Targeting Inflammatory Pathways in Chronic Low Back Pain: Opportunities for Novel Therapeutics
Abstract
1. Introduction
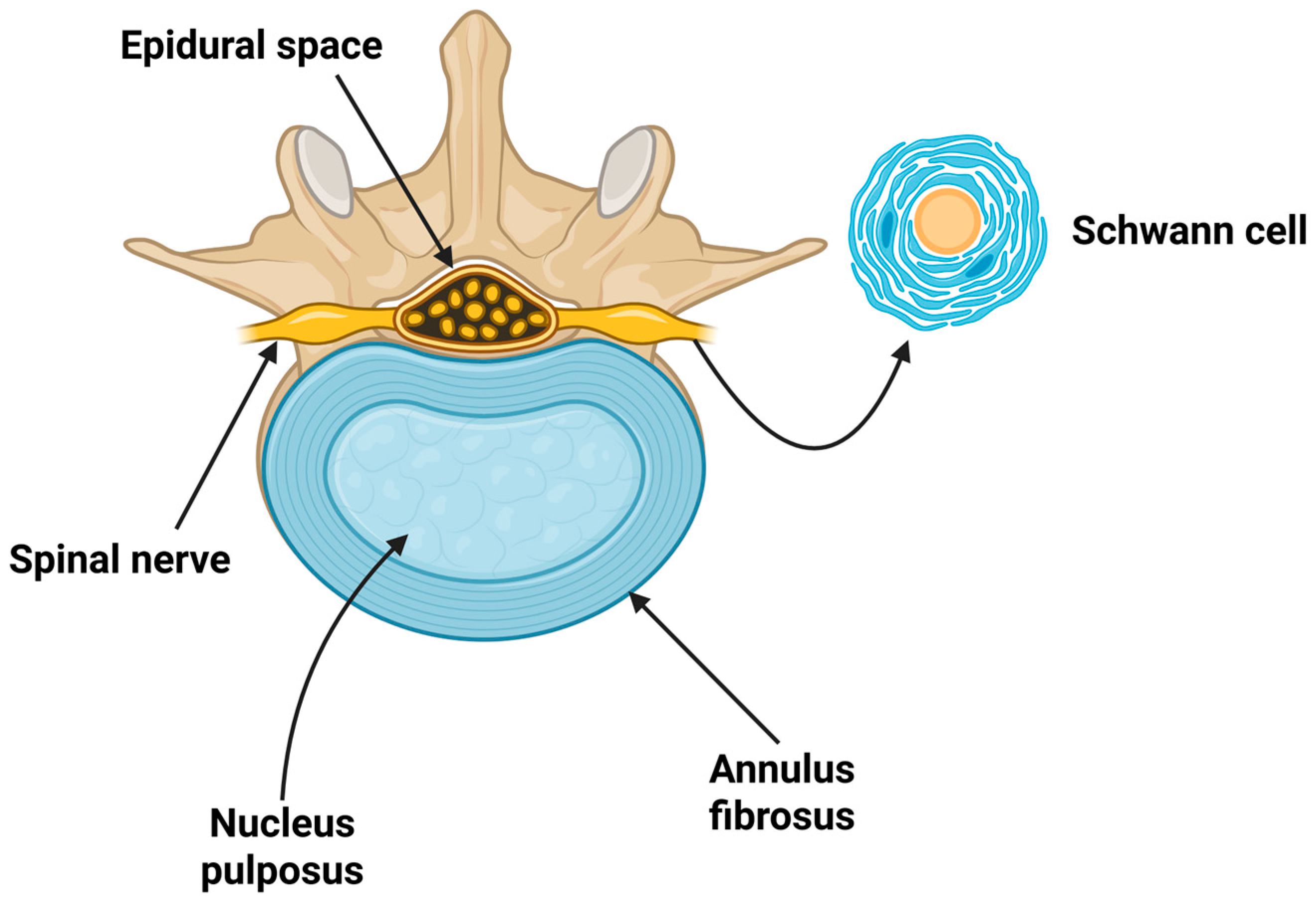
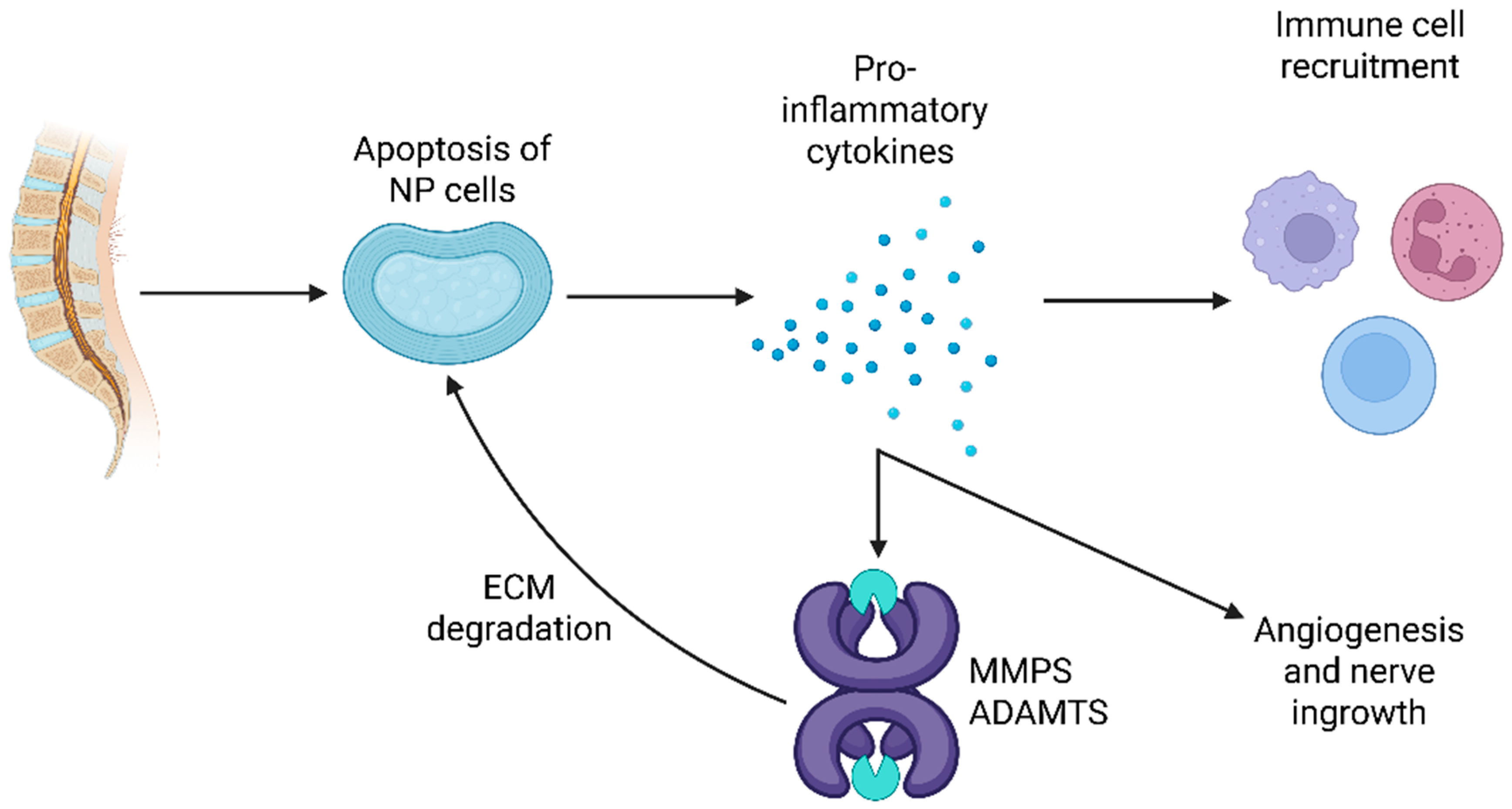
2. Anatomy and Degeneration Characteristics of Disc Herniation as a Cause of Chronic LBP
3. Systemic Inflammation in Patients with Chronic LBP
3.1. CRP and hs-CRP
3.2. Inflammatory Cytokines
4. The Role of Modern Therapies in the Management of Chronic LBP
4.1. Anti-TNF-α Inhibition
4.2. Anti-NGFs
4.3. Other Molecules
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [CrossRef]
- Grabovac, I.; Dorner, T.E. Association between low back pain and various everyday performances: Activities of daily living, ability to work and sexual function. Wien. Klin. Wochenschr. 2019, 131, 541–549. [Google Scholar] [CrossRef]
- Nazir, S.N.B.; Pereira, F.A.; Muhammad, A.; Shamsi, I.I.; Khan, M.U. The Relationship Between Fear-Avoidance Beliefs, Disability, and Physical Capacity in Patients with Chronic Low Back Pain. Mediterr. J. Rheumatol. 2022, 33, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Lui, A.; Matsoyan, A.; Safaee, M.M.; Aryan, H.; Ames, C. Comparative Review of the Socioeconomic Burden of Lower Back Pain in the United States and Globally. Neurospine 2024, 21, 487–501. [Google Scholar] [CrossRef]
- Ronnegard, A.S.; Nowak, C.; Ang, B.; Arnlov, J. The association between short-term, chronic localized and chronic widespread pain and risk for cardiovascular disease in the UK Biobank. Eur. J. Prev. Cardiol. 2022, 29, 1994–2002. [Google Scholar] [CrossRef]
- Xie, S.; Xiao, H.; Li, G.; Zheng, J.; Zhang, F.; Lan, Y.; Luo, M. Association between a body shape index and low back pain: A cross-sectional study highlighting gender-specific differences in NHANES data. BMC Public. Health 2025, 25, 753. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, X.Q.; Zhou, H.Y. Chronic Low Back Pain and Sleep Disturbance in Adults in the US: The NHANES 2009-2010 Study. Pain Physician 2024, 27, E255–E262. [Google Scholar] [PubMed]
- Klyne, D.M.; Barbe, M.F.; Hodges, P.W. Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav. Immun. 2017, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.A.; Minic, Z. Chronic Pain-Associated Cardiovascular Disease: The Role of Sympathetic Nerve Activity. Int. J. Mol. Sci. 2023, 24, 5378. [Google Scholar] [CrossRef]
- Olsen, A.M.; Fosbol, E.L.; Lindhardsen, J.; Andersson, C.; Folke, F.; Nielsen, M.B.; Kober, L.; Hansen, P.R.; Torp-Pedersen, C.; Gislason, G.H. Cause-specific cardiovascular risk associated with nonsteroidal anti-inflammatory drugs among myocardial infarction patients--a nationwide study. PLoS ONE 2013, 8, e54309. [Google Scholar] [CrossRef]
- Snowden, S.; Nelson, R. The effects of nonsteroidal anti-inflammatory drugs on blood pressure in hypertensive patients. Cardiol. Rev. 2011, 19, 184–191. [Google Scholar] [CrossRef]
- Dionne, C.E.; Dunn, K.M.; Croft, P.R.; Nachemson, A.L.; Buchbinder, R.; Walker, B.F.; Wyatt, M.; Cassidy, J.D.; Rossignol, M.; Leboeuf-Yde, C.; et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine 2008, 33, 95–103. [Google Scholar] [CrossRef]
- Patrick, N.; Emanski, E.; Knaub, M.A. Acute and chronic low back pain. Med. Clin. N. Am. 2014, 98, 777–789. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain. Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Sima, S.; Diwan, A. Contemporary clinical perspectives on chronic low back pain: The biology, mechanics, etc. underpinning clinical and radiological evaluation. JOR Spine 2025, 8, e70021. [Google Scholar] [CrossRef]
- Qaseem, A.; McLean, R.M.; O’Gurek, D.; Batur, P.; Lin, K.; Kansagara, D.L.; Clinical Guidelines Committee of the American College of Physicians; Commission on Health of the Public and Science of the American Academy of Family Physicians; Cooney, T.G.; Forciea, M.A.; et al. Nonpharmacologic and Pharmacologic Management of Acute Pain From Non-Low Back, Musculoskeletal Injuries in Adults: A Clinical Guideline From the American College of Physicians and American Academy of Family Physicians. Ann. Intern. Med. 2020, 173, 739–748, Erratum in Ann. Intern. Med. 2023, 176, 584. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.; Ostelo, R.W.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cook, K.F.; Dunn, W.; Griffith, J.W.; Morrison, M.T.; Tanquary, J.; Sabata, D.; Victorson, D.; Carey, L.M.; Macdermid, J.C.; Dudgeon, B.J.; et al. Pain assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. S3), S49–S53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783, Erratum in Ann. Rheum. Dis. 2019, 78, e59.. [Google Scholar] [CrossRef] [PubMed]
- Parreira, P.; Maher, C.G.; Steffens, D.; Hancock, M.J.; Ferreira, M.L. Risk factors for low back pain and sciatica: An umbrella review. Spine J. 2018, 18, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Krasin, E.; Hemo, Y.; Doron, R. Stop Searching under the Streetlight! A Primer and Practical Guide to the Diagnosis of Joint Pain and Inflammation. Mediterr. J. Rheumatol. 2022, 33, 291–304. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Lambe, T.; Raphael, J.H.; Kitas, G.D.; Duarte, R.V. Biologic Drugs as Analgesics for the Management of Low Back Pain and Sciatica. Pain. Med. 2019, 20, 1678–1686. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Hoyland, J.A.; Freemont, A.J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther. 2007, 9, R77. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kikuchi, S.; Shubayev, V.; Myers, R.R. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000, 25, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Olmarker, K.; Rydevik, B. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: Possible implications for future pharmacologic treatment strategies of sciatica. Spine 2001, 26, 863–869. [Google Scholar] [CrossRef]
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B., Jr.; Hartl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar] [CrossRef]
- Yang, X.; Li, X. Nucleus pulposus tissue engineering: A brief review. Eur. Spine J. 2009, 18, 1564–1572. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Fei, Q.; Jiang, L. Contribution of immune cells to intervertebral disc degeneration and the potential of immunotherapy. Connect. Tissue Res. 2023, 64, 413–427. [Google Scholar] [CrossRef]
- Tseranidou, S.; Segarra-Queralt, M.; Chemorion, F.K.; Le Maitre, C.L.; Piñero, J.; Noailly, J. Nucleus pulposus cell network modelling in the intervertebral disc. NPJ Syst. Biol. Appl. 2025, 11, 13. [Google Scholar] [CrossRef]
- Mohd Isa, I.L.; Teoh, S.L.; Mohd Nor, N.H.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 24, 208. [Google Scholar] [CrossRef]
- Cazzanelli, P.; Wuertz-Kozak, K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int. J. Mol. Sci. 2020, 21, 3601. [Google Scholar] [CrossRef]
- Yan, S.; Han, B.; Song, C.; Yan, L. Molecular mechanisms and treatment strategies for discogenic lumbar pain. Immunol. Res. 2025, 73, 111. [Google Scholar] [CrossRef]
- Sun, H.; Guo, J.; Xiong, Z.; Zhuang, Y.; Ning, X.; Liu, M. Targeting nucleus pulposus cell death in the treatment of intervertebral disc degeneration. JOR Spine 2024, 7, e70011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rannou, F.; Ouanes, W.; Boutron, I.; Lovisi, B.; Fayad, F.; Mace, Y.; Borderie, D.; Guerini, H.; Poiraudeau, S.; Revel, M. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 2007, 57, 1311–1315. [Google Scholar] [CrossRef]
- Gebhardt, K.; Brenner, H.; Sturmer, T.; Raum, E.; Richter, W.; Schiltenwolf, M.; Buchner, M. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain—A 6 months prospective longitudinal study. Eur. J. Pain. 2006, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, P.; Hashemi, S.M.; Taheri, M.; Zakeri, H. Association of Serum Minerals, Vitamin D, Total Protein, and Inflammatory Mediators and Severity of Low Back Pain. Galen. Med. J. 2020, 9, e1342. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Goyal, A.; Patel, B.C. C-Reactive Protein: Clinical Relevance and Interpretation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Ho, K.K.N.; Simic, M.; Cvancarova Smastuen, M.; de Barros Pinheiro, M.; Ferreira, P.H.; Bakke Johnsen, M.; Heuch, I.; Grotle, M.; Zwart, J.A.; Nilsen, K.B. The association between insomnia, c-reactive protein, and chronic low back pain: Cross-sectional analysis of the HUNT study, Norway. Scand. J. Pain 2019, 19, 765–777. [Google Scholar] [CrossRef]
- Hodges, S.; Guler, S.; Sacca, V.; Vangel, M.; Orr, S.; Pace-Schott, E.; Wen, Y.; Ge, T.; Kong, J. Associations among acute and chronic musculoskeletal pain, sleep duration, and C-reactive protein (CRP): A cross-sectional study of the UK biobank dataset. Sleep Med. 2023, 101, 393–400. [Google Scholar] [CrossRef]
- Kraychete, D.C.; Sakata, R.K.; Issy, A.M.; Bacellar, O.; Santos-Jesus, R.; Carvalho, E.M. Serum cytokine levels in patients with chronic low back pain due to herniated disc: Analytical cross-sectional study. Sao Paulo Med. J. 2010, 128, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liu, Z.Z.; Duan, D.P. Inflammation in low back pain may be detected from the peripheral blood: Suggestions for biomarker. Biosci. Rep. 2016, 36, e00361. [Google Scholar] [CrossRef]
- Pedersen, L.M.; Schistad, E.; Jacobsen, L.M.; Roe, C.; Gjerstad, J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav. Immun. 2015, 46, 132–136. [Google Scholar] [CrossRef]
- Luchting, B.; Heyn, J.; Woehrle, T.; Rachinger-Adam, B.; Kreth, S.; Hinske, L.C.; Azad, S.C. Differential expression of P2X7 receptor and IL-1beta in nociceptive and neuropathic pain. J. Neuroinflammation 2016, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.A.; Bezerra, R.J.; Faria, R.X.; Ferreira, L.G.; da Silva Frutuoso, V. Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules 2013, 18, 10953–10972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teodorczyk-Injeyan, J.A.; Triano, J.J.; Injeyan, H.S. Nonspecific Low Back Pain: Inflammatory Profiles of Patients with Acute and Chronic Pain. Clin. J. Pain 2019, 35, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Teodorczyk-Injeyan, J.A.; McGregor, M.; Triano, J.J.; Injeyan, S.H. Elevated Production of Nociceptive CC Chemokines and sE-Selectin in Patients with Low Back Pain and the Effects of Spinal Manipulation: A Nonrandomized Clinical Trial. Clin. J. Pain 2018, 34, 68–75. [Google Scholar] [CrossRef]
- Xu, H.W.; Zhang, S.B.; Yi, Y.Y.; Chen, H.; Hu, T.; Wang, S.J.; Wu, D.S. Relationship between Vitamin D and Nonspecific Low Back Pain May Be Mediated by Inflammatory Markers. Pain Physician 2021, 24, E1015–E1023. [Google Scholar]
- Slouma, M.; Kharrat, L.; Tezegdenti, A.; Metoui, L.; Ghazouani, E.; Dhahri, R.; Gharsallah, I.; Louzir, B. Pro-inflammatory cytokines in patients with low back pain: A comparative study. Reumatol. Clin. 2023, 19, 244–248. [Google Scholar] [CrossRef]
- Gjefsen, E.; Gervin, K.; Goll, G.; Braten, L.C.H.; Wigemyr, M.; Aass, H.C.D.; Vigeland, M.D.; Schistad, E.; Pedersen, L.M.; Pripp, A.H.; et al. Macrophage migration inhibitory factor: A potential biomarker for chronic low back pain in patients with Modic changes. RMD Open 2021, 7, e001726. [Google Scholar] [CrossRef]
- Heffner, K.L.; France, C.R.; Trost, Z.; Ng, H.M.; Pigeon, W.R. Chronic low back pain, sleep disturbance, and interleukin-6. Clin. J. Pain 2011, 27, 35–41. [Google Scholar] [CrossRef]
- Capossela, S.; Pavlicek, D.; Bertolo, A.; Landmann, G.; Stoyanov, J.V. Unexpectedly decreased plasma cytokines in patients with chronic back pain. J. Pain Res. 2018, 11, 1191–1198. [Google Scholar] [CrossRef]
- Stec-Martyna, E.; Wojtczak, K.; Nowak, D.; Stawski, R. Battle of the Biomarkers of Systemic Inflammation. Biology 2025, 14, 438. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; van Tulder, M.W. Exercise therapy for chronic low back pain. Cochrane Database Syst. Rev. 2021, 9, CD009790. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.J.; Correa, L.; Brown, B.T.; Ferreira, G.E.; Nim, C.; Aspinall, S.L.; Wareham, D.; Choi, J.; Maher, C.G.; Hancock, M.J. Long-term effectiveness of non-surgical interventions for chronic low back pain: A systematic review and meta-analysis. Lancet Rheumatol. 2025, 7, e607–e617. [Google Scholar] [CrossRef] [PubMed]
- Maharty, D.C.; Hines, S.C.; Brown, R.B. Chronic Low Back Pain in Adults: Evaluation and Management. Am. Fam. Physician 2024, 109, 233–244. [Google Scholar]
- Leung, L.; Cahill, C.M. TNF-α and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Udalova, I.; Monaco, C.; Nanchahal, J.; Feldmann, M. Anti-TNF Therapy. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630. [Google Scholar] [CrossRef]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular mechanisms of action of anti-TNF-α agents—Comparison among therapeutic TNF-α antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Karas, M.; Joubran, E.; Jara Silva, C.E.; Cordova, S.; Sinha, M.; Salam, A.; Leyva, M.M.; Quinonez, J.; Ruxmohan, S. Recent Advancements in Epidural Etanercept for Pain Management in Radiculopathy: A Literature Review. Cureus 2023, 15, e37672. [Google Scholar] [CrossRef]
- Gjefsen, E.; Braten, L.C.; Ponzi, E.; Dagestad, M.H.; Marchand, G.H.; Kadar, T.; Bakland, G.; Haugen, A.J.; Granviken, F.; Florenes, T.W.; et al. Efficacy of a Tumor Necrosis Factor Inhibitor in Chronic Low-Back Pain with Modic Type 1 Changes: A Randomized Controlled Trial. Arthritis Rheumatol. 2025, 77, 615–623. [Google Scholar] [CrossRef]
- Dagestad, M.H.; Vetti, N.; Haugli Braten, L.C.; Gjefsen, E.; Grovle, L.; Gervin, K.; Haugen, A.J.; Bakland, G.; Marchand, G.H.; Kadar, T.; et al. Modic Change Edema in Chronic Low Back Pain Treated with Infliximab or Placebo: The BackToBasic Trial. Spine 2025, 50, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Genevay, S.; Boudabbous, S.; Balague, F. Exploratory study of adalimumab in twelve patients with chronic low back pain associated with Modic I changes. Joint Bone Spine 2019, 86, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Golimumab: A Review in Inflammatory Arthritis. BioDrugs 2017, 31, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Certolizumab Pegol: A Review in Inflammatory Autoimmune Diseases. BioDrugs 2016, 30, 607–617. [Google Scholar] [CrossRef]
- Campanati, A.; Benfaremo, D.; Luchetti, M.M.; Ganzetti, G.; Gabrielli, A.; Offidani, A. Certolizumab pegol for the treatment of psoriasis. Expert. Opin. Biol. Ther. 2017, 17, 387–394. [Google Scholar] [CrossRef]
- Pedersen, S.J.; Maksymowych, W.P. Beyond the TNF-alpha Inhibitors: New and Emerging Targeted Therapies for Patients with Axial Spondyloarthritis and their Relation to Pathophysiology. Drugs 2018, 78, 1397–1418. [Google Scholar] [CrossRef]
- Cohen, S.P.; White, R.L.; Kurihara, C.; Larkin, T.M.; Chang, A.; Griffith, S.R.; Gilligan, C.; Larkin, R.; Morlando, B.; Pasquina, P.F.; et al. Epidural steroids, etanercept, or saline in subacute sciatica: A multicenter, randomized trial. Ann. Intern. Med. 2012, 156, 551–559. [Google Scholar] [CrossRef]
- Genevay, S.; Viatte, S.; Finckh, A.; Zufferey, P.; Balague, F.; Gabay, C. Adalimumab in severe and acute sciatica: A multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 2339–2346. [Google Scholar] [CrossRef]
- Okoro, T.; Tafazal, S.I.; Longworth, S.; Sell, P.J. Tumor necrosis alpha-blocking agent (etanercept): A triple blind randomized controlled trial of its use in treatment of sciatica. J. Spinal Disord. Tech. 2010, 23, 74–77. [Google Scholar] [CrossRef]
- Cohen, S.P.; Bogduk, N.; Dragovich, A.; Buckenmaier, C.C., 3rd; Griffith, S.; Kurihara, C.; Raymond, J.; Richter, P.J.; Williams, N.; Yaksh, T.L. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 2009, 110, 1116–1126. [Google Scholar] [CrossRef]
- Cohen, S.P.; Wenzell, D.; Hurley, R.W.; Kurihara, C.; Buckenmaier, C.C., 3rd; Griffith, S.; Larkin, T.M.; Dahl, E.; Morlando, B.J. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 2007, 107, 99–105. [Google Scholar] [CrossRef]
- Korhonen, T.; Karppinen, J.; Paimela, L.; Malmivaara, A.; Lindgren, K.A.; Jarvinen, S.; Niinimaki, J.; Veeger, N.; Seitsalo, S.; Hurri, H. The treatment of disc herniation-induced sciatica with infliximab: Results of a randomized, controlled, 3-month follow-up study. Spine 2005, 30, 2724–2728. [Google Scholar] [CrossRef]
- Genevay, S.; Finckh, A.; Zufferey, P.; Viatte, S.; Balagué, F.; Gabay, C. Adalimumab in acute sciatica reduces the long-term need for surgery: A 3-year follow-up of a randomised double-blind placebo-controlled trial. Ann. Rheum. Dis. 2012, 71, 560–562, Epub 2011 Oct 13. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Miyagi, M.; Eguchi, Y.; Inoue, G.; Orita, S.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Nakamura, J.; Aoki, Y.; et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: A prospective randomized study. Spine 2012, 37, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2016, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.F.; Lane, N.E. Control of arthritis pain with anti-nerve-growth factor: Risk and benefit. Curr. Rheumatol. Rep. 2012, 14, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Yavuz Saricay, L.; Gonzalez Monroy, J.E.; Fulton, A.B. Can Nerve Growth Factor (NGF) Be a Treatment Option for Pediatric Eye Diseases? Semin. Ophthalmol. 2023, 38, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Mantelli, F.; Sacchetti, M.; Rossi, S.; Aloe, L.; Bonini, S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011, 149, 283–292. [Google Scholar] [CrossRef]
- Baldini, M.; Farinelli, L.; Luciani, P.; Manzotti, S.; Salaffi, F.; Gigante, A. Synovial and serum levels of NGF in osteoarthritis and rheumatic diseases: A systematic review. J. Biol. Regul. Homeost. Agents 2020, 34, 25–32, IORS Special Issue on Orthopedics. [Google Scholar]
- Belanger, P.; West, C.R.; Brown, M.T. Development of pain therapies targeting nerve growth factor signal transduction and the strategies used to resolve safety issues. J. Toxicol. Sci. 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Carey, K. Anti-nerve growth factor drugs exonerated. Nat. Biotechnol. 2012, 30, 298. [Google Scholar] [CrossRef]
- Bannwarth, B.; Kostine, M. Targeting nerve growth factor (NGF) for pain management: What does the future hold for NGF antagonists? Drugs 2014, 74, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Sadik, O.; Ezenwa, V.; Iguh, C.; Ravichandran, V.; Ashraf, N.N.; O’Connor, E.M.; Sayabugari, R. Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients. Cureus 2023, 15, e46790. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.I.; Nikaido, T.; Markman, J.D.; Ohta, M.; Machida, T.; Isogawa, N.; Yoshimatsu, H.; Viktrup, L.; Brown, M.T.; West, C.R.; et al. Tanezumab for chronic low back pain: A long-term, randomized, celecoxib-controlled Japanese Phase III safety study. Pain. Manag. 2022, 12, 323–335. [Google Scholar] [CrossRef]
- Lian, J.; Wang, J.; Li, X.; Yang, S.; Li, H.; Zhong, Y.; Gao, H.; Chen, G. Different Dosage Regimens of Tanezumab for the Treatment of Chronic Low Back Pain: A Meta-analysis of Randomized Controlled Trials. Clin. Neuropharmacol. 2023, 46, 6–16. [Google Scholar] [CrossRef]
- Bannwarth, B.; Kostine, M. Nerve Growth Factor Antagonists: Is the Future of Monoclonal Antibodies Becoming Clearer? Drugs 2017, 77, 1377–1387. [Google Scholar] [CrossRef]
- Leite, V.F.; Buehler, A.M.; El Abd, O.; Benyamin, R.M.; Pimentel, D.C.; Chen, J.; Hsing, W.T.; Mazloomdoost, D.; Amadera, J.E. Anti-nerve growth factor in the treatment of low back pain and radiculopathy: A systematic review and a meta-analysis. Pain Physician 2014, 17, E45–E60. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Chen, B. Standardized efficacy and safety evaluation of fulranumab for osteoarthritis. Clin. Rheumatol. 2025, 44, 1417–1426. [Google Scholar] [CrossRef]
- Sanga, P.; Katz, N.; Polverejan, E.; Wang, S.; Kelly, K.M.; Haeussler, J.; Thipphawong, J. Long-Term Safety and Efficacy of Fulranumab in Patients with Moderate-to-Severe Osteoarthritis Pain: A Phase II Randomized, Double-Blind, Placebo-Controlled Extension Study. Arthritis Rheumatol. 2017, 69, 763–773. [Google Scholar] [CrossRef]
- Sanga, P.; Polverejan, E.; Wang, S.; Kelly, K.M.; Thipphawong, J. Efficacy, Safety, and Tolerability of Fulranumab as an Adjunctive Therapy in Patients with Inadequately Controlled, Moderate-to-Severe Chronic Low Back Pain: A Randomized, Double-blind, Placebo-controlled, Dose-ranging, Dose-loading Phase II Study. Clin. Ther. 2016, 38, 1435–1450. [Google Scholar] [CrossRef][Green Version]
- Dakin, P.; Kivitz, A.J.; Gimbel, J.S.; Skrepnik, N.; DiMartino, S.J.; Emeremni, C.A.; Gao, H.; Stahl, N.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Efficacy and safety of fasinumab in patients with chronic low back pain: A phase II/III randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 509–517. [Google Scholar] [CrossRef]
- Katz, N.; Borenstein, D.G.; Birbara, C.; Bramson, C.; Nemeth, M.A.; Smith, M.D.; Brown, M.T. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011, 152, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Guermazi, A. Imaging atlas for eligibility and on-study safety of potential joint adverse events in anti-NGF studies. Osteoarthr. Cartil. 2015, 23 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef][Green Version]
- Kivitz, A.J.; Gimbel, J.S.; Bramson, C.; Nemeth, M.A.; Keller, D.S.; Brown, M.T.; West, C.R.; Verburg, K.M. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013, 154, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Gimbel, J.S.; Kivitz, A.J.; Bramson, C.; Nemeth, M.A.; Keller, D.S.; Brown, M.T.; West, C.R.; Verburg, K.M. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain 2014, 155, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.D.; Bolash, R.B.; McAlindon, T.; Kivitz, A.J.; Pombo-Suarez, M.; Ohtori, S.; Roemer, F.W.; Li, D.J.; Viktrup, L.; Bramson, C.; et al. Tanezumab for chronic low back pain: A randomized, double-blind, placebo- and active-controlled, phase 3 study of efficacy and safety. Pain 2020, 161, 2068–2078. [Google Scholar] [CrossRef]
- Tiseo, P.J.; Ren, H.; Mellis, S. Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: Results of a proof-of-concept study. J. Pain Res. 2014, 7, 523–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Anyfanti, P.; Dara, A.; Angeloudi, E.; Bekiari, E.; Dimitroulas, T.; Kitas, G.D. Monitoring and Managing Cardiovascular Risk in Immune Mediated Inflammatory Diseases. J. Inflamm. Res. 2021, 14, 6893–6906. [Google Scholar] [CrossRef] [PubMed]
- Anyfanti, P.; Ainatzoglou, A.; Angeloudi, E.; Michailou, O.; Defteraiou, K.; Bekiari, E.; Kitas, G.D.; Dimitroulas, T. Cardiovascular Risk in Rheumatoid Arthritis: Considerations on Assessment and Management. Mediterr. J. Rheumatol. 2024, 35, 402–410. [Google Scholar] [CrossRef]
- Vassilakis, K.D.; Magiouf, K.; Siebert, S.; Fragoulis, G.E. Selective JAK-Inhibitors in Spondyloarthritis. Mediterr. J. Rheumatol. 2024, 35, 27–36. [Google Scholar] [CrossRef]
- Makabe, K.; Okada, H.; Tachibana, N.; Ishikura, H.; Ito, N.; Tanaka, M.; Chijimatsu, R.; Terashima, A.; Yano, F.; Asaka, M.; et al. Baricitinib ameliorates inflammatory and neuropathic pain in collagen antibody-induced arthritis mice by modulating the IL-6/JAK/STAT3 pathway and CSF-1 expression in dorsal root ganglion neurons. Arthritis Res. Ther. 2024, 26, 121. [Google Scholar] [CrossRef]
- Pohoczky, K.; Kun, J.; Szentes, N.; Aczel, T.; Urban, P.; Gyenesei, A.; Bolcskei, K.; Szoke, E.; Sensi, S.; Denes, A.; et al. Discovery of novel targets in a complex regional pain syndrome mouse model by transcriptomics: TNF and JAK-STAT pathways. Pharmacol. Res. 2022, 182, 106347. [Google Scholar] [CrossRef]
- Horbal, N.; Maksymowych, W.P. Nociplastic pain in axial spondyloarthritis and psoriatic arthritis: Role of JAK kinases in immunopathology and therapeutic impact of JAK inhibitors. Expert. Rev. Clin. Immunol. 2025, 21, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Klavdianou, K.; Papagoras, C.; Baraliakos, X. JAK Inhibitors for the Treatment of Axial Spondyloarthritis. Mediterr. J. Rheumatol. 2023, 34, 129–138. [Google Scholar] [CrossRef]
- Bechman, K.; Yates, M.; Galloway, J.B. The new entries in the therapeutic armamentarium: The small molecule JAK inhibitors. Pharmacol Res. 2019, 147, 104392, Erratum in Pharmacol. Res. 2020, 153, 104634 . [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudnik-Jansen, I.; van Kruining Kodele, S.; Creemers, L.; Joosten, B. Biomolecular therapies for chronic discogenic low back pain: A narrative review. JOR Spine 2024, 7, e1345. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Cao, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol Ameliorates Intervertebral Disc Degeneration and Neuropathic Pain by Reducing Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef]
- Krock, E.; Millecamps, M.; Anderson, K.M.; Srivastava, A.; Reihsen, T.E.; Hari, P.; Sun, Y.R.; Jang, S.H.; Wilcox, G.L.; Belani, K.G.; et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019, 43, 487–500. [Google Scholar] [CrossRef]
- de Carvalho, J.F.; Davidson, J. Oral Hyaluronic Acid in Osteoarthritis and Low Back Pain: A Systematic Review. Mediterr. J. Rheumatol. 2024, 35, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Li, K.; Chen, Y.; Lai, W.; Lian, Z.; Wei, Z.; Wang, L.; Zhang, Z.; Huang, M. Inhibition of TrkB-BDNF positive feedback loop attenuates intervertebral disc degeneration and low back pain in a composite mouse model. Brain Behav. Immun. 2025, 128, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamaji, Y.; Mori, K.; Washizu, S.; Hoshino, O.; Fujimoto, K.; Kanazawa, S.; Minegishi, Y.; Ota, N.; Mori, T.; et al. Persistent pain signaling and stress response in a mouse model of inflammatory low back pain. Pain. Rep. 2025, 10, e1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Friton, J.J.; Raffals, L.E.; Leighton, J.A.; Pasha, S.F.; Picco, M.F.; Cushing, K.C.; Monroe, K.; Nix, B.D.; Newberry, R.D.; et al. Novel Genetic Risk Variants Can Predict Anti-TNF Agent Response in Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 1036–1043. [Google Scholar] [CrossRef]
| Reference | Study Design | Number of Participants | Age (years), Mean (SD) | Female Sex, n (%) | Duration of Pain | CLBP Evaluation | Biomarkers |
|---|---|---|---|---|---|---|---|
| Gebhardt, et al., 2006 [36] | Prospective longitudinal comparative study | -72 cases (31: with ALBP, 41: CLBP) -1572 controls (from the VERA study) | -ALBP group: 44.8 (20–64) -CLBP: 42.2 (27–57) | -ALBP: 16 (51.6) -CLBP: 27 (65.8) | -ALBP: <8 weeks -CLBP: >3 months | -VAS -Hannover questionnaire | hs-CRP |
| Kraychete et al., 2010 [41] | Case–control study | -23 cases with CLBP -10 controls | -CLBP: 42.8 (7) -Controls: 39.5 (4.5) | -CLBP: 11 (47.8) -Controls: 4 (40) | ≥3 months | NRS | IL-1, IL-6, TNF-α, sTNF-R |
| Heffner et al., 2011 [51] | Case–control study | -25 cases with CLBP -25 controls | 30.8 (11.4) | -Total cohort: 30 (60) | ≥6 months | MPQ-SF | IL-6 |
| Pedersen et al., 2015 [43] | Prospective longitudinal | 127 patients with LBP due to disc herniation | 40 (10) | 65 (51.2) | >1 month | VAS | IL-6, IL-8 |
| Li et al., 2016 [42] | Case–control study | -35 cases with CLBP -35 controls | Range: 45–70 | NS | NS | NS | IL-6, IL-10, monocyte markers (CD14, CD16), β-endorphin |
| Luchting et al., 2016 [44] | Case–control | -19 cases with CLBP -19 cases with NeP -19 controls | -CLBP: 40 (11) -NeP: 47 (13) -Controls: 58 (13) | -CLBP: 15 (79) -NeP: 13 (68) -Controls: 11 (58) | NS | PainDETECT-questionnaire | P2X7R, IL-1β |
| Capossela et al., 2018 [52] | Case–control | -23 cases with CLB -30 controls | 52.5 (15.9) | -CLBP: 14 (61) -Controls: NS | NS | NS | IL-6, IL-1β, TNF-α, IL-2, IL-10, MCP1, CCL5, CXCL6, G-CSF |
| Teodorczyk-Injeyan et al., 2018 [47] | Non-randomized clinical trial | -19 cases with ALBP patients -23 cases with CLBP -21 controls | -ALBP: 35.4 (9.9) -CLBP: 31.6 (7.8) -Controls: 36.1 (11.4) | -ALBP: 7 (36.9) -CLBP: 10 (43.5) -Controls: 8 (38.1) | -ALBP: <4 weeks -CLBP: ≥12 weeks | -VAS -Oswestry disability index | CCL2, CCL3, CCL4, sE-selectin |
| Teodorczyk-Injeyan et al., 2019 [46] | Case–control | -22 cases with ALBP -25 cases with CLBP -24 controls | -ALBP: 32.8 (9.2) -CLBP: 36.5 (11.1) -Controls: 35.2 (10.4) | -ALBP: 9 (40.9) -CLBP: 11 (44) -Controls: 9 (37.5) | -ALBP: <4 weeks -CLBP: >12 weeks | -VAS -Oswestry disability index | TNFα, IL-1β, IL-6, IL-2, IL-10, IL-1 receptor antagonist, sTNF-R2 |
| Ho et al., 2019 [39] | Cross-sectional study | 6559 with CLBP | 52.6 | 16,663 (54) | >3 months | Standardized Nordic questionnaire for musculoskeletal symptoms | CRP |
| Dadkhah et al., 2020 [37] | Case–control study | -148 cases with CLBP -150 controls | -CLBP: 49.2 (6.1) -Controls: 47.57 (5.8) | -CLBP: 87 (50.3) -Controls: 86 (49.7) | >12 weeks | -MPQ -Oswestry disability index | IL-1B, IL-6, hs-CRP, TNF-α |
| Xu et al., 2021 [48] | Case–control study | -60 cases with ALBP -78 cases with CLBP -60 controls | -ALBP: 64.46 (10.45) -CLBP: 63.17 (12.49)-Controls: 62.31 (11.06) | -ALBP: 43 (71.7) -CLBP: 51 (65.4) -Controls: 32 (53.3) | -ALBP: <12 weeks -CLBP: >12 weeks | -VAS -Modified Oswestry disability index | CRP, WBCs, neutrophils, TNF-α, IL-6, IL-1, 25(OH)D, |
| Gjefsen et al., 2021 [50] | Case–control study | -46 cases with CLBP and MC1 -37 cases with CLBP and MC2 -50 controls | -MC1: 42.1 (8.3) -MC2: 45.8 (9.0) -Control: NS | -MC1: 32 (69.6) -MC2: 19 (51.4) | >6 months | -NRS -Oswestry Disability Index | 40 different cytokines |
| Slouma et al., 2023 [49] | Case–control study | -46 CLBP cases -60 controls | -CLBP: 43.17 (8.7) -Controls: NS | NS | >3 months | VAS | IL-6, IL-8, IL-17, IL-23, IL-22, TNF-α |
| Hodges et al., 2023 [40] | Case–control study | -Initial cohort: 59,208 (CLBP: 17,642, ALBP: 11,962, controls: 29,604) -Validation cohort: 2326 (CLBP: 669, ALBP: 494, Controls: 1163) | -Initial: 55.8 (8.1) -Validation: 60.7 (7.6) | Initial cohort: -CLBP: 8586 (48.7%) -ALBP: 5348 (44.7%) -Controls: 13,934 (47.1%) Validation cohort -CLBP: 303 (45.3%) -ALBP: 207 (41.9%) -Controls: 510 (43. | >3 months | Self-reported | CRP |
| Marker | Biological Role | Findings | Reference |
|---|---|---|---|
| hs-CRP | Acute-phase protein Sensitive marker of low-grade systemic inflammation | Increased Association with pain severity | [36] |
| CRP | Acute-phase protein | Increased Association with sleep disturbance | [39] |
| TNF-α | Pro-inflammatory cytokine Induces of production of IL-1β, IL-6, chemokines, and adhesion molecules | Increased Association with pain severity | [37] |
| sTNF-R | Cleavage of the extracellular domains of TNF receptors circulating in plasma | Increased | [41] |
| IL-1β | Pro-inflammatory cytokine promoting prostaglandin synthesis and leukocyte recruitment | Increased Association with pain severity | [37] |
| IL-6 | Pro-inflammatory cytokine Key stimulator of hepatic CRP production | Increased Association with pain severity, disability rate, and sleep disturbance | [37] |
| IL-8 | Chemokine recruiting neutrophils | Increased Association with pain severity | [43] |
| MCP-1 | CC chemokine recruiting monocytes and memory T cells Role in neuroinflammation | Increased | [42] |
| CCL4 | CC chemokine attracting monocytes, NK cells, and T cells | Increased | [42] |
| CD16+ monocytes | Pro-inflammatory monocytes with enhanced capacity for producing pro-inflammatory cytokines | Increased | [42] |
| sE-selectin | Adhesion molecule Mediation of leukocyte adhesion and migration | Increased | [47] |
| IL-4 | Anti-inflammatory cytokine | Decreased | [52] |
| IL-10 | Anti-inflammatory cytokine | Decreased | [42] |
| Author/Year | Population | Study Design | Anti-TNF Agent | Main Outcomes | Key Findings |
|---|---|---|---|---|---|
| Cohen et al., 2012 [69] | 84 adults with lumbosacral radiculopathy of less than 6 months’ duration | Multicenter, triple-arm RCT | Etanercept (epidural) | The primary outcome measure was leg pain 1 month after the second injection. | -Small differences favoring steroids compared with saline and etanercept were observed for back pain. -Etanercept fared worse for functional capacity than the other treatments (steroids and placebo) |
| Genevay et al., 2010 [70] | 61 patients with acute (duration of <12 weeks) and severe (ODI score of >50) radicular leg pain and imaging-confirmed lumbar disc herniation | RCT, placebo-controlled | Etanercept (subcutaneous, repeated) | The primary outcome was the VAS score for leg pain. | -Small but significant improvement in leg pain. -Patients receiving adalimumab demonstrated significantly better outcomes in terms of back pain and a reduced rate of surgery. |
| Okoro et al., 2010 [71] | 15 patients with acute unilateral radicular leg pain secondary to a herniated nucleus pulposus, confirmed on magnetic resonance imaging scan | Triple blind, placebo-controlled RCT | Etanercept (subcutaneous injection in the perispinal area) | Primary outcome measures were VAS pain, ODI score, modified somatic perception, and modified Zung depression index | No benefit to the use of etanercept over placebo, but small numbers of trial participants limited statistical analysis. |
| Cohen et al., 2009 [72] | 24 patients with subacute lumbosacral radiculopathy | RCT, placebo-controlled | Etanercept (epidural) | The primary outcome was a numerical rating scale leg pain score reflecting pain. Secondary outcome measures included ODI score, numerical rating scale back pain score, reduction in analgesic medications, and global perceived effect. | Significant improvements in leg and back pain for the etanercept-treated patients, but not for the placebo group, one month after treatment. |
| Cohen et al., 2007 [73] | 36 patients with chronic lumbosacral radiculopathy or discogenic LBP | RCT, placebo-controlled | Etanercept intradiscally | The primary outcome was the VAS pain score. Secondary outcome measures included ODI score, reduction in analgesic medications, and global perceived effect. | -A single low dose of intradiscal etanercept was not effective for chronic radicular or discogenic LBP according to pain scores or disability scores, which did not differ between or within groups for any dose range or subgroup of patients. |
| Korhonen et al., 2005 [74] | 40 patients with unilateral moderate to severe sciatic pain with an MRI-confirmed disc herniation | Randomized, double-blind, placebo-controlled | Infliximab IV (single dose) | The primary endpoint was a reduction in leg pain through 12 weeks. | -No differences vs. placebo in the primary endpoint. -No differences were observed in the secondary outcomes (reduction in back pain and ODI score, improvement of straight leg restriction, differences in the number of days on sick leave, and the number of discectomies). |
| Gjefsen et al., 2025 [62] | 128 patients with moderate to severe chronic low-back pain and Modic type 1 changes | Randomized, triple-blind, placebo-controlled, multicenter trial | Infliximab (four IV infusions 5 mg/kg) over ~5 months | No significant difference in the primary outcome between infliximab and placebo at 5 months. | -Secondary outcomes reported no effect. -Adverse event rates were similar between groups. |
| Genevay et al., 2012 [75] | 61 patients were enrolled (31 in the adalimumab group and 30 in the placebo group) with acute and severe sciatica | Multicenter, randomized, double-blind, placebo-controlled trial | Adalimumab (40 mg, 2 subcutaneous injections, within 2 weeks) | Primary short-term outcome: leg pain (measured by visual analogue scale, VAS 0–100 mm), daily for 10 days, then at 6 weeks and 6 months. Long-term (3-year) outcome: incidence of discectomy | -Over time, the adalimumab group had a more favorable course of leg pain vs. placebo. -Fewer surgical discectomies in the adalimumab group vs. placebo (6 vs. 13) in the short/medium-term follow-up. |
| Ohtori et al., 2012 [76] | 80 patients with lumbar spinal stenosis (40 patients received etanercept, 40 patients received dexamethasone) | Prospective, randomized, controlled study | Etanercept (epidural administration onto the spinal nerves) | Epidural administration of etanercept was more effective than dexamethasone for leg pain, low back pain, and leg numbness. No adverse event was reported in either group. | Epidural administration of a TNF inhibitor onto the spinal nerve led to pain relief, without adverse events. |
| Author/Year | Population | Study Design | Anti-NGF Agent | Main Outcomes | Key Findings |
|---|---|---|---|---|---|
| Sanga et al., 2016 [93] | 183 patients with chronic LBP | Phase II, randomized, double-blind, placebo-controlled, dose-ranging study | Fulranumab (SC, every 4 weeks) | Primary endpoint: pain improvement at week 12 | -Fulranumab was characterized as well-tolerated, but significant results compared with placebo were not reported. -Neurologic TEAEs were more frequent in fulranumab group (25%) vs. placebo (14%) |
| Katz et al., 2011 [95] | 217 adult patients, with chronic LBP (72 received tanezumab, 73 received oral naproxen, 72 placebo) | Randomized, double-blind, multicenter, placebo-controlled trial. | Tanezumab, IV (200 µg/kg–single dose) | LBPI at week 6 | -Tanezumab was more effective against pain and reported improvements in disability. -No severe TEAEs were mentioned, but 9/72 patients discontinued. |
| Kivitz et al., 2013 [97] | 1347 patients with chronic LBP | Phase IIb randomized, placebo-controlled trial | Tanezumab IV (5, 10, or 20 mg every 8 weeks) | The primary endpoint was the mean change in daily average LBPI from baseline to week 16 | -10 and 20 mg of tanezumab had similar efficacy and improved LBPI. -Arthralgia and paresthesia were the most frequent TEAEs, resulting in discontinuation. |
| Gimbel et al., 2014 [98] | 849 patients with chronic LBP | Non-controlled, randomized, multicenter study | Tanezumab (IV injections of 10 or 20 mg) | The primary outcome was average pain | -A dose of 10 mg proved better tolerated than 20 mg. -No severe TEAEs were reported. |
| Markman et al., 2020 [99] | 1825 participants were enrolled (406: placebo group, 407: tanezumab 5 mg, 407: tanezumab 10 mg, 605: tramadol prolonged-release 100–300 mg/day) | Randomized, double-blind, placebo- and active-controlled, phase 3 study | Tanezumab (5 or 10 mg SC every 8 weeks) | Reported efficacy with ≥50% LBPI reduction at week 16 | -Tanezumab 5 mg did not meet the primary endpoint. -TEAEs were more frequent at the 10 mg dose. |
| Dakin et al., 2021 [94] | 563 patients with moderate to severe chronic LBP | Phase II/III, double-blind, placebo-controlled study | Fasinumab (SC 6 mg or 9 mg or IV 9 mg) | The primary outcome was the change from baseline to week 16 in average daily LBPI | -Significant placebo-adjusted LBP reductions at week 16 for fasinumab 9 mg SC and 9 mg IV. -6 mg dose was not significantly better. -Joint TEAEs were more common in fasinumab vs. placebo. |
| Tiseo et al., 2014 [100] | 159 adults with moderate-to-severe unilateral sciatica pain | Randomized, double-blind, placebo-controlled, parallel-group proof-of-concept trial | Fasinumab (0.1 or 0.3 mg/kg SC) | AUC of average leg pain scores (measured by numerical rating scale) from baseline to Week 4 | -No notable difference in average leg pain reduction compared with placebo. -More TEAEs occurred with fasinumab, especially at the higher dose. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyfanti, P.; Evangelidis, P.; Tragiannidis, K.; Antza, C.; Poulis, D.; Dimitroulas, T.; Kotsis, V. Targeting Inflammatory Pathways in Chronic Low Back Pain: Opportunities for Novel Therapeutics. Pharmaceuticals 2025, 18, 1612. https://doi.org/10.3390/ph18111612
Anyfanti P, Evangelidis P, Tragiannidis K, Antza C, Poulis D, Dimitroulas T, Kotsis V. Targeting Inflammatory Pathways in Chronic Low Back Pain: Opportunities for Novel Therapeutics. Pharmaceuticals. 2025; 18(11):1612. https://doi.org/10.3390/ph18111612
Chicago/Turabian StyleAnyfanti, Panagiota, Paschalis Evangelidis, Konstantinos Tragiannidis, Christina Antza, Dimitrios Poulis, Theodoros Dimitroulas, and Vasilios Kotsis. 2025. "Targeting Inflammatory Pathways in Chronic Low Back Pain: Opportunities for Novel Therapeutics" Pharmaceuticals 18, no. 11: 1612. https://doi.org/10.3390/ph18111612
APA StyleAnyfanti, P., Evangelidis, P., Tragiannidis, K., Antza, C., Poulis, D., Dimitroulas, T., & Kotsis, V. (2025). Targeting Inflammatory Pathways in Chronic Low Back Pain: Opportunities for Novel Therapeutics. Pharmaceuticals, 18(11), 1612. https://doi.org/10.3390/ph18111612







