The SP1-SuperEnhancer-SPHK1 Axis Mediates Niraparib Resistance in TNBC
Abstract
1. Introduction
2. Results
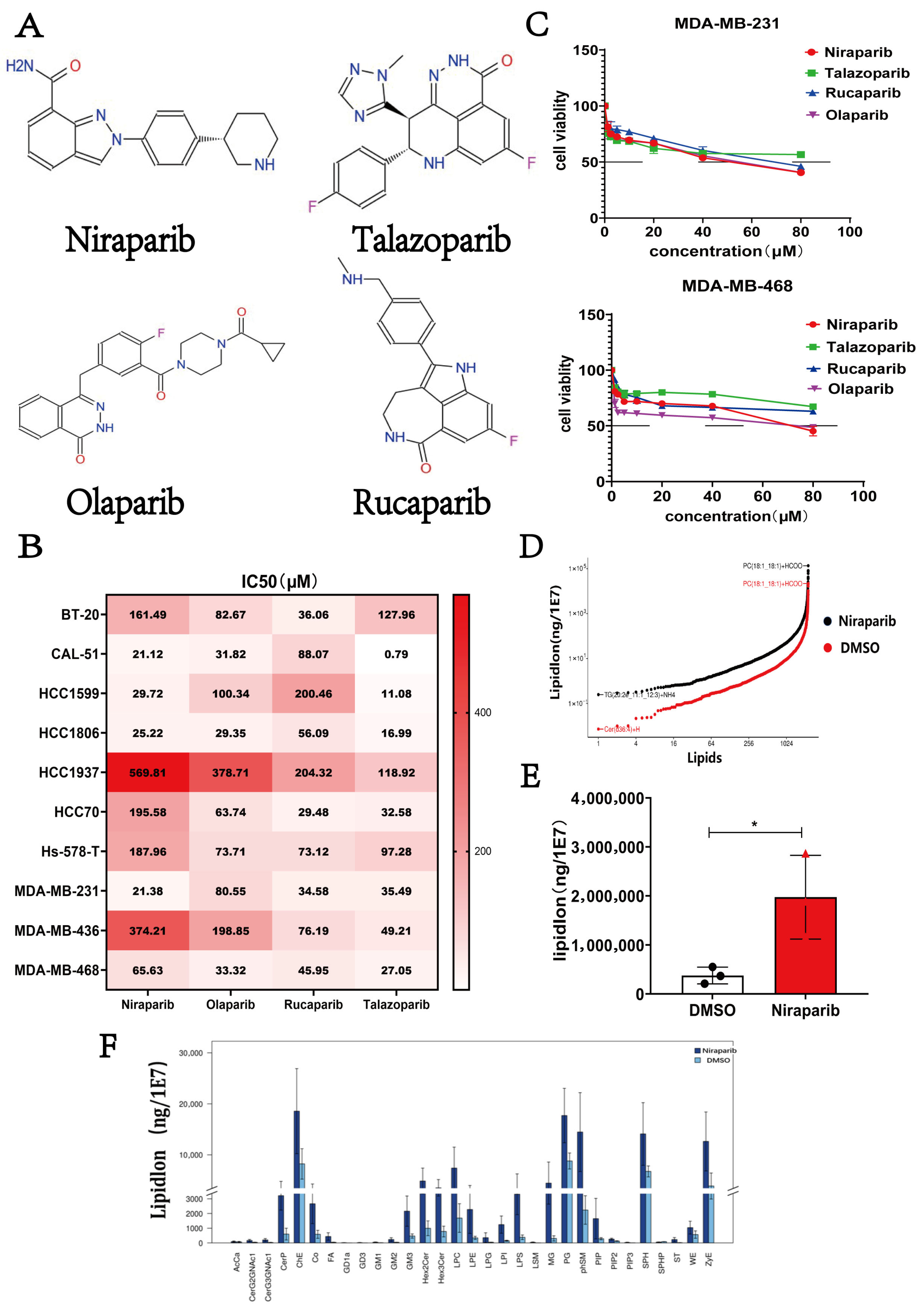
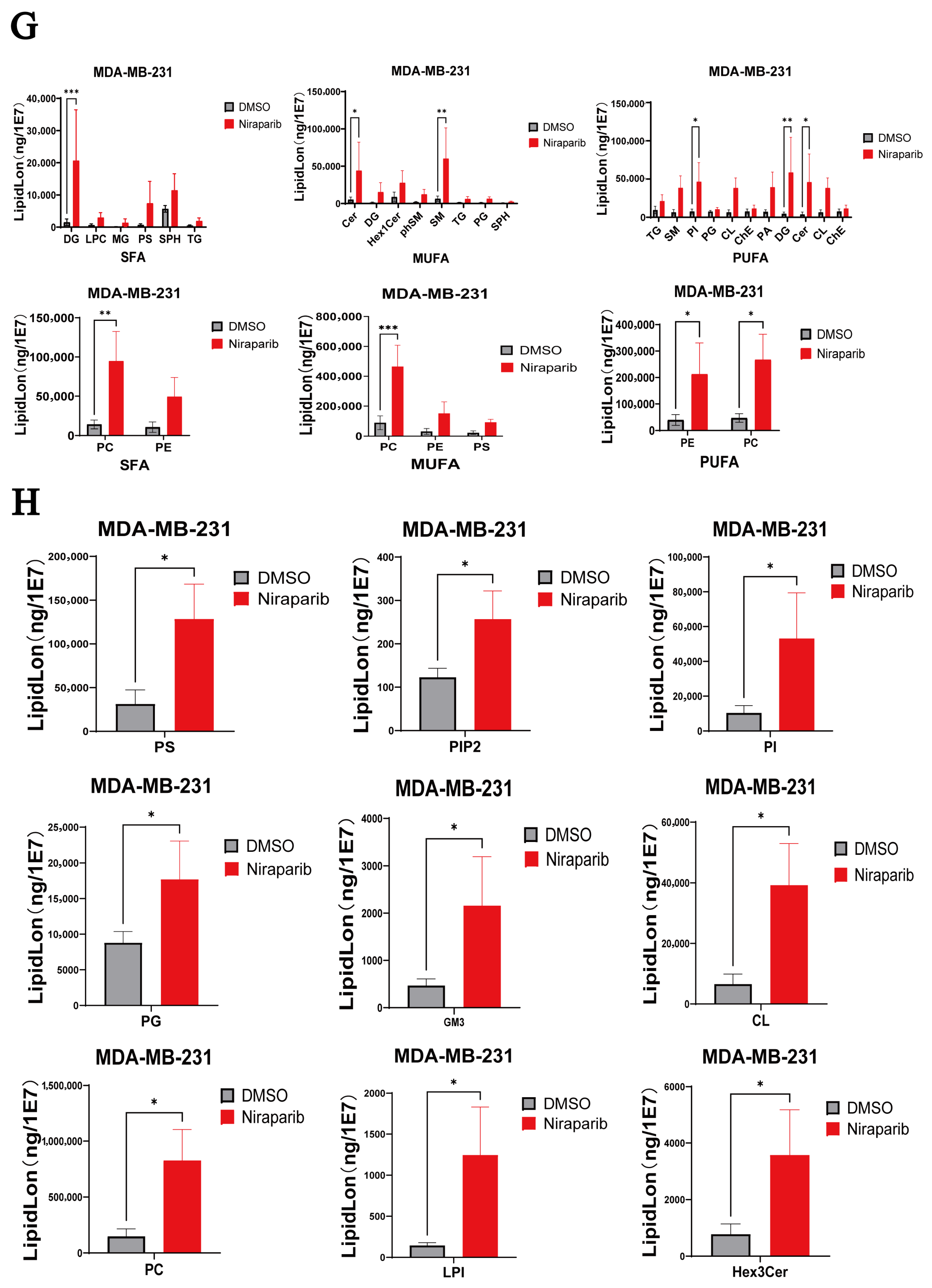
2.1. Niraparib Exhibits Limited Efficacy and Induces Lipid Metabolic Reprogramming in TNBC Cells
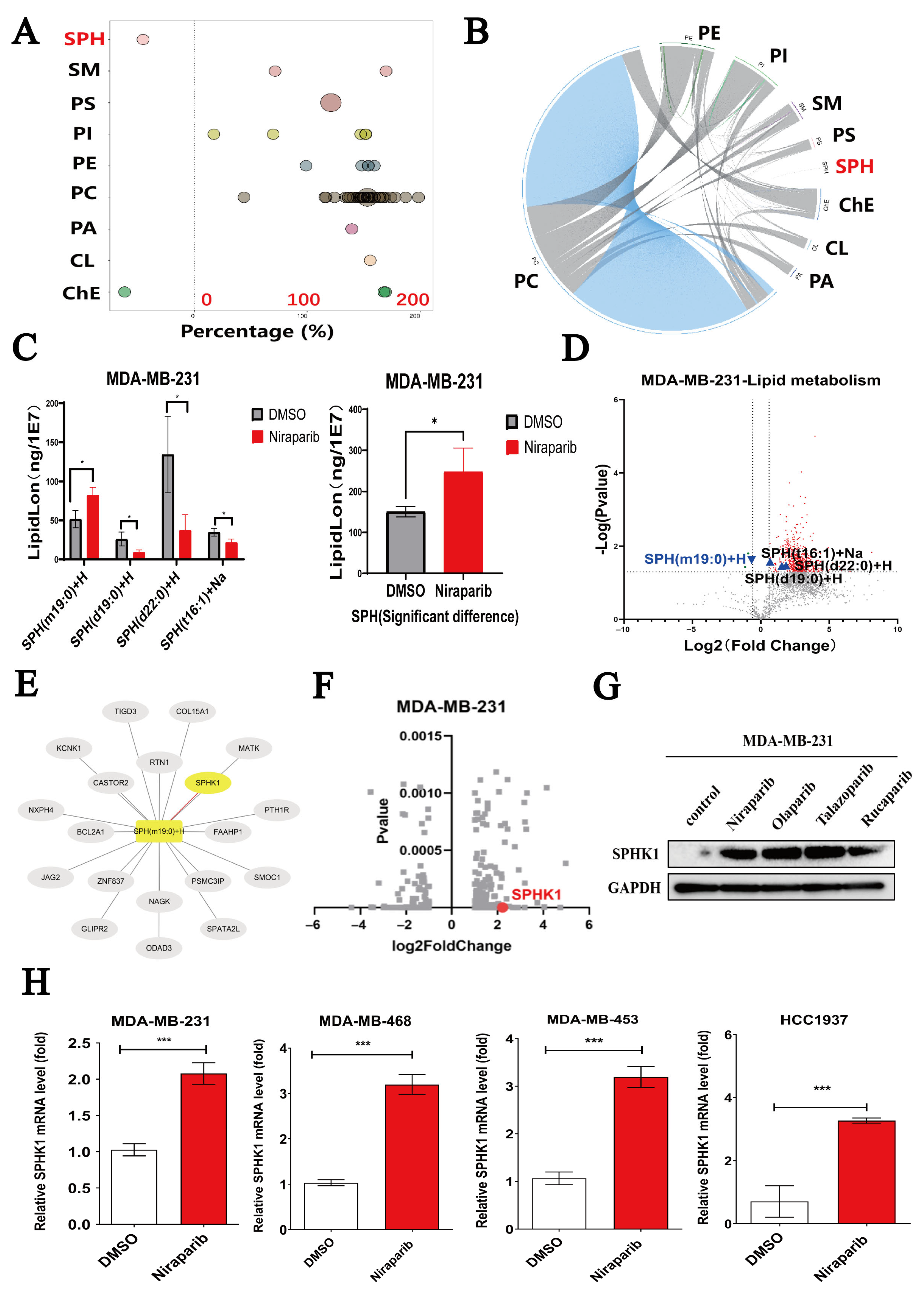
2.2. Niraparib Induces Dysregulation of Sphingolipid SPH Metabolism and Activates SPHK1
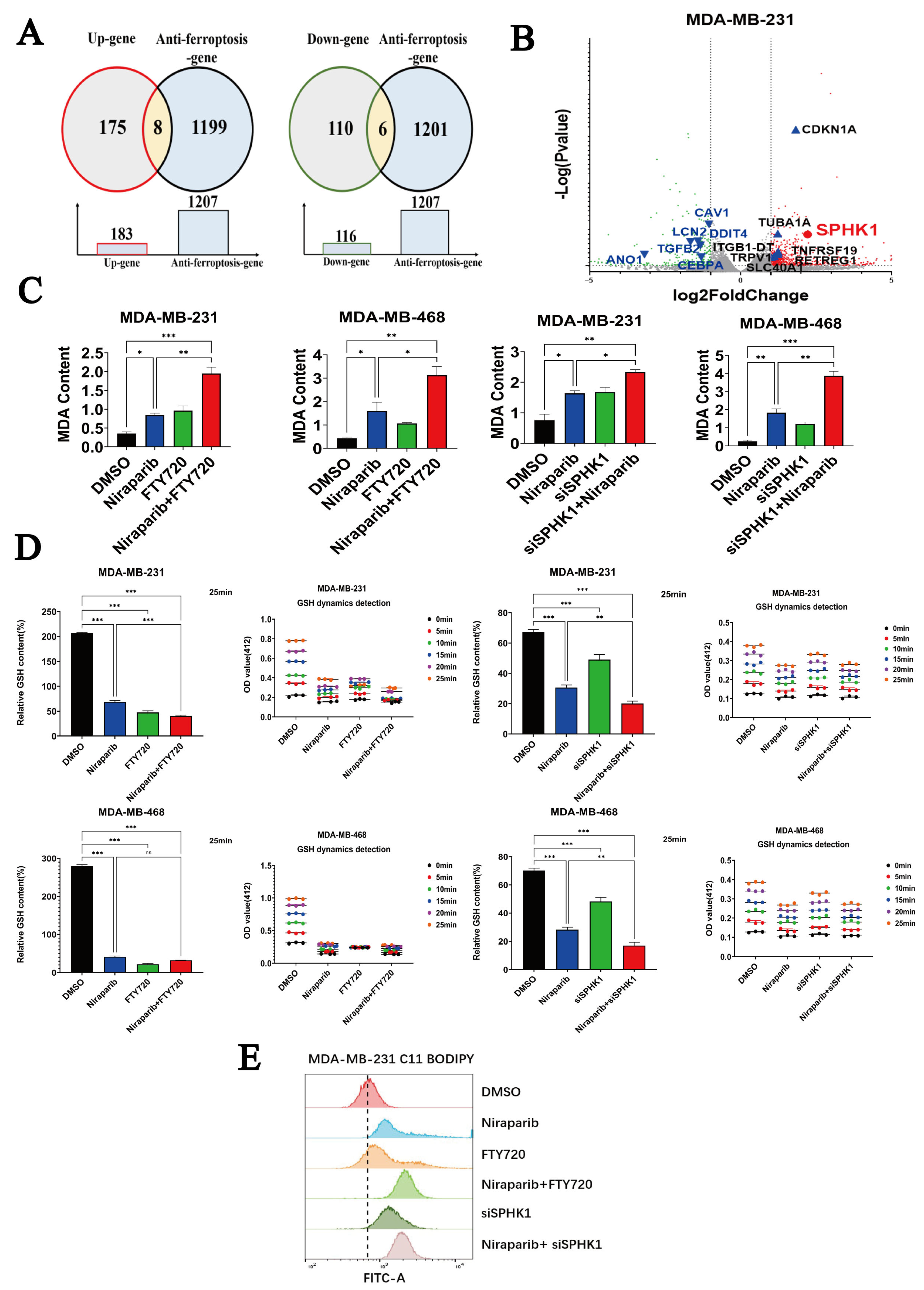
2.3. Niraparib Potentiates Ferroptosis in TNBC Cells by Targeting SPHK1
2.4. Niraparib-Induced Activation of PI3K-AKT and MAPK Pathways Is Significantly Reversed by SPHK1 Knockdown
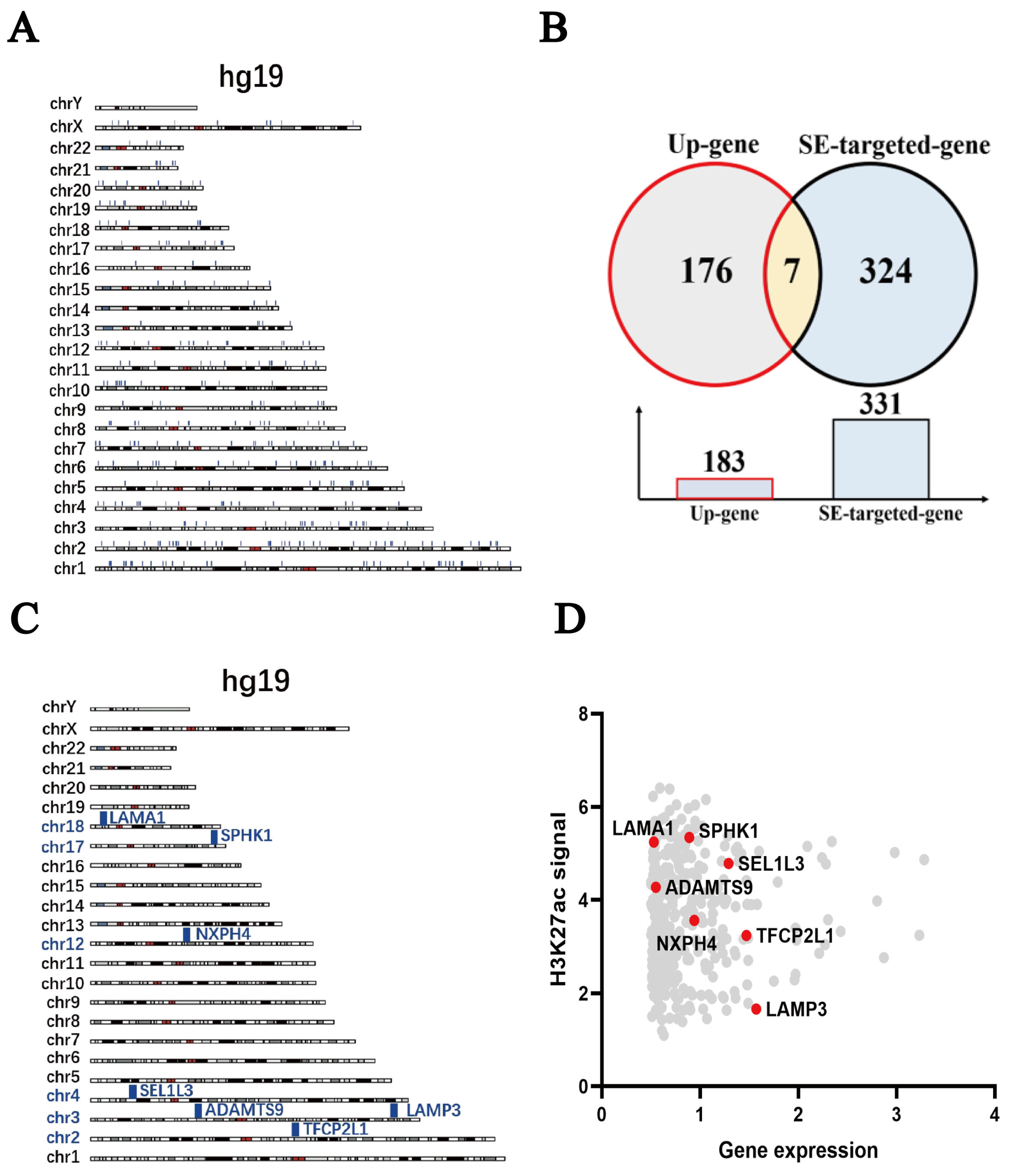
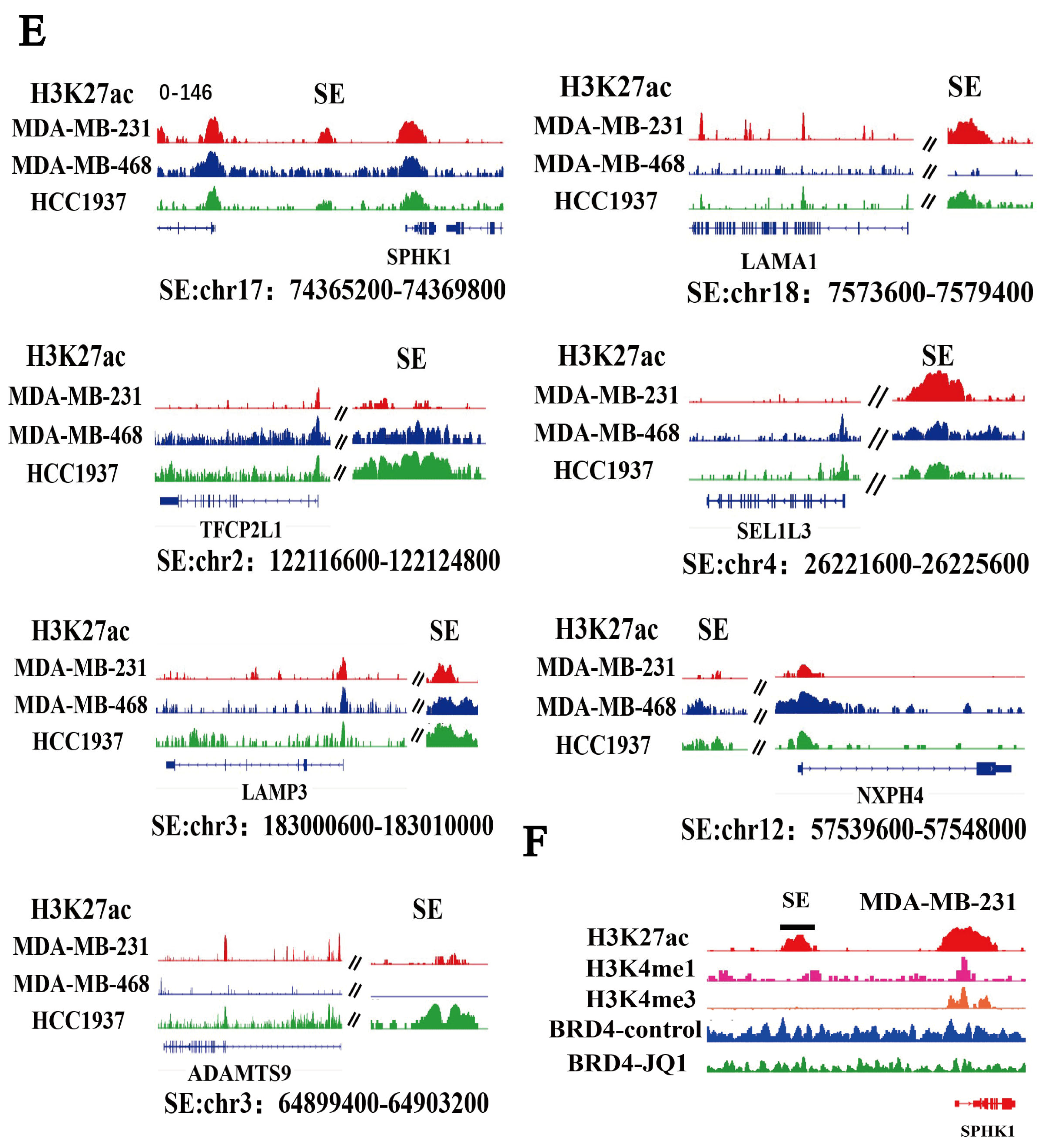
2.5. SPHK1 Is Identified as a Super-Enhancer-Driven Gene in TNBC
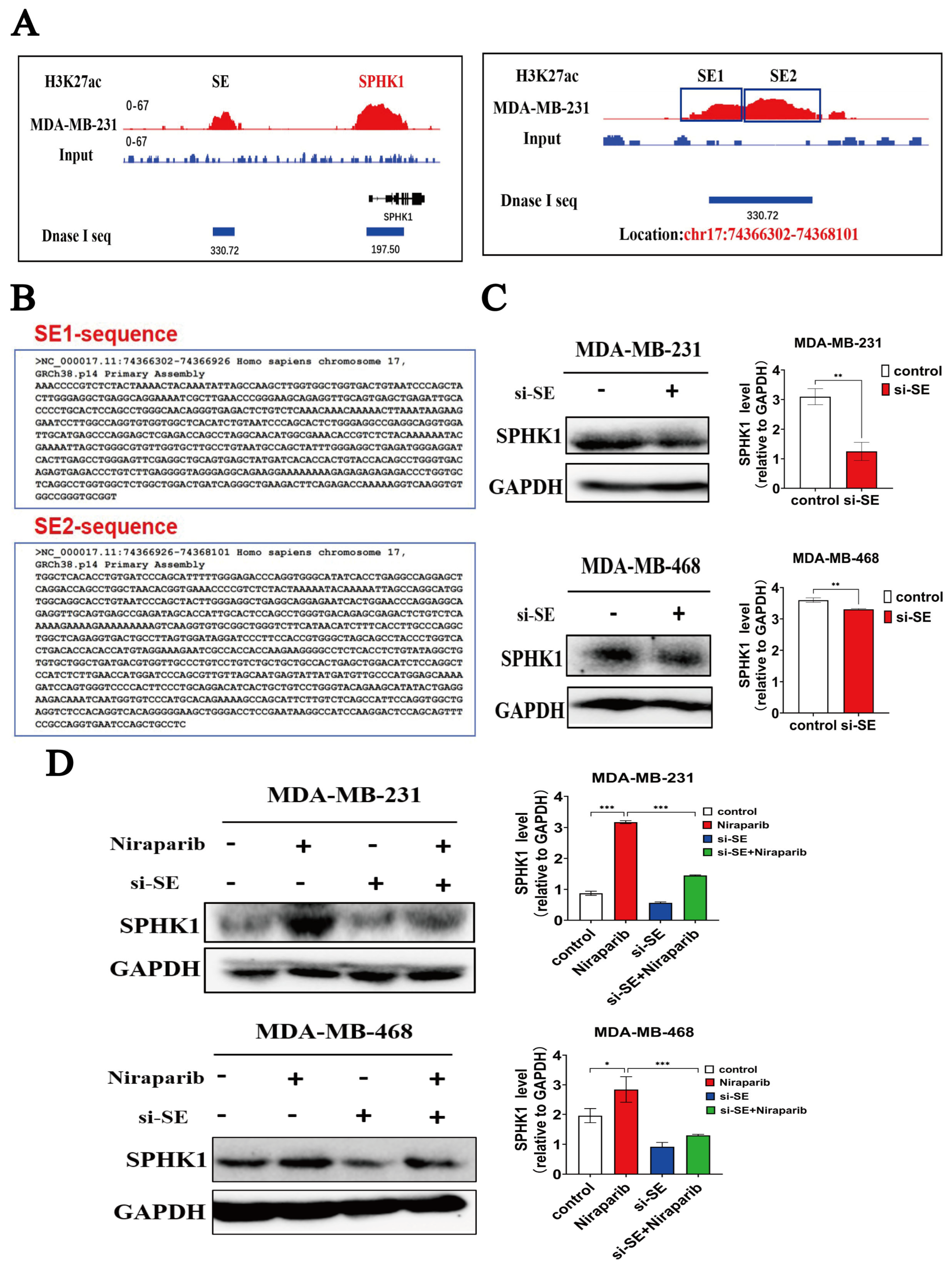
2.6. Niraparib-Induced SPHK1 Activation Is Significantly Reversed by Targeted Inhibition of Super-Enhancer Activity
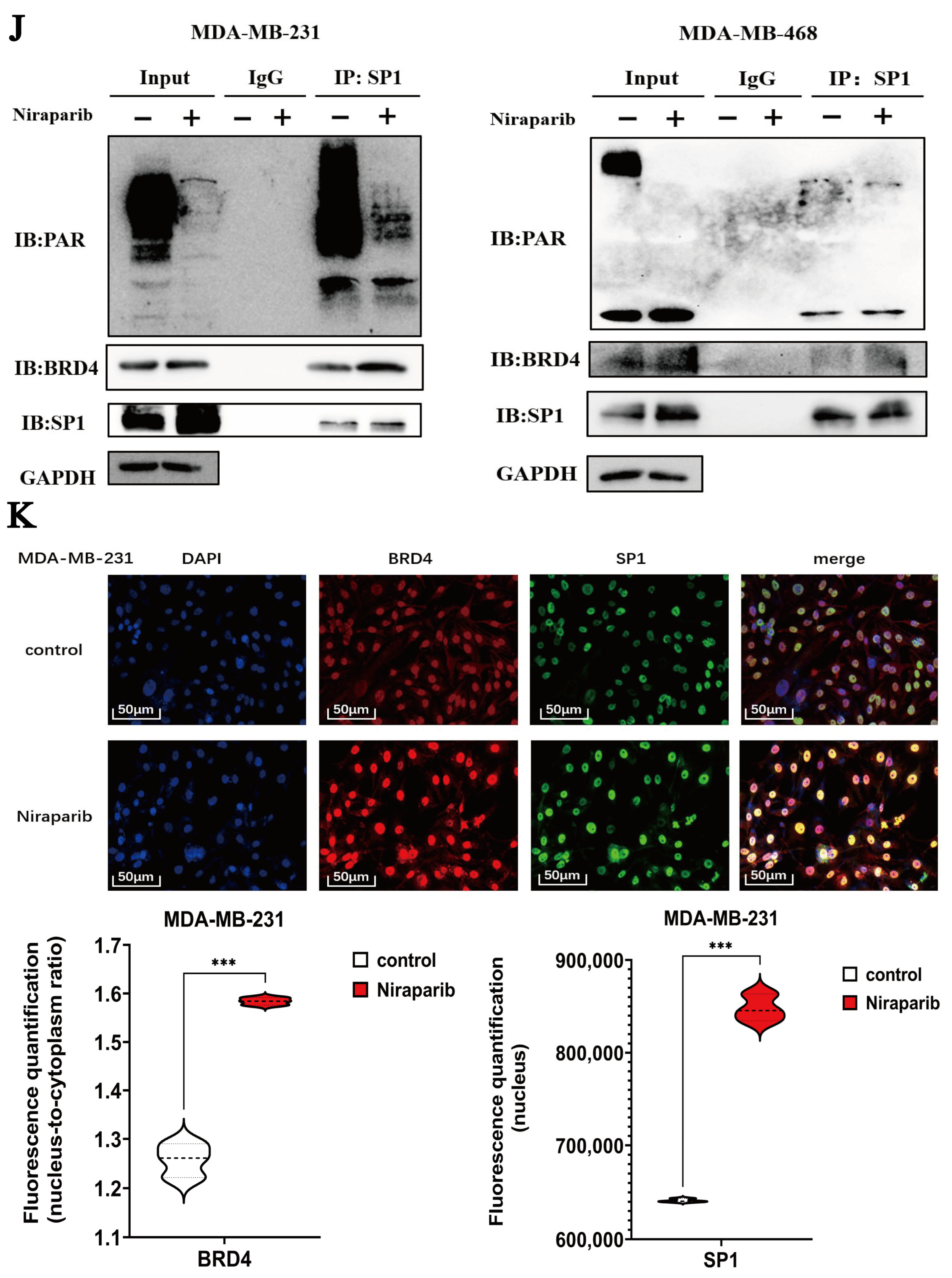
2.7. Niraparib Promotes SPHK1 Transcriptional Activation Through SP1 Recruitment and Super-Enhancer Engagement
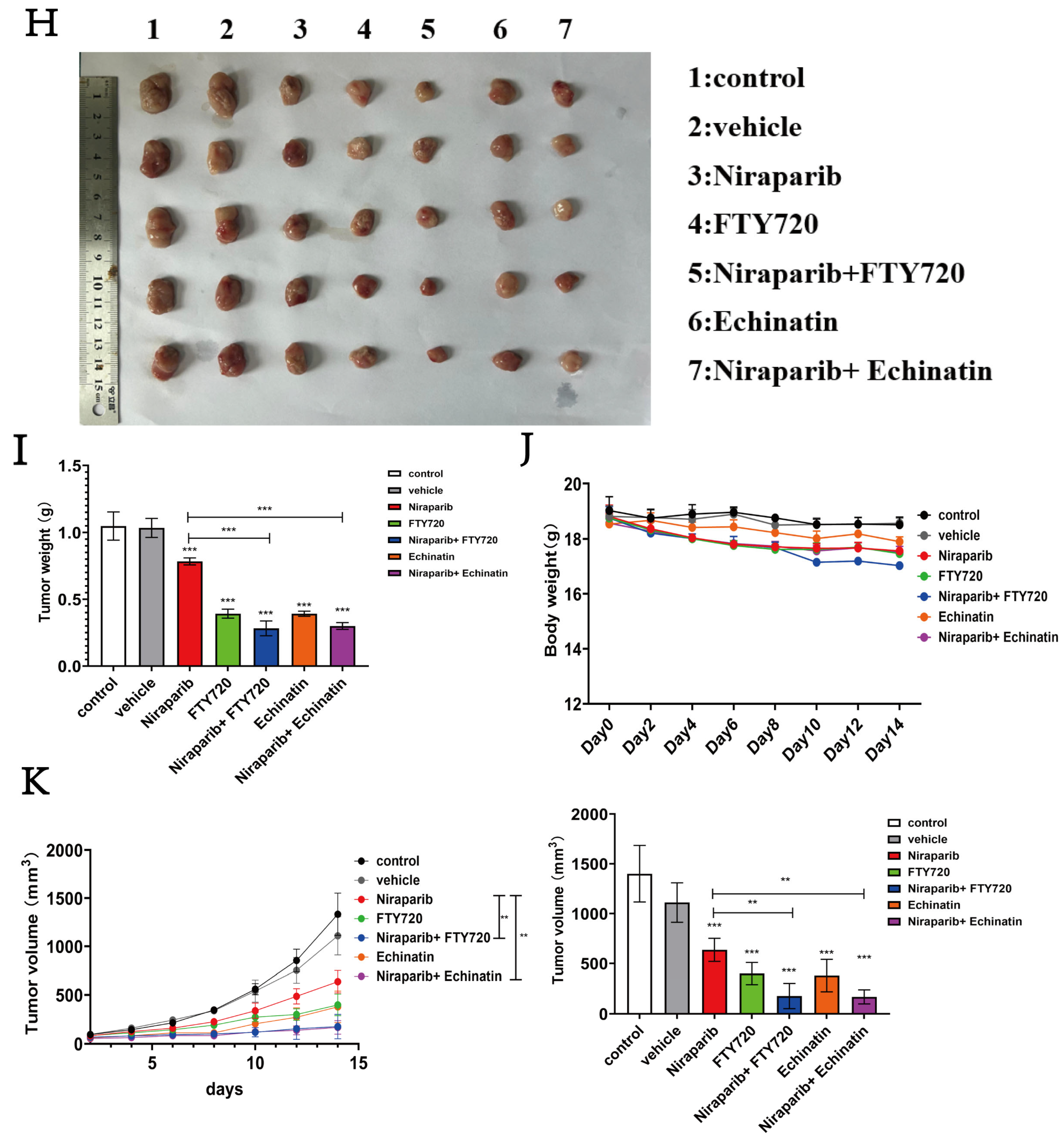
2.8. Niraparib Efficacy Is Significantly Enhanced by Combination Therapy with SP1 and SPHK1 Inhibitors
3. Discussion
4. Materials and Methods
4.1. Compounds and Reagents
4.2. Cell Culture
4.3. Lipidomics Sequencing Analysis
4.4. Transcriptomics Sequencing Analysis
4.5. RT-qPCR Experiment
4.6. Assessment of GSH Levels and MDA Content
4.7. Measurement of Lipid Peroxidation
4.8. Western Blot
4.9. Cell Viability Assay
4.10. siRNA Transfection
4.11. Multi-Dimensional Public Data Analysis
4.12. Chromatin Immunoprecipitation (ChIP)-qPCR
4.13. Co-Immunoprecipitation, Co-IP
4.14. Immunofluorescence (IF) Experiment
4.15. Molecular Docking with AutoDock
4.16. Applications of AlphaFold
4.17. Molecular Dynamics (MD) Simulation
4.18. Syngeneic 4T1 Mammary Tumor Model
4.19. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Full name | abbreviation |
| PARP inhibitors | PARPi |
| homologous recombination deficiency | HRD |
| super-enhancers | SEs |
| triple-negative breast cancer | TNBC |
| pathological complete response rate | pCR |
| prolonging progression-free survival | PFS |
| Sphingosine Kinase 1 | SPHK1 |
| sphingosine | SPH |
| sphingosine-1-phosphate | S1P |
| transcription factors | TFs |
| Artificial intelligence | AI |
| short tandem repeat | STR |
| fetal bovine serum | FBS |
| glutathione | GSH |
| oxidized glutathione | GSSG |
| malondialdehyde | MDA |
| thiobarbituric acid | TBA |
| polyvinylidene fluoride | PVDF |
| Tris-buffered saline containing 0.1% Tween-20 | TBST |
| quantitative PCR | qPCR |
| dimethyl sulfoxide | DMSO |
| Chromatin immunoprecipitation | ChIP |
| Co-Immunoprecipitation | Co-IP |
| Immunofluorescence | IF |
| molecular dynamics | MD |
| standard deviation | SD |
| honestly significant difference | HSD |
| least significant difference | LSD |
| Genomics of Drug Sensitivity in Cancer | GDSC |
| saturated fatty acids | SFAs |
| monounsaturated fatty acids | MUFAs |
| polyunsaturated fatty acids | PUFAs |
| Fingolimod | FTY720 |
| multiple sclerosis | MS |
| sphingomyelin | SM |
| hexosylceramides | HexCer |
| ceramide | Cer |
| root-mean-square deviation | RMSD |
| root-mean-square fluctuation | RMSF |
| secondary structure elements | SSEs |
| phosphatidylserine | PS |
| phosphatidylinositol 4,5-bisphosphate | PIP2 |
| phosphatidylinositol | PI |
| phosphatidylglycerol | PG |
| ganglioside GM3 | GM3 |
| cardiolipin | CL |
| phosphatidylcholine | PC |
| lyso-phosphatidylinositol | LPI |
References
- Mason, S.R.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum-based chemotherapy for early triple-negative breast cancer. Cochrane Database Syst. Rev. 2023, 9, CD014805. [Google Scholar]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP inhibitors: Enhancing efficacy through rational combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef]
- Gou, R.; Dong, H.; Lin, B. Application and reflection of genomic scar assays in evaluating the efficacy of platinum salts and PARP inhibitors in cancer therapy. Life Sci. 2020, 261, 118434. [Google Scholar] [CrossRef]
- Bondar, D.; Karpichev, Y. Poly(ADP-Ribose) Polymerase (PARP) Inhibitors for Cancer Therapy: Advances, Challenges, and Future Directions. Biomolecules 2024, 14, 1269. [Google Scholar] [CrossRef]
- Pham, M.M.; Ngoi, N.Y.L.; Peng, G.; Tan, D.S.P.; Yap, T.A. Development of poly(ADP-ribose) polymerase inhibitor and immunotherapy combinations: Progress, pitfalls, and promises. Trends Cancer 2021, 7, 958–970. [Google Scholar] [CrossRef]
- Xiao, Q.; Xia, M.; Tang, W.; Zhao, H.; Chen, Y.; Zhong, J. The lipid metabolism remodeling: A hurdle in breast cancer therapy. Cancer Lett. 2024, 582, 216512. [Google Scholar] [CrossRef]
- Janneh, A.H.; Atkinson, C.; Tomlinson, S.; Ogretmen, B. Sphingolipid metabolism and complement signaling in cancer progression. Trends Cancer 2023, 9, 782–787. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yuen, T.; Qingchuan, C.; Jianjun, Z.; Yefu, L.; Shulan, S. Melanophilin inhibit the growth and lymph node metastasis of triple negative breast cancer via the NONO-SPHK1-S1P axis. J. Transl. Med. 2025, 23, 284. [Google Scholar] [CrossRef] [PubMed]
- Reddi, K.K.; Chava, S.; Chabattula, S.C.; Edwards, Y.J.K.; Singh, K.; Gupta, R. ASAH1 facilitates TNBC by DUSP5 suppression-driven activation of MAP kinase pathway and represents a therapeutic vulnerability. Cell Death Dis. 2024, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: A promising therapeutic target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef]
- Bhadwal, P.; Randhawa, V.; Vaiphei, K.; Dahiya, D.; Agnihotri, N. Clinical relevance of CERK and SPHK1 in breast cancer and their association with metastasis and drug resistance. Sci. Rep. 2022, 12, 18239. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Q.; Wang, S.; Xie, H.; Liu, J.; Huang, R.; Xiang, Y.; Jiang, Y.; Tian, D.; Bian, E. Super-enhancers complexes zoom in transcription in cancer. J. Exp. Clin. Cancer Res. 2023, 42, 183. [Google Scholar] [CrossRef]
- Bacabac, M.; Xu, W. Oncogenic super-enhancers in cancer: Mechanisms and therapeutic targets. Cancer Metastasis Rev. 2023, 42, 471–480. [Google Scholar] [CrossRef]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A Phase Separation Model for Transcriptional Control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Tsang, F.H.; Law, C.T.; Tang, T.C.; Cheng, C.L.; Chin, D.W.; Tam, W.V.; Wei, L.; Wong, C.C.; Ng, I.O.; Wong, C.M. Aberrant Super-Enhancer Landscape in Human Hepatocellular Carcinoma. Hepatology 2019, 69, 2502–2517. [Google Scholar] [CrossRef]
- Yang, Y.S.; Jin, X.; Li, Q.; Chen, Y.Y.; Chen, F.; Zhang, H.; Su, Y.; Xiao, Y.; Di, G.H.; Jiang, Y.Z.; et al. Superenhancer drives a tumor-specific splicing variant of MARCO to promote triple-negative breast cancer progression. Proc. Natl. Acad. Sci. USA 2022, 119, e2207201119. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef] [PubMed]
- Magtanong, L.; Ko, P.J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e429. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Reznik, E.; Santer, M.; Fongheiser, M.A.; Smith, N.; Hirschhorn, T.; Zandkarimi, F.; Soni, R.K.; Dafre, A.L.; Miranda-Vizuete, A.; et al. Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury. Cell Chem. Biol. 2024, 31, 249–264.e247. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.Y.; Oh, M.; Lee, E.W. An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, H.; Wang, X.; Li, G.; Liu, N.; Zhang, M.; Shen, Y. The application of approaches in detecting ferroptosis. Heliyon 2024, 10, e23507. [Google Scholar] [CrossRef]
- Jiang, Y.; Glandorff, C.; Sun, M. GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors. Antioxidants 2024, 13, 697. [Google Scholar] [CrossRef]
- Pournajaf, S.; Dargahi, L.; Javan, M.; Pourgholami, M.H. Molecular Pharmacology and Novel Potential Therapeutic Applications of Fingolimod. Front. Pharmacol. 2022, 13, 807639. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Li, H.; Huang, Z.; Wang, Y. Sphingosine kinase 1 counteracts chemosensitivity and immune evasion in diffuse large B cell lymphoma cells via the PI3K/AKT/PD-L1 axis. Int. Immunopharmacol. 2024, 143 Pt 2, 113361. [Google Scholar] [CrossRef]
- Sim, N.; Carter, J.M.; Deka, K.; Tan, B.K.T.; Sim, Y.; Tan, S.M.; Li, Y. TWEAK/Fn14 signalling driven super-enhancer reprogramming promotes pro-metastatic metabolic rewiring in triple-negative breast cancer. Nat. Commun. 2024, 15, 5638. [Google Scholar] [CrossRef]
- Huang, H.; Hu, J.; Maryam, A.; Huang, Q.; Zhang, Y.; Ramakrishnan, S.; Li, J.; Ma, H.; Ma, V.W.S.; Cheuk, W.; et al. Defining super-enhancer landscape in triple-negative breast cancer by multiomic profiling. Nat. Commun. 2021, 12, 2242. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Jiang, Y.; Wang, Z.; Li, H.; Wang, W.; Wang, C.; Hou, J. KLF7 regulates super-enhancer-driven IGF2BP2 overexpression to promote the progression of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 69. [Google Scholar] [CrossRef]
- Choi, H.I.; An, G.Y.; Baek, M.; Yoo, E.; Chai, J.C.; Lee, Y.S.; Jung, K.H.; Chai, Y.G. BET inhibitor suppresses migration of human hepatocellular carcinoma by inhibiting SMARCA4. Sci. Rep. 2021, 11, 11799. [Google Scholar] [CrossRef] [PubMed]
- Funaki, M.; Kitabayashi, J.; Shimakami, T.; Nagata, N.; Sakai, Y.; Takegoshi, K.; Okada, H.; Murai, K.; Shirasaki, T.; Oyama, T.; et al. Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo. Sci. Rep. 2017, 7, 16978. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Wang, W.; Du, L.; Li, D.; Wang, Y.; Xie, Y.; Li, H.; Wang, J.; Shi, Z.; Zhou, Y.; et al. SP1 undergoes phase separation and activates RGS20 expression through super-enhancers to promote lung adenocarcinoma progression. Proc. Natl. Acad. Sci. USA 2024, 121, e2401834121. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, K.; Li, X.; Du, M.; Kang, X.; Luo, X.; Gao, L.; Wang, C.; Zhang, Y.; Zhang, C.; et al. Identification of poly(ADP-ribose) polymerase-1 as a cell cycle regulator through modulating Sp1 mediated transcription in human hepatoma cells. PLoS ONE 2013, 8, e82872. [Google Scholar] [CrossRef]
- Chen, W.; Qiukai, Q.; Sun, B.; Zhang, P.; Li, N.; Fei, S.; Wang, Y.; Liu, S.; Liu, X.; Zhang, X. PARP1-catalyzed PARylation of YY1 mediates endoplasmic reticulum stress in granulosa cells to determine primordial follicle activation. Cell Death Dis. 2023, 14, 524. [Google Scholar] [CrossRef]
- Guo, C.; Si, S.; Fang, H.; Shuai, S.; Zhang, Y.; Du, X.; Duan, B.; Wu, J.; Yao, H.; Ge, Z.; et al. LEDGF/p75 promotes transcriptional pausing through preventing SPT5 phosphorylation. Sci. Adv. 2025, 11, eadr2131. [Google Scholar] [CrossRef]
- Quarni, W.; Dutta, R.; Green, R.; Katiri, S.; Patel, B.; Mohapatra, S.S.; Mohapatra, S. Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Sci. Rep. 2019, 9, 15202. [Google Scholar] [CrossRef]
- Gan, J.H.; Liu, J.X.; Liu, Y.; Chen, S.W.; Dai, W.T.; Xiao, Z.X.; Cao, Y. DrugRep: An automatic virtual screening server for drug repurposing. Acta Pharmacol. Sin. 2023, 44, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Song, J.; Hsieh, C.Y.; Cao, D.; Kang, Y.; Ye, W.; Wu, Z.; Wang, J.; Zhang, O.; Zhang, X.; et al. DrugFlow: An AI-Driven One-Stop Platform for Innovative Drug Discovery. J. Chem. Inf. Model. 2024, 64, 5381–5391. [Google Scholar] [CrossRef] [PubMed]
- Szanto, M.; Gupte, R.; Kraus, W.L.; Pacher, P.; Bai, P. PARPs in lipid metabolism and related diseases. Prog. Lipid Res. 2021, 84, 101117. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Z.; Wang, X.R.; Wang, J.; Xie, C.; Wang, X.X.; Pan, H.D.; Meng, W.Y.; Liang, T.L.; Li, J.X.; Yan, P.Y.; et al. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front. Oncol. 2022, 12, 941643. [Google Scholar] [CrossRef]
- Schiffmann, S.; Sandner, J.; Birod, K.; Wobst, I.; Angioni, C.; Ruckhaberle, E.; Kaufmann, M.; Ackermann, H.; Lotsch, J.; Schmidt, H.; et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 2009, 30, 745–752. [Google Scholar] [CrossRef]
- Kawakita, Y.; Motoyama, S.; Sato, Y.; Koyota, S.; Wakita, A.; Liu, J.; Saito, H.; Minamiya, Y. Sphingosine-1-phosphate/sphingosine kinase 1-dependent lymph node metastasis in esophageal squamous cell carcinoma. Surg. Today 2017, 47, 1312–1320. [Google Scholar] [CrossRef]
- Ruckhaberle, E.; Rody, A.; Engels, K.; Gaetje, R.; von Minckwitz, G.; Schiffmann, S.; Grosch, S.; Geisslinger, G.; Holtrich, U.; Karn, T.; et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res. Treat. 2008, 112, 41–52. [Google Scholar] [CrossRef]
- Jozefczuk, E.; Guzik, T.J.; Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol. Res. 2020, 156, 104793. [Google Scholar] [CrossRef]
- Teng, K.; Ma, H.; Gai, P.; Zhao, X.; Qi, G. SPHK1 enhances olaparib resistance in ovarian cancer through the NFkappaB/NRF2/ferroptosis pathway. Cell Death Discov. 2025, 11, 29. [Google Scholar] [CrossRef]
- Jin, N.; Qian, Y.Y.; Jiao, X.F.; Wang, Z.; Li, X.; Pan, W.; Jiang, J.K.; Huang, P.; Wang, S.Y.; Jin, P.; et al. Niraparib restricts intraperitoneal metastases of ovarian cancer by eliciting CD36-dependent ferroptosis. Redox Biol. 2025, 80, 103528. [Google Scholar] [CrossRef]
- Oei, S.L.; Griesenbeck, J.; Schweiger, M.; Ziegler, M. Regulation of RNA polymerase II-dependent transcription by poly(ADP-ribosyl)ation of transcription factors. J. Biol. Chem. 1998, 273, 31644–31647. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.; Ozerov, I.V.; Pun, F.W.; Tretina, K.; Fokina, T.; Chen, S.; Naumov, V.; Long, X.; Lin, S.; Korzinkin, M.; et al. PandaOmics: An AI-Driven Platform for Therapeutic Target and Biomarker Discovery. J. Chem. Inf. Model. 2024, 64, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Glod, J.; Peer, C.J.; Sissung, T.M.; Arnaldez, F.I.; Long, L.; Figg, W.D.; Whitcomb, P.; Helman, L.J.; Widemann, B.C. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother. Pharmacol. 2017, 80, 645–652. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Alshaker, H.; Cooper, C.; Winkler, M.; Pchejetski, D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016, 7, 23106–23127. [Google Scholar] [CrossRef]
- Hong, P.; Liu, Q.W.; Xie, Y.; Zhang, Q.H.; Liao, L.; He, Q.Y.; Li, B.; Xu, W.W. Echinatin suppresses esophageal cancer tumor growth and invasion through inducing AKT/mTOR-dependent autophagy and apoptosis. Cell Death Dis. 2020, 11, 524. [Google Scholar] [CrossRef]
- Wang, X.; Luo, L.; Xu, J.; Lu, Q.; Xia, H.; Huang, Y.; Zhang, L.; Xie, L.; Jiwa, H.; Liang, S.; et al. Echinatin inhibits tumor growth and synergizes with chemotherapeutic agents against human bladder cancer cells by activating p38 and suppressing Wnt/beta-catenin pathways. Genes. Dis. 2024, 11, 1050–1065. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, H.; Wang, X.; Luo, L.; Xia, H.; Zhang, L.; Xu, J.; Huang, Y.; Luo, X.; Luo, J. Echinatin inhibits the growth and metastasis of human osteosarcoma cells through Wnt/beta-catenin and p38 signaling pathways. Pharmacol. Res. 2023, 191, 106760. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Han, X.; Xie, Z.; Hou, Y.; Liu, M.; Cheng, Y.; Lu, Q.; Luo, J.; Wang, H. Echinatin inhibits the growth and metastasis of human hepatocellular carcinoma cells through p38 and JNK signaling pathways. Tissue Cell 2025, 95, 102907. [Google Scholar] [CrossRef]






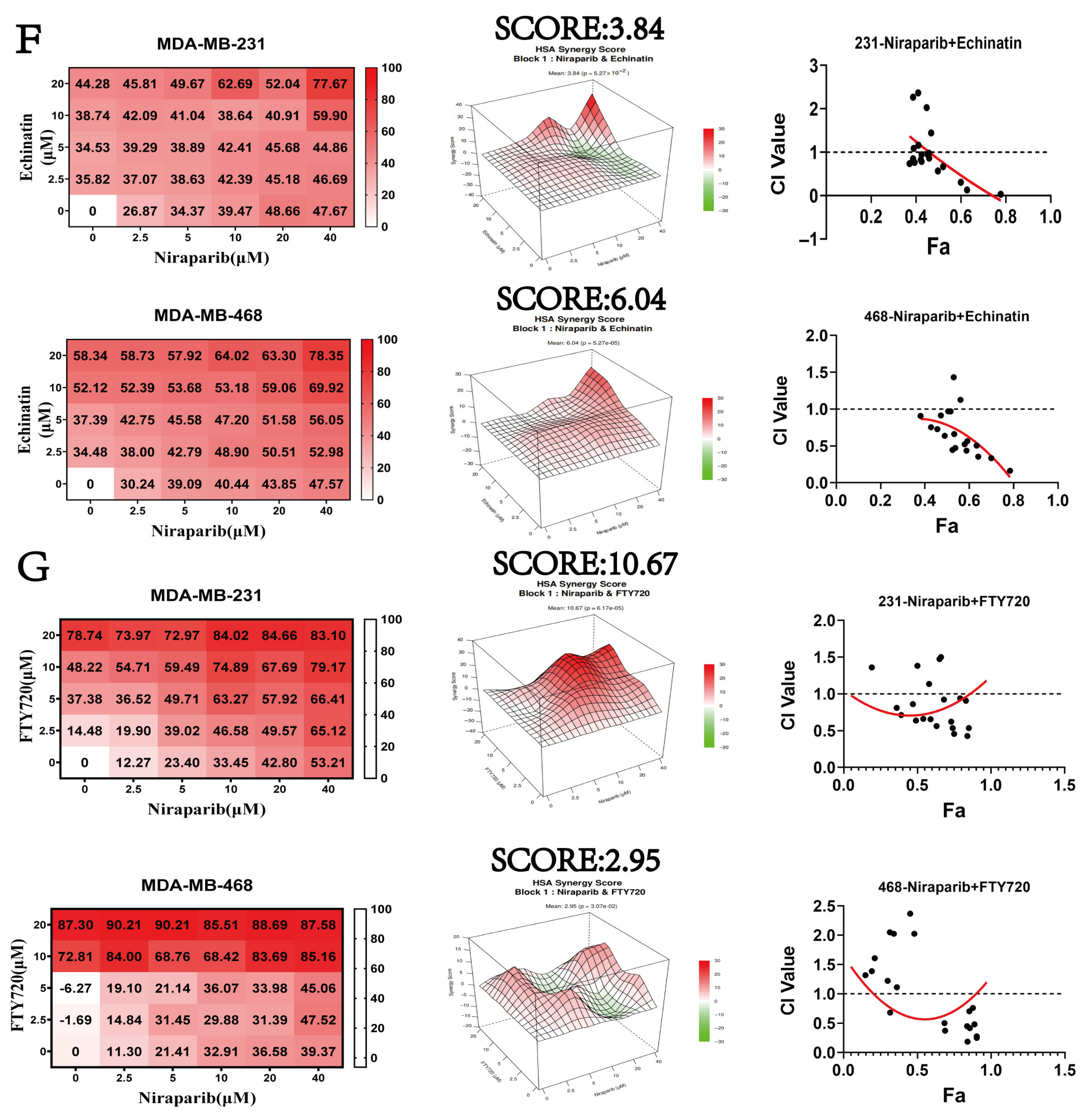
| Cell Lines | Niraparib (μM) + FTY720 (μM) ED50 | Niraparib (μM) + Echinatin (μM) ED50 |
|---|---|---|
| MDA-MB-231 | 0.75 | 0.60 |
| MDA-MB-468 | 0.65 | 0.64 |
| Cell Lines | Niraparib (μM) | Echinatin (μM) | FTY720 (μM) | Niraparib (μM) + Echinatin (μM) | Niraparib (μM) + FTY720 (μM) |
|---|---|---|---|---|---|
| MDA-MB-231 | 35.90 | 32.31 | 8.54 | 18.32/9.16 | 8.11/4.05 |
| MDA-MB-468 | 52.91 | 6.61 | 13.23 | 9.37/4.68 | 9.74/4.87 |
| Gene | Primer Direction | Sequence (5′ → 3′) |
|---|---|---|
| SPHK1-(homo) | Forward | CCTTCACGCTGATGCTCACT |
| Reverse | GTTCACCACCTCGTGCATCA | |
| SE-(homo) | Forward | CCTGTGTGACAGCCTTGGTTGAAACCC |
| Reverse | GAGTGTGGACATTAGGGTCGAGAAACTC | |
| actin-(homo) | Forward | GAGAAAATCTGGCACCACACC |
| Reverse | GGATAGCACAGCCTGGATAGCAA |
| Gene | Direction | Sequence (5′ → 3′) |
|---|---|---|
| SPHK1-Homo | sense | GCGUCAUGCAUCUGUUCUATT |
| antisense | UAGAACAGAUGCAUGACGCTT | |
| SE-Homo | sense | GAUGUUGCCCAUGGAGCAATT |
| antisense | UUGCUCCAUGGGCAACAUCTT | |
| SP1-Homo | sense | GCCGUUGGCUAUAGCAAAUTT |
| antisense | AUUUGCUAUAGCCAACGGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.-X.; Chen, R.-J.; Tu, G.-H.; Li, C.-Q.; Xu, L.-L.; Lu, Y.-L.; Wu, L.-X. The SP1-SuperEnhancer-SPHK1 Axis Mediates Niraparib Resistance in TNBC. Pharmaceuticals 2025, 18, 1622. https://doi.org/10.3390/ph18111622
Yuan Y-X, Chen R-J, Tu G-H, Li C-Q, Xu L-L, Lu Y-L, Wu L-X. The SP1-SuperEnhancer-SPHK1 Axis Mediates Niraparib Resistance in TNBC. Pharmaceuticals. 2025; 18(11):1622. https://doi.org/10.3390/ph18111622
Chicago/Turabian StyleYuan, Yu-Xia, Rui-Jia Chen, Gui-Hui Tu, Chao-Qi Li, Long-Long Xu, Yi-Ling Lu, and Li-Xian Wu. 2025. "The SP1-SuperEnhancer-SPHK1 Axis Mediates Niraparib Resistance in TNBC" Pharmaceuticals 18, no. 11: 1622. https://doi.org/10.3390/ph18111622
APA StyleYuan, Y.-X., Chen, R.-J., Tu, G.-H., Li, C.-Q., Xu, L.-L., Lu, Y.-L., & Wu, L.-X. (2025). The SP1-SuperEnhancer-SPHK1 Axis Mediates Niraparib Resistance in TNBC. Pharmaceuticals, 18(11), 1622. https://doi.org/10.3390/ph18111622







