The Effects of Frondanol, a Non-Polar Extract of the Atlantic Sea Cucumber, in Colon Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Profiling of Frondanol
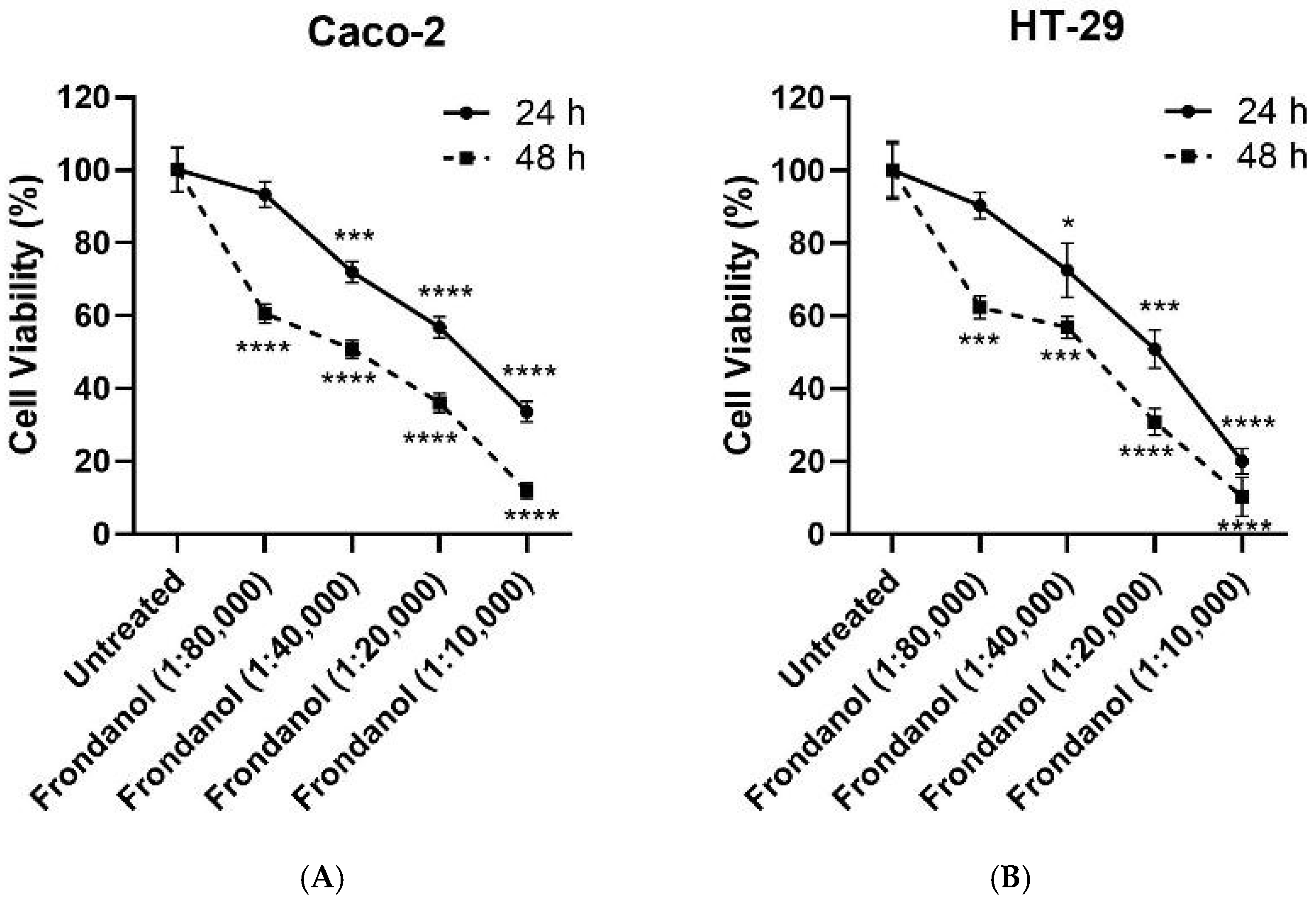
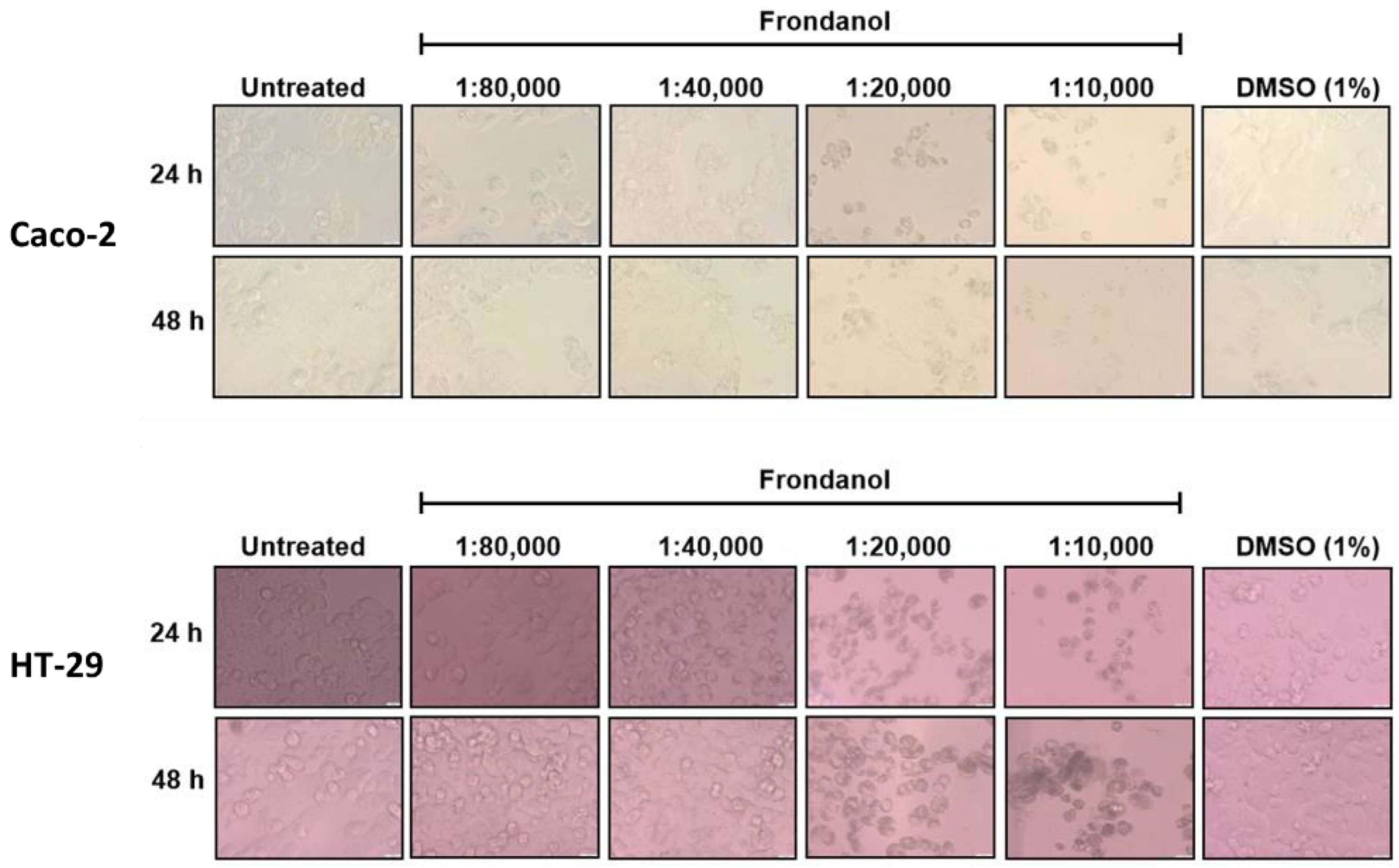
2.2. Frondanol Suppressed Colon Cancer Cell Proliferation in a Dose-Dependent Manner
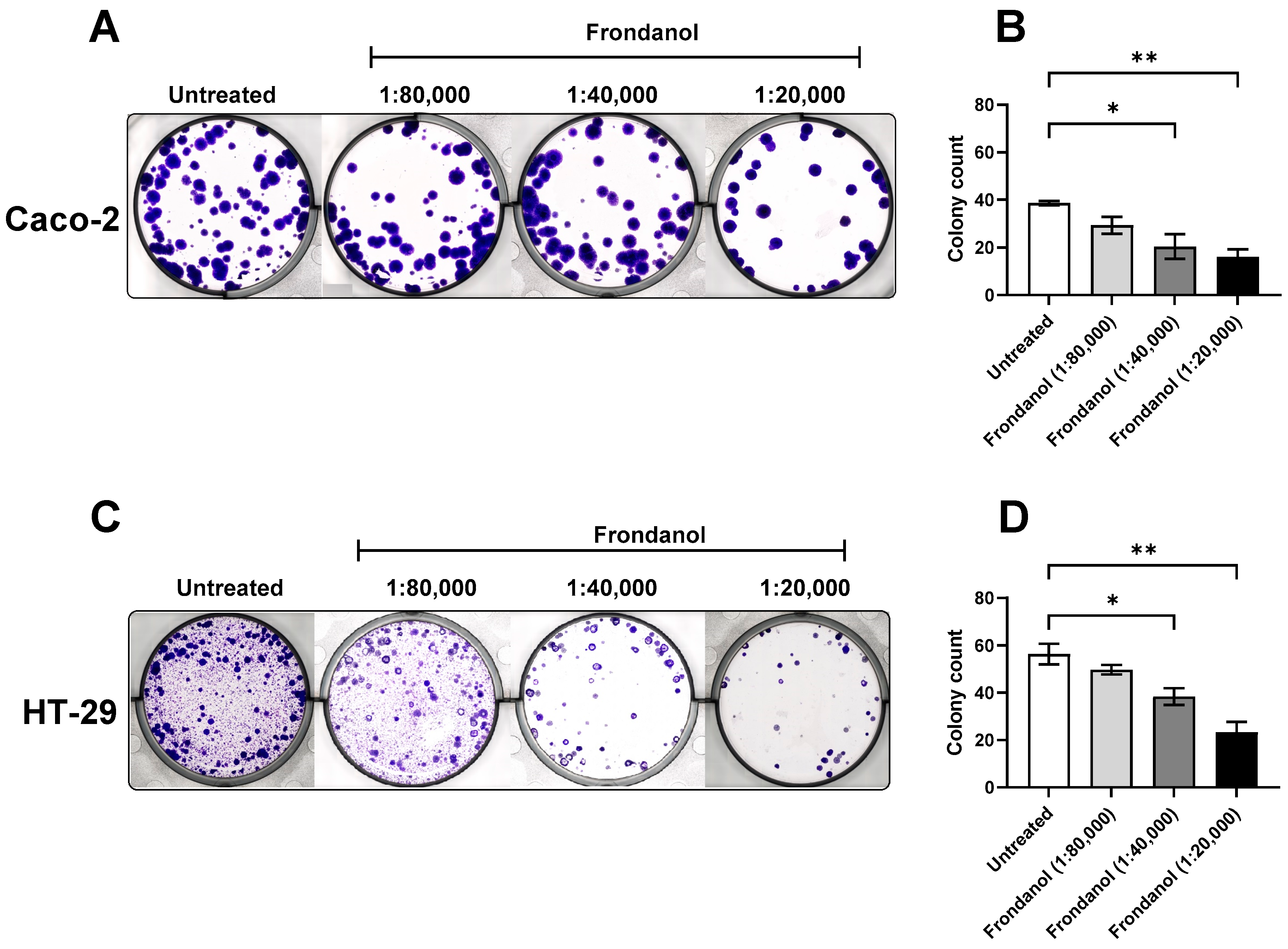
2.3. Frondanol Reduced CRC Cell Lines’ Clonogenic Ability
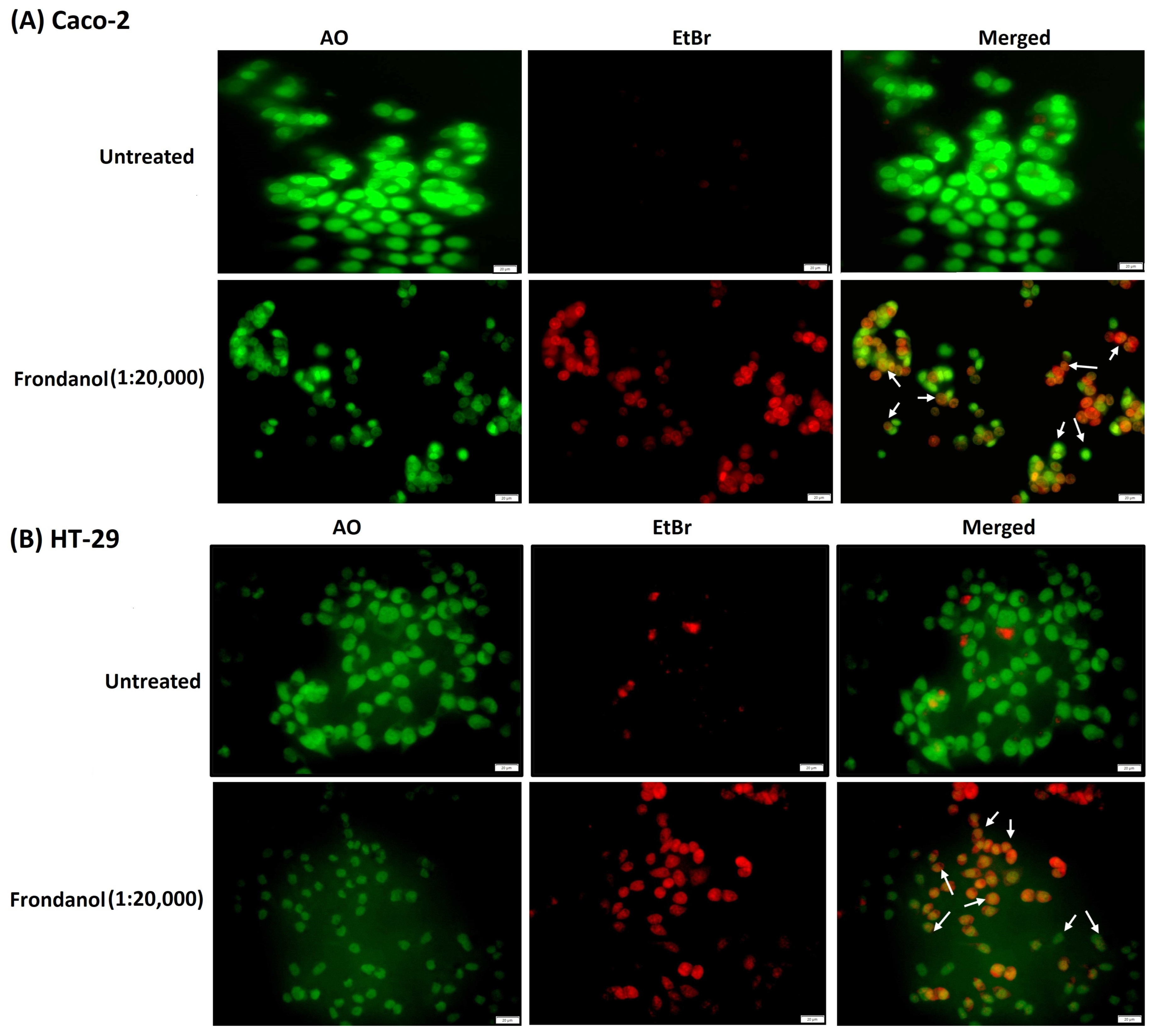
2.4. Frondanol Induced Apoptosis in AO/EtBr Staining Assay
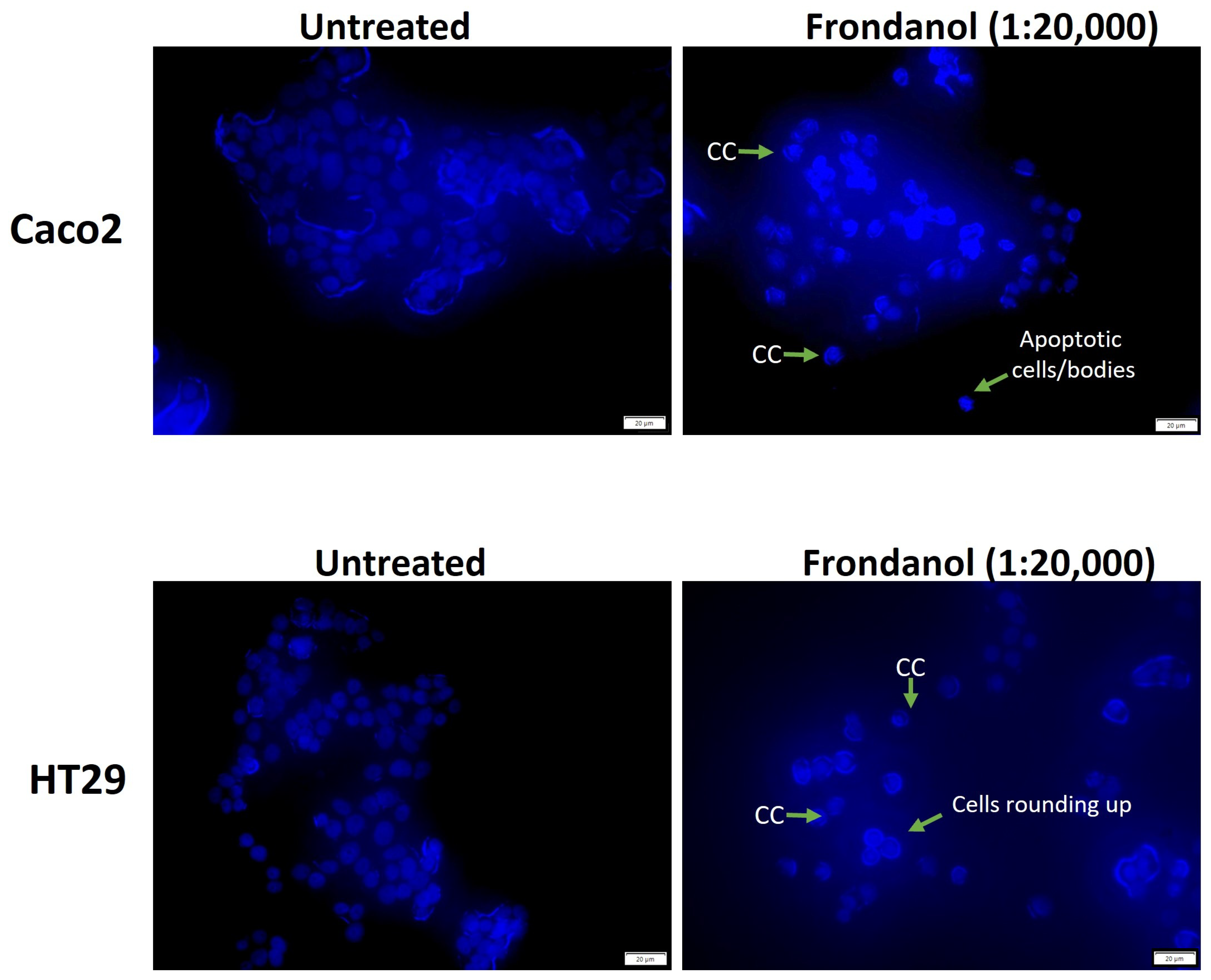
2.5. Frondanol Induced Apoptosis in Hoechst 33342 Staining Assay
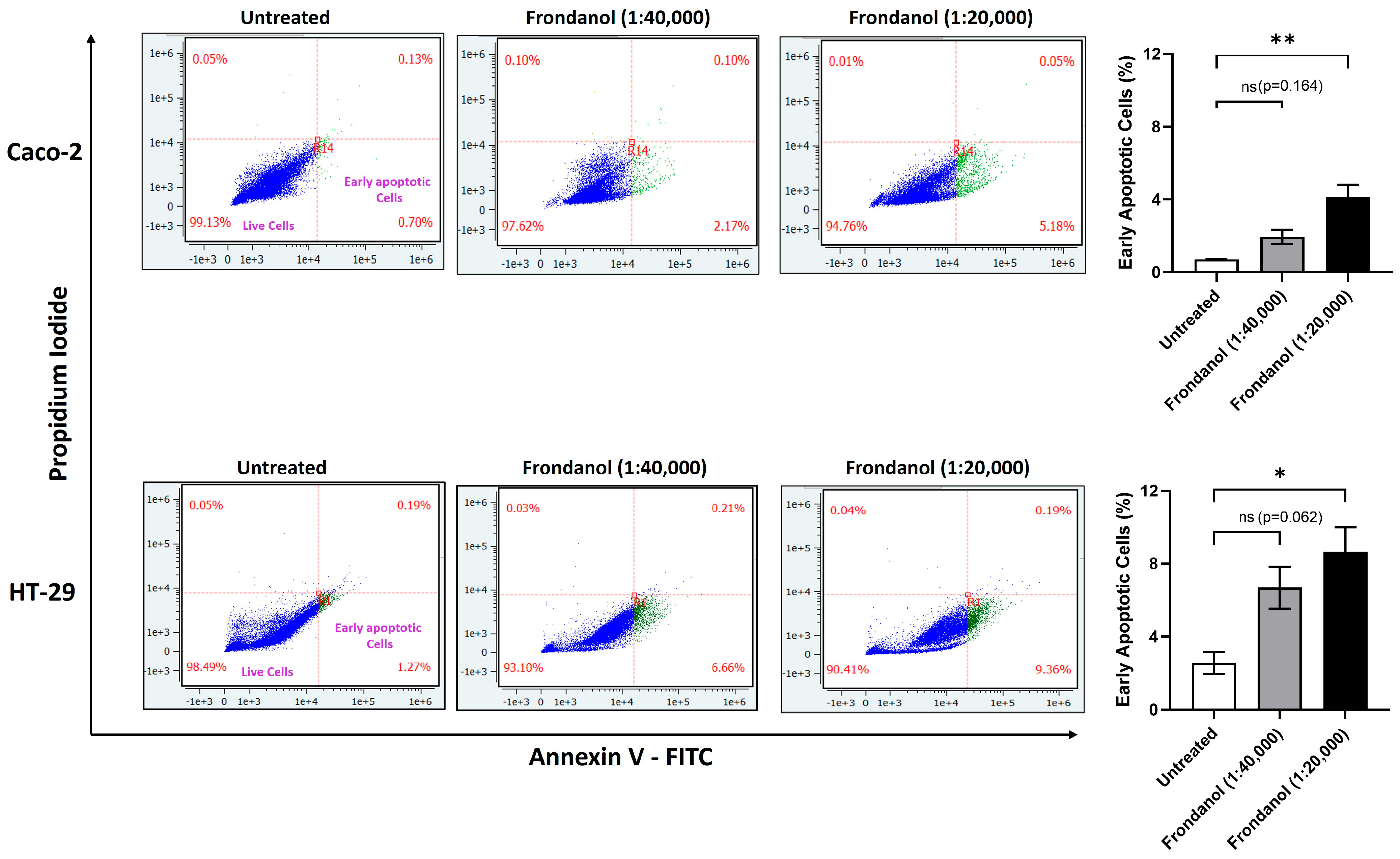
2.6. Frondanol Induced Early Apoptosis in Annexin V-PI Assay
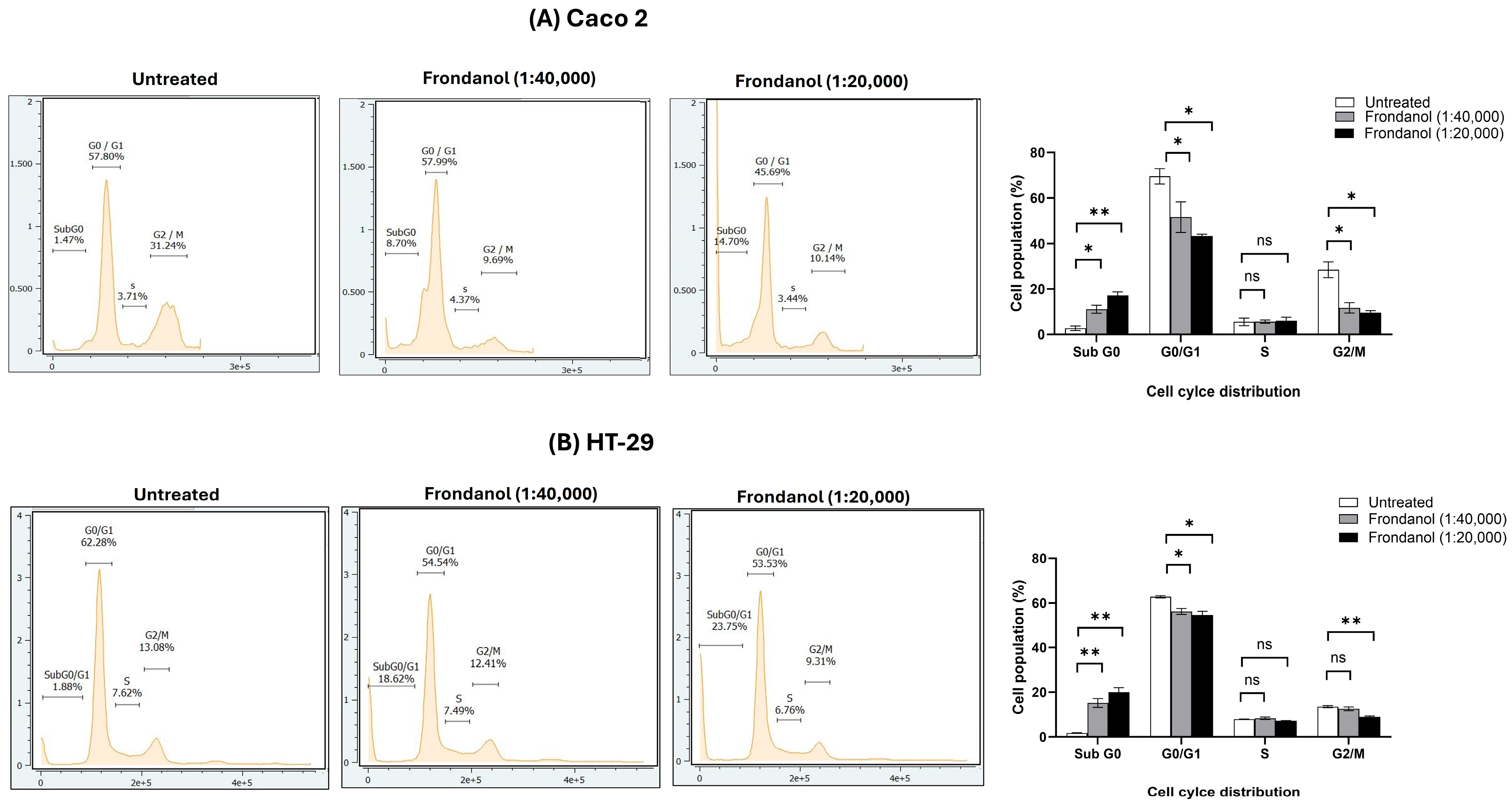
2.7. Frondanol Inhibited Cell Proliferation Through Induction of Cell Cycle Arrest
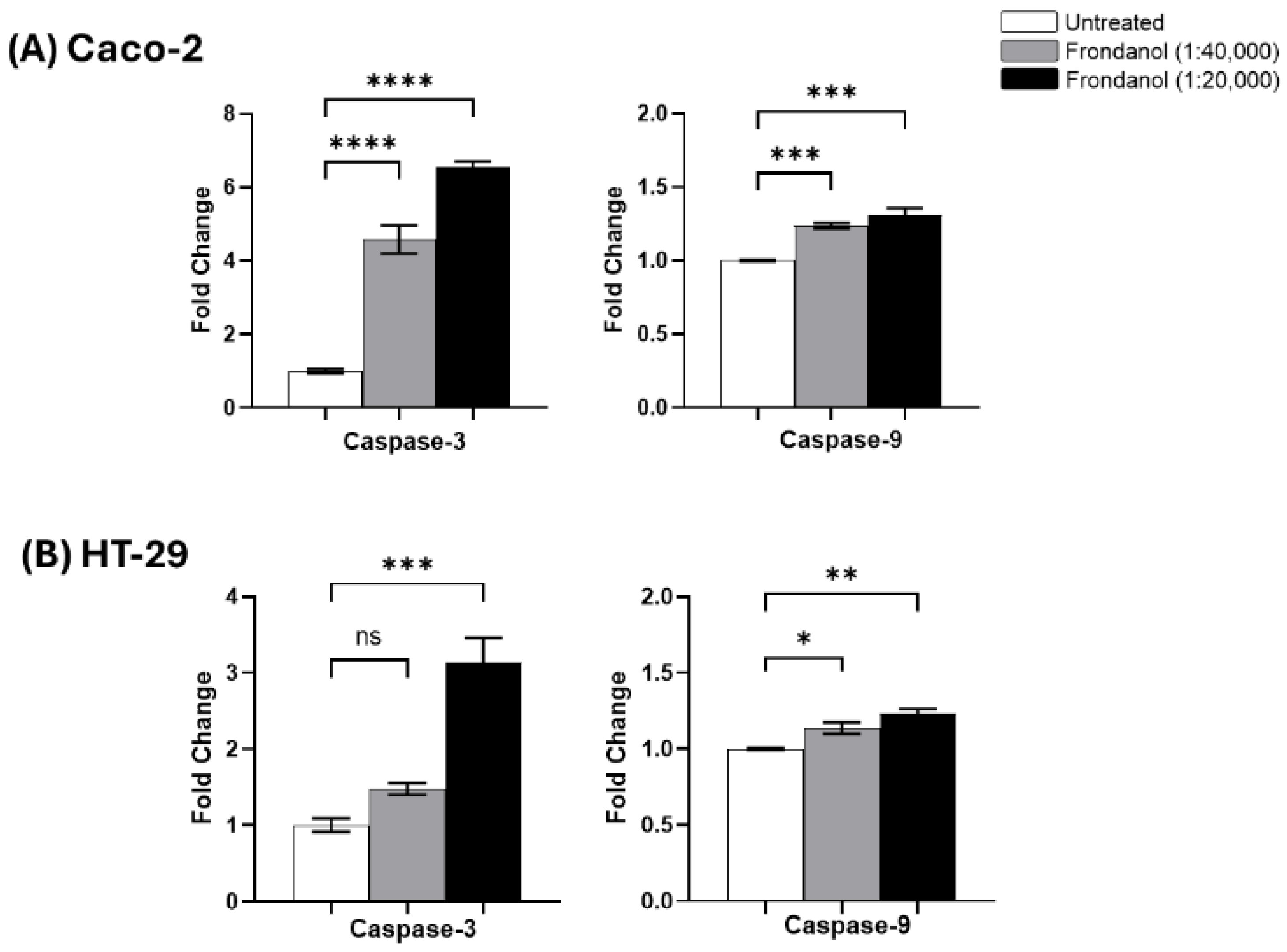
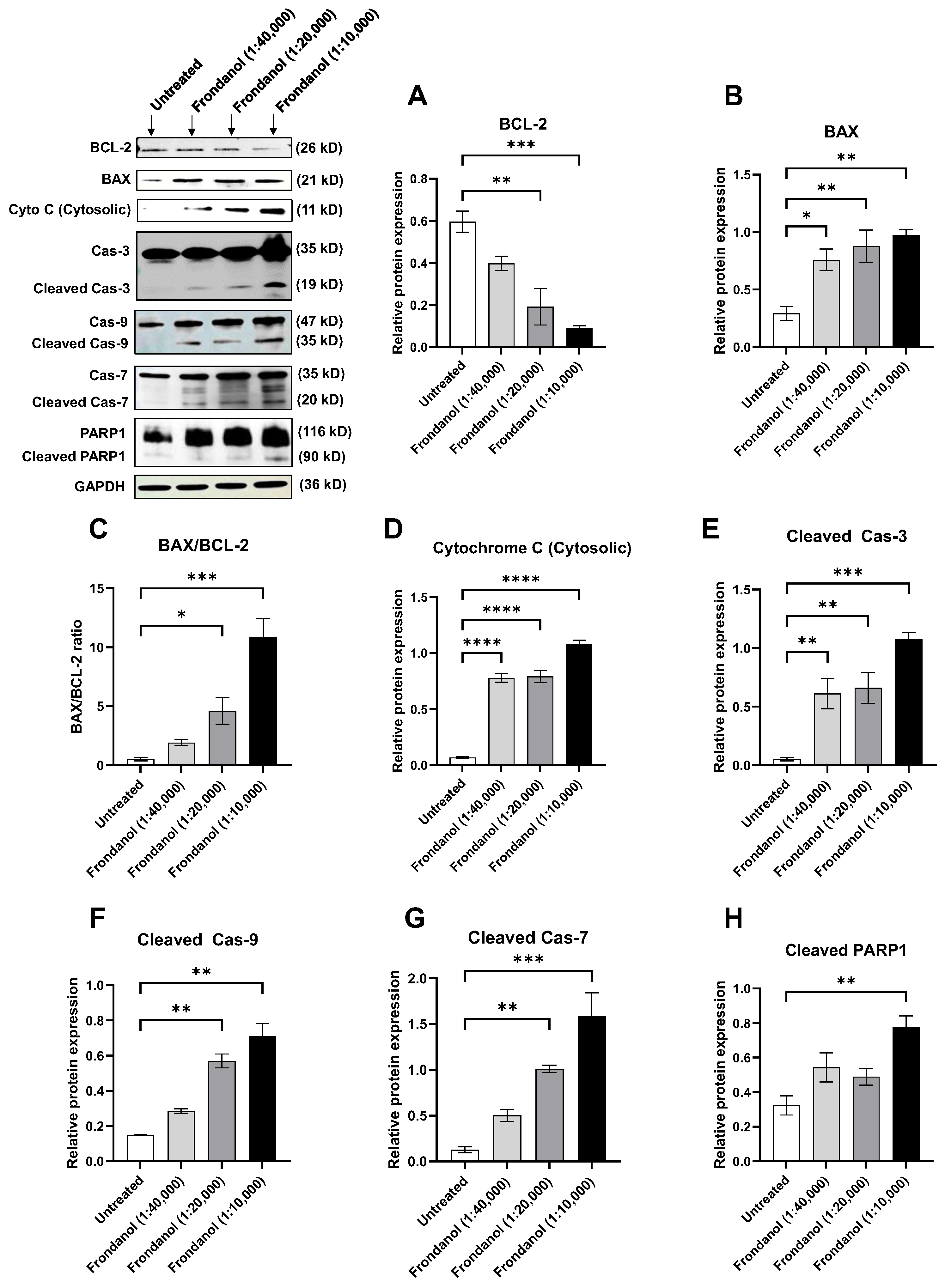
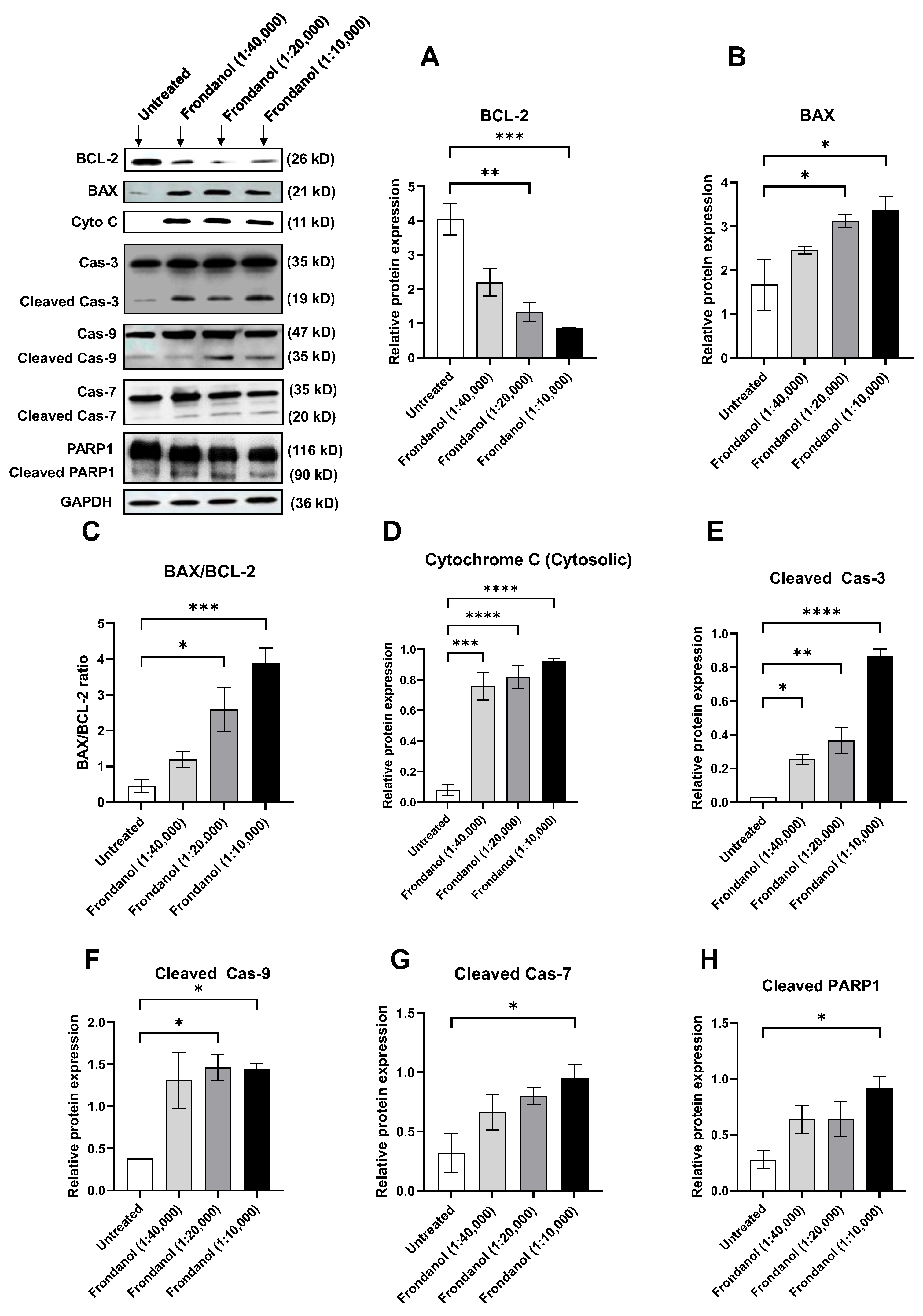
2.8. Frondanol Induced Apoptosis via Caspase Activation
2.9. Frondanol Modulated Anti-Apoptotic, Pro-Apoptotic, and Caspase Protein Expression
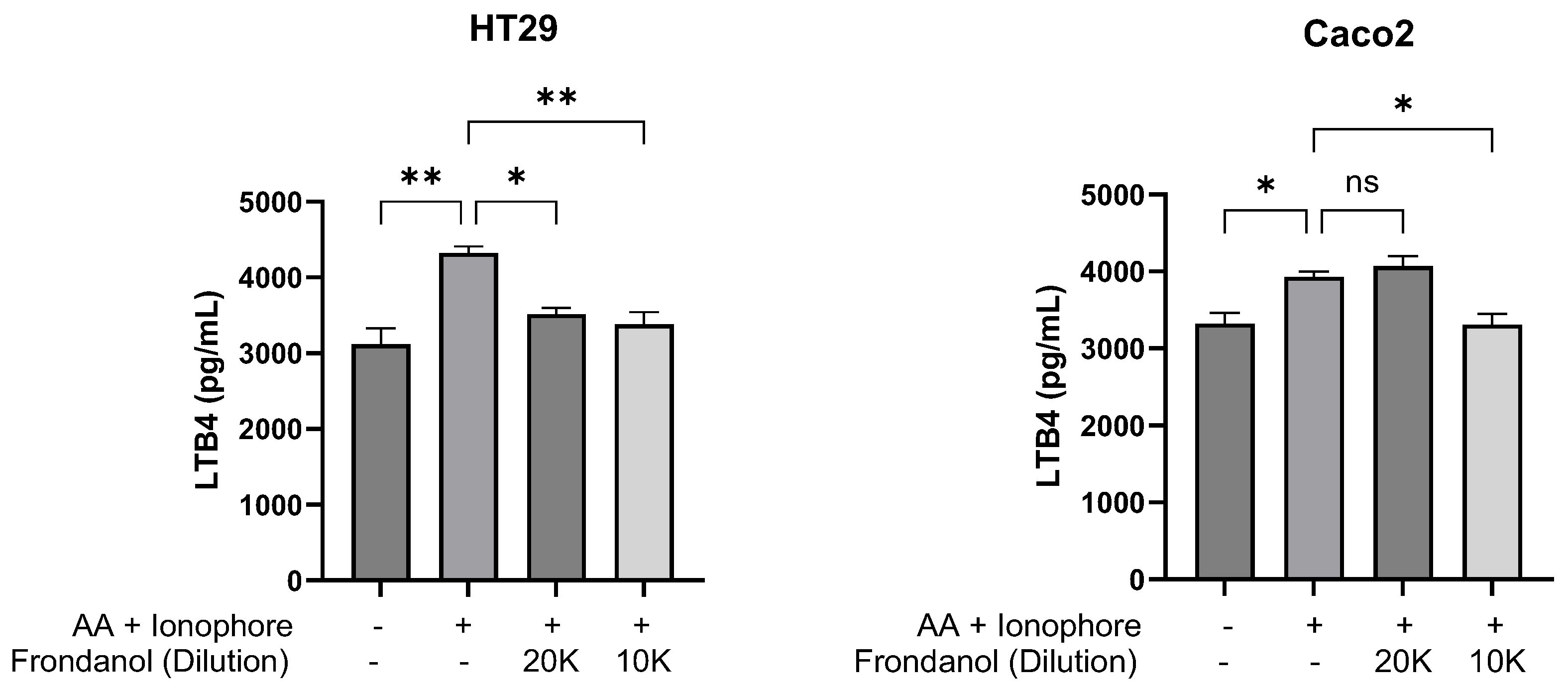
2.10. Frondanol Inhibited 5-LOX Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Frondanol and Its Preparation
4.2. Gas Chromatography-Flame Ionization Total Fatty Acid Analysis of Frondanol
4.3. Cell Lines and Cultures
4.4. Cell Viability Assay and Morphological Analysis
4.5. Clonogenic Assay
4.6. Qualitative Staining for Apoptosis Assessment
- AO/EtBr double staining
- 2.
- Hoechst 33342 staining
4.7. Early Apoptosis Assay (Annexin V/Propidium Iodide Assay)
4.8. Caspase Activity Assays
4.9. Cell Cycle Analysis
4.10. Western Blotting
4.11. 5-LOX Enzyme Activity
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide variations in colorectal cancer. CA Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Lynch Syndrome-Associated Colorectal Cancer. N. Engl. J. Med. 2018, 379, 764–773. [Google Scholar] [CrossRef]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Khan, S.Z.; Lengyel, C.G. Challenges in the management of colorectal cancer in low- and middle-income countries. Cancer Treat. Res. Commun. 2023, 35, 100705. [Google Scholar] [CrossRef]
- Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Nowak-Sliwinska, P. Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment. Molecules 2020, 25, 2614. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef]
- Ghelani, H.; Adrian, T.E.; Ho, S.B.; Akhras, J.; Azar, A.J.; Jan, R.K. Study protocol for a pilot randomized, double-blind, placebo-controlled trial to investigate the anti-inflammatory effects of Frondanol in adults with inflammatory bowel disease. Contemp. Clin. Trials Commun. 2023, 31, 101046. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Chandran, S.; Almarzooqi, S.; Raj, V.; Al Zahmi, A.S.; Al Katheeri, R.A.; Al Zadjali, S.A.; Collin, P.D.; Adrian, T.E. Frondanol, a Nutraceutical Extract from Cucumaria frondosa, Attenuates Colonic Inflammation in a DSS-Induced Colitis Model in Mice. Mar. Drugs 2018, 16, 148. [Google Scholar] [CrossRef]
- Collin, P.D. Sea Cucumber Carotenoid Lipid Fraction Products and Methods of Use. U.S. Patent 6,399,105B1, 4 June 2002. [Google Scholar]
- Ghelani, H.; Hanspal, K.; Talo, L.; Talo, S.; Altaher, H.; Sarsour, H.; Tabbal, M.; Badawi, S.; Collin, P.; Adrian, T.E.; et al. Anti-inflammatory effects of Frondanol, a nutraceutical extract from Cucumaria frondosa, via modulation of NF-κB and MAPK pathways in LPS-induced RAW 264.7 cells. Front. Pharmacol. 2025, 16, 1683630. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Qian, H.R.; Shi, Z.Q.; Zhu, H.P.; Gu, L.H.; Wang, X.F.; Yang, Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget 2017, 8, 62759–62768. [Google Scholar] [CrossRef]
- Collin, P.; Adrian, T.E.; Roginsky, A.; Newman, R.A.; Woodward, C.; Mehta, R.; Ding, Z.; Pinegin, B.; Kalinin, V.; Aminin, D.; et al. Frondanol® A5: A novel nutrapreventive and therapeutic agent derived from sea cucumber showing promising antiproliferative, antiinflammatory and antiangiogenic activities. Cancer Epidemiol. Biomark. Prev. 2006, 15, A80. [Google Scholar]
- Roginsky, A.B.; Ding, X.-Z.; Singh, B.; Ujiki, M.; Salabat, M.R.; Chan, C.-Y.; Bell, R.H.; Collin, P.; Adrian, T.E. Frondanol-A5 from cucumaria frondosa induces cell cycle arrest and apoptosis in pancreatic cancer cells. J. Am. Coll. Surg. 2004, 199, 91. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Liu, Y.; Bodmer, W.F. Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc. Natl. Acad. Sci. USA 2006, 103, 976–981. [Google Scholar] [CrossRef]
- Li, J.-M.; Huang, Y.-C.; Kuo, Y.-H.; Cheng, C.-C.; Kuan, F.-C.; Chang, S.-F.; Lee, Y.-R.; Chin, C.-C.; Shi, C.-S. Flavopereirine Suppresses the Growth of Colorectal Cancer Cells through P53 Signaling Dependence. Cancers 2019, 11, 1034. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Tong, J.H.M.; Chan, A.W.H.; Yu, J.; Kang, W.; To, K.F. Targeting the Oncogenic p53 Mutants in Colorectal Cancer and Other Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5999. [Google Scholar] [CrossRef]
- Lee, K.H.; Seong, H.J.; Kim, G.; Jeong, G.H.; Kim, J.Y.; Park, H.; Jung, E.; Kronbichler, A.; Eisenhut, M.; Stubbs, B.; et al. Consumption of Fish and ω-3 Fatty Acids and Cancer Risk: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2020, 11, 1134–1149. [Google Scholar] [CrossRef]
- Yu, X.F.; Zou, J.; Dong, J. Fish consumption and risk of gastrointestinal cancers: A meta-analysis of cohort studies. World J. Gastroenterol. 2014, 20, 15398–15412. [Google Scholar] [CrossRef]
- Kobayashi, N.; Barnard, R.J.; Henning, S.M.; Elashoff, D.; Reddy, S.T.; Cohen, P.; Leung, P.; Hong-Gonzalez, J.; Freedland, S.J.; Said, J.; et al. Effect of Altering Dietary ω-6/ω-3 Fatty Acid Ratios on Prostate Cancer Membrane Composition, Cyclooxygenase-2, and Prostaglandin E2. Clin. Cancer Res. 2006, 12, 4662–4670. [Google Scholar] [CrossRef]
- Luo, J.; Peng, S.; Jiang, Z.; Wang, Q.; Zhang, M.; Zeng, Y.; Yuan, Y.; Xia, M.; Hong, Z.; Yan, Y.; et al. Roles and therapeutic opportunities of ω-3 long-chain polyunsaturated fatty acids in lung cancer. iScience 2025, 28, 111601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Cai, W.; Guo, Y.; Xue, C.; Wang, J. DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice. J. Agric. Food Chem. 2021, 69, 676–685. [Google Scholar] [CrossRef]
- Yang, P.; Collin, P.; Madden, T.; Chan, D.; Sweeney-Gotsch, B.; McConkey, D.; Newman, R.A. Inhibition of proliferation of PC3 cells by the branched-chain fatty acid, 12-methyltetradecanoic acid, is associated with inhibition of 5-lipoxygenase. Prostate 2003, 55, 281–291. [Google Scholar] [CrossRef]
- Wright, K.C.; Yang, P.; Van Pelt, C.S.; Hicks, M.E.; Collin, P.; Newman, R.A. Evaluation of targeted arterial delivery of the branched chain fatty acid 12-methyltetradecanoic acid as a novel therapy for solid tumors. J. Exp. Ther. Oncol. 2005, 5, 55–68. [Google Scholar]
- Zuo, W.; Kwok, H.F. Development of Marine-Derived Compounds for Cancer Therapy. Mar. Drugs 2021, 19, 342. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Bryant, T.; Lightfoot, S.; Collin, P.D.; Steele, V.E.; Rao, C.V. Improved innate immune responses by Frondanol A5, a sea cucumber extract, prevent intestinal tumorigenesis. Cancer Prev. Res. 2015, 8, 327–337. [Google Scholar] [CrossRef]
- Attoub, S.; Arafat, K.; Khalaf, T.; Sulaiman, S.; Iratni, R. Frondoside A Enhances the Anti-Cancer Effects of Oxaliplatin and 5-Fluorouracil on Colon Cancer Cells. Nutrients 2018, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Castro-Carvalho, B.; Ramos, A.A.; Prata-Sena, M.; Malhão, F.; Moreira, M.; Gargiulo, D.; Dethoup, T.; Buttachon, S.; Kijjoa, A.; Rocha, E. Marine-derived Fungi Extracts Enhance the Cytotoxic Activity of Doxorubicin in Nonsmall Cell Lung Cancer Cells A459. Pharmacogn. Res. 2017, 9, s92–s98. [Google Scholar] [CrossRef]
- Adina, A.B.; Goenadi, F.A.; Handoko, F.F.; Nawangsari, D.A.; Hermawan, A.; Jenie, R.I.; Meiyanto, E. Combination of Ethanolic Extract of Citrus aurantifolia Peels with Doxorubicin Modulate Cell Cycle and Increase Apoptosis Induction on MCF-7 Cells. Iran. J. Pharm. Res. 2014, 13, 919–926. [Google Scholar] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. Cold Spring Harb. Protoc. 2006, 2006, pdb-rot4493. [Google Scholar] [CrossRef]
- Harada, K.; Kawaguchi, S.-i.; Supriatno; Kawashima, Y.; Yoshida, H.; Sato, M. S-1, an oral fluoropyrimidine anti-cancer agent, enhanced radiosensitivity in a human oral cancer cell line in vivo and in vitro: Involvement possibility of inhibition of survival signal, Akt/PKB. Cancer Lett. 2005, 226, 161–168. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011, 50, 2597. [Google Scholar] [CrossRef]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Tong, W.-G.; Ding, X.-Z.; Witt, R.C.; Adrian, T.E. Lipoxygenase Inhibitors Attenuate Growth of Human Pancreatic Cancer Xenografts and Induce Apoptosis through the Mitochondrial Pathway1. Mol. Cancer Ther. 2002, 1, 929–935. [Google Scholar]
| Fatty Acid | % Weight |
|---|---|
| 12-Methyltetradecanoic acid (12-MTA) | 19.6 |
| C08:0 Octanoic (Caprylic) | <0.1 |
| C10:0 Decanoic (Capric) | <0.1 |
| C11:0 Undecanoic (Hendecanoic) | <0.1 |
| C12:0 Dodecanoic (Lauric) | <0.1 |
| C14:0 Tetradecanoic (Myristic) | 2.54 |
| C14:1 Tetradecenoic (Myristoleic) | 2.71 |
| C15:0 Pentadecanoic | 1.07 |
| C15:1 Pentadecenoic | <0.1 |
| C16:0 Hexadecanoic (Palmitic) | 1.97 |
| C16:1 Hexadecenoic (Palmitoleic) | 12.7 |
| C16:2 Hexadecadienoic | <0.1 |
| C16:3 Hexadecatrienoic | <0.1 |
| C16:4 Hexadecatetraenoic | <0.1 |
| C17:0 Heptadecanoic (Margaric) | 0.56 |
| C17:1 Heptadecenoic Margaroleic | <0.1 |
| C18:0 Octadecanoic (Stearic) | 2.84 |
| C18:1 Octadecenoic (Oleic) | 4.49 |
| C18:2 Octadecadienoic (Linoleic) | 2.54 |
| C18:3 Octadecatrienoic (Linolenic) | 0.28 |
| C18:4 Octadecatetraenoic | <0.1 |
| C19:0 Nonadecanoic | <0.1 |
| C20:0 Eicosanoic (Arachidic) | 0.33 |
| C20:1 Eicosenoic (Gadoleic) | 0.39 |
| C20:2 Eicosadienoic | <0.1 |
| C20:3 Eicosatrienoic | 0.55 |
| C20:4 Eicosatetraenoic (Arachidonic) | 0.60 |
| C20:5 Eicosapentaenoic (EPA) | 17.9 |
| C21:0 Heneicosanoic | <0.1 |
| C21:5 Heneicosapentaenoic | <0.1 |
| C22:0 Docosanoic (Behenic) | 0.21 |
| C22:1 Docosenoic (Erucic) | 0.30 |
| C22:2 Docosadienoic | 0.80 |
| C22:3 Docosatrienoic | <0.1 |
| C22:4 Docosatetraenoic | <0.1 |
| C22:5 Docosapentaenoic | 0.46 |
| C22:6 Docosahexaenoic (DHA) | 0.60 |
| C24:0 Tetracosanoic (Lignoceric) | <0.1 |
| C24:1 Tetracosenoic (Nervonic) | 0.99 |
| Unidentified Fatty acids | 25.6 |
| Fatty acid Composition | % Weight |
|---|---|
| Saturated | 9.52 |
| Monosaturated | 21.68 |
| Polyunsaturated | 23.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghelani, H.; Altaher, H.; Sarsour, H.; Tabbal, M.; Badawi, S.; Adrian, T.E.; Jan, R.K. The Effects of Frondanol, a Non-Polar Extract of the Atlantic Sea Cucumber, in Colon Cancer Cells. Pharmaceuticals 2025, 18, 1714. https://doi.org/10.3390/ph18111714
Ghelani H, Altaher H, Sarsour H, Tabbal M, Badawi S, Adrian TE, Jan RK. The Effects of Frondanol, a Non-Polar Extract of the Atlantic Sea Cucumber, in Colon Cancer Cells. Pharmaceuticals. 2025; 18(11):1714. https://doi.org/10.3390/ph18111714
Chicago/Turabian StyleGhelani, Hardik, Hala Altaher, Hadil Sarsour, Marah Tabbal, Sally Badawi, Thomas E. Adrian, and Reem K. Jan. 2025. "The Effects of Frondanol, a Non-Polar Extract of the Atlantic Sea Cucumber, in Colon Cancer Cells" Pharmaceuticals 18, no. 11: 1714. https://doi.org/10.3390/ph18111714
APA StyleGhelani, H., Altaher, H., Sarsour, H., Tabbal, M., Badawi, S., Adrian, T. E., & Jan, R. K. (2025). The Effects of Frondanol, a Non-Polar Extract of the Atlantic Sea Cucumber, in Colon Cancer Cells. Pharmaceuticals, 18(11), 1714. https://doi.org/10.3390/ph18111714










