] [CrossRef]
- Petit, A.M.; Rak, J.; Hung, M.-C.; Rockwell, P.; Goldstein, N.; Fendly, B.; Kerbel, R.S. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: Angiogenic implications for signal transduction therapy of solid tumors. Am. J. Pathol. 1997, 151, 1523–1530. [Google Scholar] [PubMed]
- Maloney, C.; Kallis, M.P.; Edelman, M.; Tzanavaris, C.; Lesser, M.; Soffer, S.Z.; Symons, M.; Steinberg, B.M. Gefitinib Inhibits Invasion and Metastasis of Osteosarcoma via Inhibition of Macrophage Receptor Interacting Serine-Threonine Kinase 2. Mol. Cancer Ther. 2020, 19, 1340–1350. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdollah, M.R.A.; Elsesy, M.E.; Abou El Ella, D.A.; Zada, S.K.; Tolba, M.F. The natural isoflavone Biochanin-A synergizes 5-fluorouracil anticancer activity in vitro and in vivo in Ehrlich solid-phase carcinoma model. Phytother. Res. 2022, 36, 1310–1325. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Shi, H.; Pan, W.; Yu, H.; Zhu, J. Combined inhibition of epidermal growth factor receptor and cyclooxygenase-2 leads to greater anti-tumor activity of docetaxel in advanced prostate cancer. PLoS ONE 2013, 8, e76169. [Google Scholar]
- Tan, W.; Zhang, W.; Strasner, A.; Grivennikov, S.; Cheng, J.Q.; Hoffman, R.M.; Karin, M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 2011, 470, 548–553. [Google Scholar] [PubMed]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462. [Google Scholar] [PubMed]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Diao, D.; Zhai, J.; Yang, J.; Wu, H.; Jiang, J.; Dong, X.; Passaro, A.; Aramini, B.; Rao, S.; Cai, K. Delivery of gefitinib with an immunostimulatory nanocarrier improves therapeutic efficacy in lung cancer. Transl. Lung Cancer Res. 2021, 10, 926. [Google Scholar] [CrossRef]
- Gad, S.; Awd, H.; Abdelwahab, N.; Nasr, A.; Ghorab, M. Bilosomes as a Versatile Drug Delivery System: Preparation Techniques and Biomedical Application. Rec. Pharm. Biomed. Sci. 2024, 8, 67–86. [Google Scholar] [CrossRef]
- Boyd, B.J.; Rizwan, S.B.; Dong, Y.D.; Hook, S.; Rades, T. Self-assembled geometric liquid-crystalline nanoparticles imaged in three dimensions: Hexosomes are not necessarily flat hexagonal prisms. Langmuir 2007, 23, 12461–12464. [Google Scholar] [CrossRef]
- Patra, S.R.; Bali, A.; Saha, M. Derivative Spectrophotometric Methods for Determination of Gefitinib in Bulk and in Formulation. J. Appl. Spectrosc. 2021, 88, 1088–1094. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Khedr, N.F.; Khalil, R.M. Effect of hesperidin on mice bearing Ehrlich solid carcinoma maintained on doxorubicin. Tumor Biol. 2015, 36, 9267–9275. [Google Scholar] [CrossRef]
- Rosenberg, I.; Russell, C.W.; Giles, G. Cell viability studies on the exfoliated colonic cancer cell. J. Br. Surg. 1978, 65, 188–190. [Google Scholar] [CrossRef]
- El-Sisi, A.E.; Sokkar, S.S.; Ibrahim, H.A.; Hamed, M.F.; Abu-Risha, S.E. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3, and Ki-67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam. Clin. Pharmacol. 2020, 34, 458–475. [Google Scholar] [CrossRef]
- Keith, R.L.; Karoor, V.; Mozer, A.B.; Hudish, T.M.; Le, M.; Miller, Y.E. Chemoprevention of murine lung cancer by gefitinib in combination with prostacyclin synthase overexpression. Lung Cancer 2010, 70, 37–42. [Google Scholar] [CrossRef] [PubMed]
- El-Sisi, A.E.; Sokar, S.S.; Ibrahim, H.A.; Abu-Risha, S.E. Enhanced anticancer activity of combined treatment of imatinib and dipyridamole in solid Ehrlich carcinoma-bearing mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.E.-M.M.; Ahmed, M.M.S.; Khayyal, M.T.E.-D.; El-Merzabani, M.M. Hyperthermic potentiation of cisplatin cytotoxicity on solid Ehrlich carcinoma. Tumori J. 1993, 79, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Szasz, G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin. Chem. 1969, 15, 124–136. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.; Morfin, R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T., Jr.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Doumas, B.T.; Biggs, H.G.; Arends, R.L.; Pinto, P.V. Determination of serum albumin. In Standard Methods of Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 1972; Volume 7, pp. 175–188. [Google Scholar]
- Walters, M.I.; Gerarde, H.W. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem. J. 1970, 15, 231–243. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 421–431. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Corso, C.R.; Stipp, M.C.; Adami, E.R.; da Silva, L.M.; Mariott, M.; de Andrade, S.F.; de Souza Ramos, E.A.; Klassen, G.; Beltrame, O.C.; Queiroz-Telles, J.E. Salvia lachnostachys Benth has antitumor and chemopreventive effects against solid Ehrlich carcinoma. Mol. Biol. Rep. 2019, 46, 4827–4841. [Google Scholar] [CrossRef]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- David, J.D. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Vis, A.N.; Kranse, R.; Nigg, A.L.; Van Der Kwast, T.H. Quantitative analysis of the decay of immunoreactivity in stored prostate needle biopsy sections. Am. J. Clin. Pathol. 2000, 113, 369–373. [Google Scholar] [CrossRef]
- Stirling, J.W.; Woods, A.E. Resin (plastic) embedding for microscopy and tissue analysis. In Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 96–113. [Google Scholar]

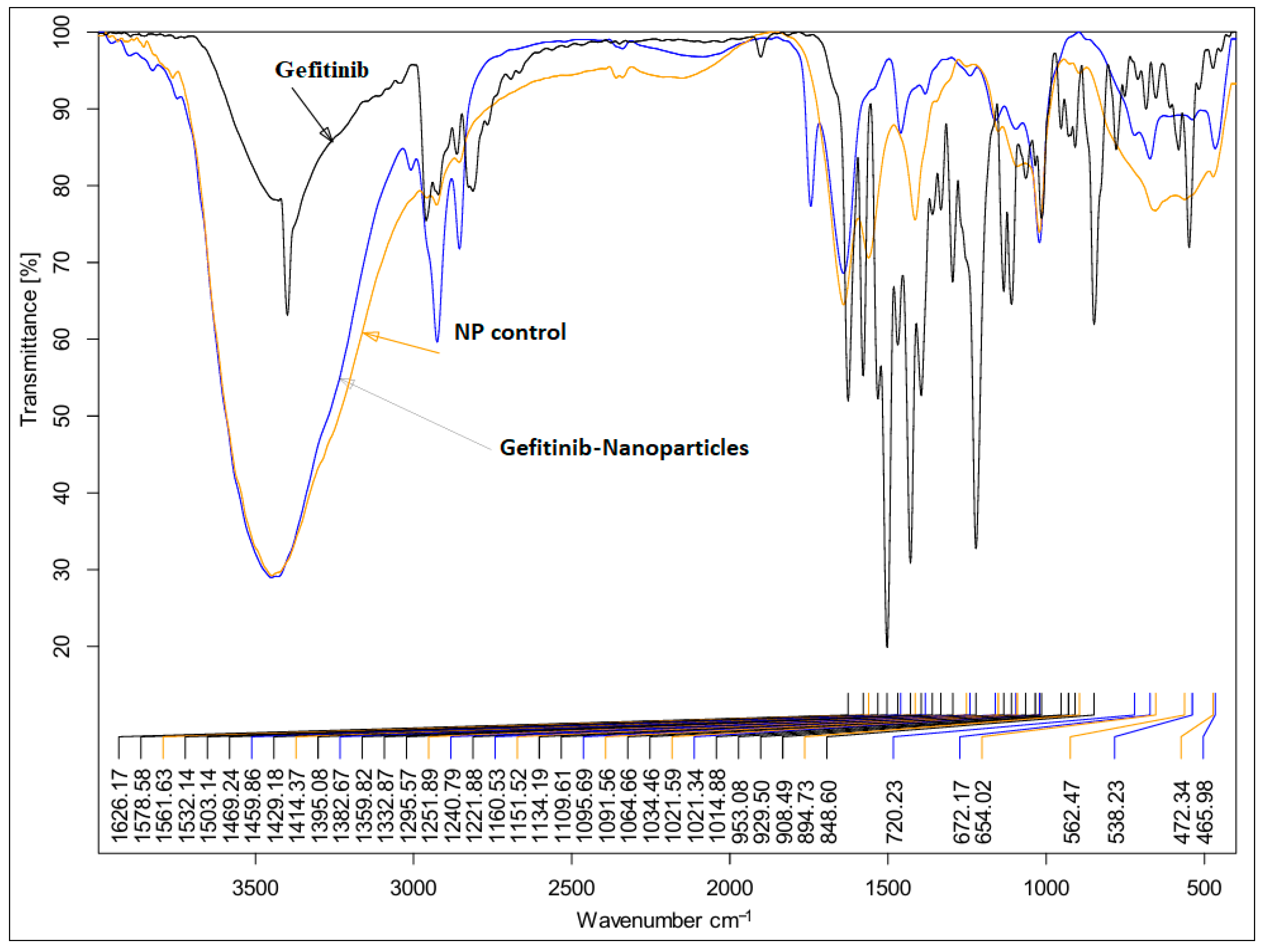
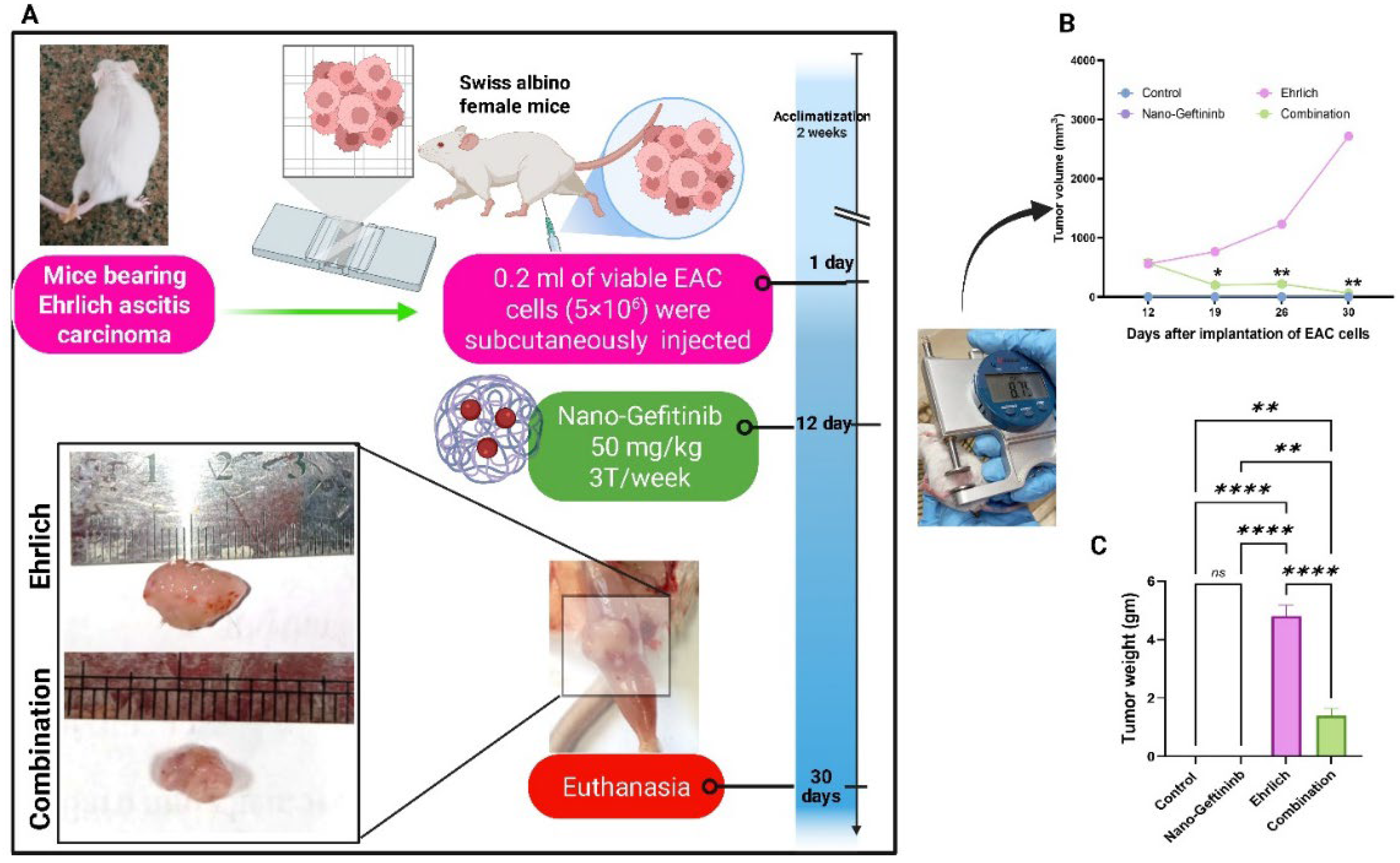
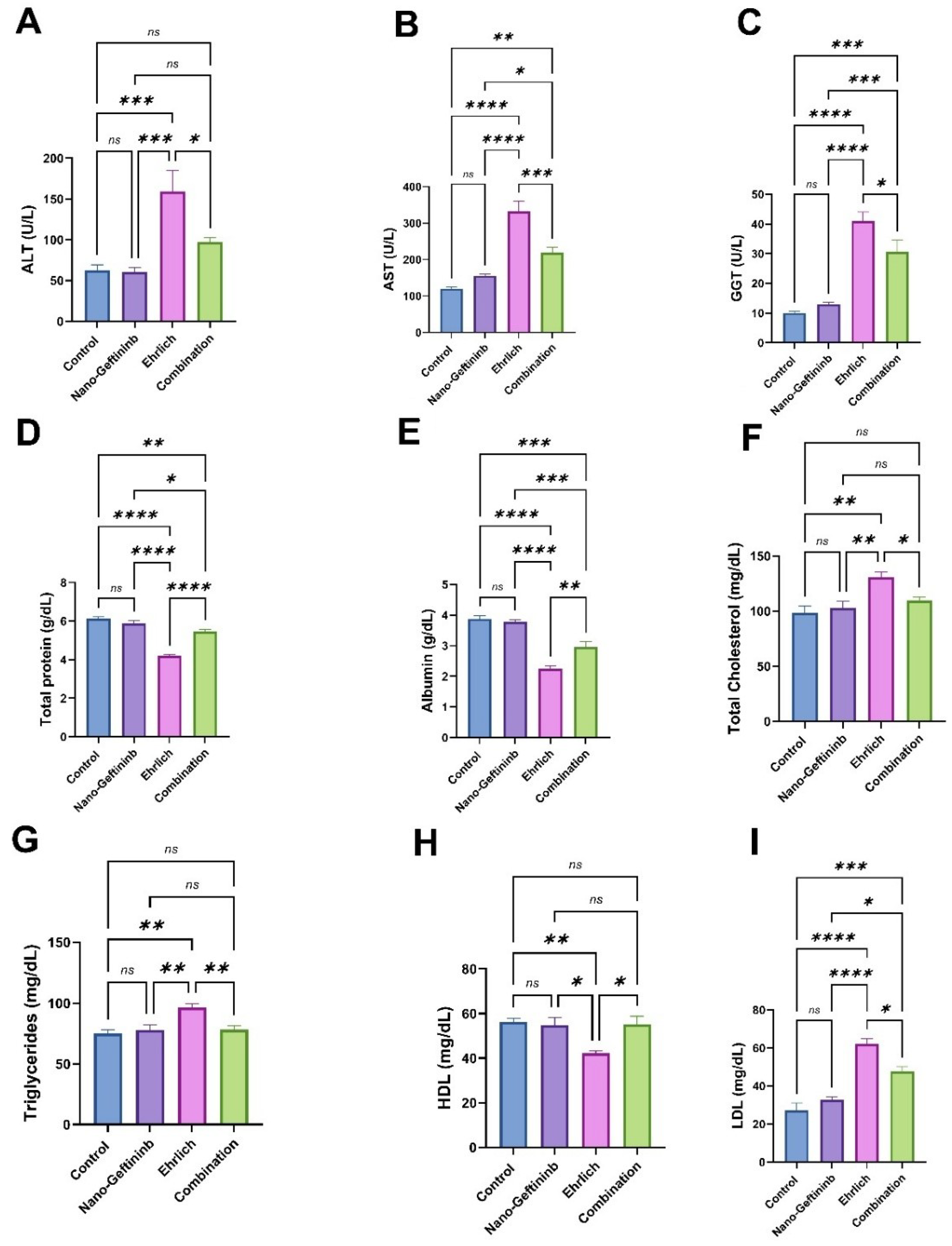
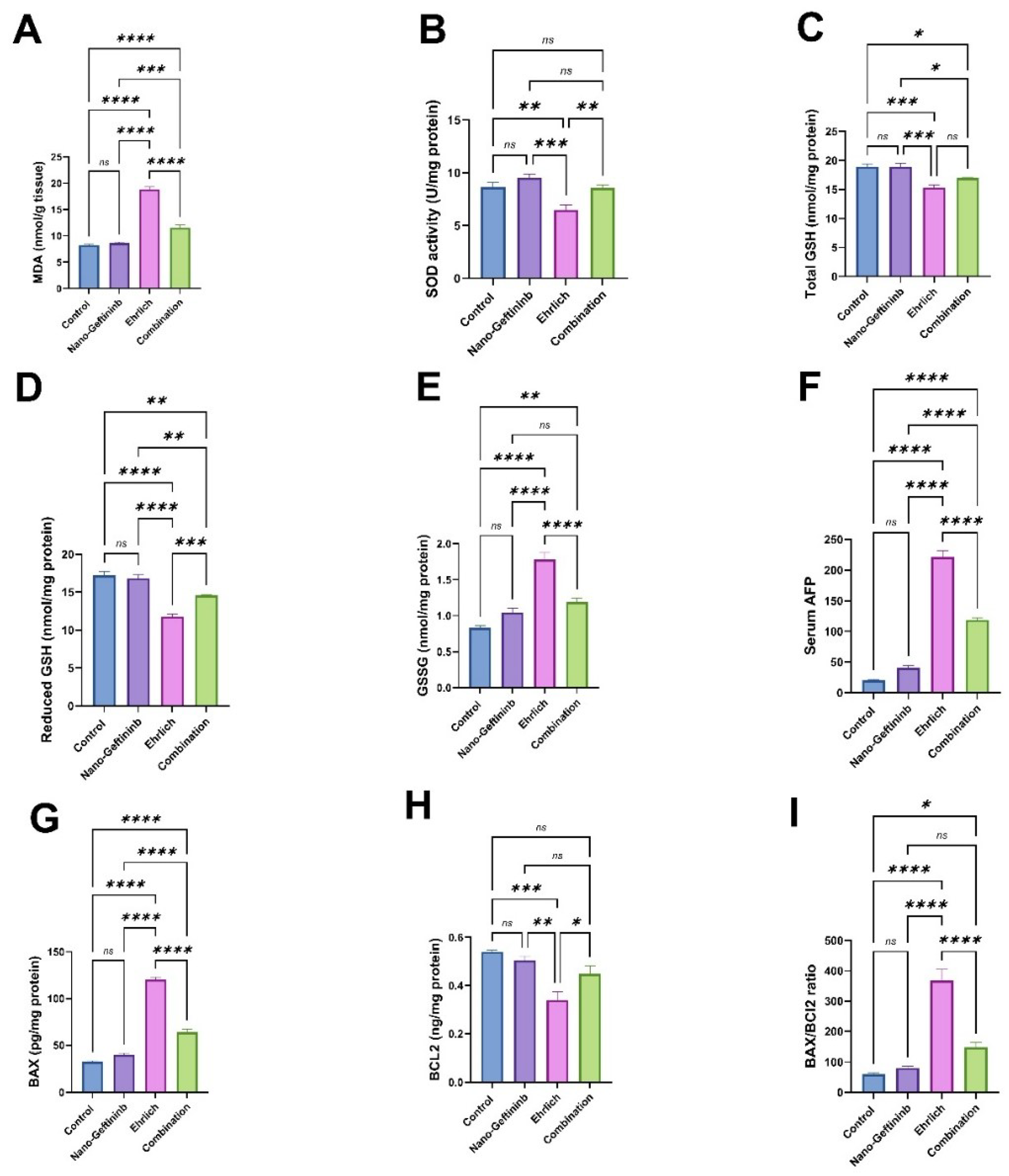
| Parameter | Mean | Range |
|---|---|---|
| Particle size (nm) | 113.66 ± 2.23 | 112 to 116.2 |
| Zeta potential (mV) | −53.68 ± 1.36 | −54.91 to −52.22 |
| PDI | 0.31 ± 0.02 | 0.294 to 0.333 |
| Drug content (mg/mL) | 3.13 ± 0.24 | 3.31 to 2.87 |
| %EE | 94.03 ± 7.05 | 86.02 to 99.31 |
| Parameters | Control | Nano-Gefitinib | Ehrlich | Combination |
|---|---|---|---|---|
| Necrosis score | − | − | 0 | 4 |
| Cytological features | − | − | +++ | + |
| Apoptosis | − | − | + | +++ |
| Angiogenesis | − | − | +++ | + |
| Inflammatory infiltrates | − | − | +++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashoura, N.R.; Saad, H.M.; El Zahaby, E.I.; Salama, A.R.; Amer, N.E.; Elhussieny, O.; Edres, H.A.; Nematalla, H.A.; Mohammed, S.A.A. Effect of Nano-Gefitinib on Solid Ehrlich Carcinoma via Targeting EGFR, RIPK2 Pathways, and Macrophage Reprogramming. Pharmaceuticals 2025, 18, 1747. https://doi.org/10.3390/ph18111747
Ashoura NR, Saad HM, El Zahaby EI, Salama AR, Amer NE, Elhussieny O, Edres HA, Nematalla HA, Mohammed SAA. Effect of Nano-Gefitinib on Solid Ehrlich Carcinoma via Targeting EGFR, RIPK2 Pathways, and Macrophage Reprogramming. Pharmaceuticals. 2025; 18(11):1747. https://doi.org/10.3390/ph18111747
Chicago/Turabian StyleAshoura, Neveen R., Hebatallah M. Saad, Enas I. El Zahaby, Alyaa R. Salama, Nihal E. Amer, Omnya Elhussieny, Hanan A. Edres, Hisham A. Nematalla, and Salman A. A. Mohammed. 2025. "Effect of Nano-Gefitinib on Solid Ehrlich Carcinoma via Targeting EGFR, RIPK2 Pathways, and Macrophage Reprogramming" Pharmaceuticals 18, no. 11: 1747. https://doi.org/10.3390/ph18111747
APA StyleAshoura, N. R., Saad, H. M., El Zahaby, E. I., Salama, A. R., Amer, N. E., Elhussieny, O., Edres, H. A., Nematalla, H. A., & Mohammed, S. A. A. (2025). Effect of Nano-Gefitinib on Solid Ehrlich Carcinoma via Targeting EGFR, RIPK2 Pathways, and Macrophage Reprogramming. Pharmaceuticals, 18(11), 1747. https://doi.org/10.3390/ph18111747






