The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma
Abstract
1. Introduction
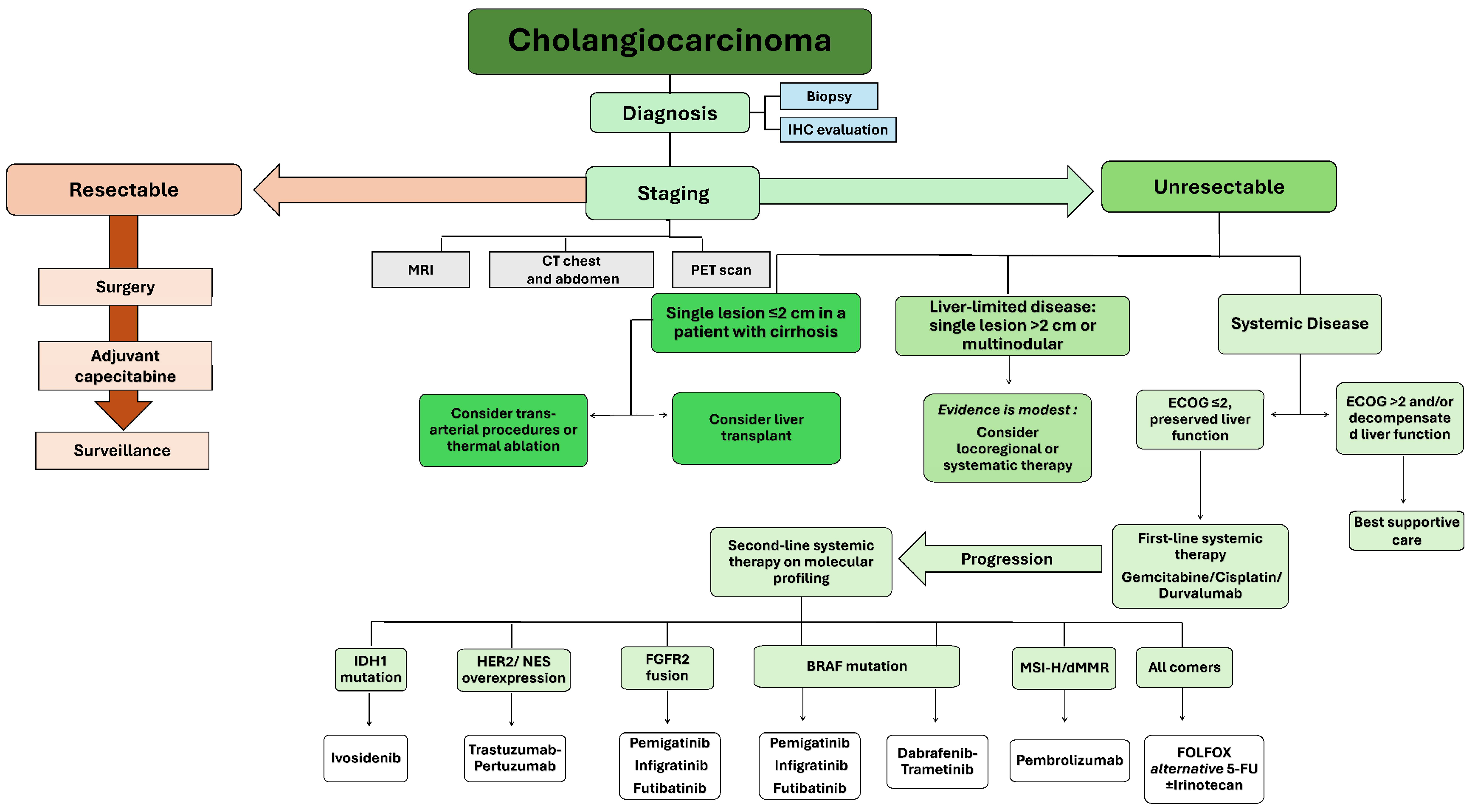
2. Systemic Therapy for CCA
2.1. Targeted Therapy
- IDH1 mutations are present in around 10–20% of CCA cases and are responsible for the accumulation of oncometabolites, which leads to epigenetic changes affecting various signaling pathways. The FDA has approved ivosidenib for treating adult patients with unresectable or metastatic IDH1-mutated CCA as detected by an FDA-approved test, specifically in cases where the disease has progressed after one to two prior lines of systemic therapy for advanced disease. This approval was based on data from the phase III ClarIDHy study, a double-blind placebo-controlled trial. Ivosidenib, the first IDH1 inhibitor, has shown significant improvements in both PFS (2.7 vs. 1.4 months) and OS (10.3 vs. 5.1 months [adjusted median OS]) compared to placebo in patients with chemorefractory IDH1-mutated CCA [67].
- HER2 overexpression: In 15–20% of extrahepatic CCA and gallbladder carcinomas, there is observed overexpression or amplification of the EGFR family receptor tyrosine-protein kinase erbB-2 (HER2) [21]. Several investigations into HER2 alterations’ targeting initially yielded discouraging findings. Between 2021 and 2023, a total of eleven studies examined outcomes from seven distinct HER2-targeted medications in BTC. Among these, one of the most recent trials, the phase II trial NCT02091141, explored trastuzumab with pertuzumab. The study enrolled 39 patients, resulting in an ORR of 23% (95% CI 11–329) and a median OS of 10.9 months (95% CI 5.2–15.6) [68]. The phase II open-label ROAR trial demonstrated a 47% objective response rate with the BRAF inhibitor dabrafenib combined with trametinib [69,70]. Up to 20% of CCA, particularly dCCA, have amplifications of HER2, and accumulating evidence supports the benefit of HER2-targeted therapies, such as trastuzumab plus pertuzumab or lapatinib, or trastuzumab deruxtecan, in such cases [71,72,73].
- FGFR2 fusions: Mutations in FGFR2 are present in 13–20% of CCAs, often involving activating translocations through fusion or rearrangements. These mutations drive increased cell proliferation, metastasis, and angiogenesis. Pemigatinib received FDA approval for the treatment of adults facing previously treated, unresectable locally advanced or metastatic CCA [74]. This approval targets patients with an FGFR2 fusion or rearrangement, as identified through an FDA-approved test. The approval was obtained through the FIGHT-202 (NCT02924376) trial, a multicenter open-label single-arm study. Within this trial, 107 patients with locally advanced unresectable or metastatic CCA, whose disease had progressed post at least one prior therapy, were examined. These patients exhibited an ORR of 36% (95% confidence interval: 27–45), with a median DOR of 9.1 months. Moreover, the FDA granted approval to infigratinib, an inhibitor targeting FGFR1–3. This approval was primarily influenced by its favorable outcomes in a phase II trial (NCT02150967). In this trial, patients with previously treated advanced CCA demonstrated an ORR of 23.1%. The trial revealed a median DoR of 5 months and a median PFS of 7.3 months [21].On the other hand, futibatinib received accelerated approval from the FDA to address the treatment needs of adult patients confronting previously treated, unresectable, locally advanced, or metastatic iCCA exhibiting FGFR2 fusions or other rearrangements. This decision stemmed from the study TAS-120–101, a multicenter open-label, single-arm trial. Notably, the median DoR in this trial was recorded at 9.7 months [75].
- BRAf mutation: In the phase II “VE basket” trial, patients aged 18 years or older with BRAFV600E-mutated BTC, whether it was unresectable, metastatic, locally advanced, or recurrent, were included. Over a median follow-up of 10 months, 22 out of 43 patients achieved an investigator-assessed overall response, yielding a response rate of 51% (95% CI 36–67). Additionally, the median overall survival (mOS) was 14 months (95% CI 10–33). These promising outcomes led to the FDA approval of the dabrafenib and trametinib combination for treating advanced BRAF V600E-mutated solid malignancies in patients who had undergone prior therapy but experienced progression [76].
- MSI-H: Pembrolizumab has received FDA approval as a treatment for patients facing metastatic or inoperable solid tumors characterized by high microsatellite instability (MSI) or mismatch repair (MMR) deficiency. This approval is supported by findings from two pivotal studies: KEYNOTE-158 (NCT02628067) and KEYNOTE-028 (NCT02054806) [77].
- Second-line therapy initiation is contingent upon the tumor’s mutational profile, as delineated in the targeted therapy section. For patients lacking actionable alterations or facing contraindications to targeted treatment, chemotherapy remains the preferred treatment modality. In contrast to first-line chemotherapy, very limited RCTs support an optimal second-line regimen. Moreover, there is no well-established stratification or selection system for second-line therapy candidates. Among some possible regimens, short-term infusional FU plus leucovorin (LV) and oxaliplatin (FOLFOX) is an active regimen for second-line therapy, particularly in patients initially treated with GC [78,79,80]. Danmei Zhang and colleagues conducted a systematic review study, which revealed that while not formally approved, FOLFOX is widely recognized as a standard second-line therapy, supported by findings from the British phase III ABC-06 study [81]. Nevertheless, ongoing debate surrounds whether commencing oxaliplatin-based therapy immediately following cisplatin failure represents the most effective approach, considering both mechanism of action and toxicity. Data from a randomized phase II trial unveiled no discernible difference in OS between FOLFOX and 5-FU, LV, and irinotecan (FOLFIRI). However, the toxicity profiles of these regimens exhibited notable disparities. Neuropathy and thrombocytopenia were more prevalent in the FOLFOX arm, while vomiting and cholangitis were more common in the FOLFIRI arm. Alternatively, the South Korean NIFTY study showcased the superior efficacy of the combination of liposomal irinotecan and 5-FU compared to 5-FU alone [81].
2.2. Immunotherapies
3. Neoadjuvant Therapy for CCA
4. Adjuvant Therapy for CCA
5. Interventional Therapy
5.1. Intra-Arterial Therapies
5.1.1. Hepatic Arterial Infusion Chemotherapy (HAIC)
5.1.2. Conventional Transcatheter Arterial Chemoembolization (TACE)
5.1.3. Drug-Eluting Bead-Transcatheter Arterial Chemoembolization (dbTACE)
5.1.4. Radioembolization (RE)
5.2. Ablation
5.3. Radiation Therapy (RT)
6. Conclusions
Funding
Conflicts of Interest
References
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bismuth, H.; Nakache, R.; Diamond, T. Management strategies in resection for hilar cholangiocarcinoma. Ann. Surg. 1992, 215, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Wood, H.; Logan, R.F.; Quinn, M.; Aithal, G.P. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br. J. Cancer 2006, 94, 1751–1758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Benipal, B. Incidence of Cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer Statistics Analysis of 50 States. Cureus 2019, 11, e3962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaib, Y.H.; El-Serag, H.B.; Davila, J.A.; Morgan, R.; McGlynn, K.A. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology 2005, 128, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Broomé, U.; Olsson, R.; Lööf, L.; Bodemar, G.; Hultcrantz, R.; Danielsson, A.; Prytz, H.; Sandberg-Gertzén, H.; Wallerstedt, S.; Lindberg, G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996, 38, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F. Peripheral cholangiocarcinoma (cholangiocellular carcinoma): Clinical features, diagnosis and treatment. J. Gastroenterol. Hepatol. 1999, 14, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-R.; Lee, C.-U.; Park, H.-J.; Seol, S.-Y.; Chung, J.-M.; Choi, H.-C.; Ahn, Y.-O.; Shigemastu, T. Hepatitis B and C Virus, Clonorchis sinensis for the Risk of Liver Cancer: A Case-Control Study in Pusan, Korea. Int. J. Epidemiol. 1996, 25, 933–940. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D’Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Peng, W.; Feng, X.; Teng, F.; Tang, Y.; Kong, Q.; Chen, Z. Extrahepatic morbidities and mortality of NAFLD: An umbrella review of meta-analyses. Aliment. Pharmacol. Ther. 2022, 56, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2016. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 12 November 2022).
- Mihalache, F.; Tantau, M.; Diaconu, B.; Acalovschi, M. Survival and quality of life of cholangiocarcinoma patients: A prospective study over a 4 year period. J. Gastrointest. Liver Dis. JGLD 2010, 19, 285–290. [Google Scholar] [PubMed]
- Abboud, K.; Umoru, G.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Al-Shamsi, H.O.; Javle, M.; Saharia, A.; Connor, A.A.; Kodali, S. Immune checkpoint inhibitors for solid tumors in the adjuvant setting: Current progress, future directions, and role in transplant oncology. Cancers 2023, 15, 1433. [Google Scholar] [CrossRef]
- Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-approved targeted therapy for cholangiocarcinoma. Cancers 2022, 14, 2641. [Google Scholar] [CrossRef]
- Zhang, Y.; Esmail, A.; Mazzaferro, V.; Abdelrahim, M. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers 2022, 14, 5074. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Tisnado, D.; Malin, J.; Kahn, K.; Landrum, M.B.; Fletcher, R.; Klabunde, C.; Clauser, S.; Rogers, S.O., Jr.; Keating, N.L. Variations in Oncologist Recommendations for Chemotherapy for Stage IV Lung Cancer: What Is the Role of Performance Status? J. Oncol. Pract. 2016, 12, 653–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Furuse, J.; Okusaka, T.; Boku, N.; Ohkawa, S.; Sawaki, A.; Masumoto, T.; Funakoshi, A. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: A multicenter phase II study. Cancer Chemother. Pharmacol. 2008, 62, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Tsumura, T.; Asada, M.; Suzuki, C.; Niimi, M.; Matsumoto, S.; Nishimura, T.; Nitta, T.; Yasuchika, K.; et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2011, 67, 1429–1434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasaki, T.; Isayama, H.; Nakai, Y.; Ito, Y.; Kogure, H.; Togawa, O.; Toda, N.; Yasuda, I.; Hasebe, O.; Maetani, I.; et al. Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2010, 65, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401-MITSUBA). J. Hepatobiliary Pancreat. Sci. 2023, 30, 102–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Hoffman, K.; Sjödén, P.O.; Jacobsson, G.; Sellström, H.; Enander, L.K.; Linné, T.; Svensson, C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996, 7, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bridgewater, J.; Lopes, A.; Palmer, D.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Valle, J.; Wasan, H. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br. J. Cancer 2016, 114, 965–971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Buechner-Steudel, P.; Moehler, M.; Schmalenberg, H.; Behrens, R.; Fahlke, J.; Wein, A.; Behl, S.; Kuss, O.; Kleber, G.; et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: Two parallel, multicentre phase-II trials. Br. J. Cancer 2009, 101, 1846–1852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, R.; Wang, B.; Chen, Y.J.; Li, H.B.; Hu, J.B.; Zou, S.Q. Efficacy of gemcitabine plus platinum agents for biliary tract cancers: A meta-analysis. Anti-Cancer Drugs 2013, 24, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kang, J.H.; Lee, J.; Lee, H.W.; Oh, S.Y.; Jang, J.S.; Lee, M.A.; Sohn, B.S.; Yoon, S.Y.; Choi, H.J.; et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 2019, 30, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nehls, O.; Oettle, H.; Hartmann, J.T.; Hofheinz, R.D.; Hass, H.G.; Horger, M.S.; Koppenhöfer, U.; Hochhaus, A.; Stieler, J.; Trojan, J.; et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: A prospective multicentre phase II trial. Br. J. Cancer 2008, 98, 309–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iqbal, S.; Rankin, C.; Lenz, H.J.; Gold, P.J.; Ahmad, S.A.; El-Khoueiry, A.B.; Messino, M.J.; Holcombe, R.F.; Blanke, C.D. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother. Pharmacol. 2011, 68, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Sahai, V.; Catalano, P.J.; Zalupski, M.M.; Lubner, S.J.; Menge, M.R.; Nimeiri, H.S.; Munshi, H.G.; Benson, A.B., 3rd; O’Dwyer, P.J. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 1707–1712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Elsayed, Z.; Elhalawani, H. Gemcitabine-based chemotherapy for advanced biliary tract carcinomas. Cochrane Database Syst. Rev. 2018, 4, Cd011746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, C.W.; Choi, I.K.; Seo, J.H.; Kim, B.S.; Kim, J.S.; Kim, C.D.; Um, S.H.; Kim, J.S.; Kim, Y.H. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am. J. Clin. Oncol. 2000, 23, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Altamira, P.M.; Ferrante, K.; Jenkins, R.L.; Lewis, W.D.; Huberman, M.S.; Stuart, K.E. A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer 1998, 82, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.A.; Aziz, Z. Prospective evaluation of efficacy and toxicity of 5-fu and folinic acid (Mayo Clinic regimen) in patients with advanced cancer of the gallbladder. Am. J. Clin. Oncol. 2003, 26, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Kato, H.; Matsushiro, T.; Nimura, Y.; Nagakawa, T.; Nakayama, T. Comparison of 5-fluorouracil, doxorubicin and mitomycin C with 5-fluorouracil alone in the treatment of pancreatic-biliary carcinomas. Oncology 1994, 51, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Rougier, P.; Fandi, A.; Clavero-Fabri, M.C.; Villing, A.L.; Fassone, F.; Fandi, L.; Zarba, J.; Armand, J.P. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann. Oncol. 1998, 9, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Patt, Y.Z.; Hassan, M.M.; Aguayo, A.; Nooka, A.K.; Lozano, R.D.; Curley, S.A.; Vauthey, J.N.; Ellis, L.M.; Schnirer, I.I.; Wolff, R.A.; et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 2004, 101, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Kapacee, Z.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J. Clin. Med. 2020, 9, 2854. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizvi, S.; Borad, M.J. The rise of the FGFR inhibitor in advanced biliary cancer: The next cover of time magazine? J. Gastrointest. Oncol. 2016, 7, 789–796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walter, D.; Hartmann, S.; Waidmann, O. Update on cholangiocarcinoma: Potential impact of genomic studies on clinical management. Z. Gastroenterol. 2017, 55, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliveira, D.V.; Zhang, S.; Chen, X.; Calvisi, D.F.; Andersen, J.B. Molecular profiling of intrahepatic cholangiocarcinoma: The search for new therapeutic targets. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Farshidfar, F.; Zheng, S.; Gingras, M.C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakravarty, D.; Johnson, A.; Sklar, J.; Lindeman, N.I.; Moore, K.; Ganesan, S.; Lovly, C.M.; Perlmutter, J.; Gray, S.W.; Hwang, J.; et al. Somatic Genomic Testing in Patients With Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2022, 40, 1231–1258. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mody, K.; Jain, P.; El-Refai, S.M.; Azad, N.S.; Zabransky, D.J.; Baretti, M.; Shroff, R.T.; Kelley, R.K.; El-Khouiery, A.B.; Hockenberry, A.J.; et al. Clinical, Genomic, and Transcriptomic Data Profiling of Biliary Tract Cancer Reveals Subtype-Specific Immune Signatures. JCO Precis. Oncol. 2022, 6, e2100510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavacchi, D.; Caliman, E.; Rossi, G.; Buttitta, E.; Botteri, C.; Fancelli, S.; Pellegrini, E.; Roviello, G.; Pillozzi, S.; Antonuzzo, L. Ivosidenib in IDH1-mutated cholangiocarcinoma: Clinical evaluation and future directions. Pharmacol. Ther. 2022, 237, 108170. [Google Scholar] [CrossRef] [PubMed]
- ten Haaft, B.H.E.A.; Pedregal, M.; Prato, J.; Klümpen, H.-J.; Moreno, V.; Lamarca, A. Revolutionizing anti-HER2 therapies for extrahepatic cholangiocarcinoma and gallbladder cancer: Current advancements and future perspectives. Eur. J. Cancer 2024, 199, 113564. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients With Tumors With BRAF(V600E) Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, P.Y.; Stein, A.; van den Bent, M.; De Greve, J.; Wick, A.; de Vos, F.; von Bubnoff, N.; van Linde, M.E.; Lai, A.; Prager, G.W.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022, 23, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.R.; Kim, J.W.; Cha, Y.; Ha, H.; Park, J.E.; Bang, J.H.; Jin, M.H.; Lee, K.H.; Kim, T.Y.; Han, S.W.; et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 2016, 7, 58007–58021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.H.; Marcus, L.; Horiba, M.N.; Donoghue, M.; Chatterjee, S.; Mishra-Kalyani, P.S.; Schuck, R.N.; Li, Y.; Zhang, X.; Fourie Zirkelbach, J.; et al. FDA Approval Summary: Pemigatinib for Previously Treated, Unresectable Locally Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusion or Other Rearrangement. Clin. Cancer Res. 2023, 29, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Gandhy, S.U.; Casak, S.J.; Mushti, S.L.; Cheng, J.; Subramaniam, S.; Zhao, H.; Zhao, M.; Bi, Y.; Liu, G.; Fan, J.; et al. FDA Approval Summary: Futibatinib for Unresectable Advanced or Metastatic, Chemotherapy Refractory Intrahepatic Cholangiocarcinoma with FGFR2 Fusions or Other Rearrangements. Clin. Cancer Res. 2023, 29, 4027–4031. [Google Scholar] [CrossRef] [PubMed]
- Storandt, M.H.; Kurniali, P.C.; Mahipal, A.; Jin, Z. Targeted Therapies in Advanced Cholangiocarcinoma. Life 2023, 13, 2066. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, T.; Isayama, H.; Nakai, Y.; Ito, Y.; Yasuda, I.; Toda, N.; Kogure, H.; Hanada, K.; Maguchi, H.; Sasahira, N.; et al. A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2013, 71, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Casadei Gardini, A.; Brieau, B.; Vivaldi, C.; Smolenschi, C.; Brandi, G.; Tougeron, D.; Filippi, R.; Vienot, A.; Silvestris, N.; et al. Prediction of survival with second-line therapy in biliary tract cancer: Actualisation of the AGEO CT2BIL cohort and European multicentre validations. Eur. J. Cancer 2019, 111, 94–106. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Shen, J.; Sun, X.; Liu, L.; Dong, J. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J. Chemother. 2014, 26, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dorman, K.; Westphalen, C.B.; Haas, M.; Ormanns, S.; Neumann, J.; Seidensticker, M.; Ricke, J.; De Toni, E.N.; Klauschen, F.; et al. Unresectable biliary tract cancer: Current and future systemic therapy. Eur. J. Cancer 2024, 203, 114046. [Google Scholar] [CrossRef]
- Kim, B.J.; Yoo, C.; Kim, K.P.; Hyung, J.; Park, S.J.; Ryoo, B.Y.; Chang, H.M. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: Retrospective analysis of 321 patients. Br. J. Cancer 2017, 116, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Guion-Dusserre, J.F.; Lorgis, V.; Vincent, J.; Bengrine, L.; Ghiringhelli, F. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2015, 21, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Lubner, S.J.; Mahoney, M.R.; Kolesar, J.L.; Loconte, N.K.; Kim, G.P.; Pitot, H.C.; Philip, P.A.; Picus, J.; Yong, W.P.; Horvath, L.; et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: A phase II Consortium study. J. Clin. Oncol. 2010, 28, 3491–3497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bréchon, M.; Dior, M.; Dréanic, J.; Brieau, B.; Guillaumot, M.A.; Brezault, C.; Mir, O.; Goldwasser, F.; Coriat, R. Addition of an antiangiogenic therapy, bevacizumab, to gemcitabine plus oxaliplatin improves survival in advanced biliary tract cancers. Investig. New Drugs 2018, 36, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Borbath, I.; Van den Eynde, M.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.G.; Yu, Y.; Eathiraj, S.; Wang, Y.; Savage, R.E.; Lapierre, J.M.; Schwartz, B.; Abbadessa, G. Preclinical Activity of ARQ 087, a Novel Inhibitor Targeting FGFR Dysregulation. PLoS ONE 2016, 11, e0162594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perera, T.P.S.; Jovcheva, E.; Mevellec, L.; Vialard, J.; De Lange, D.; Verhulst, T.; Paulussen, C.; Van De Ven, K.; King, P.; Freyne, E.; et al. Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor. Mol. Cancer Ther. 2017, 16, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.O.; Feng, Y.-H.; Chen, Y.-Y.; Su, W.-C.; Oh, D.-Y.; Shen, L.; Kim, K.-P.; Liu, X.; Bai, Y.; Liao, H.; et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J. Clin. Oncol. 2019, 37 (Suppl. 15), 4117. [Google Scholar] [CrossRef]
- Liu, P.C.C.; Koblish, H.; Wu, L.; Bowman, K.; Diamond, S.; DiMatteo, D.; Zhang, Y.; Hansbury, M.; Rupar, M.; Wen, X.; et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS ONE 2020, 15, e0231877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Food and Drug Administration. FDA Grants Accelerated Approval to Pemigatinib for Cholangiocarcinoma with an FGFR2 Rearrangement or Fusion. Available online: www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (accessed on 11 November 2022).
- Guagnano, V.; Furet, P.; Spanka, C.; Bordas, V.; Le Douget, M.; Stamm, C.; Brueggen, J.; Jensen, M.R.; Schnell, C.; Schmid, H.; et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J. Med. Chem. 2011, 54, 7066–7083. [Google Scholar] [CrossRef] [PubMed]
- Nogova, L.; Sequist, L.V.; Perez Garcia, J.M.; Andre, F.; Delord, J.P.; Hidalgo, M.; Schellens, J.H.; Cassier, P.A.; Camidge, D.R.; Schuler, M.; et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1–3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J. Clin. Oncol. 2017, 35, 157–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39 (Suppl. 3), 265. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Maggie, L.S. FOENIX Update: A New Era in Cholangiocarcinoma Precision Medicine. Evid. -Based Oncol. 2022, 28, SP251. [Google Scholar]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berlin, J.; Hong, D.S.; Deeken, J.F.; Boni, V.; Oh, D.-Y.; Patel, J.D.; Nanda, S.; Brega, N.; Childs, B.H.; Hyman, D.M.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion gastrointestinal cancer. J. Clin. Oncol. 2020, 38 (Suppl. S4), 824. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Nathenson, M.; Demetri, G.; Lassen, U.; Hong, D.; Boni, V.; Deeken, J.; Dowlati, A.; Cox, M.; Ku, N.; Cruickshank, S.; et al. Activity of larotrectinib in patients with TRK fusion GI malignancies. Ann. Oncol. 2018, 29 (Suppl. S5), v107. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rolfo, C.D.; Braud, F.G.D.; Doebele, R.C.; Drilon, A.E.; Siena, S.; Patel, M.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; Chiu, C.-H.; et al. Efficacy and safety of entrectinib in patients (pts) with NTRK-fusion positive (NTRK-fp) solid tumors: An updated integrated analysis. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3605. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. FDA Grants Accelerated Approval to Dabrafenib in Combination with Trametinib for Unresectable or Metastatic Solid Tumors with BRAF V600E Mutation; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2022. [Google Scholar]
- Meric-Bernstam, F.; Hainsworth, J.; Bose, R., III.; Howard, A.B.; Friedman, C.F.; Kurzrock, R.; Swanton, C.; Wang, Y.; Levy, J.; Schulze, K.; et al. MyPathway HER2 basket study: Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J. Clin. Oncol. 2021, 39 (Suppl. S15), 3004. [Google Scholar] [CrossRef]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II study of erlotinib in patients with advanced biliary cancer. J. Clin. Oncol. 2006, 24, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kinoshita, M.; Takemura, S.; Tanaka, S.; Hamano, G.; Nakamori, S.; Fujikawa, M.; Sugawara, Y.; Yamamoto, T.; Arimoto, A.; et al. The PD-1/PD-L1 axis may be aberrantly activated in occupational cholangiocarcinoma. Pathol. Int. 2017, 67, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piha-Paul, S.A.; Oh, D.-Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Endres, S.; Kobold, S. Enhancing tumor T cell infiltration to enable cancer immunotherapy. Immunotherapy 2019, 11, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, J. T cell-redirecting bispecific antibodies in cancer immunotherapy: Recent advances. J. Cancer Res. Clin. Oncol. 2019, 145, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Scholler, J.; Schalk, D.L.; June, C.H.; Lum, L.G. Enhanced cytotoxicity against solid tumors by bispecific antibody-armed CD19 CAR T cells: A proof-of-concept study. J. Cancer Res. Clin. Oncol. 2020, 146, 2007–2016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral Immunotherapy-Update 2019. Oncologist 2020, 25, e423–e438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lange, S.; Lampe, J.; Bossow, S.; Zimmermann, M.; Neubert, W.; Bitzer, M.; Lauer, U.M. A novel armed oncolytic measles vaccine virus for the treatment of cholangiocarcinoma. Hum. Gene Ther. 2013, 24, 554–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Z.B.; Chen, Y.; Makhija, S.K.; Lu, B.; Wang, M.; Rivera, A.A.; Yamamoto, M.; Wang, S.; Siegal, G.P.; Curiel, D.T.; et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int. J. Oncol. 2006, 29, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–608; discussion 608-609. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; He, A.R.; Khushman, M.d.; Rayyan, Y. Advances in Immunotherapy for Transplant Oncology. Cancers 2024, 16, 2369. [Google Scholar] [CrossRef]
- Esmail, A.; Badheeb, M.; Alnahar, B.; Almiqlash, B.; Sakr, Y.; Khasawneh, B.; Al-Najjar, E.; Al-Rawi, H.; Abudayyeh, A.; Rayyan, Y. Cholangiocarcinoma: The Current Status of Surgical Options including Liver Transplantation. Cancers 2024, 16, 1946. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, W.A.; Fairfield, C.; Powell, J.J.; Harrison, E.M.; Søreide, K.; Wigmore, S.J.; Guest, R.V. Meta-analysis and Meta-regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann. Surg. 2021, 273, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Xie, H.; Bin-Riaz, I.; Sharma, P.; Durani, U.; Goyal, G.; Borah, B.; Borad, M.J.; Smoot, R.L.; Roberts, L.R.; et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019, 45, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Victor, D.; Kodali, S.; Cheah, Y.L.; Simon, C.J.; Noureddin, M.; Connor, A. Transplant Oncology: An Emerging Discipline of Cancer Treatment. Cancers 2023, 15, 5337. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A. P-168 Combination of gemcitabine plus cisplatin compared to non-gemcitabine and cisplatin regimens as neo-adjuvant treatment in liver transplant recipients with cholangiocarcinoma. Ann. Oncol. 2022, 33, S309–S310. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abudayyeh, A.; Victor, D.; McMillan, R.; Kodali, S. Gemcitabine plus cisplatin versus non-gemcitabine and cisplatin regimens as neoadjuvant treatment for cholangiocarcinoma patients prior to liver transplantation: An institution experience. Front. Oncol. 2022, 12, 908687. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abdelrahim, M. P-169 Feasibility of gemcitabine plus cisplatin as neo-adjuvant in cholangiocarcinoma patients prior to liver transplantation. Ann. Oncol. 2022, 33, S310. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Al-Rawi, H.; Esmail, A.; Xu, J.; Umoru, G.; Ibnshamsah, F.; Abudayyeh, A.; Victor, D.; Saharia, A.; McMillan, R.; et al. Gemcitabine and Cisplatin as Neo-Adjuvant for Cholangiocarcinoma Patients Prior to Liver Transplantation: Case-Series. Curr. Oncol. 2022, 29, 3585–3594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maithel, S.K.; Keilson, J.M.; Cao, H.S.T.; Rupji, M.; Mahipal, A.; Lin, B.S.; Javle, M.M.; Cleary, S.P.; Akce, M.; Switchenko, J.M.; et al. NEO-GAP: A Single-Arm, Phase II Feasibility Trial of Neoadjuvant Gemcitabine, Cisplatin, and Nab-Paclitaxel for Resectable, High-Risk Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 30, 6558–6566. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ikai, I.; Fujii, H.; Hatano, E.; Shimahara, Y. Surgical resection of hilar cholangiocarcinoma: Analysis of survival and postoperative complications. World J. Surg. 2007, 31, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R.; Ruo, L.; Little, S.A.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Wagman, R.; Blumgart, L.H.; Fong, Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: Implications for adjuvant therapeutic strategies. Cancer 2003, 98, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Mizusawa, J.; Katayama, H.; Sano, Y.; Uesaka, K.; Yanagimoto, H.; Morinaga, S.; Wada, H. Adjuvant S-1 vs. Observation for Resected Biliary Tract Cancer: 5-Year Follow-Up of the JCOG1202/ASCOT; American Society of Clinical Oncology: Alexandria, VG, USA, 2024. [Google Scholar]
- Nakachi, K.; Ikeda, M.; Konishi, M.; Nomura, S.; Katayama, H.; Kataoka, T.; Todaka, A.; Yanagimoto, H.; Morinaga, S.; Kobayashi, S.; et al. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2023, 401, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Ben-Josef, E.; Guthrie, K.A.; El-Khoueiry, A.B.; Corless, C.L.; Zalupski, M.M.; Lowy, A.M.; Thomas, C.R., Jr.; Alberts, S.R.; Dawson, L.A.; Micetich, K.C.; et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J. Clin. Oncol. 2015, 33, 2617–2622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.H.; Kim, K.P.; Park, D.H.; Moon, S.H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009, 3, 298–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hare, A.E.; Makary, M.S. Locoregional Approaches in Cholangiocarcinoma Treatment. Cancers 2022, 14, 5853. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.H.; Yoon, H.J.; Lee, I.S.; Yoon, H.K.; Kim, K.P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Gusani, N.J.; Balaa, F.K.; Steel, J.L.; Geller, D.A.; Marsh, J.W.; Zajko, A.B.; Carr, B.I.; Gamblin, T.C. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): A single-institution experience. J. Gastrointest. Surg. 2008, 12, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, H.-K.; Sung, K.-B.; Ko, G.-Y.; Gwon, D.I.; Shin, J.H.; Song, H.-Y. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma. Cancer 2008, 113, 1614–1622. [Google Scholar] [CrossRef]
- Boehm, L.M.; Jayakrishnan, T.T.; Miura, J.T.; Zacharias, A.J.; Johnston, F.M.; Turaga, K.K.; Gamblin, T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, N.; Daly, J.; Oderman, P.; Shike, M.; Chun, H.; Petroni, G.; Geller, N. Hepatic artery pump infusion: Toxicity and results in patients with metastatic colorectal carcinoma. J. Clin. Oncol. 1984, 2, 595–600. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; McArdle, C.S. Epidemiology of colorectal liver metastases. Surg. Oncol. 2007, 16, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Boerner, T.; Tan, B.R.; Chou, J.F.; Gönen, M.; Boucher, T.M.; Hauser, H.F.; Do, R.K.G.; Lowery, M.A.; Harding, J.J.; et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 60–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantore, M.; Mambrini, A.; Fiorentini, G.; Rabbi, C.; Zamagni, D.; Caudana, R.; Pennucci, C.; Sanguinetti, F.; Lombardi, M.; Nicoli, N. Phase II study of hepatic intraarterial epirubicin and cisplatin, with systemic 5-fluorouracil in patients with unresectable biliary tract tumors. Cancer 2005, 103, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Lorgis, V.; Vincent, J.; Ladoire, S.; Guiu, B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: Preliminary experience. Chemotherapy 2013, 59, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, W.D.; Gyves, J.W. Clinical pharmacology of hepatic arterial chemotherapy. Semin. Oncol. 1983, 10, 176–182. [Google Scholar] [PubMed]
- Cohen, A.D.; Kemeny, N.E. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 2003, 8, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Aramaki, O.; Moriguchi, M.; Nakayama, H.; Midorikawa, Y.; Takayama, T. Arterial infusion of cisplatin plus S-1 against unresectable intrahepatic cholangiocarcinoma. Biosci. Trends 2018, 12, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Herber, S.; Otto, G.; Schneider, J.; Manzl, N.; Kummer, I.; Kanzler, S.; Schuchmann, A.; Thies, J.; Düber, C.; Pitton, M. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc. Interv. Radiol. 2007, 30, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Burger, I.; Hong, K.; Schulick, R.; Georgiades, C.; Thuluvath, P.; Choti, M.; Kamel, I.; Geschwind, J.F. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: Initial experience in a single institution. J. Vasc. Interv. Radiol. JVIR 2005, 16, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Liapi, E.; Geschwind, J.F. Transcatheter and ablative therapeutic approaches for solid malignancies. J. Clin. Oncol. 2007, 25, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Stark, S.; Wang, C.; Savic, L.J.; Letzen, B.; Schobert, I.; Miszczuk, M.; Murali, N.; Oestmann, P.; Gebauer, B.; Lin, M.; et al. Automated feature quantification of Lipiodol as imaging biomarker to predict therapeutic efficacy of conventional transarterial chemoembolization of liver cancer. Sci. Rep. 2020, 10, 18026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiefer, M.V.; Albert, M.; McNally, M.; Robertson, M.; Sun, W.; Fraker, D.; Olthoff, K.; Christians, K.; Pappas, S.; Rilling, W.; et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer 2011, 117, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Z.G.; Ke, Q.; Lou, J.Y.; Zheng, S.G.; Bi, X.Y.; Wang, J.M.; Guo, W.; Li, F.Y.; Wang, J.; et al. Adjuvant transarterial chemoembolization following radical resection for intrahepatic cholangiocarcinoma: A multi-center retrospective study. J. Cancer 2020, 11, 4115–4122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, W.H.; Yu, T.; Luo, Y.H.; Wang, Y.; Liu, Y.F.; Hua, X.D.; Lin, J.; Ma, Z.H.; Ai, F.L.; Wang, T.L. Clinical efficacy of gemcitabine and cisplatin-based transcatheter arterial chemoembolization combined with radiotherapy in hilar cholangiocarcinoma. World J. Gastrointest. Oncol. 2019, 11, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Khwaja, A.; Liapi, E.; Torbenson, M.S.; Georgiades, C.S.; Geschwind, J.F. New intra-arterial drug delivery system for the treatment of liver cancer: Preclinical assessment in a rabbit model of liver cancer. Clin. Cancer Res. 2006, 12, 2563–2567. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, G.; Zhang, Y.; Zhou, T.; Nie, C.; Zhu, T.; Chen, S.; Wang, B.; Yu, Z.; Wang, H.; et al. Comprehensive analysis of common safety profiles and their predictive factors in 520 records of liver cancer patients treated by drug-eluting beads transarterial chemoembolization. Medicine 2018, 97, e11131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poggi, G.; Amatu, A.; Montagna, B.; Quaretti, P.; Minoia, C.; Sottani, C.; Villani, L.; Tagliaferri, B.; Sottotetti, F.; Rossi, O.; et al. OEM-TACE: A new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc. Interv. Radiol. 2009, 32, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, J.; Shi, C.; Fang, J.; Peng, Z.; Huang, J.; Sun, J.; Zhou, G.; Li, T.; Zhu, D.; et al. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: A preliminary result from CTILC study. Medicine 2020, 99, e19276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hori, A.; Toei, H.; Nakamura, T.; Makitani, K.; Hori, S. Successful control of a large intrahepatic cholangiocarcinoma treated by transarterial chemo-embolization; a single case report. BJR Case Rep. 2022, 8, 20210186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gates, V.L.; Atassi, B.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; Nemcek, A.A.; Omary, R.; Salem, R. Radioembolization with Yttrium-90 microspheres: Review of an emerging treatment for liver tumors. Future Oncol. 2007, 3, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, G.M.; Levi Sandri, G.B.; Laurenzi, A.; Colasanti, M.; Meniconi, R.L.; Lionetti, R.; Santoro, R.; Lepiane, P.; Sciuto, R.; Pizzi, G.; et al. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J. Surg. 2017, 41, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H. Current status of transarterial radioembolization. World J. Radiol. 2016, 8, 449–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houle, S.; Yip, T.K.; Shepherd, F.A.; Rotstein, L.E.; Sniderman, K.W.; Theis, E.; Cawthorn, R.H.; Richmond-Cox, K. Hepatocellular carcinoma: Pilot trial of treatment with Y-90 microspheres. Radiology 1989, 172, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Mulcahy, M.F.; Lewandowski, R.J.; Sato, K.T.; Ryu, R.K.; Masterson, E.J.; Newman, S.B.; Benson, A., 3rd; Omary, R.A.; Salem, R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: Results from a pilot study. Cancer 2008, 113, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Ali, A.; Ljuboja, D.; Weinstein, J.L.; Shenoy-Bhangle, A.S.; Nasser, I.A.; Morrow, M.K.; Faintuch, S.; Curry, M.P.; Bullock, A.J.; et al. Neoadjuvant Yttrium-90 Transarterial Radioembolization with Resin Microspheres Prescribed Using the Medical Internal Radiation Dose Model for Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2021, 32, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Chotipanich, C.; Choo, S.P.; Kwang, S.W.; Mo, F.; Worakitsitisatorn, A.; Tai, D.; Sundar, R.; Ng, D.C.E.; Loke, K.S.H.; et al. Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres Followed by Gemcitabine plus Cisplatin for Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Single-Arm Multicenter Clinical Trial. Liver Cancer 2022, 11, 451–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, T.Y.; Zhou, G.H.; Zhang, Y.L.; Nie, C.H.; Zhu, T.Y.; Wang, H.L.; Chen, S.Q.; Wang, B.Q.; Yu, Z.N.; Wu, L.M.; et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 4534–4541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aliberti, C.; Carandina, R.; Sarti, D.; Pizzirani, E.; Ramondo, G.; Mulazzani, L.; Mattioli, G.M.; Fiorentini, G. Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer. Res. 2017, 37, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Georgiades, C. Radiofrequency ablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. JVIR 2010, 21 (Suppl. S8), S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.; Nurkin, S.J.; Hochwald, S.N. Ablative therapy for esophageal dysplasia and early malignancy: Focus on RFA. BioMed Res. Int. 2014, 2014, 642063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanabe, K.K.; Kulu, Y. Radiofrequency Ablation for Colon and Rectal Carcinoma Liver Metastases: What’s Missing? Gastrointest. Cancer Res. GCR 2007, 1 (Suppl. S2), S42–S46. [Google Scholar] [PubMed] [PubMed Central]
- Han, K.; Ko, H.K.; Kim, K.W.; Won, H.J.; Shin, Y.M.; Kim, P.N. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. J. Vasc. Interv. Radiol. JVIR 2015, 26, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.A.; Kim, P.N. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Calisti, G.; De Stefano, G.; Farella, N.; Di Sarno, A.; Amendola, F.; Scognamiglio, U.; Giorgio, V. Radiofrequency ablation for intrahepatic cholangiocarcinoma: Retrospective analysis of a single centre experience. Anticancer. Res. 2011, 31, 4575–4580. [Google Scholar] [PubMed]
- Brandi, G.; Rizzo, A.; Dall’Olio, F.G.; Felicani, C.; Ercolani, G.; Cescon, M.; Frega, G.; Tavolari, S.; Palloni, A.; De Lorenzo, S.; et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int. J. Hyperth. 2020, 37, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Li, W.M.; Li, H.C.; Ao, G.K.; Zheng, F.; Lin, H. Percutaneous Intraductal Radiofrequency Ablation for Extrahepatic Distal Cholangiocarcinoma: A Method for Prolonging Stent Patency and Achieving Better Functional Status and Quality of Life. Cardiovasc. Interv. Radiol. 2017, 40, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tarek, M. Membrane electroporation: A molecular dynamics simulation. Biophys. J. 2005, 88, 4045–4053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delemotte, L.; Tarek, M. Molecular dynamics simulations of lipid membrane electroporation. J. Membr. Biol. 2012, 245, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Reginelli, A.; Maggialetti, N.; Carbone, M.; Giovine, S.; Laporta, A.; Urraro, F.; Nardone, V.; Grassi, R.; Cappabianca, S.; et al. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: Feasibility, safety and efficacy. Med. Oncol. 2020, 37, 45. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.K.; Bhutiani, N.; Egger, M.E.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Kelly, L.R.; Vitale, G.C.; Martin, R.C.G. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB 2018, 20, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.C.; van Veldhuisen, E.; Ruarus, A.H.; Coelen, R.J.S.; Roos, E.; van Delden, O.M.; Besselink, M.G.; Klümpen, H.J.; van Lienden, K.P.; van Gulik, T.M.; et al. Outcomes of Irreversible Electroporation for Perihilar Cholangiocarcinoma: A Prospective Pilot Study. J. Vasc. Interv. Radiol. JVIR 2022, 33, 805–813.e801. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Makary, M.S.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 2384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubinsky, B.; Lee, C.Y.; Bastacky, J.; Onik, G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology 1990, 27, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Glazer, D.I.; Tatli, S.; Shyn, P.B.; Vangel, M.G.; Tuncali, K.; Silverman, S.G. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. AJR Am. J. Roentgenol. 2017, 209, 1381–1389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, K.R.; Sannapaneni, S.; Mullikin, T.C.; Harmsen, W.S.; Petersen, M.M.; Antharam, P.; Laughlin, B.; Mahipal, A.; Halfdanarson, T.R.; Merrell, K.W.; et al. Chemoradiotherapy for patients with locally advanced or unresectable extra-hepatic biliary cancer. J. Gastrointest. Oncol. 2020, 11, 1408–1420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nag, S.; DeHaan, M.; Scruggs, G.; Mayr, N.; Martin, E.W. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.D.; Guo, J.H.; Huang, M.; Ji, J.S.; Xu, H.; Lu, J.; Li, H.L.; Wang, W.H.; Li, Y.L.; Ni, C.F.; et al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial. J. Hepatol. 2018, 68, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, S.; Zheng, Y.B.; Song, X.P.; Sun, B.L.; Jiang, W.J.; Wang, L.G. Clinical Study on Using (125)I Seeds Articles Combined with Biliary Stent Implantation in the Treatment of Malignant Obstructive Jaundice. Anticancer. Res. 2017, 37, 4649–4653. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, E.T.; Guo, M.; Mitra, N.; Metz, J.M. Brachytherapy in the treatment of cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.D.; Guo, J.H.; Zhu, G.Y.; He, S.C.; Fang, W.; Deng, G.; Qin, Y.L.; Li, G.Z.; Coldwell, D.M.; Teng, G.J. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: Preliminary results versus a conventional biliary stent. J. Hepatol. 2012, 56, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Khosla, D.; Zaheer, S.; Gupta, R.; Madan, R.; Goyal, S.; Kumar, N.; Kapoor, R. Role of intraluminal brachytherapy in palliation of biliary obstruction in cholangiocarcinoma: A brief review. World J. Gastrointest. Endosc. 2022, 14, 106–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baak, R.; Willemssen, F.; van Norden, Y.; Eskens, F.; Milder, M.T.W.; Heijmen, B.J.M.; Koerkamp, B.G.; Sprengers, D.; van Driel, L.; Klümpen, H.J.; et al. Stereotactic Body Radiation Therapy after Chemotherapy for Unresectable Perihilar Cholangiocarcinoma: The STRONG Trial, a Phase I Safety and Feasibility Study. Cancers 2021, 13, 3991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smart, A.C.; Goyal, L.; Horick, N.; Petkovska, N.; Zhu, A.X.; Ferrone, C.R.; Tanabe, K.K.; Allen, J.N.; Drapek, L.C.; Qadan, M.; et al. Hypofractionated Radiation Therapy for Unresectable/Locally Recurrent Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Upadhyay, R.; Liao, K.; Kumala, T.; Shi, C.; Dodoo, G.; Abi Jaoude, J.; Corrigan, K.L.; Manzar, G.S.; Marqueen, K.E.; et al. Definitive Liver Radiotherapy for Intrahepatic Cholangiocarcinoma with Extrahepatic Metastases. Liver Cancer 2023, 12, 198–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
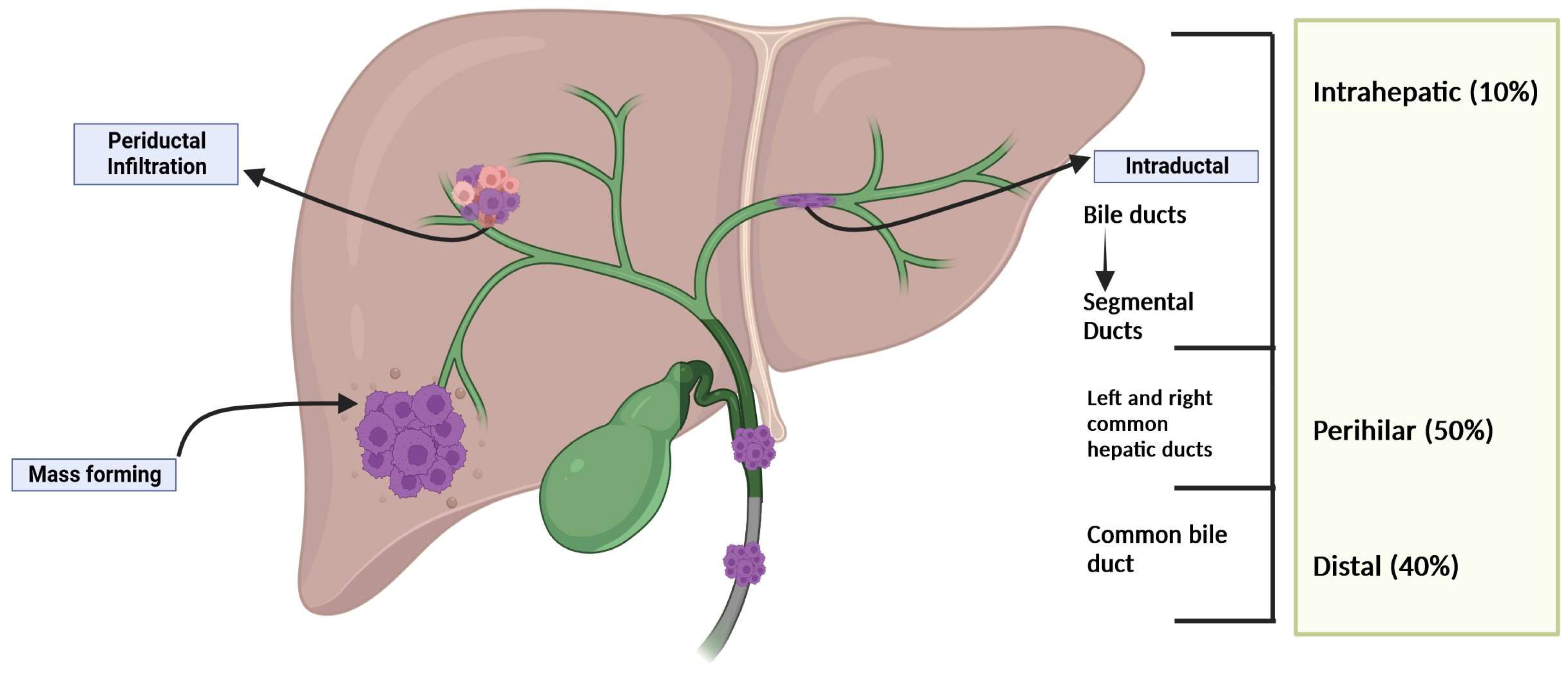
 High mortality (>2 cases per 100,000).
High mortality (>2 cases per 100,000).  Intermediate mortality (1–2 cases per 100,000).
Intermediate mortality (1–2 cases per 100,000).  Low mortality (<1 cases per 100 000).
Low mortality (<1 cases per 100 000).
 High mortality (>2 cases per 100,000).
High mortality (>2 cases per 100,000).  Intermediate mortality (1–2 cases per 100,000).
Intermediate mortality (1–2 cases per 100,000).  Low mortality (<1 cases per 100 000).
Low mortality (<1 cases per 100 000).| Country | iCCA | eCCA |
|---|---|---|
| Republic of Korea | 2.6 | 2.45 |
| Japan | 0.97 | 2.14 |
| Ireland | 2.66 | 0.05 |
| Austria | 1.43 | 0.90 |
| Hong Kong | 2.27 | 0.04 |
| Malta | 2.03 | 0.25 |
| Germany | 1.31 | 0.94 |
| UK | 2.04 | 0.04 |
| Australia | 1.96 | 0.03 |
| Hungary | 0.48 | 1.51 |
| Spain | 1.81 | 0.16 |
| Portugal | 1.79 | 0.12 |
| Canada | 1.82 | 0.07 |
| Switzerland | 1.48 | 0.40 |
| Singapore | 1.77 | 0.10 |
| France | 1.79 | 0.06 |
| Sweden | 0.90 | 0.81 |
| Belgium | 1.61 | 0.09 |
| Netherlands | 1.30 | 0.34 |
| Croatia | 0.96 | 0.57 |
| USA | 1.32 | 0.16 |
| Norway | 1.35 | 0.13 |
| Lithuania | 1.04 | 0.29 |
| Slovakia | 0.95 | 0.37 |
| Czech Republic | 0.78 | 0.52 |
| New Zealand | 0.99 | 0.27 |
| Italy | 1.08 | 0.17 |
| Denmark | 1.08 | 0.14 |
| Latvia | 0.96 | 0.17 |
| Israel | 0.95 | 0.11 |
| Romania | 0.55 | 0.29 |
| Turkey | 0.72 | 0.11 |
| Poland | 0.29 | 0.14 |
| First-Line Therapy for CCA | ||||
|---|---|---|---|---|
| Good performance status and no hyperbilirubinemia | Regimen | Trial/Evidence | Benefit(s) | Limitation(s) |
| Gemcitabine-based regimens | ||||
| Gemcitabine plus cisplatin | ABC-02 trial [24] Okusaka et al. RCT [33] Pooled analysis of RCTs [34] |
| Higher rates of Grade 3 or 4 neutropenia [35] | |
| Gemcitabine plus cisplatin and durvalumab | TOPAZ-1 trial [36] |
| Immune-mediated side effects in 13% of patients | |
| Gemcitabine plus S-1 * | FUGA-BT trial [28] |
| Limited availability outside the US | |
| Gemcitabine plus Oxaliplatin (GEMOX) | FUGA-BT trial [28]. Wagner et al. phase III trial [37]. |
| Results were less favorable in patients who had a poorer performance status or a higher bilirubin level | |
| GEMOX plus Bevacizumab | Kim et al. phase III RCT [39] Zhu et al. RCT [40] |
| Bevacizumab-related toxicities | |
| Gemcitabine plus capecitabine | Zhu et al. RCT [40] Nehls et al. RCT [41] | May be an alternative option | Needs RCTs to determine the effectiveness compared to cisplatin | |
| Gemcitabine plus Nabpaclitaxel | Iqbal et al. RCT [42] Sahai et al. RCT [43] | Neutropenia (43%) and fatigue (14%) | ||
| Non-gemcitabine-based regimens | ||||
| Modified FOLFIRINOX (Oxaliplatin, LV, Irinotecan and FU) | Phelip et al. RCT [44] Abdel-Rahman et al. review [45] | No advantages in terms of median OS, PFS, or six-month PFS | ||
| Special Circumstances |
| Choi et al. trial [46] | ||
| ||||
| LV-modulated FU | Sanz-Altamira et al. [47] Mayo Clinic regimen [48] | Survival benefits are unclear [47,49,50] | ||
| Capecitabine | Patt et al. [51] | Alternative to LV-modulated FU | Capecitabine as a single agent appears to be relatively less active for CCA than for gallbladder cancer | |
| Year 2020 | Year 2022 | ||
|---|---|---|---|
| Gene Mutations | Percentage | Gene Mutations | Percentage |
| FGFR2 | 15–20% | FGFR2 | 4–9% |
| IDH1 | 15–20% | IDH1 | 3–14% |
| HER2 | 10–15% | IDH2 | 4.0% |
| NTRK | <5% | TMB high | 3.7% |
| RNF43 | <5% | MDM | 4.3% |
| MMR | <5% | BRACA1/2 | 3.4% |
| BRAF | <5% | ERBB2 | 3.8% |
| BRAF | 2.3% | ||
| ERBB3 | 1.6% | ||
| MSI high | 1.2% | ||
| KRAS | 1.1% | ||
| Targeted Therapy for CCA | |||
|---|---|---|---|
| Medication | Mechanism | Trial(s) | Results |
| FGFR-targeted therapy | |||
| Derazantinib | ATP-competitive, pan-FGFR inhibitor. “FGFR1–3 kinases selectivity” [87] | Phase I RCT (NCT01752920) Phase I/II, open-label RCT (NCT01752920) |
|
| Phase II FIDES-01 (NCT03230318) | Ongoing | ||
| Erdafitinib | Pan-FGFR inhibitor [88] | Phase IIa study (NCT02699606) |
|
| Pemigatinib | Selective FGFR1–3 inhibitor [90] | FIGHT-202 trial | |
| Infigratinib | Selective inhibitor of FGFR1–3 [92] | Phase I study (NCT01004224) | Identification of a recommended phase II dose for infigratinib as 125 mg QD given on a 3-weeks-on/1-week-off schedule [93] |
| Phase II study (NCT02150967) |
| ||
| Futibatinib | Pan-FGFR inhibitor | Phase I (FOENIX-101; NCT02052778) |
|
| FOENIX-CCA2 phase II trial (NCT02052778) |
| ||
| IDH- targeted therapy | |||
| Ivosidenib | IDH1 inhibitor | Combined phase I/II study | |
| TRK fusion- targeted therapy | |||
| Larotrectinib | Tropomyosin receptor kinase (TRK) inhibitor | Analysis of (NCT02122913 and NCT02576431) trials | |
| Analysis of (NCT02122913, NCT02637687, and NCT02576431) trials |
| ||
| Entrectinib | Inhibitor of NTRK1/2/3, ROS1, and ALK [101] | An updated integrated analysis phase I/II studies (ALKA, STARTRK-1, STARTRK-2; EudraCT 2012-000148-88; NCT02097810; NCT02568267) |
|
| BRAF V600E- targeted therapy | |||
| Combination of dabrafenib plus trametinib | Reversibly and selectively inhibits mitogen-activated extracellular kinase [MEK], a downstream effector of BRAF | Phase II (ROAR) basket trial |
|
| NCI-MATCH Trial Subprotocol H | |||
| HER2 overexpression- targeted therapy | |||
| Combination of pertuzumab plus trastuzumab | MyPathway HER2 basket study |
| |
| EGFR-targeted therapy | |||
| Erlotinib | Oral tyrosine kinase inhibitor | Phase II RCT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Badheeb, M.; Alnahar, B.W.; Almiqlash, B.; Sakr, Y.; Al-Najjar, E.; Awas, A.; Alsayed, M.; Khasawneh, B.; Alkhulaifawi, M.; et al. The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma. Pharmaceuticals 2024, 17, 910. https://doi.org/10.3390/ph17070910
Esmail A, Badheeb M, Alnahar BW, Almiqlash B, Sakr Y, Al-Najjar E, Awas A, Alsayed M, Khasawneh B, Alkhulaifawi M, et al. The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma. Pharmaceuticals. 2024; 17(7):910. https://doi.org/10.3390/ph17070910
Chicago/Turabian StyleEsmail, Abdullah, Mohamed Badheeb, Batool Wael Alnahar, Bushray Almiqlash, Yara Sakr, Ebtesam Al-Najjar, Ali Awas, Mohammad Alsayed, Bayan Khasawneh, Mohammed Alkhulaifawi, and et al. 2024. "The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma" Pharmaceuticals 17, no. 7: 910. https://doi.org/10.3390/ph17070910
APA StyleEsmail, A., Badheeb, M., Alnahar, B. W., Almiqlash, B., Sakr, Y., Al-Najjar, E., Awas, A., Alsayed, M., Khasawneh, B., Alkhulaifawi, M., Alsaleh, A., Abudayyeh, A., Rayyan, Y., & Abdelrahim, M. (2024). The Recent Trends of Systemic Treatments and Locoregional Therapies for Cholangiocarcinoma. Pharmaceuticals, 17(7), 910. https://doi.org/10.3390/ph17070910







