Determination of Nitrosamine Drug Substance-Related Impurities Derived from Nortriptyline and Sertraline Using LC-MS/MS: A Comparative Evaluation of Chromatographic Separation and Pharmaceutical Application
Abstract
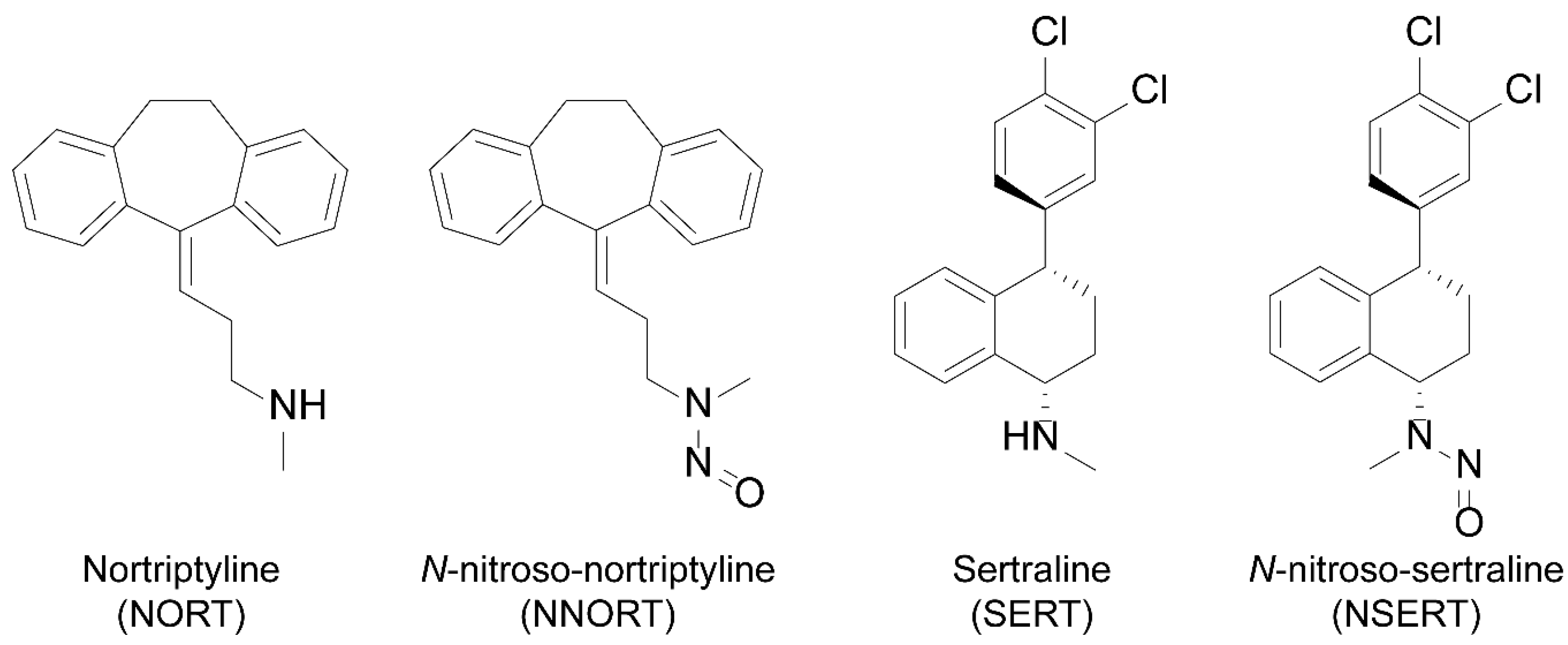
1. Introduction
2. Results and Discussion
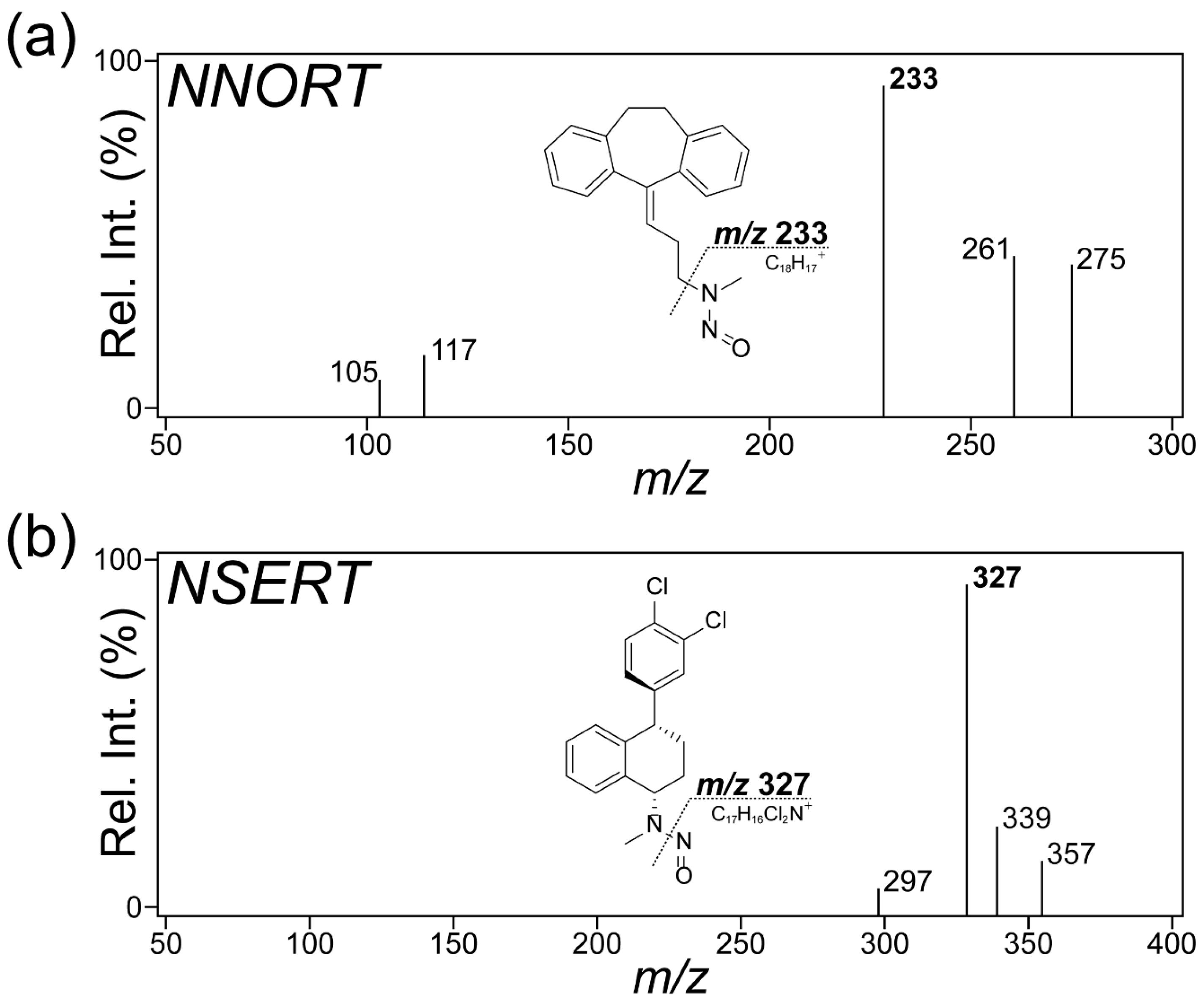
2.1. Optimization of MS Conditions for API and NDSRI of Two Antidepressant Drugs
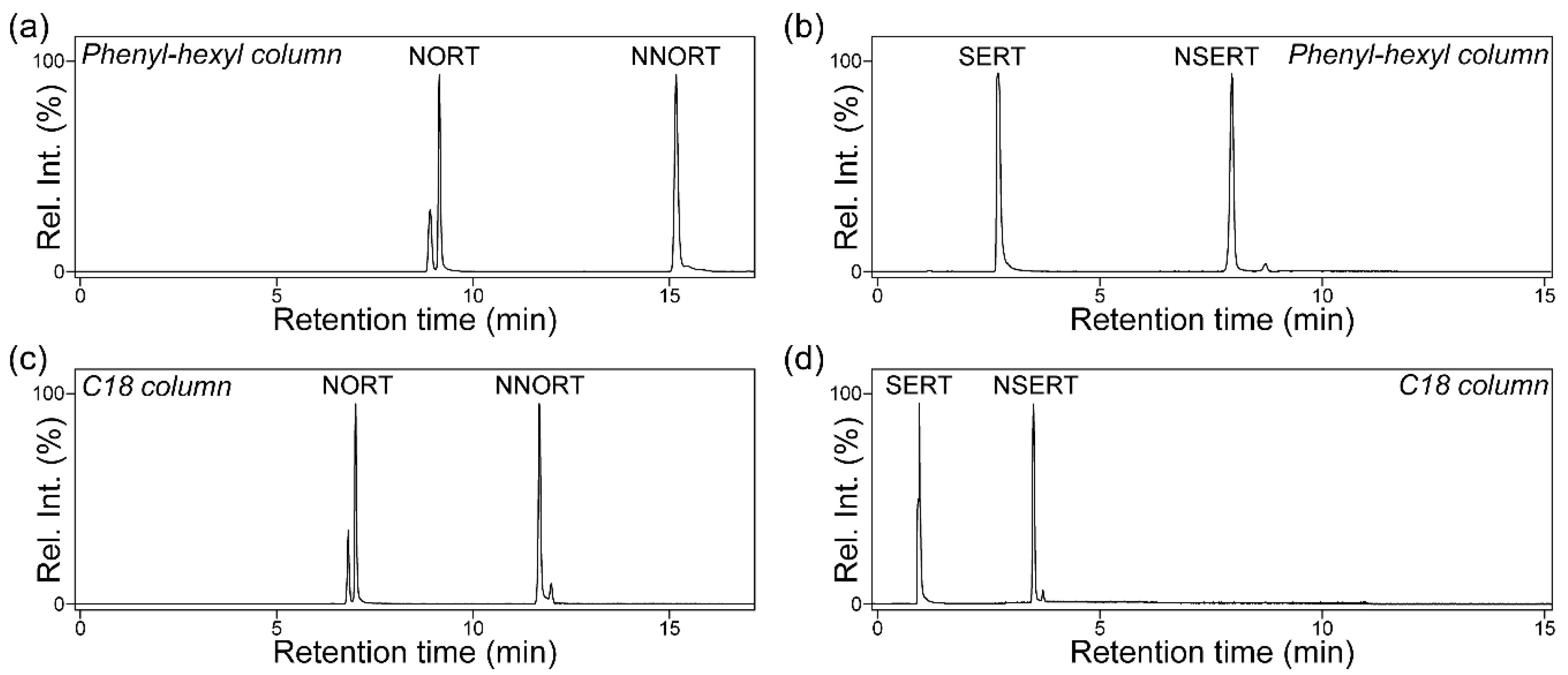
2.2. Comparative Evaluation of Reversed-Phase Stationary Phases for Optimal NDSRI/API Separation
2.3. Method Validation and Assessment of Performance, Applicability and Environmental Compatibility
2.4. Monitoring of NNORT and NSERT in Their Respective Drug Products
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. LC-MS/MS Analysis
3.3.1. LC-MS/MS Analysis of NORT
3.3.2. LC-MS/MS Analysis of SERT
3.4. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NDSRIs | nitrosamine drug substance-related impurities |
| NORT | nortriptyline |
| SERT | sertraline |
| NNORT | N-nitroso-nortriptyline |
| NSERT | N-nitroso-sertraline |
| LC | liquid chromatography |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| API | active pharmaceutical ingredient |
| CPCA | Carcinogenic Potency Categorization Approach |
| AI | acceptable intake |
| MRM | multiple reaction monitoring |
| RT | retention time |
| Rs | peak resolution |
| LOQ | limit of quantitation |
| RSD | relative standard deviation |
| STD/IS | standard-to-IS |
| TF | tailing factor |
| ESI | electrospray ionization |
| LOD | limit of detection |
| HPLC | high-performance LC |
| S/N | signal-to-noise |
References
- EMA. Nitrosamine Impurities in Human Medicines: The Response of the European Medicines Regulatory Network; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Aishwarya, D.; Dhampalwar, V.R.; Pallaprolu, N.; Peraman, R. Nitrosamine Drug Substance-Related Impurities (NDSRIs) in Pharmaceuticals: Formation, Mitigation Strategies, and Emphasis on Mutagenicity Risks. Pharm. Res. 2025, 42, 547–578. [Google Scholar] [CrossRef]
- Schlingemann, J.; Burns, M.J.; Ponting, D.J.; Avila, C.M.; Romero, N.E.; Jaywant, M.A.; Smith, G.F.; Ashworth, I.W.; Simon, S.; Saal, C.; et al. The Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals. J. Pharm. Sci. 2023, 112, 1287–1304. [Google Scholar] [CrossRef]
- Vogel, M.; Escher, S.E.; Weiler, E.; Londenberg, A.; Deppenmeier, U.; Whomsley, R. Quantitative Investigation of Nitrosamine Drug Substance-Related Impurities (NDSRIs) Under Artificial Gastric Conditions by Liquid Chromatography-Tandem Mass Spectrometry and Structure-Activity Relationship Analysis. Drug Test. Anal. 2025, 17, 1772–1784. [Google Scholar] [CrossRef]
- Ashworth, I.W.; Curran, T.; Dirat, O.; Zheng, J.J.; Whiting, M.; Lee, D. A Consideration of the Extent That Tertiary Amines Can Form N-Nitroso Dialkylamines in Pharmaceutical Products. Org. Process Res. Dev. 2023, 27, 1714–1718. [Google Scholar] [CrossRef]
- Akkaraju, H.; Tatia, R.; Mane, S.S.; Khade, A.B.; Dengale, S.J. A comprehensive review of sources of nitrosamine contamination of pharmaceutical substances and products. Regul. Toxicol. Pharm. 2023, 139, 105355. [Google Scholar] [CrossRef]
- Cioc, R.C.; Joyce, C.; Mayr, M.; Bream, R.N. Formation of N-Nitrosamine Drug Substance Related Impurities in Medicines: A Regulatory Perspective on Risk Factors and Mitigation Strategies. Org. Process Res. Dev. 2023, 27, 1736–1750. [Google Scholar] [CrossRef]
- FDA. US Updates on Possible Mitigation Strategies to Reduce the Risk of Nitrosamine Drug Substance-Related Impurities; Food and Drug Administration: Silver Spring, MD, USA, 2023.
- American Pharmaceutical Review. Using the Carcinogenic Potency Categorization Approach (CPCA) to Classify N-Nitrosamine Impurities. 15 March 2024. Available online: https://documents.lgcstandards.com/MediaGallery/Catalogues_Publications/LGC_CPCA_Infographic.pdf (accessed on 10 October 2025).
- Kruhlak, N.L.; Schmidt, M.; Froetschl, R.; Graber, S.; Haas, B.; Horne, I.; Horne, S.; King, S.T.; Koval, I.A.; Kumaran, G.; et al. Determining recommended acceptable intake limits for N-nitrosamine impurities in pharmaceuticals: Development and application of the Carcinogenic Potency Categorization Approach (CPCA). Regul. Toxicol. Pharmacol. 2024, 150, 105640. [Google Scholar] [CrossRef]
- FDA. US Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities; Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Seo, J.E.; Xu, H.; Li, X.; Atrakchi, A.H.; McGovern, T.; Davis Bruno, K.L.; Keire, D.A.; Mei, N.; Heflich, R.H.; Guo, X. Genotoxicity evaluation of ten nitrosamine drug substance-related impurities using 2D and 3D HepaRG cell models. Regul. Toxicol. Pharmacol. 2025, 162, 105906. [Google Scholar] [CrossRef]
- Cross, K.P.; Ponting, D.J. Developing Structure-Activity Relationships for N-Nitrosamine Activity. Comput. Toxicol. 2021, 20. [Google Scholar] [CrossRef]
- Holm, R.; Elder, D.P. Analytical advances in pharmaceutical impurity profiling. Eur. J. Pharm. Sci. 2016, 87, 118–135. [Google Scholar] [CrossRef]
- Snodin, D.J. Mutagenic impurities in pharmaceuticals: A critical assessment of the cohort of concern with a focus on N-nitrosamines. Regul. Toxicol. Pharmacol. 2023, 141, 105403. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nantaphol, S.; Noppakhunsomboon, K.; Rojsitthisak, P. An update on the current status and prospects of nitrosation pathways and possible root causes of nitrosamine formation in various pharmaceuticals. Saudi Pharm. J. 2023, 31, 295–311. [Google Scholar] [CrossRef]
- Muijsers, R.B.; Plosker, G.L.; Noble, S. Sertraline: A review of its use in the management of major depressive disorder in elderly patients. Drugs Aging 2002, 19, 377–392. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, Y.; Wang, S.; Song, J.; Ding, Y.; Wang, Y.; Dong, C.; Liu, J.; Qiu, W.; Qi, W. Nortriptyline hydrochloride, a potential candidate for drug repurposing, inhibits gastric cancer by inducing oxidative stress by triggering the Keap1-Nrf2 pathway. Sci. Rep. 2024, 14, 6050. [Google Scholar] [CrossRef]
- Horne, S.; Vera, M.D.; Nagavelli, L.R.; Sayeed, V.A.; Heckman, L.; Johnson, D.; Berger, D.; Yip, Y.Y.; Krahn, C.L.; Sizukusa, L.O.; et al. Regulatory Experiences with Root Causes and Risk Factors for Nitrosamine Impurities in Pharmaceuticals. J. Pharm. Sci. 2023, 112, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Nitrosamine Impurities in Medications: Overview. 1 August 2025. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities.html (accessed on 10 October 2025).
- Government of Canada. Amitriptyline (10&50 mg): NDMA and NNORT Impurity. 16 August 2023. Available online: https://recalls-rappels.canada.ca/en/alert-recall/amitriptyline-10-50mg-ndma-and-nnort-impurity (accessed on 10 October 2025).
- Department of Health, Drug Office, Canada. Recall: Apo-Amitriptyline Tablets: NNORT Impurity. 14 August 2023. Available online: https://recalls-rappels.canada.ca/en/alert-recall/apo-amitriptyline-tablets-nnort-impurity (accessed on 10 October 2025).
- Manchuri, K.M.; Shaik, M.A.; Gopireddy, V.S.R.; Naziya, S.; Gogineni, S. Analytical Methodologies to Detect N-Nitrosamine Impurities in Active Pharmaceutical Ingredients, Drug Products and Other Matrices. Chem. Res. Toxicol. 2024, 37, 1456–1483. [Google Scholar] [CrossRef]
- Chagarlamudi, K.; Damarapurapu, R.; Gurram, V.S.S.; Manglige, V.K.; Boppana, S.S.S.K. High-Sensitivity LC-MS/MS Method for Precise Quantification of N-Nitroso Sertraline in the Antidepressant Sertraline Drug Products. Sep. Sci. Plus 2025, 8, e70048. [Google Scholar] [CrossRef]
- Asare, S.O.; Hoskins, J.N.; Blessing, R.A.; Hertzler, R.L. Mass spectrometry based fragmentation patterns of nitrosamine compounds. Rapid Commun. Mass. Spectrom. 2022, 36, e9261. [Google Scholar] [CrossRef]
- Cooney, J.W.A.a.J.D. Some Aspects of the Mass Spectra of.N-Nitrosamines. Can. J. Chem. 1971, 49, 3584–3589. [Google Scholar] [CrossRef]
- Ganesh, V.; Poorna Basuri, P.; Sahini, K.; Nalini, C.N. Retention behaviour of analytes in reversed-phase high-performance liquid chromatography-A review. Biomed. Chromatogr. 2023, 37, e5482. [Google Scholar] [CrossRef]
- Valko, K.; Bevan, C.; Reynolds, D. Chromatographic Hydrophobicity Index by Fast-Gradient RP-HPLC: A High-Throughput Alternative to log P/log D. Anal. Chem. 1997, 69, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Satti, P.; Vallamkonda, B.; Sharma, S.; Vinod. Quantitative analysis of nitrosamine impurities using liquid chromatography tandem mass spectrometry. Pure Appl. Chem. 2025. [Google Scholar] [CrossRef]
- Timmer, B.J.J.; Mooibroek, T.J. Intermolecular pi-pi Stacking Interactions Made Visible. J. Chem. Educ. 2021, 98, 540–545. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). CDER Nitrosamine Impurity Acceptable Intake Limits; Food and Drug Administration: Silver Spring, MD, USA, 2025.
- European Medicines Agency. ICH Guideline Q2(R2) on Validation of Analytical Procedures; ICH: Geneva, Switzerland, 2022; Volume 1. [Google Scholar]
| Compound | Retention Time (min) | Conc. Range (ng/g) | Calibration Curve | R2 | LOD (ng/g) | LOQ (ng/g) | Spiked Conc. (ng/g) | Intra-Day | Inter-Day | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | ||||||||
| NNORT | 15.19 | 20–4000 | Y = 1.641x − 0.07364 | 0.9984 | 6.7 | 20 | 20 | 99.4 ± 3.82% | 3.84% | 97.4 ± 3.86% | 3.96% |
| 1000 | 96.6 ± 0.72% | 0.74% | 97.1 ± 2.08% | 2.14% | |||||||
| 4000 | 99.4 ± 1.76% | 1.77% | 99.1 ± 0.78% | 0.79% | |||||||
| NSERT | 7.95 | 125–600 | Y = 0.00249x − 0.0291 | 0.9979 | 42.5 | 125 | 125 | 99.3 ± 0.23% | 0.23% | 99.2 ± 0.42% | 0.43% |
| 375 | 99.4 ± 1.87% | 1.88% | 98.6 ± 0.53% | 0.54% | |||||||
| 600 | 98.6 ± 1.19% | 1.21% | 99.3 ± 0.44% | 0.44% | |||||||
| NNORT | NSERT | |||||||
|---|---|---|---|---|---|---|---|---|
| RT RSD | STD/IS Ratio RSD | TF (±STDEV) | Rs (±STDEV) | RT RSD | STD/IS Ratio RSD | TF (±STDEV) | Rs (±STDEV) | |
| Column temperature −4 °C | 0.05% | 2.32% | 1.46 (±0.34) | 2.67 (±0.16) | 0.38% | 1.61% | 0.98 (±0.07) | 5.70 (±2.31) |
| Column temperature +4 °C | 0.05% | 3.00% | 1.55 (±0.14) | 2.78 (±0.11) | 0.44% | 2.50% | 1.09 (±0.06) | 6.01 (±0.70) |
| Flow rate −0.05 mL/min | 0.06% | 0.06% | 1.62 (±0.18) | 2.58 (±0.06) | 0.16% | 2.03% | 0.99 (±0.05) | 10.89 (±2.55) |
| Flow rate +0.05 mL/min | 0.08% | 4.21% | 1.46 (±0.46) | 2.87 (±0.11) | 0.16% | 5.75% | 1.01 (±0.08) | 12.15 (±1.17) |
| NNORT Concentration (ng/g) | NSERT Concentration (ng/g) | ||
|---|---|---|---|
| Batch_01 | 203.0 ± 1.2 | Product_01 | 84.8 ± 13.6 |
| Batch_02 | 188.3 ± 19.1 | Product_02 | 196.5 ± 15.1 |
| Batch_03 | 180.9 ± 28.9 | Product_03 | 469.4 ± 91.7 |
| Product_04 | 54.7 ± 8.6 | ||
| Product_05 | 176.4 ± 27.0 | ||
| Product_06 | 50.4 ± 5.4 | ||
| Compound | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|
| NNORT | 293.2 | 233.2 1 | 10 |
| 293.2 | 117.2 | 20 | |
| 293.2 | 199.2 | 30 | |
| NNORT-d3 | 296.2 | 233.2 1 | 10 |
| 296.2 | 105.2 | 30 | |
| NSERT | 357.1 | 326.9 1 | 8 |
| 357.1 | 265.0 | 23 | |
| 357.1 | 241.0 | 30 | |
| NSERT-13C,15N,d3 | 362.0 | 332.0 1 | 8 |
| 362.0 | 280.0 | 23 |
| Calibration Point | Concentration (ng/g) | |
|---|---|---|
| NNORT | NSERT | |
| CS1 | 20 | 125 |
| CS2 | 200 | 250 |
| CS3 | 1000 | 375 |
| CS4 | 2000 | 500 |
| CS5 | 4000 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, M.; Kim, J.Y.; Jung, S.; Hong, M.; Han, S.B.; Lee, D.-K. Determination of Nitrosamine Drug Substance-Related Impurities Derived from Nortriptyline and Sertraline Using LC-MS/MS: A Comparative Evaluation of Chromatographic Separation and Pharmaceutical Application. Pharmaceuticals 2025, 18, 1673. https://doi.org/10.3390/ph18111673
Shim M, Kim JY, Jung S, Hong M, Han SB, Lee D-K. Determination of Nitrosamine Drug Substance-Related Impurities Derived from Nortriptyline and Sertraline Using LC-MS/MS: A Comparative Evaluation of Chromatographic Separation and Pharmaceutical Application. Pharmaceuticals. 2025; 18(11):1673. https://doi.org/10.3390/ph18111673
Chicago/Turabian StyleShim, Minki, Ji Yeon Kim, Seungjin Jung, Minkyeong Hong, Sang Beom Han, and Dong-Kyu Lee. 2025. "Determination of Nitrosamine Drug Substance-Related Impurities Derived from Nortriptyline and Sertraline Using LC-MS/MS: A Comparative Evaluation of Chromatographic Separation and Pharmaceutical Application" Pharmaceuticals 18, no. 11: 1673. https://doi.org/10.3390/ph18111673
APA StyleShim, M., Kim, J. Y., Jung, S., Hong, M., Han, S. B., & Lee, D.-K. (2025). Determination of Nitrosamine Drug Substance-Related Impurities Derived from Nortriptyline and Sertraline Using LC-MS/MS: A Comparative Evaluation of Chromatographic Separation and Pharmaceutical Application. Pharmaceuticals, 18(11), 1673. https://doi.org/10.3390/ph18111673








