Vitamins A and D and Their Combinations for Breast and Colorectal Cancers: Analysis of the Clinical, Epidemiological, Preclinical and Transcriptomic Data
Abstract
1. Introduction
2. Results and Discussion
2.1. Vitamin A Effects in Breast Cancer: Incidence, Risk, Metastasis, and Survival
2.2. Vitamin D Effects in Breast Cancer: Risk, Prognosis, Metastasis, and Survival
2.3. Effects of Vitamin D in Colorectal Cancer (CRC): Risk, Prognosis, and Survival
2.4. Vitamin A and D Dosing in Cancer
2.4.1. Vitamin D Dosing in Cancer
2.4.2. Vitamin A Dosing in Cancer
2.5. Supporting Preclinical and Molecular Studies
2.5.1. Vitamins A and D Impact Molecular Signaling and Induction of Apoptosis
2.5.2. Vitamins A and D Are Antioxidants and Reduce Reactive Oxygen Species (ROS)
2.5.3. Vitamin D Induction of Autophagy
2.5.4. Vitamins A and D Downregulate Estrogen Signaling Pathways
2.6. Transcriptomic and Proteomics Studies of Vitamins A and D in Colon and Breast Cancers
2.7. Synergistic Effects of the Combination of Vitamins A and D and Transcriptomic Analyses in Breast and Colon Cancers
2.8. Vitamins A and D Stimulate Immune Responses
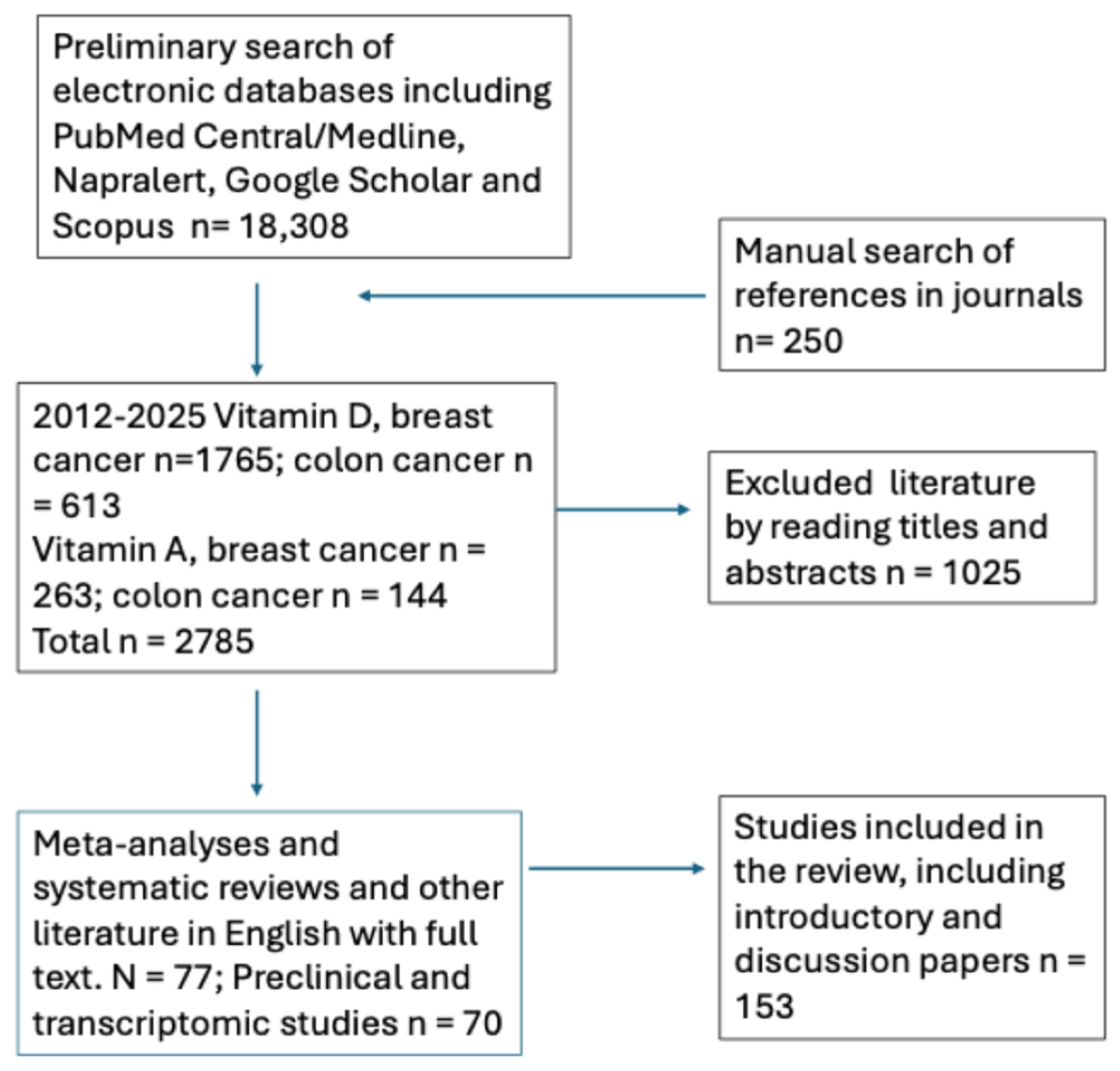
3. Materials and Methods
4. Conclusions and Future Research
5. Limitations of the Review
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A Disintegrin and Metalloproteases | ADAMs |
| All-trans-retinoic acid | ATRA |
| Breast cancer | BC |
| Bone morphogenetic protein and activin membrane-bound inhibitor | BAMBI |
| Calciferol | 25-OH-D |
| Calcitriol | 1,25-OH-D |
| Cathelicidin | CAMP |
| Cholecalciferol | vitamin D3 |
| Collapsin response mediator protein-1 | CRMP1 |
| Colorectal cancer | CRC |
| Cyclin D1 | CCND1 |
| Cyclin E1 | CCNE1 |
| Cyclin E2 | CCNE2 |
| Epithelial–mesenchymal transition | EMT |
| Ergocalciferol | vitamin D2 |
| Estrogen receptor | ER |
| Hormone responsive | HR |
| Inducible nitric oxide synthase | iNOS |
| Interleukin-12 | IL-12 |
| Interleukin 15 | IL-15 |
| Integrin | ITBG |
| Mammalian target of rapamycin | mTOR |
| Non-adjuvant chemotherapy | NAC |
| Nuclear Factor kappa beta | NF-κβ |
| Phosphoinositide 3-kinase | P13K |
| Protein kinase RNA-like endoplasmic reticulum kinase | PERK |
| Randomized controlled clinical trial | RCT |
| Reactive oxygen species | ROS |
| Retinaldehyde dehydrogenase 1A3 | ALDH1A3 |
| Retinoid receptor | RXR, RAR |
| Reverse Transcription polymerase chain reaction | RT-PCR |
| Transforming growth factor-1beta | TGF1β |
| Vitamin D binding protein | VDBP |
| Vitamin D receptor | VDR |
| Vitamin D response elements | VDREs |
References
- Martirosyan, D.M.; Lampert, T.; Lee, M. A comprehensive review on the role of food bioactive compounds in functional food science. Funct. Food Sci. 2022, 3, 64–79. [Google Scholar] [CrossRef]
- Aghajanpour, M.; Nazer, M.; Obeidav, Z.; Kor, M.N. Functional foods and their role in cancer prevention and health promotion: A comprehensive review. Am. J. Can. Res. 2017, 7, 740–769. [Google Scholar]
- Bikle, D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. The vitamin D Endocrine system: Manipulation of structure-function relationships to provide opportunities for development of new cancer chemopreventive and immunosuppressive agents. J. Cell. Biochem. Suppl. 1995, 22, 218–225. [Google Scholar] [CrossRef]
- Norman, A.W. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Salehi, S.S.; Rabizadeh, S.; Karimpour, S.; Mouodi, M.; Nakhjavani, M.; Esteghamati, A.; Mirmiranpour, H. Leptin, hs-CRP and HOMA-IR in patients with type 2 diabetes: The role of different levels of vitamin D deficiency. Funct. Foods Health Dis. 2019, 9, 695–705. [Google Scholar] [CrossRef]
- Salehi, S.S.; Karimpour, S.; Rabizadeh, S.; Esteghamati, A.; Rajab, A.; Salehi, S.S.; Nakhjavani, M.; Mirmiranpour, H. The effect of vitamin D deficiency state on oxidized low-density lipoprotein alteration in patients with type 2 diabetes. Funct. Foods Health Dis. 2021, 11, 357–367. [Google Scholar] [CrossRef]
- Barghi, M.; Ranjbar, A.S.; Moazen, H.; Eskandari-Roozbahani, N. Serum levels of vitamin D, calcium, phosphorus, and oxidative parameters in healthy and diabetic people. Funct. Foods Health Dis. 2021, 11, 238–245. [Google Scholar] [CrossRef]
- Velangi, M.; Mandalika, S.; Shukla, S.; Pradhan, V. Effect of vitamin D3 and virgin coconut oil on cartilage inflammation and functional abilities in early knee osteoarthritis. Funct. Foods Health Dis. 2019, 9, 662–677. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Haskell, M.J. The challenge to reach nutritional adequacy for vitamin a: Β-carotene bioavailability and conversion--evidence in humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S. [Google Scholar] [CrossRef]
- Siddikuzzaman, G.C.; Berlin, G.V.M. All-trans retinoic acid and cancer. Immunopharmacol. Immunotoxicol. 2011, 33, 241–249. [Google Scholar] [CrossRef]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: Genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef]
- Fujimaki, Y. Formation of gastric carcinoma in albino rats fed on deficient diets. J. Can. Res. 1926, 10, 469–477. [Google Scholar]
- Bouriez, D.; Giraud, J.; Gronnier, C.; Varon, C. Efficiency of all-trans retinoic acid on gastric cancer: A narrative literature review. Int. J. Mol. Sci. 2018, 19, 3388–3401. [Google Scholar] [CrossRef]
- Wei, S.; Kozono, S.; Kats, L.; Nechama, M.; Li, W.; Guarnerio, J.; Luo, M.; You, M.-H.; Yao, Y.; Kondo, A.; et al. Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer. Nat. Med. 2015, 21, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Apperly, F.L. The relation of solar radiation to cancer mortality in North American. Cancer Res. 1941, 1, 191–195. [Google Scholar]
- Garland, C.F.; Garland, F.C. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int. J. Epidemiol. 1980, 9, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Comstock, G.W.; Garland, F.C.; Helsing, K.J.; Shaw, E.K.; Gorham, E.D. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 1989, 2, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Grant, W.B.; Giovannucci, E.L. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007, 103, 708–711. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Hanchette, C.L.; Schwartz, G.G. Geographic patterns of prostate cancer mortality: Evidence for a protective effect of ultraviolet radiation. Cancer 1992, 70, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Vitamin D status and cancer incidence and mortality. Adv. Exp. Med. Biol. 2008, 624, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Grant, W. A review of the evidence supporting the vitamin D-cancer hypothesis in 2017. Anticancer Res. 2018, 38, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, A.; Karishma; Choudhury, B.K.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S. Vitamin D and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef]
- Schwartz, G.G. Vitamin D, sunlight, and the epidemiology of prostate cancer. Anticancer Agents Med. Chem. 2013, 13, 45–57. [Google Scholar] [CrossRef]
- Yan, B.; Lu, M.-S.; Wang, L.; Mo, X.-F.; Luo, W.-P.; Du, Y.-F.; Zhang, C.-X. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar] [CrossRef]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Furtado, J.D.; Campos, H.; McCullough, M.L. Plasma carotenoids and breast cancer risk in the cancer prevention study ii nutrition cohort. Cancer Causes Control 2015, 26, 1233–1244. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Hankinson, S.E.; Campos, H.; Spiegelman, D.; Zhang, S.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am. J. Epidemiol. 2005, 161, 153–160. [Google Scholar] [CrossRef]
- Rosa, C.; Franca, C.; Lanes Vieira, S.; Carvalho, A.; Penna, A.; Nogueira, C.; Lessa, S.; Ramalho, A. Reduction of serum concentrations and synergy between retinol, β-carotene, and zinc according to cancer staging and different treatment modalities prior to radiation therapy in women with breast cancer. Nutrients. 2019, 11, 2953. [Google Scholar] [CrossRef]
- Toniolo, P.; Van Kappel, A.L.; Akhmedkhanov, A.; Ferrari, P.; Kato, I.; Shore, R.E.; Riboli, E. Serum carotenoids and breast cancer. Am. J. Epidemiol. 2001, 153, 1142–1147. [Google Scholar] [CrossRef]
- Peng, C.; Gao, C.; Lu, D.; Rosner, B.A.; Zeleznik, O.; Hankinson, S.E.; Kraft, P.; Eliassen, A.H.; Tamimi, R.M. Circulating carotenoids and breast cancer among high-risk individuals. Am. J. Clin. Nutr. 2021, 113, 525–533. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Natarajan, L.; Thomson, C.A.; Bardwell, W.A.; Newman, V.A.; Hollenbach, K.A.; Jones, L.; Caan, B.J.; Pierce, J.P. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J. Clin. Oncol. 2005, 23, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.-F.; Tsai, L.Y.; Huang, C.J.; Huang, Y.S.; Hsieh, J.S.; Huang, T.J.; Chen, H.M.; Wang, J.Y. Serum vitamin a level in breast cancer patients. Kaohsiung J. Med. Sci. 1998, 14, 673–678. [Google Scholar] [PubMed]
- Kim, J.A.; Choi, R.; Won, H.; Kim, S.; Choi, H.J.; Ryu, J.M.; Lee, S.K.; Yu, J.; Kim, S.W.; Lee, J.E.; et al. Serum vitamin levels and their relationships with other biomarkers in Korean breast cancer patients. Nutrients 2020, 12, 2831. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ahn, S.H.; Lee-Kim, Y.C. Relationship of serum α-tocopherol, carotenoids and retinol with the risk of breast cancer. Nutr. Res. 2001, 21, 797–809. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Zhang, L.; Li, Y.; Liu, B.; Kang, H.; Fan, Z.; Tian, Y.; Liu, S.; Li, T. Metabolomics research on potential role for 9-cis-retinoic acid in breast cancer progression. Cancer Sci. 2018, 109, 2315–2326. [Google Scholar] [CrossRef]
- Matos, A.; Nogueira, C.; Franca, C.; Carvalho, A.; Lannes Vieira, S.; Penna, A.; Ramalho, A. The relationship between serum vitamin A and breast cancer staging before and after radiotherapy. Nutr. Hosp. 2014, 29, 136–139. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Hendrickson, S.J.; Brinton, L.A.; Buring, J.E.; Campos, H.; Dai, Q.; Dorgan, J.F.; Franke, A.A.; Gao, Y.T.; Goodman, M.T.; et al. Circulating carotenoids and risk of breast cancer: Pooled analysis of eight prospective studies. J. Natl. Cancer Inst. 2012, 104, 1905–1916. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Zhang, S.M.; Norkus, E.P.; Gaziano, J.M. Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Dorjgochoo, T.; Gao, Y.-T.; Chow, W.-H.; Shu, X.-O.; Li, H.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Franke, A.A.; et al. Plasma carotenoids, tocopherols, retinol and breast cancer risk: Results from the Shanghai women health study (swhs). Breast Cancer Res. Treat. 2009, 117, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Maillard, V.; Kuriki, K.; Lefebvre, B.; Boutron-Ruault, M.C.; Lenoir, G.M.; Joulin, V.; Clavel-Chapelon, F.; Chajès, V. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the e3n-epic study. Int. J. Cancer 2010, 127, 1188–1196. [Google Scholar] [CrossRef]
- Epplein, M.; Shvetsov, Y.B.; Wilkens, L.R.; Franke, A.A.; Cooney, R.V.; Le Marchand, L.; Henderson, B.E.; Kolonel, L.N.; Goodman, M.T. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the multiethnic cohort study: A nested case-control study. Breast Cancer Res. 2009, 11, R49. [Google Scholar] [CrossRef]
- Han, X.; Zhao, R.; Wang, Y.; Ma, H.; Yu, M.; Chen, X.; Zhang, D.; Ma, S.; Liu, B.; Cai, H. Dietary vitamin A intake and circulating vitamin A concentrations and the risk of three common cancers in women: A meta-analysis. Oxid. Med. Cell Longev. 2022, 2022, 7686405. [Google Scholar] [CrossRef]
- Hu, F.; Wu, Z.; Li, G.; Teng, C.; Liu, Y.; Wang, F.; Zhao, Y.; Pang, D. The plasma level of retinol, vitamins A, C and α-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. J. Cancer Res. Clin. Oncol. 2015, 141, 601–614. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and breast cancer survival: A systematic review and meta-analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Q.; Lu, X.; Li, W. Post-Diagnosis use of antioxidant vitamin supplements and breast cancer prognosis: A systematic review and meta-analysis. Clin. Breast Can. 2021, 21, 477–485. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, J.H.; Lee, S.Y. An updated comprehensive review on vitamin A and carotenoids in breast cancer: Mechanisms, genetics, assessment, current evidence, and future clinical implications. Nutrients 2021, 13, 3162. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Biological evidence to define a vitamin A deficiency cutoff using total liver vitamin A reserves. Exp. Biol. Med. 2021, 246, 1045–1053. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Vitamin A: Is it a risk factor for osteoporosis and bone fracture? Nutr. Rev. 2007, 65, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Imoesi, P.I.; Olarte-Sánchez, C.M.; Croce, L.; Blaner, W.S.; Morgan, P.J.; Heisler, L.; McCaffery, P. Control by the brain of vitamin A homeostasis. iScience 2023, 26, 107373. [Google Scholar] [CrossRef] [PubMed]
- Glasziou, P.P.; Mackerras, D.E. Vitamin A supplementation in infectious diseases: A meta-analysis. BMJ 1993, 306, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef]
- Este Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and risk of breast cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Yao, S.; Sheng, H.Y.; Kwan, M.L.; Zhu, Q.Q.; Roh, J.; D’addario, L.; Ergas, I.J.; Schultz, E.; Cheng, T.Y.; Davis, W.; et al. Clinically sufficient vitamin D levels at breast cancer diagnosis and survival outcomes in a prospective cohort of 3,995 patients after a median follow-up of 10 years. J. Clin. Oncol. 2021, 39, 105. [Google Scholar] [CrossRef]
- Rosso, C.; Fera, N.; Murugan, N.J.; Voutsadakis, I.A. Vitamin D levels in newly diagnosed breast cancer patients according to tumor sub-types. J. Diet. Suppl. 2022, 20, 926–938. [Google Scholar] [CrossRef]
- Mackey, J.D.; Young, P.; Zimmerer, R.; Miles, B. Vitamin D deficiency as a risk factor for breast cancer development. J. Clin. Oncol. 2023, 41, 10559. [Google Scholar] [CrossRef]
- Ottaiano, A.; Facchini, B.A.; Iacovino, M.; Santorsola, M.; Facchini, S.; Di Mauro, G.; Toscano, E.; Montopoli, M.; Di Mauro, A.; Quagliariello, V.; et al. Impact of vitamin D levels on progression-free survival and response to neoadjuvant chemotherapy in breast cancer patients: A systematic review and meta-analysis. Cancers 2024, 16, 4206. [Google Scholar] [CrossRef]
- Shu, C.; Yang, Q.; Huang, J.; Xie, X.; Li, H.; Wu, H.; Wang, X.; Chen, X.; Xie, Y.; Zhou, Y.; et al. Pretreatment plasma vitamin D and response to neoadjuvant chemotherapy in breast cancer: Evidence from pooled analysis of cohort studies. Int. J. Surg. 2024, 110, 8126–8135. [Google Scholar] [CrossRef]
- Zhao, M.; Ye, M.; Zhao, Y. Causal link between dietary antioxidant vitamins intake, oxidative stress injury biomarkers and colorectal cancer: A Mendelian randomization study. Medicine 2025, 104, e41531. [Google Scholar] [CrossRef]
- Heine-Bröring, R.C.; Winkels, R.M.; Renkema, J.M.; Kragt, L.; van Orten-Luiten, A.C.; Tigchelaar, E.F.; Chan, D.S.; Norat, T.; Kampman, E. Dietary supplement use and colorectal cancer risk: A systematic review and meta-analyses of prospective cohort studies. Int. J. Cancer 2015, 136, 2388–2401. [Google Scholar] [CrossRef]
- Dou, R.; Ng, K.; Giovannucci, E.; Manson, J.E.; Qian, R.; Ogino, S. Vitamin D and colorectal cancer: Molecular, epidemiological, and clinical evidence. Br. J. Nutr. 2016, 115, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Meyerhardt, J.A.; Wu, K.; Feskanich, D.; Hollis, B.W. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J. Clin. Oncol. 2008, 26, 2984–2991. [Google Scholar] [CrossRef] [PubMed]
- Morales-Oyarvide, V.; Meyerhardt, J.A.; Ng, K. Vitamin D and physical activity in patients with colorectal cancer: Epidemiological Evidence and Therapeutic Implications. Cancer J. 2016, 22, 223–231. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Bours, M.J.L.; de Wilt, J.H.W.; Aquarius, M.; Breukink, S.O.; Hansson, B.; Keulen, E.T.P.; Kok, D.E.; van den Ouweland, J.; van Roekel, E.H.; et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J. Steroid Biochem. Mol. Biol. 2020, 199, 105577. [Google Scholar] [CrossRef]
- Klampfer, L. Vitamin D and colon cancer. World J. Gastrointest. Oncol. 2014, 15, 430–437. [Google Scholar] [CrossRef]
- Wu, G.; Xue, M.; Zhao, Y.; Han, Y.; Zhang, S.; Zhang, J.; Li, C.; Xu, J. Low circulating 25-hydroxyvitamin D level is associated with increased colorectal cancer mortality: A systematic review and dose-response meta-analysis. Biosci. Rep. 2020, 40, BSR20201008. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomás, N.; Fernández de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvadó, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D intake and the risk of colorectal cancer: An updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lei, S.; Wu, Y.; Weng, M.; Zhou, Y.; Xu, J.; Xia, D.; Xu, E.; Lai, M.; Zhang, H. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2525–2538. [Google Scholar] [CrossRef]
- Ottaiano, A.; Iacovino, M.L.; Santorsola, M.; Facchini, S.; Iervolino, D.; Perri, F.; Nasti, G.; Quagliariello, V.; Maurea, N.; Ronchi, A.; et al. Circulating vitamin D level before initiating chemotherapy impacts on the time-to-outcome in metastatic colorectal cancer patients: Systematic review and meta-analysis. J. Transl. Med. 2024, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D intake, serum 25-hydroxyvitamin-D (25(OH)D) levels, and cancer risk: A comprehensive meta-meta-analysis including meta-analyses of randomized controlled trials and observational epidemiological studies. Nutrients 2023, 15, 2722. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between vitamin D supplementation and cancer mortality: A systematic review and meta-analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 8428–8442. [Google Scholar] [CrossRef]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomized controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef]
- Beer, T.M.; Myrthue, A. Calcitriol in cancer treatment: From the lab to the clinic. Mol. Cancer Ther. 2004, 3, 373–381. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Shane, E.; Cremers, S.; McMahon, D.J.; Irani, D.; Hershman, D.L. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J. Clin. Oncol. 2008, 27, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Kimler, B.F.; Sharma, P.; Reddy, P.; Baxa, S.; Klemp, J.R.; Fabian, C.J. Vitamin D levels during and after high-dose vitamin D supplementation in women with early-stage breast cancer. J. Clin. Oncol. 2009, 27 (Suppl. S15), e20561. [Google Scholar] [CrossRef]
- Peppone, L.J.; Huston, A.J.; Reid, M.E.; Rosier, R.N.; Zakharia, Y.; Trump, D.L.; Mustian, K.M.; Janelsins, M.C.; Purnell, J.Q.; Morrow, G.R. The effect of various vitamin D supplementation regimens in breast cancer patients. Breast Cancer Res. Treat. 2011, 127, 171–177. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥ 60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Torres, A.; Cameselle, C.; Otero, P.; Simal-Gandara, J. The impact of vitamin D and its dietary supplementation in breast cancer prevention: An integrative review. Nutrients 2024, 16, 573. [Google Scholar] [CrossRef]
- Alberts, D.; Ranger-Moore, J.; Einspahr, J.; Saboda, K.; Bozzo, P.; Liu, Y.; Xu, X.C.; Lotan, R.; Warneke, J.; Salasche, S.; et al. Safety and efficacy of dose-intensive oral vitamin A in subjects with sun-damaged skin. Clin. Cancer Res. 2004, 10, 1875–1880. [Google Scholar] [CrossRef]
- Moon, T.E.; Levine, N.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: A randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 949–956. [Google Scholar] [PubMed]
- Cartmel, B.; Moon, T.E.; Levine, N. Effects of long-term intake of retinol on selected clinical and laboratory indexes. Am. J. Clin. Nutr. 1999, 69, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.E.; Alberts, D.S.; Earnest, D.L.; Meyskens, F.L. Phase I trial of retinol in cancer patients. J. Clin. Oncol. 1983, 1, 394–399. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 to 50,000 international units a day in long-term hospitalized patients: Insights from a seven-year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-kB and wnt/β-catenin signaling pathways via inactivation of ERK/MAPK and PI3k/AKT to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Khoshnan, A.; Tindell, C.; Laux, I.; Bae, D.; Bennett, B.; Nel, A.E. The NF-kappa b cascade is important in Bcl-xl expression and for the anti-apoptotic effects of the cd28 receptor in primary human cd4+ lymphocytes. J. Immunol. 2000, 165, 1743–1754. [Google Scholar] [CrossRef]
- Muto, A.; Kizaki, M.; Yamato, K.; Kawai, Y.; Kamata-Matsushita, M.; Ueno, H.; Ohguchi, M.; Nishihara, T.; Koeffler, H.P.; Ikeda, Y. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1). Blood 1999, 93, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, X.; Chakrabarty, S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int. J. Cancer 2010, 126, 631–639. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Retinoic acids in the treatment of most lethal solid cancers. J. Clin. Med. 2020, 9, 360. [Google Scholar] [CrossRef]
- Hu, X.T.; Zuckerman, K.S. Role of cell cycle regulatory molecules in retinoic acid- and vitamin D3-induced differentiation of acute myeloid leukemia cells. Cell Prolif. 2014, 47, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Y.; Guan, X.; Shu, X.; Li, C. Advanced progress on the relationship between RA and its receptors and malignant tumors. Crit. Rev. Oncol. Hematol. 2014, 91, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Giraud, J.; Staedel, C.; Chambonnier, L.; Dubus, P.; Chevret, E.; Boeuf, H.; Gauthereau, X.; Rousseau, B.; Fevre, M.; et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene 2016, 35, 5619–5628. [Google Scholar] [CrossRef] [PubMed]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, cancer treatment and prevention: The new role of cellular retinol binding proteins. Biomed. Res. Int. 2015, 2015, 624627. [Google Scholar] [CrossRef]
- Takahashi, N.; Saito, D.; Hasegawa, S.; Yamasaki, M.; Imai, M. Vitamin A in health care: Suppression of growth and induction of differentiation in cancer cells by vitamin A and its derivatives and their mechanisms of action. Pharmacol. Ther. 2022, 230, 107942. [Google Scholar] [CrossRef]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Abdel-Samad, R.; Aouad, P.; Darwiche, N. Natural and synthetic retinoids in preclinical colorectal cancer models. Anticancer. Drugs 2019, 30, e0802. [Google Scholar] [CrossRef]
- Tong, W.M.; Hofer, H.; Ellinger, A. Mechanism of antimitogenic action of vitamin D in human colon carcinoma cells: Relevance for suppression of epidermal growth factor-stimulated cell growth. Oncolog. Res. 1999, 11, 77–84. [Google Scholar] [PubMed]
- Diaz, G.D.; Paraskeva, C.; Thomas, M.G. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: Possible implications for prevention and therapy. Cancer Res. 2000, 60, 2304–2312. [Google Scholar] [PubMed]
- Talib, W.H.; Ahmed Jum’AH, D.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front. Nutr. 2024, 10, 1281879. [Google Scholar] [CrossRef]
- Veeresh, P.K.M.; Basavaraju, C.G.; Dallavalasa, S.; Anantharaju, P.G.; Natraj, S.M.; Sukocheva, O.A.; Madhunapantula, S.V. Vitamin D3 inhibits the viability of breast cancer cells in vitro and Ehrlich ascites carcinomas in mice by promoting apoptosis and cell cycle arrest and by impeding tumor angiogenesis. Cancers 2023, 15, 4833. [Google Scholar] [CrossRef]
- Refaat, B.; El-Shemi, A.G.; Kensara, O.A.; Mohamed, A.M.; Idris, S.; Ahmad, J.; Khojah, A. Vitamin D3 enhances the tumoricidal effects of 5-Fluorouracil through multi-pathway mechanisms in azoxymethane rat model of colon cancer. J. Exp. Clin. Cancer Res. 2015, 34, 71–75. [Google Scholar] [CrossRef]
- Najafzadeh, N.; Mazani, M.; Abbasi, A.; Farassati, F.; Amani, M. Low-dose all-trans retinoic acid enhances cytotoxicity of cisplatin and 5-fluorouracil on CD44(+) cancer stem cells. Biomed. Pharmacother. 2015, 74, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.S.; Li, H.X.; Li, C.; Zhang, S.; Chen, J.; Wang, Q.L.; Gao, J. Synergistic antitumor activity of vitamin D3 combined with metformin in human breast carcinoma MDA-MB-231 cells involves m-TOR related signaling pathways. Pharmazie 2015, 70, 117–122. [Google Scholar] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lim, J.Y.S.; Eu, J.Q.; Chan, A.K.M.H.; Goh, B.C.; Wang, L.; Wong, A.L. Reactive oxygen species modulation in the current landscape of anticancer therapies. Antioxid. Redox Signal 2024, 41, 322–341. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Verfaillie, T.; Salazar, M.; Velasco, G.; Agostinis, P. Linking ER stress to autophagy: Potential implications for cancer therapy. Int. J. Cell Biol. 2010, 2010, 930509. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and macro-autophagy in inflammation and infection. Discov. Med. 2011, 11, 325–335. [Google Scholar] [PubMed]
- Miro Estruch, I.; de Haan, L.H.J.; Melchers, D.; Houtman, R.; Louisse, J.; Groten, J.P.; Rietjens, I.M. The effects of all-trans retinoic acid on estrogen receptor signaling in the estrogen sensitive MCF/BUS subline. J. Recept. Signal Transduct. Res. 2018, 38, 112–121. [Google Scholar] [CrossRef]
- Stoica, A.; Saceda, M.; Fakhro, A.; Solomon, H.B.; Fenster, B.D.; Martin, M. Regulation of estrogen receptor-alpha gene expression by 1,25-dihydroxyvitamin D in MCF-7 cells. J. Cell Biochem. 1999, 75, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Aruna, V.; Krishnan, S.S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-selective regulation of aromatase expression by calcitriol: Implications for breast cancer therapy. Endocrinology 2010, 151, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Vitamin D and the risk for cancer: A molecular analysis. Biochem. Pharmacol. 2022, 196, 114735. [Google Scholar] [CrossRef] [PubMed]
- Coyle, K.M.; Maxwell, S.; Thomas, M.L.; Marcato, P. Profiling of the transcriptional response to all-trans retinoic acid in breast cancer cells reveals RARE-independent mechanisms of gene expression. Sci. Rep. 2017, 7, 16684. [Google Scholar] [CrossRef]
- Carrier, M.; Joint, M.; Lutzing, R.; Page, A.; Rochette-Egly, C. Phosphoproteome and Transcriptome of RA-Responsive and RA-Resistant Breast Cancer Cell Lines. PLoS ONE 2016, 11, e0157290. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; Grimes, G.; Blackmur, J.P.; Timofeeva, M.; Walker, M.; Ooi, L.Y.; Svinti, V.; Donnelly, K.; Din, F.V.N.; Farrington, S.M.; et al. Oral vitamin D supplementation induces transcriptomic changes in rectal mucosa that are linked to anti-tumour effects. BMC Med. 2021, 19, 174. [Google Scholar] [CrossRef]
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.; Amin, A.; Aquilano, K. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2011, 35, S276–S304. [Google Scholar] [CrossRef]
- Block, K.I.; Block, P.B.; Gyllenhaal, C. Integrative therapies in cancer: Modulating a broad spectrum of targets for cancer management. Integr. Cancer Ther. 2015, 14, 113–118. [Google Scholar] [CrossRef]
- Crawford, S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: A new therapeutic approach to disease progression and recurrence. Ther. Adv. Med. Oncol. 2014, 6, 52068. [Google Scholar] [CrossRef]
- Lu, H.Q.; Zheng, J. Synergistic inhibitory effect of all-trans retinoic acid and 1,25-dihydroxy vitamin D3 on growth of human hepatoma cell line HepG2. Ai Zheng 2006, 25, 1470–1476. [Google Scholar]
- Sha, J.; Pan, J.; Ping, P.; Xuan, H.; Li, D.; Bo, J.; Liu, D.; Huang, Y. Synergistic effect and mechanism of vitamin A and vitamin D on inducing apoptosis of prostate cancer cells. Mol. Biol. Rep. 2013, 40, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Lawal, T.O.; Patel, S.; Raut, N.; Wicks, S.M. Combination of vitamins A, D2 and D3 have synergistic effects in gastric and colon cancer cells. Funct. Foods Health Dis. 2019, 9, 749–771. [Google Scholar] [CrossRef]
- Mahady, G.B.; Kanabar, P.; Los, N.; Lawal, T.; Patel, S.; Maienshein-Cline, M.; Arbieva, Z. Transcriptomic analysis reveals that combinations of vitamins A, D2 and D3 have synergistic effects in HCT-116 colon cancer cells by altering the expression of genes involved in multiple canonical pathways including apoptosis, regulation of the epithelial mesenchymal transition and immunity. Funct. Foods Health Dis. 2021, 11, 154–178. [Google Scholar] [CrossRef]
- Arbeiva, Z.; Cabada-Aguirre, P.; Garay Beunrostro, K.D.; Kanabar, P.N.; Lawal, T.O.; Lopez, A.M.; Los, N.S.; Maienschein-Cline, M.; Ostos Mendoza, K.C.; Patel, S.M.; et al. Combination of vitamins A, D2 and D3 reduce tumor-load and alter the expression of miRNAs that regulate genes involved with apoptosis, tumor suppression, and the epithelial mesenchymal transition in HCT-116 colon cancer cells. Funct. Foods Health Dis. 2022, 12, 216–241. [Google Scholar] [CrossRef]
- Kanabar, P.; Los, N.; Arbieva, Z.; Cline, M.; Patel, S.; Lawal, T.O.; Mahady, G.B. Combinations of vitamin A and D are synergistic in breast cancer cells and alter gene expression in the endoplasmic reticulum stress, unfolded protein and estrogen signaling canonical pathways. Funct. Foods Health Dis. 2023, 13, 135–155. [Google Scholar] [CrossRef]
- Raut, N.A.; Lawal, T.O.; Adeniyi, B.A.; Kanabar, P.N.; Maienschein-Cline, M.; Los, N.S.; Arbieva, Z.; Mahady, G.B. Vitamin A and D3 combinations reduce breast cancer tumor load in a postmenopausal MCF-7 xenograft mouse model in a dose- and time- dependent manner. Funct. Foods Health Dis. 2024, 14, 984–1003. [Google Scholar] [CrossRef]
- Jia, Z.; Ragoonanan, D.; Mahadeo, K.M.; Gill, J.; Gorlick, R.; Shpal, E.; Li, S. IL12 immune therapy clinical trial review: Novel strategies for avoiding CRS-associated cytokines. Front. Immunol. 2022, 13, 952231. [Google Scholar] [CrossRef]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Schetter, A.J.; Okayama, H.; Harris, C.C. The role of microRNAs in colorectal cancer. Cancer J. 2012, 18, 244–252. [Google Scholar] [CrossRef]
- Padi, S.K.; Zhang, Q.; Rustum, Y.M.; Morrison, C.; Guo, B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology 2013, 145, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zou, H.; Mo, J.; Jin, C.; Jiang, H.; Yu, C.; Jiang, Z.; Yang, Y.; He, B.; Wang, K. Micro1278 leads to tumor growth arrest, enhanced sensitivity to Oxaliplatin and Vitamin D and inhibits metastasis via KIF5B, CYP24A1, and BTG2, respectively. Front. Oncol. 2021, 11, 637878. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Song, Y.; Zhang, C.; Gao, P.; Huang, B.; Yang, J. The protective role of all-trans-retinoic acid (ATRA) against colorectal cancer development is achieved via increasing miR-3666 expression and decreasing E2F7 expression. Biomed. Pharmacother. 2018, 104, 94–101. [Google Scholar] [CrossRef]
- Rehó, B.; Fadel, L.; Brazda, P.; Benziane, A.; Hegedüs, É.; Sen, P.; Gadella, T.W.J.; Tóth, K.; Nagy, L.; Vámosi, G. Agonist-controlled competition of RAR and VDR nuclear receptors for heterodimerization with RXR is manifested in their DNA binding. J. Biol. Chem. 2023, 299, 102896. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; White, J.H. The pleiotropic actions of vitamin D. Bioessays 2004, 26, 21–28. [Google Scholar] [CrossRef]
- Uchida, H.; Hasegawa, Y.; Takahashi, H.; Makishima, M. 1α-dihydroxy-vitamin D3 and retinoic acid increase nuclear vitamin D receptor expression in monocytic THP-1 cells. Anticancer. Res. 2016, 36, 6297–6301. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take center stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Tomita, A.; Kiyoi, H.; Naoe, T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int. J. Hematol. 2013, 97, 717–725. [Google Scholar] [CrossRef]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Müller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Freemantle, S.J.; Spinella, M.J.; Dmitrovsky, E. Retinoids in cancer therapy and chemoprevention: Promise meets resistance. Oncogene 2003, 22, 7305–7315. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
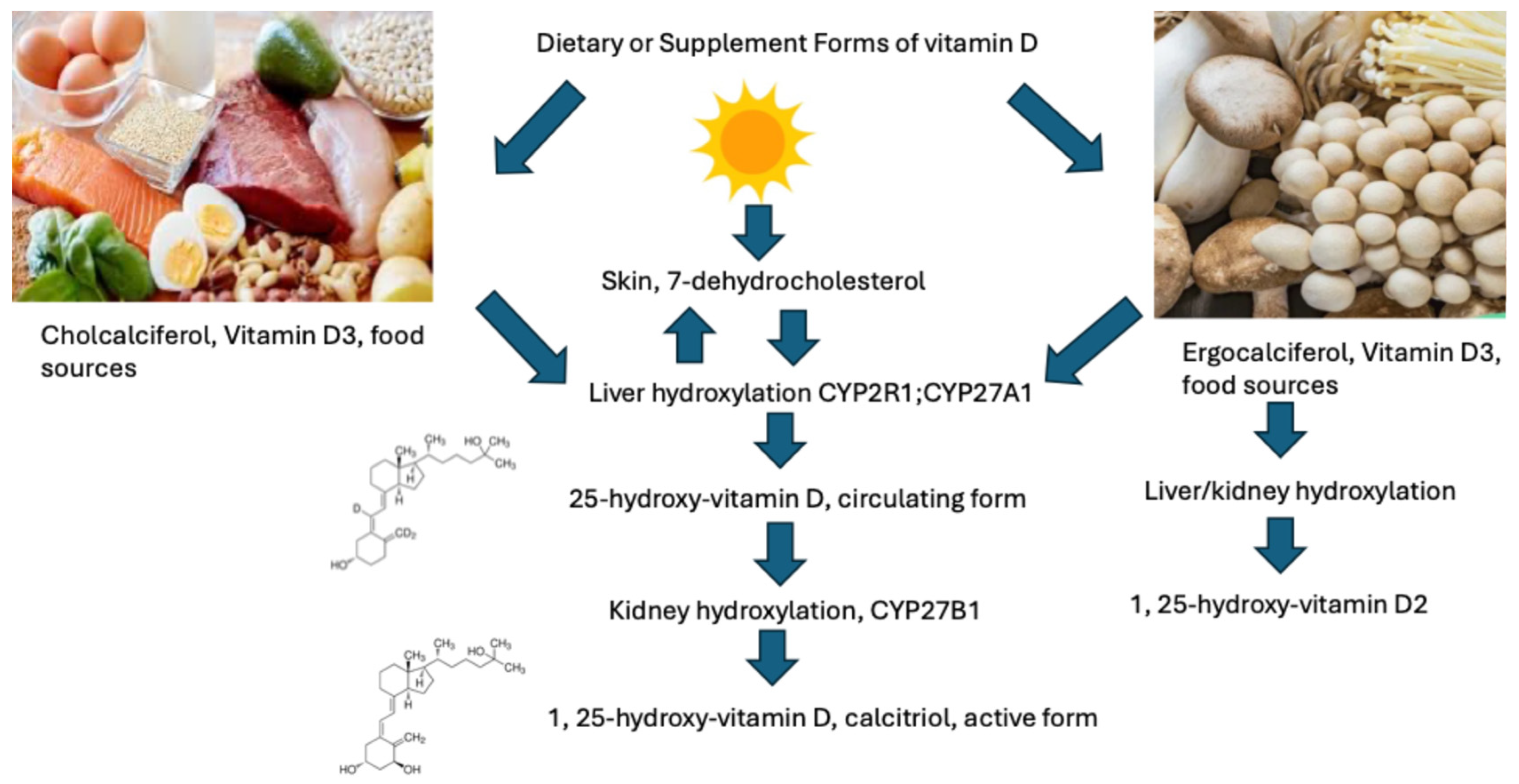

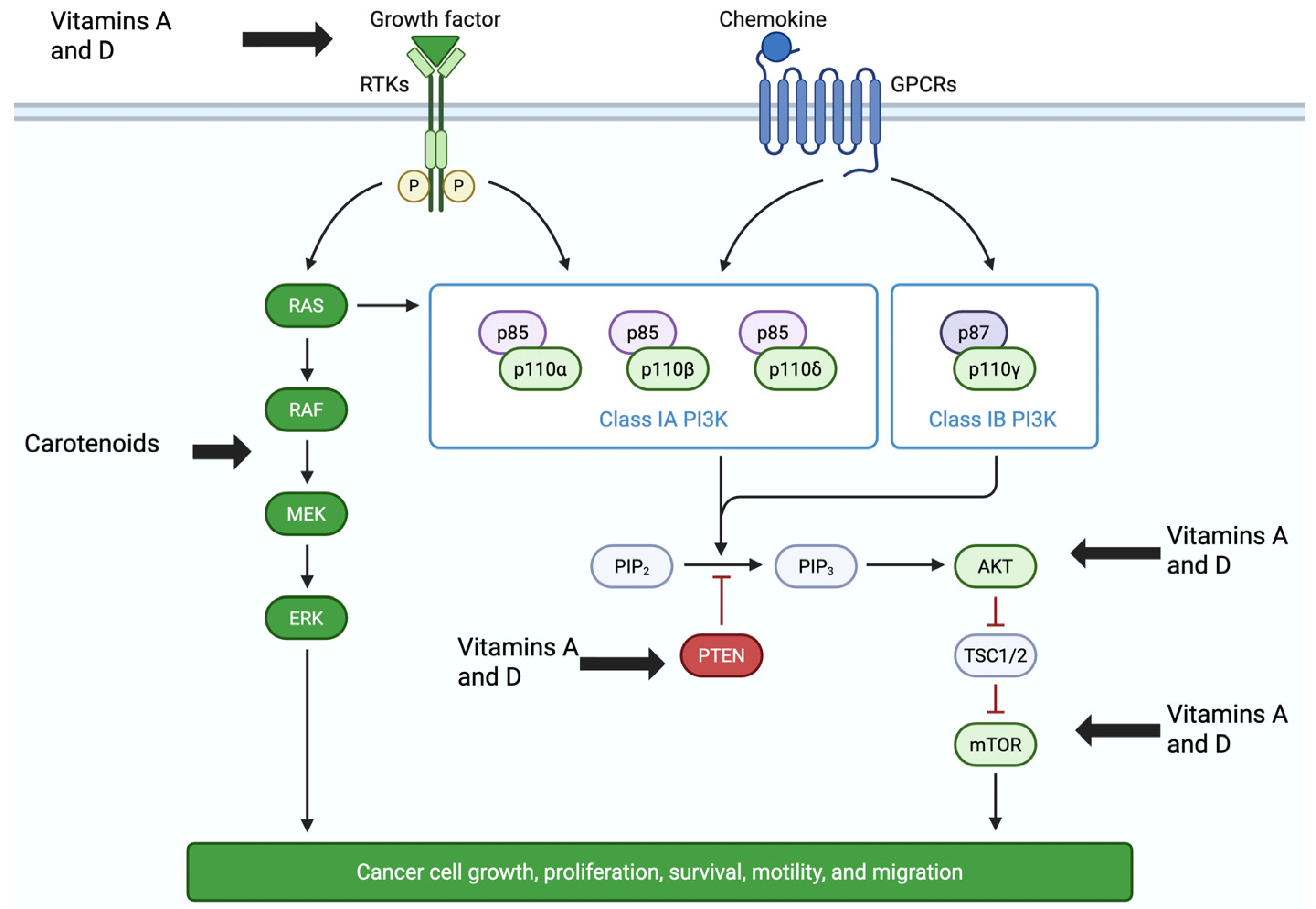
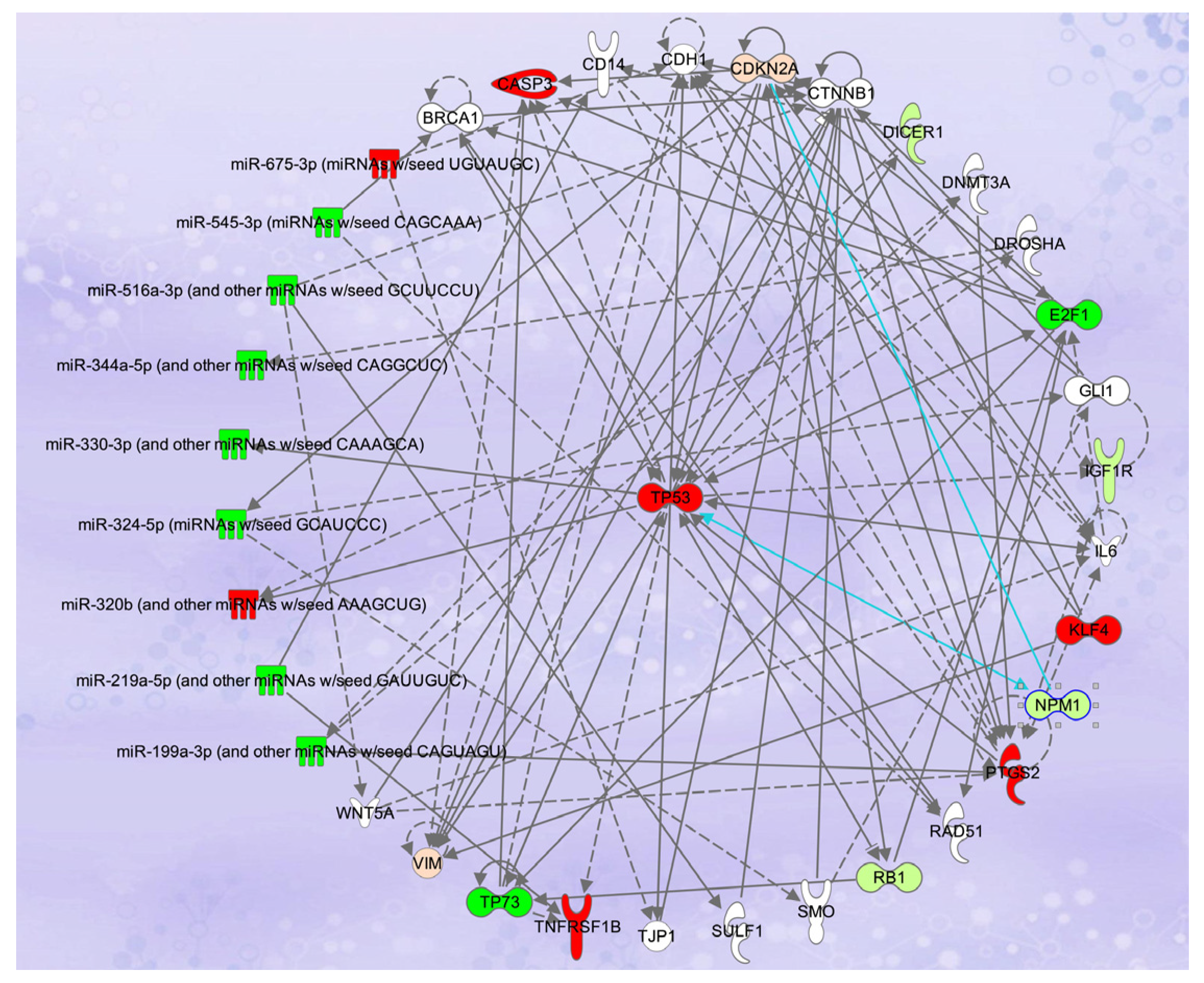
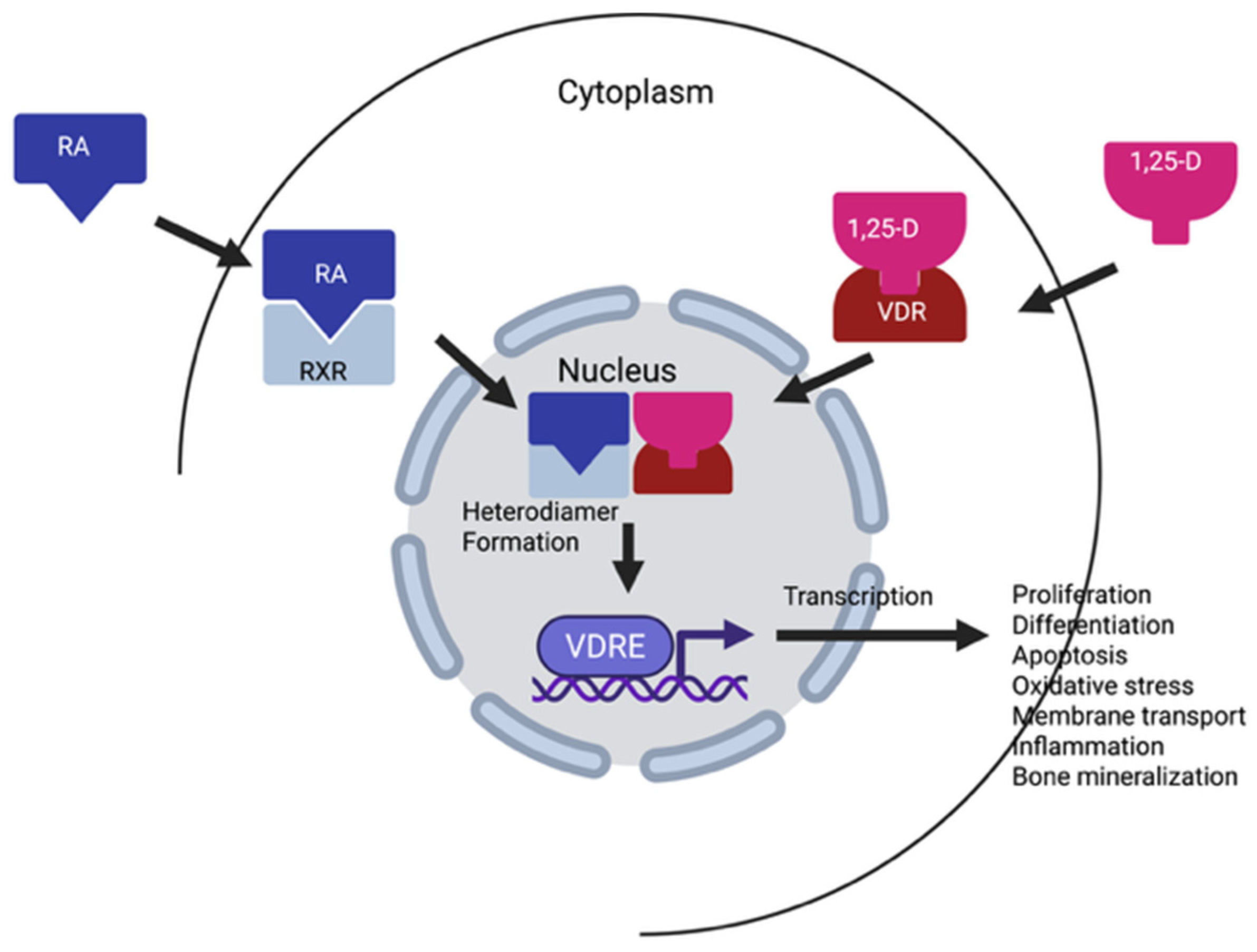
| Reference | Type of Studies | Subjects | Size Effect * | Limitations | Clinical Outcomes |
|---|---|---|---|---|---|
| Eliassen et al. [40] | Cohort/case–control | 3055 cases 3055 controls | α-carotene RR = 0.87 β-carotene RR = 0.83 Total carotenoids RR = 0.81 | Confounded by other dietary compounds. Only one blood sample per participant. | Higher circulating carotenoids reduced BC risk. |
| Eliassen et al. [41] | Nested case–control | 2188 cases 2188 controls Pre- and post- menopausal | α-carotene RR = 0.54 β-carotene RR = 0.32 Total carotenoids RR = 0.48 | Confounded by other dietary compounds. Carotenoid measurement inconsistencies. | Higher circulating carotenoids reduced BC risk. |
| Sesso et al. [42] | Prospective cohort/nested case–control | 508 BC cases 508 controls | Lycopene RR = 0.95 | Single baseline measurements. Did not assess the long-term stability of the lycopene samples. Higher dietary lycopene was associated with lower BMI, smoking rates, reduced familial BC history, increased exercise, and fruits and vegetable intake, thus confounded. | Increasing lycopene doses did not reduce BC risk |
| Han et al. [46] | Cohort/case–control | 25,363 BC cases, 42,281 controls | High dietary Vitamin A OR = 0.83, Dietary plus supplements OR = 0.81 High circulating vitamin A OR = 1.0 | Different ethnicities, Publication biases, language issues, and vitamin A measurement differences. | Higher dietary intake and supplementation of vitamin A lowered the incidence of BC in North American and Asian women, but not women from Oceania or Europe |
| Hu et al. [47] | Nested case/case–control | NS Pre- and post-menopausal | Highest plasma retinol versus lowest OR = 0.81 Plasma retinol OR = NS | Possible measurement issues, unmeasured or residual confounders, moderate to high heterogenicity in analysis. Lack of original data. | No significant association between plasma retinol and vitamin A levels and BC was observed. |
| He et al. [48] | Epidemiological studies | 19,450 Pre-diagnosis 2990 BC cases, 502 deaths; post-diagnosis 16, 460 BC cases, 1823 deaths | Pre-diagnosis α-carotene HR = 0.9 β-carotene HR = 0.7 Post-diagnosis HR 0.92–1.17 | Misclassification of vitamin A intake. The food questionnaire used introduced measurement errors. Heterogenicity in pre-versus post-diagnosis intakes, variation in study methods, no adjustment for tumor stages, and few studies adjusted for BMI, physical activities, or healthy lifestyles. | Higher dietary ingestion of β-carotene increased breast cancer survival by 30%, but other vitamin A derivatives, including α-carotene, β-cryptoxanthin, lycopene, retinol, or lutein, had no effect. |
| Li et al. [49] | Cohort | 17,062 | HR = 0.92 | Multiple vitamins were used, including vitamin A; small sample sizes, heterogenicity in study designs; different countries and lifestyles; no staging of BC; descriptions of vitamins were lacking. | No significant difference in vitamin A or E use in breast cancer survival, and only vitamin C intake after breast cancer diagnosis was significantly associated with better overall survival |
| Kim et al. [50] | 150 Cohort/nested case–control studies; randomized clinical trials | NS | NS | Some of the studies included had poor design and protocols; variations in the vitamin A derivative used and doses; variations in the length of the trial study period, and the heterogeneity of breast cancer; the methodology for measuring serum levels of vitamin A was inconsistent. | Inverse relationship between concentrations of retinoids and carotenoids and the risk of BC and premalignant breast disease; Inverse relationship between incidence, recurrence, and survival of aggressive tumors |
| Reference | Type of Studies | Subjects | Size Effect | Limitations | Clinical Outcomes |
|---|---|---|---|---|---|
| Este Estébanez et al. [56] | Cohort (CO)/case–control (CC) | Not reported | CO RR = 0.85 CC RR = 0.65 For the relationship between vitamin D and breast cancer. for premenopausal women only RR = 0.67, no effect for menopausal women | Variability in the literature used. Different cutoffs for vitamin D levels in studies. No dose response. | Analysis showed a protective effect between higher serum levels of 25 (OH) D and BC in both cohort studies and case–control studies. But when menopausal status was included, the protective effect of vitamin D was only significant for premenopausal women if the analysis was restricted to nested case-control studies. No conclusions could be made for vitamin D intake or supplements. |
| Hossain et al. [57] | Case–control/nested case–control | 229,597 | Vitamin D deficiency RR = 1.91 Serum vitamin D levels vs. BC occurrence RR = 0.99 Vitamin D intake RR = 0.99 Vitamin D supplements RR = 0.97 | Studies used were restricted to three public databases; no cross-referencing was performed; low-powered studies used; evidence from observational studies included. | Vitamin D deficiency is directly related to BC occurrence. No significant effect found for vitamin D intake or 1,25-OH-D2. |
| Yao et al. [58] | Cohort | 3995 women with BC Stratified for 25-OH-D levels <20 ng/mL deficient; 20–30 ng/mL insufficient; ≥30 ng/mL sufficient | Survival Sufficient at diagnosis HR = 0.73; BC specific HR = 0.78; recurrence free survival HR = 0.79 | Different ethnicities; publication biases; and different measurement methods | African American women with BC had the lowest serum 25-OH-D levels, as well as the worst prognosis and poorer survival. The analysis showed a protective effect between higher serum levels of 25-OH-D and BC, versus lower levels of 25-OH-D. |
| Rosso et al. [59] | Cohort | 292 pre- and post-menopausal women with BC | RR = Not reported | Single-center study; variations in BMI; co-morbidities were not included in analysis; lack of healthy controls; vitamin D levels only measured at time of diagnosis. | Approximately 65% of newly diagnosed women with BC were vitamin D deficient, including 56% of younger women. Vitamin D deficiency in newly diagnosed patients is associated with higher tumor grade and advanced stage BC. |
| Mackey et al. [60] | Cohort, women over 40 yrs of age | Stratified for vitamin D levels <20 ng/mL 73,659 ≥30 ng/mL 73,659 | <20 ng/mL RR = 1.45 postmenopausal RR = 1.18 pre-menopausal | Possible measurement issues, unmeasured or residual confounders, moderate to high heterogenicity in analysis. | Low vitamin D levels (≤20 ng/mL) in postmenopausal women were associated with a significant 45% increase in the risk of developing BC. Pre-menopausal women with deficient vitamin D levels had a significant 18% increased risk. |
| Ottaiano et al. [61] | Cohort | 722 for NACT response; 1033 for PFS; Stratified for low/deficient vitamin D levels versus high/sufficient vitamin D levels | NACT high levels OR = 0.78 High vs. low for disease progression HR = 0.65 | Variation in study heterogenicity and methods; no adjustment for tumor stages; limited vitamin D assessments. | Adequate baseline 25-OH-D levels are associated with a significant 22% reduction in the risk of a non-response to Neoadjuvant Chemotherapy and a significant 35% reduction in the risk of disease progression. |
| Reference | Type of Studies | Subjects | Size Effect | Study Limitations | Clinical Outcomes |
|---|---|---|---|---|---|
| Hernández-Alonso et al. [72] | Cohort (CC)/case–control (CO) | 140,112 | OR = 0.61 in CC OR = 0.8 in CO | Variability in the international literature used, and no lifestyle issues were addressed. Only two public databases were used. Different methods and cutoffs for vitamin D analyses and levels; No dose response. | A 39% risk reduction in CRC was found in the highest vs. the lowest levels of total 25(OH)D; a 20% reduced CRC risk was seen in prospective cohort studies. Results in women were significant, while results in men were non-significant |
| Huang et al. [75] | Case–control/nested case–control | >800,000 | Women RR = 0.63 Men RR = 0.89 Asian populations RR = 0.67 European and USA RR = 0.82 Vitamin D intake and CRC risk RR = 0.81 High vitamin D overall survival outcomes HR = 0.69 CRC survival RR = 0.64 Left-sided CRC RR = 0.6 | Studies used were restricted to three public databases; no cross-referencing. | High circulating 25-OH-D reduced CRC risk. Vitamin D and calcium have an additive effect on CRC incidence, transformation, and progression, especially for women and left-sided CRC patients |
| Ottaiano et al. [76] | Randomized controlled clinical trials (RCTs) | >1800 | Lower 25-OH-D Risk of death HR = 1.47 Risk of progression HR = 1.38 | Different analytical methods and cutoffs for vitamin D levels and vitamin D concentrations were expressed using different measurement units. Vitamin D level cutoff values (low vs. high) also varied. Chemotherapy regimens were not discussed, nor were prognostic factors such as the initial disease burden, treatment responses, and toxicity. | Low vitamin D levels increased the overall risk of disease progression and mortality in metastatic CRC patients |
| Keum et al. [77] | RCTs | 6537 | CRC incidence RR = 0.98 Mortality RR = 0.87 with daily dosing | Included RCTs were not designed to test the hypothesis that vitamin D influenced the risk of cancer incidence or mortality. Over sampling of Caucasians versus other ethnic groups. Lack of data on site-specific cancers. | A significant 13% decrease in cancer mortality and 7% reduction in total mortality over 3–10 years of follow-up, which was attributed to daily and not bolus dosing of vitamin D. |
| Arayici et al. [78] | Meta-analyses of RCTs, observational, and epidemiological studies | >1,000,000 | Higher 25-OH-D levels associated with reduced cancer risk OR = 0.93 Cancer mortality OR = 0.67 Vitamin D intake and CRC-specific risk OR = 0.89 Vitamin D intake and overall cancer mortality OR = 0.89 | A large portion of the studies included in the research (77.1%) were also observational studies. The primary endpoints of many studies included in the meta-analyses of RCTs were not focused on cancer incidence or cancer-related deaths. RCTs did not measure vitamin D levels at the conclusion of the studies. Differences in dosing protocols between studies. | Higher Vit-D intake and serum 25(OH)D levels were associated with lower cancer risk and cancer-related mortality. In subgroup analysis, Vit-D intake was associated with a significant decrease in CRC incidence. |
| Zhang et al. [79] | RCTs | 72,669 | RR = 0.96 | A limited number of databases were used for the study. Limited number of studies and sample sizes. | Vitamin D supplementation did not reduce overall cancer mortality. |
| Guo et al. [80] | RCTs | 60,876 Vitamin D intervention; 60,653 controls | RR = 0.98 Vit D supplementation mortality RR = 0.88 | Due to the wide variations in vitamin D treatments and dosing, the analysis could not effectively assess the equivalent daily dose of vitamin D supplementation. Total cancer incidence or mortality were secondary outcomes of the included RCTs. Not all RCTs reported associations between different doses of vitamin D intake and total cancer incidence and mortality. While RCTs reported country information, the longitude and latitude were not reported. This may be a confounder, as it is well known that people in lower latitudes tend to have higher 25-OH-D levels. | Pooled data from RCTs showed that vitamin D supplementation, with/without calcium, did not reduce total cancer incidence of breast or colon cancers. However, vitamin D supplementation significantly reduced total cancer mortality by 10%. Total cancer mortality was observed when 25-OH-D levels were <40 ng/mL and the baseline mean < 20 ng/mL. |
| Serum 25-OH-D ng/mL | Vitamin D Threshold and Treatment Advice |
|---|---|
| <30 | Deficient-treat |
| 30–50 | Sufficient for most healthy people. Treatment is advised for patients with fragility fracture, osteoporosis, or high fracture risk, patients taking antiresorptive drug therapy, symptoms of vitamin D deficiency, reduced exposure to sunlight, darker skin tones, religious/cultural dress, and for those patients taking parathyroid medications, antiepileptic drugs, oral glucocorticoids, or malabsorption syndrome. |
| >50 | Sufficient for the whole population, but levels need to be maintained by dietary supplementation or safe sunlight exposure |
| Reference | Vitamin D Dose | Serum Level | Clinical Outcomes |
|---|---|---|---|
| Giovannucci et al. [84] | Each 100 IU D3 raised the serum level of 25-OH-D by 1.75 nmol/L (0.7 ng/mL) | Increase of 25 nmol/L | 17% reduction in total cancer in men |
| 29% reduction in total cancer mortality in men | |||
| 43% reduction in GI cancers in men 45% reduction in GI cancer mortality | |||
| Crew et al. [85] | 400 IU/day D3 for one year | Increased serum levels of 25-OH-D by less than 3 ng/mL over one year | Administration of 400 IU/day D3 did not increase 25-OH-D levels into the sufficient range in women with breast cancer taking zoledronate |
| Khan et al. [86] | 50,000 IU/week D3 for 12 weeks, then 600–1000 IU daily for 3–6 months | Increased serum 25-OH-D to 66 ng/mL | High-dose vitamin D3 increased the 25-OH-D levels into the sufficient range in breast cancer patients taking letrozole. But maintenance doses of 600–1000 IU/day were not high enough, and the levels dropped by 7%/month |
| Peppone et al. [87] | Low dose: 1000 IU/day High dose: 50,000 IU/week D3 | Increased serum 25-OH-D by 24.3 ng/mL after 8–16 weeks | High-dose vitamin D3 increased the 25-OH-D levels into the sufficient range in women with BC, while the low dose did not |
| Reference | Vitamin A Dose | Serum Level | Clinical Outcome |
|---|---|---|---|
| Alberts et al. [90] | 50,000 to 75,000 IU/day for 12 months | Not tested | Reduced squamous cell carcinoma. High doses of vitamin A were safe in patients with severe sun damage. No toxicity observed |
| Moon et al. [91] | 25,000 IU/day | Not tested | Reduced squamous cell carcinoma but had no effect on basal cell carcinoma. No adverse effects reported. |
| Cartmel et al. [92] | 25,000 IU/day for 3.8 years | Not tested | High-dose vitamin A intake for an extended period of time was associated with an increased alkaline phosphatase level (7%); higher triacylglycerols (11%), higher cholesterol (3%), and a lower HDL (1%) in the retinol group than in the placebo group |
| Goodman et al. [93] | Up to 200,000 U/m2 (~350,000 IU presuming a 150-pound person) | Not tested | High dose vitamin A increased triglycerides, increased headaches and emotional instability, mild skin and mucous membrane dryness, and, in some cases of hepatomegaly associated with vitamin A toxicity |
| Life Stage | Recommended Amount |
|---|---|
| Birth–12 months | 10 mcg (400 IU) |
| Children 1–13 years | 15 mcg (600 IU) |
| Teens 14–18 years | 15 mcg (600 IU) |
| Adults 19–70 years | 15 mcg (600 IU) |
| Adults > 70 years | 20 mcg (800 IU) |
| Pregnant and breastfeeding women | 15 mcg (600 IU) |
| Cell Line Tested and Vitamin Treatment | Transcripts Altered |
|---|---|
| Breast, colon, vitamin D [124] | Oncogenes: MYC, JUN, AP-1, JUNB, JUND, FOS Tumor suppressor genes: CCNC (cyclin C), CCND1, CDKN1A, CDKN1B and G0S2 (G0/G1 switch 2) |
| MDA-MB-231 and MDA-MB-468, all-trans-retinoic acid (ATRA) [125] | ATRA-regulated genes: keratin 7 (KRT7), prostaglandin E synthase (PTGES), dehydrogenase reductase 3 (DHRS3), nuclear receptor interaction protein 1 (NRIP1), and cytochrome p450 family 26A1 (CYP26A1)52. Transcription factors: interferon regulatory factor 1 (IRF1) and myocyte enhancer factor 2 (MEF2) |
| MCF7 cell line, and a RA-resistant BT474 cell lines, ATRA [126] | STE20-like protein kinase 4 in MCF7 cells, Ppp4r3a protein phosphatase 4 regulatory subunit 3A (SMEK1) in BT474 cells; Transforming growth factor-1beta (TGF1β) in MCF-7 cells, bone morphogenetic protein and activin membrane-bound inhibitor (BAMBI) in BT474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawal, T.O.; Adeniyi, B.A.; Mahady, G.B. Vitamins A and D and Their Combinations for Breast and Colorectal Cancers: Analysis of the Clinical, Epidemiological, Preclinical and Transcriptomic Data. Pharmaceuticals 2025, 18, 1684. https://doi.org/10.3390/ph18111684
Lawal TO, Adeniyi BA, Mahady GB. Vitamins A and D and Their Combinations for Breast and Colorectal Cancers: Analysis of the Clinical, Epidemiological, Preclinical and Transcriptomic Data. Pharmaceuticals. 2025; 18(11):1684. https://doi.org/10.3390/ph18111684
Chicago/Turabian StyleLawal, Temitope O., Bolanle A. Adeniyi, and Gail B. Mahady. 2025. "Vitamins A and D and Their Combinations for Breast and Colorectal Cancers: Analysis of the Clinical, Epidemiological, Preclinical and Transcriptomic Data" Pharmaceuticals 18, no. 11: 1684. https://doi.org/10.3390/ph18111684
APA StyleLawal, T. O., Adeniyi, B. A., & Mahady, G. B. (2025). Vitamins A and D and Their Combinations for Breast and Colorectal Cancers: Analysis of the Clinical, Epidemiological, Preclinical and Transcriptomic Data. Pharmaceuticals, 18(11), 1684. https://doi.org/10.3390/ph18111684





