Evaluating the Safety and Efficacy of PD-1 Inhibitors in HIV Patients Diagnosed with Lung Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Research Questions
2.4. Trials Selection
2.5. Data Extraction
2.6. Outcome Measures
2.7. Quality Assessment
2.8. Data Analysis
3. Results
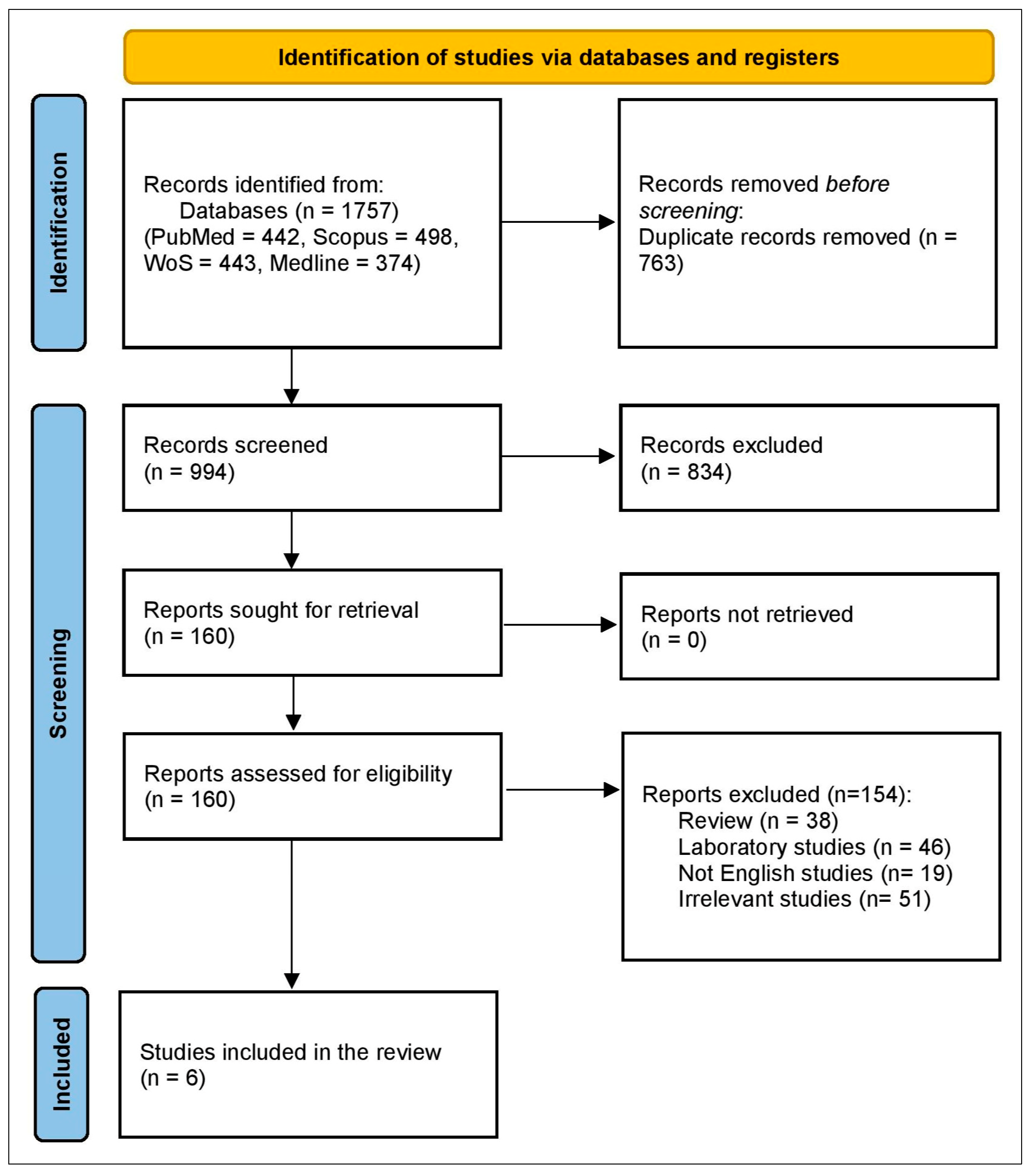
3.1. Study Selection
3.2. Study Characteristics
3.3. Efficacy Outcomes
3.4. Quality Assessment Results
| Study (Year) | Design/ Duration | Sample Size (n) | Median Age (Years) | Male, n (%) | Cancer Type/Stage | PD-1/PD-L1 Inhibitor(s) | Combination or Prior Therapy | Median Treatment Duration (Months) | ART at Baseline | HIV/AIDS Status | Autoimmune Disease (If Any) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assoumou et al., 2024 [29] | Prospective observational cohort (Jan 2018–Dec 2023) | 65 | 59 (IQR 54–64) | 48 (74%) | Lung cancer (mixed histology, stage not specified) | Pembrolizumab, nivolumab, cemiplimab, atezolizumab, durvalumab; ± ipilimumab or bevacizumab | Prior chemo/radiotherapy/targeted therapy in some patients | 5.4 (IQR 2.1–12.7) | All on ART | Majority asymptomatic; AIDS data not detailed | Autoimmune hemolytic anemia, Crohn’s disease, myositis, rheumatoid arthritis, psoriasis, ITP, ankylosing spondylitis |
| El Zarif et al., 2023 [30] | Retrospective multicenter cohort (2015–2021) | 111 | 58 (51–63) | Not reported | NSCLC (mixed histology/stage) | Nivolumab, pembrolizumab, atezolizumab, durvalumab | Prior chemotherapy, TKIs, antiangiogenic therapy, or dual ICIs | Not reported | All on ART | AIDS status not reported | Not reported |
| Lavole et al., 2021 [22] | Nonrandomized, open-label phase 2 trial (2017–2019) | 16 | 58 (44–71) | 14 (88%) | NSCLC: adenocarcinoma 63%, squamous 31%; stage IIIB–IVB | Nivolumab 3 mg/kg IV q2wk | None | 3.5 (0.5–26.5) | All on cART | All with controlled HIV (VL < 200 copies/mL) | Not reported |
| Cortellini et al., 2019 [31] | Retrospective (2013–2018) | 492 | 69 (24–92) | Not reported | NSCLC (advanced) | Pembrolizumab, nivolumab | None | Not reported | Not reported | HIV+ subgroup; AIDS data not detailed | Thyroid, dermatologic, rheumatologic, GI/hepatic, neurologic disorders |
| Shah et al., 2019 [32] | Retrospective (2011–2018) | 22 | 62 (29–85) | Not reported | NSCLC (mixed) | Nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab | None | Not reported | Most on ART (various regimens) | Not reported | Not reported |
| Leonardi et al., 2018 [33] | Retrospective cohort (2015–2017) | 56 | 67 (45–90) | 21 (38%) | NSCLC: adenocarcinoma 73%, squamous 25%; stage IIIB–IV | Nivolumab (80%), pembrolizumab (18%), atezolizumab (2%) | None | 3.1 (95% CI 1.8–5.1) | Majority on ART | 20% symptomatic AIDS; 11 receiving immunosuppressive therapy | Rheumatologic (45%), dermatologic (29%), endocrine (16%), GI (11%), neurologic (5%) |
| Study (Year) | Point of Assessment | Progression-Free Survival (Months) | Overall Survival (Months) | Deaths n (%) | Any irAE n (%) | Grade 1–2 irAEs n (%) | Grade 3–4 irAEs n (%) | Treatment Required for irAEs n (%) | CD4 Count (Cells/µL) | CD4 Nadir (Cells/µL) | CD4:CD8 Ratio | HIV RNA (Copies/mL) | Response (ORR/DCR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assoumou et al., 2024 [29] | 6, 12, 18 months | 6 mo: 48.8 (40.1–57.0); 12 mo: 32.3 (24.3–40.5); 18 mo: 25.5 (17.8–33.5) | 6 mo: 66.0 (52.8–76.4); 12 mo: 45.1 (31.8–57.6); 18 mo: 36.4 (23.7–49.3) | 81/140 (58%) | Not available | Not available | 20 (15.0% at 12 mo; 18.7% at 18 mo) | 41/140 (29.3%) received glucocorticoids | 336 (210–598) | 117 (51–240) | 0.7 (0.3–1.0) | VL < 50 in 84%; viremic median 460 (IQR: 106–39,550) | ORR 22% (PR 11/50); DCR 53% (SD 15/50) |
| El Zarif et al., 2023 [30] | Not reported | 6.3 (4.3–10.1) | 16.0 (10.6–40.2) | 53/111 (47.7%) | 12 (20%) | Not reported | 7 (12%) | 6/61 (9.8%) | 314 (206–472) | Not available | Not available | VL <400 in 96% | ORR 31% (32/102); 95% CI 23–41% |
| Lavole et al., 2021 [22] | Median follow-up: 23.6 mo | 3.4 (1.8–5.6) | 10.9 (2.2–NR) | Not stated (based on 10 patients) | 12 (75%) | 11 (69%) | 1 (6%) | One serious AE, managed clinically | 385 (187–778) | 274 (32–778) | Not reported | All controlled (VL < 50) | DCR 62.5% (PR 2, SD 8, PD 5) |
| Cortellini et al., 2019 [31] | Not reported | 3.0 (2.0–4.1) | 10.0 (6.6–13.4) | 23/30 (77%) | 16 (53%) | 10 (33%) | 6 (20%) | 6 (20%) received corticosteroids; 9 (30%) discontinued ICIs | 491 ±182 | 196 ±125 | 0.7 ±0.3 | All undetectable (<50 copies/mL) | ORR 20%; DCR 47% |
| Shah et al., 2019 [32] | Not reported | Not available | Not available | Not available | 3 (25%) | 1 (8%)—rash (ICIs + chemo) | 2 (17%)—pneumonitis (ICIs mono) | Not reported | Not reported | Not reported | Not reported | 4/6 undetectable VL pre-ICIs | ICIs monotherapy ORR 13% (1 CR); ICIs + chemo ORR 75% (3 PR) |
| Leonardi et al., 2018 [33] | Not reported | 3.1 (1.8–5.1) | Not available | Not available | 22 (38%) | 15 (27%) | 6 (11%) | 7 (13%) corticosteroids; 14% discontinued ICIs | Not reported | Not reported | Not reported | Not reported | ORR 22% (11 PRs); DCR 53% |
| Study ID | Leonardi et al., 2018 [33] | Cortellini et al., 2019 * [31] | Shah et al. (2019) [32] | Lavole et al., 2021 [22] | El Zarif et al., 2023 [30] | Assoumou et al., 2024 [29] |
|---|---|---|---|---|---|---|
| Were There Clear Criteria for Inclusion in the Case Series? | Yes | Yes | Not clear | Not clear | Yes | Not clear |
| Was the Condition Measured in a Standard, Reliable Way for All Participants Included in the Case Series? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were Valid Methods Used for Identification of the Condition for all Participants Included in the Case Series? | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the Case Series Have Consecutive Inclusion of Participants? | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the Case Series Have Complete Inclusion of Participants? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was There Clear Reporting of the Demographics of the Participants in the Study? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was There Clear Reporting of Clinical Information of the Participants? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the Outcomes or Follow-up Results of Cases Clearly Reported? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was There Clear Reporting of the Demographics of the Participants in the Study? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was Statistical Analysis Appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; d’Arminio Monforte, A.; Egger, M.; Gill, M.J.; Grabar, S.; Guest, J.L.; Jarrin, I.; et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: A collaborative analysis of cohort studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef] [PubMed]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.P. Immune checkpoint inhibitors in people living with HIV: What about anti-HIV effects? AIDS 2020, 34, 167–175. [Google Scholar] [CrossRef]
- Guiguet, M.; Boué, F.; Cadranel, J.; Lang, J.-M.; Rosenthal, E.; Costagliola, D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): A prospective cohort study. Lancet Oncol. 2009, 10, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Sigel, K.; Wisnivesky, J.; Justice, A.; Brown, S.; Butt, A.; Crystal, S.; Rimland, D.; Rodriguez-Barradas, M.; Gibert, C. HIV infection is an independent risk factor for lung cancer. In Proceedings of the 17th Conference on Retroviruses and Opportunistic Infections (CROI), San Francisco, CA, USA, 16–19 February 2010; pp. 16–19. [Google Scholar]
- Hunt, P.W. HIV and inflammation: Mechanisms and consequences. Curr. HIV/AIDS Rep. 2012, 9, 139–147. [Google Scholar] [CrossRef]
- Ji, W.-T.; Liu, H.J. PI3K-Akt signaling and viral infection. Recent Pat. Biotechnol. 2008, 2, 218–226. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Y.; Shu, X.; Li, Y.; Zhang, X.; Wang, C.; He, S.; Li, J.; Li, T.; Liu, T. Molecular mechanisms of viral oncogenesis in haematological malignancies: Perspectives from metabolic reprogramming, epigenetic regulation and immune microenvironment remodeling. Exp. Hematol. Oncol. 2025, 14, 69. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J. Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef]
- Guihot, A.; Cadranel, J.; Lambotte, O.; Lavolé, A.; Autran, B.; Spano, J.P. Biological follow-up of patients with HIV treated with anti-PD-1 or anti-PD-L1 for non-small cell bronchial carcinoma: A task group proposal. Rev. Mal. Respir. 2016, 33, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Makinson, A.; Tenon, J.-C.; Eymard-Duvernay, S.; Pujol, J.-L.; Allavena, C.; Cuzin, L.; Poizot-Martin, I.; de la Tribonnière, X.; Cabié, A.; Pugliese, P.; et al. Human Immunodeficiency Virus Infection and Non-small Cell Lung Cancer: Survival and Toxicity of Antineoplastic Chemotherapy in a Cohort Study. J. Thorac. Oncol. 2011, 6, 1022–1029. [Google Scholar] [CrossRef]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 2016, 27, 559–574. [Google Scholar] [CrossRef]
- Vora, K.B.; Ricciuti, B.; Awad, M.M. Exclusion of patients living with HIV from cancer immune checkpoint inhibitor trials. Sci. Rep. 2021, 11, 6637. [Google Scholar] [CrossRef]
- Hleyhel, M.; Belot, A.; Bouvier, A.M.; Tattevin, P.; Pacanowski, J.; Genet, P.; De Castro, N.; Berger, J.L.; Dupont, C.; Lavolé, A.; et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: Results from the FHDH-ANRS CO4 cohort. Clin. Infect. Dis. 2013, 57, 1638–1647. [Google Scholar] [CrossRef]
- Hleyhel, M.; Hleyhel, M.; Bouvier, A.M.; Belot, A.; Tattevin, P.; Pacanowski, J.; Genet, P.; De Castro, N.; Berger, J.L.; Dupont, C.; et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: Results from a French cohort. AIDS 2014, 28, 2109–2118. [Google Scholar] [CrossRef]
- Hunt, P.W.; Lee, S.A.; Siedner, M.J. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J. Infect. Dis. 2016, 214 (Suppl. 2), S44–S50. [Google Scholar] [CrossRef]
- Alexandrova, Y.; Costiniuk, C.T.; Jenabian, M.A. Pulmonary Immune Dysregulation and Viral Persistence During HIV Infection. Front. Immunol. 2021, 12, 808722. [Google Scholar] [CrossRef] [PubMed]
- Iordache, L.; Launay, O.; Bouchaud, O.; Jeantils, V.; Goujard, C.; Boue, F.; Cacoub, P.; Hanslik, T.; Mahr, A.; Lambotte, O.; et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun. Rev. 2014, 13, 850–857. [Google Scholar] [CrossRef]
- Bourgarit, A.; Carcelain, G.; Martinez, V.; Lascoux, C.; Delcey, V.; Gicquel, B.; Vicaut, E.; Lagrange, P.H.; Sereni, D.; Autran, B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006, 20, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009, 458, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cao, M.; Morán, T.; Dalmau, J.; Garcia-Corbacho, J.; Bracht, J.W.; Bernabe, R.; Juan, O.; De Castro, J.; Blanco, R.; Drozdowskyj, A. Assessment of the feasibility and safety of durvalumab for treatment of solid tumors in patients with HIV-1 infection: The phase 2 DURVAST study. JAMA Oncol. 2020, 6, 1063–1067. [Google Scholar] [CrossRef]
- Lavole, A.; Mazieres, J.; Schneider, S.; Brosseau, S.; Kiakouama, L.; Greillier, L.; Guihot, A.; Abbar, B.; Baron, M.; Makinson, A.; et al. Assessment of nivolumab in HIV-Infected patients with advanced non-small cell lung cancer after prior chemotherapy. The IFCT-1602 CHIVA2 phase 2 clinical trial. Lung Cancer 2021, 158, 146–150. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Gonçalves, P.H.; Abdul-Hay, M.; Claeys, A.J.; Emu, B.; Ernstoff, M.S.; Fling, S.P.; Fong, L.; Kaiser, J.C.; Lacroix, A.M.; et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced cancer—A Phase 1 Study. JAMA Oncol. 2019, 5, 1332–1339. [Google Scholar] [CrossRef]
- Zarif, T.; Nassar, A.; Adib, E.; Fitzgerald, B.; Huang, J.; Mouhieddine, T.; Nonato, T.; McKay, R.; Li, M.; Mittra, A.; et al. 437 Safety and efficacy of immune checkpoint inhibitors (ICI) in patients living with HIV (PLWH) and metastatic non-small cell lung cancer (NSCLC): A matched cohort study from the international CATCH-IT consortium. J. Immunother. Cancer 2022, 10, A457–A458. [Google Scholar]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Assoumou, L.; Baldé, R.; Katlama, C.; Abbar, B.; Delobel, P.; Allegre, T.; Lavole, A.; Makinson, A.; Zaegel-Faucher, O.; Greillier, L.; et al. Safety and tolerability of immune checkpoint inhibitors in people with HIV infection and cancer: Insights from the national prospective real-world OncoVIHAC ANRS CO24 cohort study. J. Immunother. Cancer 2024, 12, e009728. [Google Scholar] [CrossRef]
- El Zarif, T.; Nassar, A.H.; Adib, E.; Fitzgerald, B.G.; Huang, J.; Mouhieddine, T.H.; Rubinstein, P.G.; Nonato, T.; McKay, R.R.; Li, M. Safety and activity of immune checkpoint inhibitors in people living with HIV and cancer: A real-world report from the cancer therapy using checkpoint inhibitors in people living with HIV-international (CATCH-IT) consortium. J. Clin. Oncol. 2023, 41, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Buti, S.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Bersanelli, M.; Michiara, M.; Grassadonia, A.; Brocco, D.; et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019, 24, e327–e337. [Google Scholar] [CrossRef]
- Shah, N.J.; Al-Shbool, G.; Blackburn, M.; Cook, M.; Belouali, A.; Liu, S.V.; Madhavan, S.; He, A.R.; Atkins, M.B.; Gibney, G.T.; et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J. Immunother. Cancer 2019, 7, 353. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Gainor, J.F.; Altan, M.; Kravets, S.; Dahlberg, S.E.; Gedmintas, L.; Azimi, R.; Rizvi, H.; Riess, J.W.; Hellmann, M.D.; et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J. Clin. Oncol. 2018, 36, 1905–1912. [Google Scholar] [CrossRef]
- Wei, K.; Sun, T.; Feng, X.; Chen, Y.; Liu, Q.; Tang, H. PD-1/L1 immune checkpoint inhibitors for KRAS-mutant non-small cell lung cancer: A multicenter retrospective real-world study. BMC Cancer 2025, 25, 444. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ali, M.A.S.; Alqifari, S.F.; Salem, H.A.; Qasem, K.A.; Alanazi, F.E.; Alhowiti, A.; Alatawi, A.M.; Mirghani, H.; Alrasheed, T.; et al. Is Anlotinib and Radiotherapy Combination Effective for Non-Small-Cell Lung Cancer with Brain Metastases? A Systematic Scoping Review and Meta-Analysis. Pharmaceuticals 2025, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Spano, J.-P.; Massiani, M.-A.; Bentata, M.; Rixe, O.; Friard, S.; Bossi, P.; Rouges, F.; Katlama, C.; Breau, J.-L.; Morere, J.-F. Lung cancer in patients with HIV infection and review of the literature. Med. Oncol. 2004, 21, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Frega, S.; Ferro, A.; Bonanno, L.; Guarneri, V.; Conte, P.; Pasello, G. Lung cancer (LC) in HIV positive patients: Pathogenic features and implications for treatment. Int. J. Mol. Sci. 2020, 21, 1601. [Google Scholar] [CrossRef]
- Takaishi, S.; Okumura, T.; Wang, T.C. Gastric cancer stem cells. J. Clin. Oncol. 2008, 26, 2876–2882. [Google Scholar] [CrossRef]
- Hetta, H.F.; Alqifari, S.F.; Alshehri, K.; Alhowiti, A.; Alharbi, S.S.; Mirghani, H.; Alrasheed, T.; Mostafa, M.E.A.; Sheikh, M.; Elodemi, M.; et al. Efficacy of Anlotinib Plus Docetaxel in Advanced NSCLC Previously Treated with Platinum-Based Chemotherapy: A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 652. [Google Scholar] [CrossRef]
- Hetta, H.F.; Aljohani, H.M.; Sirag, N.; Elfadil, H.; Salama, A.; Al-Twalhy, R.; Alanazi, D.; Al-johani, M.D.; Albalawi, J.H.; Al-Otaibi, R.M.; et al. Synergizing Success: The Role of Anlotinib Combinations in Advanced Non-Small Cell Lung Cancer Treatment. Pharmaceuticals 2025, 18, 585. [Google Scholar] [CrossRef]
- Stankov, M.; Behrens, G. HIV-therapy associated lipodystrophy: Experimental and clinical evidence for the pathogenesis and treatment. Endocr. Metab. Immune Disord.-Drug Targets 2007, 7, 237–249. [Google Scholar]
- Willig, A.L.; Overton, E.T. Metabolic consequences of HIV: Pathogenic insights. Curr. HIV/AIDS Rep. 2014, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Wang, P.-F.; Chen, Y.; Song, S.-Y.; Wang, T.-J.; Ji, W.-J.; Li, S.-W.; Liu, N.; Yan, C.-X. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front. Pharmacol. 2017, 8, 730. [Google Scholar] [CrossRef]
- Naigeon, M.; Roulleaux Dugage, M.; Danlos, F.-X.; Boselli, L.; Jouniaux, J.-M.; de Oliveira, C.; Ferrara, R.; Duchemann, B.; Berthot, C.; Girard, L. Human virome profiling identified CMV as the major viral driver of a high accumulation of senescent CD8+ T cells in patients with advanced NSCLC. Sci. Adv. 2023, 9, eadh0708. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef]
- Sahin, I.H.; Kane, S.R.; Brutcher, E.; Guadagno, J.; Smith, K.E.; Wu, C.; Lesinski, G.B.; Gunthel, C.J.; El-Rayes, B.F. Safety and efficacy of immune checkpoint inhibitors in patients with cancer living with HIV: A perspective on recent progress and future needs. JCO Oncol. Pract. 2020, 16, 319–325. [Google Scholar] [CrossRef]
- Alatawi, A.D.; Alaqyl, A.B.; Alalawi, R.J.; Alqarni, R.S.; Sufyani, R.A.; Alqarni, G.S.; Alqarni, R.S.; Albalawi, J.H.; Alsharif, R.A.; Alatawi, G.I.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Human Immunodeficiency Virus-Associated Cancer: A Systematic Scoping Review. Diseases 2025, 13, 230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetta, H.F.; Alatawi, Y.; Alanazi, F.E.; Alattar, A.; Alshaman, R.; Alshareef, H.; Alatawi, Z.; Alatawi, M.S.; Albalawi, J.H.; Alosaimi, G.A.; et al. Evaluating the Safety and Efficacy of PD-1 Inhibitors in HIV Patients Diagnosed with Lung Cancer: A Systematic Review. Pharmaceuticals 2025, 18, 1654. https://doi.org/10.3390/ph18111654
Hetta HF, Alatawi Y, Alanazi FE, Alattar A, Alshaman R, Alshareef H, Alatawi Z, Alatawi MS, Albalawi JH, Alosaimi GA, et al. Evaluating the Safety and Efficacy of PD-1 Inhibitors in HIV Patients Diagnosed with Lung Cancer: A Systematic Review. Pharmaceuticals. 2025; 18(11):1654. https://doi.org/10.3390/ph18111654
Chicago/Turabian StyleHetta, Helal F., Yasser Alatawi, Fawaz E. Alanazi, Abdullah Alattar, Reem Alshaman, Hanan Alshareef, Zinab Alatawi, Majd S. Alatawi, Jumana H. Albalawi, Ghadeer A. Alosaimi, and et al. 2025. "Evaluating the Safety and Efficacy of PD-1 Inhibitors in HIV Patients Diagnosed with Lung Cancer: A Systematic Review" Pharmaceuticals 18, no. 11: 1654. https://doi.org/10.3390/ph18111654
APA StyleHetta, H. F., Alatawi, Y., Alanazi, F. E., Alattar, A., Alshaman, R., Alshareef, H., Alatawi, Z., Alatawi, M. S., Albalawi, J. H., Alosaimi, G. A., Sayad, R., & Nageeb, W. M. (2025). Evaluating the Safety and Efficacy of PD-1 Inhibitors in HIV Patients Diagnosed with Lung Cancer: A Systematic Review. Pharmaceuticals, 18(11), 1654. https://doi.org/10.3390/ph18111654







