Efficacy and Safety of Non-Surgical Treatments for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Literature Search and Information Sources
2.3. Data Extraction and Data Items
2.4. Quality Appraisal
2.5. Data Synthesis
3. Results
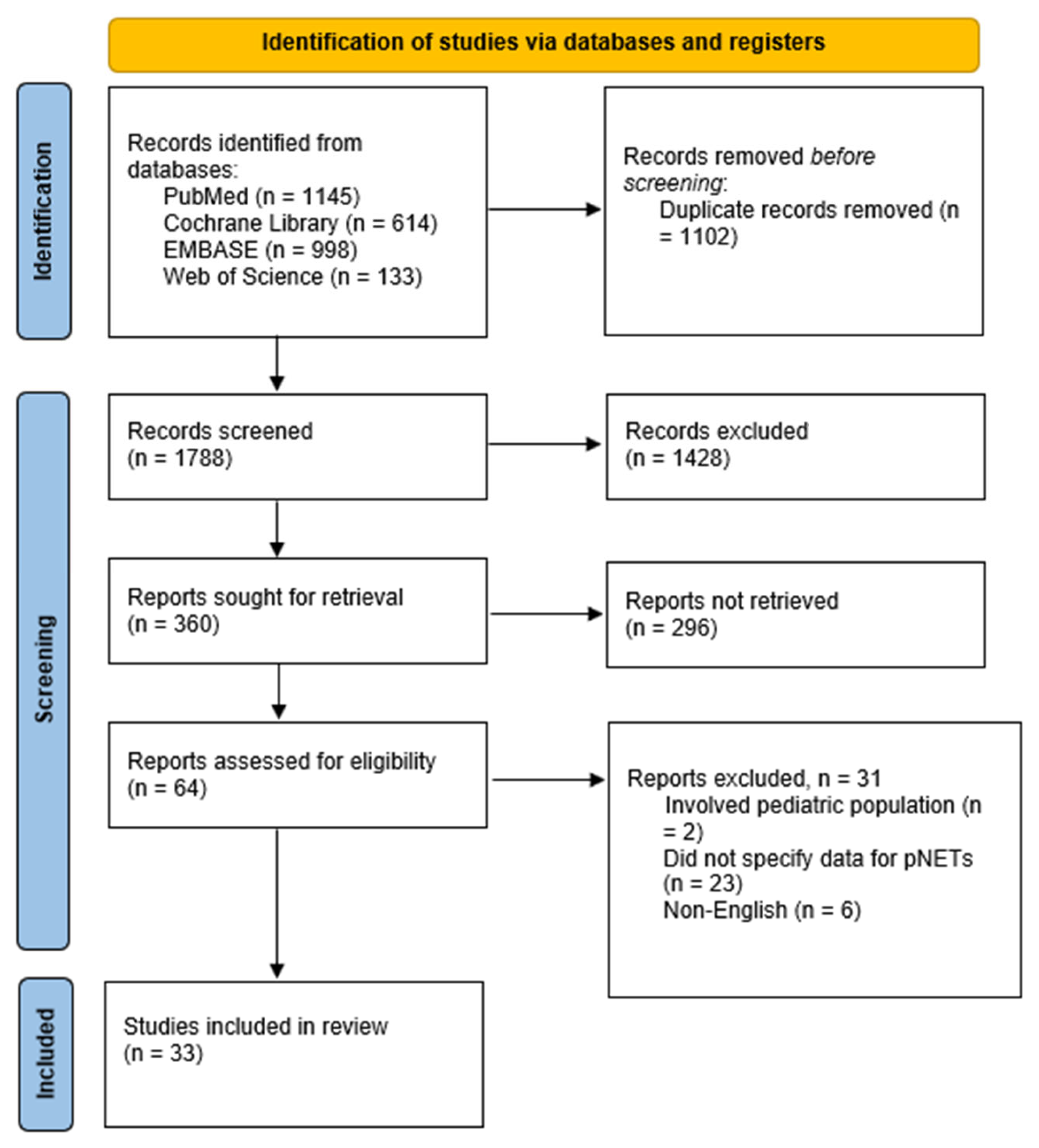
3.1. Search Results
3.2. Characteristics of Included Studies
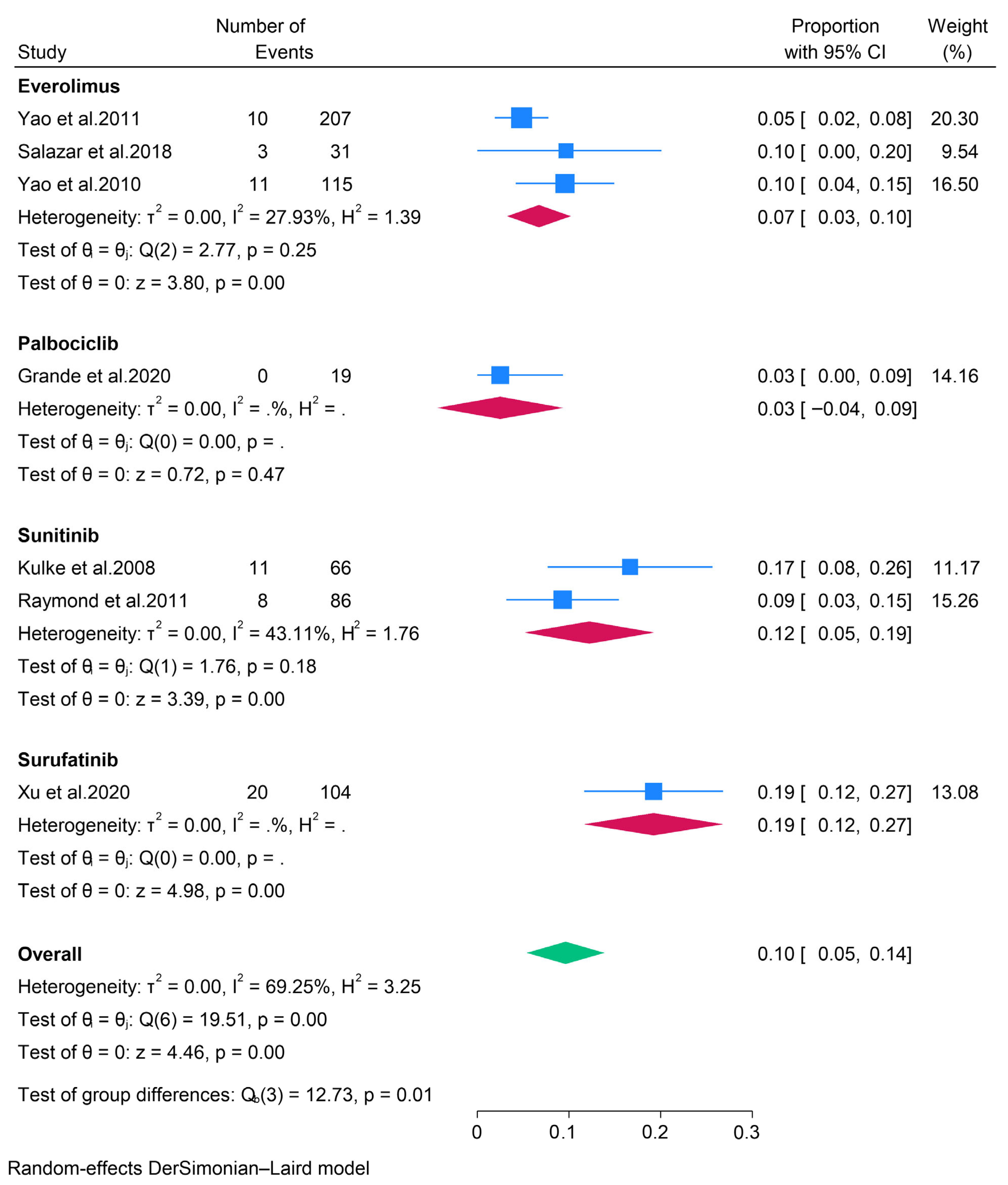
3.2.1. Targeted Therapies
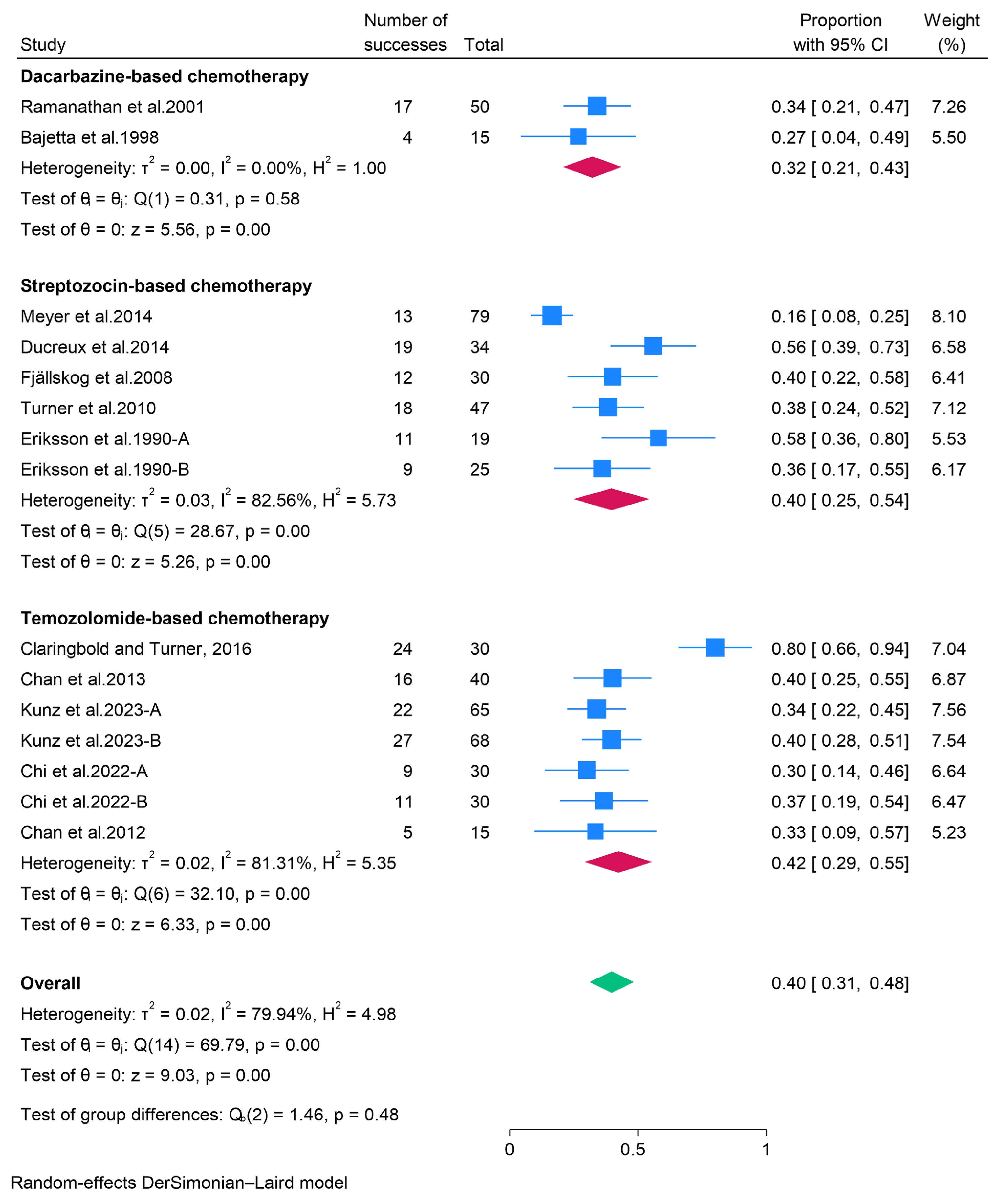
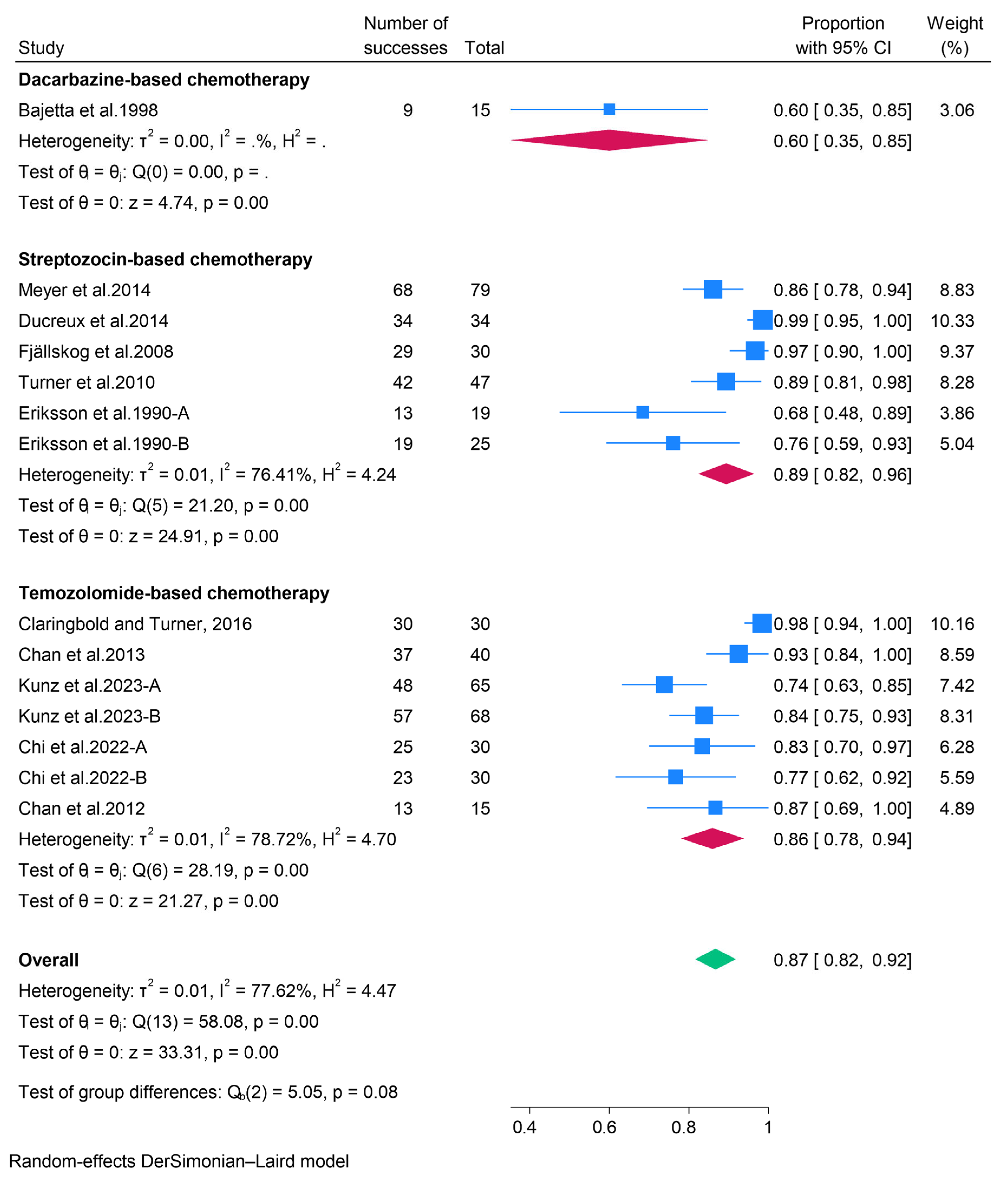
3.2.2. Cytotoxic Chemotherapy
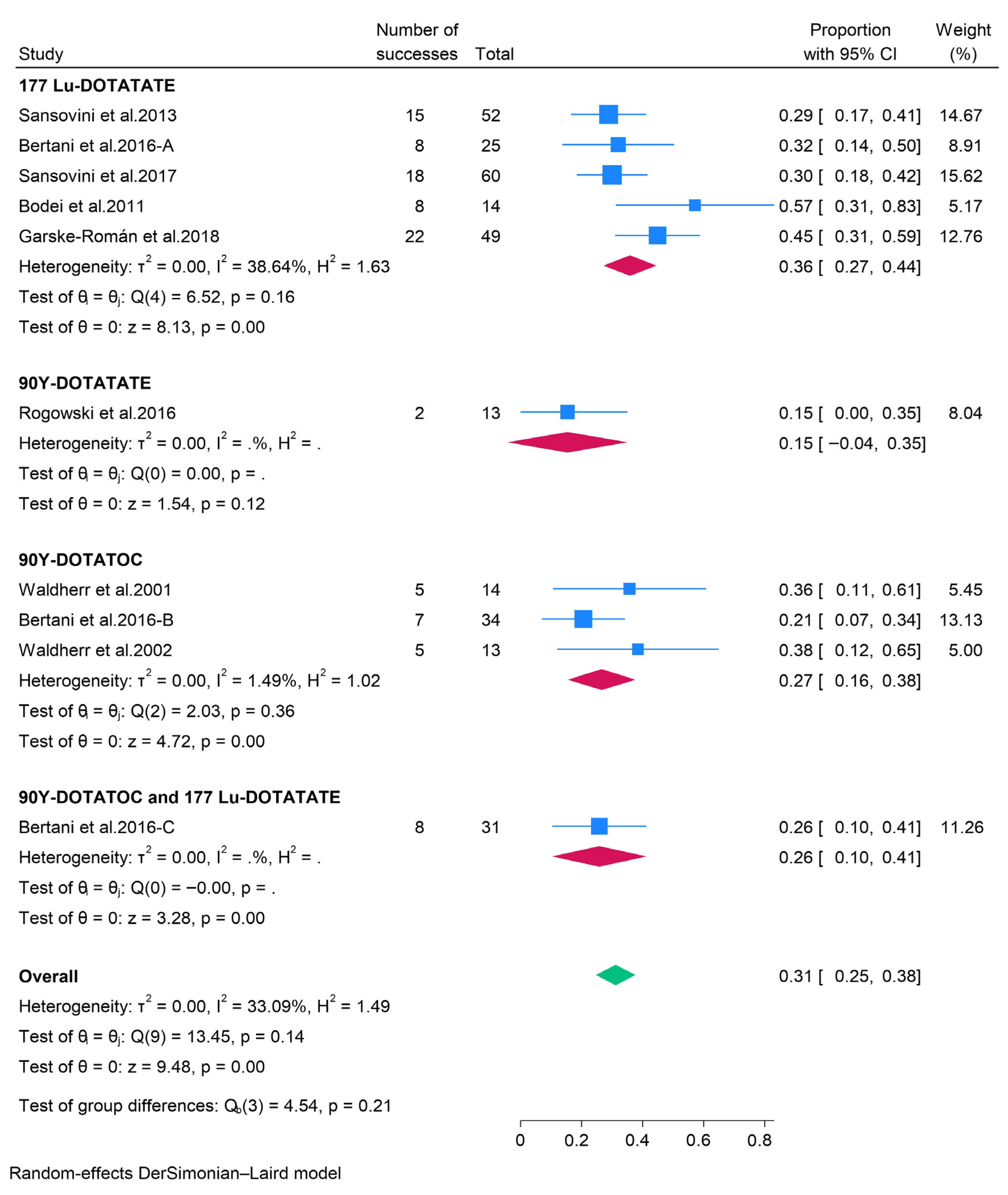
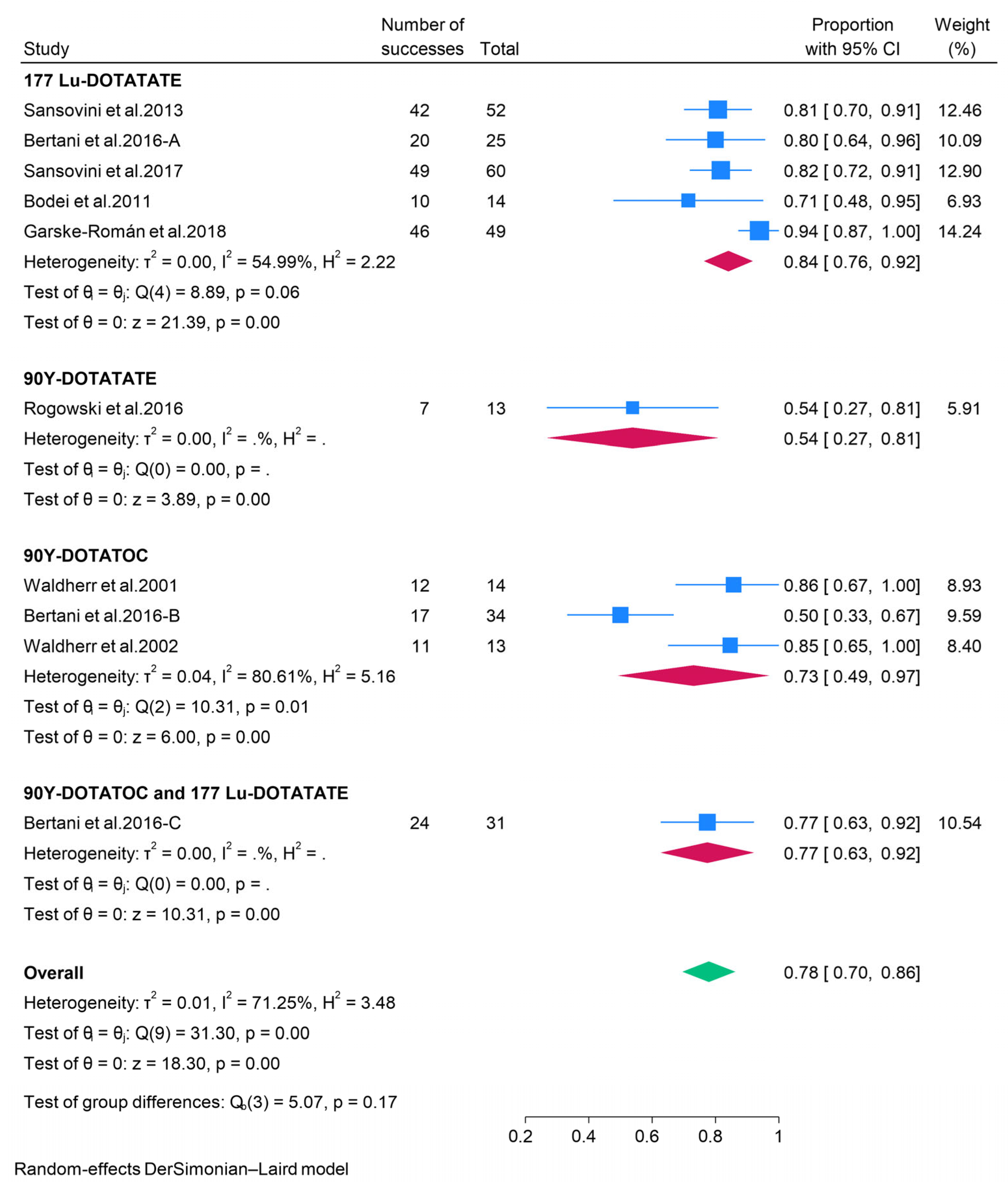
3.2.3. Peptide Receptor Radionuclide Therapy (PRRT)
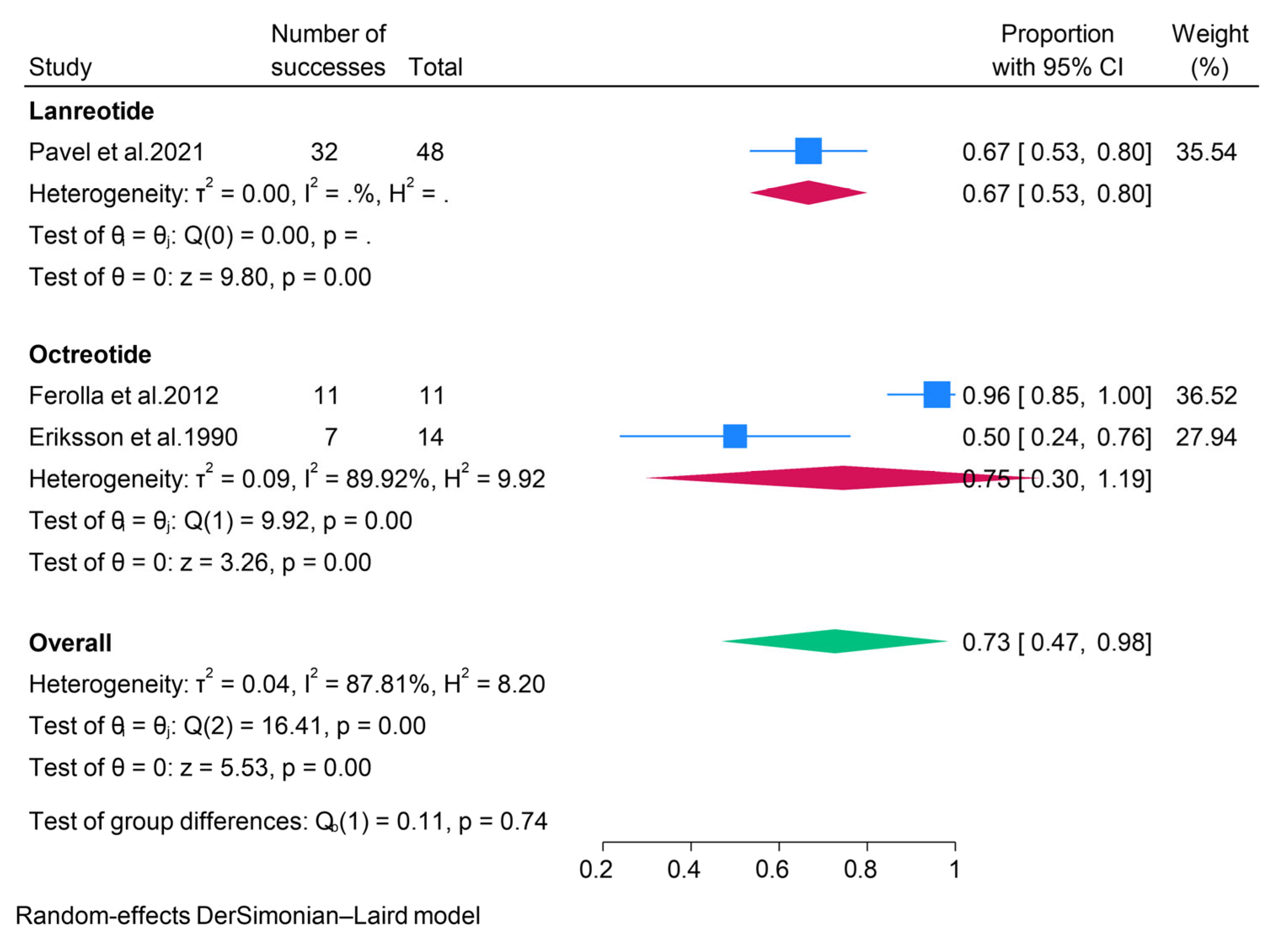
3.2.4. Somatostatin Analogues (SSAs)
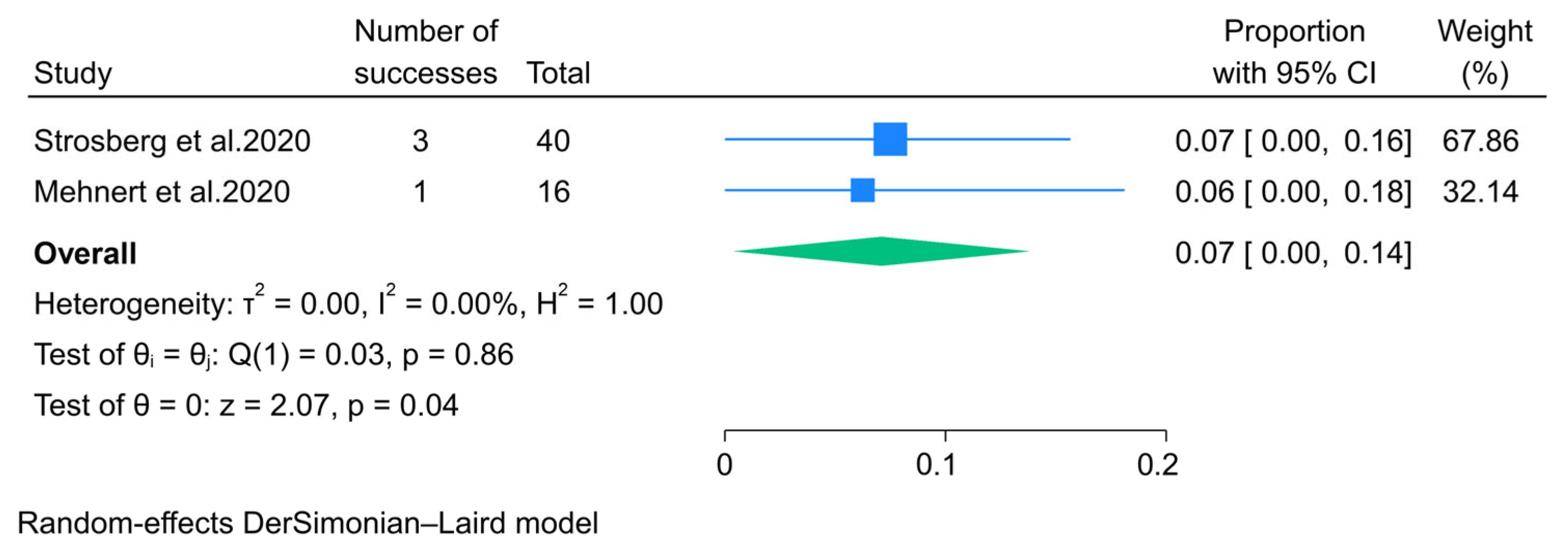
3.2.5. Immunotherapy
3.3. Meta-Regression Analysis
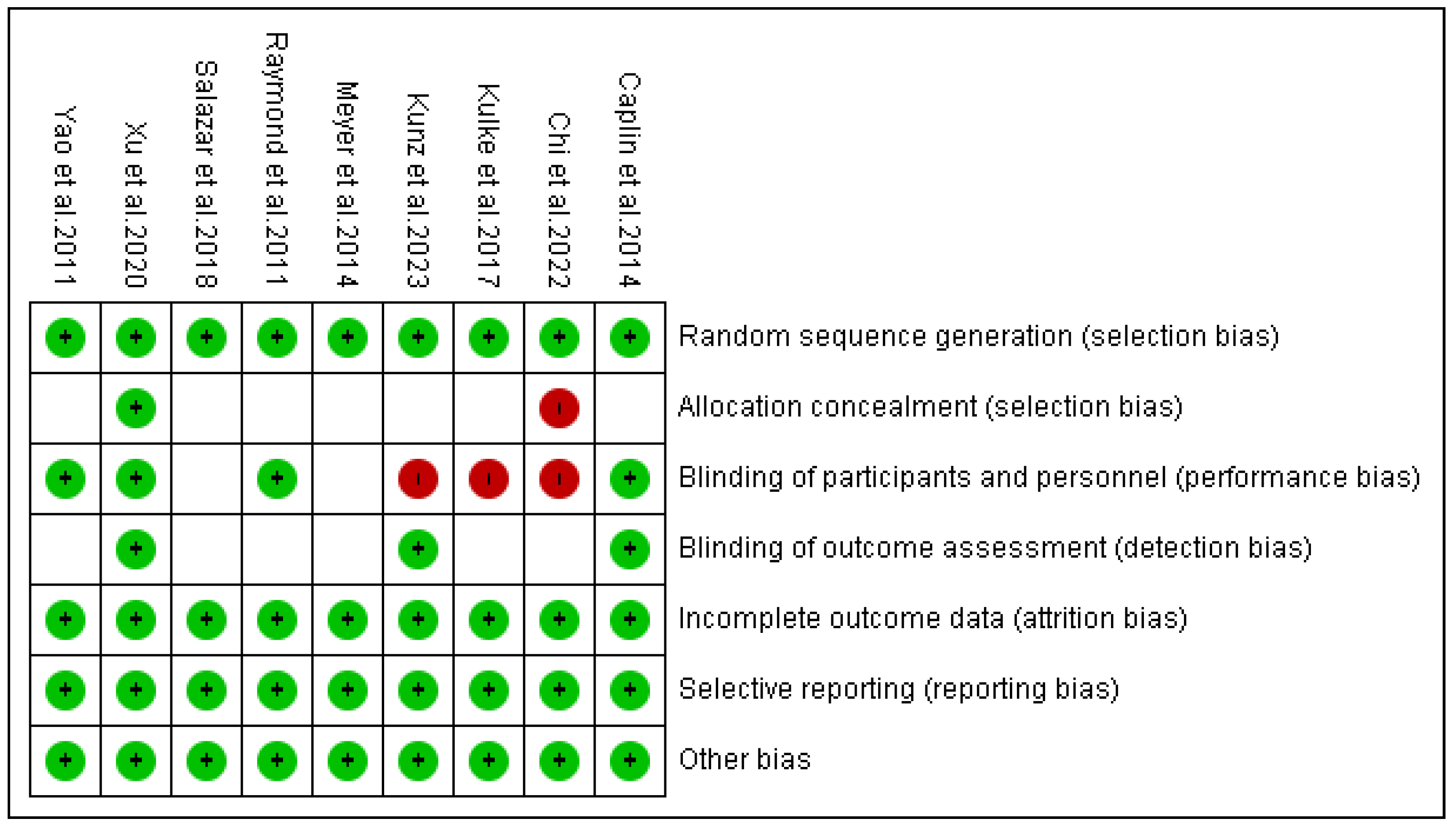
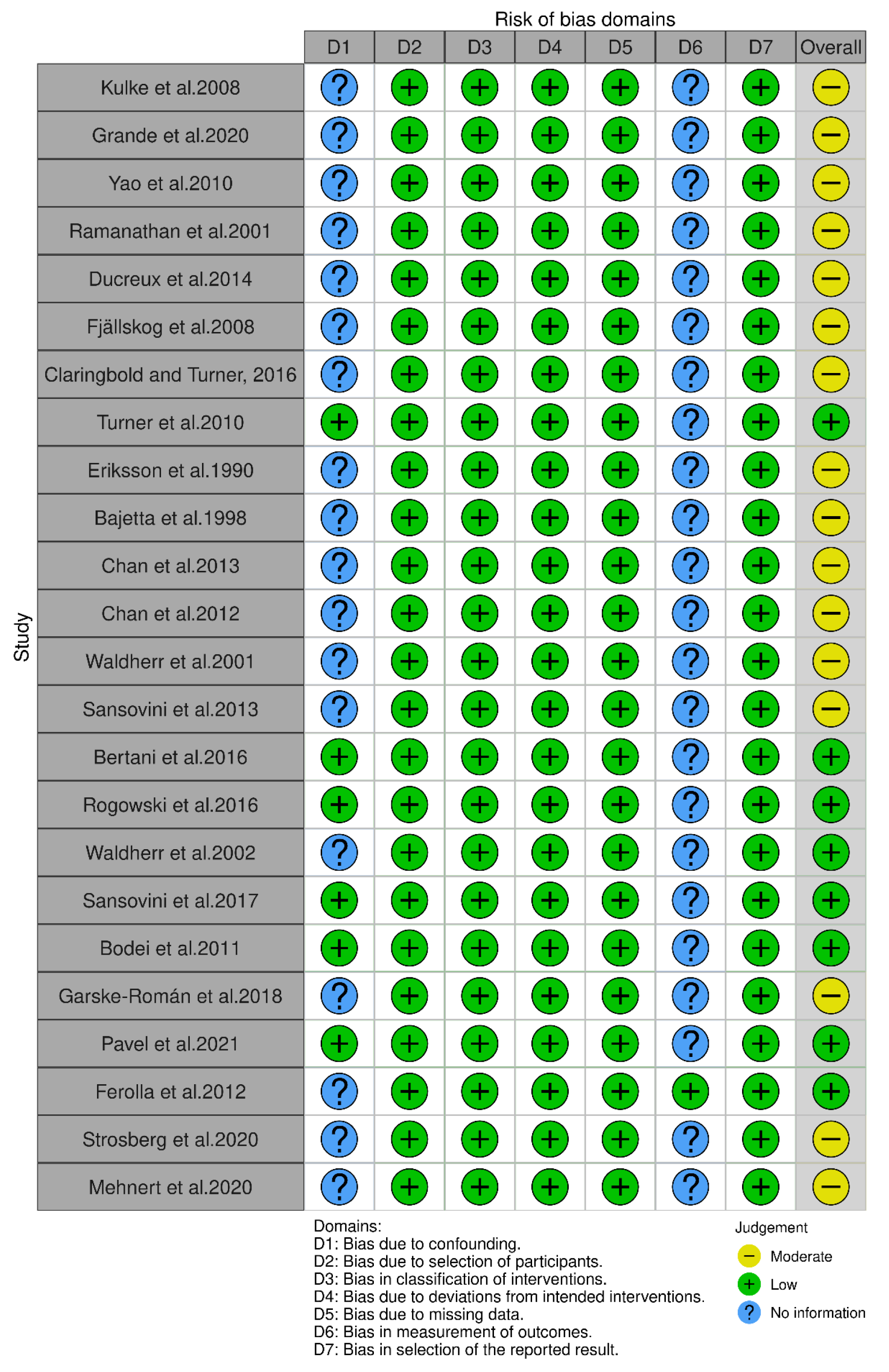
3.4. Risk of Bias Outcomes
4. Discussion
4.1. Comparison with Existing Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| CI | Confidence Interval |
| CR | Complete Response |
| DCR | Disease Control Rate |
| DOTATATE | DOTA-Tyr3-Octreotate |
| DOTATOC | DOTA-Tyr3-Octreotide |
| FDA | Food and Drug Administration |
| IV | Intravenous |
| LAR | Long-Acting Release |
| Lu | Lutetium |
| N/A | Not Applicable |
| NET | Neuroendocrine Tumor |
| NR | Not Reported |
| ORR | Objective Response Rate |
| pNET | Pancreatic Neuroendocrine Tumor |
| PICOS | Population, Intervention, Comparison, Outcomes, and Study Design |
| PR | Partial Response |
| PRRT | Peptide Receptor Radionuclide Therapy |
| RCT | Randomized Controlled Trial |
| RoB-2 | Cochrane Risk of Bias tool version 2 |
| ROBINS-I | Risk Of Bias In Non-randomized Studies-of Interventions |
| SD | Stable Disease |
| SSA | Somatostatin Analog |
| SSTR | Somatostatin Receptor |
| WHO | World Health Organization |
| Y | Yttrium |
Appendix A. Search Strategy
- PubMed
- Embase
- Cochrane Library
- Web of Science
References
- Ramage, J.K.; Ahmed, A.; Ardill, J.; Bax, N.; Breen, D.J.; Caplin, M.E.; Corrie, P.; Davar, J.; Davies, A.H.; Lewington, V.; et al. Guidelines for the Management of Gastroenteropancreatic Neuroendocrine (Including Carcinoid) Tumours. Gut 2005, 54, iv1–iv16. [Google Scholar] [CrossRef] [PubMed]
- Goldin, S.B.; Aston, J.; Wahi, M.M. Sporadically occurring functional pancreatic endocrine tumors: Review of recent literature. Curr. Opin. Oncol. 2008, 20, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Pape, U.F.; Böhmig, M.; Berndt, U.; Tiling, N.; Wiedenmann, B.; Plöckinger, U. Survival and Clinical Outcome of Patients with Neuroendocrine Tumors of the Gastroenteropancreatic Tract in a German Referral Center. Ann. N. Y. Acad. Sci. 2004, 1014, 222–233. [Google Scholar] [CrossRef]
- Oberg, K. Pancreatic endocrine tumors. Semin. Oncol. 2010, 37, 594–618. [Google Scholar] [CrossRef]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef]
- McKenna, L.R.; Edil, B.H. Update on pancreatic neuroendocrine tumors. Gland Surg. 2014, 3, 258–275. [Google Scholar]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic neuroendocrine tumors: Biology, diagnosis, and treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef]
- Perren, A.; Couvelard, A.; Scoazec, J.Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Ruszniewski, P.; Van Cutsem, E.; Lombard-Bohas, C.; Valle, J.W.; De Herder, W.W.; Pavel, M.; Degtyarev, E.; Brase, J.C.; Bubuteishvili-Pacaud, L.; et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann. Oncol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, L.; Bai, C.; Wang, W.; Li, J.; Yu, X.; Li, Z.; Li, E.; Yuan, X.; Chi, Y.; et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Kulke, M.H.; Lenz, H.J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Salazar, R.; Garcia-Carbonero, R.; Libutti, S.K.; Hendifar, A.E.; Custodio, A.; Guimbaud, R.; Lombard-Bohas, C.; Ricci, S.; Klümpen, H.J.; Capdevila, J.; et al. Phase II Study of BEZ235 versus Everolimus in Patients with Mammalian Target of Rapamycin Inhibitor-Naïve Advanced Pancreatic Neuroendocrine Tumors. Oncologist 2017, 23, 766-e90. [Google Scholar] [CrossRef]
- Grande, E.; Teulé, A.; Alonso-Gordoa, T.; Jiménez-Fonseca, P.; Benavent, M.; Capdevila, J.; Custodio, A.; Vera, R.; Munarriz, J.; La Casta, A.; et al. The PALBONET Trial: A Phase II Study of Palbociclib in Metastatic Grade 1 and 2 Pancreatic Neuroendocrine Tumors (GETNE-1407). Oncologist 2020, 25, 745-e1265. [Google Scholar] [CrossRef]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St Peter, J.; Haas, T.; Lebwohl, D.; et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: A phase II trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Cnaan, A.; Hahn, R.G.; Carbone, P.P.; Haller, D.G. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann. Oncol. 2001, 12, 1139–1143. [Google Scholar] [CrossRef]
- Meyer, T.; Qian, W.; Caplin, M.E.; Armstrong, G.; Lao-Sirieix, S.H.; Hardy, R.; Valle, J.W.; Talbot, D.C.; Cunningham, D.; Reed, N.; et al. Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur. J. Cancer 2014, 50, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Dahan, L.; Smith, D.; O’Toole, D.; Lepère, C.; Dromain, C.; Vilgrain, V.; Baudin, E.; Lombard-Bohas, C.; Scoazec, J.Y.; et al. Bevacizumab combined with 5-FU/streptozocin in patients with progressive metastatic well-differentiated pancreatic endocrine tumours (BETTER trial)—A phase II non-randomised trial. Eur. J. Cancer 2014, 50, 3098–3106. [Google Scholar] [CrossRef] [PubMed]
- Fjallskog, M.L.H.; Janson, E.T.; Falkmer, U.G.; Vatn, M.H.; Oberg, K.E.; Eriksson, B.K. Treatment with combined streptozotocin and liposomal doxorubicin in metastatic endocrine pancreatic tumors. Neuroendocrinology 2008, 88, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Claringbold, P.G.; Turner, J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2015, 103, 432–439. [Google Scholar] [CrossRef]
- Turner, N.C.; Strauss, S.J.; Sarker, D.; Gillmore, R.; Kirkwood, A.; Hackshaw, A.; Papadopoulou, A.; Bell, J.; Kayani, I.; Toumpanakis, C.; et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br. J. Cancer 2010, 102, 1106–1112. [Google Scholar] [CrossRef]
- Eriksson, B.; Skogseid, B.; Lundqvist, G.; Wide, L.; Wilander, E.; Oberg, K. Medical treatment and long-term survival in a prospective study of 84 patients with endocrine pancreatic tumors. Cancer 1990, 65, 1883–1890. [Google Scholar] [CrossRef]
- Bajetta, E.; Rimassa, L.; Carnaghi, C.; Seregni, E.; Ferrari, L.; Di Bartolomeo, M.; Regalia, E.; Cassata, A.; Procopio, G.; Mariani, L. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer 1998, 83, 372–378. [Google Scholar] [CrossRef]
- Chan, J.A.; Blaszkowsky, L.; Stuart, K.; Zhu, A.X.; Allen, J.; Wadlow, R.; Ryan, D.P.; Meyerhardt, J.; Gonzalez, M.; Regan, E.; et al. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. Cancer 2013, 119, 3212–3218. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Chi, Y.; Song, L.; Liu, W.; Zhou, Y.; Miao, Y.; Fang, W.; Tan, H.; Shi, S.; Jiang, H.; Xu, J.; et al. S-1/temozolomide versus S-1/temozolomide plus thalidomide in advanced pancreatic and non-pancreatic neuroendocrine tumours (STEM): A randomised, open-label, multicentre phase 2 trial. eClinicalMedicine 2022, 54, 101667. [Google Scholar] [CrossRef]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef]
- Waldherr, C.; Pless, M.; Maecke, H.R.; Haldemann, A.; Mueller-Brand, J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: A clinical phase II study. Ann. Oncol. 2001, 12, 941–945. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ambrosetti, A.; Monti, M.; Nanni, O.; Sarnelli, A.; Bodei, L.; Garaboldi, L.; Bartolomei, M.; Paganelli, G. Treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 2013, 97, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Grana, C.; Bodei, L.; Funicelli, L.; Ferrari, C.; Spada, F.; Partelli, S.; et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 981–989. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, W.; Wachuła, E.; Lewczuk, A.; Buscombe, J.R.; Seklecka, N.; Sankowski, A. Long-term efficacy of (90)Y-DOTATATE in patients with nonresectable pancreatic and small bowel neuroendocrine neoplasms. Future Oncol. 2016, 12, 1877–1885. [Google Scholar] [CrossRef]
- Waldherr, C.; Pless, M.; Maecke, H.R.; Schumacher, T.; Crazzolara, A.; Nitzsche, E.U.; Haldemann, A.; Mueller-Brand, J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J. Nucl. Med. 2002, 43, 610–616. [Google Scholar]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Grana, C.M.; Fazio, N.; Iodice, S.; Baio, S.M.; Bartolomei, M.; Lombardo, D.; Ferrari, M.E.; Sansovini, M.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: The IEO phase I-II study. Eur. J. Nucl. Med. 2011, 38, 2125–2135. [Google Scholar] [CrossRef]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. 2018, 45, 970–988. [Google Scholar] [CrossRef]
- Pavel, M.; Ćwikła, J.B.; Lombard-Bohas, C.; Borbath, I.; Shah, T.; Pape, U.F.; Capdevila, J.; Panzuto, F.; Truong Thanh, X.M.; Houchard, A.; et al. Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur. J. Cancer 2021, 157, 403–414. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Ferolla, P.; Faggiano, A.; Grimaldi, F.; Ferone, D.; Scarpelli, G.; Ramundo, V.; Severino, R.; Bellucci, M.C.; Camera, L.M.; Lombardi, G.; et al. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J. Endocrinol. Investig. 2011, 35, 326–331. [Google Scholar]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Fazio, N.; Buzzoni, R.; Delle Fave, G.; Tesselaar, M.E.; Wolin, E.; Van Cutsem, E.; Tomassetti, P.; Strosberg, J.; Voi, M.; Bubuteishvili-Pacaud, L.; et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci. 2017, 109, 174–181. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Cortazar, P.; Zhang, J.J.; Tang, S.; Sridhara, R.; Murgo, A.; Justice, R.; Pazdur, R. FDA approval summary: Sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist 2012, 17, 1108–1113. [Google Scholar] [CrossRef]
- Merola, E.; Alonso Gordoa, T.; Zhang, P.; Al-Toubah, T.; Pellè, E.; Kolasińska-Ćwikła, A.; Zandee, W.; Laskaratos, F.; de Mestier, L.; Lamarca, A.; et al. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is ≥10%? Oncologist 2020, 26, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Bogdani, C.; O’Dwyer, E.; Siolas, D. Emerging innovations in theranostics for pancreatic neuroendocrine tumors. NPJ Precis. Oncol. 2025, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Scoazec, J.Y.; Couvelard, A. Réseau TENpath. [Classification of pancreatic neuroendocrine tumours: Changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future]. Ann. Pathol. 2017, 37, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Mittal, B.R. 177Lu-DOTATATE peptide receptor radionuclide therapy versus Everolimus in advanced pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Nucl. Med. Commun. 2019, 40, 1195–1203. [Google Scholar] [CrossRef]


| Criterion | Included | Excluded |
|---|---|---|
| Population (P) | Adults (age ≥ 18 years) with pancreatic neuroendocrine tumors and without restriction on previous treatment. | Children (<18 years) or patients with non-pancreatic neuroendocrine tumors |
| Interventions (I) | Surgery, chemotherapy, targeted therapies, somatostatin analogs, immunotherapy, or peptide receptor radionuclide therapy. | Liver-directed therapies include chemoembolization, radioembolization, radiofrequency ablation, liver transplant, and arterial embolization. |
| Control (C) | Placebo, usual care, or no treatment | - |
| Outcomes (O) | Efficacy-related outcomes, such as objective response rate (ORR) and disease control rate (DCR) Safety outcomes, i.e., adverse events | - |
| Study Design (S) | Randomized controlled trials (RCTs), non-randomized clinical trials, or single-arm prospective studies. | Retrospective studies, case series, narrative reviews, conference/meeting abstracts, letters to the editor, case reports, meta-analyses, or commentaries. |
| Treatment | Outcome | Covariate | Regression Coefficient | p-Value | Residual Heterogeneity Test |
|---|---|---|---|---|---|
| Streptozocin-based therapy | ORR | Sample size | −0.281 | 0.0002 | Q_res: 4.99 p = 0.289 |
| Study design | −0.281 | 0.0002 | Q_res: 4.99 p = 0.289 | ||
| Response assessment criteria | −0.085 | 0.577 | Q_res: 22.80 p = 0.0001 | ||
| Line of treatment | −0.097 | 0.558 | Q_res: 24.41 p = 0.0001 | ||
| DCR | Sample size | −0.041 | 0.649 | Q_res: 15.98 p = 0.003 | |
| Study design | −0.041 | 0.649 | Q_res: 15.98 p = 0.003 | ||
| Response assessment criteria | 0.073 | 0.392 | Q_res: 20.41 p = 0.0004 | ||
| Line of treatment | 0.205 | 0.01 | Q_res: 10.49 p = 0.033 | ||
| Temozolomide-based therapy | ORR | Sample size | −0.0799 | 0.599 | Q_res: 24.48 p < 0.001 |
| Study design | −0.181 | 0.124 | Q_res: 19.54 p = 0.002 | ||
| Line of treatment | −0.439 | <0.0001 | Q_res: 1.36 p = 0.929 | ||
| DCR | Sample size | −0.105 | 0.134 | Q_res: 14.01 p = 0.016 | |
| Study design | −0.162 | <0.0001 | Q_res: 5.31 p = 0.380 | ||
| Line of treatment | −0.150 | 0.016 | Q_res: 8.63 p = 0.125 | ||
| 177Lu-DOTATATE | ORR | Sample size | −0.133 | 0.048 | Q_res: 2.62 p = 0.454 |
| Response assessment criteria | 0.133 | 0.048 | Q_res: 2.62 p = 0.454 | ||
| DCR | Sample size | −0.058 | 0.425 | Q_res: 5.20 p = 0.157 | |
| Response assessment criteria | 0.058 | 0.425 | Q_res: 5.20 p = 0.157 | ||
| 90Y-DOTATOC | ORR | Response assessment criteria | −0.164 | 0.156 | Q_res: 0.02 p = 0.883 |
| DCR | Response assessment criteria | −0.352 | 0.001 | Q_res: 0.01 p = 0.936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQahtani, M.S.; Miutescu, B.; Domilescu, I.; Negru, S.; Popovici, D.; Gadour, E. Efficacy and Safety of Non-Surgical Treatments for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 1650. https://doi.org/10.3390/ph18111650
AlQahtani MS, Miutescu B, Domilescu I, Negru S, Popovici D, Gadour E. Efficacy and Safety of Non-Surgical Treatments for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(11):1650. https://doi.org/10.3390/ph18111650
Chicago/Turabian StyleAlQahtani, Mohammed Saad, Bogdan Miutescu, Ielmina Domilescu, Serban Negru, Dorel Popovici, and Eyad Gadour. 2025. "Efficacy and Safety of Non-Surgical Treatments for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 11: 1650. https://doi.org/10.3390/ph18111650
APA StyleAlQahtani, M. S., Miutescu, B., Domilescu, I., Negru, S., Popovici, D., & Gadour, E. (2025). Efficacy and Safety of Non-Surgical Treatments for Pancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Pharmaceuticals, 18(11), 1650. https://doi.org/10.3390/ph18111650