The ErChen Decoction and Its Active Compounds Ameliorate Non-Alcoholic Fatty Liver Disease Through Activation of the AMPK Signaling Pathway
Abstract
1. Introduction
2. Results
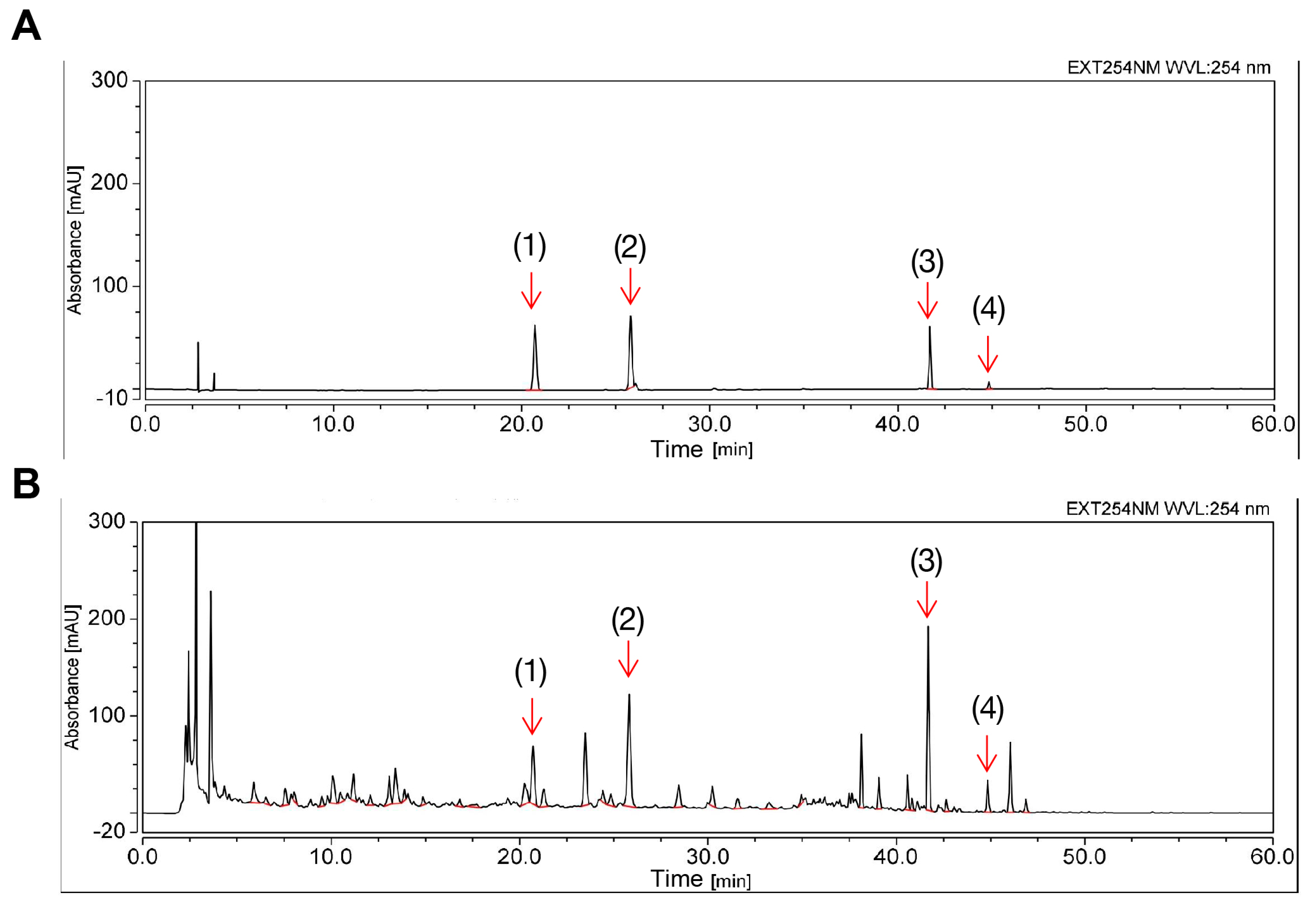
2.1. Determination of the Chemical Profile of ECD Using HPLC
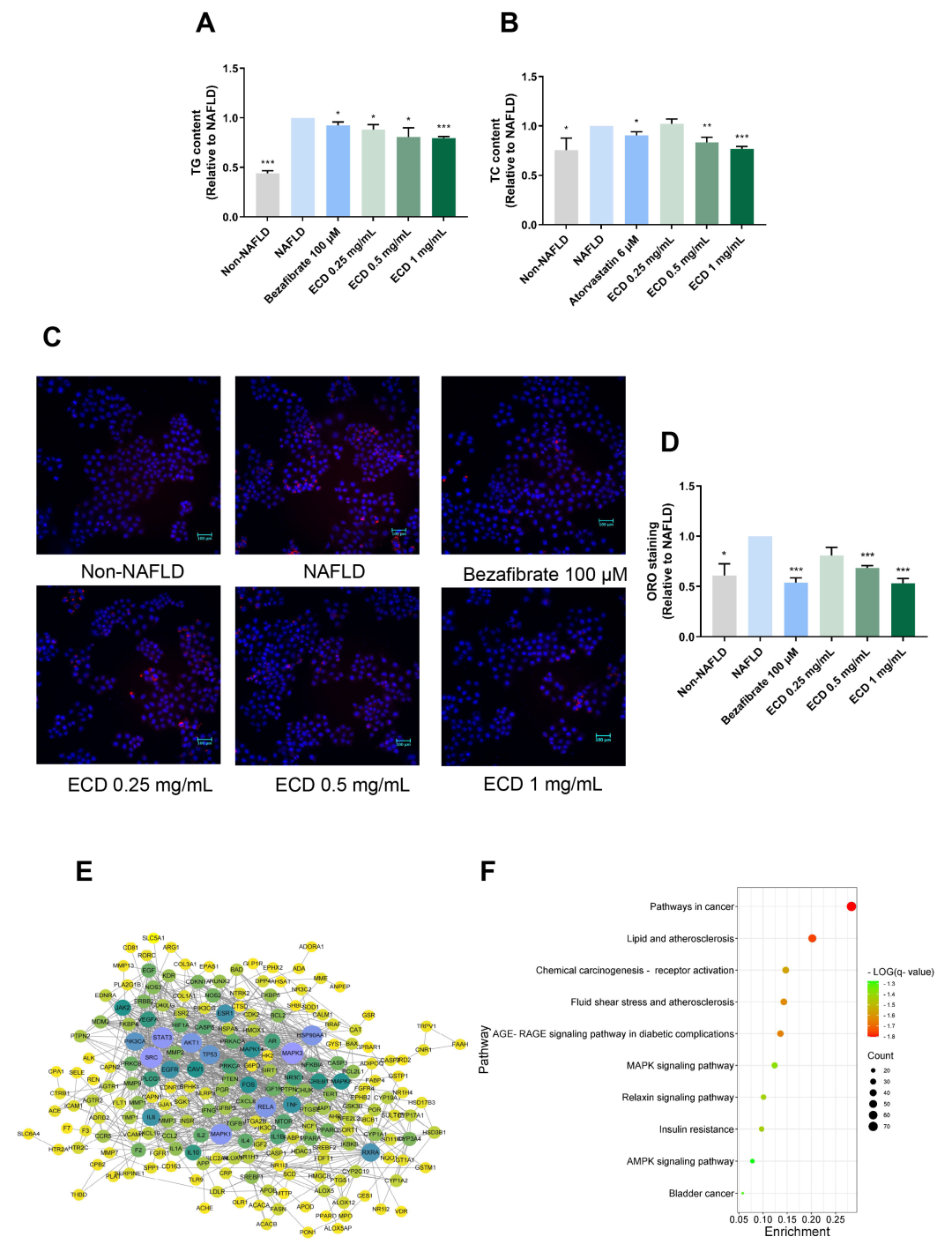
2.2. ECD Reduced Lipid Accumulation in HepG2 NAFLD Cells
2.3. Network Pharmacology Study of ECD
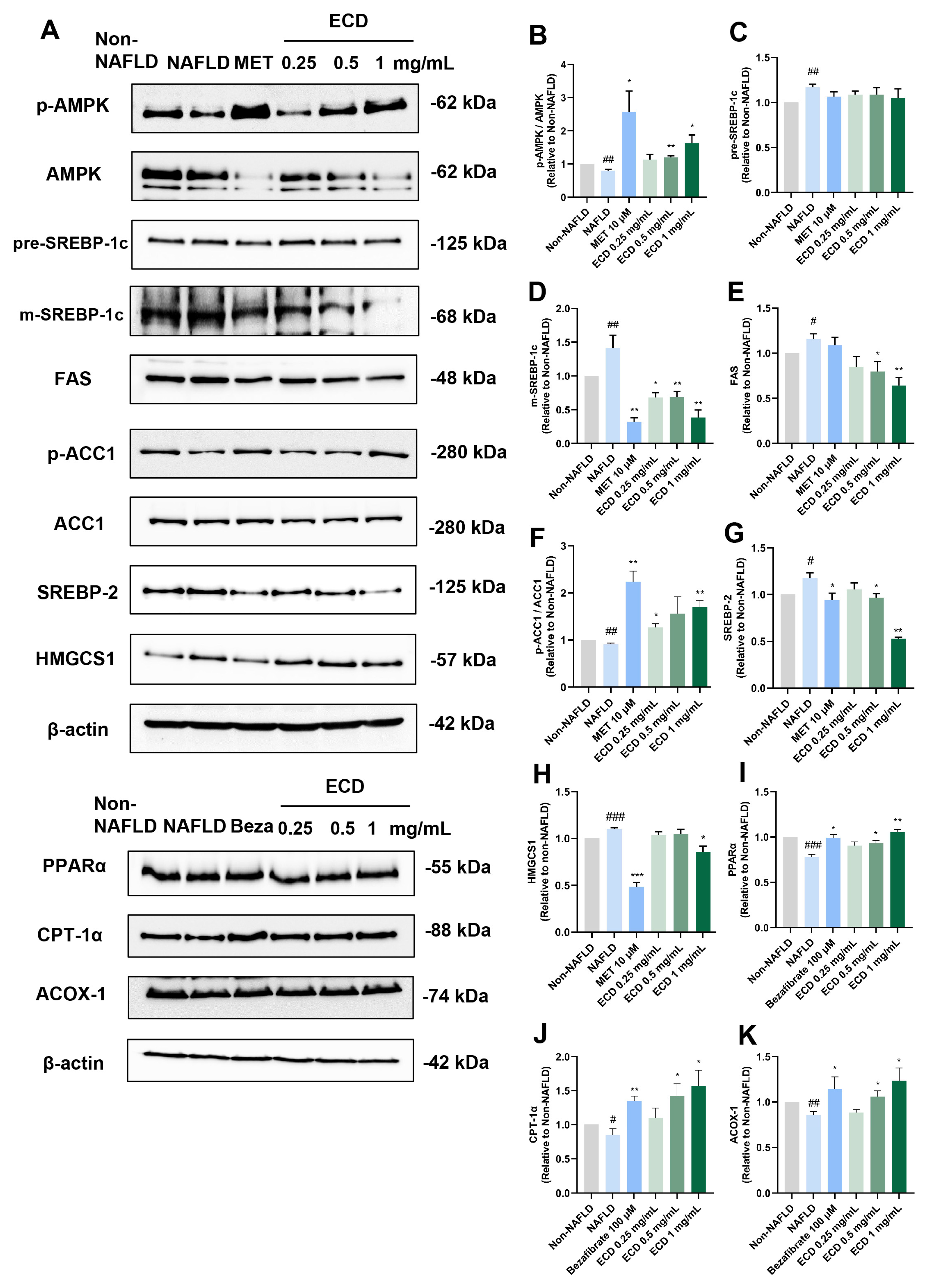
2.4. ECD Activated AMPK and PPARα Signaling Pathways to Inhibit Lipid Synthesis and Promote β-Oxidation
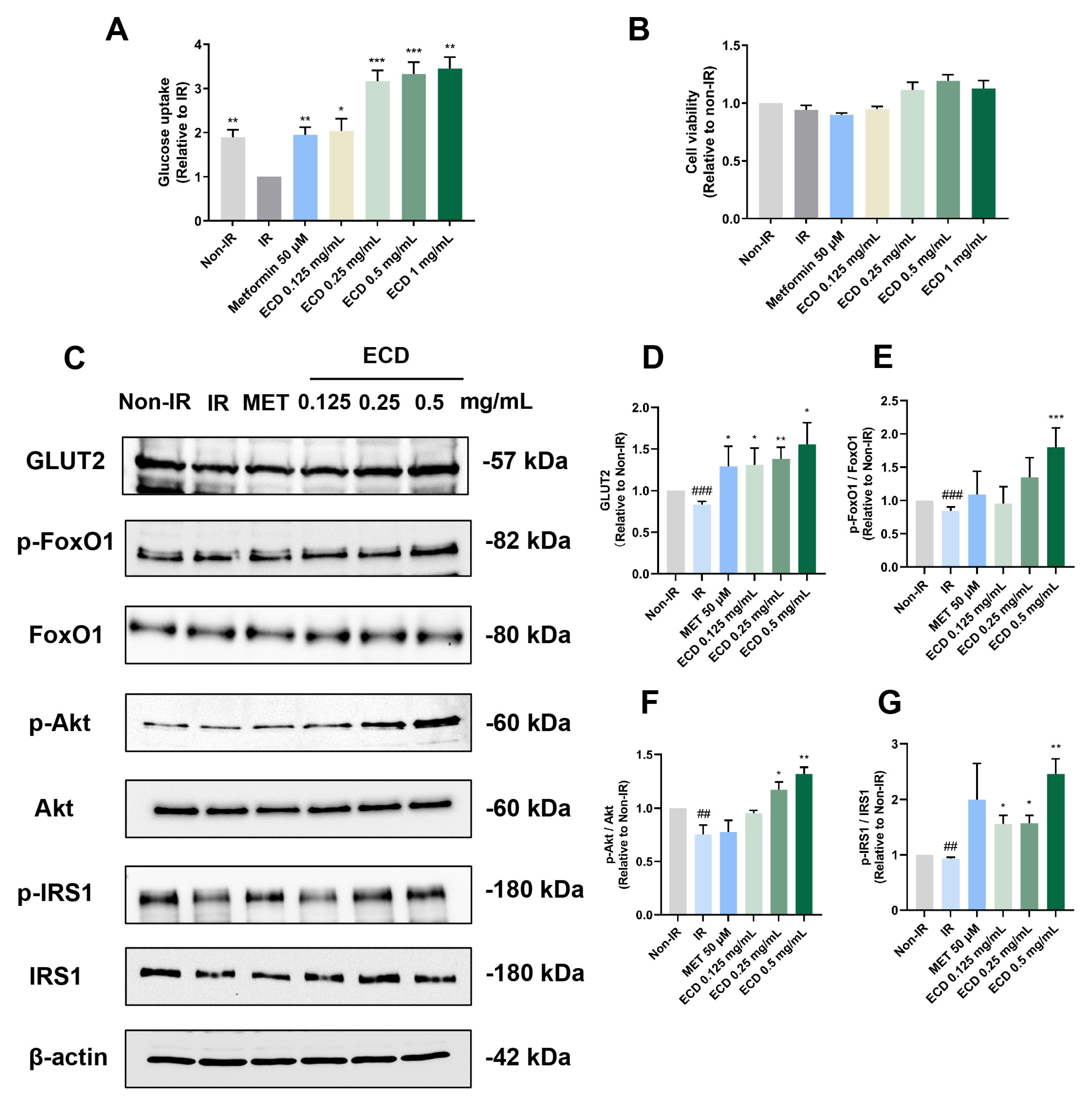
2.5. ECD Increased Insulin Sensitivity and Promoted Glucose Uptake in HepG2 IR Cells
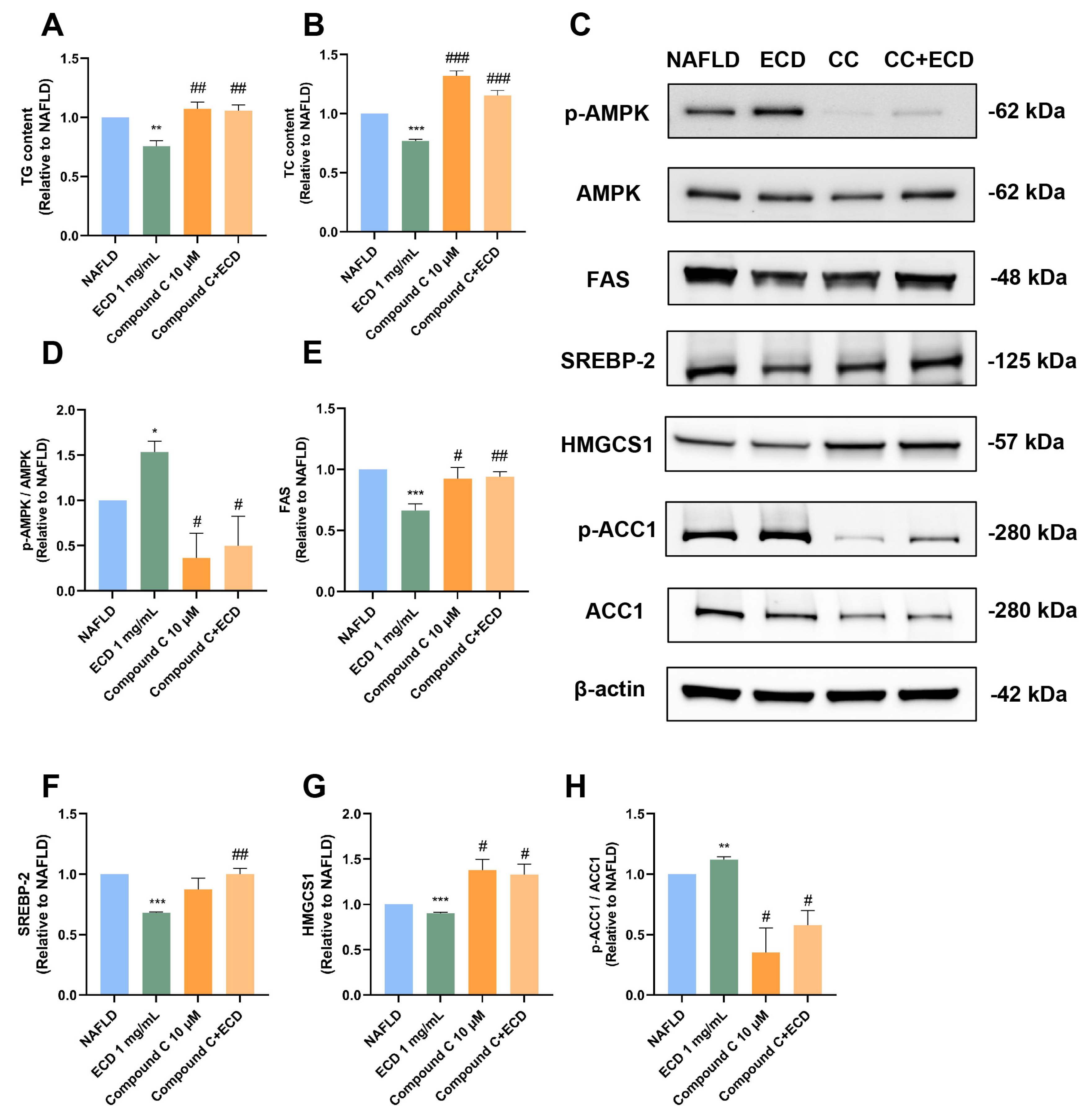
2.6. Inhibition of De Novo Lipogenesis by ECD Depends on AMPK Activation
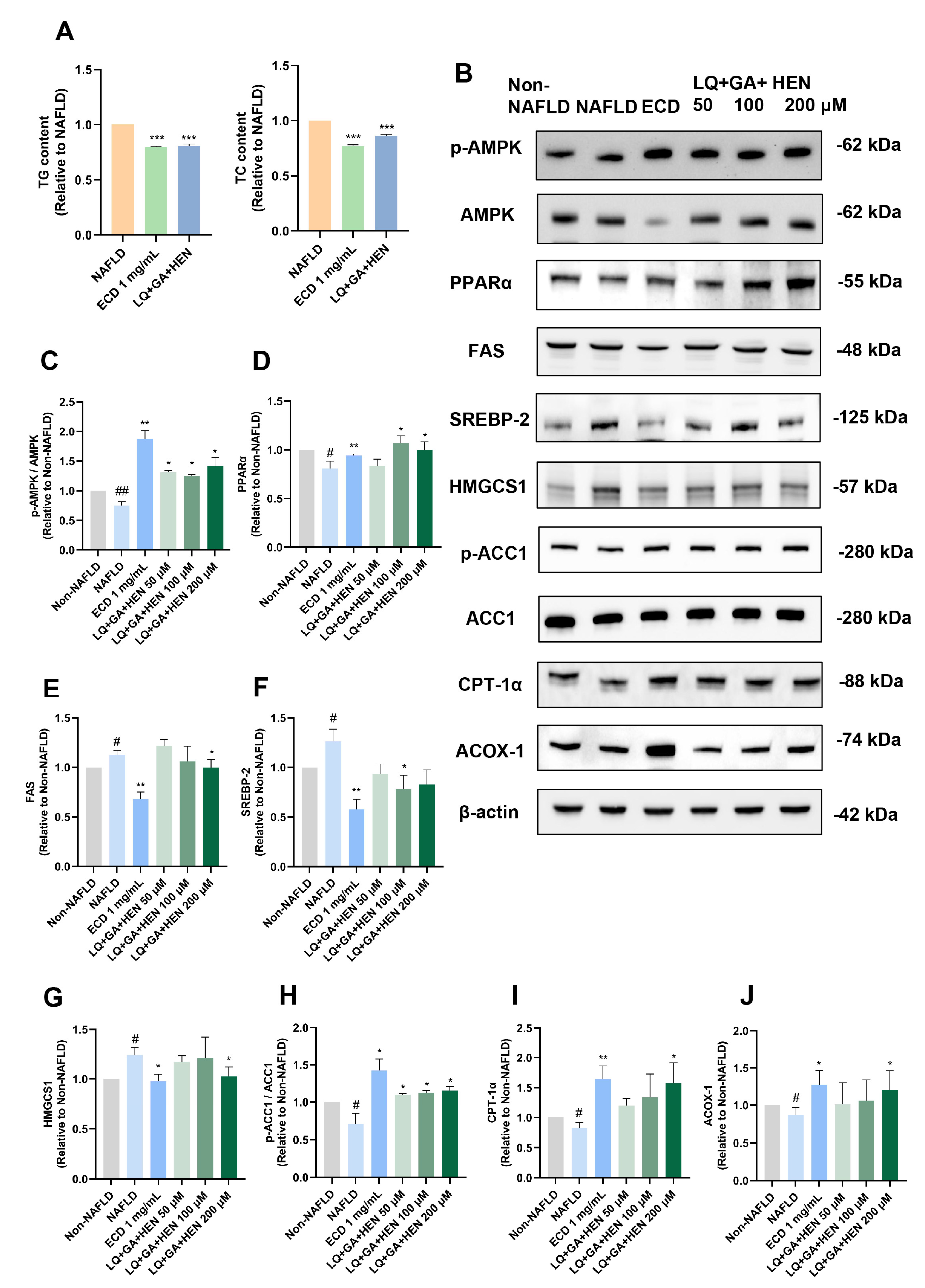
2.7. Combined Treatment of HepG2 NAFLD Model Cells with LQ, GA and HEN Significantly Reduced De Novo Lipogenesis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. ECD Extract Preparation
4.3. HPLC Identification of the Chemical Composition of ECD Extract
4.4. Network Pharmacology Analysis
4.5. NAFLD Model Cells and Cell Viability of ECD-Treated Hepatocytes
4.6. Oil Red O Staining
4.7. Measurement of Intracellular Triglyceride (TG) and Total Cholesterol (TC) Levels
4.8. Quantification of Glucose Uptake
4.9. Orthogonal Experimental Design
4.10. Immunoblotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC1 | Acetyl-CoA Carboxylase 1 |
| ACOX-1 | peroxisomal acyl-coenzyme A oxidase 1 |
| AMPK | AMP-activated protein kinase |
| Akt | protein kinase B |
| CPT-1α | carnitine palmitoyltransferase 1α |
| ECD | ErChen decoction |
| FAS | fatty acid synthase |
| FoxO1 | Forkhead box protein O1 |
| GA | glycyrrhizic acid |
| GLUT2 | glucose transporter type 2 |
| HEN | hesperidin |
| HMGCS1 | 3-Hydroxy-3-Methylglutaryl-CoA Synthase 1 |
| IR | insulin resistance |
| LQ | liquiritin |
| NAFLD | Non-alcoholic fatty liver disease |
| PPARα | peroxisome proliferator-activated receptor α |
| SREBP-1c | sterol regulatory element-binding protein 1c |
| SREBP-2 | sterol regulatory element-binding protein 2 |
| TCM | traditional Chinese medicine |
| T2DM | type II diabetes |
References
- Parameswaran, M.; Hasan, H.A.; Sadeque, J.; Jhaveri, S.; Avanthika, C.; Arisoyin, A.E.; Dhanani, M.B.; Rath, S.M.; Hasan, H.A., Jr. Factors that predict the progression of non-alcoholic fatty liver disease (NAFLD). Cureus 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic fatty liver disease in adults: Current concepts in etiology, outcomes, and management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S90–S102. [Google Scholar] [CrossRef]
- Boliaki, N.; Henin, G.; Bale, G.; Lanthier, N. Impact of peroxisome proliferator-activated receptor agonists on myosteatosis in the context of metabolic dysfunction-associated steatotic liver disease. Discov. Med. 2024, 36, 1139–1153. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, J.; Li, H.; Zhang, L.; Xu, P. Network pharmacology-based investigation to explore the effect and mechanism of Erchen decoction against the nonalcoholic fatty liver disease. Anat. Rec. 2021, 304, 2605–2619. [Google Scholar] [CrossRef]
- Zhao, T.; Zhan, L.; Zhou, W.; Chen, W.; Luo, J.; Zhang, L.; Weng, Z.; Zhao, C.; Liu, S. The effects of erchen decoction on gut microbiota and lipid metabolism disorders in zucker diabetic fatty rats. Front. Pharmacol. 2021, 12, 647529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, R.J.; Peng, S.Y.; Yu, W.C.; Chang, V.H.S. Therapeutic targeting of nonalcoholic fatty liver disease by downregulating SREBP-1C expression via AMPK-KLF10 axis. Front. Mol. Biosci. 2021, 8, 751938. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef]
- Ni, H.Y.; Yu, L.; Zhao, X.L.; Wang, L.T.; Zhao, C.J.; Huang, H.; Zhu, H.L.; Efferth, T.; Gu, C.B.; Fu, Y.J. Seed oil of Rosa roxburghii Tratt against non-alcoholic fatty liver disease in vivo and in vitro through PPARα/PGC-1α-mediated mitochondrial oxidative metabolism. Phytomedicine 2022, 98, 153919. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Kim, H.-G.; Liangpunsakul, S.; Dong, X.C. FOXO transcription factors protect against the diet-induced fatty liver disease. Sci. Rep. 2017, 7, 44597. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Cao, H. Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol. 2019, 127, 182–187. [Google Scholar] [CrossRef]
- Scoditti, E.; Sabatini, S.; Carli, F.; Gastaldelli, A. Hepatic glucose metabolism in the steatotic liver. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 319–334. [Google Scholar] [CrossRef]
- Dall’Agnese, A.; Platt, J.M.; Zheng, M.M.; Friesen, M.; Dall’Agnese, G.; Blaise, A.M.; Spinelli, J.B.; Henninger, J.E.; Tevonian, E.N.; Hannett, N.M.; et al. The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat. Commun. 2022, 13, 7522. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: A comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM. org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, A.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Cherry, J.M. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Wang, J.; Amin, A.; Cheung, M.H.; Shi, L.; Liang, C. Targeted inhibition of the expression of both MCM5 and MCM7 by miRNA-214 impedes DNA replication and tumorigenesis in hepatocellular carcinoma cells. Cancer Lett. 2022, 539, 215677. [Google Scholar] [CrossRef]
- Cheung, M.H.; Amin, A.; Wu, R.; Qin, Y.; Zou, L.; Yu, Z.; Liang, C. Human NOC3 is essential for DNA replication licensing in human cells. Cell Cycle 2019, 18, 605–620. [Google Scholar] [CrossRef] [PubMed]
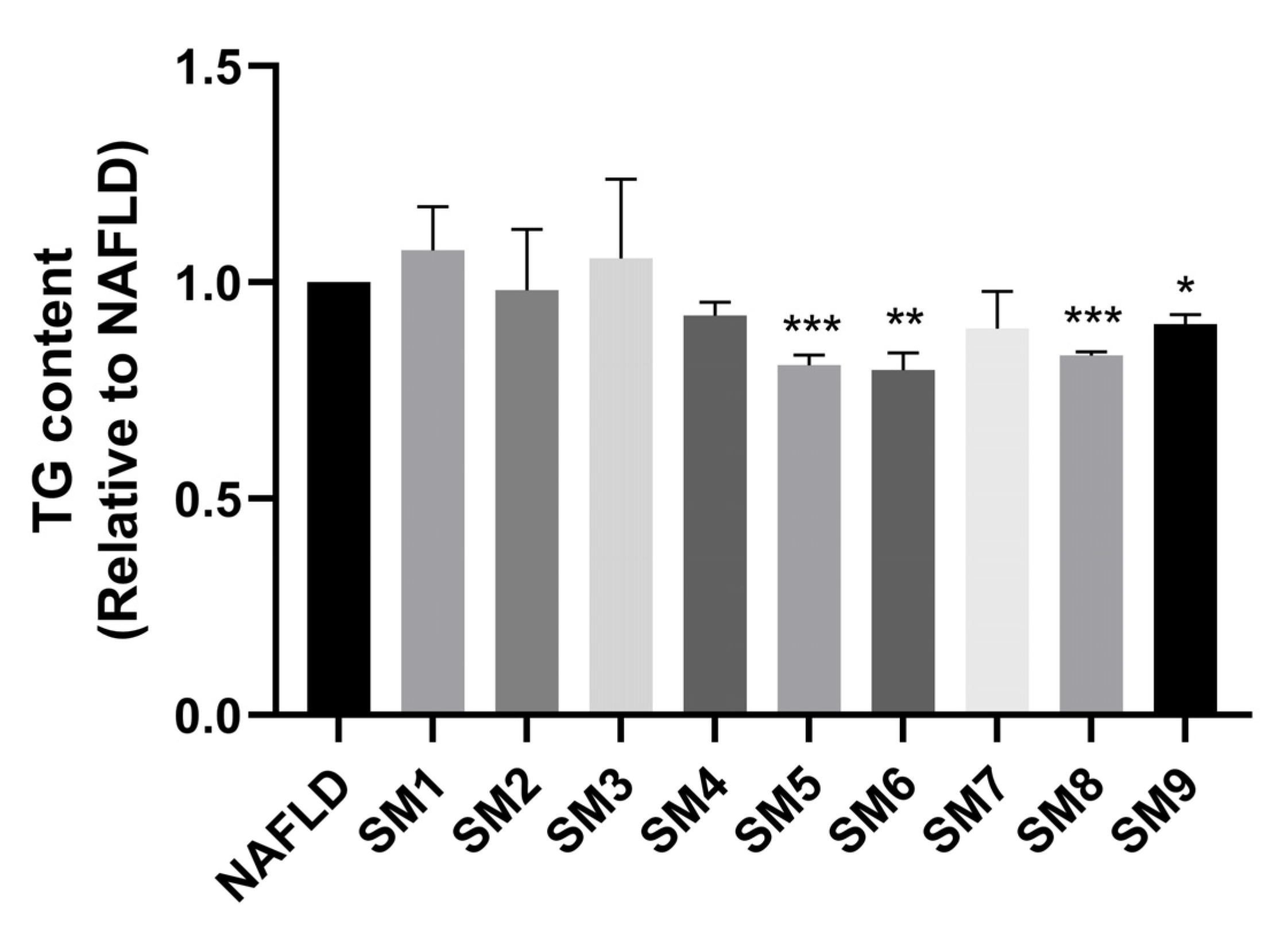
| Group | A: LQ (μM) | B: GA (μM) | C: HEN (μM) | TG (Relative) |
|---|---|---|---|---|
| SM1 | 50 | 50 | 50 | 1.074 |
| SM2 | 50 | 100 | 200 | 0.982 |
| SM3 | 50 | 200 | 100 | 1.056 |
| SM4 | 100 | 50 | 100 | 0.923 |
| SM5 | 100 | 100 | 50 | 0.810 |
| SM6 | 100 | 200 | 200 | 0.799 |
| SM7 | 200 | 50 | 200 | 0.893 |
| SM8 | 200 | 100 | 100 | 0.831 |
| SM9 | 200 | 200 | 50 | 0.903 |
| K1 | 3.112 | 2.890 | 2.714 | |
| K2 | 2.531 | 2.695 | 2.882 | |
| K3 | 2.627 | 2.685 | 2.684 | |
| R | 0.194 | 0.068 | 0.066 |
| * Herbs | Place of Origin | Lot Number | Quantity |
|---|---|---|---|
| PT | Guizhou, China | 20210801 | 15 g |
| CR | Guangdong, China | 190601 | 15 g |
| PC | Hunan, China | 201101 | 9 g |
| GU | Gansu, China | 191005341 | 4.5 g |
| ZO | Guangdong, China | 202108 | 7 pieces |
| PM | Sichuan, China | 191001C085 | 1 piece |
| Factor | A: LQ | B: GA | C: HEN | |
|---|---|---|---|---|
| Level | ||||
| 1 | 50 μM | 50 μM | 50 μM | |
| 2 | 100 μM | 100 μM | 100 μM | |
| 3 | 200 μM | 200 μM | 200 μM | |
| Group | A: LQ (μM) | B: GA (μM) | C: HEN (μM) |
|---|---|---|---|
| SM1 | 50 | 50 | 50 |
| SM2 | 50 | 100 | 200 |
| SM3 | 50 | 200 | 100 |
| SM4 | 100 | 50 | 100 |
| SM5 | 100 | 100 | 50 |
| SM6 | 100 | 200 | 200 |
| SM7 | 200 | 50 | 200 |
| SM8 | 200 | 100 | 100 |
| SM9 | 200 | 200 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liang, Y.; Cheung, M.H.; Wang, X.; Mo, H.; Gan, J.; Yang, W.; Guo, J.; Liang, C. The ErChen Decoction and Its Active Compounds Ameliorate Non-Alcoholic Fatty Liver Disease Through Activation of the AMPK Signaling Pathway. Pharmaceuticals 2025, 18, 1707. https://doi.org/10.3390/ph18111707
Wang Y, Liang Y, Cheung MH, Wang X, Mo H, Gan J, Yang W, Guo J, Liang C. The ErChen Decoction and Its Active Compounds Ameliorate Non-Alcoholic Fatty Liver Disease Through Activation of the AMPK Signaling Pathway. Pharmaceuticals. 2025; 18(11):1707. https://doi.org/10.3390/ph18111707
Chicago/Turabian StyleWang, Ye, Yanting Liang, Man Hei Cheung, Xinran Wang, Huimei Mo, Jiehua Gan, Wei Yang, Jianmin Guo, and Chun Liang. 2025. "The ErChen Decoction and Its Active Compounds Ameliorate Non-Alcoholic Fatty Liver Disease Through Activation of the AMPK Signaling Pathway" Pharmaceuticals 18, no. 11: 1707. https://doi.org/10.3390/ph18111707
APA StyleWang, Y., Liang, Y., Cheung, M. H., Wang, X., Mo, H., Gan, J., Yang, W., Guo, J., & Liang, C. (2025). The ErChen Decoction and Its Active Compounds Ameliorate Non-Alcoholic Fatty Liver Disease Through Activation of the AMPK Signaling Pathway. Pharmaceuticals, 18(11), 1707. https://doi.org/10.3390/ph18111707






