1. Introduction
According to the World Health Organization (WHO), Neglected Tropical Diseases (NTDs) are a group of diseases that mainly affect the poorest regions of the world [
1]. They are in the tropical zone of the planet and arouse limited commercial interest on the part of large pharmaceutical companies, since many of these diseases are practically nonexistent in the richer regions of the world. Currently, nearly one billion individuals, i.e., one sixth of the world’s population, are affected by some of these diseases, most of them concentrated in Africa and Latin America. Many of these diseases are also associated with a lack of hygiene in the population, which in turn is related to a lack of education, something very common in the poorest regions of the world. Currently, some 17 diseases are considered as NTDs, many of which are not well known. They are characterized by being endemic and cannot spread outside tropical regions; for example, dracunculiasis (Guinea worm), American trypanosomiasis (Chagas disease), African trypanosomiasis (sleeping sickness), dengue, malaria, leishmaniasis, leprosy, lymphatic filariasis, and schistosomiasis, among others.
NTDs represent a significant health burden in large parts of the world. Some
Croton species are notable for their production of alkaloids, which are biogenetically related to benzylisoquinolines, such as morphinandienones, apomorphine, and tetrahydroprotoberberine alkaloids [
2,
3].
Croton linearis Jacq is an evergreen shrub that grows in Caribbean regions (Cuba, Jamaica), the Bahamas, and Florida [
4]. In the Bahamas, the crushed leaves have a small effect on bronchial and head colds when inhaled, owing to the release of fragrant oils. Tea eases symptoms of colds, flu, and influenza. Also, the decoction of leaves is used by oral route or in baths to relieve pains related to the menstrual period, after birth, and rheumatism [
4]. Asprey and Thornton reported that it is usually used by Jamaican peasants as a hair wash, as in Browne’s day, it is still used in baths for fever and colds, for the treatment of which a tea made with the plant may also be taken [
5].
Alkaloids have been documented as major components for this plant. The studies conducted in Jamaica by Haynes, Farnsworth et al. in the 1960s report the isolation of benzylisoquinoline type alkaloids hernovine, nuciferine, wilsonirine, jacularine, crotonosine, and linearisine, as well as morphinandienone type alkaloids 8,14-dihydrosalutaridine and 8,14-dihydronorsalutaridine. Recently Garcia et al. [
6], reported six alkaloids isolated from
C. linearis leaves: laudanidine, laudanosine, reticuline, corydine, glaucine, and cularine. Recent studies on
C. linearis have demonstrated its significant antiprotozoal potential, particularly against
Leishmania species, while revealing negligible antibacterial or antifungal activity. For instance, the essential oil derived from
C. linearis stems, featuring major components such as 1,8-cineole, α-pinene, and epi-γ-eudesmol, exhibited potent in vitro efficacy with IC
50 values of 21.4 μg/mL (promastigotes) and 18.9 μg/mL (amastigotes) of
Leishmania amazonensis, alongside acceptable selectivity indexes of 2–3. Moreover, molecular docking studies revealed that epi-γ-eudesmol interacts effectively with
Leishmania target enzymes, supporting its mechanistic plausibility [
7].
Additionally, isoquinoline alkaloids isolated from
C. linearis leaves, namely reticuline, laudanidine, and 8,14-dihydrosalutaridine, displayed moderate antiplasmodial activity against chloroquine-resistant
Plasmodium falciparum K1 strains, with IC
50 values of 46.8 ± 0.6, 17.7 ± 0.6, and 16.0 ± 0.5 μM, respectively, and no cytotoxicity up to 64 μM [
8]. This underscores the versatility of alkaloids as antiparasitic scaffolds, particularly for malaria. Further supporting evidence comes from bioassay-guided fractionation of
C. linearis leaf extracts, which revealed IC
50 values in the range of 1–26 μg/mL against various protozoa, including
Leishmania infantum,
Trypanosoma cruzi, and
Leishmania species, thereby, substantiating the antiprotozoal efficacy of both crude extracts and isolated alkaloids [
7]. Together, these findings suggest that
C. linearis produces a diverse chemical arsenal of monoterpenoid-rich essential oils and isoquinoline alkaloids with selective antiparasitic activity. Such evidence supports the hypothesis that these compounds could serve as valuable lead structures for the design and synthesis of novel semisynthetic antiprotozoal agents.
Natural products have long been recognized as valuable sources of bioactive com-pounds with therapeutic potential, especially in the search for new treatments for NTDs. A variety of studies have consistently underscored the significance of their diverse chemical structures and biological activities, which position them as optimal candidates for the identification of new pharmaceutical agents. Although conventional in vitro methods re-main paramount for evaluating antiparasitic effects, the integration of computational modeling has become progressively advantageous for predicting molecular targets and elucidating potential mechanisms of action at the nascent stages of research. Natural products have long been recognized as valuable sources of bioactive compounds with therapeutic potential, especially in the search for new treatments for NTDs. Numerous studies continue to highlight their diverse chemical structures and biological activities, which make them ideal candidates for drug discovery. While traditional in vitro approaches remain important for assessing antiparasitic effects, the incorporation of computational modeling has become increasingly advantageous for predicting molecular targets and understanding potential mechanisms of action at earlier stages of research [
9].
Comprehensive phytochemical reviews further emphasize the importance of integrating computational tools into natural product research. The wide range of pharmaco-logical activities observed in plant-derived compounds suggests that many of these metabolites may interact with multiple biological pathways relevant to NTDs. Molecular modeling approaches, including virtual screening and docking simulations, provide valuable insights into these complex interactions, supporting the selection of promising com-pounds for further pharmacological development [
10].
The growing use of artificial intelligence and cheminformatics has significantly enhanced the efficiency of natural product-based drug discovery. These advanced technologies allow researchers to analyze large datasets, predict bioactivity and toxicity profiles, and accelerate the identification of drug-like candidates. As highlighted in recent work, such approaches help streamline the discovery process by complementing traditional methods and enabling a more targeted exploration of nature-derived chemical diversity [
11]. Molecular docking is a modeling technique to study how a small molecule (ligand/drug) interacts with a macro-molecule (protein/enzyme, DNA, etc.). This molecular modeling technique basically addresses three key goals—virtual screening, pose prediction, and binding affinity estimation. Taking into account the in vitro antiprotozoal potentialities showed by extract of
C. linearis, which different alkaloids were identified, in this work, we have studied the potential bio-chemical interaction of identified alkaloids from
C. linearis leaves on different protozoan NTDs targets using a molecular docking approach to suggest possible mechanisms of these metabolites.
2. Results and Discussion
To explore the therapeutic potential of natural alkaloids derived from
C. linearis, a comprehensive molecular docking study was conducted against a panel of validated enzymatic targets implicated in various NTDs, including leishmaniasis, Chagas disease, sleeping sickness, and malaria [
10]. The selection criteria for these alkaloids (
Figure 1) are based on the previously reported in vitro antiprotozoal activities of
C. linearis extracts [
8,
12]. The selected alkaloids were docked into the active sites of key parasite enzymes using a structure-based approach to predict their binding affinity and interaction profiles.
The results revealed notable differences in binding energies across targets, with several alkaloids demonstrating comparable or superior affinity to that of co-crystallized reference ligands. These findings provide valuable insights into the structure-activity relationships of these compounds and support their potential as lead molecules for the development of novel antiparasitic agents.
To assess the inhibitory potential of alkaloids isolated from
C. linearis against protozoan targets implicated in NTDs, molecular docking was performed against a panel of enzymes from
Trypanosoma (
Table 1),
Leishmania, and
Plasmodium species (
Table 2). The binding affinity (Δ
Gdock) values revealed that norsalutaridine, reticuline, and salutardine consistently displayed strong interactions across multiple targets, with particularly high affinity for
Leishmania infantum Cyp51 (−9.3, −8.8, and −9.0 kcal/mol, respectively), outperforming the co-crystallized ligand TPF (−8.0 kcal/mol).
These alkaloids also exhibited favorable docking scores against T. cruzi DHFR-TS and T. brucei ADKinase. Reticuline, for instance, reached −8.5 kcal/mol in DHFR-TS and −8.1 kcal/mol in ADKinase, indicating potential multitarget activity. Such results support the idea that benzylisoquinoline alkaloids may interfere with multiple metabolic pathways, enhancing their pharmacological relevance.
Among the tested alkaloids from C. linearis, reticuline, salutaridine, and norsalutaridine emerged as top candidates across multiple enzyme targets from L. infantum, T. cruzi, T. brucei, and P. falciparum. Against L. infantum Cyp51, the most promising target for leishmaniasis, norsalutaridine achieved the best docking score (−9.3 kcal/mol), outperforming both reticuline (−8.8) and the co-crystallized ligand TPF (−8.0). This suggests a strong and specific interaction, potentially driven by aromatic stacking with the heme group and polar contacts within the enzyme’s hydrophobic pocket.
Similarly, reticuline demonstrated superior binding to T. cruzi DHFR-TS (−8.4) and T. brucei ADKinase (−8.1), indicating its broad inhibitory potential.
For PTR1 from
L. major, wilsonirine demonstrated the highest docking score (−8.9), exceeding the reference ligand (−7.9). This compound’s extended aromatic structure may enable deeper engagement with the catalytic cleft, forming multiple stabilizing interactions. Interestingly, the enzyme DHODH from
P. falciparum, a target in antimalarial therapy, was best inhibited by reticuline (−8.3), with linearisine (−8.2) following closely, while the co-crystallized ligand DZB scored −8.4 kcal/mol (
Table 2). These values indicate that certain alkaloids can closely mimic, or even rival, the binding efficiency of synthetic drugs in terms of predicted binding energy
The comparative chart underscores the fact that while the co-crystallized ligands generally perform well, several alkaloids approach or surpass their affinity, particularly in Leishmania and Trypanosoma targets. Notably, norsalutaridine and reticuline consistently achieve strong binding across all selected enzymes. This cross-target activity, combined with their natural origin and traditional medicinal use, positions these compounds as valuable leads for further optimization. Homolinearisine and linearisine also exhibit moderate but consistent affinity, suggesting a favorable chemical scaffold.
Likewise, García Díaz et al. [
6] reported significant in vitro antiparasitic activity for
C. linearis extracts, attributing part of this efficacy to the presence of reticuline, glaucine, and related alkaloids. Together, these results support the computational predictions and reinforce the pharmacological relevance of these natural compounds as promising scaffolds for future drug development.
The docking score results provided an initial indication of the binding affinity of the alkaloids toward each target enzyme; however, a deeper understanding was achieved through the analysis of molecular interactions within the active sites. Compounds with the most favorable docking scores, such as reticuline and norsalutaridine, consistently established key stabilizing interactions, such as hydrogen bonds, hydrophobic contacts, and π–π stacking, mirroring or surpassing those formed by the co-crystallized ligands. These interactions not only validate the docking predictions but also highlight the structural features responsible for their strong enzyme binding.
2.1. Interaction T. cruzi 14α-Demethylase (Cyp51) with Reticuline, Linearisine, and Norsalutari-Dine
Sterol 14α-demethylase is a cytochrome P450 monooxygenase (Cyp51) that catalyzes oxidative removal of the 14α-methyl group from sterols and is an essential enzyme in sterol biosynthesis. According to Lepesheva et al. in 2010 [
13], the active site involves the amino acids Tyr103, Met106, Phe110, Tyr116, Leu127, Ala297, Phe290, Ala291, Thr295, Leu356, Leu357, Met358, Met360, Val461, Met460, and Ala287. The interaction of alkaloids with
T. cruzi Cyp51 revealed that several metabolites from
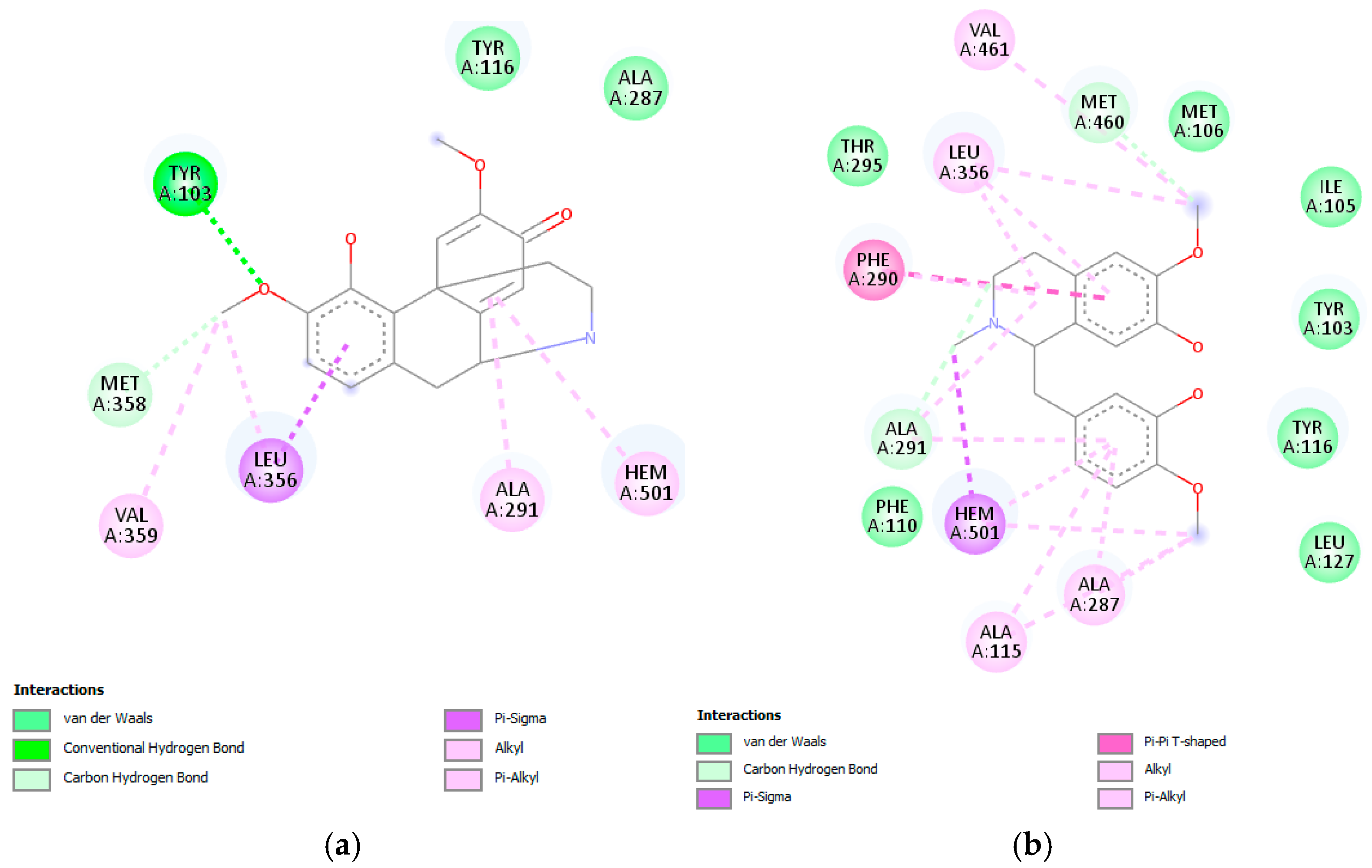
C. linearis displayed docking scores very close to or comparable with the co-crystallized ligand NEE (−9.1 kcal/mol). Notably, norsalutaridine (−8.8 kcal/mol) and reticuline (−8.3 kcal/mol) demonstrated strong affinities, forming multiple stabilizing interactions within the enzyme’s active site (
Figure 2), slightly better than co-crystallized ligand TPF (−8.0 kcal/mol). From a structural point of view, these alkaloids maintain aromatic moieties and basic nitrogen atoms that enable π-stacking and hydrogen bonding interactions with key residues in the heme-containing active pocket, like standard azole-based inhibitors.
These interactions are supported by visualizations of docked poses, where both (norsalutaridine and reticuline) are stabilized by contacts with Met105, Tyr116, and the heme group. Literature from recent years supports the role of Cyp51 as a validated target for Chagas disease chemotherapy. For instance, azole derivatives like posaconazole and ravuconazole have shown nanomolar affinities in vitro and in vivo [
14]. The binding energies of alkaloids in this study, although slightly less potent, demonstrate promising leads for natural product-based inhibitors. Furthermore, homolinearisine and wilsonirine also perform comparably to the reference compound, reinforcing the potential of these molecules in lead optimization pipelines.
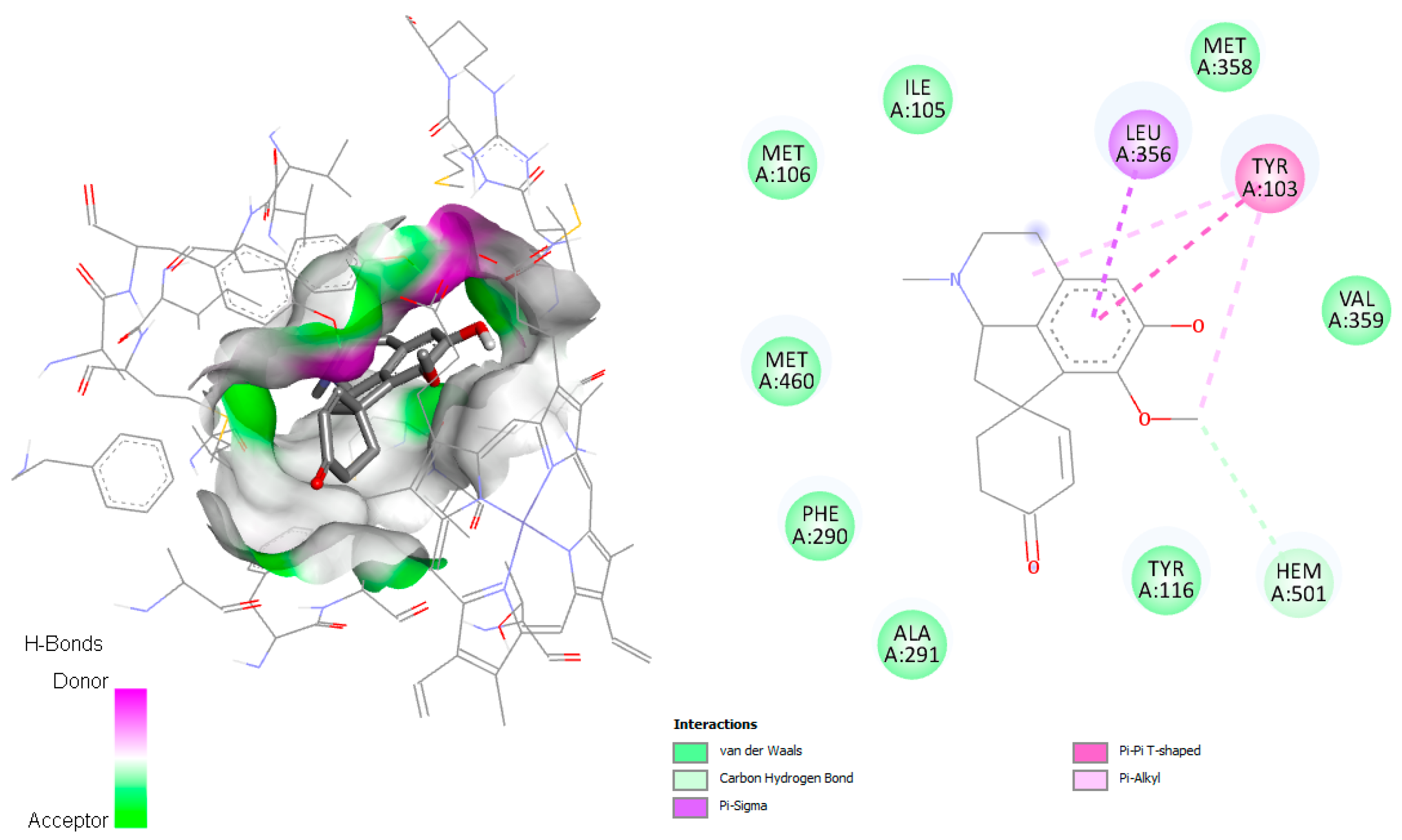
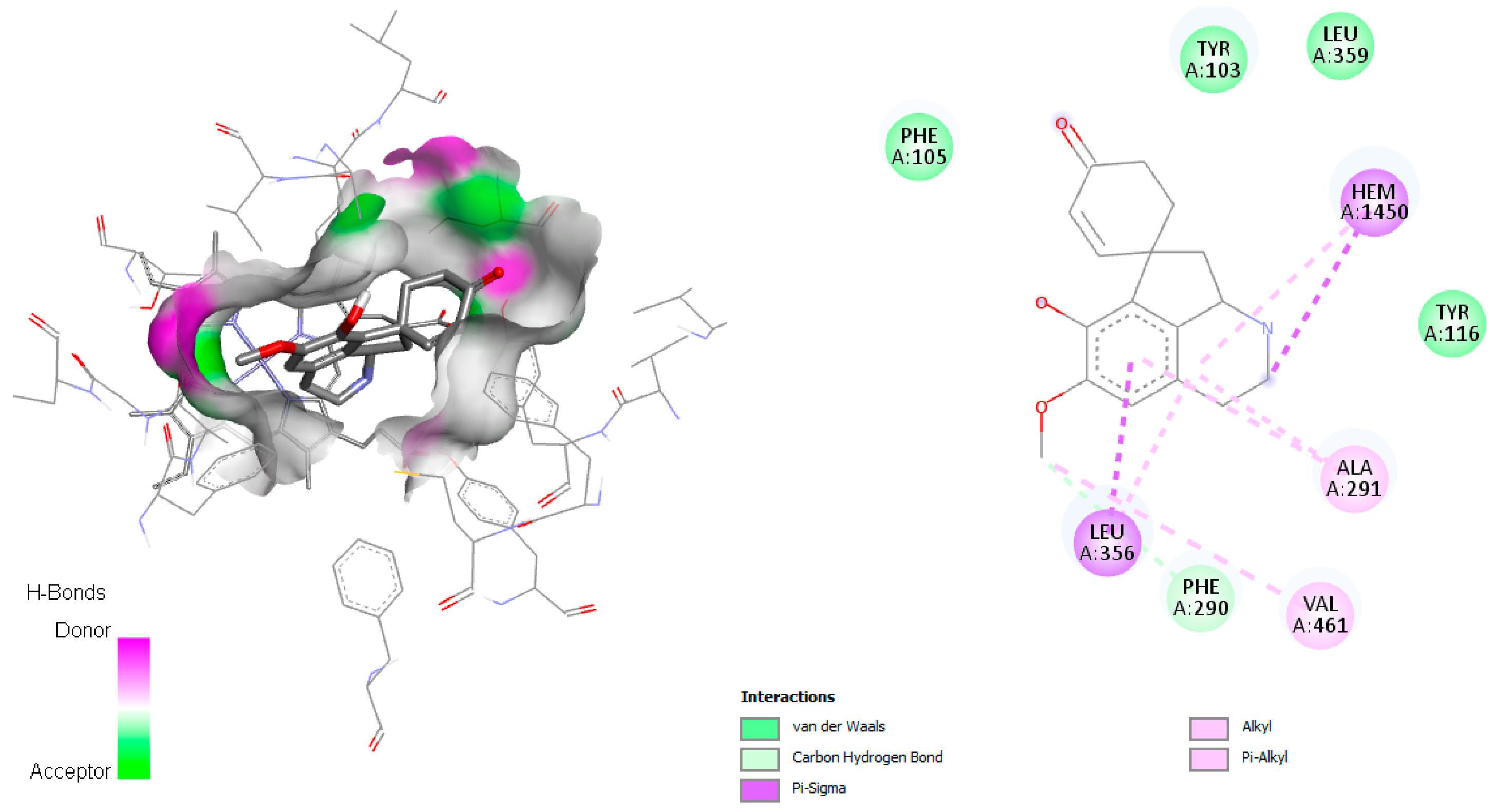
On the other hand, linearisine was found to interact with Leu356 by π–σ interaction and π–π T-shaped interaction with Tyr103, both involving the active site (
Figure 3), in addition to many hydrophobic interactions involving different amino acid residues, including Met358, Val359, Ile105, Met106, Met460, Phe290, Ala291, and Ty116 present in the active site.
Co-crystallized ligand NEE was found to establish π–π interactions with Tyr116 and Phe290 in
T.cruzi Cyp51 and demonstrated a π-σ interaction with Leu127. Moreover, for this complex, alkyl interactions with the amino acid residues Hem501, Ala287, Tyr103, Leu356, Ala115, Ala291, and Val461 were observed (
Figure S1). Co-crystallized ligand NEE demonstrated similar interaction as norlutaridine, reticuline, and linearisine alkaloids; they share the same residues involved in the active site as Ty116, Met106, Met460, Phe290, Ala291, etc.
2.2. Interaction T. cruzi DHFR-TS with Laudanidine and Reticuline
Dihydrofolate reductase (DHFR) is a global enzyme that has been a well-known and effective target in medicinal chemistry not only due to its anticancer, antibacterial, and antimalarial treatments, but also due to its central role in cellular metabolism and DNA synthesis in
T. cruzi [
15].
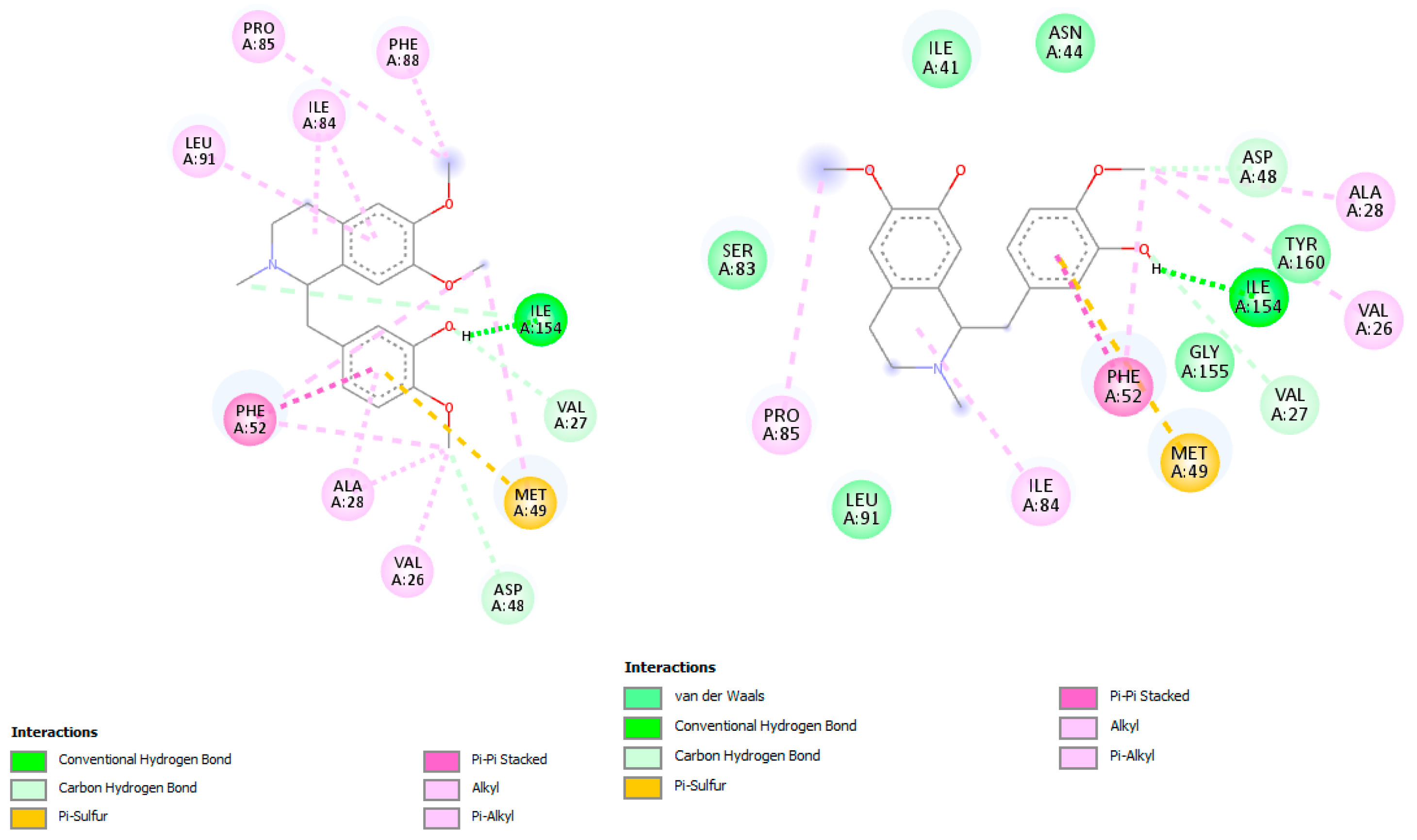
In docking simulations against dihydrofolate reductase-thymidylate synthase (DHFR-TS), several
C. linearis alkaloids outperformed the co-crystallized ligand 2CY. The top scorers, laudanidine and reticuline, exhibited stronger predicted binding affinities than the reference, suggesting a more stable enzyme-inhibitor complex. Structural docking analysis (
Figure 4) shows these compounds fitting tightly into the folate-binding pocket, engaging in hydrogen bonds and hydrophobic interactions with active site residues such as Phe52, Phe-88, Asp48, Ala28, Gly156, Gly157, and Tyr16. Other interactions with hydrophobic contact and weak bonds involved Ile41, Ile35, Lys79, Tyr160, Thr80, Pro85, and Ile84 (
Figure 5). These findings align with recent drug discovery efforts [
16]. For example, the repurposing of antifolates for trypanosomatids has regained interest due to cross-species conserved domains in DHFR [
17]. Some studies have also reported activity of natural hemisynthetic alkaloids against DHFR in
Plasmodium species, which supports the plausibility of these findings [
18].
The
T. cruzi DHFR enzymes differ from other enzymes in that they are found as a bifunctional homodimeric protein with the thymidylate synthase (TS) domain located at the C-terminal. The overall structure of DHFR-TS is very flexible, and the DHFR domain is at the N-terminus and is separated from the TS domain (C-terminus) by a peptide linker that varies in size. In most organisms, DHFR and TS exist as separate monofunctional enzymes. According to Beltran-Hortelano et al. in 2022 [
15], DHFR-TS has three regions with different characteristics: region I is composed of alkyl and aromatic side chains, region II has a hydrophilic character formed by hydrophilic amino acids and carbonyl (C=O) or amine (NH) groups of the peptide bonds, and region III has an arginine capable of bridging hydrogen.
2.3. Interaction T. brucei 14α-Demethylase (Cyp51) with Jacularine
According to Choi et al., 2013 [
19], the
T. brucei 14α-demethylase (Cyp51) has 85% sequence identity to
T. cruzi 14α-demethylase (Cyp51). Many of the first-tier active-site residues in these two parasite Cyp51 enzymes are identical, except for amino acid residue Phe105, which is isoleucine in the
T. cruzi counterpart.
The Cyp51-jacularine complex demonstrated π–σ with Leu356 residue and heme group (
Figure 5). Other favorable interactions of different types were formed, as van der Waals and π–alkyl interactions with several residues (Phe105, Tyr103, Leu359, Tyr116, Phe290, Ala291, and Val461) involved in the active site [
13].
2.4. Interaction T. brucei Adenosine Kinase (ADkinase) with Reticuline
Adenosine kinase (ADkinase) catalyzes the phosphorylation of adenosine-to-adenosine monophosphate (AMP) in presence of Mg2+ and utilizing ATP as the preferred phosphoryl donor. The corresponding adenosine kinase of T. b. rhodesiense (TbrAK) is an enzyme that functions as a monomer, a common characteristic of other adenosine kinases. In general, the sequence identity among adenosine kinases of different species is moderate (17% to 40%); however, their structures are remarkably similar. The catalytic site of ADkinase involved Asn13, Ile38, Ser64, Phe169, Thr172, Asp299 (adenosine binding pocket), R132, N222, T264, G296, A297 (pentaphosphate moiety), and Arg265, Glu268, Thr270 (ATP binding pocket).
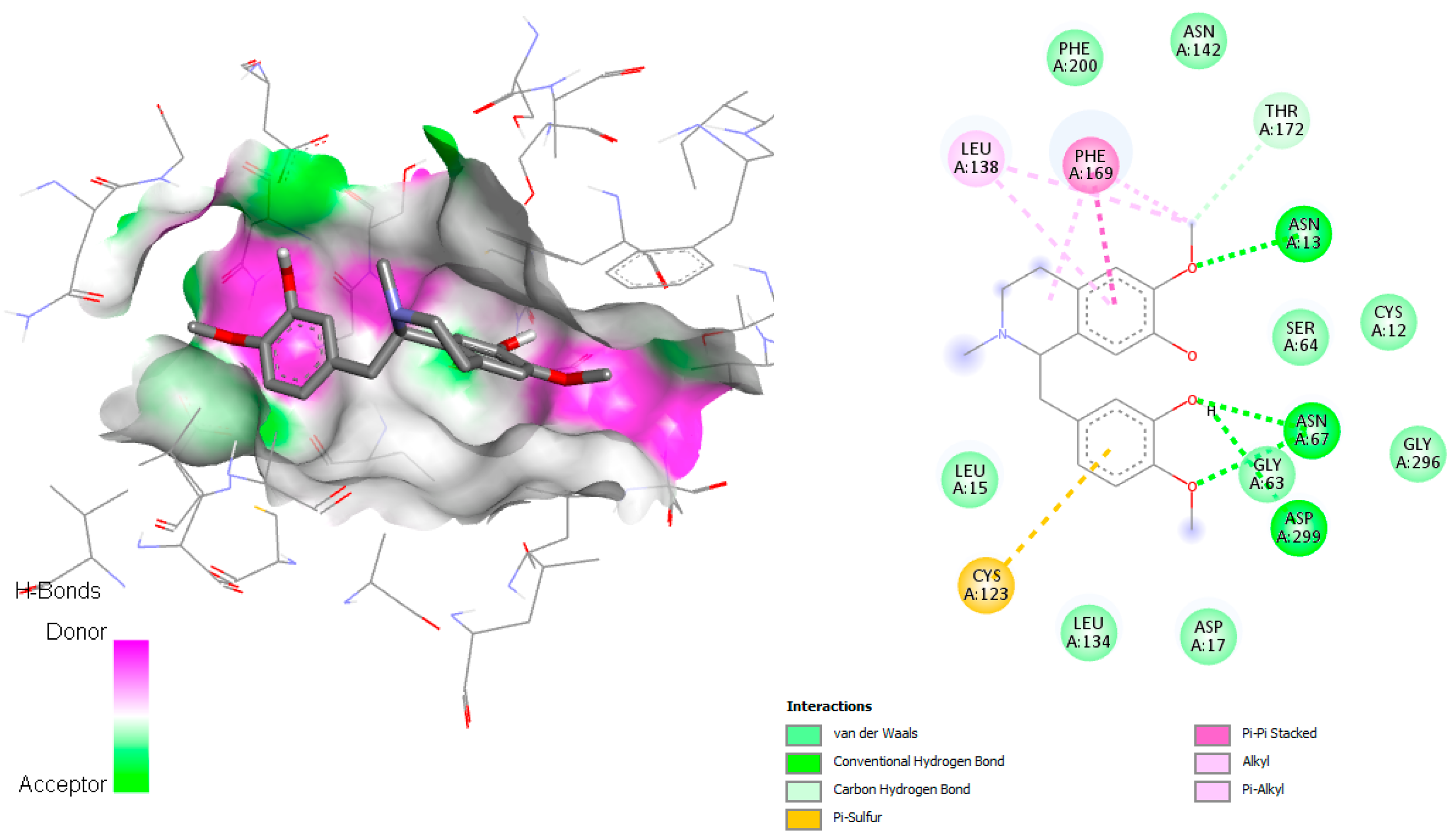
The docking simulation of reticuline in
T. brucei ADkinase target exhibited an H-bonding interaction with Asn13, Asn67, and Asp299 through the oxygen atom of the hydroxyl group present in reticuline involved in the adenosine binding pocket reported by Kuettel et al., 2011 [
20] (
Figure 6). Two interactions with Phe169 (π–π stacked) and Cys123 (π–sulfur) were observed, as well as hydrophobic interactions, mainly van der Waals interactions (Phe200, Asn142, Thr172, Cy12, Ser64, Gly296, Gly63, Asp17, Leu134, Leu15), which are involved in the active site.
The docking interaction of the co-crystallized ligand KRM demonstrated π–σ interaction with Leu138, π–π interaction with Phe200, Phe204, and Phe169 (
Figure S2). On the other hand, different hydrophobic interactions in the active site were observed, mainly van der Waals and alkyl interactions with the amino acids Leu15, Leu39, Ser64, Gly63, Cys12, Asn13, Asn142, Thr172, and Phe205. The reticuline and co-crystallized ligand KRM share some residues, Phe169, Leu138, and Phe200, involved in the active site, according to Kuettel et al., 2011 [
20].
The docking simulations against
T. brucei adenosine kinase (ADKinase) revealed that reticuline had the most favorable binding among the alkaloids, although it did not outperform the co-crystallized ligand KRM (–8.8 kcal/mol). The active site interactions shown in
Figure 6 demonstrate hydrogen bonding and van der Waals contacts, particularly with key residues like Asp299 and Phe169 [
21,
22]. Despite slightly lower docking scores than the control, these metabolites may still offer biological relevance, especially considering the known flexibility and plasticity of the ADKinase pocket. Previous studies have shown that selective inhibitors for trypanosomal ADKinase can block purine salvage, critical for parasite survival [
21]. Some alkaloids, such as crotonosine and linearisine, approach −7.8 kcal/mol, suggesting that chemical optimization (e.g., adding hydrogen bond donors or planar groups) could enhance binding affinity further. Moreover, these molecules may offer structural diversity compared to existing ADKinase inhibitors like tubercidin analogs [
22].
2.5. Interaction L. infantum 14α-Demethylase (Cyp51) with Reticuline and Wilsonirine
Both compounds established key stabilizing interactions within the catalytic site of the enzyme (
Figure 6 and
Figure 7), including hydrogen bonds and π–π stacking with residues such as His99, Tyr116, and the heme prosthetic group, which plays a critical role in the enzyme’s function. These interactions suggest that the aromatic moieties of the alkaloids are well-oriented within the active site, mimicking the binding mode of the co-crystallized ligand.
The structural comparison with the native ligand revealed that while the co-crystallized TPF (
Figure S3) primarily stabilizes through coordination with the heme iron and polar interactions with residues in the active channel, reticuline and norsalutaridine extend further into the hydrophobic pocket, enabling additional van der Waals contacts that may enhance binding stability. This extended coverage could confer greater inhibition potential, especially considering the structural rigidity and electron-rich features of their benzylisoquinoline scaffolds. Importantly, the docking poses retained the orientation required for interaction with the central heme group, a characteristic essential for successful Cyp51 inhibition.
Recent literature continues to underscore the potential of isoquinoline alkaloids as inhibitors of sterol biosynthesis in
Leishmania. A study by Bessa et al. (2024) identified a range of isoquinoline and indole alkaloids from Amaryllidaceae showing potent
in vitro activity against
L. infantum [
23], with molecular docking indicating strong binding to the parasite’s sterol 14α-demethylase (CYP51) and key π–π interactions within the heme pocket [
24]. Additionally, research by Tanwar et al. (2024) [
25] highlighted that antifungal azoles and azasterols, known to engage both π–π stacking and iron coordination, effectively inhibited
L. amazonensis sterol biosynthesis, disrupting membrane integrity and resulting in parasite death [
23]. These findings reinforce the concept that alkaloids like reticuline and norsalutaridine, with aromatic scaffolds capable of similar interactions, represent promising leads for Cyp51-focused drug development against
Leishmania. Thus, the alkaloids identified in this study, particularly reticuline and norsalutaridine, may serve as promising scaffolds for further optimization against
L. infantum Cyp51.
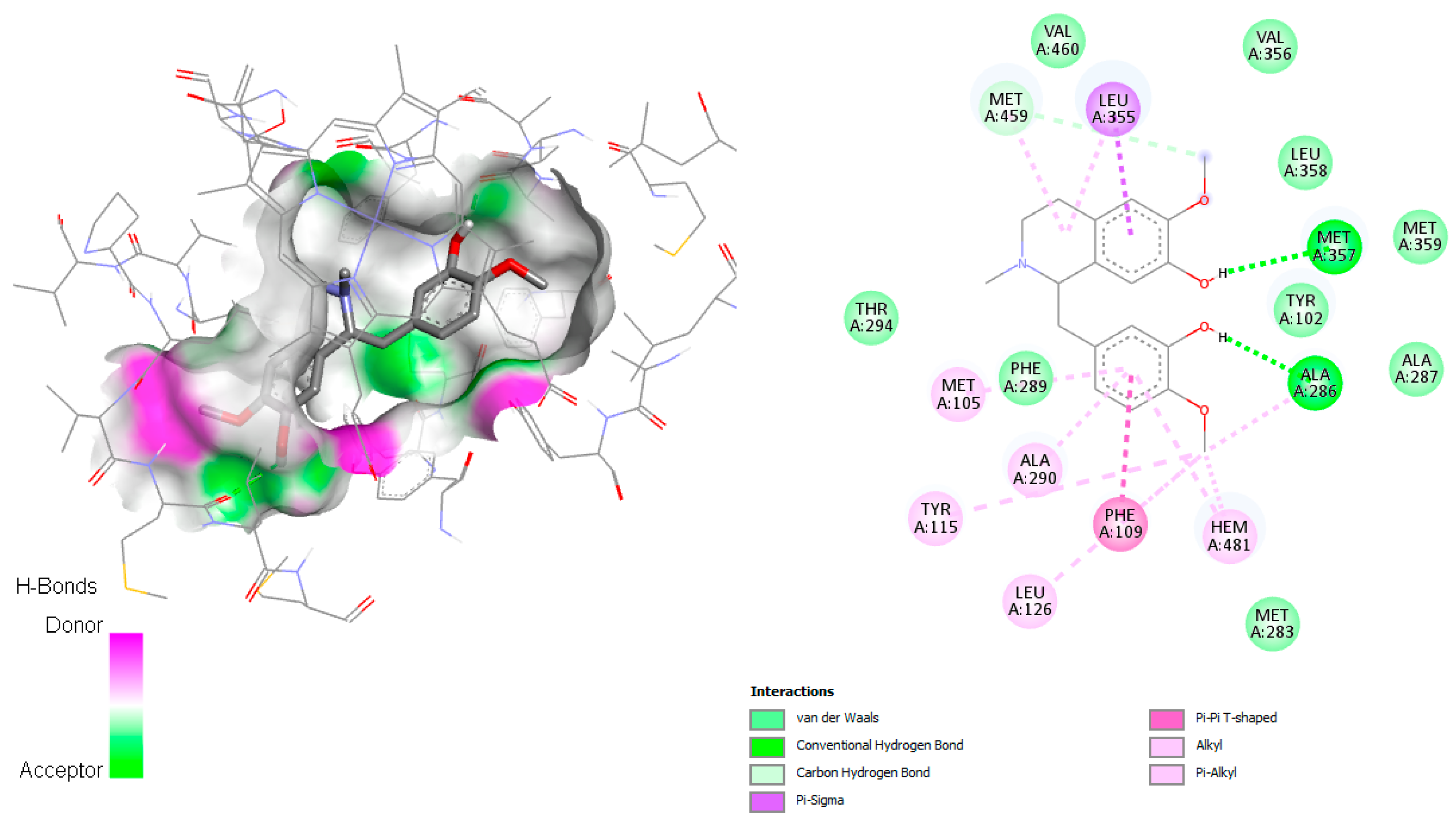
According to Hargrove et al. in 2011 [
26], the active site involved the amino acids Leu355, Met357, Leu358, Met459, Val212, Phe104, Met105, Tyr115, Ala114, Ala286, Phe109, Gly282, Met283, Phe289, Leu129, Ala290, and Val460. Reticuline was found to establish hydrogen bonds with Met357 and Ala286 (
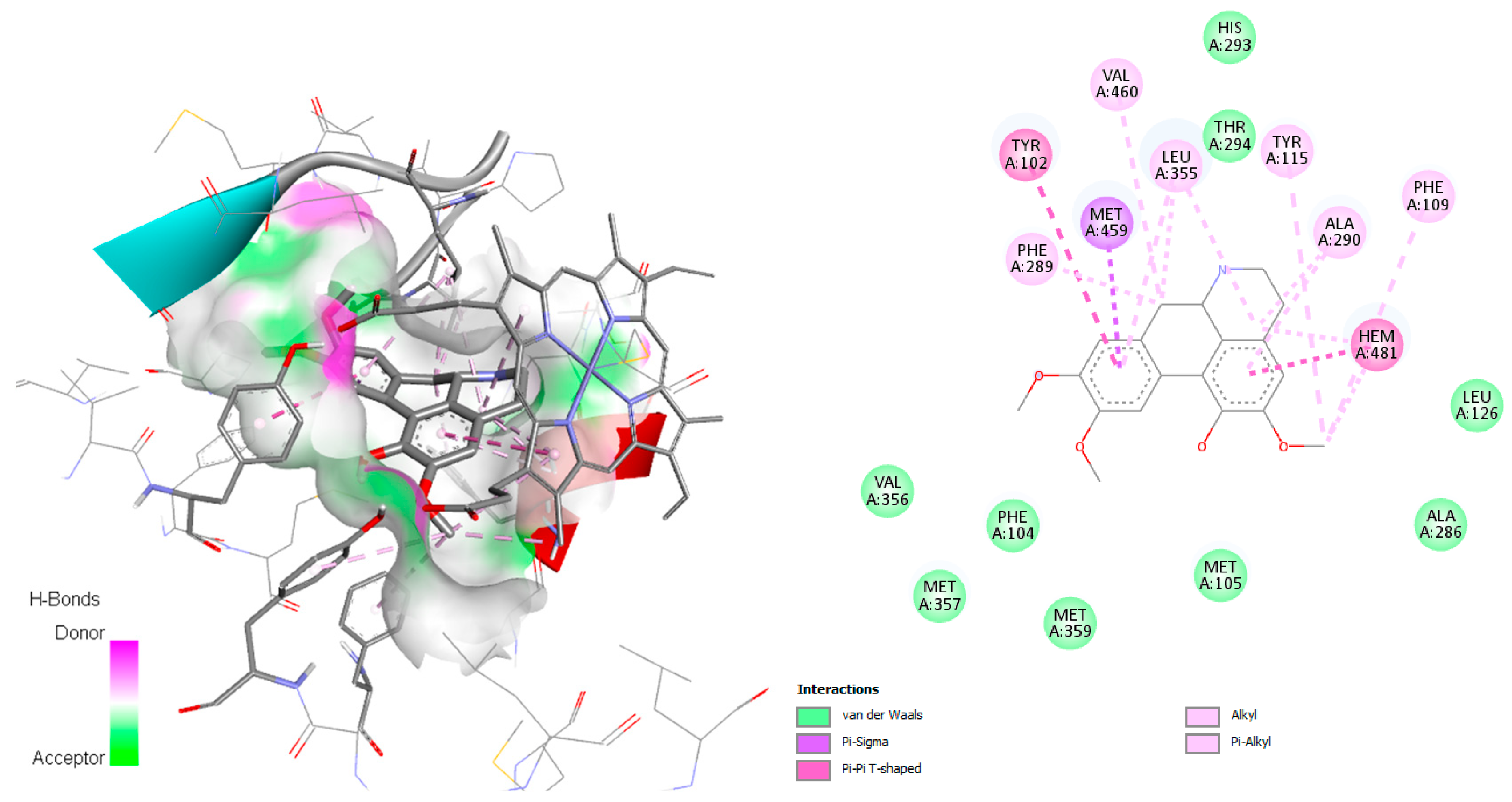
Figure 7), both involved in the active site. Additionally, for this complex, π–π T-shaped with Phe109, π–σ interaction with Leu355, and Van der Waals and alkyl interactions with the amino acid residues Hem371, Leu358, Val 460, Val356, Leu358, Met359, Tyr102, Ala287, Met283, Ala290, Met205, Tyr115, Phe289, and Leu126 were observed. Wilsonirine demonstrated π–π T-shaped interaction with Hem481 and Tyr102 (
Figure 8), besides hydrophobic interaction in the active site, van der Waals and alkyl interactions were observed mainly with Val460, Phe109, Val356, Leu355, Ala286, Leu358, Met359, Tyr102, Ala287, Met283, Ala290, Met205, Tyr115, Met357, Phe289, and Leu126, all involved in the active site [
26].
The co-crystallized ligand TPF was found to establish halogen (fluorine) interaction with Ala290 and Hem481 (
Figure S3), demonstrate π–σ interaction with Leu355 and Met459, van der Waals and alkyl interaction with Phe104, Met105, Phe289, Phe109, Tyr115, Tyr102, Leu126, Thr294, and Ala286. Reticuline and wilsonirine share some residues with the co-crystallized ligand, as Ala290, Phe109, Hem481.
In the case of
L. infantum, norsalutaridine again emerged as the top-performing alkaloid with a docking score of −9.3 kcal/mol, surpassing the co-crystallized ligand TPF. This result indicates a potential dual inhibitory profile against
T. cruzi and
Leishmania Cyp51 enzymes. Docked conformations (
Figure 6,
Figure 7 and
Figure S3) show consistent interaction networks, with hydrophobic contacts and hydrogen bonding contributing to binding stability. These findings are notable given the limited number of approved treatments for visceral leishmaniasis and the known limitations of azole-based drugs, including resistance and toxicity [
26]. The high binding affinities and favorable interactions of wilsonirine and salutaridine support their potential as scaffolds for novel CYP51 inhibitors.
Recent docking studies by Bessa et al. (2024) [
23] revealed that isoquinoline alkaloids isolated from
Hippeastrum aulicum exhibit strong binding affinity toward sterol 14α-demethylase (Cyp51) in
L. infantum, primarily through π–π stacking interactions and hydrogen bonding within the heme-binding site. However, their predicted binding energies were generally less favorable compared to those of the
C. linearis alkaloids evaluated in the present study. These findings support the hypothesis that
C. linearis metabolites, particularly reticuline and norsalutaridine, may constitute a distinct subclass of alkaloids with superior structural compatibility and binding strength against Cyp51 enzymes.
2.6. Interaction L. major Pteridine Reductase 1 (PTR1) with Reticuline and Wilsonirine
The pteridine reductase 1 (PTR1) enzyme plays an adaptive role in antifolate resistance, making it a validated drug target in
Leishmania species. In this study, several
C. linearis alkaloids demonstrated superior binding to PTR1 compared to the reference compound. Notably, laudanosine and reticuline yielded docking scores of −8.8 and −8.7 kcal/mol, respectively, which indicates higher predicted affinity.
Figure 9 and
Figure 10 show that these alkaloids form extensive hydrogen bonds and π–π stacking interactions with conserved residues such as Ser111 and Phe113, involved in substrate recognition. This is consistent with known interactions reported for methotrexate analogs and benzoquinolines [
27], which also demonstrated strong binding to
Leishmania PTR1 through hydrogen bonds with Ser111 and π–π stacking with Phe113. Singh et al. [
28] demonstrated that these interactions are critical for inhibitor efficacy, as they stabilize the ligand within the enzyme’s active site. The similar binding patterns observed in our study with
C. linearis alkaloids, particularly laudanosine and reticuline, suggest that these natural compounds may exploit the same mechanistic pathways as synthetic inhibitors, further validating PTR1’s conserved binding features as a target for antifolate resistance. Given that PTR1 inhibitors are scarce in the clinical pipeline, these results highlight the potential of natural isoquinoline scaffolds to serve as new leads. Moreover, wilsonirine also demonstrated strong binding (−8.9), warranting further evaluation through in vitro enzyme inhibition studies.
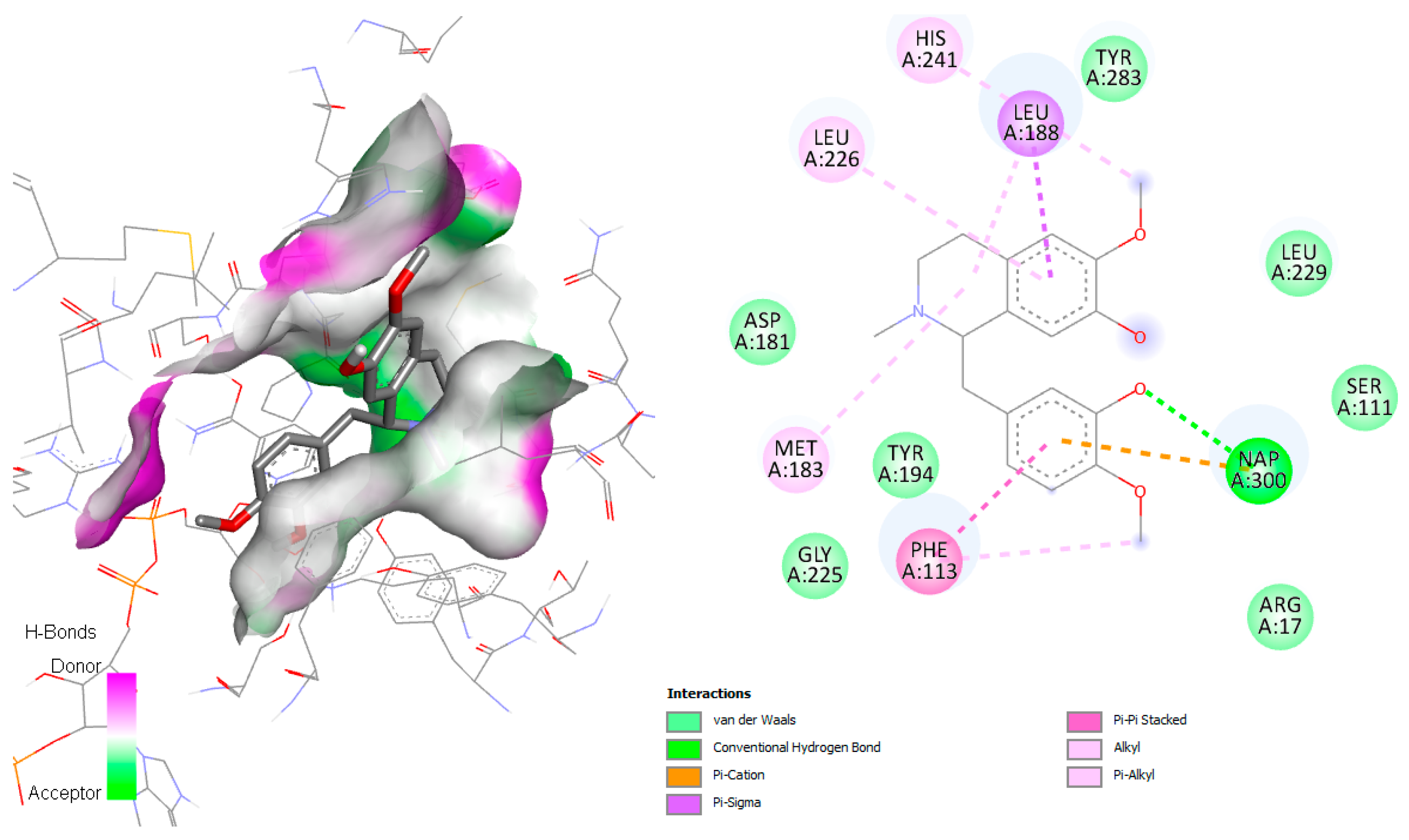
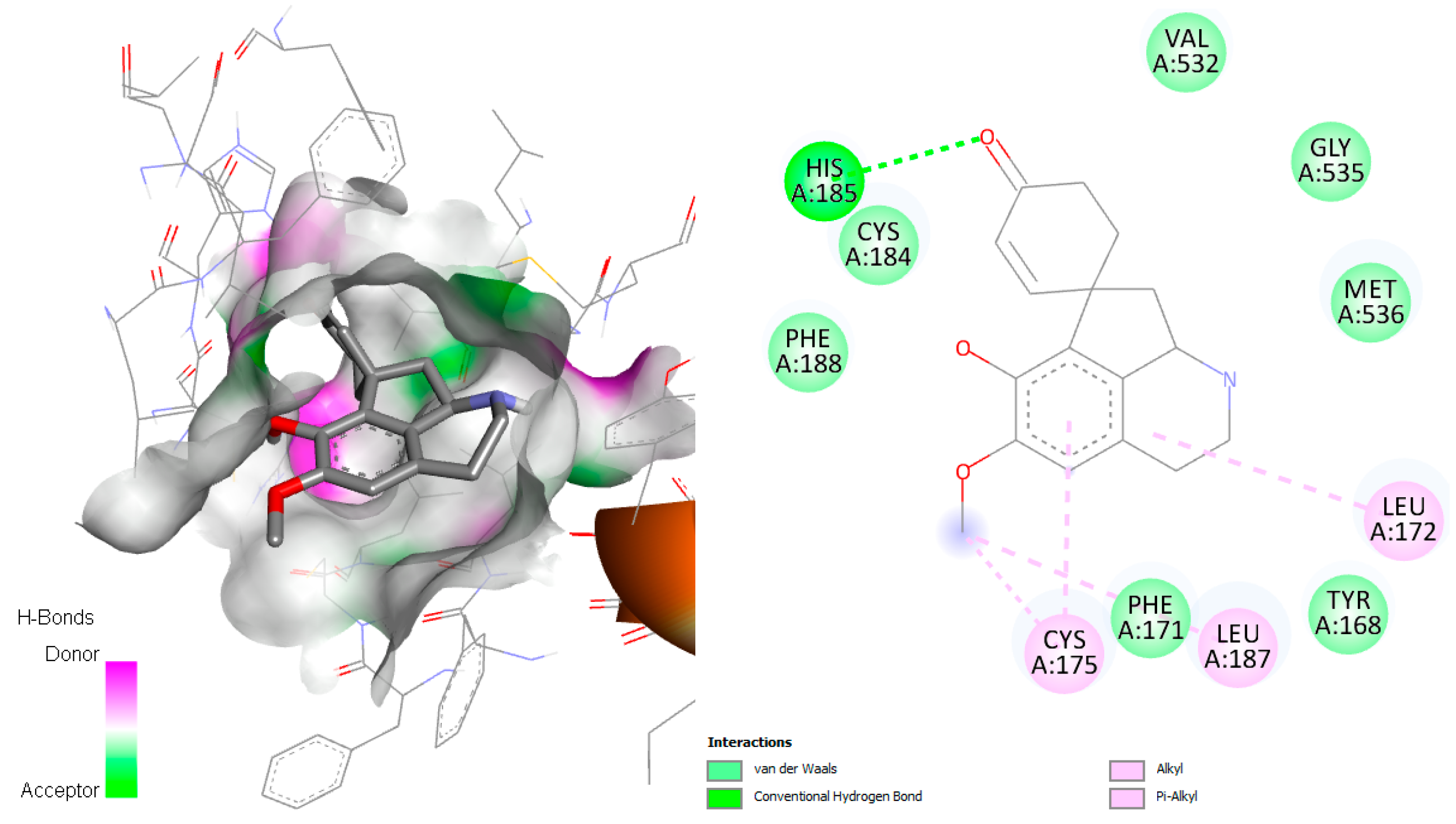
2.7. Interaction P. falciparum Dihydroorotate Dehydrogenase (DHODH) with Jacularine, Reticuline, and Linearisine
The target of P. falciparum dihydroorotate dehydrogenase (PfDHODH) is an enzyme involved in the catalysis of the fourth step in de novo pyrimidine biosynthesis. The de novo pyrimidine biosynthesis pathway is crucial to the survival of the parasite. Unlike human cells, which can utilize the salvage pathway for pyrimidine acquisition,
Plasmodium species can only access de novo-synthesized pyrimidines. According to Pippione et al., 2019 [
29], the active site of this enzyme contains numerous amino acid residues, including Tyr168, Phe171, Leu172, Ile176, Leu187, Phe188, Tyr528, Phe227, Phe188, Cys175, Leu531, Ile263, His185, Ile272, Arg265, and Met536. The
P. falciparum_DHODH-jacularine complex demonstrated hydrogen with His185, similar to the result obtained by Pippione et al. [
29]. In addition, different hydrophobic interactions in the active site were observed, mainly van der Waals and alkyl interactions with the amino acids Val532, Cys184, Phe188, Gly535, Met536, Leu172, Tyr168, Leu187, Phe171, and Cys175 (
Figure 11).
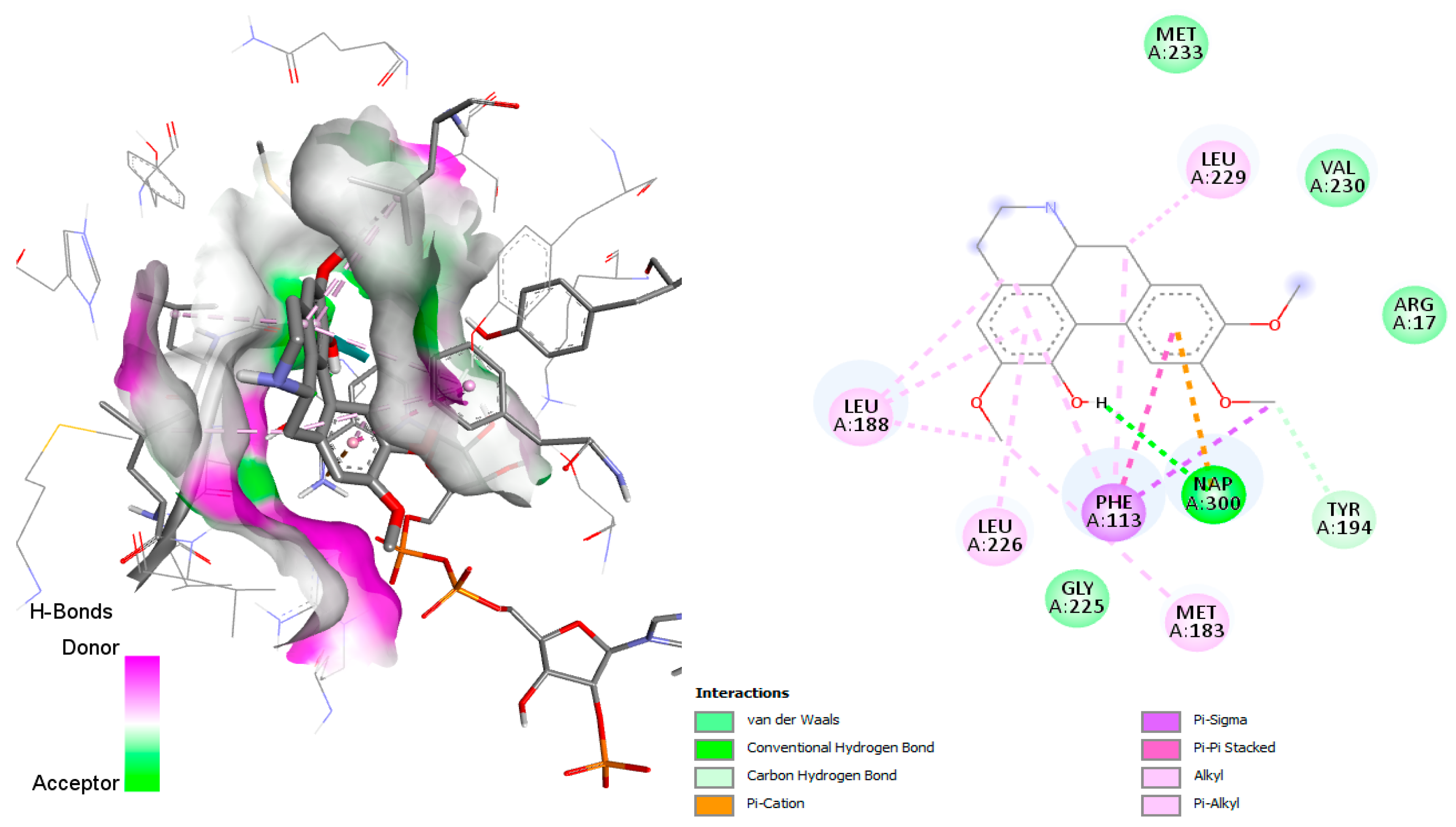
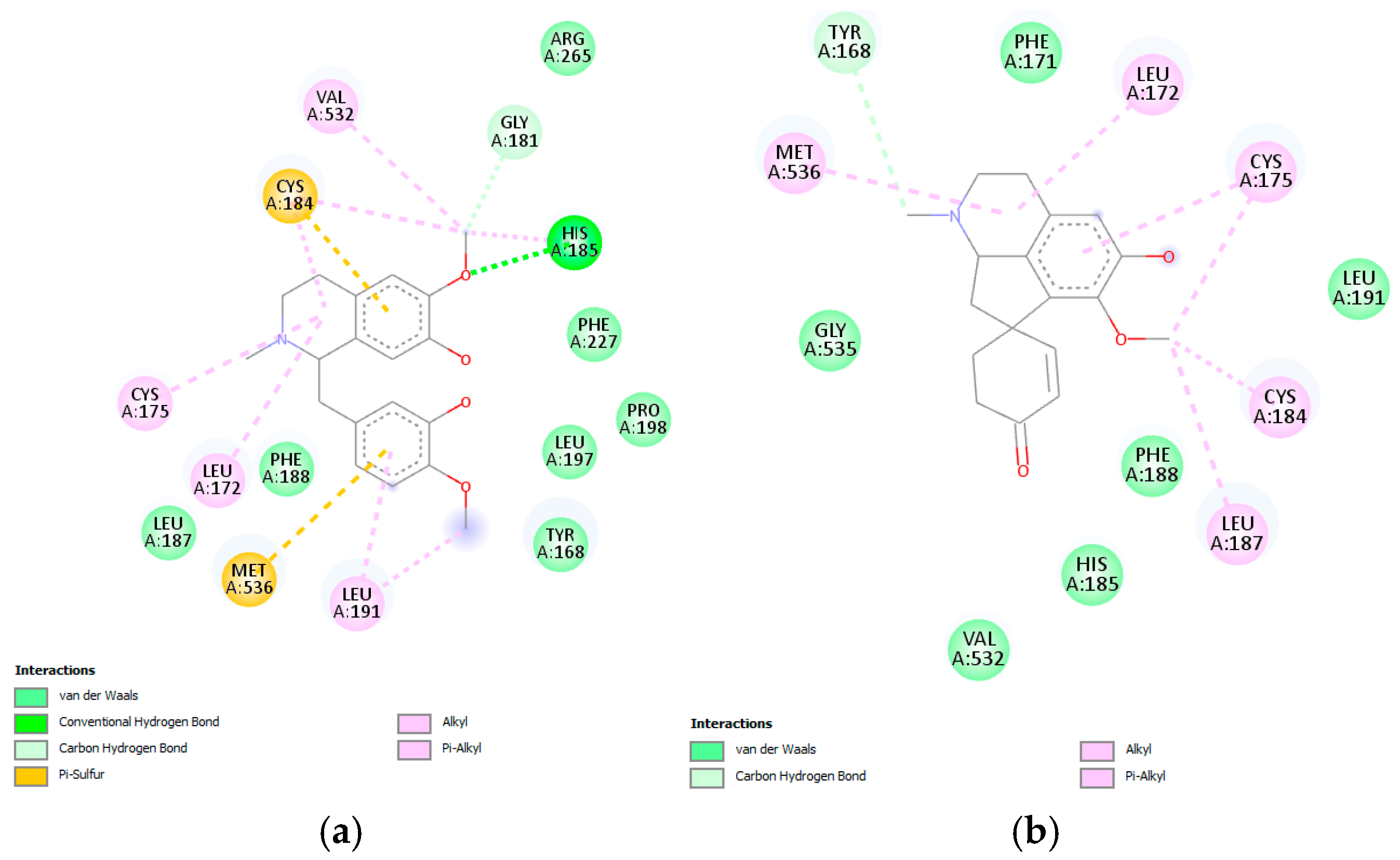
Reticuline and linearisine demonstrated similar interactions like jacularine (
Figure 12); however, only the reticuline was found to establish hydrogen bonds with His185, and π–sulfur interactions with Cys184 and Met536 were observed (
Figure 12a). On the other hand, linearisine demonstrated no hydrogen bond interactions; however, it exhibits several hydrophobic interactions, as van der Waals interactions with the Gly535, Phe171, Leu191, Phe188, His185, and Val532, alkyl and π–alkyl interactions with Met536, Leu172, Cys175, Cys184, Leu187 residues (
Figure 12b). Reticuline and linearisine share several amino acids—Phe171, Leu172, Leu187, Phe188, Phe188, and Met536—involved in the active site of the
P. falciparum DHODH enzyme.
Inhibitors of
P. falciparum DHODH are effective antimalarial agents due to their role in pyrimidine biosynthesis. The best-performing alkaloids in this study, reticuline and jacularine, demonstrated binding energies very close to the reference compound DZB (−8.4 kcal/mol), as observed in
Figure 10 and
Figure 11. These compounds aligned well with the ubiquinone-binding pocket of
PfDHODH, forming key interactions with residues such as Arg265, Tyr528, and Phe188. These findings align with studies on triazolopyrimidine-based compounds (DSM265) analogs [
30], which report similar hydrophobic and π-stacking contacts. Moreover, linearisine and homolinearisine exhibited good binding (−8.1 to −8.2 kcal/mol), quite similar to the value achieved with the co-crystallized ligand (−8.4 kcal/mol), suggesting that multiple alkaloids share a common pharmacophore motif.
The comparative analysis of docking scores across parasite enzymes and their human homologs revealed a heterogeneous pattern of selectivity among the tested alkaloids (
Table S3a,b). Compounds such as reticuline, laudanidine, and linearisine consistently displayed positive ΔScore values in several targets, particularly against
Trypanosoma cruzi cruzain,
Leishmania FPPS, and
Plasmodium falciparum DHODH. Positive ΔScore values suggest stronger predicted binding to the parasite enzyme compared to the human counterpart, highlighting these molecules as potential candidates with favorable selectivity. Conversely, several compounds, including corydine and glaucine, presented predominantly negative ΔScore values in multiple comparisons, reflecting either non-selective binding or stronger interactions with human homologs, thus raising potential concerns about off-target effects.
In
Trypanosoma targets (
Table S3a), the selectivity profile was variable. While compounds like reticuline and laudanidine exhibited promising selectivity against cruzain and DHFR-TS, molecules such as pronuciferine and jacularine showed negative ΔScore values for the same enzymes, suggesting stronger interactions with human cathepsins and DHFS. Interestingly, n-methylhernovine and litseglutine B displayed extreme variations, with strongly positive ΔScore values against
T. cruzi cruzain but markedly negative ones against other targets, emphasizing their context-dependent selectivity. These findings underscore the complexity of achieving broad parasite selectivity within this alkaloid series.
For
Leishmania enzymes (
Table S3b), particularly FPPS, TryR, and cyp51, the results highlighted reticuline, linearisine, and homolinearisine as molecules with favorable parasite-biased binding, as reflected in positive ΔScore values. However, a recurring observation was the loss of selectivity in
L. infantum TryR and
L. mexicana ArgI, where most compounds displayed strongly negative ΔScore values, showing preferential affinity toward the human homologs. This suggests that enzyme conservation between parasite and human homologs may limit the therapeutic window for certain targets. In the case of
P. falciparum, compounds like cularine, wilsonirine, and crotonosine exhibited notable selectivity toward DHODH and falcipain, consistent with previous studies finding these enzymes as exploitable selective targets in antimalarial drug discovery.
Overall, the ΔScore analysis shows that while several alkaloids, including reticuline, laudanidine, and linearisine, show promising selectivity for parasite enzymes, none of the tested molecules achieved uniform selectivity across all species and targets. The observed variability highlights the necessity of a target-specific approach, where alkaloid scaffolds may be customized for distinct parasite enzymes rather than seeking broad-spectrum selectivity. Future optimization should focus on compounds with consistently positive ΔScore values while also integrating ADMET predictions to mitigate the risks of off-target interactions with human homologs.
2.8. Overall ADMET Profile and Prioritization of Alkaloids Candidates
In silico ADMET evaluation of the
C. linearis derived alkaloids (
Table S4) provided critical insights into their pharmacokinetic behavior and potential safety liabilities, complementing the molecular docking analysis against neglected tropical disease targets. The objective of the present study was to identify candidate molecules with favorable bioavailability, minimal toxicity risks, and optimal drug-like properties. This was achieved by assessing absorption, distribution, metabolism, excretion, and toxicity parameters. The integration of these predictive data with binding affinity profiles offers a more comprehensive understanding of each alkaloid’s therapeutic potential, thereby guiding the prioritization of compounds for future in vitro and in vivo validation.
Across the 18 alkaloids, physicochemical properties cluster tightly within an “orally developable” window: MW 283–357 g/mol (median ≈ 327), XLogP3 1.48–3.67 (median ≈ 2.55), and TPSA 38.8–71.0 Å2 (median ≈ 54.9). It is notable that none of the compounds under examination violate the Lipinski, Veber, Egan, or Muegge filters, thereby aligning with established correlates of good oral exposure (≤5 HBD, ≤10 HBA, MW ≤ 500, cLogP ≤ 5; ≤10 rotatable bonds and TPSA ≤ 140 Ų). The findings of this study align with the hypothesis that consistently high GI absorption predictions further support oral suitability. All molecules are designated as BBB-permeant, a classification that is consistent with their low TPSA (<90 Ų) and moderate lipophilicity. These physicochemical characteristics are well-documented as being associated with CNS access. These observations are consistent with the established principles of classical oral drug-likeness literature (Lipinski; Veber) [
31] and with SwissADME’s BOILED-Egg rationale [
32], which establishes a correlation between lipophilicity/polarity and GI and brain permeation.
However, predictions concerning transport and metabolism introduce a degree of complexity to this interpretation. The P-gp [
33] substrate status was found to be positive for 15 out of 18 compounds, with only laudanosine, laudanidine, and pronuciferine predicted to be non-substrates. This suggests the possibility of efflux-limited brain exposure despite passive BBB permeability [
34]. This finding aligns with the established role of P-gp as a gatekeeper at the BBB, as well as with clinical imaging and PK evidence demonstrating reduced unbound brain partitioning for P-gp substrates. With regard to the subject of metabolism, CYP2D6 inhibition (16/18) and CYP3A4 inhibition (11/18) have been observed, indicating the potential for drug–drug interactions (DDIs) with substrates of these polymorphic/high-clearance pathways. In contrast, fewer compounds have been found to inhibit CYP2C9 (2/18) or CYP2C19 (1/18). Such inhibition patterns are consistent with the high clinical interaction liability of 2D6/3A4, as highlighted in recent reviews of CYP-mediated DDIs [
35].
From a medicinal chemistry quality perspective, the set is considered to be clean, as it does not contain any PAINS (Potential Adverse Drug Reactions) [
36] or Brenk structural alerts. This characteristic supports the notion that there is a reduced risk of assay interference and toxicity, and it is well-suited for screening purposes. Lead-likeness is also strong (16/18 with zero violations), with only glaucine (1 violation) and laudanosine (2 violations) flagged. This is useful for prioritization if fragment/lead-like progression is desired. The presence of a bioavailability score (typically 0.55 in SwissADME for Ro5-compliant, nonpolar zwitterions) complements the absorption predictions. Collectively, these results mirror widely utilized quality filters (PAINS/Brenk) and SwissADME guidance for early triage [
37].
4. Conclusions
Integrated docking and ADMET analyses of C. linearis Jacq alkaloids highlight their potential as versatile scaffolds in the discovery of drugs for neglected tropical diseases (NTDs). The docking simulations revealed potent multitarget inhibitory profiles, with metabolites such as reticuline, norsalutaridine, laudanosine, and jacularine exhibiting binding affinities comparable to or even greater than those of co-crystallized ligands against key protozoan enzymes, including Cyp51, DHFR-TS, and PTR1. Importantly, the calculated ΔScore values (ΔG_human − ΔG_parasite) consistently favored parasite enzymes, indicating a higher selectivity towards the protozoan targets over their human homologues. This trend strongly suggests that these alkaloids could exert specific antiparasitic activity through mechanisms consistent with the disruption of ergosterol biosynthesis and antifolate-like pathways.
In addition, the chemical diversity of alkaloids, one of the most widely studied and distributed classes of secondary metabolites, reinforces their potential as a source of novel therapeutic scaffolds. Taking into account present results, could be interesting to assay other known alkaloids against the studied parasite targets. The current findings suggest that the selective binding identification through ΔScore analysis could be extrapolated to other known alkaloids, thereby broadening the spectrum of candidates worth evaluating against the studied parasite targets. Nevertheless, different limitations of the present study require continuing further experiments. First, cross-docking experiments with X-ray structures of human orthologues should be prioritized, since most of the targeted parasite enzymes belong to conserved metabolic pathways. The identification of more favorable binding energies in parasites compared to humans, as supported by the present ΔScore results, would significantly strengthen the translational potential of these compounds for clinical applications. Second, in silico results should be corroborated by in vitro assays, preferably enzymatic and cellular models.
Complementary ADMET predictions reinforced the drug-like potential of the analyzed alkaloids, highlighting favorable oral bioavailability, high gastrointestinal absorption, strong drug-likeness scores, and the absence of major structural alerts. However, CYP450 inhibition, particularly of CYP2D6, remains a concern. Despite this limitation, several alkaloids (notably jacularine, linearicine, salutaridine, nor-salutaridine, and reticuline) showed balanced pharmacokinetic and toxicity profiles, making them the most promising candidates for further experimental validation. Overall, the combination of docking, ΔScore selectivity analysis, and ADMET profiling provides a rational basis for advancing selected alkaloids into in vitro and in vivo studies. Future research should validate the proposed molecular mechanisms using enzymatic and cellular models, while also focusing on metabolism, solubility optimization, and polypharmacological strategies to address the complexity of NTDs.