Dietary Polyphenol Combinations Have a Multifaceted Inhibitory Effect on Metabolic Rewiring and Signaling Pathways in Neuroblastoma
Abstract
1. Introduction
2. Results
2.1. Screening Some Dietary Polyphenols for Their Cytotoxic Properties in Human Neuroblastoma and Mesenchymal Stem Cell Lines
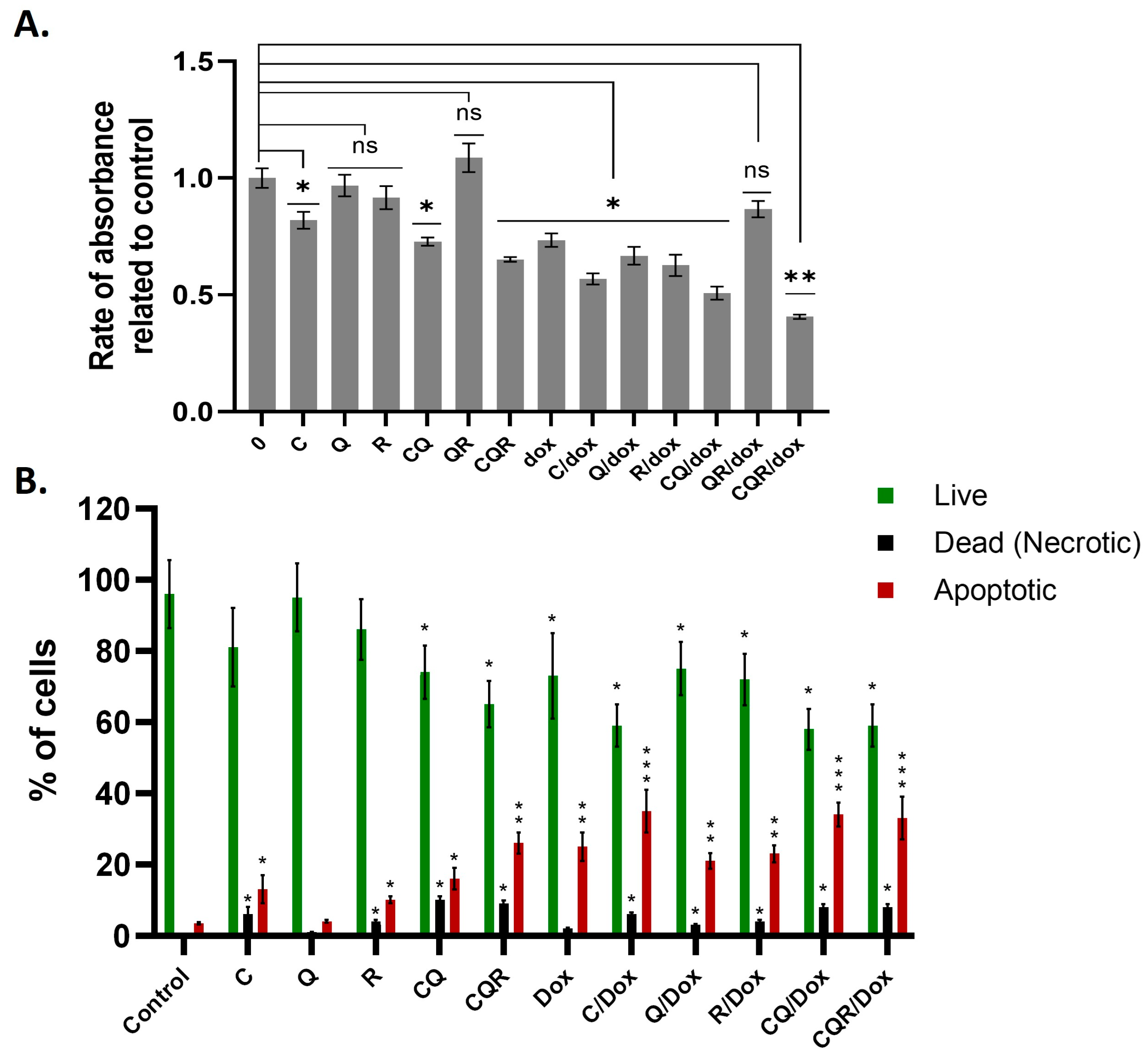
2.2. Curcumin, Quercetin, and Resveratrol in Combination Display Synergistic Down-Regulation of Neuroblastoma Cell Lines
2.3. The Combination of Curcumin, Quercetin, and Resveratrol Arrests the Cell Cycle and Induces Cell Death of Neuroblastoma Cells
2.4. The Polyphenol Combinations Inhibit Energy Metabolism in Neuroblastoma Cells
2.5. The Polyphenol Combinations Have Multiple Inhibitory Impacts on Metabolic Rewiring in Neuroblastoma Cells
2.6. The Polyphenol Combinations Inhibit Different Signaling Pathways in Neuroblastoma Cell Lines
2.7. The Polyphenol Combinations Suppress Total Protein Biosynthesis in Neuroblastoma Cell Lines
2.8. Polyphenols and Their Combinations Increase the Susceptibility of SH-SY5Y to Doxorubicin
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. MTT Assay
4.3. Assessment of Drug Synergy
4.4. Crystal Violet Assay
4.5. Western Blot
4.6. SeaHorse Energy Profiling
4.7. Cell Cycle Analysis
4.8. Annexin V Test
4.9. Total Protein Biosynthesis Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Curcumin |
| Q | Quercetin |
| EGCG | Epigallocatechin gallate |
| R | Resveratrol |
| CQ | Curcumin and Quercetin |
| CQR | Curcumin, Quercetin, and Resveratrol |
References
- Kirdeeva, Y.; Fedorova, O.; Daks, A.; Barlev, N.; Shuvalov, O. How should the worldwide knowledge of traditional cancer healing be integrated with herbs and mushrooms into modern molecular pharmacology? Pharmaceuticals 2022, 15, 868. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Fefilova, E.; Kirdeeva, Y.; Parfenyev, S.; Daks, A.; Fedorova, O.; Sorokina, M.; Ha, N.X.; Huong, T.T.; Loc, V.T.; Hai, P.T. MDM2 up-regulates the energy metabolism in NSCLC in a p53-independent manner. Biochem. Biophys. Res. Commun. 2025, 743, 151169. [Google Scholar] [CrossRef] [PubMed]
- Kiyimba, T.; Yiga, P.; Bamuwamye, M.; Ogwok, P.; Van der Schueren, B.; Matthys, C. Efficacy of dietary polyphenols from whole foods and purified food polyphenol extracts in optimizing cardiometabolic health: A meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary polyphenols and obesity: A review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis, and starch digestibility and absorption. Plant Foods Hum. Nutr. 2023, 78, 1–12. [Google Scholar] [CrossRef]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols rich diets and risk of type 2 diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The protective effect of polyphenols for colorectal cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A review of registered clinical trials on dietary (poly) phenols: Past efforts and possible future directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A neural crest derived embryonal malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Bansal, M.; Gupta, A.; Ding, H.-F. MYCN and metabolic reprogramming in neuroblastoma. Cancers 2022, 14, 4113. [Google Scholar] [CrossRef]
- Otte, J.; Dyberg, C.; Pepich, A.; Johnsen, J.I. MYCN function in neuroblastoma development. Front. Oncol. 2021, 10, 624079. [Google Scholar] [CrossRef] [PubMed]
- Benchia, D.; Bîcă, O.D.; Sârbu, I.; Savu, B.; Farcaș, D.; Miron, I.; Postolache, A.L.; Cojocaru, E.; Abbo, O.; Ciongradi, C.I. Targeting Pathways in Neuroblastoma: Advances in Treatment Strategies and Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 4722. [Google Scholar] [CrossRef] [PubMed]
- Kafoud, A.; Salahuddin, Z.; Ibrahim, R.S.; Al-Janahi, R.; Mazurakova, A.; Kubatka, P.; Büsselberg, D. Potential treatment options for neuroblastoma with polyphenols through anti-proliferative and apoptotic mechanisms. Biomolecules 2023, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Leis, K.; Baska, A.; Bereźnicka, W.; Marjańska, A.; Mazur, E.; Lewandowski, B.T.; Kałużny, K.; Gałązka, P. Resveratrol in the treatment of neuroblastoma: A review. Rev. Neurosci. 2020, 31, 873–881. [Google Scholar] [CrossRef]
- Shuvalov, O.; Kirdeeva, Y.; Daks, A.; Fedorova, O.; Parfenyev, S.; Simon, H.-U.; Barlev, N.A. Phytochemicals target multiple metabolic pathways in cancer. Antioxidants 2023, 12, 2012. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Corich, L.; Aranda, A.; Carrassa, L.; Bellarosa, C.; Ostrow, J.D.; Tiribelli, C. The cytotoxic effect of unconjugated bilirubin in human neuroblastoma SH-SY5Y cells is modulated by the expression level of MRP1 but not MDR1. Biochem. J. 2009, 417, 305–312. [Google Scholar] [CrossRef]
- Kaur, B.; Sohrabi, Y.; Achreja, A.; Lisanti, M.P.; Martinez-Outschoorn, U.E. Hallmark of cancer: Reprogramming of cellular metabolism. Front. Media SA 2023, 12, 1126913. [Google Scholar] [CrossRef]
- Deaver, J.W.; López, S.M.; Ryan, P.J.; Nghiem, P.P.; Riechman, S.E.; Fluckey, J.D. Regulation of cellular anabolism by mTOR: Or how I learned to stop worrying and love translation. Sports Med. Health Sci. 2020, 2, 195–201. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.-H.; Xu, X. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J. Pharm. Biomed. Anal. 2021, 201, 114129. [Google Scholar] [CrossRef]
- Pawar, C.S.; Balamurugan, K.; Baskar, S.; Prasad, N.R.; Khan, H.A. Enhancing chemosensitivity in drug-resistant breast cancer cells using β-cyclodextrin-loaded quercetin and doxorubicin inclusion complex via modulating SRC/PI3K/akt pathway. Appl. Biochem. Biotechnol. 2025, 197, 4068–4095. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Bagherian, M.; Torabi, S.M.; Sharifzadeh, S.O.; Hushmandi, K.; Fives, K.R.; Khan, H. Resveratrol augments doxorubicin and cisplatin chemotherapy: A novel therapeutic strategy. Curr. Mol. Pharmacol. 2023, 16, 280–306. [Google Scholar]
- Liu, Y.; Fang, M.; Tu, X.; Mo, X.; Zhang, L.; Yang, B.; Wang, F.; Kim, Y.-B.; Huang, C.; Chen, L. Dietary Polyphenols as Anti-Aging Agents: Targeting the Hallmarks of Aging. Nutrients 2024, 16, 3305. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Ahmad, N.; Qamar, M.; Yuan, Y.; Nazir, Y.; Wilairatana, P.; Mubarak, M.S. Dietary polyphenols: Extraction, identification, bioavailability, and role for prevention and treatment of colorectal and prostate cancers. Molecules 2022, 27, 2831. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Daks, A.; Fedorova, O.; Petukhov, A.; Barlev, N. Linking metabolic reprogramming, plasticity and tumor progression. Cancers 2021, 13, 762. [Google Scholar] [CrossRef]
- Nong, S.; Han, X.; Xiang, Y.; Qian, Y.; Wei, Y.; Zhang, T.; Tian, K.; Shen, K.; Yang, J.; Ma, X. Metabolic reprogramming in cancer: Mechanisms and therapeutics. MedComm 2023, 4, e218. [Google Scholar] [CrossRef]
- Shuvalov, O.; Petukhov, A.; Daks, A.; Fedorova, O.; Vasileva, E.; Barlev, N.A. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget 2017, 8, 23955. [Google Scholar] [CrossRef]
- Tjaden, B.; Baum, K.; Marquardt, V.; Simon, M.; Trajkovic-Arsic, M.; Kouril, T.; Siebers, B.; Lisec, J.; Siveke, J.T.; Schulte, J.H. N-Myc-induced metabolic rewiring creates novel therapeutic vulnerabilities in neuroblastoma. Sci. Rep. 2020, 10, 7157. [Google Scholar] [CrossRef]
- Pouliou, M.; Koutsi, M.A.; Champezou, L.; Giannopoulou, A.-I.; Vatsellas, G.; Piperi, C.; Agelopoulos, M. MYCN amplifications and metabolic rewiring in neuroblastoma. Cancers 2023, 15, 4803. [Google Scholar] [CrossRef]
- Jahangiri, L. Metabolic targeting of neuroblastoma, an update. Cancer Lett. 2024, 611, 217393. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.; Parfenyev, S.; Shuvalov, O.; Frolova, K.; Naminat, E.; Nevzorov, I.; Petukhov, A.; Karpova, N.; Fedorova, O.; Barlev, N. Effects of n-Myc and c-Myc on the expression of p53 family members and their transcriptional targets in human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2025, 769, 151944. [Google Scholar] [CrossRef]
- Cormerais, Y.; Lapp, S.C.; Kalafut, K.C.; Cissé, M.Y.; Shin, J.; Stefadu, B.; Personnaz, J.; Schrötter, S.; Freed, J.; D’Amore, A. AKT-mediated phosphorylation of TSC2 controls stimulus-and tissue-specific mTORC1 signaling and organ growth. Dev. Cell, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.; Lam, H.Y.; Yap, K.C.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signalling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef]
- Shuvalov, O.; Kirdeeva, Y.; Fefilova, E.; Daks, A.; Fedorova, O.; Parfenyev, S.; Nazarov, A.; Vlasova, Y.; Krasnov, G.S.; Barlev, N.A. 20-Hydroxyecdysone Boosts Energy Production and Biosynthetic Processes in Non-Transformed Mouse Cells. Antioxidants 2024, 13, 1349. [Google Scholar] [CrossRef]
- Majumder, A.; Bano, S.; Nayak, K.B. The Pivotal Role of One-Carbon Metabolism in Neoplastic Progression During the Aging Process. Biomolecules 2024, 14, 1387. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Cui, H. Serine–glycine-one-carbon metabolism: Vulnerabilities in MYCN-amplified neuroblastoma. Oncogenesis 2020, 9, 14. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Chen, X.; Shen, Y.; Lian, G.; Shah, N.; Davidoff, A.M.; Yang, J.; Wang, R. MYCN drives glutaminolysis in neuroblastoma and confers sensitivity to an ROS augmenting agent. Cell Death Dis. 2018, 9, 220. [Google Scholar] [CrossRef]
- Ruiz-Pérez, M.V.; Sainero-Alcolado, L.; Oliynyk, G.; Matuschek, I.; Balboni, N.; Ubhayasekera, S.K.A.; Snaebjornsson, M.T.; Makowski, K.; Aaltonen, K.; Bexell, D. Inhibition of fatty acid synthesis induces differentiation and reduces tumor burden in childhood neuroblastoma. IScience 2021, 24, 102128. [Google Scholar] [CrossRef]
- Kling, M.J.; Griggs, C.N.; McIntyre, E.M.; Alexander, G.; Ray, S.; Challagundla, K.B.; Joshi, S.S.; Coulter, D.W.; Chaturvedi, N.K. Synergistic efficacy of inhibiting MYCN and mTOR signaling against neuroblastoma. BMC Cancer 2021, 21, 1061. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Jamialahmadi, T.; Kesharwani, P.; Sahebkar, A. Bioinformatic analysis of the molecular targets of curcumin in colorectal cancer. Pathol. Res. Pract. 2024, 262, 155533. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.Q.; Zheng, S.Y.; Sun, Z.; Luo, Y.; Wang, Y.T.; Yi, P.; Li, Y.S.; Huang, C.; Xiao, W.F. Resveratrol: Molecular Mechanisms, Health Benefits, and Potential Adverse Effects. MedComm 2025, 6, e70252. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Yoon, D.; Choi, Y.; Kim, Y.; Cha, J.S.; Yoo, J. Quercetin: Molecular Insights into Its Biological Roles. Biomolecules 2025, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.; Husain, M. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin induces a fatal energetic impairment in tumor cells in vitro and in vivo by inhibiting ATP-synthase activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef]
- Yang, R.; Fang, X.-L.; Zhen, Q.; Chen, Q.-Y.; Feng, C. Mitochondrial targeting nano-curcumin for attenuation on PKM2 and FASN. Colloids Surf. B Biointerfaces 2019, 182, 110405. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, X.; Li, N.; Liu, H.; Dong, Q.; Zhang, J.; Yang, C.; Liu, Y.; Liang, Q.; Zhang, S. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp. Cell Res. 2016, 349, 320–327. [Google Scholar] [CrossRef]
- Wu, H.; He, L.; Shi, J.; Hou, X.; Zhang, H.; Zhang, X.; An, Q.; Fan, F. Resveratrol inhibits VEGF-induced angiogenesis in human endothelial cells associated with suppression of aerobic glycolysis via modulation of PKM 2 nuclear translocation. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Wu, M.-L.; Chen, X.-Y.; Kong, Q.-Y.; Wang, X.-W.; Sun, Y.; Wen, S.; Liu, J. c-Myc downregulation: A critical molecular event in resveratrol-induced cell cycle arrest and apoptosis of human medulloblastoma cells. J. Neuro-Oncol. 2006, 80, 123–131. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-Y.; Hong, S.-C.; Chang, C.-M.; Chen, Y.-H.; Liao, P.-C.; Huang, C.-Y. Oral squamous cell carcinoma cells with acquired resistance to erlotinib are sensitive to anti-cancer effect of quercetin via pyruvate kinase M2 (PKM2). Cells 2023, 12, 179. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Sturza, A.; Pavel, I.; Ancușa, S.; Danciu, C.; Dehelean, C.; Duicu, O.; Muntean, D. Quercetin exerts an inhibitory effect on cellular bioenergetics of the B164A5 murine melanoma cell line. Mol. Cell. Biochem. 2018, 447, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Mao, J.M.; Zhang, S.Y.; Zhou, Z.Q.; Tan, Y.; Zhang, Y. Quercetin induces HepG2 cell apoptosis by inhibiting fatty acid biosynthesis. Oncol. Lett. 2014, 8, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Zenkov, R.G.; Kirsanov, K.I.; Ogloblina, A.M.; Vlasova, O.A.; Naberezhnov, D.S.; Karpechenko, N.Y.; Fetisov, T.I.; Lesovaya, E.A.; Belitsky, G.A.; Dolinnaya, N.G. Effects of G-quadruplex-binding plant secondary metabolites on c-MYC expression. Int. J. Mol. Sci. 2022, 23, 9209. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, H.; Xiang, J.; Shen, G.; Yang, F.; Wang, F.; Wang, J.; Tang, Y. Advances and prospects of natural dietary polyphenols as G-quadruplex stabilizers in biomedical applications. Int. J. Biol. Macromol. 2024, 254, 127825. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets 2024, 2, 307–326. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the management of human health: How far have we come? A systematic review of resveratrol clinical trials to highlight gaps and opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a therapeutic product: Evaluation of its pharmacological action and clinical applications—A review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Elgar, K. Curcumin: A review of clinical use and efficacy. Nutr. Med. J. 2022, 1, 10–31. [Google Scholar]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.X.; Ou, Q.J.; Lin, F.T.; Zhao, Y.T.; Zhou, R.H.; Zhou, R.L.; Fang, Y.J.; Zhang, C.X. Higher Intake of Resveratrol Is Associated with a Lower Risk of Colorectal Cancer: A Large-Scale Case–Control Study. Phytother. Res. 2025, 39, 2776–2789. [Google Scholar] [CrossRef]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef]
- Perkins, S.; Verschoyle, R.D.; Hill, K.; Parveen, I.; Threadgill, M.D.; Sharma, R.A.; Williams, M.L.; Steward, W.P.; Gescher, A.J. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomark. Prev. 2002, 11, 535–540. [Google Scholar]
- Liu, Y.; Wu, Y.-M.; Zhang, P.-Y. Protective effects of curcumin and quercetin during benzo (a) pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Mérida, D.M.; Vitelli-Storelli, F.; Moreno-Franco, B.; Rodríguez-Ayala, M.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Polyphenol intake and mortality: A nationwide cohort study in the adult population of Spain. Clin. Nutr. 2023, 42, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Lisco, G.; Corbo, F.; Crupi, P.; Sardone, R.; Panza, F.; Lozupone, M.; Rondanelli, M.; Clodoveo, M.L. Dietary Intake of Polyphenols and All-Cause Mortality: A Systematic Review with Meta-Analysis. Metabolites 2024, 14, 404. [Google Scholar] [CrossRef]
- Kishino, M.; Kanehara, R.; Mori, N.; Ishihara, J.; Takachi, R.; Yamaji, T.; Iwasaki, M.; Tsugane, S.; Sawada, N. Dietary polyphenol intake and risk of overall and site-specific cancers: The Japan Public Health Center-based Prospective Study. J. Nutr. 2025, 155, 1987–1998. [Google Scholar] [CrossRef]
- Fan, L.; Fike, L.T.; Munro, H.; Yu, D.; Si, H.; Shrubsole, M.J.; Dai, Q. Dietary polyphenols and risk of breast cancer in a predominantly low-income population: A prospective analysis in the Southern Community Cohort Study (SCCS). Am. J. Clin. Nutr. 2025, 121, 1335–1345. [Google Scholar] [CrossRef]
- Shuvalov, O.; Kirdeeva, Y.; Fefilova, E.; Netsvetay, S.; Zorin, M.; Vlasova, Y.; Fedorova, O.; Daks, A.; Parfenyev, S.; Barlev, N. 20-Hydroxyecdysone Confers Antioxidant and Antineoplastic Properties in Human Non-Small Cell Lung Cancer Cells. Metabolites 2023, 13, 656. [Google Scholar] [CrossRef]
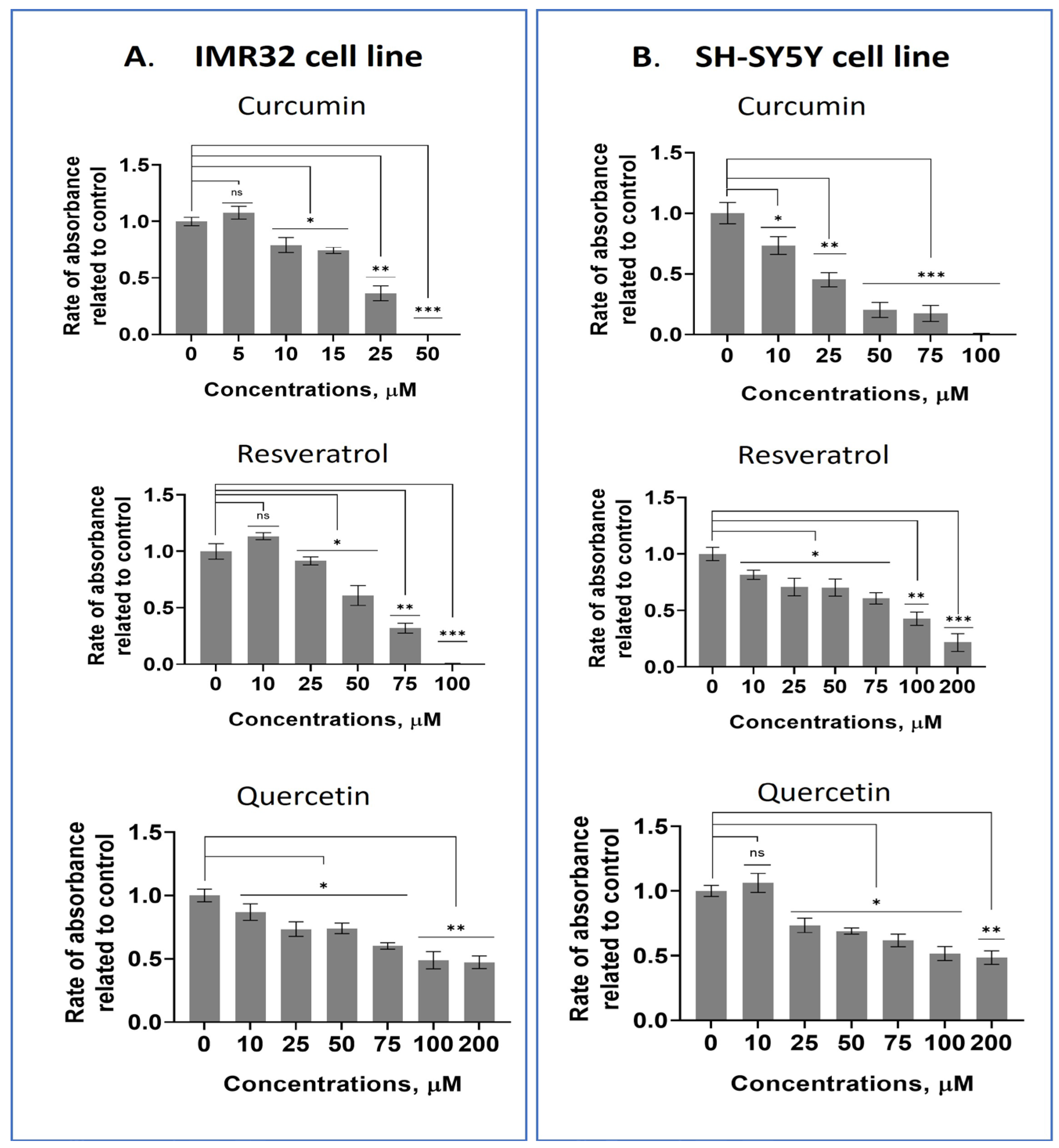









| Cell line | Substance/IC50 (µM) | ||||
|---|---|---|---|---|---|
| Curcumin | Quercetin | Resveratrol | EGCG | Kaempferol | |
| IMR32 | 19.7 ± 2.2 | 140.5 ± 12.1 | 54.6 ± 4.8 | 43.7 ± 5.1 | >200 |
| SH-SY5Y | 20.8 ± 2.4 | 121 ± 9.9 | 94.2 ± 10.4 | 182 ± 17.3 | 167 ± 15.6 |
| DF2 | 54.6 ± 4.9 | >200 | 151 ± 16.1 | 151 ± 14.2 | >200 |
| FRSN | 79.5 ± 8.7 | >200 | >200 | >200 | >200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpova, N.; Fefilova, E.; Daks, A.; Parfenyev, S.; Nazarov, A.; Barlev, N.A.; Shuvalov, O. Dietary Polyphenol Combinations Have a Multifaceted Inhibitory Effect on Metabolic Rewiring and Signaling Pathways in Neuroblastoma. Pharmaceuticals 2025, 18, 1717. https://doi.org/10.3390/ph18111717
Karpova N, Fefilova E, Daks A, Parfenyev S, Nazarov A, Barlev NA, Shuvalov O. Dietary Polyphenol Combinations Have a Multifaceted Inhibitory Effect on Metabolic Rewiring and Signaling Pathways in Neuroblastoma. Pharmaceuticals. 2025; 18(11):1717. https://doi.org/10.3390/ph18111717
Chicago/Turabian StyleKarpova, Natalia, Elizaveta Fefilova, Alexandra Daks, Sergey Parfenyev, Alexander Nazarov, Nick A. Barlev, and Oleg Shuvalov. 2025. "Dietary Polyphenol Combinations Have a Multifaceted Inhibitory Effect on Metabolic Rewiring and Signaling Pathways in Neuroblastoma" Pharmaceuticals 18, no. 11: 1717. https://doi.org/10.3390/ph18111717
APA StyleKarpova, N., Fefilova, E., Daks, A., Parfenyev, S., Nazarov, A., Barlev, N. A., & Shuvalov, O. (2025). Dietary Polyphenol Combinations Have a Multifaceted Inhibitory Effect on Metabolic Rewiring and Signaling Pathways in Neuroblastoma. Pharmaceuticals, 18(11), 1717. https://doi.org/10.3390/ph18111717










