Clinical Studies on Topical Anesthesia in Dentistry: A Bibliometric Analysis
Abstract
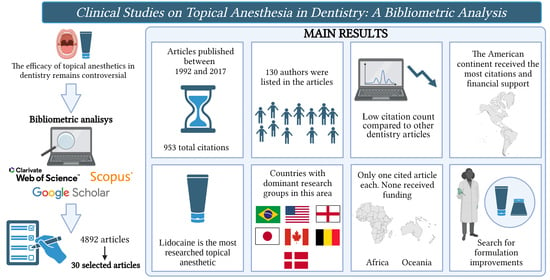
1. Introduction
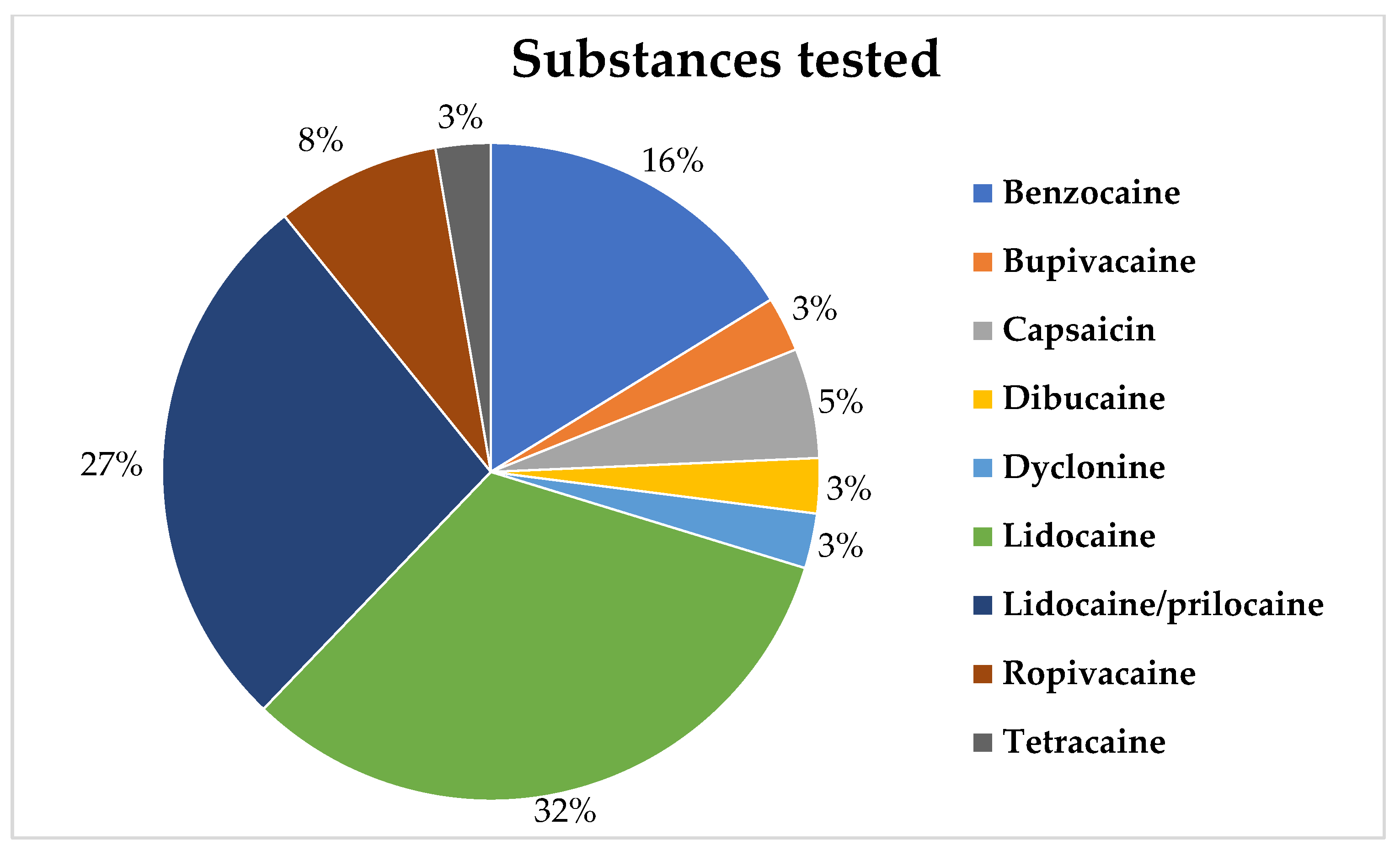
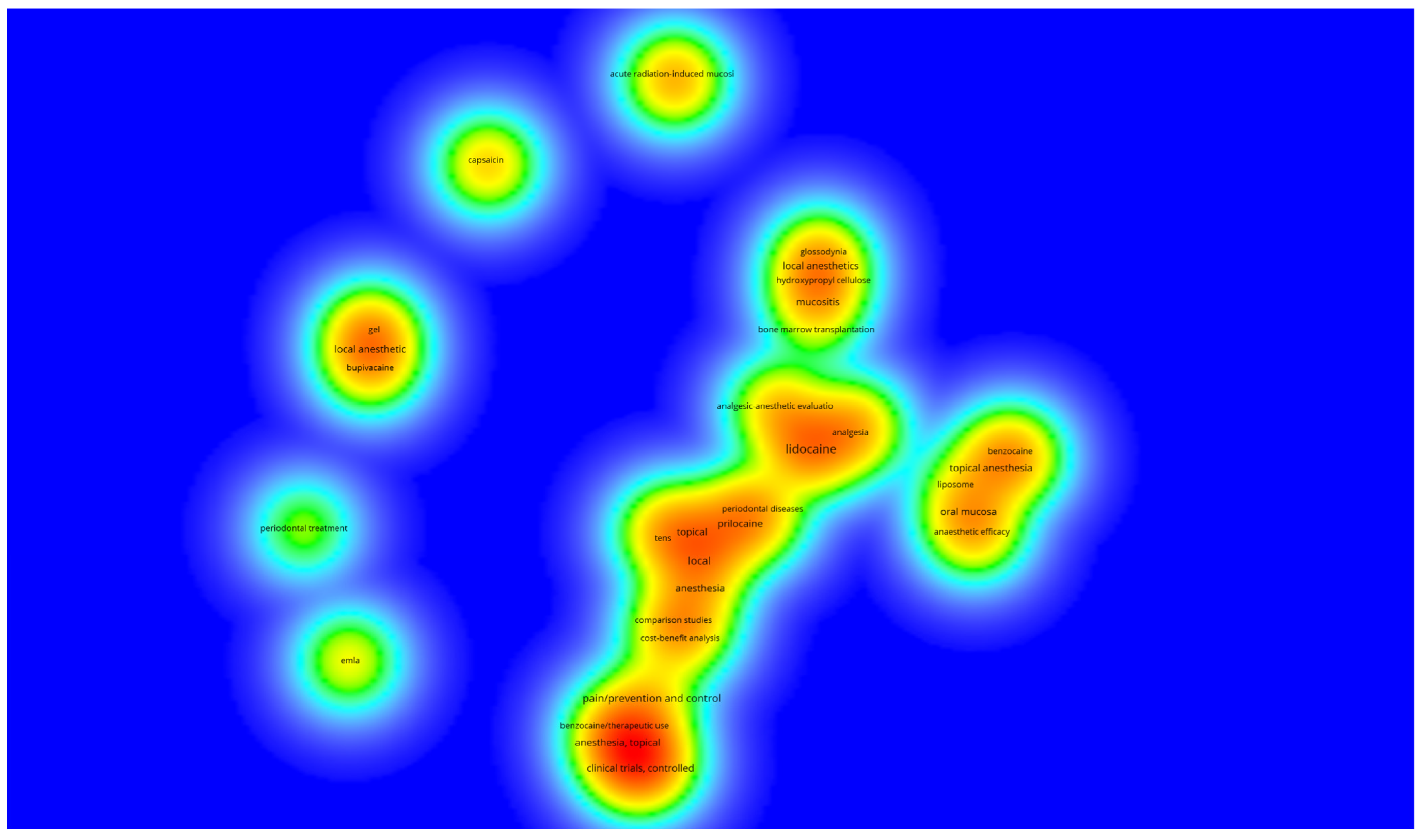
2. Results
| Rank | Article Title | Corresponding Author | Country (According to the Address of the Corresponding Author) | Year of Publication | Total Citations (Citation Density *) | ||
|---|---|---|---|---|---|---|---|
| Web of Science—Core Collection | Scopus | Google Scholar | |||||
| 1 | Efficacy of a wax containing benzocaine in the relief of oral mucosal pain caused by orthodontic appliances [33] | Kluemper, GT. | USA | 2002 | 70 (3.5) | 86 (4.3) | 178 (8.9) |
| 2 | The effects of topical anesthesia on oral burning in burning mouth syndrome [35] | Formaker, BK. | USA | 1998 | 60 (2.5) | 82 (3.42) | 115 (4.8) |
| 3 | Analgesic efficacy and safety of an intraoral lidocaine patch [9] | Hersh, EV. | USA | 1996 | 48 (1.85) | 68 (2.62) | 121 (4.66) |
| 4 | Liposomal lidocaine gel for topical use at the oral mucosa: characterization, in vitro assays and in vivo anesthetic efficacy in humans [23] | Franz-Montan, M. | Brazil | 2015 | 46 (6.57) | 44 (6.29) | 68 (9.72) |
| 5 | The effect of clove and benzocaine versus placebo as topical anesthetics [34] | Alqareer, A. | Kuwait | 2006 | 42 (2.63) | 73 (4.56) | 171 (10.67) |
| 6 | The anesthetic onset and duration of a new lidocaine/prilocaine gel intra-pocket anesthetic (Oraqix® for periodontal scaling/root planing [36] | Friskopp, J. | Switzerland | 2001 | 42 (2.0) | 47 (2.24) | 71 (3.38) |
| 7 | Liposome-encapsulated ropivacaine for topical anesthesia of human oral mucosa [27] | Groppo, FC. | Brazil | 2007 | 41 (2.73) | 37 (2.47) | 73 (4.87) |
| 8 | A placebo-controlled multi-centred evaluation of an anaesthetic gel (Oraqix®) for periodontal therapy [21] | Donaldson, D. | Canada | 2003 | 40 (2.11) | 53 (2.79) | 82 (4.32) |
| 9 | Intrapocket anesthesia for scaling and root planing: Results of a double-blind multicenter trial using lidocaine prilocaine dental gel [37] | Jeffcoat, MK. | USA | 2001 | 37 (1.76) | 45 (2.14) | 78 (3.72) |
| 10 | Mucosa-adhesive water-soluble polymer film for treatment of acute radiation-induced oral mucositis [20] | Oguchi, M. | Japan | 1998 | 35 (1.46) | 46 (1.92) | 66 (2.75) |
| 11 | Comparison of topical anesthesia of 20% benzocaine and 60% lidocaine gel [38] | Fukayama, H. | Japan | 2002 | 34 (1.7) | 45 (2.25) | 80 (4.0) |
| 12 | Patient evaluation of a novel non-injectable anesthetic gel: A multicenter crossover study comparing the gel to infiltration anesthesia during scaling and root planing [2] | van Steenberghe, D. | Belgium | 2004 | 32 (1.78) | 39 (2.17) | 63 (2.5) |
| 13 | Formulation of an antispasmodic drug as a topical local anesthetic [39] | Abdel-Hamid, SM. | Egypt | 2006 | 29 (1.81) | 36 (2.25) | 52 (3.25) |
| 14 | A comparison of topical anaesthesia and electronic nerve stimulation for reducing the pain of intra-oral injections [40] | Meechan, JG. | England | 1996 | 29 (1.16) | 42 (1.62) | 82 (3.16) |
| 15 | Liposomal delivery system for topical anaesthesia of the palatal mucosa [26] | Franz-Montan, M. | Brazil | 2012 | 28 (2.8) | 30 (3.0) | 56 (5.6) |
| 16 | The efficacy of EMLA® and 5% lignocaine gel for anaesthesia of human gingival mucosa [41] | McMillan, AS. | China | 2000 | 28 (1.27) | 36 (1.64) | 61 (2.77) |
| 17 | Onset and duration of hypoalgesia of lidocaine spray applied to oral mucosa—A dose–response study [42] | Van der Burght, M | Denmark | 1992 | 26 (0.87) | 27 (0.9) | 40 (1.33) |
| 18 | Effect of a local anesthetic lozenge in relief of symptoms in burning mouth syndrome [22] | Treldal, C. | Denmark | 2016 | 25 (4.17) | 28 (4.67) | 44 (7.33) |
| 19 | Intrapocket anesthesia for scaling and root planing in pain-sensitive patients [43] | Magnusson, I. | USA | 2003 | 25 (1.32) | 33 (1.74) | 62 (3.26) |
| 20 | A human oral capsaicin pain model to assess topical anesthetic-analgesic drugs [44] | Dallel, R. | France | 2001 | 25 (1.19) | 29 (1.38) | 41 (1.95) |
| 21 | Plasma levels of lidocaine and prilocaine after application of Oraqix®, a new intrapocket anesthetic, in patients with advanced periodontitis [45] | Friskopp, J. | Switzerland | 2001 | 25 (1.19) | 36 (1.72) | 43 (2.05) |
| 22 | Analgesic effect of topical oral capsaicin gel in burning mouth syndrome [46] | Jørgensen, MR | Denmark | 2017 | 24 (4.0) | 28 (5.6) | 56 (9.33) |
| 23 | Oral mucosal adhesive film containing local anesthetics: In vitro and clinical evaluation [47] | Yamamura, K. | Japan | 1998 | 24 (1.0) | 25 (1.04) | 49 (2.04) |
| 24 | The use of patient-controlled transcutaneous electronic nerve stimulation (TENS) to decrease the discomfort of regional anaesthesia in dentistry: Randomized controlled clinical trial [48] | Meechan, JG. | England | 1998 | 24 (1.0) | 34 (1.42) | 63 (2.63) |
| 25 | The intraoral use of EMLA cream in children—A clinical investigation [49] | Meechan, JG. | England | 1994 | 24 (0.86) | 34 (1.22) | 50 (1.79) |
| 26 | Topical Lidocaine to Improve Oral Intake in Children With Painful Infectious Mouth Ulcers: A Blinded, Randomized, Placebo-Controlled Trial [50] | Hopper, SM. | Australia | 2014 | 23 (2.56) | 26 (3.25) | 72 (8.0) |
| 27 | Liposome-encapsulated ropivacaine for intraoral topical anesthesia [25] | Franz-Montan, M. | Brazil | 2010 | 23 (1.92) | 25 (2.08) | 43 (3.58) |
| 28 | Clinical evaluation and comparison of 2 topical anesthetics for pain caused by needle sticks and scaling and root planing [51] | Carr, MP. | USA | 2001 | 23 (1.1) | 30 (1.43) | 46 (2.19) |
| 29 | Influence of topical application of capsaicin, menthol and local anesthetics on intraoral somatosensory sensitivity in healthy subjects: temporal and spatial aspects [52] | Naganawa, T. | Denmark | 2015 | 21 (3.0) | 19 (2.72) | 25 (3.57) |
| 30 | Systemic absorption of lidocaine after topical application for the treatment of oral mucositis in bone marrow transplantation patients [53] | Elad, S | Israel | 1999 | 21 (0.91) | 32 (1.39) | 42 (1.83) |
| Rank | Author (Year) | Main Objective | Drugs Used | Type of Formulations | Mouth Region | Time of Application | No. of Volunteers | Age | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kluemper et al., (2002) [33] | Determine the effectiveness of an orthodontic wax containing benzocaine, which is released over time in a controlled manner, for reducing pain related to trauma resulting from friction of orthodontic appliances on the oral mucosa. | Benzocaine (20%) | Wax | Oral mucosa | The participants used the wax during the 53 h pain assessment. | 70 | Adults and children, with no information about the age range. | The wax was significantly effective in reducing discomfort in the mucosa associated with orthodontic appliances. |
| 2 | Formaker et al., (1998) [35] | Test the effectiveness of a topical anesthetic in patients with oral burning and taste alterations. | Dyclonine HCl 1% | Solution | Oral mucosa | 2 min | 33 | 35–83 years | Dyclonine promoted an increase, reduction, and absence of change in patients with burning mouth syndrome. Regarding taste, dyclonine led to a reduction in dysgeusia. |
| 3 | Hersh et al., 1996 [9] | Test the effectiveness of intraoral lidocaine patches in the pain experience during the puncture of a 25 G needle. | Lidocaine 10% and 20% | patches | 2 mm apical to the mucogingival junction in both the maxillary and mandibular premolar region. | 2 min | 101 | 18–65 years | Lidocaine patches at both concentrations were effective and safe in reducing needle insertion pain. |
| 4 | Franz-Montan et al., 2015 [23] | Characterize liposomal formulations of lidocaine for topical use on the oral mucosa and compare their in vitro permeation and in vivo anesthetic efficacy. | 5% lidocaine encapsulated in liposomes, 2.5% lidocaine encapsulated in liposomes, 5% lidocaine ointment, eutectic mixture of lidocaine and prilocaine 2.5% (EMLA)® | Gel | Palatal mucosa of the upper canines. | 2 min | 40 | 18–29 years | 5% lidocaine encapsulated in liposomes and EMLA showed the best in vitro results, as well as the best topical anesthetic efficacy in vivo. |
| 5 | Alqareer et al., 2006 [34] | Verify if natural clove can replace benzocaine as a topical anesthetic in reducing pain during puncture. | Clove gel (prepared with commercially available clove powder mixed with glycerin), 20% benzocaine, and placebo. | Gel | Buccal mucosa of the upper canines. | 4 min | 73 | 19–25 years | Both clove gel and benzocaine showed lower pain scores compared to the placebo, with no statistical difference between the two active agents. |
| 6 | Friskopp et al., 2001 [36] | Determine the onset and duration of the anesthetic gel (Oraqix®) in patients with periodontitis during scaling and root planing procedures. | Lidocaine (25 mg/g) + Prilocaine (25 mg/g) (Oraqix®) | Gel | Periodontal pocket | 30 s, 2 min and 5 min. | 30 | 18–60 years | Oraqix® provides anesthesia 30 s after application and has a duration of effect close to 17–20 min. |
| 7 | Franz-Montan et al., 2007 [27] | Evaluate the effectiveness of ropivacaine encapsulated in liposomes for topical anesthesia. | 1% ropivacaine encapsulated in liposomes, 1% plain ropivacaine, a mixture of 2.5% lidocaine and 2.5% prilocaine (EMLA®), and 20% benzocaine | Gel | Buccal fold of the upper-right canine | 2 min | 30 | 18–26 years | The 1% ropivacaine gel encapsulated in liposomes was equivalent to EMLA in reducing pain during needle insertion and for the duration of anesthesia in soft tissue. None of the topical anesthetics was effective in inducing pulp anesthesia. |
| 8 | Donaldson et al., 2003 [21] | The ability of a thermosetting gel to produce analgesia in periodontal pockets during scaling and root planing. | Lidocaine (25 mg/g) + Prilocaine (25 mg/g) (Oraqix®) | Gel | Periodontal pocket | 30 s–2 min | 130 | 18+ years | The 5% anesthetic gel was statistically more effective than the placebo in reducing pain during periodontal debridement surgery. |
| 9 | Jeffcoat et al., 2001 [37] | Evaluate the effectiveness of a new anesthetic gel for periodontal pocket anesthesia. | Lidocaine 25 mg/g with prilocaine 25 mg/g | Gel | Periodontal pocket | 30 s–2 min | 122 | 18+ years | The gel showed a significant reduction in pain compared to the placebo, indicating its potential effectiveness/utility during scaling and root planing procedures. |
| 10 | Oguchi et al., 1998 [20] | Examine the usefulness and safety of a water-soluble and mucoadhesive polymeric film containing anesthetics and antibiotics for the treatment of radiation-induced acute oral mucositis. | Hydroxypropyl cellulose (600 mg), Tetracaine Hydrochloride (5 mg), Ofloxacin (5 mg), Miconazole (5 mg), Guaiazulene (0.6 mg), Triacetin (24 mg), ethyl alcohol (100 mL) | Adhesive | Oral mucosa | 1 h | 52 | 34–87 years | It proved to be useful for relieving pain resulting from acute radiation-induced oral mucositis, maintaining good oral nutrition, and preventing secondary oral infections, without inducing adverse reactions. |
| 11 | Fukayama et al., 2002 [38] | Determine the effectiveness of 2 commonly used topical anesthetics in dentistry. | Benzocaine 20% and lidocaine 60% | Gel | Alveolar mucosa of the apices of the upper incisors. | 20 min | 20 | 23–34 years | The 20% benzocaine showed no change in pain perception when compared to the placebo. However, the 60% lidocaine significantly reduced this perception. |
| 12 | van Steenberghe et al., 2004 [2] | Evaluate the preferences of volunteers for the use of non-injectable or injectable anesthetics during scaling and/or root planing. | Lidocaine 25 mg/g and prilocaine 25 mg/g versus injection anesthesia (lidocaine 2% with adrenaline) | Gel and solution | Periodontal pocket of upper and lower quadrants. | 30 s | 170 | 25–72 years | The majority of patients (70%) preferred topical anesthesia. 96% of patients reported satisfactory anesthesia with the injection, and 80% with the gel. Post-procedure issues, such as numbness and pain, were lower when the gel was used compared to the injection. |
| 13 | Abdel-Hamid et al., 2006 [39] | Formulate mebeverine HCl into a gel that could be used locally in the treatment of various oral painful conditions. | Mebeverine HCl and Lidocaine HCl 20%. | Gel | Oral Cavity | 3 to 4 times a day for 2 days. | 25 | 18–65 years | The gel formulation has shown a better pain reduction efficiency and longer duration than Lidocaine HCl gel®. |
| 14 | Meechan et al., 1996 [40] | Investigate the effectiveness of using EMLA and TENS in relieving pain from intraoral injections prior to extractions of maxillary teeth. | EMLA®, TENS®, and placebo, before the injection of 2% lidocaine. | Cream in patches; TENS (electrodes); | Palatal mucosa | EMLA and placebo: 5 min; TENS: 2 min | 100 | 18+ years | EMLA reduced the pain of the palatal injection compared to the placebo group. TENS did not differ from the placebo group. Therefore, EMLA may be useful for reducing the pain of injection in the palatal region. |
| 15 | Franz-Montan et al., 2012 [26] | Evaluate the efficiency of ropivacaine encapsulated in liposomes at different concentrations for topical anesthesia in the palate. | Ropivacaine 2% encapsulated in liposomes; ropivacaine 1% encapsulated in liposomes; placebo liposomes; and lidocaine 2.5% with prilocaine 2.5% (EMLA®) | Gel | Palatal mucosa of the upper canines. | 5 min | 40 | 19–29 years | The ropivacaine formulations encapsulated in liposomes were not effective in reducing puncture pain, with no differences compared to the placebo. EMLA was effective in reducing pain during puncture. However, none of the formulations were effective in reducing pain from local anesthetic injection when compared to the placebo. |
| 16 | McMillan et al., 2000 [41] | Compare the analgesic effect of a mixture of 2.5% lidocaine and 2.5% prilocaine (EMLA®) with 5% lidocaine gel alone for minor gum operations. | EMLA® (Lidocaine 2.5% and prilocaine 2.5%) | Gel | Palatal mucosa | 10 min | 10 | 20–21 years | EMLA showed a longer duration of anesthesia and a better area under the pressure-pain threshold curve than lidocaine alone. |
| 17 | Schønemann et al., 1992 [42] | Determine the ideal time interval for dental procedures involving pain after the application of lidocaine spray. Pain was tested through stimulation with argon laser. | Lidocaine (30 and 60 mg) | Spray | Lower lip mucosa | 5 s | 24 | Lidocaine 30 mg group: average age of 29.5 years. Lidocaine 60 mg group: average age of 34 years. | There was an increase in the pain threshold for both dosages, with no difference between the groups. Local oral application did not produce complete analgesia. Maximum hypoalgesia occurred between 4 and 5 min, with a duration of up to 14 min. However, the ideal time interval for procedures was between 3-8 min. |
| 18 | Treldal et al., 2016 [22] | Evaluate the effect of a bupivacaine lozenge on oral mucosa pain, xerostomia, and taste alterations in patients with Burning Mouth Syndrome. | Bupivacaine | Lozenge | Oral cavity | Use until the tablet is completely dissolved | 18 | 39–71 years | Bupivacaine lozenge significantly reduced burning oral pain, increased the sensation of taste disturbances, and had no impact on xerostomia. |
| 19 | Magnusson et al., 2003 [43] | To evaluate the anesthetic effect of the mixture of lidocaine and prilocaine in pain-sensitive patients using a visual analogue scale (VAS) and a verbal rating scale (VRS). | Lidocaine 2.5 mg/g with prilocaine 2.5 mg/g (Oraqix®) | Gel | Periodontal pocket | 30–45 s | 85 | 21–77 years | The overall median VAS pain score was 11 mm in the treated group and 27 mm in the placebo group. No pain or only mild pain was reported by 70% in the anesthetic group and by 48% in the placebo group. Two patients in the treated group and 7 patients in the placebo group required additional anesthesia. |
| 20 | Ngom et al., 2001 [44] | To evaluate the reliability of the oral pain model of response to repeated applications of capsaicin | 0.25, 0.5 and 1% lidocaine | Mouthwash (30 mL) | Oral mucosa (tongue) | 3 min | 29 | 21–26 years | The capsaicin oral pain model has good reliability and sensitivity and allows safe evaluation of candidate topical analgesics and anesthetics. |
| 21 | Friskopp et al., 2001 [45] | To describe the plasma profiles of lidocaine and prilocaine after a single dose of Oraqix® for patients with advanced periodontitis. | Lidocaine/prilocaine (Oraqix®) (0.9 to 3.5 g) | Gel | Periodontal pocket | Oraqix® was applied in the pockets around all the teeth. Directly there after all the pockets were probed, teeth were subjected to SRP, and the mouth was then rinsed out with a glass of water | 10 | 38–56 years | Maximum plasma concentrations of lidocaine (99–266 ng/mL) and prilocaine (46–118 ng/mL) occurred 20–40 min after the start of application (below toxicity levels in the Central Nervous System). Therefore, there is a large margin of safety when it comes to applying up to 3.5 g of Oraqix® in periodontal pockets. |
| 22 | Jørgensen et al., 2017 [46] | To investigate the efficacy of repeated topical application of capsaicin oral gel at two different concentrations to alleviate burning/stinging sensations in patients with burning mouth syndrome (BMS) | 0.01% and 0.025% capsaicin | Gel | Dorsum of the tongue | Three times daily for 14 days, followed by 14 days wash-out period, and finally treatment with the other concentration of oral gel three times daily for 14 days. | 22 | +18 years | Topical capsaicin may be an alternative for short-term treatment of BMS. However, more studies are needed to investigate, especially the gastrointestinal side effects that may limit its long-term use. |
| 23 | Yamamura et al., 1998 [47] | To evaluate the effectiveness of an adhesive film for oral mucosa containing dibucaine (DC) in relieving pain caused by oral erosion. | Dibucaine | Adhesive film containing two concentrations: 0.113 and 0.225 mg/cm2 | Left buccal mucosa | No information | 23 | 29–66 years | The film showed good adhesion to the mucosa. The onset of the anesthetic effect was less than 5 min for both concentrations tested. The duration of anesthesia was 2.2 ± 0.21 h for the 0.113 mg/cm2 group and 4.3 ± 0.25 h for the 0.225 mg/cm2 group. |
| 24 | Meechan et al., 1998 [48] | Compare the use of topical anesthesia and transcutaneous electrical stimulation (TENS) as a means of reducing discomfort from lower tooth block injections | 20% Benzocaine, TENS and no pretreatment | Benzocaine: Gel; TENS: electrodes. | Oral mucosa | 2 min | 100 | +18 years | The discomfort of the long buccal injection did not differ between the three groups of patients. For lower tooth block anesthesia, the injection discomfort after TENS was lower than that in other groups. Topical anesthetic did not produce a significant change in injection discomfort compared to no pretreatment. |
| 25 | Meechan et al., 1994 [49] | Efficacy of EMLA® in Local Topical Anesthesia on the Palate Compared to Other Local Anesthetics | EMLA® and lidocaine 5%. | Gel | Fold of the oral mucosa | 5 min | 20 | 6–15 years | There is no advantage to be gained from using EMLA compared to conventional intraoral topical anesthetics when placed in the buccal fold in children. |
| 26 | Hopper et al., 2014 [50] | To establish the effectiveness of viscous 2% lidocaine in increasing oral intake in children with oral infectious conditions compared to placebo. | 2% lidocaine | Viscous lidocaine | Oral mucosa | 60 min | 110 | 6 months–8 years | Viscous lidocaine is not superior to a flavored gel placebo in improving oral intake in children with painful infectious mouth ulcers. |
| 27 | Franz-Montan et al., 2010 [25] | To evaluate the efficacy of 2% ropivacaine encapsulated in liposomes in topical anesthesia and its influence on pulp response. | 2% ropivacaine encapsulated in liposomes; 20% benzocaine, placebo encapsulated in liposomes; placebo liposomal gel; and placebo gel. | Gel | Vestibular groove of the lateral region of the maxilla-incisive region (bilateral) | 30 min | 40 | 18–43 years | Ropivacaine gel 2% and benzocaine 20% showed a lower response to needle insertion pain and long anesthesia in soft tissues, when compared to placebo, with no differences between the first two formulations. However, none were able to induce pulpal anesthesia. |
| 28 | Carr et al., 2001 [51] | To evaluate the effectiveness of the transoral lidocaine delivery system and compare it with a benzocaine gel preparation in reducing pain for needle stick and scaling and root planing. | 60% Lidocaine and 20% benzocaine | Adhesive and gel | Maxillary and mandibular molar-bicuspid areas | 15 min (lidocaine) 30 s (gel benzocaine) | 40 | 21–70 years | The transoral lidocaine delivery system is more effective than benzocaine gel in topical pain suppression for needle sticks and root planing in both arches. |
| 29 | Naganawa et al., 2015 [52] | To investigate temporal and spatial aspects of somatosensory changes after topical application of capsaicin, menthol and local anesthetics to the gums using intraoral palpometers and thermal devices. | EMLA® (lidocaine with prilocaine 5%) | Cream | Gum in the region of the upper first premolar | 15 min | 16 | Average age = 29 years | Topical application of capsaicin, but not menthol, increased thermal, but not mechanical, sensitivity at the application site and adjacent test sites, indicating thermal hyperalgesia in the primary zone, and for the first time also in the secondary gingival zone. |
| 30 | Elad et al., 1999 [53] | To evaluate the absorption of lidocaine by the oral mucosa after its topical administration in the symptomatic treatment of oral mucositis induced by bone marrow transplantation. | 2% lidocaine | Mouthwash | Oral mucosa | 1 min | 10 | 6–48 years | Lidocaine prescribed as an anesthetic mouthwash in patients with bone marrow transplant and oral mucositis, results in less systemic absorption of the drug. |
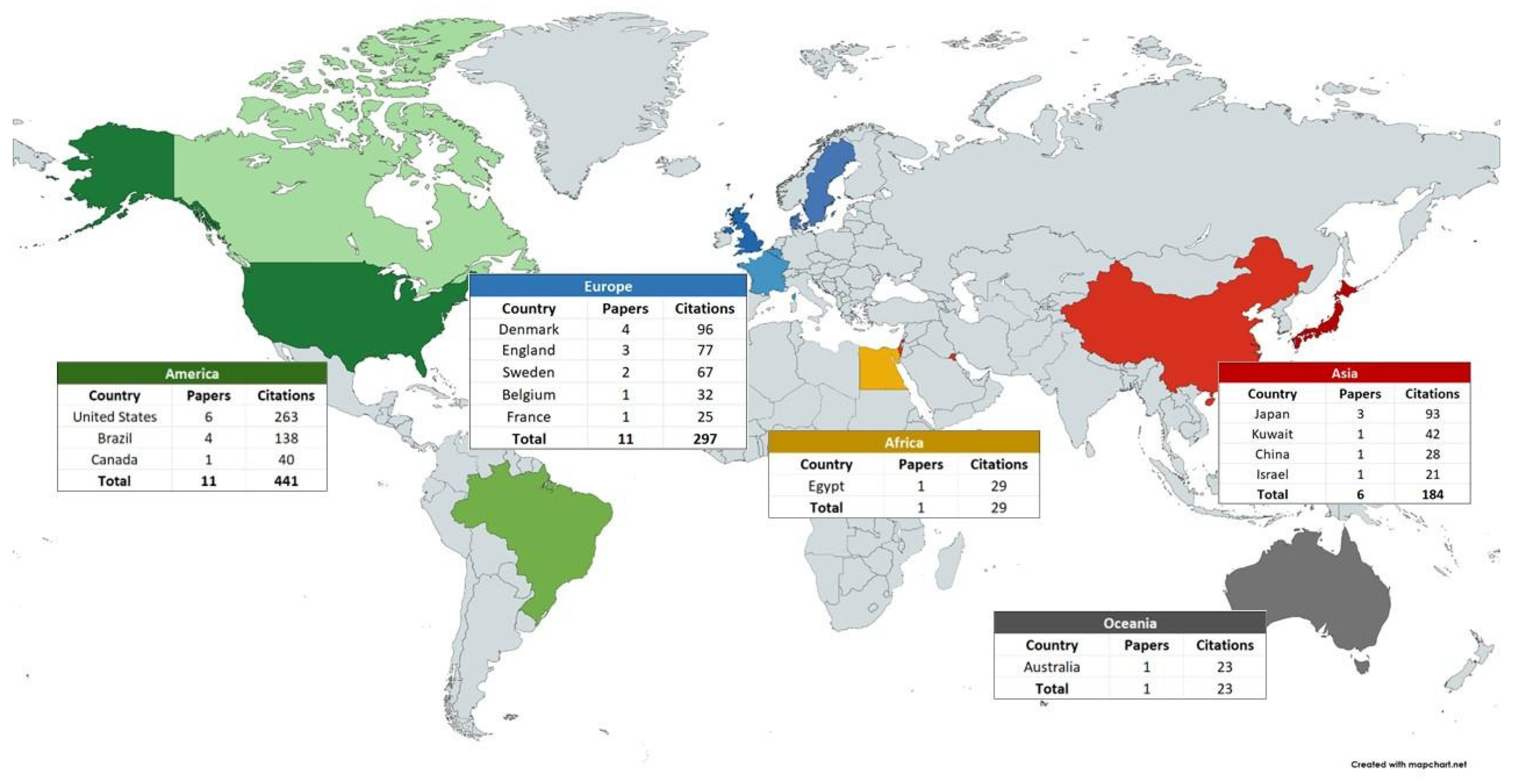
| Characteristics | N | Citations | |
|---|---|---|---|
| Main Institutions | |||
 | Universidade Estadual de Campinas | 4 | 138 |
 | University of Newcastle | 3 | 77 |
 | Public Dental Services of Sweden | 2 | 67 |
| Main journals | |||
| Journal of Periodontology | 4 | 117 | |
| Journal of Clinical Periodontology | 3 | 107 | |
| Journal of Dentistry | 2 | 66 | |
| Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology | 2 | 57 | |
| British Journal of Oral and Maxillofacial Surgery | 2 | 56 | |
| Funding/continent | |||
| America | |||
| Funded | 8 | 282 | |
| Not Funded | 3 | 159 | |
| Europe | |||
| Funded | 4 | 102 | |
| Not Funded | 7 | 195 | |
| Asia | |||
| Funded | 2 | 63 | |
| Not Funded | 4 | 121 | |
| Africa | |||
| Funded | 0 | - | |
| Not Funded | 1 | 29 | |
| Oceania | |||
| Funded | 0 | - | |
| Not Funded | 1 | 23 | |
| Number of Authors/Articles | N | ||
| 1– 4 | 16 | ||
| 5–8 | 10 | ||
| >8 | 4 | ||
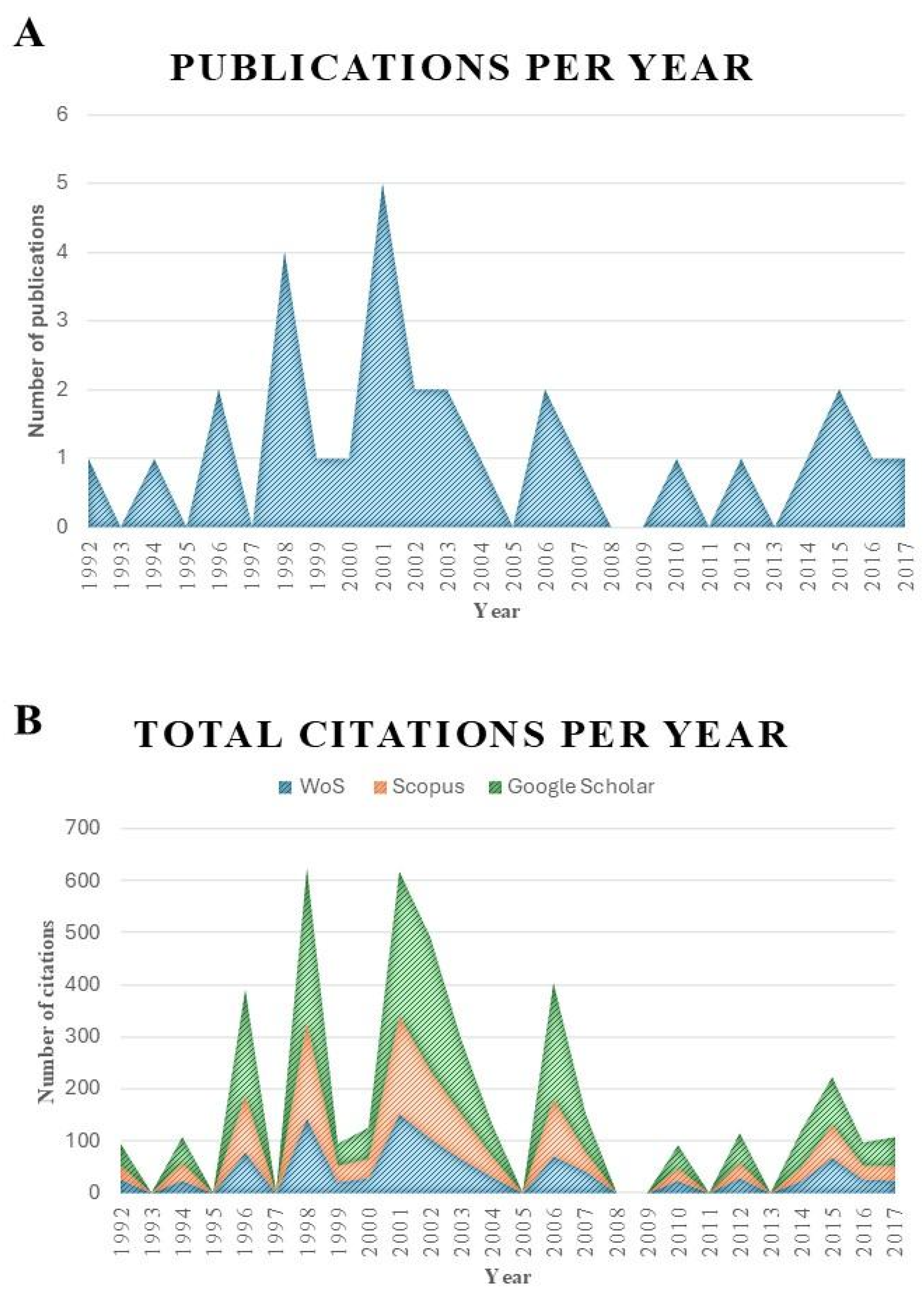
| Publication Time Range | N | ||
| 1992–1996 | 4 | ||
| 1997–2001 | 11 | ||
| 2002–2006 | 7 | ||
| 2007–2011 | 2 | ||
| 2012–2017 | 6 | ||
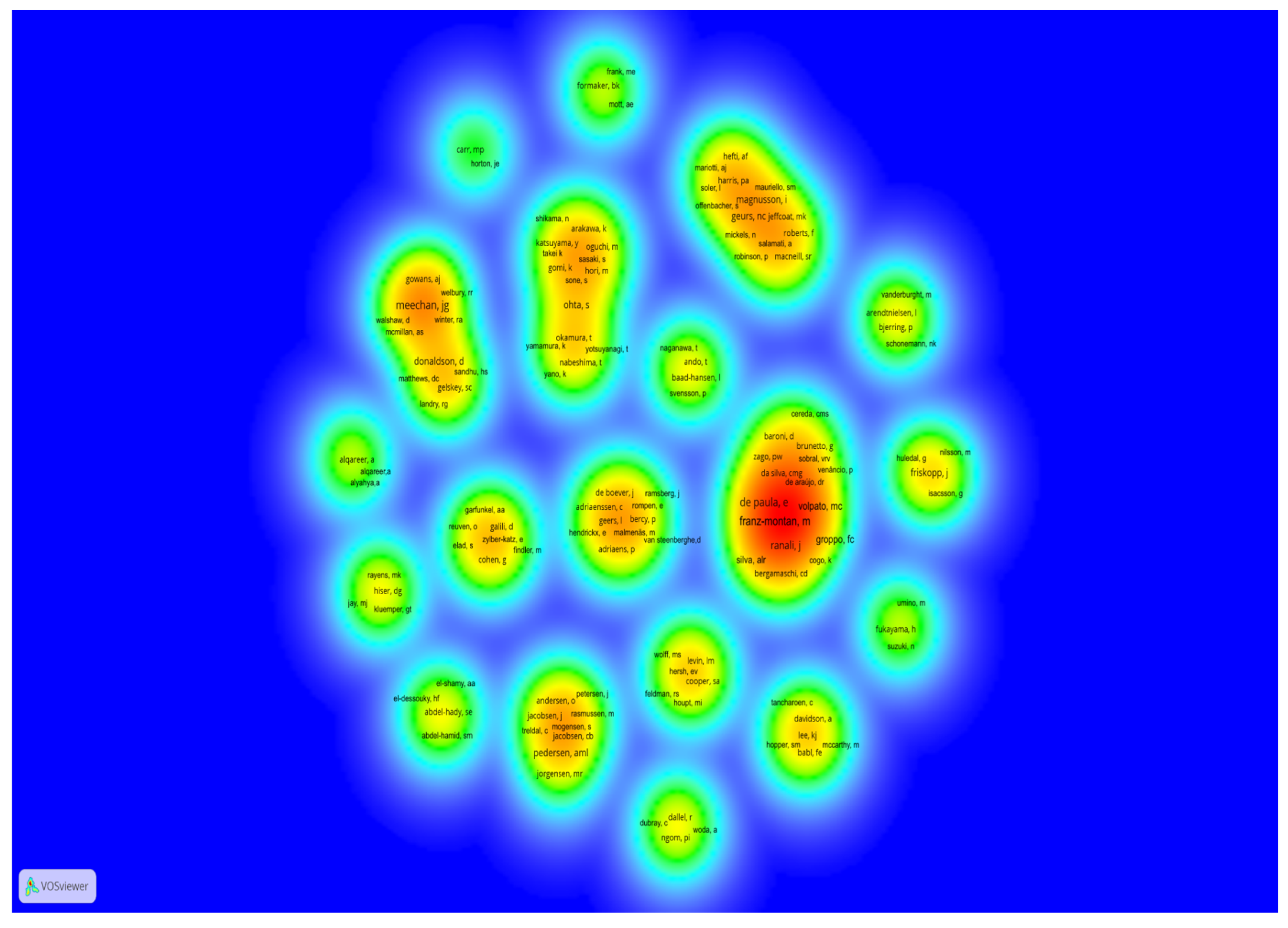
| Authors | Number of Articles Among the 30 Most Cited | Total Citations in the 30 Most Cited Articles | Authorship Position | Number of WoS-CC Articles | Number of WoS-CC Citations | H-Index | |
|---|---|---|---|---|---|---|---|
 | Franz-Montan M. | 4 | 138 | First author on all | 65 | 1413 | 23 |
 | Groppo FC. | 4 | 138 | Co-author: 2 Last author: 2 | 137 | 1781 | 22 |
 | Volpato MC. | 4 | 138 | Co-author: 2 Last author: 2 | 66 | 835 | 18 |
 | de Paula E. | 4 | 138 | Co-author on all | 220 | 5248 | 39 |
 | Meechan JG. * | 4 | 105 | First author: 3 Last author: 1 | 164 | 2386 | 27 |
 | Ranali J * | 3 | 92 | Co-author on all | 37 | 411 | 12 |
 | Kluemper GT * | 1 | 70 | First author: 1 | 27 | 656 | 13 |
 | Jay MJ | 1 | 70 | Last author: 1 | 138 | 3602 | 33 |
 | Rayens MK * | 1 | 70 | Co-author: 1 | 295 | 4810 | 36 |
 | Hiser DG * | 1 | 70 | Co-author: 1 | 2 | 77 | 2 |
3. Discussion
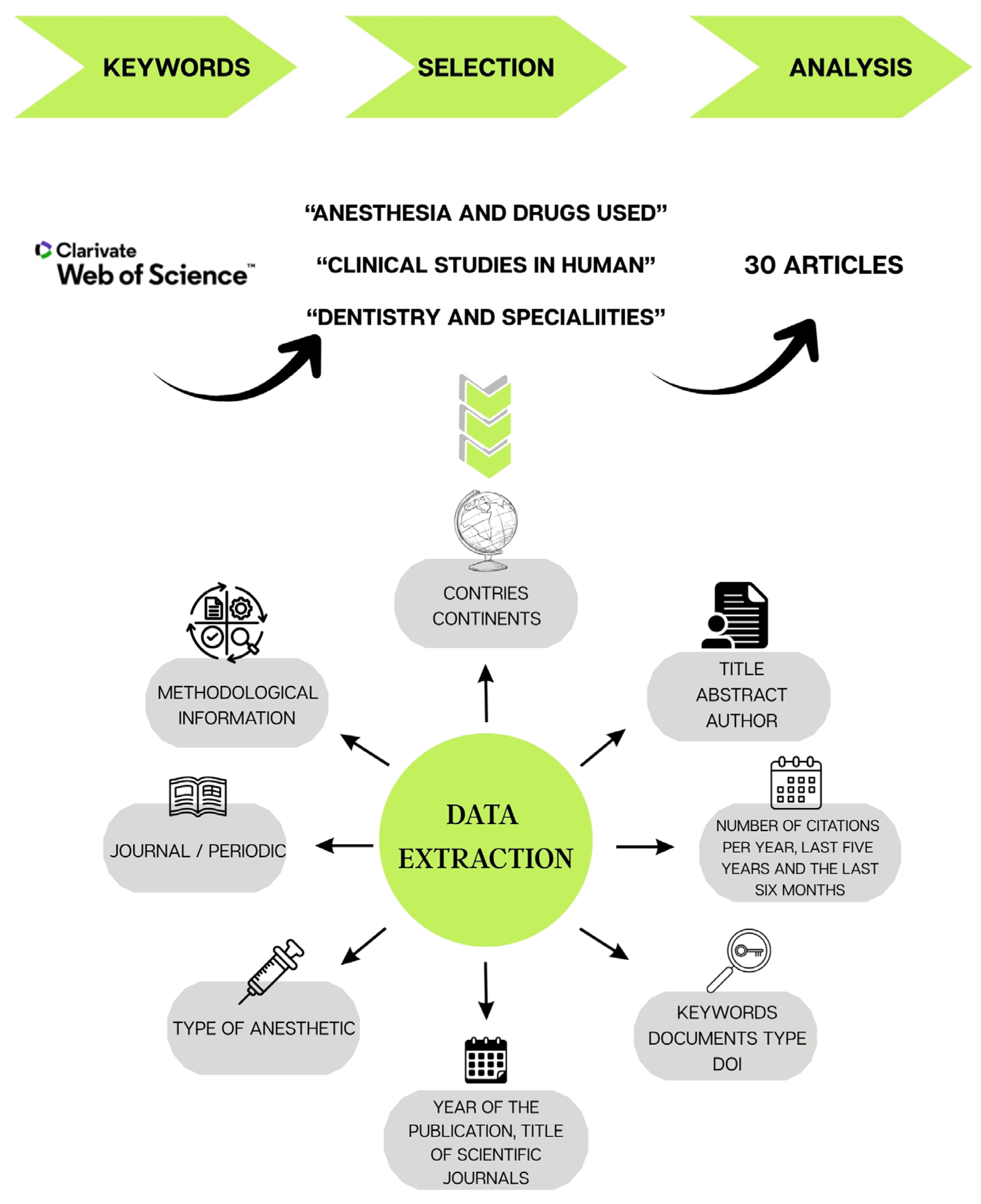
4. Materials and Methods
4.1. Search Strategy and Determination of Search Terms
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mundiya, J.; Woodbine, E. Updates on topical and local anesthesia agents. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Van Steenberghe, D.; Garmyn, P.; Geers, L.; Hendrickx, E.; Maréchal, M.; Huizar, K.; Kristofferson, A.; Meyer-Rosberg, K.; Vandenhoven, G. Patient evaluation of a novel non-injectable anesthetic gel: A multicenter crossover study comparing the gel to infiltration anesthesia during scaling and root planing. J. Periodontol. 2004, 75, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Al-Melh, M.A.; Andersson, L. Comparison of topical anesthetics (EMLA/Oraqix vs. benzocaine) on pain experienced during palatal needle injection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, e16–e20. [Google Scholar] [CrossRef]
- Canakci, C.F.; Canakci, V. Pain experienced by patients undergoing different periodontal therapies. J. Am. Dent. Assoc. 2007, 138, 1563–1573. [Google Scholar] [CrossRef]
- Wambier, L.M.; de Geus, J.L.; Chibinski, A.C.; Wambier, D.S.; Rego, R.O.; Loguercio, A.D.; Reis, A. Intra-pocket anaesthesia and pain during probing, scaling and root planing: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 754–766. [Google Scholar] [CrossRef]
- Fernández-Castellano, E.R.; Blanco-Antona, L.A.; Vicente-Galindo, P.; Amor-Esteban, V.; Flores-Fraile, J. Pain Experienced during Various Dental Procedures: Clinical Trial Comparing the Use of Traditional Syringes with the Controlled-Flow Delivery Dentapen® Technique. Medicina 2021, 57, 1335. [Google Scholar] [CrossRef]
- Van Wijk, A.J.; Hoogstraten, J. Anxiety and pain during dental injections. J. Dent. 2009, 37, 700–704. [Google Scholar] [CrossRef] [PubMed]
- De Freiras, G.C.; Pozzobon, R.T.; Blaya, D.S.; Moreira, C.H. Efficacy of Benzocaine 20% Topical Anesthetic Compared to Placebo Prior to Administration of Local Anesthesia in the Oral Cavity: A Randomized Controlled Trial. Anesth. Prog. 2015, 62, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hersh, E.V.; Houpt, M.I.; Cooper, S.A.; Feldman, R.S.; Wolff, M.S.; Levin, L.M. Analgesic efficacy and safety of an intraoral lidocaine patch. J Am. Dent. Assoc. 1996, 127, 1626–1634, quiz 1665–1666. [Google Scholar] [CrossRef]
- Lee, H.S. Recent advances in topical anesthesia. J. Dent. Anesth. Pain. Med. 2016, 16, 237–244. [Google Scholar] [CrossRef]
- Yaacob, H.; Noor, G.; Malek, S.N. The pharmacological effect of xylocaine topical anaesthetic—A comparison with a placebo. Singap. Dent. J. 1981, 6, 55–57. [Google Scholar]
- Schmidt, A.C. Solid-state characterization of falicaine hydrochloride and isomorphic dyclonine hydrochloride. Part IV. Crystal polymorphism of local anaesthetic drugs. Eur. J. Pharm. Sci. 2005, 25, 407–416. [Google Scholar] [CrossRef]
- Haas, D.A. An update on local anesthetics in dentistry. J. Can. Dent. Assoc. 2002, 68, 546–551. [Google Scholar]
- Malamed, S.F. Handbook of Local Anesthesia, 7th ed.; Elviser: Amsterdam, The Netherlands, 2019; p. 464. [Google Scholar]
- Miroshnychenko, A.; Ibrahim, S.; Azab, M.; Roldan, Y.; Diaz Martinez, J.P.; Tamilselvan, D.; He, L.; Urquhart, O.; Tampi, M.; Polk, D.E.; et al. Injectable and topical local anesthetics for acute dental pain: 2 systematic reviews. J. Am. Dent. Assoc. 2023, 154, 53–64. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Ribeiro, L.N.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; de Araújo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef]
- Meechan, J.G. Effective topical anesthetic agents and techniques. Dent. Clin. N. Am. 2002, 46, 759–766. [Google Scholar] [CrossRef]
- Meechan, J.G. Intraoral topical anesthesia. Periodontology 2000 2008, 46, 56–79. [Google Scholar] [CrossRef]
- Ogle, O.E.; Mahjoubi, G. Local anesthesia: Agents, techniques, and complications. Dent. Clin. N. Am. 2012, 56, 133–148. [Google Scholar] [CrossRef]
- Oguchi, M.; Shikama, N.; Sasaki, S.; Gomi, K.; Katsuyama, Y.; Ohta, S.; Hori, M.; Takei, K.; Arakawa, K.; Sone, S. Mucosa-adhesive water-soluble polymer film for treatment of acute radiation-induced oral mucositis. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.; Gelskey, S.; Landry, R.; Matthews, D.; Sandhu, H. A placebo-controlled multi-centred evaluation of an anaesthetic gel (Oraqix®) for periodontal therapy. J. Clin. Periodontol. 2003, 30, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Treldal, C.; Jacobsen, C.B.; Mogensen, S.; Rasmussen, M.; Jacobsen, J.; Petersen, J.; Lynge Pedersen, A.; Andersen, O. Effect of a local anesthetic lozenge in relief of symptoms in burning mouth syndrome. Oral Dis. 2016, 22, 123–131. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Baroni, D.; Brunetto, G.; Sobral, V.R.; da Silva, C.M.; Venâncio, P.; Zago, P.W.; Cereda, C.M.; Volpato, M.C.; de Araújo, D.R.; et al. Liposomal lidocaine gel for topical use at the oral mucosa: Characterization, in vitro assays and in vivo anesthetic efficacy in humans. J. Liposome Res. 2015, 25, 11–19. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Cereda, C.M.; Gaspari, A.; da Silva, C.M.; de Araújo, D.R.; Padula, C.; Santi, P.; Narvaes, E.; Novaes, P.D.; Groppo, F.C.; et al. Liposomal-benzocaine gel formulation: Correlation between in vitro assays and in vivo topical anesthesia in volunteers. J. Liposome Res. 2013, 23, 54–60. [Google Scholar] [CrossRef]
- Franz-Montan, M.; de Paula, E.; Groppo, F.; Silva, A.; Ranali, J.; Volpato, M. Liposome-encapsulated ropivacaine for intraoral topical anesthesia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 50, 800–804. [Google Scholar] [CrossRef]
- Franz-Montan, M.; de Paula, E.; Groppo, F.C.; Silva, A.L.; Ranali, J.; Volpato, M.C. Liposomal delivery system for topical anaesthesia of the palatal mucosa. Br. J. Oral Maxillofac. Surg. 2012, 50, 60–64. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Silva, A.L.R.; Cogo, K.; Bergamaschi, C.D.; Volpato, M.C.; Ranali, J.; de Paula, E.; Groppo, F.C. Liposome-encapsulated ropivacaine for topical anesthesia of human oral mucosa. Anesth. Analg. 2007, 104, 1528–1531. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Silva, A.L.R.; Fraceto, L.F.; Volpato, M.C.; de Paula, E.; Ranali, J.; Groppo, F.C. Liposomal encapsulation improves the duration of soft tissue anesthesia but does not induce pulpal anesthesia. J. Clin. Anesth. 2010, 22, 313–317. [Google Scholar] [CrossRef]
- Yagiela, J.A. Local anesthetics: A century of progress. Anesth. Prog. 1985, 32, 47. [Google Scholar] [CrossRef]
- Ruetsch, Y.A.; Boni, T.; Borgeat, A. From cocaine to ropivacaine: The history of local anesthetic drugs. Curr. Top. Med. Chem. 2001, 1, 175–182. [Google Scholar] [CrossRef]
- Tobe, M.; Suto, T.; Saito, S. The history and progress of local anesthesia: Multiple approaches to elongate the action. J. Anesth. 2018, 32, 632–636. [Google Scholar] [CrossRef]
- Celeste, R.K.; Broadbent, J.M.; Moyses, S.J. Half-century of Dental Public Health research: Bibliometric analysis of world scientific trends. Community Dent. Oral Epidemiol. 2016, 44, 557–563. [Google Scholar] [CrossRef]
- Kluemper, G.T.; Hiser, D.G.; Rayens, M.K.; Jay, M.J. Efficacy of a wax containing benzocaine in the relief of oral mucosal pain caused by orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 359–365. [Google Scholar] [CrossRef]
- Alqareer, A.; Alyahya, A.; Andersson, L. The effect of clove and benzocaine versus placebo as topical anesthetics. J. Dent. 2006, 34, 747–750. [Google Scholar] [CrossRef]
- Formarker, B.; Mott, A.; Frank, M. The Effects of Topical Anesthesia on Oral Burning in Burning Mouth Syndrome. Ann. N. Y. Acad. Sci. 1998, 855, 776–780. [Google Scholar] [CrossRef]
- Friskopp, J.; Nilsson, M.; Isacsson, G. The anesthetic onset and duration of a new lidocaine/prilocaine gel intra-pocket anesthetic (Oraqix) for periodontal scaling/root planing. J. Clin. Periodontol. 2001, 28, 453–458. [Google Scholar] [CrossRef]
- Jeffcoat, M.K.; Geurs, N.C.; Magnusson, I.; MacNeill, S.R.; Mickels, N.; Roberts, F.; Robinson, P.; Salamati, A.; Yukna, R. Intrapocket anesthesia for scaling and root planing: Results of a double-blind multicenter trial using lidocaine prilocaine dental gel. J. Periodontol. 2001, 72, 895–900. [Google Scholar] [CrossRef]
- Fukayama, H.; Suzuki, N.; Umino, M. Comparison of topical anesthesia of 20% benzocaine and 60% lidocaine gel. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 157–161. [Google Scholar] [CrossRef]
- Abdel-Hamid, S.M.; Abdel-Hady, S.E.; El-Shamy, A.A.; El-Dessouky, H.F. Formulation of an antispasmodic drug as a topical local anesthetic. Int. J. Pharm. 2006, 326, 107–118. [Google Scholar] [CrossRef]
- Meechan, J.G.; Winter, R.A. A comparison of topical anaesthesia and electronic nerve stimulation for reducing the pain of intra-oral injections. Br. Dent. J. 1996, 181, 333–335. [Google Scholar] [CrossRef]
- McMillan, A.S.; Walshaw, D.; Meechan, J.G. The efficacy of Emla and 5% lignocaine gel for anaesthesia of human gingival mucosa. Br. J. Oral Maxillofac. Surg. 2000, 38, 58–61. [Google Scholar] [CrossRef]
- Schønemann, N.; Van Der Burght, M.; Arendt-Nielsen, L.; Bjerring, P. Onset and duration of hypoalgesia of lidocaine spray applied to oral mucosa–a dose response study. Acta Anaesthesiol. Scand. 1992, 36, 733–735. [Google Scholar] [CrossRef]
- Magnusson, I.; Geurs, N.C.; Harris, P.A.; Hefti, A.F.; Mariotti, A.J.; Mauriello, S.M.; Soler, L.; Offenbacher, S. Intrapocket anesthesia for scaling and root planing in pain-sensitive patients. J. Periodontol. 2003, 74, 597–602. [Google Scholar] [CrossRef]
- Ngom, P.I.; Dubray, C.; Woda, A.; Dallel, R. A human oral capsaicin pain model to assess topical anesthetic–analgesic drugs. Neurosci. Lett. 2001, 316, 149–152. [Google Scholar] [CrossRef]
- Friskopp, J.; Huledal, G. Plasma levels of lidocaine and prilocaine after application of Oraqix, a new intrapocket anesthetic, in patients with advanced periodontitis. J. Clin. Periodontol. 2001, 28, 425–429. [Google Scholar] [CrossRef]
- Jørgensen, M.R.; Pedersen, A.M.L. Analgesic effect of topical oral capsaicin gel in burning mouth syndrome. Acta Odontol. Scand. 2017, 75, 130–136. [Google Scholar] [CrossRef]
- Yamamura, K.; Ohta, S.; Yano, K.; Yotsuyanagi, T.; Okamura, T.; Nabeshima, T. Oral mucosal adhesive film containing local anesthetics: In vitro and clinical evaluation. J. Biomed. Mater. Res. 1998, 43, 313–317. [Google Scholar] [CrossRef]
- Meechan, J.; Gowans, A.; Welbury, R. The use of patient-controlled transcutaneous electronic nerve stimulation (TENS) to decrease the discomfort of regional anaesthesia in dentistry: A randomised controlled clinical trial. J. Dent. 1998, 26, 417–420. [Google Scholar] [CrossRef]
- Meechan, J.G.; Donaldson, D. The intraoral use of EMLA cream in children: A clinical investigation. ASDC J. Dent. Child. 1994, 61, 260–262. [Google Scholar]
- Hopper, S.M.; McCarthy, M.; Tancharoen, C.; Lee, K.J.; Davidson, A.; Babl, F.E. Topical lidocaine to improve oral intake in children with painful infectious mouth ulcers: A blinded, randomized, placebo-controlled trial. Ann. Emerg. Med. 2014, 63, 292–299. [Google Scholar] [CrossRef]
- Carr, M.P.; Horton, J.E. Clinical evaluation and comparison of 2 topical anesthetics for pain caused by needle sticks and scaling and root planing. J. Periodontol. 2001, 72, 479–484. [Google Scholar] [CrossRef]
- Naganawa, T.; Baad-Hansen, L.; Ando, T.; Svensson, P. Influence of topical application of capsaicin, menthol and local anesthetics on intraoral somatosensory sensitivity in healthy subjects: Temporal and spatial aspects. Exp. Brain Res. 2015, 233, 1189–1199. [Google Scholar] [CrossRef]
- Elad, S.; Cohen, G.; Zylber-Katz, E.; Findler, M.; Galili, D.; Garfunkel, A.A.; Or, R. Systemic absorption of lidocaine after topical application for the treatment of oral mucositis in bone marrow transplantation patients. J. Oral Pathol. Med. 1999, 28, 170–172. [Google Scholar] [CrossRef]
- Baldiotti, A.L.P.; Amaral-Freitas, G.; Barcelos, J.F.; Freire-Maia, J.; Perazzo, M.d.F.; Freire-Maia, F.B.; Paiva, S.M.; Ferreira, F.M.; Martins-Júnior, P.A. The top 100 most-cited papers in cariology: A bibliometric analysis. Caries Res. 2021, 55, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kammer, P.V.; Moro, J.S.; Martins-Júnior, P.A.; Cardoso, M.; Bolan, M.; Santana, C.M. The 100 most-cited papers in dentistry for individuals with neurodevelopmental disorders: Bibliometric profile of scientific research. Spec. Care Dent. 2022, 42, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Antonio Alarcon, M.; Esparza, D.; Montoya, C.; Monje, A.; Faggion, C.M., Jr. The 300 Most-Cited Articles in Implant Dentistry. Int. J. Oral Maxillofac. Implant. 2017, 32, e1–e8. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr.; Málaga, L.; Monje, A.; Trescher, A.-L.; Listl, S.; Alarcón, M.A. The 300 most cited articles published in periodontology. Clin. Oral Investig. 2017, 21, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Daryakenari, G.; Batooli, Z. A bibliometric and subject analysis of 3300 most-cited articles in dentistry. Clin. Exp. Dent. Res. 2022, 8, 1302–1310. [Google Scholar] [CrossRef]
- Iftikhar, P.M.; Ali, F.; Faisaluddin, M.; Khayyat, A.; De Sa, M.D.G.; Rao, T. A bibliometric analysis of the top 30 most-cited articles in gestational diabetes mellitus literature (1946–2019). Cureus 2019, 11, e4131. [Google Scholar] [CrossRef] [PubMed]
- Karobari, M.I.; Maqbool, M.; Ahmad, P.; Abdul, M.S.M.; Marya, A.; Venugopal, A.; Shaik, G.M.; Scardina, G.A.; Messina, P.; Noorani, T.Y. Endodontic microbiology: A bibliometric analysis of the top 50 classics. BioMed Res. Int. 2021, 2021, 6657167. [Google Scholar] [CrossRef]
- Ahmad, P.; Asif, J.A.; Alam, M.K.; Slots, J. A bibliometric analysis of periodontology 2000. Periodontology 2000 2020, 82, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Slots, J. A bibliometric analysis of periodontology. Periodontology 2000 2021, 85, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yeung, A.W.; Pow, E.H.; Tsoi, J.K. Current status and research trends of lithium disilicate in dentistry: A bibliometric analysis. J. Prosthet. Dent. 2021, 126, 512–522. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.-I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, J.F.; Limeres, J.; Fernández-Varela, M.; Ramos, I.; Diz, P. The 100 most cited articles in dentistry. Clin. Oral Investig. 2014, 18, 699–706. [Google Scholar] [CrossRef]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Eriksson, B.; Lekholm, U.; Brånemark, P.-I.; Jemt, T. A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implant. 1990, 5, 347–359. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral. Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Löe, H. The gingival index, the plaque index and the retention index systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Theilade, E.; Jensen, S.B. Experimental gingivitis in man. J. Periodontol. 1965, 36, 177–187. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) Technique. J. Oral Pathol. Med. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1998, 85, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.W. New fields of application for xylocaine as applied to dentistry: I. Xylocaine ointment as a topical anesthetic in dentistry. II. Current research regarding toxicity of xylocaine. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1952, 5, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hs, S.; Deckelbaum, R.; Bk, F. Lidocaine hydrochloride as a topical anesthetic in operative dentistry: Effect on pulp tissue. Bulletin. Georget. Univ. Med. Cent. 1954, 7, 228–230. [Google Scholar] [PubMed]
- Haasio, J.; Jokinen, T.; Numminen, M.; Rosenberg, P.H. Topical anaesthesia of gingival mucosa by 5% eutectic mixture of lignocaine and prilocaine or by 10% lignocaine spray. Br. J. Oral Maxillofac. Surg. 1990, 28, 99–101. [Google Scholar] [CrossRef]
- Svensson, P.; Bjerring, P.; Arendt-Nielsen, L.; Kaaber, S. Hypoalgesic effect of EMLA and lidocaine gel applied on human oral mucosa: Quantitative evaluation by sensory and pain thresholds to argon laser stimulation. Anesth. Prog. 1992, 39, 4–8. [Google Scholar]
- Svensson, P.; Petersen, J.K. Anesthetic effect of EMLA occluded with Orahesive oral bandages on oral mucosa. A placebo-controlled study. Anesth. Prog. 1992, 39, 79–82. [Google Scholar]
- Pere, P.; Iizuka, T.; Rosenberg, P.; Lindqvist, C. Topical application of 5% eutectic mixture of lignocaine and prilocaine (EMLA®) before removal of arch bars. Br. J. Oral Maxillofac. Surg. 1992, 30, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Vickers, E.R.; Punnia-Moorthy, A. A clinical evaluation of three topical anaesthetic agents. Aust. Dent. J. 1992, 37, 267–270. [Google Scholar] [CrossRef]
- Alam, B.F.; Najmi, M.A.; Qasim, S.B.; Almulhim, K.S.; Ali, S. A bibliometric analysis of minimally invasive dentistry: A review of the literature from 1994 to 2021. J. Prosthet. Dent. 2023, 130, 179–186. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, H.C.G.; de Sousa, I.T.C.; Pestana, A.M.; Bezerra, A.A.C.; Lucas, A.L.M.; Martins-Júnior, P.A.; Franz-Montan, M. Clinical Studies on Topical Anesthesia in Dentistry: A Bibliometric Analysis. Pharmaceuticals 2025, 18, 1611. https://doi.org/10.3390/ph18111611
dos Santos HCG, de Sousa ITC, Pestana AM, Bezerra AAC, Lucas ALM, Martins-Júnior PA, Franz-Montan M. Clinical Studies on Topical Anesthesia in Dentistry: A Bibliometric Analysis. Pharmaceuticals. 2025; 18(11):1611. https://doi.org/10.3390/ph18111611
Chicago/Turabian Styledos Santos, Helena Carla Gonçalves, Iago Torres Cortês de Sousa, Aylla Mesquita Pestana, Arthur Antunes Costa Bezerra, Ana Luiza Martins Lucas, Paulo Antônio Martins-Júnior, and Michelle Franz-Montan. 2025. "Clinical Studies on Topical Anesthesia in Dentistry: A Bibliometric Analysis" Pharmaceuticals 18, no. 11: 1611. https://doi.org/10.3390/ph18111611
APA Styledos Santos, H. C. G., de Sousa, I. T. C., Pestana, A. M., Bezerra, A. A. C., Lucas, A. L. M., Martins-Júnior, P. A., & Franz-Montan, M. (2025). Clinical Studies on Topical Anesthesia in Dentistry: A Bibliometric Analysis. Pharmaceuticals, 18(11), 1611. https://doi.org/10.3390/ph18111611