Generating and Modeling Virtual Patient Data from Published Population Pharmacokinetic Analyses: A Vancomycin Case Study
Abstract
1. Introduction
2. Results
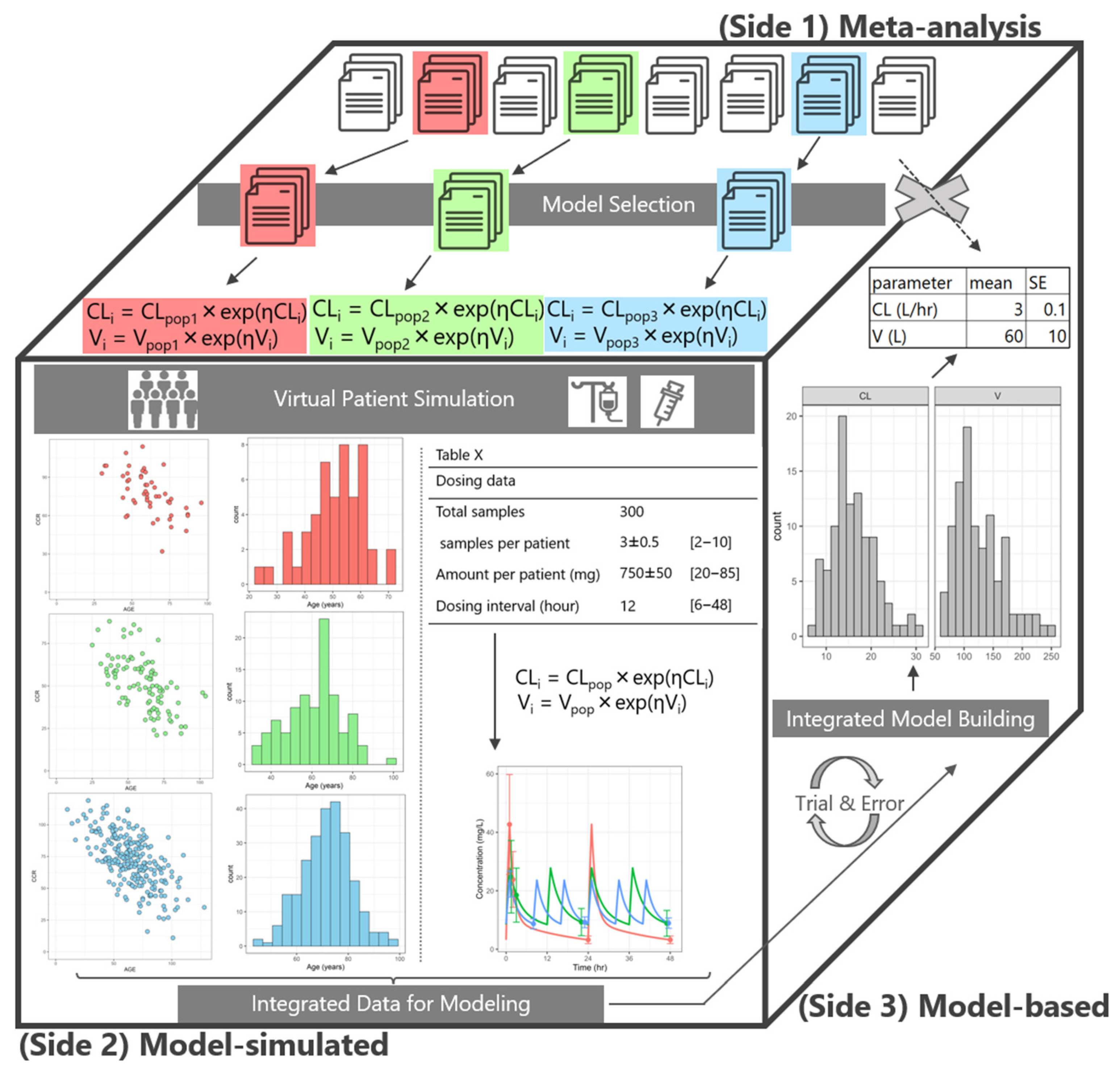
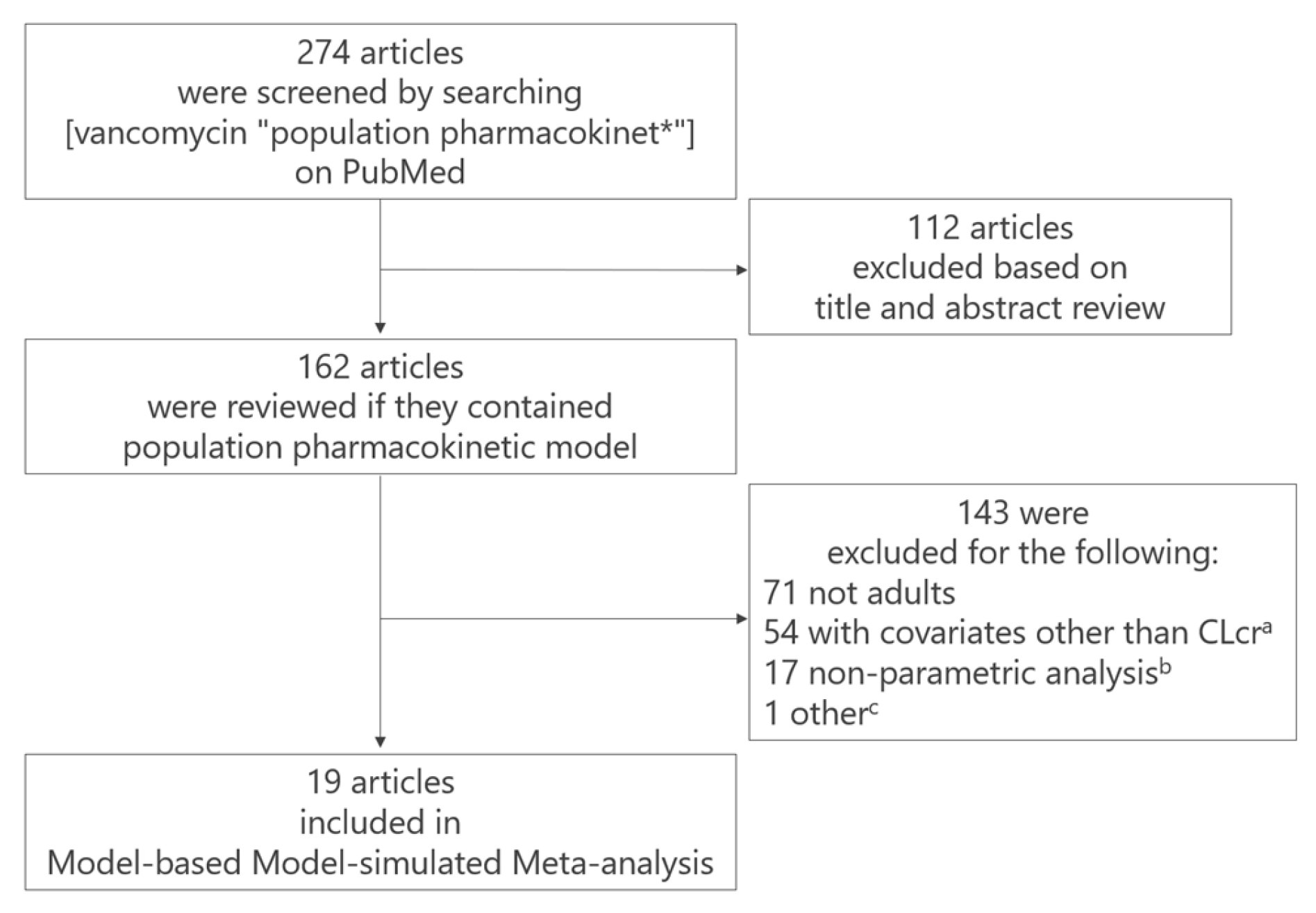
2.1. M-Cube Side 1
- (a)
- 54 studies were excluded because their CL models included covariates other than creatinine clearance (CLcr) estimated using the Cockcroft–Gault equation [11]. Although the Cockcroft–Gault equation for CLcr is common in clinical settings worldwide, estimated glomerular filtration rate (eGFR) formulas (e.g., [12,13]) vary among studies, making standardization impossible. This is the reason why we included models which had CLcr as a covariate.
- (b)
- 17 studies were excluded due to the use of nonparametric methods,
- (c)
- one article was excluded because the reported estimates of clearance differed greatly from those reported in other studies due to unknown reasons.
| Study | Number of Patients | Purpose of the popPK Model | Characteristics a–d | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| No. | 1st Author (year) | Sex (Male/Female) | Age (year) | Body Weight (kg) | Creatinine Clearance (mL/min) | |||
| 1 | Yasuhara (1998) | 190 | hospitalized MRSA-infected patients | 131/59 | 64.3 ± 13.8 [19.3–89.6] | 52.3 ± 9.6 [25.5–75] | 77.1 ± 50.9 [6.85–not rereported in the original publication] | [3] |
| 2 | Buelga (2005) | 215 | hematological malignancies | 119/96 | 51.5 ± 15.9 | 64.7 ± 11.3 | 89.4 ± 39.2 | [14] |
| 3 | Staatz (2006) | 102 | unstable renal function following cardiothoracic surgery | 71/31 | 66 b [17–87] | 74 b [44–110] | 60 b [12–172] | [15] |
| 4 | Yamamoto (2009) | 100 | adult patients with Gram-positive infections | 64/36 | 65.4 ± 15.1 [25.8–99.7] | 52.6 ± 12.7 [28.7–97] | 79.6 ± 41.8 [15.3–218.8] | [16] |
| 5 | Thomson (2009) | 398 | adult patients (age ≥ 16) | 251/147 | 66 b [16–97.0] | 72 b [40–159] | 64 b [12–216] | [17] |
| 6 | Dolton (2010) | 33 | suspected or confirmed serious infection | 26/7 | 72 b [38–95] | 67 b [48.9–111] | 75.0 ± 47.8 | [18] |
| 7 | Roberts (2011) | 206 | critically ill patients | 127/79 | 58.1 ± 14.8 | 74.8 ± 15.8 | 90.7 ± 60.4 [30–250] c | [19] |
| 8 | Purwonugroho (2012) | 212 | Thai patients (age > 18) | 112/100 | 66.62 ± 18.38 | 57.64 ± 11.62 | 35.07 ± 29.83 | [20] |
| 9 | Adane (2015) | 29 | extremely obese f | 19/10 | 43 b [38.5–53] d | 147.9 b [142.8–178.3] d | 124.8 b [106–133.9] c,d | [21] |
| 10 | Moore (2016) | 14 | ECMO e | 11/3 | 47 ± 16 [19–72] | 95 ± 27 | 84 ± 37 | [22] |
| 11 | Lin (2016) | 100 | with post-craniotomy meningitis | 66/34 | 51.6 ± 16.9 [18–86] | 59.1 ± 10.0 [38–85] | 104.7 ± 43.9 [9.5–216.9] | [23] |
| 12 | Okada (2018) | 75 | undergoing allogeneic hematopoietic stem cell transplantation | 49/26 | 49 b [17–69] | 59.4 [39.4–104.5] b | 113 [47–253] c | [24] |
| 13 | Usman (2018) | 144 | adult patients (age > 16) | 93/51 | 62 b [16–88] | 79.5 b [40–177] | 89.8 b [11.3–313.6] | [25] |
| 14 | Zhou (2019) | 70 | geriatric patients with pulmonary infections (age ≥ 65 years) | 49/21 | 78.3 ± 6.96 | 60.7 ± 10.2 | 56.3 ± 22.1 | [26] |
| 15 | Dorajoo (2019) | 80 | chronic kidney disease | 51/29 | 71.7 ± 13 [31–97] | 57.8 ± 15.7 [33.6–103.8] | 33.8 ± 10.3 [−60] c | [27] |
| 16 | Jaisue (2020) | 180 | patients with heterogeneous and unstable renal function | 102/78 | 60.8 ± 17.5 [17–97] | 54.2 ± 11.7 [30–103] | 66.2 ± 56.2 [7.3–281] | [28] |
| 17 | Kovacevic (2020) | 73 | critically ill septic patients | 40/33 | 56.9 ± 17 [20–87] | 78.2 ± 14.2 [30–120] | 80 ± 44 [14.28–192.9] | [29] |
| 18 | Masich (2020) | 16 | obese with sepsis or septic shock | 9/7 | 62 b [30–78] | 112.7 b [72.6–129.1] | 46 b [14–123] g | [30] |
| 19 | Jalusic (2021) | 29 | external ventricular drain-associated ventriculitis | 14/15 | 52 b [44–61] d | 80 b [70–85] d | 152 b [109–174] c,d | [31] |
2.2. M-Cube Side 2
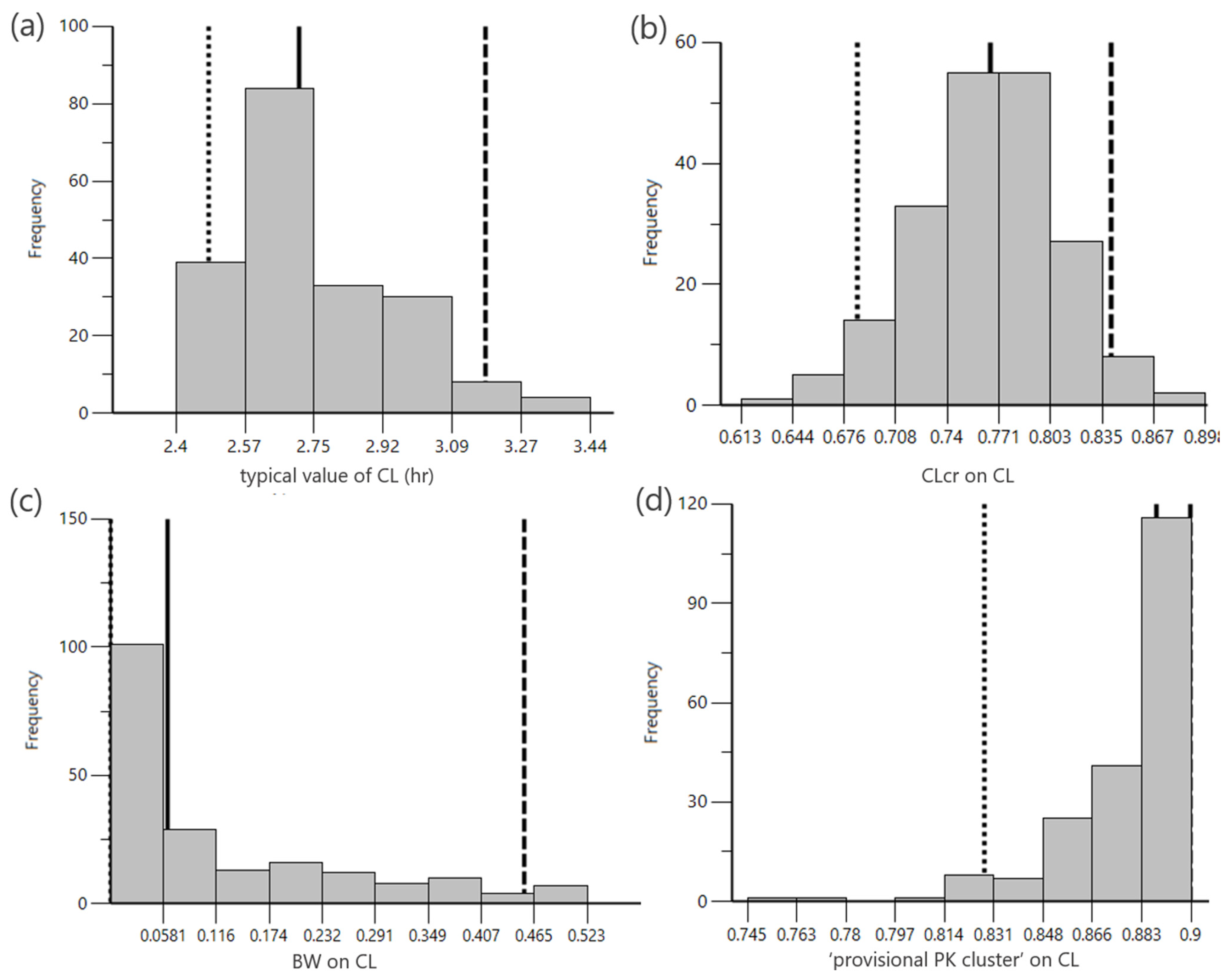
2.3. M-Cube Side 3
3. Discussion
4. Materials and Methods
4.1. An Overview of Integrating Multiple popPK Models
4.2. M-Cube Side 1: Selection of popPK Models for Integration
4.3. M-Cube Side 2: Generation of Virtual Patients
4.3.1. Generation of Virtual Patient Background
4.3.2. Generation of Vancomycin Plasma Concentration
4.4. M-Cube Side 3: popPK Modeling of the Integrated Virtual Patients
4.5. PopPK Model Building
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BW | Body weight |
| CLcr | Creatinine clearance |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NLME | Nonlinear Mixed Effects Model |
| PK | Pharmacokinetics |
| popPK | Population pharmacokinetics |
| OFV | Objective function value |
| TDM | Therapeutic drug monitoring |
| VCM | Vancomycin |
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 2011, 7, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, M.; Iga, T.; Zenda, H.; Okumura, K.; Oguma, T.; Yano, Y.; Hori, R. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther. Drug Monit. 1998, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Hashiguchi, Y.; Kimura, T.; Tsuji, Y.; Shoji, K.; Takahashi, Y.; Matsumoto, K.; Kawamura, H.; Saito, H.; Takesue, Y. Performance of Area under the Concentration-Time Curve Estimations of Vancomycin with Limited Sampling by a Newly Developed Web Application. Pharm. Res. 2021, 38, 637–646. [Google Scholar] [CrossRef]
- Upreti, V.V.; Venkatakrishnan, K. Model-Based Meta-Analysis: Optimizing Research, Development, and Utilization of Therapeutics Using the Totality of Evidence. Clin. Pharmacol. Ther. 2019, 106, 981–992. [Google Scholar] [CrossRef]
- Yang, Z.; He, H.; Wang, R.; Liu, D.; Li, G.; Sun, F. Application and Quality of Model-Based Meta-Analysis in Pharmaceutical Research: A Systematic Cross-Sectional Analysis and Practical Considerations. Clin. Pharmacol. Ther. 2024, 116, 397–407. [Google Scholar] [CrossRef]
- van Os, W.; O’JEanson, A.; Troisi, C.; Liu, C.; Brooks, J.T.; Hughes, J.H.; Tong, D.M.H.; Keizer, R.J. Machine Learning-Based Model Selection and Averaging Outperform Single-Model Approaches for a Priori Vancomycin Precision Dosing. CPT Pharmacomet. Syst. Pharmacol. 2025, 14, 1650–1660. [Google Scholar] [CrossRef]
- Taylor, Z.L.; Poweleit, E.A.; Paice, K.; Somers, K.M.; Pavia, K.; Vinks, A.A.; Punt, N.; Mizuno, T.; Girdwood, S.T. Tutorial on model selection and validation of model input into precision dosing software for model-informed precision dosing. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1827–1845. [Google Scholar] [CrossRef]
- Wicha, S.G.; Märtson, A.-G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.-W.; Minichmayr, I.K.; International Society of Anti-Infective Pharmacology (ISAP); the PK/PD study group of the European Society of Clinical Microbiology; Infectious Diseases (EPASG). From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef]
- Minichmayr, I.; Dreesen, E.; Centanni, M.; Wang, Z.; Hoffert, Y.; Friberg, L.; Wicha, S. Model-informed precision dosing: State of the art and future perspectives. Adv. Drug Deliv. Rev. 2024, 215, 115421. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: Accuracy and use for population estimates. Am. J. Kidney Dis. 2010, 56, 32–38. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Buelga, D.S.; del Mar Fernandez de Gatta, M.; Herrera, E.V.; Dominguez-Gil, A.; Garcia, M.J. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob. Agents Chemother. 2005, 49, 4934–4941. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Byrne, C.; Thomson, A.H. Population pharmacokinetic modelling of gentamicin and vancomycin in patients with unstable renal function following cardiothoracic surgery. Br. J. Clin. Pharmacol. 2006, 61, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kuzuya, T.; Baba, H.; Yamada, K.; Nabeshima, T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J. Clin. Pharm. Ther. 2009, 34, 473–483. [Google Scholar] [CrossRef]
- Thomson, A.H.; Staatz, C.E.; Tobin, C.M.; Gall, M.; Lovering, A.M. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J. Antimicrob. Chemother. 2009, 63, 1050–1057. [Google Scholar] [CrossRef]
- Dolton, M.; Xu, H.; Cheong, E.; Maitz, P.; Kennedy, P.; Gottlieb, T.; Buono, E.; McLachlan, A.J. Vancomycin pharmacokinetics in patients with severe burn injuries. Burns 2010, 36, 469–476. [Google Scholar] [CrossRef]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef]
- Purwonugroho, T.A.; Chulavatnatol, S.; Preechagoon, Y.; Chindavijak, B.; Malathum, K.; Bunuparadah, P. Population pharmacokinetics of vancomycin in Thai patients. Sci. World J. 2012, 2012, 762649. [Google Scholar] [CrossRef]
- Adane, E.D.; Herald, M.; Koura, F. Pharmacokinetics of vancomycin in extremely obese patients with suspected or confirmed Staphylococcus aureus infections. Pharmacotherapy 2015, 35, 127–139. [Google Scholar] [CrossRef]
- Moore, J.; Healy, J.; Thoma, B.; Peahota, M.; Ahamadi, M.; Schmidt, L.; Cavarocchi, N.; Kraft, W. A Population Pharmacokinetic Model for Vancomycin in Adult Patients Receiving Extracorporeal Membrane Oxygenation Therapy. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 495–502. [Google Scholar] [CrossRef]
- Lin, W.-W.; Wu, W.; Jiao, Z.; Lin, R.-F.; Jiang, C.-Z.; Huang, P.-F.; Liu, Y.-W.; Wang, C.-L. Population pharmacokinetics of vancomycin in adult Chinese patients with post-craniotomy meningitis and its application in individualised dosage regimens. Eur. J. Clin. Pharmacol. 2016, 72, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Kariya, M.; Irie, K.; Okada, Y.; Hiramoto, N.; Hashimoto, H.; Kajioka, R.; Maruyama, C.; Kasai, H.; Hamori, M.; et al. Population Pharmacokinetics of Vancomycin in Patients Undergoing Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Pharmacol. 2018, 58, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Fobker, M.; Hempel, G. Investigation of the age dependency of vancomycin clearance by population pharmacokinetic modeling. Int. J. Clin. Pharmacol. Ther. 2018, 56, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, F.; Chen, C.; Ma, L.; Yang, T.; Liu, X.; Liu, Y.; Wang, X.; Zhao, X.; Que, C.; et al. Development of a Population Pharmacokinetic Model of Vancomycin and its Application in Chinese Geriatric Patients with Pulmonary Infections. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 361–370. [Google Scholar] [CrossRef]
- Dorajoo, S.R.; Winata, C.L.; Goh, J.H.F.; Ooi, S.T.; Somani, J.; Yeoh, L.Y.; Lee, S.Y.; Yap, C.W.; Chan, A.; Chae, J.-W. Optimizing Vancomycin Dosing in Chronic Kidney Disease by Deriving and Implementing a Web-Based Tool Using a Population Pharmacokinetics Analysis. Front. Pharmacol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Jaisue, S.; Pongsakul, C.; D’Argenio, D.Z.; Sermsappasuk, P. Population Pharmacokinetic Modeling of Vancomycin in Thai Patients With Heterogeneous and Unstable Renal Function. Ther. Drug Monit. 2020, 42, 856–865. [Google Scholar] [CrossRef]
- Kovacevic, T.; Miljkovic, B.; Kovacevic, P.; Dragic, S.; Momcicevic, D.; Avram, S.; Jovanovic, M.; Vucicevic, K. Population pharmacokinetic model of Vancomycin based on therapeutic drug monitoring data in critically ill septic patients. J. Crit. Care 2020, 55, 116–121. [Google Scholar] [CrossRef]
- Masich, A.M.; Kalaria, S.N.; Gonzales, J.P.; Heil, E.L.; Tata, A.L.; Claeys, K.C.; Patel, D.; Gopalakrishnan, M. Vancomycin Pharmacokinetics in Obese Patients with Sepsis or Septic Shock. Pharmacotherapy 2020, 40, 211–220. [Google Scholar] [CrossRef]
- Jalusic, K.O.; Hempel, G.; Arnemann, P.; Spiekermann, C.; Kampmeier, T.; Ertmer, C.; Gastine, S.; Hessler, M. Population pharmacokinetics of vancomycin in patients with external ventricular drain-associated ventriculitis. Br. J. Clin. Pharmacol. 2021, 87, 2502–2510. [Google Scholar] [CrossRef]
- Winter, M.A.; Guhr, K.N.; Berg, G.M. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy 2012, 32, 604–612. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical Practice Guidelines for Therapeutic Drug Monitoring of Vancomycin in the Framework of Model-Informed Precision Dosing: A Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef]
- Claisse, G.; Zufferey, P.J.; Trone, J.C.; Maillard, N.; Delavenne, X.; Laporte, S.; Ollier, E. Predicting the dose of vancomycin in ICU patients receiving different types of RRT therapy: A model-based meta-analytic approach. Br. J. Clin. Pharmacol. 2019, 85, 1215–1226. [Google Scholar] [CrossRef]
- Colin, P.J.; Allegaert, K.; Thomson, A.H.; Touw, D.J.; Dolton, M.; de Hoog, M.; Roberts, J.A.; Adane, E.D.; Yamamoto, M.; Santos-Buelga, D.; et al. Vancomycin Pharmacokinetics Throughout Life: Results from a Pooled Population Analysis and Evaluation of Current Dosing Recommendations. Clin. Pharmacokinet. 2019, 58, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Uster, D.W.; Stocker, S.L.; Carland, J.E.; Brett, J.; Marriott, D.J.; Day, R.O.; Wicha, S.G. A Model Averaging/Selection Approach Improves the Predictive Performance of Model-Informed Precision Dosing: Vancomycin as a Case Study. Clin. Pharmacol. Ther. 2021, 109, 175–183. [Google Scholar] [CrossRef] [PubMed]
- NCHS. National Health and Nutrition Examination Survey Data; Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2019–2020. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 1 February 2025).
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Kasai, H.; Aoyama, T.; Tsuji, Y. Generating and Modeling Virtual Patient Data from Published Population Pharmacokinetic Analyses: A Vancomycin Case Study. Pharmaceuticals 2025, 18, 1748. https://doi.org/10.3390/ph18111748
Suzuki M, Kasai H, Aoyama T, Tsuji Y. Generating and Modeling Virtual Patient Data from Published Population Pharmacokinetic Analyses: A Vancomycin Case Study. Pharmaceuticals. 2025; 18(11):1748. https://doi.org/10.3390/ph18111748
Chicago/Turabian StyleSuzuki, Moeko, Hidefumi Kasai, Takahiko Aoyama, and Yasuhiro Tsuji. 2025. "Generating and Modeling Virtual Patient Data from Published Population Pharmacokinetic Analyses: A Vancomycin Case Study" Pharmaceuticals 18, no. 11: 1748. https://doi.org/10.3390/ph18111748
APA StyleSuzuki, M., Kasai, H., Aoyama, T., & Tsuji, Y. (2025). Generating and Modeling Virtual Patient Data from Published Population Pharmacokinetic Analyses: A Vancomycin Case Study. Pharmaceuticals, 18(11), 1748. https://doi.org/10.3390/ph18111748








