Therapeutic Impact of Zanubrutinib in Chronic Lymphocytic Leukemia: Evidence from a Systematic Review and Single-Arm Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Protocol and Registration
2.2. Search Strategy and Data Collection
2.3. Eligibility Criteria
2.4. Data Extraction and Outcome Measurement
2.5. Quality Assessment
2.6. Data Analysis
3. Results
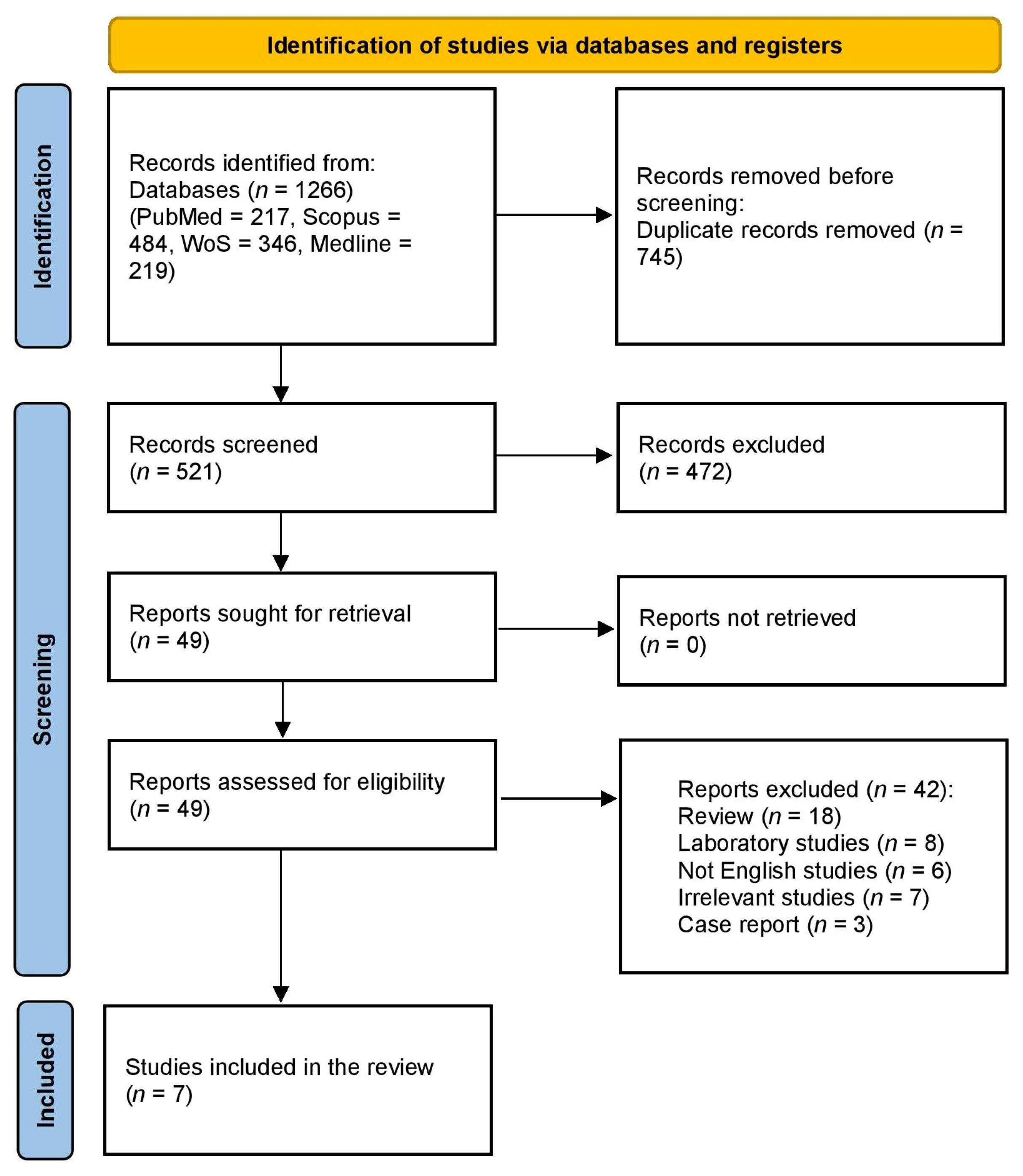
3.1. Study Selection
3.2. Study Characteristics
3.3. Patient Characteristics
3.4. Quality Assessment
3.5. Primary and Secondary Outcomes
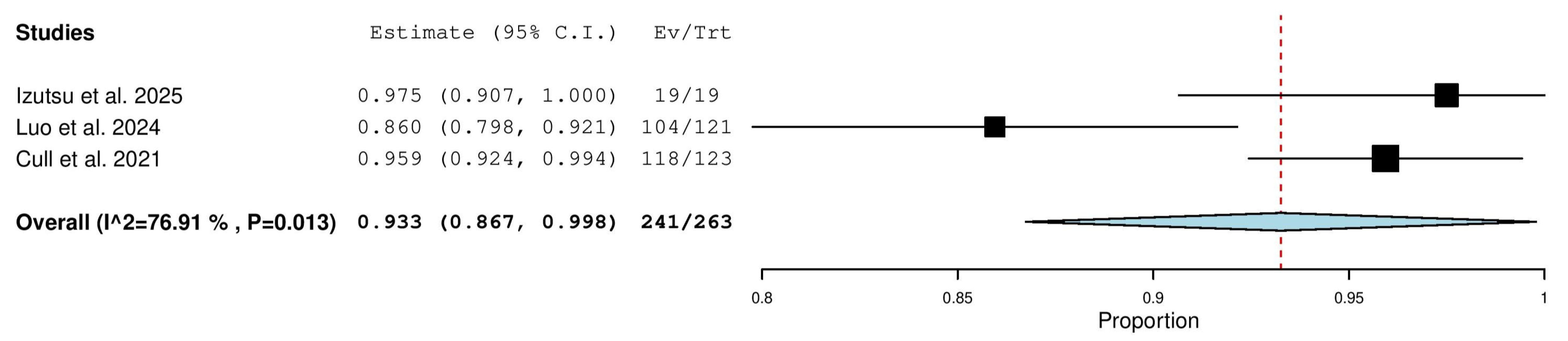
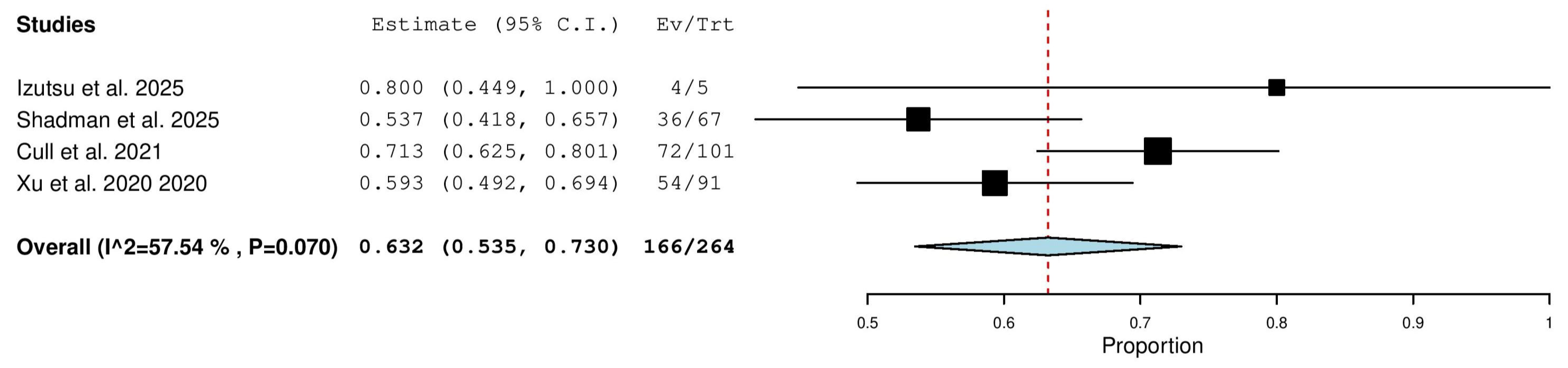
3.5.1. Overall Response Rate (ORR) in Combined (TN & R/R) Patients
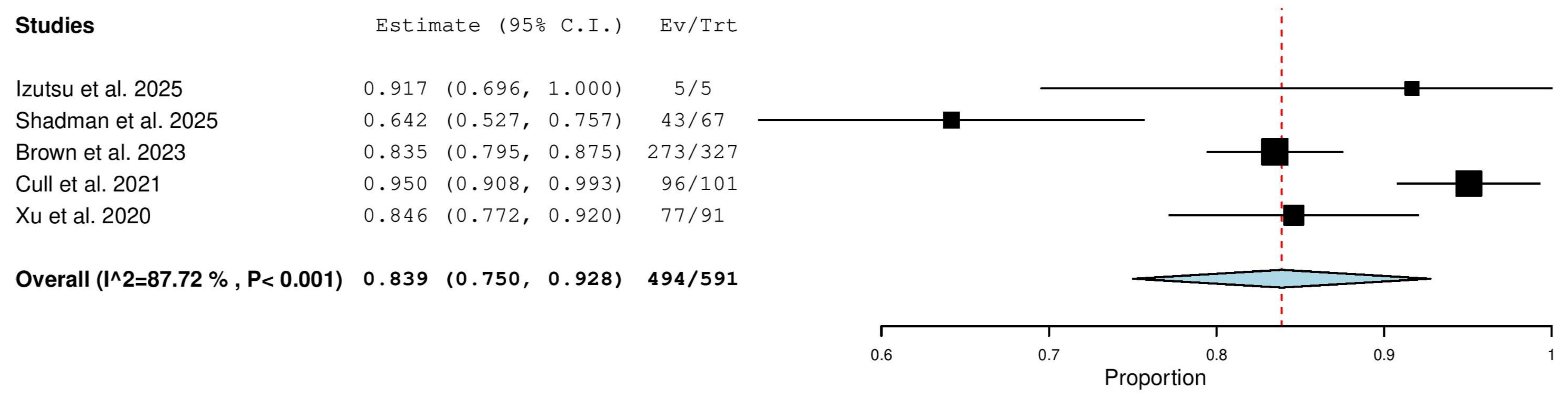
3.5.2. Overall Response Rate (ORR) in Relapsed/Refractory (R/R) Patients
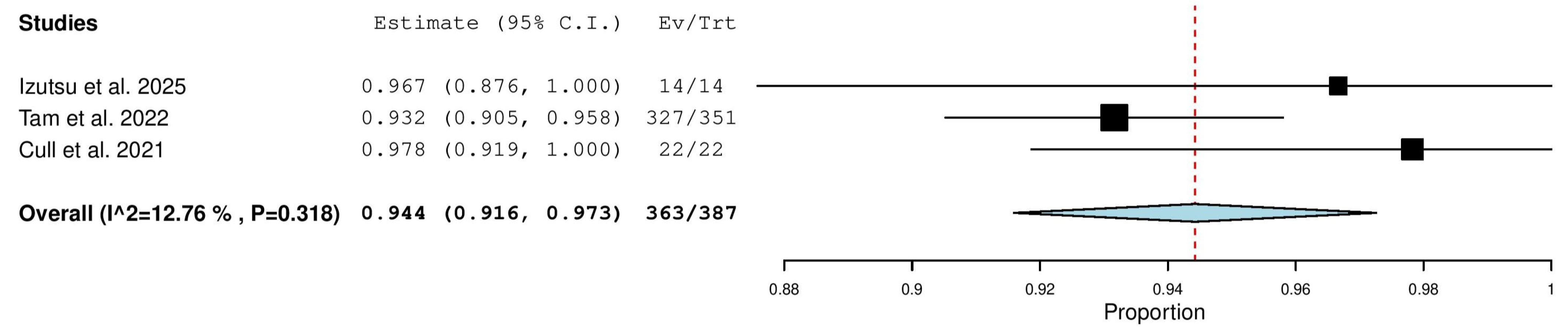
3.5.3. Overall Response Rate (ORR) in Treatment-Naïve (TN) Patients
3.5.4. Complete Response (CR) Rate in Combined (TN & R/R) Patients
3.5.5. Complete Response (CR) Rate in Relapsed/Refractory (R/R) Patients
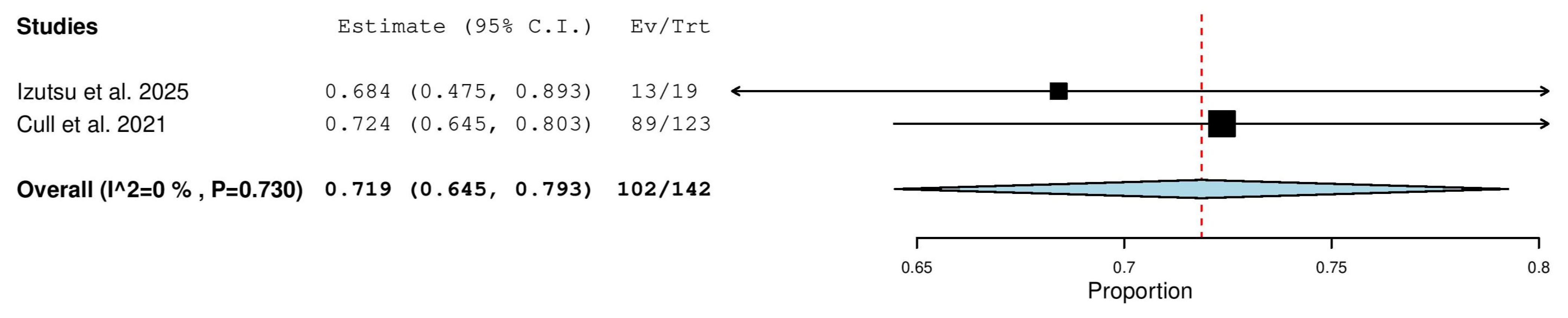
3.5.6. Complete Response (CR) Rate in Treatment-Naïve (TN) Patients
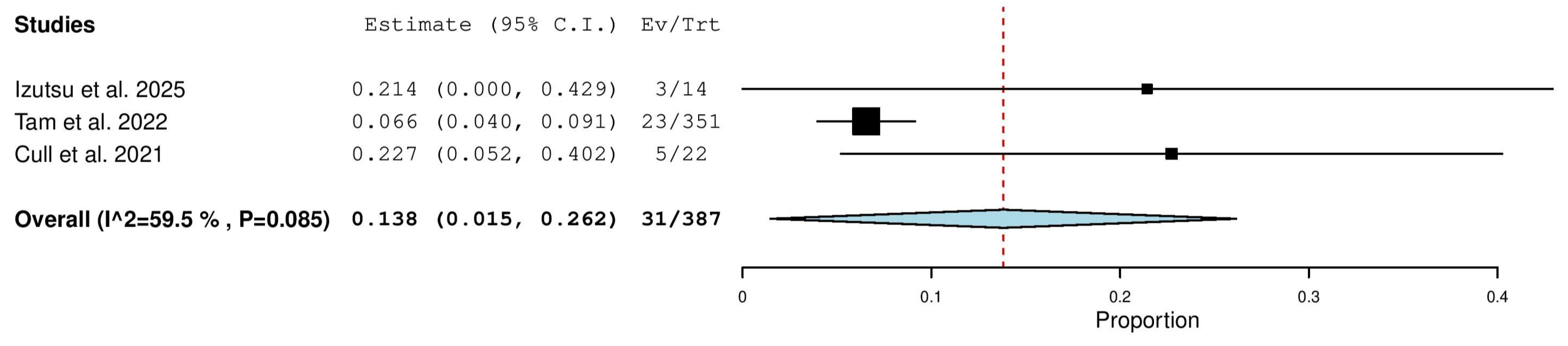
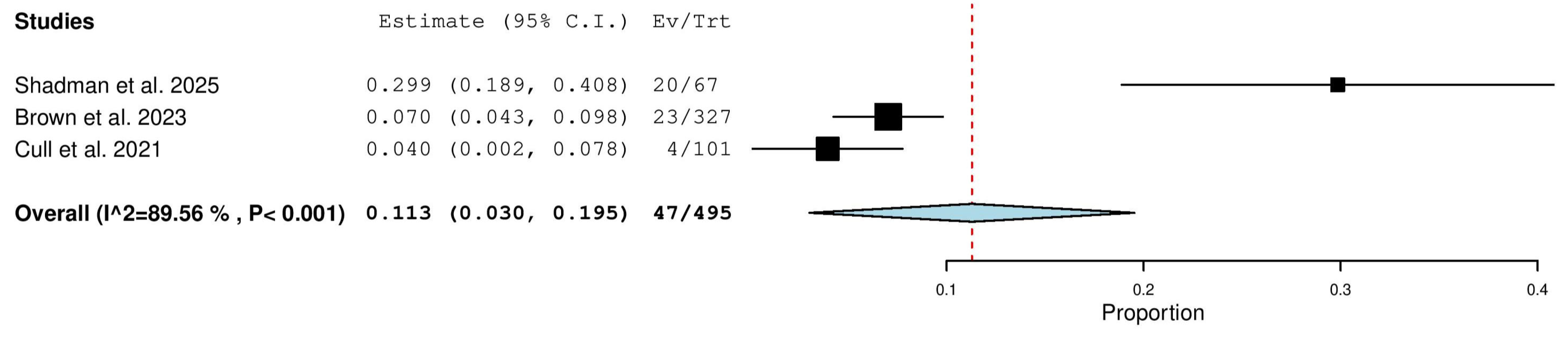
3.5.7. Nodular Partial Response (nPR) Rate in Relapsed/Refractory (R/R) Patients
3.5.8. Partial Response (PR) Rate in Combined (TN & R/R) Patients
3.5.9. Partial Response (PR) in Relapsed/Refractory (R/R) Patients
3.5.10. Partial Response (PR) in Treatment-Naïve (TN) Patients
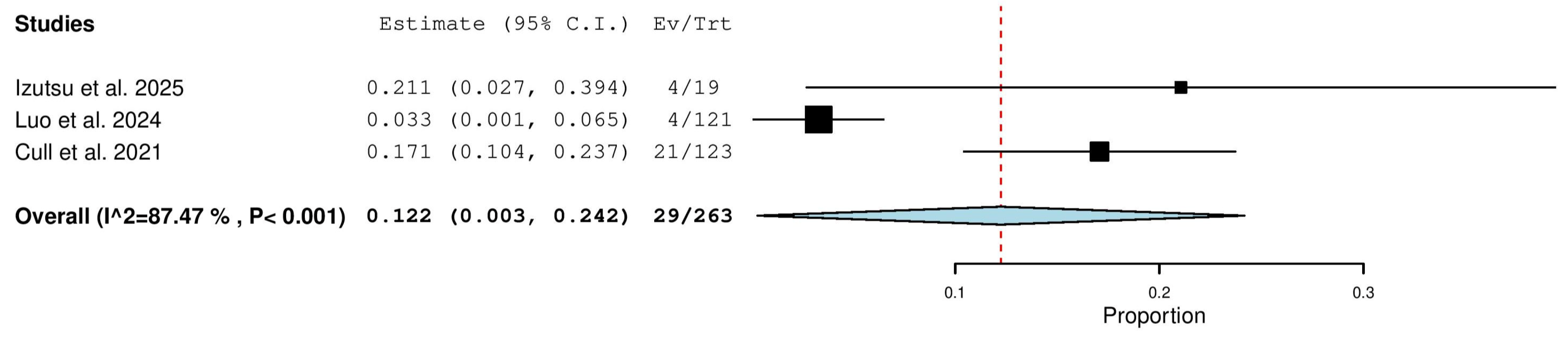
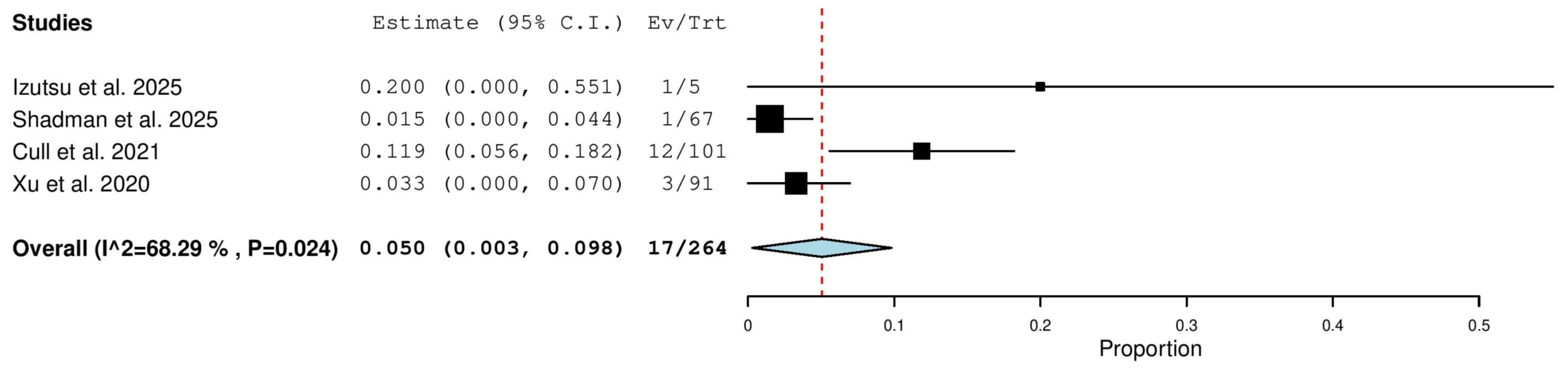
3.5.11. Progressive Disease (PD) Rate in Combined (TN & R/R) Patients
3.5.12. Progressive Disease (PD) Rate in Relapsed/Refractory (R/R) Patients
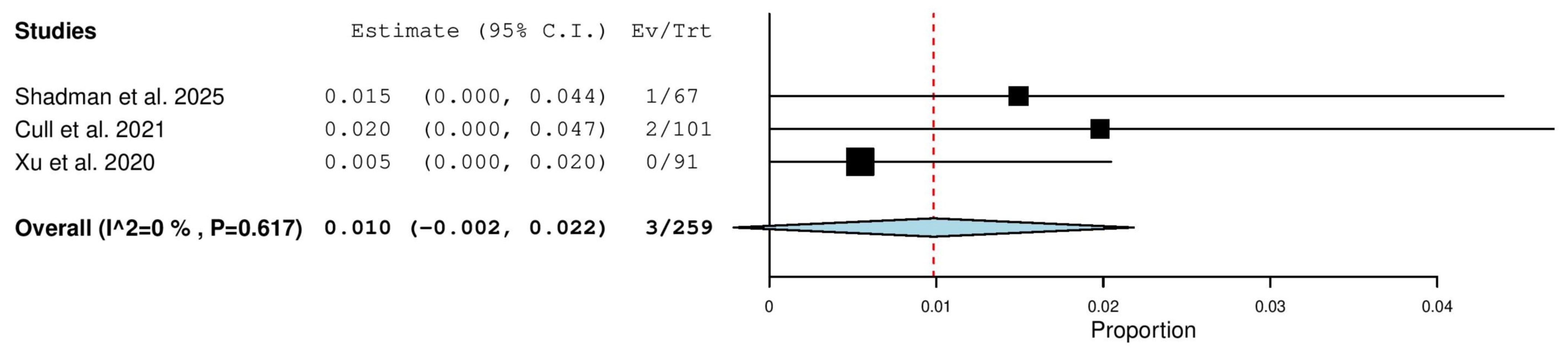
3.5.13. Stable Disease (SD) Rate in Relapsed/Refractory (R/R) Patients
4. Discussion
4.1. Efficacy Findings
4.2. Sources of Heterogeneity
4.3. Strengths and Limitations
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, Y.; Long, Y.C.; Ji, L.L.; Zhan, Y.X.; Qiao, T.K.; Wang, X.D.; Chen, H.; Cheng, Y.F. Trends in Disease Burden of Chronic Lymphocytic Leukemia at the Global, Regional, and National Levels From 1990 to 2019, and Projections Until 2030: A Population-Based Epidemiologic Study. Front. Oncol. 2022, 12, 840616. [Google Scholar] [CrossRef]
- Tomlinson, R. Chronic lymphocytic leukaemia: An updated approach to diagnosis and management in general practice. Aust. Fam. Physician 2017, 46, 493–496. [Google Scholar] [PubMed]
- Moreno, C. Standard treatment approaches for relapsed/refractory chronic lymphocytic leukemia after frontline chemoimmunotherapy. Hematol.-Am. Soc. Hematol. Educ. Program 2020, 2020, 33–40. [Google Scholar] [CrossRef]
- Kim, H.O. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch. Pharmacal Res. 2019, 42, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Buggy, J.J.; Elias, L. Bruton Tyrosine Kinase (BTK) and Its Role in B-cell Malignancy. Int. Rev. Immunol. 2012, 31, 119–132. [Google Scholar] [CrossRef]
- Wiestner, A. Targeting B-Cell Receptor Signaling for Anticancer Therapy: The Bruton’s Tyrosine Kinase Inhibitor Ibrutinib Induces Impressive Responses in B-Cell Malignancies. J. Clin. Oncol. 2013, 31, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Ibrutinib: Pediatric first approval. Pediatr. Drugs 2023, 25, 127–133. [Google Scholar] [CrossRef]
- Guo, Y.H.; Liu, Y.; Hu, N.; Yu, D.S.; Zhou, C.Y.; Shi, G.Y.; Zhang, B.; Wei, M.; Liu, J.H.; Luo, L.S.; et al. Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton’s Tyrosine Kinase. J. Med. Chem. 2019, 62, 7923–7940. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Simkovic, M.; Shadman, M.; Osterborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kazmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef]
- Castillo, J.J.; Kingsley, E.C.; Narang, M.; Yimer, H.A.; Dasanu, C.A.; Melear, J.M.; Coleman, M.; Farber, C.M.; Shulman, J.; Mantovani, E.H. Zanubrutinib in patients with treatment-naïve or relapsed/refractory Waldenström macroglobulinemia: An expanded-access study of 50 patients in the United States. EJHaem 2023, 4, 301. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technologies in Health (CADTH). Zanubrutinib (Brukinsa). Can. J. Health Technol. 2022, 2, 123. [Google Scholar]
- Izutsu, K.; Ishikawa, T.; Shimada, K.; Kubo, K.; Kondo, T.; Fujimoto, K.; Fujisaki, T.; Kurahashi, S.; Nagafuji, K.; Sakai, R.; et al. Zanubrutinib in Japanese treatment-naive and relapsed/refractory patients with Waldenstrom macroglobulinemia and CLL/SLL. Int. J. Hematol. 2025, 121, 483–493. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration John Wiley Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Shadman, M.; Burke, J.M.; Cultrera, J.; Yimer, H.A.; Zafar, S.F.; Misleh, J.; Rao, S.S.; Farber, C.M.; Cohen, A.; Yao, H.; et al. Zanubrutinib is well tolerated and effective in patients with CLL/SLL intolerant of ibrutinib/acalabrutinib: Updated results. Blood Adv. 2025, 9, 4100–4110. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J.; Liu, L.; Wei, R.; Yao, Y.; Xu, M.; Shi, J.; Yang, J.; Hou, J.; Wang, J.; et al. Efficacy and safety of zanubrutinib monotherapy for chronic lymphocytic leukemia/small lymphocytic lymphoma: A multicenter, real-world study in China. Am. J. Hematol. 2024, 100, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Cull, G.; Burger, J.A.; Opat, S.; Gottlieb, D.; Verner, E.; Trotman, J.; Marlton, P.; Munoz, J.; Johnston, P.; Simpson, D. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: Long-term follow-up of the phase I/II AU-003 study. Br. J. Haematol. 2021, 196, 1209–1218. [Google Scholar] [CrossRef]
- Xu, W.; Yang, S.; Zhou, K.; Pan, L.; Li, Z.; Zhou, J.; Gao, S.; Zhou, D.; Hu, J.; Feng, R.; et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: Phase 2, single-arm, multicenter study. J. Hematol. Oncol. 2020, 13, 48. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2008; Volume 2. [Google Scholar]
- Hillmen, P.; Eichhorst, B.; Brown, J.R.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.G.; Kazmierczak, M.; Zhou, K.S.; Simkovic, M.; et al. Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: Interim Analysis of a Randomized Phase III Trial. J. Clin. Oncol. 2023, 41, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.G.; Jurczak, W.; Zhou, K.S.; Simkovic, M.; Mayer, J.; et al. Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: Final comparative analysis of ALPINE. Blood 2024, 144, 2706–2717. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Type of Study | Country | Duration | Trial Registration | Type of Patients | Diagnostic Criteria | Zanubrutinib Regimen (Dose, Route of Administration, Regimen) | No. of Patients in the Zanubrutinib Group | Follow-Up, Months, Median (Range) |
|---|---|---|---|---|---|---|---|---|---|
| Izutsu et al., 2025 [13] | Phase I/II, multicenter, open-label study | Japan | From 30 January 2020 to 31 October 2022. | NCT04172246 | Japanese adults (age ≥ 20 years) with TN or R/R CLL/SLL or WM. Also included patients with other B-cell malignancies in Part 1 and an R/R MCL cohort in Part 2 for safety analysis. | CLL/SLL: 2018 iwCLL guidelines; SLL: Lugano classification; WM: 6th International Workshop on WM criteria | Dose: 160 mg twice daily Route: orally Regimen: continuously until disease progression, unacceptable toxicity, or other discontinuation criteria | CLL/SLL: 19 (TN: 14, R/R: 5) | 27.9 |
| Shadman et al., 2025 [18] | Phase II, single-arm, open-label clinical trial | United States | Data Cut-off: 1 May 2024 | NCT04116437 | Patients with CLL or SLL who are intolerant of prior ibrutinib and/or acalabrutinib therapy | iwCLL criteria | Dose: 160 mg twice daily or 320 mg once daily Route: Oral (implied) Regimen: Continuous dosing; patients/investigators selected a regimen and could not switch | 71 | 34.5 (0.1–51.1) |
| Luo et al., 2024 [19] | Multicenter, real-world, retrospective observational study | China | The last follow-up time was 15 September 2024. | NR (real-world study) | Adult patients with CLL/SLL. TN: 90 patients (65.2%) R/R: 48 patients (34.8%) | iwCLL 2018 guidelines. | Standard Dose: 160 mg twice daily (orally), dose reduction to 80 mg twice daily | 138 | 36.8 (95% CI: 34.5–39.1) |
| Brown et al., 2023 [10] | Phase III, randomized, open-label, active-controlled, multinational, clinical Trial | Multinational (15 countries across North America, Europe, and the Asia Pacific) | From 1 November 2018, through 14 December 2020 | NCT03734016 | Relapsed or refractory CLL or SLL | iwCLL criteria | Dose: 160 mg twice daily Route: orally Regimen: until disease progression or unacceptable toxicity | 327 patients in the zanubrutinib group (intention-to-treat population) | 29.6 |
| Tam et al., 2022 [9] | Randomized, controlled, phase III trial | 14 countries and regions (multicenter) | From 31 October 2017 to 22 July 2019 | NCT03336333 | Patients aged ≥65 years, or ≥18 years with comorbidities, with untreated CLL or SLL requiring treatment per iwCLL criteria | iwCLL criteria | Dose: 160 mg twice per day Route: Orally Regimen: in 28-day cycles until disease progression or unacceptable toxicity | Group A (without del(17p)): 241, Group C (with del(17p)): 111 | 26.2 (IQR 23·7–29·6) |
| Cull et al., 2021 [20] | Phase I/II, open-label, single-arm study | Multinational: Australia, USA, New Zealand, China | From September 2014 to November 2018 (last first dose) Data cut-off: March 2021 (study closure) | NCT02343120 | TN CLL/SLL: 22 patients (17.9%) R/R CLL/SLL: 101 patients (82.1%) | CLL/SLL diagnosed according to the WHO classifications. Requirement for treatment per the iwCLL criteria. | Dose: 160 mg twice daily (81 patients) or 320 mg once daily (40 patients). Two patients received 160 mg once daily (in the initial dose escalation phase). Route: Orally Regimen: Administered in 28-day cycles until disease progression or unacceptable toxicity. | Total: 123 patients (118 CLL, 5 SLL) | 47.2 |
| Xu et al., 2020 [21] | Phase II, single-arm, multicenter study | China | From 9 March to 14 December 2017 | CTR20160890 (7 December 2016) NCT03206918 (2 July 2017) | R/R CLL or SLL | CLL: iwCLL guidelines (2008); SLL: WHO classification (2008), histologically confirmed by central pathologic review | 160 mg orally twice daily in 28-day cycles until disease progression or intolerance | 91 | 15.1 (0.8–21.2) |
| Study ID | Age, Median (range) | Female n (%) | ECOG PS, No. (%) | Type of Cancer No. (%) | Binet Stage at CLL Diagnosis, n (%) | Prior Treatment Status No. (%) | Cytopenia at Baseline, No. (%) | Chromosomal Mutation Status, No. (%) | IGHV Mutational Status—No. (%) | Bulky Disease—No. (%)¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| Izutsu et al., 2025 [13] | 71.0 (38–77) | 5 (26.3) | 0: 17 (89.5) 1: 2 (10.5) | CLL/SLL: 19 (100) | NR | TN: 14 (73.7%) R/R: 5 (26.3%) R/R CLL/SLL (n = 5) | NR | CLL/SLL: del(17p): 0 (0) | Mutated: 12 (63.2) Unmutated: 7 (36.8) | NR |
| Shadman et al., 2025 [18] | 71 (49–91) | 35 (49.3) | 0: 44 (62) 1: 25 (35.2) 2: 2 (2.8) | CLL: 63 (88.7) SLL: 8 (11.3) | A: 12 (16.9) B: 6 (8.5) C: 4 (5.6) Unknown: 41 (57.7) | R/R: 71 (100%) | Hemoglobin ≤ 110 g/L: 11 (15.5) Platelet count ≤ 100 × 109/L: 6 (8.5) ANC ≤ 1.5 × 109/L: 3 (4.2) | del(11q): 10 (14.1) del(17p): 6 (8.5) del(13q): 17 (23.9) TP53 mutation: 16 (22.5) | Unmutated: 13 (18.3) Mutated: 12 (16.9) Unknown/Missing: 46 (64.8) | Largest diameter < 5 cm: 49 (69) Largest diameter > 5 cm: 10 (14.1) No measurable disease: 12 (16.9) |
| Luo et al., 2024 [19] | 68 (IQR: 37–87) | 46 (33.3) | NR | CLL: 132 (95.7) SLL: 6 (4.3) | A: 18 (13.6) B: 55 (41.7) C: 59 (44.7) | TN: 90 (65.2) R/R: 48 (34.8) | NR | TP53 status (deletion and/or mutation): 27/109 (24.8) del(11q): 9/84 (10.7) del(17p): 15/105 (14.3) Complex Karyotype (≥3 abnormalities): 6/76 (7.9) | Unmutated IGHV: 29/67 (43.3) | NR |
| Brown et al., 2023 [10] | 67 (35–90) ≥65 and <75: 127 (38.8) ≥75: 74 (22.6) | 114 (34.9) | ≥1: 198 (60.6) | CLL or SLL: 327 (100) | A/B: 182 (55.7) C: 145 (44.3) | 1 line: Zanubrutinib 192 (58.7) 2 lines: 86 (26.3) 3 lines: 25 (7.6) 3 lines: Zanubrutinib 24 (7.3) | NR | 17p deletion and/or TP53 mutation: 75 (22.9) 11q deletion: Zanubrutinib 91 (27.8%), IComplex karyotype (≥3 abnormalities): 56 (17.1) | Unmutated: 239 (73.1) Mutated: 79 (24.2) Missing:9 (2.8) | Yes (tumor ≥ 5 cm): 145 (44.3) |
| Tam et al., 2022 [9] | 70 (IQR: 66–74) | 119 (33.8) | 0: 154/352 (43.8) 1: 169/352 (48.0) 2: 29/352 (8.2) | CLL: 321/352 (91.2) SLL: 31/352 (8.8) | A/B: 243 (69) C: 109 (31) | TN: 352 (100) | 163 (46.4) | del(17p): 112 (31.8) del(11q): 80 (22.7) del(13q): 210 (59.7) Trisomy 12: 65 (18.5) No FISH abnormalities: 56 (15.9) | Unmutated: 192 (57.0) Mutated: 145 (43.0) | Bulky disease (≥5 cm): 113 (32.1%) |
| Cull et al., 2021 [20] | 67 (24–87) | 31 (25.2) | 0: 57 (46.3) 1: 61 (49.6) 2: 5 (4.1) | CLL: 118 (95.9) SLL: 5 (4.1) | NR | TN: 22 (17.8) R/R: 101 (82.2) 1 line: 49 (39.8) ≥3 lines: 28 (22.76) | Hemoglobin ≤ 110 g/L OR platelet count ≤ 100 × 109/L OR absolute neutrophil count ≤ 1.5 × 109/L Present: 66 (53.7) | del(17p): 16/99 (16.2) TP53 mutation: 14/43 (32.6) del(11q): 23/98 (23.5) del(13q): 45/98 (45.9) Trisomy 12: 15/97 (15.5) Both del(17p) and TP53 mutation: 6/101 (5.9) | Unmutated: 29/42 (69.0%) Mutated: 13/42 (31.0) | Defined as: Any target lesion with the longest diameter ≥ 5 cm, Present: 47 patients (38.2) ≥10 cm: 5 patients (4.1) |
| Xu et al., 2020 [21] | 61 (35–87) | 39 (42.9) | 0/1: 88 (96.7) 2: 3 (3.3) | CLL: 82 (90.1) SLL: 9 (9.9) | A/B: 27 (32.9) C: 55 (67.1) | Refractory to last systemic therapy: 72 (79.1) Non-refractory: 19 (20.9) ≥2 prior lines: 45 (49.5) | Present: 66 (72.5) had a baseline serum ẞ2-microglobulin level > 3.5 mg/L Absent: 25 (27.5) had a baseline absolute neutrophil count < 1.5 × 109/L. | del(17p) or TP53 mutation: 22 (24.2) del(11q): 20 (22.0) del(13q): 41 (45.1) Trisomy 12: 21 (23.1) | Unmutated: 51 (56.0) Mutated: 23 (25.3) Not available: 17 (18.7) | Present (≥1 lesion with LDI ≥ 5 cm): 40 (44.0) Absent: 51 (56.0) |
| Study ID | Izutsu et al., 2025 [13] | Shadman et al., 2025 [18] | Luo et al. 2024 [19] | Cull et al. 2021 [20] | Xu et al., 2020 [21] |
|---|---|---|---|---|---|
| 1. Were there clear criteria for inclusion in the case series? | Yes | Yes | Yes | Yes | Yes |
| 2. Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | Yes | Yes | Yes | Yes |
| 3. Were valid methods used for identification of the condition for all participants included in the case series? | Yes | Yes | Yes | Yes | Yes |
| 4. Did the case series have consecutive inclusion of participants? | Unclear | Yes | Unclear | Yes | Yes |
| 5. Did the case series have complete inclusion of participants? | Yes | Yes | Yes | Yes | Yes |
| 6. Was there clear reporting of the demographics of the participants included in the study? | Yes | Yes | Yes | Yes | Yes |
| 7. Was there clear reporting of clinical information of the participants? | Yes | Yes | Yes | Yes | Yes |
| 8. Were the outcomes or follow-up results of cases clearly reported? | Yes | Yes | Yes | Yes | Yes |
| 9. Was there clear reporting of the presenting sites’/clinics’ demographic information? | Unclear | Unclear | Unclear | Yes | Unclear |
| 10. Was statistical analysis appropriate? | Yes | Yes | Yes | Yes | Yes |
| Study ID | Brown et al., 2023 [10] | Tam et al., 2022 [9] |
|---|---|---|
| Was true randomization used for assignment of participants to treatment groups? | YES | YES |
| Was allocation to treatment groups concealed? | YES | UNCLEAR |
| Were treatment groups similar at the baseline? | YES | YES |
| Were participants blind to treatment assignment? | NO | NO |
| Were those delivering treatment blind to treatment assignment? | NO | NO |
| Were outcomes assessors blind to treatment assignment? | YES | UNCLEAR |
| Were treatment groups treated identically other than the intervention of interest? | YES | NO |
| Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed? | YES | YES |
| Were participants analyzed in the groups to which they were randomized? | YES | YES |
| Were outcomes measured in the same way for treatment groups? | YES | YES |
| Were outcomes measured in a reliable way? | YES | YES |
| Was appropriate statistical analysis used? | YES | YES |
| Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? | YES | YES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatawi, Y.; Alanazi, F.E.; Alattar, A.; Alshaman, R.; Ramadan, Y.N.; Sayad, R.; Hetta, H.F. Therapeutic Impact of Zanubrutinib in Chronic Lymphocytic Leukemia: Evidence from a Systematic Review and Single-Arm Meta-Analysis. Pharmaceuticals 2025, 18, 1674. https://doi.org/10.3390/ph18111674
Alatawi Y, Alanazi FE, Alattar A, Alshaman R, Ramadan YN, Sayad R, Hetta HF. Therapeutic Impact of Zanubrutinib in Chronic Lymphocytic Leukemia: Evidence from a Systematic Review and Single-Arm Meta-Analysis. Pharmaceuticals. 2025; 18(11):1674. https://doi.org/10.3390/ph18111674
Chicago/Turabian StyleAlatawi, Yasser, Fawaz E. Alanazi, Abdullah Alattar, Reem Alshaman, Yasmin N. Ramadan, Reem Sayad, and Helal F. Hetta. 2025. "Therapeutic Impact of Zanubrutinib in Chronic Lymphocytic Leukemia: Evidence from a Systematic Review and Single-Arm Meta-Analysis" Pharmaceuticals 18, no. 11: 1674. https://doi.org/10.3390/ph18111674
APA StyleAlatawi, Y., Alanazi, F. E., Alattar, A., Alshaman, R., Ramadan, Y. N., Sayad, R., & Hetta, H. F. (2025). Therapeutic Impact of Zanubrutinib in Chronic Lymphocytic Leukemia: Evidence from a Systematic Review and Single-Arm Meta-Analysis. Pharmaceuticals, 18(11), 1674. https://doi.org/10.3390/ph18111674