Anticancer Effects and Phytochemical Profile of Lavandula stoechas
Abstract
1. Introduction
2. Results
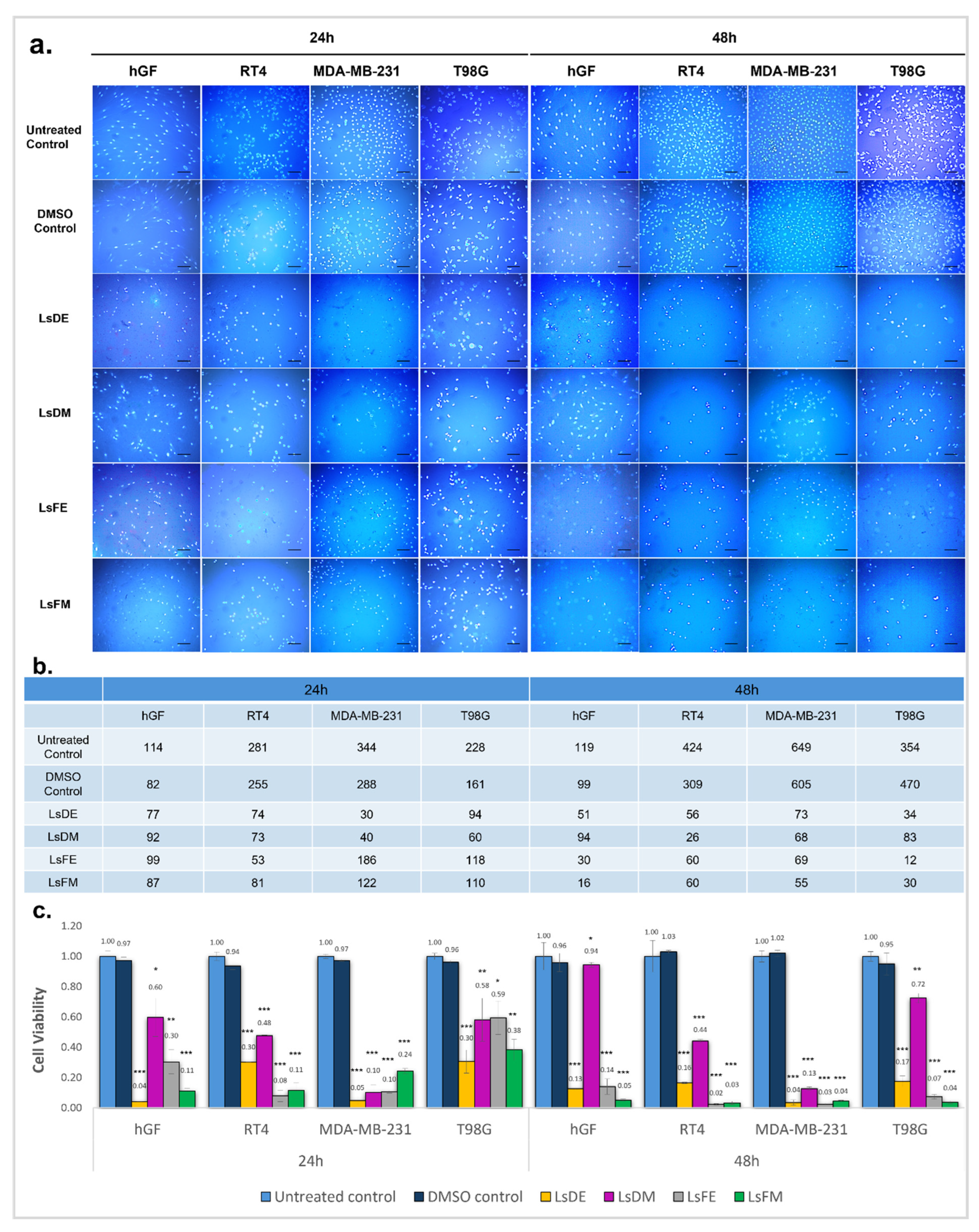
2.1. Cytotoxic Effects of L. stoechas L. Extracts on Non-Cancerous and Cancer Cell Lines: Nuclear Morphology and Cell Viability Analysis
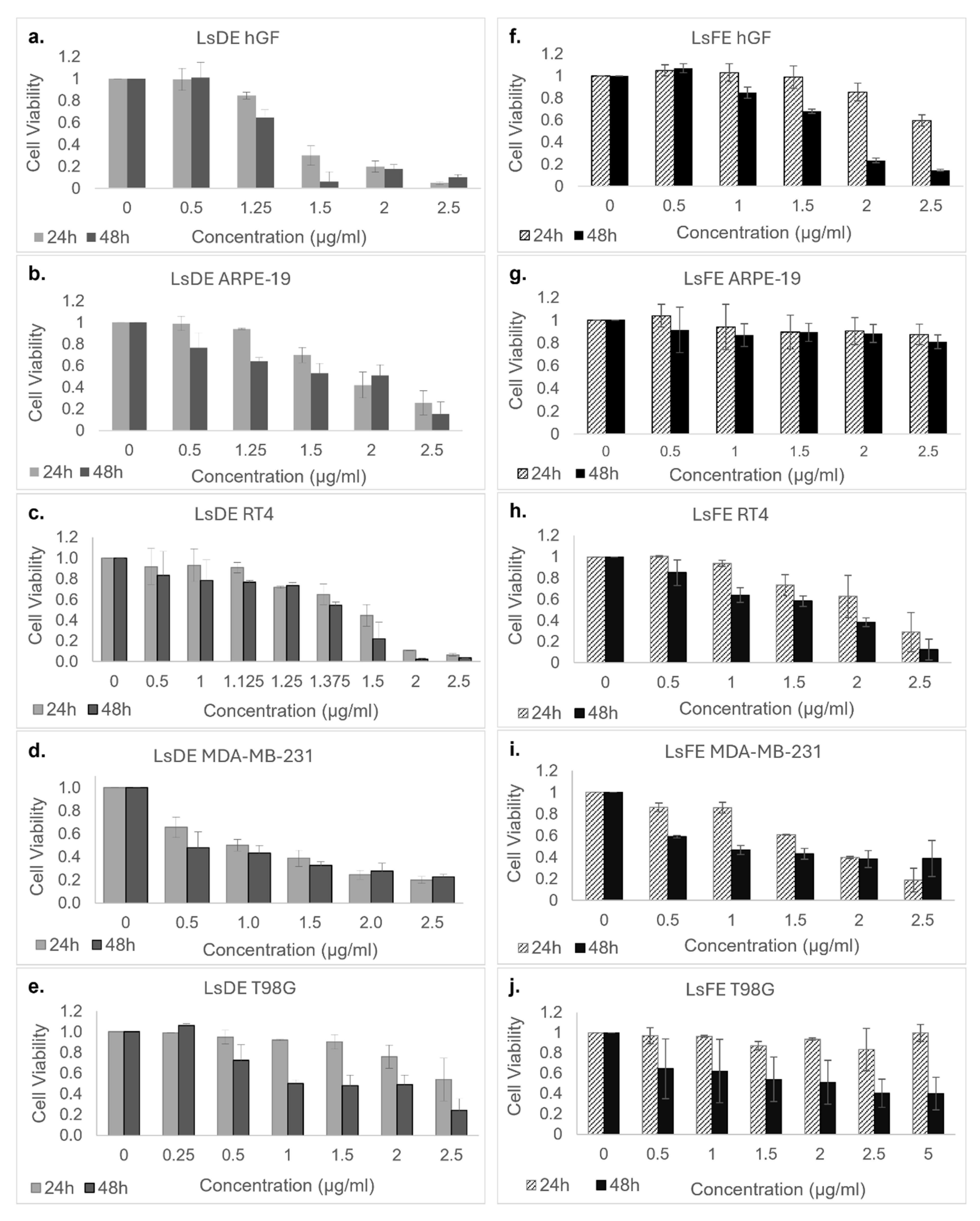
2.2. Dose-Dependent Cytotoxicity of L. stoechas L. Ethanol Extracts on Non-Cancerous and Cancer Cell Lines
2.3. Phytochemical Composition of LsDE and LsFE Extract Reveals Bioactive Compounds
3. Discussion
4. Methods
4.1. Extraction of L. stoechas L. Plant Extract
4.2. Culturing of Cells
4.3. Treatment with L. stoechas L. Extracts, Hoechst Staining, and Cytotoxicity Assays
4.4. Q-TOF Analysis for Compounds
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, B.; Bray, F.; Beltrán-Sánchez, H.; Ginsburg, O.; Soneji, S.; Soerjomataram, I. Benchmarking life expectancy and cancer mortality: Global comparison with cardiovascular disease 1981–2010. BMJ 2017, 357, j2765. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Chatterjee, S.; Nimesh, S. Natural Plant Extracts as Potential Therapeutic Agents for the Treatment of Cancer. Curr. Top. Med. Chem. 2017, 17, 96–106. [Google Scholar] [CrossRef]
- Pavan, A.R.; Silva, G.D.; Jornada, D.H.; Chiba, D.E.; Fernandes, G.F.; Man Chin, C.; Dos Santos, J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients 2016, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef]
- Carrasco, A.; Ortiz-Ruiz, V.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography–mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind. Crops Prod. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Gilani, A.H.; Aziz, N.; Khan, M.A.; Shaheen, F.; Jabeen, Q.; Siddiqui, B.S.; Herzig, J.W. Ethnopharmacological evaluation of the anticonvulsant, sedative and antispasmodic activities of Lavandula stoechas L. J. Ethnopharmacol. 2000, 71, 161–167. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Garrido-Mesa, J.; Garrido-Mesa, N.; Utrilla, M.P.; Gonzalez-Tejero, M.R.; Casares-Porcel, M.; Molero-Mesa, J.; Del Mar Contreras, M.; et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016, 190, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Gohar, U.F.; Ahmad, M.; Marc, R.A.; Mureşan, C.C.; Irimie, M.; Bobescu, E. Biomolecular Evaluation of Lavandula stoechas L. for Nootropic Activity. Plants 2021, 10, 1259. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Goren, A.; Topcu, G.; Bilsel, G.; Bilsel, M.; Aydogmus, Z.; Pezzuto, J.M. The chemical constituents and biological activity of essential oil of Lavandula stoechas ssp. stoechas. Z. Naturforsch. C J. Biosci. 2002, 57, 797–800. [Google Scholar] [CrossRef]
- Ahamad, R.; Kumar, S.; Akhtar, M.; Aqil, M.; Yar, M.S.; Akram, M.; Ismail, M.V.; Mujeeb, M. A Comprehensive Review of Lavandula stoechas L. (Ustukhuddus) Plant: Phytochemistry, Pharmacology, and In Silico Studies. Chem. Biodivers. 2025, 22, e202401996. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.R.; Işık, M.; Kavaz Yüksel, A.; Dikici, E. Phenolic Content Analysis of Two Species Belonging to the Lamiaceae Family: Antioxidant, Anticholinergic, and Antibacterial Activities. Molecules 2024, 29, 480. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef]
- Syaj, H.; Aboalhaija, N.; Afifi, F.; Abu-Dahab, R.; Abusulieh, S.; Amro, R. Phytochemistry and Antiproliferative Potential of a Naturalized Plant in Jordan: Lavandula stoechas. Chem. Biodivers. 2025, 22, e202500181. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Siddiqui, H.H.; Mishra, A.; Usmani, A. Evaluation of Cytotoxic Activity of Lavandula stoechas Aerial Parts Fractions against HepG2 Cell Lines. Curr. Bioact. Compd. 2020, 16, 1281–1289. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Santiago-Osorio, E.; Sánchez-Flores, N.; Salazar-Aguilar, S.; Soto-Hernández, R.M.; Riviello-Flores, M.L.; Macías-Zaragoza, V.M.; Aguiñiga-Sánchez, I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules 2024, 29, 1439. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.; Paul, K.; Bakshi, P.; Bajaj, P.; Kumar, M.; Dhiman, S.; Jasrotia, S.; Kumar, P.; Dutta, R. Hydroxytyrosol in cancer research: Recent and historical insights on discoveries and mechanisms of action. Futur. J. Pharm. Sci. 2024, 10, 129. [Google Scholar] [CrossRef]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef]
- Majid Rasheed, H.; Farooq, U.; Bashir, K.; Wahid, F.; Khan, T.; Khusro, A.; Gajdács, M.; Alghamdi, S.; Amer Alsaiari, A.; Almehmadi, M.; et al. Isolation of oleanolic acid from Lavandula stoechas and its potent anticancer properties against MCF-7 cancer cells via induced apoptosis. J. King Saud Univ. Sci. 2023, 35, 102454. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Markowska, A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. Int. J. Mol. Sci. 2021, 22, 8437. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, Y. Methyl jasmonate induces the apoptosis of human colorectal cancer cells via downregulation of EZH2 expression by microRNA-101. Mol. Med. Rep. 2017, 15, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Mill, R.R. Flora of Turkey and the East Aegean Islands, 1st ed.; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 76–78. [Google Scholar]
- Küçük, S.; Altıntaş, A.; Demirci, B.; Koca, F.; Başer, K.H.C. Morphological, Anatomical and Phytochemical Characterizations of Lavandula stoechas L. subsp. stoechas Growing in Turkey. Nat. Volatiles Essent. Oils 2019, 6, 9–19. [Google Scholar]
- Nyffeler, R.G.A. Occurrence Dataset. Available online: https://www.gbif.org/occurrence/3383283857 (accessed on 12 November 2021).
- Nalkiran, H.S. Digital Voucher Specimen of Lavandula stoechas L. subsp. Stoechas Collected from Türkiye. Available online: https://doi.org/10.5281/zenodo.17374963 (accessed on 17 October 2025).
- Nalkiran, I.; Sevim Nalkiran, H. Phytochemical Profile and Anticancer Potential of Helichrysum arenarium Extracts on Glioblastoma, Bladder Cancer, and Breast Cancer Cells. Pharmaceuticals 2025, 18, 144. [Google Scholar] [CrossRef]
- Sevim Nalkiran, H.; Akcora Yildiz, D.; Saydam, F.; Guzel, A.I.; Nalkiran, I. Targeting the anaphase-promoting complex/cyclosome (APC/C) enhanced antiproliferative and apoptotic response in bladder cancer. Saudi J. Biol. Sci. 2023, 30, 103564. [Google Scholar] [CrossRef]
| hGF | ARPE-19 | RT4 | MDA-MB-231 | T98G | ||
|---|---|---|---|---|---|---|
| LsDE | 24 h | 1.28 | 1.73 | 1.33 | 1.48 | 2.50 |
| 48 h | 1.01 | 1.90 | 1.40 | 0.63 | 0.58 | |
| LsFE | 24 h | 2.49 | >2.50 | 2.05 | 1.75 | >5.00 |
| 48 h | 1.68 | >2.50 | 1.72 | 0.28 | 0.43 |
| Extract | Time | hGF/RT4 | hGF/MDA-MB-231 | hGF/T98G | ARPE-19/RT4 | ARPE-19/MDA-MB-231 | ARPE-19/T98G |
|---|---|---|---|---|---|---|---|
| LsDE | 24 h | 0.96 | 0.86 | 0.51 | 1.30 | 1.17 | 0.69 |
| 48 h | 0.72 | 1.60 | 1.74 | 1.36 | 3.02 | 3.28 | |
| LsFE | 24 h | 1.21 | 1.42 | 0.50 | 1.22 | 1.43 | 0.50 |
| 48 h | 0.98 | 6.00 | 3.91 | 1.45 | 8.93 | 5.81 |
| No | m/z | z | Abundance | Name | Formula | Ion | Score (DB) | Hits (DB) |
|---|---|---|---|---|---|---|---|---|
| 1 | 225.149 | −1 | 128,791.64 | Riesling acetal | C13 H22 O3 | (M–H)− | 95.83 | 10 |
| 2 | 207.13926 | −1 | 79,761.27 | (5alpha,8beta,9beta)-5,9-Epoxy-3,6-megastigmadien-8-ol | C13 H20 O2 | (M–H)− | 98.83 | 10 |
| 3 | 239.12915 | −1 | 14,831.12 | (S)-p-Mentha-1,8-dien-10-yl acetate | C12 H18 O2 | (M+HCOO)- | 93.91 | 10 |
| 4 | 315.18099 | −1 | 10,437.16 | (S)-alpha-Terpinyl glucoside | C16 H28 O6 | (M–H)− | 97.02 | 4 |
| 5 | 283.06114 | −1 | 8447.77 | 7,3′-Dihydroxy-4′-methoxy-4-phenylcoumarin | C16 H12 O5 | (M–H)− | 85.35 | 10 |
| 6 | 293.21184 | −1 | 7284.23 | γ-9(10)-EpODE | C18 H30 O3 | (M–H)− | 95.03 | 10 |
| No | m/z | z | Abundance | Name | Formula | Ion | Score (DB) | Hits (DB) |
|---|---|---|---|---|---|---|---|---|
| 1 | 295.18755 | 1 | 158,475.45 | 3-methyl-tetradecanedioic acid | C15 H28 O4 | (M+Na)+ | 96.53 | 4 |
| 2 | 275.1614 | 1 | 16,567.18 | 3(4→5)-Abeo-4,11:4,12-diepoxy-3-eudesmanol | C15 H24 O3 | (M+Na)+ | 94.1 | 10 |
| 3 | 177.0522 | 1 | 12,840.17 | 3,4-Dihydroxyphenyl ethanol | C8 H10 O3 | (M+Na)+ | 95.81 | 8 |
| 4 | 277.17698 | 1 | 12,793.35 | Kikkanol A | C15 H26 O3 | (M+Na)+ | 77.72 | 10 |
| No | m/z | z | Abundance | Name | Formula | Ion | Score (DB) | Hits (DB) |
|---|---|---|---|---|---|---|---|---|
| 1 | 207.1387 | −1 | 145,460.26 | 3-Hydroxy-beta-ionone | C13 H20 O2 | (M–H)− | 99.11 | 10 |
| 2 | 225.1492 | −1 | 141,806.06 | Riesling acetal | C13 H22 O3 | (M–H)− | 98.77 | 10 |
| 3 | 279.124 | −1 | 79,414.87 | Crispolide | C15 H20 O5 | (M–H)− | 98.92 | 10 |
| 4 | 313.0713 | −1 | 72,412.8 | Luteolin 5,3′-dimethyl ether | C17 H14 O6 | (M–H)− | 98.61 | 10 |
| 5 | 207.0666 | −1 | 71,270.55 | 2,5-Dimethoxycinnamic acid | C11 H12 O4 | (M–H)− | 98.35 | 10 |
| 6 | 209.0448 | −1 | 28,402.86 | Vanilpyruvic acid | C10 H10 O5 | (M–H)− | 95.24 | 10 |
| 7 | 153.0186 | −1 | 26,201.32 | 3,4-Dihydroxybenzoic acid | C7 H6 O4 | (M–H)− | 96.75 | 7 |
| 8 | 177.0188 | −1 | 15,438.27 | 5,7-Dihydroxychromone | C9 H6 O4 | (M–H)− | 85.62 | 6 |
| 9 | 283.0611 | −1 | 11,233.25 | 7,3′-Dihydroxy-4′-methoxy-4-phenylcoumarin | C16 H12 O5 | (M–H)− | 98.58 | 10 |
| 10 | 225.1125 | −1 | 10,204.81 | 12-hydroxyjasmonic acid | C12 H18 O4 | (M–H)− | 95.56 | 10 |
| No | m/z | z | Abundance | Name | Formula | Ion | Score (DB) | Hits (DB) |
|---|---|---|---|---|---|---|---|---|
| 1 | 359.2187 | 1 | 72,189.0 | 5,12-dihydroxy-6,8,10,14-eicosatetraenoic acid | C20 H32 O4 | (M+Na)+ | 98.77 | 10 |
| 2 | 277.1774 | 1 | 33,388.4 | Kikkanol A | C15 H26 O3 | (M+Na)+ | 86.44 | 10 |
| 3 | 275.1614 | 1 | 31,302.5 | 3(4->5)-Abeo-4,11:4,12-diepoxy-3-eudesmanol | C15 H24 O3 | (M+Na)+ | 85.37 | 10 |
| 4 | 335.2188 | 1 | 30,372.3 | 9(S)-HpODE | C18 H32 O4 | (M+Na)+ | 83.37 | 10 |
| 5 | 249.1463 | 1 | 29,522.2 | Dihydrojasmonic Acid, Methyl Ester | C13 H22 O3 | (M+Na)+ | 84.04 | 3 |
| 6 | 177.0522 | 1 | 16,426.0 | 3,4-Dihydroxyphenyl ethanol | C8 H10 O3 | (M+Na)+ | 99.35 | 8 |
| 7 | 317.2081 | 1 | 11,726.0 | α-9(10)-EpODE | C18 H30 O3 | (M+Na)+ | 96.35 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevim Nalkiran, H.; Nalkiran, I. Anticancer Effects and Phytochemical Profile of Lavandula stoechas. Pharmaceuticals 2025, 18, 1706. https://doi.org/10.3390/ph18111706
Sevim Nalkiran H, Nalkiran I. Anticancer Effects and Phytochemical Profile of Lavandula stoechas. Pharmaceuticals. 2025; 18(11):1706. https://doi.org/10.3390/ph18111706
Chicago/Turabian StyleSevim Nalkiran, Hatice, and Ihsan Nalkiran. 2025. "Anticancer Effects and Phytochemical Profile of Lavandula stoechas" Pharmaceuticals 18, no. 11: 1706. https://doi.org/10.3390/ph18111706
APA StyleSevim Nalkiran, H., & Nalkiran, I. (2025). Anticancer Effects and Phytochemical Profile of Lavandula stoechas. Pharmaceuticals, 18(11), 1706. https://doi.org/10.3390/ph18111706






