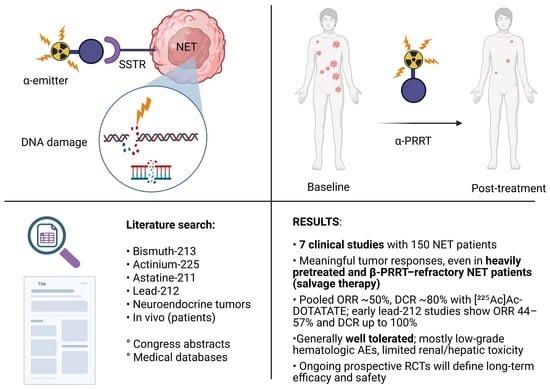
Clinical Experience with Targeted Alpha-Emitter Peptide Receptor Radionuclide Therapy (α-PRRT) for Somatostatin Receptor-Positive Neuroendocrine Tumors
Abstract
1. Introduction
2. Methods
Search Strategy
3. Results
3.1. Population Characteristics
3.2. Efficacy and Safety of [213Bi]Bi-DOTATOC
3.3. Efficacy and Safety of [225Ac]Ac-DOTATOC/[225Ac]Ac-DOTATATE
3.4. Efficacy and Safety of [212Pb]Pb-DOTAMTATE/[212Pb]Pb-VMT-α-NET
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef]
- Hiller-Sturmhöfel, S.; Bartke, A. The Endocrine System An Overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.; Ezzat, S.; de Herder, W.; Klimstra, D.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1 SST5 of somatostatin radio tracers selected for scinitgraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Klomp, M.; Dalm, S.U.; De Jong, M.; Feelders, R.; Hofland, J.; Hofland, L. Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer. Rev. Endocr. Metab. Disord. 2020, 22, 495–510. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.; Arnold, R. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long-term survival. Neuroendocrinology 2016, 104, 26–32. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands-the next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar] [PubMed]
- Leupe, H.; Ahenkorah, S.; Dekervel, J.; Unterrainer, M.; Van Cutsem, E.; Verslype, C.; Cleeren, F.; Deroose, C.M. 18F-Labeled Somatostatin Analogs as PET Tracers for the Somatostatin Receptor: Ready for Clinical Use. J. Nucl. Med. 2023, 64, 835–841. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Fani Bozkurt, M.; Graham, M.; Heaney, A.; Herrman, K.; Howe, J.; Kulke, M.; Kunz, P.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Bidakhvidi, N.A.; Goffin, K.; Dekervel, J.; Baete, K.; Nackaerts, K.; Clement, P.; Van Cutsem, E.; Verslype, C.; Deroose, C.M. Peptide receptor radionuclide therapy targeting the somatostatin receptor: Basic principles, clinical applications and optimization strategies. Cancers 2022, 14, 129. [Google Scholar] [CrossRef]
- Buscombe, J.R.; Caplin, M.E.; Hilson, A.J. Long-Term Efficacy of High-Activity 111 In-Pentetreotide Therapy in Patients with Disseminated Neuroendocrine Tumors. J. Nucl. Med. 2003, 44, 1–6. [Google Scholar]
- Bodei, L.; Pepe, G.; Paganelli, G. Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors with somatostatin analogues. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 347–351. [Google Scholar] [PubMed]
- Cwikla, J.B.; Sankowski, A.; Seklecka, N.; Buscome, J.; Nasierowska-Guttmejer, A.; Jeziorski, K.; Mikolajczak, R.; Pawlak, D.; Stepien, K.; Walecki, J. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): A phase II study. Ann. Oncol. 2009, 21, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Navalkissoor, S.; Grossman, A. Somatostatin receptor-linked α-particle therapy in neuroendocrine tumours. J. Neuroendocrinol. 2025, 37, e13463. [Google Scholar] [CrossRef]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Mäcke, H.; Rochlitz, C.; Müller-Brand, J.; Walter, M. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.; Kulke, M.; Jacena, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrman, K.; Pavel, M.; Kunz, P.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2–3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated with 177Lu-Dotatate in the Phase III NETTER-1 Tria. J. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.; Bodei, L.; Hendifar, A.; Mitrra, E.; Wolin, E.; Yao, J.; Pavel, M.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The first FDA-and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sgouros, G. Alpha and Beta Radiation for Theragnostics. PET Clin. 2024, 19, 307–323. [Google Scholar] [CrossRef]
- Uccelli, L.; Boschi, A.; Cittanti, C.; Martini, P.; Panareo, S.; Tonini, E.; Nieri, A.; Urso, L.; Caracciolo, M.; Lodi, L.; et al. 90y/177lu-dotatoc: From preclinical studies to application in humans. Pharmaceutics 2021, 13, 1463. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Baum, R.; Pavel, M.; Hörsch, D.; O’Dorisio, M.; O’Dorisio, T.; Howe, J.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Bergsma, H.; Van Lom, K.; Raaijmakers, M.H.G.P.; Konijnenberg, M.; Boen, B.; Teunissen, J.; de Herder, W.; Krenning, E.; Kwekkeboom, D. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with 177Lu-DOTATATE: Incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2018, 59, 452–458. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Damle, N.A.; Sahoo, R.K.; Bal, C. Concomitant 177Lu-DOTATATE and Capecitabine Therapy in Patients with Advanced Neuroendocrine Tumors: A Long-term-Outcome, Toxicity, Survival, and Quality-of-Life Study. Clin. Nucl. Med. 2017, 42, e457–e466. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Therapeutic Radionuclides: Biophysical and Radiobiologic Principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, D.A.; Mcdevit, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the most promising radionuclides for targeted alpha therapy: The “hopeful eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-world Scenario Management Based Long-term Outcome Study. J. Nucl. Med. 2023, 64, 211–218. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Nunez, R. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Demirci, E.; Alan Selçuk, N.; Beydağı, G.; Ocak, M.; Toklu, T.; Akcay, K.; Kabasakal, L. Initial Findings on the Use of [225Ac]Ac-DOTATATE Therapy as a Theranostic Application in Patients with Neuroendocrine Tumors. Mol. Imaging Radionucl. Ther. 2023, 32, 226–232. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Li, H.; Zhang, Y.; Feng, Y.; Yang, X.; Chen, Y. Efficacy and Safety of 225Ac-DOTATATE in the Treatment of Neuroendocrine Neoplasms with High SSTR Expression. Clin. Nucl. Med. 2024, 49, 505–512. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef]
- Michler, E.; Kästner, D.; Pretze, M.; Hartmann, H.; Freudenberg, R.; Schultz, M.; Bundschuh, R.; Kotzerke, J.; Brogsitter, C. [203/212Pb]Pb-VMT-α-NET as a novel theranostic agent for targeted alpha radiotherapy—First clinical experience. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 4171–4183. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schartz, L.; Sargent, D.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; Rubinstein, L.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, A.; Kulkarni, H.R.; Schudardt, C.; Müller, D.; Wester, H.; Maina, T.; Rösch, F.; van der Meulen, N.; Müller, C.; et al. From Bench to Bedside—The Bad Berka Experience with First-in-Human Studies. Semin. Nucl. Med. 2019, 49, 422–437. [Google Scholar] [CrossRef]
- Chandekar, K.R.; Bal, C. Advances with 225Ac-DOTATATE Targeted Alpha Therapy in Somatostatin Receptor Positive Neuroendocrine Tumors. Semin. Nucl. Med. 2025; Epub Ahead of Print. [Google Scholar] [CrossRef]
- Kratochwil, C.; Gruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC—An empiric dose finding for alpha aparticle meitter based radionuclide therapy of neuroendocrine tumors. J. Nucl. Med. 2015, 56, 1232. [Google Scholar]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.; Haberkorn, U.; Giesel, F. Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.; Morris, M.; Ulaner, G.A.; Strosberg, J.; Mehr, S.; Li, D.; Soares, H.; Anthony, L.; Kotiah, S.; Jacene, H.; et al. Phase 1b portion of the ACTION-1 phase 1b/3 trial of RYZ101 in gastro-enteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu so-matostatin analogue (SSA) therapy: Safety and efficacy findings. J. Clin. Oncol. 2025, 43, 661. [Google Scholar]
- RadioMedix and Orano Med Receive FDA Breakthrough Therapy Designation for AlphaMedixTM in Gastroenteropancreatic Neuroendocrine Tumors. Available online: https://www.businesswire.com/news/home/20240212199538/en/RadioMedix-and-Orano-Med-receive-FDA-Breakthrough-Therapy-Designation-for-AlphaMedixTM-in-gastroenteropancreatic-neuroendocrine-tumors (accessed on 12 September 2025).
- Strosberg, J.R.; Naqvi, S.; Lee Cohn, A.; Delpassand, E.; Wagner, V.; Tworowska, I.; Torgue, J.; Woloski, R.; Manuel, A.; Maluccio, M. Safety, tolerability and efficacy of 212Pb-DOTAMTATE as a targeted alpha therapy for subjects with unresectable or metastatic somatostatin receptor-expressing gastroenteropancreatic neuroendocrine tumors (SSTR+ GEP-NETs): A phase 2 study. J. Clin. Oncol. 2024, 42, 4020. [Google Scholar] [CrossRef]
- Ragnar Halfdanarson, T.; Solnes, L.B.; Robert Mancini, B.; Sibley, G.; Anthony, L.; Liao, C.; Starr, J.; Balaraman, S.; Prasad, V.; Baratto, L.; et al. [212Pb]VMT-a-NET therapy in somatostatin receptor 2 (SSTR2) expressing neu-roendocrine tumors (NETs): Dose-limiting toxicity (DLT) observation participants after 1 year follow-up and preliminary report for expansion participants. J. Clin. Oncol. 2025, 43, 3005. [Google Scholar] [CrossRef]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef]
- Gillis, A.; Zheng-Pywell, R.; McLeod, C.; Wang, D.; Ness, J.; Guenter, R.; Whitt, J.; Prolla, T.; Chen, H.; Gonzalez, M.; et al. Somatostatin Receptor Type 2 and Thyroid-Stimulating Hormone Receptor Expression in Oncocytic Thyroid Neoplasms: Implications for Prognosis and Treatment. Mod. Pathol. 2023, 36, 100332. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.; Baum, R.; Krenning, E.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Liepe, K.; Shinto, A. From palliative therapy to prolongation of survival: 223RaCl2 in the treatment of bone metastases. Ther. Adv. Med. Oncol. 2016, 8, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant prostate cancer patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Hwang, E.; Lin, F.; Clift, R.; Kim, D.; Guest, M.; Bischoff, E.; Moran, S.; Li, G. RYZ101 (Ac-225 DOTATATE) Opportunity beyond Gastroenteropancreatic Neuroendocrine Tumors: Preclinical Efficacy in Small-Cell Lung Cancer. Mol. Cancer Ther. 2023, 22, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Yang, Y.; Zhang, J.; Yin, Y.; Liu, W.; Zhang, H.; Fan, X.; Yang, M.; Yu, F. Effective Treatment of SSTR2-Positive Small Cell Lung Cancer Using 211At-Containing Targeted α-Particle Therapy Agent Which Promotes Endogenous Antitumor Immune Response. Mol. Pharm. 2023, 20, 5543–5553. [Google Scholar] [CrossRef]
- Ulaner, G.A.; VanderMolen, L.A.; Li, G.; Ferreira, D. Dotatate PET/CT and 225Ac-Dotatate Therapy for Somatostatin Receptor–expressing Metastatic Breast Cancer. Radiology 2024, 312, e233408. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Finn, R.D.; Sgouros, G.; Ma, D.; Scheinberg, D.A. An 225Ac/213Bi generator system for therapeutic clinical applications: Construction and operation. Appl. Radiat. Isot. 1999, 50, 895–904. [Google Scholar] [CrossRef]
- Zimmermann, R. Is Actinium Really Happening? J. Nucl. Med. 2023, 64, 1516–1518. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Type of Study | Sex/Mean Age (y) | ECOG Score or KPS Score | Site of Primary NET, n | Site of Metastases, n | Treatment Regimen | |
|---|---|---|---|---|---|---|---|---|
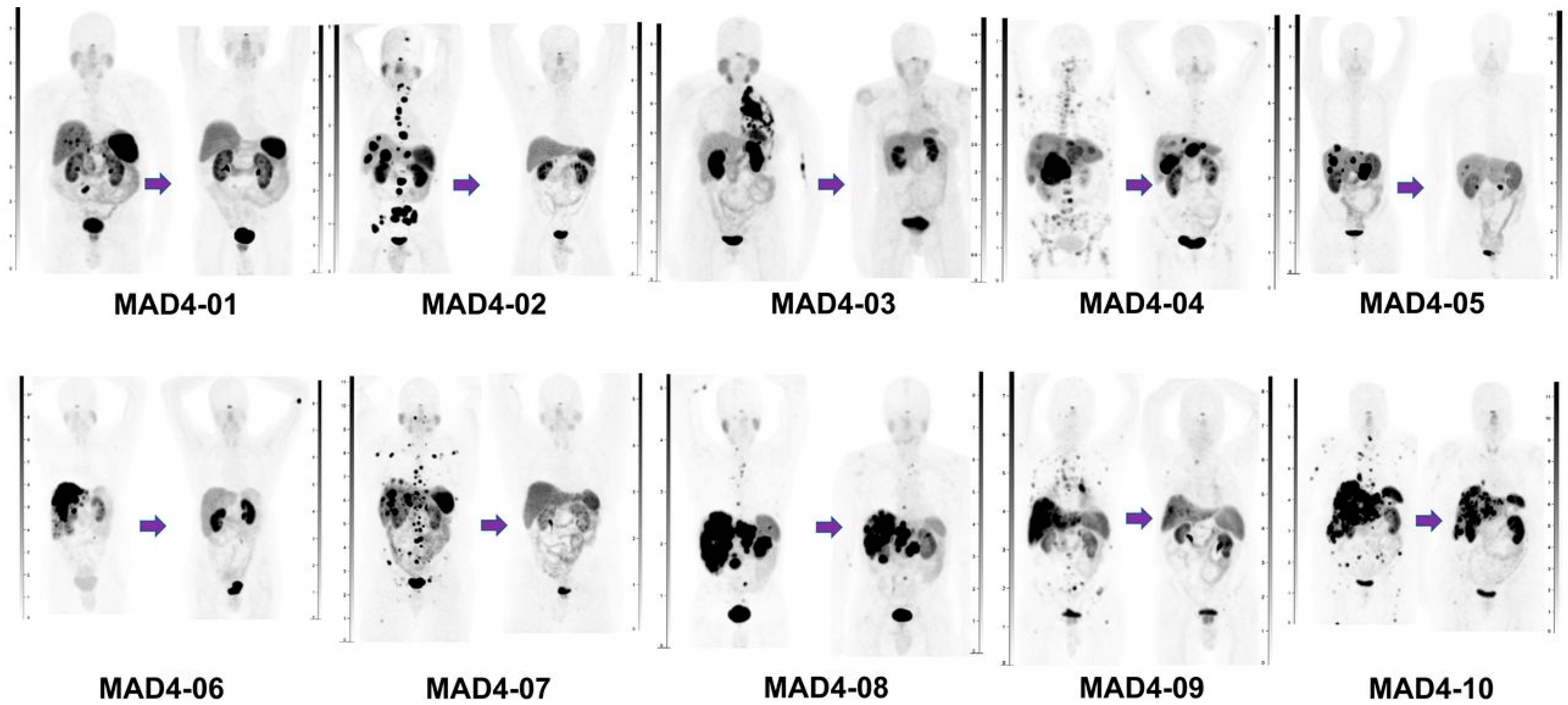
| Ballal et al. [33] | 2022 | Prospective (n = 91) | M 54 (59.4%) F 37 (40.6%) Mean 54.3 ± 11.6 (range: 25–75) | Median ECOG score = 2 Median KPS score = 60 | Foregut: 47 Midgut: 20 Hindgut: 8 Unknown:16 | Liver: 88 Lymph nodes: 66 Bone: 25 Lung: 6 Brain: 2 Adrenal glands: 6 Other: 9 | [225Ac]Ac-DOTATATE cycle every 8 weeks. Mean cumulative activity of 35.52 MBq (range: 21.64–59.47). Median 4 cycles per patient (range: 1–10). Radiosensitizer d0–14. | |
| Ballal et al. [32] | 2020 | Prospective (n = 32) | M 15 (47%) F 17 (53%) Mean 52 ± 9.2 (range: 35–72) | Baseline ECOG score < 2 Baseline KPS score > 50 | Pancreatic: 16 Foregut: 7 Midgut: 3 Hindgut: 1 Unknown: 5 | Liver: 29 Lymph nodes: 24 Bone: 12 Other sites: 6 | [225Ac]Ac-DOTATATE cycle every 8 weeks. Mean cumulative activity of 22.550 kBq ± 9842 (range: 7770–44,400 kBq). Median 3 cycles per patient (range: 1–5). | |
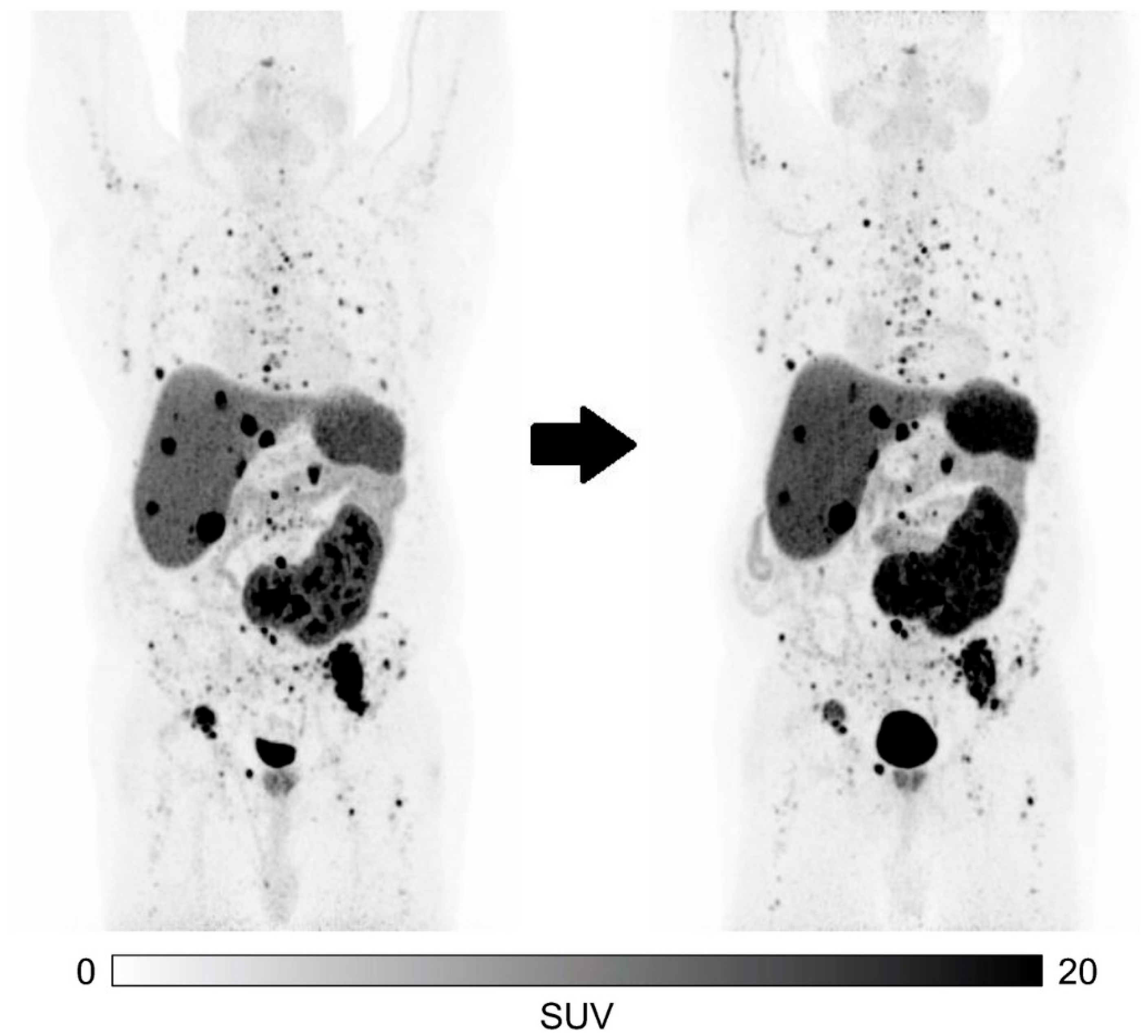
| Delpassand et al. [34] | 2022 | Prospective (n = 10) | M 6 (50%) F 4 (50%) Range: 39–80 | Baseline ECOG score < 2 | Pulmonary: 4 Pancreatic: 2 Small bowel: 2 Rectal: 2 | NR | [212Pb]Pb-DOTAMTATE cycle every 8 weeks. Mean cumulative activity of 791 MBq (range: 681–873). 4 cycles per patient. | |
| Demirci et al. [35] | 2023 | Retrospective (n = 11) | M 8 (73%) F 3 (27%) Mean 59 ± 11.9 (range: 43–79) | ECOG score not reported Baseline KPS score > 50 | Pulmonary: 1 Pancreatic: 3 Non-pancreatic GEP: 1 Paraganglioma: 1 Unknown: 3 | Liver: 10 Lymph nodes: 8 Bone: 8 Lung: 4 | [225Ac]Ac-DOTATATE cycle every 8 weeks. Mean activity of 8.2 MBq ± 0.6 (range: 7.5–10.0 MBq). Median 1 cycle per patient (range: 1–3). | |
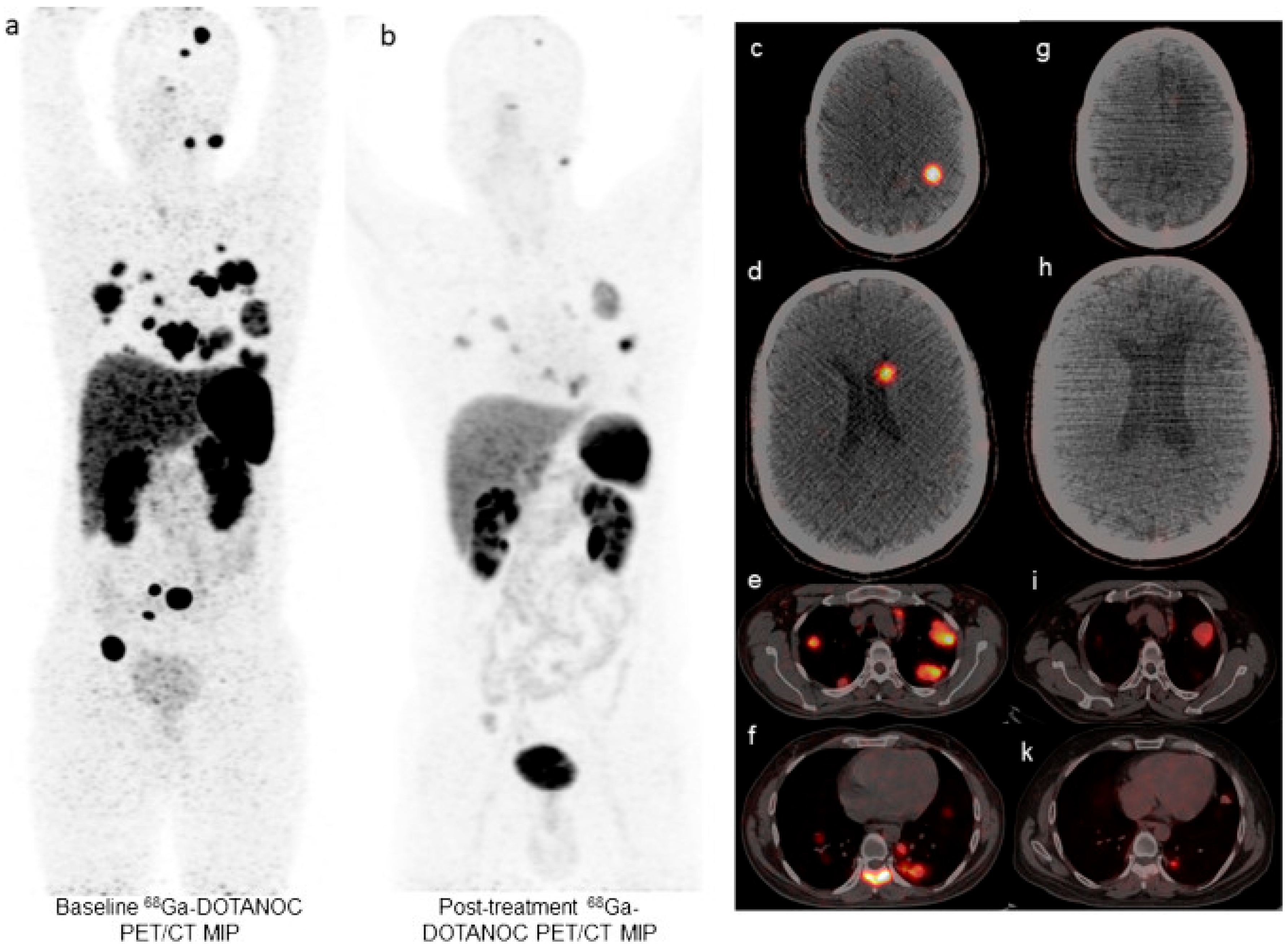
| Kratochwil et al. [36] | 2014 | Retrospective (n = 7) | M 3 (43%) F 4 (57%) Age not reported | Not reported | Pulmonary: 1 Carcinoid: 1 Pancreatic: 4 Unknown: 2 | Liver: 7 Bone: 1 | [213Bi]Bi-DOTATOC cycle every 2 months. Cumulative activity range: 13.3 GBq–20.8 GBq Median 5 cycles per patient (range: 2–5). | |
| Michler et al. [39] | 2025 | Retrospective (n = 12) | M 9 (75%) F 3 (25%) Mean 71 (range: 60–84) | Baseline ECOG score < 2 | Pancreatic: 3 Small bowel: 4 CUP: 3 Rectal: 1 Carcinoid: 1 | NR | [212Pb]Pb-VMT-α-NET single dose 1.2 MBq/kg | |
| Yadav et al. [38] | 2022 | Prospective (n = 9) | M 6 (67%) F 3 (33%) Mean 41 ± 10.5 (range: 23–65) | 9 | Baseline ECOG score < 3 Baseline KPS score > 40 | Sympathetic paraganglioma: 3 Parasympathetic paraganglioma: 6 | Liver: 2 Lymph nodes: 8 Bone: 6 Lung: 3 Brain: 1 Duodenum: 1 | [225Ac]Ac-DOTATATE cycle every 8 weeks. Mean cumulative activity of 42.4 MBq ± 27 (range: 15.54–86.6). Median 3 cycles per patient (range: 2–9). Radiosensitizer. |
| Yang et al. [37] | 2024 | Prospective (n = 10) | M 7 (70%) F 3 (30%) Mean 47.5 ± 14.1 (range: 26–66) | 10 | Baseline ECOG score < 3 Baseline KPS score > 40 | Pulmonary: 1 Pancreatic: 1 Tonsillar: 1 Paraganglioma: 4 Pheochromocytoma: 3 | Liver: 4 Lymph nodes: 9 Bone: 8 Lung: 4 Adrenal glands: 2 Subcutaneous: 1 Muscle: 1 | [225Ac]Ac-DOTATATE cycle every 8 weeks. Mean cumulative activity of 22.9 MBq ± 9.5 (range: 14.8–44.4 MBq). Median 3 cycles per patient (range: 2–6). |
| Authors | 177Lu-DOTATATE, n (%) | 90Y/177Lu-DOTATOC, n (%) | 225Ac-DOTATATE | Surgery, n (%) | Chemotherapy, n (%) | EBRT, n (%) | SSA, n (%) | Targeted Therapies, n (%) | 131I MIBG, n (%) | TARE/TACE, n (%) | IO, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ballal et al. [33] | 57 (63%) | NR | NR | 21 (23%) | 14 (15%) | NR | 70 (77%) | 10 (11%) | NR | NR | NR |
| Ballal et al. [32] | 32 (100%) | NR | NR | 10 (31%) | 12 (37%) | NR | 28 (87%) | NR | NR | NR | NR |
| Delpassand et al. [34] | 0 (0%) | 0 (0%) | NR | NR | NR | 0 (0%) | NR | NR | NR | NR | NR |
| Demirci et al. [35] | 10 (91%) | NR | NR | NR | 11 (100%) | NR | 10 (91%) | NR | 2 (18%) | 6 (55%) | NR |
| Kratochwil et al. [36] | 0 (0%) | 7 (100%) | NR | 1 (14%) | 3 (43%) | 1 (14%) | 6 (86%) | 1 (14%) | NR | NR | NR |
| Michler et al. [39] | 12 (100%) | NR | 6 (50%) | NR | 4 (33.3%) | NR | 7 (58.3%) | 4 (33.3%) | NR | 1 (8.3%) | NR |
| Yadav et al. [38] | 7 (78%) | NR | NR | 6 (67%) | 1 (11%) | 5 (55%) | NR | NR | 2 (22%) | NR | NR |
| Author (Year) | Number of Patients, n | Median Follow-Up, Months | Morphologic/Molecular ꝉ Imaging Response, n (%) | ORR, % | DCR, % | Overall Median PFS, Months | Median OS, Months |
|---|---|---|---|---|---|---|---|
| Ballal et al. [33] | 91 | 24 (range: 5–41) | CR: 2 (2.5%) PR: 38 (48.1%) SD: 23 (29.1%) PD: 16 (20.3%) NA: 12 | 50.6% | 79.7% | Not reached | Not reached |
| Ballal et al. [32] | 32 | 8 (range: 2–13) | PR: 15 (62.5%) SD: 9 (37.5%) NA: 8 | 62.5% | 100% | Not reached | Not reached |
| Delpassand et al. [34] | 10 | 17.4 (range: 9–26) | CR: 1 (10%) PR: 7 (70%) SD: 2 (20%) | 80% | 100% | Not reached | Not reached |
| Demirci et al. [35] | 11 | NR | PR: 4 (44.4%) SD: 4 (44.4%) PD: 1 (11.1%) NA: 2 | 44.4% | 88.9% | 12 | Not reported |
| Kratochwil et al. [36] | 7 | Range: 12–34 | CR: 1 (16.7%) PR: 2 (33.3%) SD: 3 (50.0%) NA: 1 | 50% | 100% | Not reached ‡ | Not reached |
| Michler et al. [39] | 12 | 3 | SD: 8 (100.0%) NA: 4 | 0% | 100% | Not reached | Not reached |
| Yadav et al. [38] | 9 | 22.5 (range: 18–28) | PR: 4 (50.0%) SD: 3 (37.5%) PD: 1 (12.5%) NA: 1 | 50% | 87.5% | Not reached | Not reached |
| Yang et al. [37] | 10 | 14 (range: 7–22) | PR: 4 (40%) ꝉ SD: 4 (40%) ꝉ PD: 2 (20%) ꝉ | 40% | 80% | Not reached | Not reached |
| Author/Sponsor (Year) | Trial No. | Study Type | Radiopharmaceutical | Treatment Regimen | Patient Characteristics | Estimated Enrollment | Outcomes |
|---|---|---|---|---|---|---|---|
| Yang Zhi, Peking University Cancer Hospital and Institute (2024) | NCT06732505 | Phase I | [225Ac]Ac-DOTATATE | Dose escalation: Co 1: 90 kBq/kg/cycle Co 2: 120 kBq/kg/cycle Interval of 8 weeks Up to 4 cycles | Inoperable, locally advanced or metastatic, progressive, well differentiated, SSTR+ GEP-NETs (PRRT naïve or with previous PRRT) | 36 | Safety, optimal IA, ORR, PFS |
| RayzeBio, ACTION-1 (2022) | NCT05477576 | Phase Ib/III | [225Ac]Ac-DOTATATE (RYZ101) vs. SoC | Dose escalation: 120 kBq/kg/cycle Interval of 8 weeks Up to 4 cycles | Inoperable, advanced, well-differentiated, SSTR+ GEP-NETs, that have progressed following 177Lu-PRRT | 288 | Safety, optimal IA, PFS |
| Orano Med, Alphamedix02 (2021) | NCT05153772 | Phase II | [212Pb]Pb-DOTAMTATE (AlphaMedix) | 2.5 MBq/kg/cycle | Inoperable or metastatic, progressive, well differentiated, SSTR+ NETs (PRRT naïve or with previous PRRT) | 69 | Safety, mPFS, OS, QoL |
| Markus Puhlmann, Perspective Therapeutics (2023) | NCT05636618 | Phase I/IIa | [212Pb]Pb-VMT-α-NET | Dose escalation: Co 1: 111 MBq/cycle Co 2: 185 MBq/cycle Co 3: 370 MBq/cycle Co 4: 555 MBq/cycle Interval of 8 weeks Up to 4 cycles | Inoperable or metastatic, well differentiated, SSTR+ NETs (PRRT naïve) | 280 | Safety, optimal IA, PFS, OS, dosimetry |
| Joy Zou, Frank Lin, National Cancer Institute (2025) | NCT06479811 NCT06427798 | Phase I/II | [212Pb]Pb-VMT-α-NET | Dose escalation: No injected activity specified Interval of 8 weeks Up to 4 cycles | Inoperable or metastatic, SSTR+ tumors (GEP-NETs, pheo/pgl, SCLC, RCC, H&N cancers) | 120 | Safety, optimal IA, ORR, PFS, OS, dosimetry |
| David Bushnell (2023) | NCT06148636 | Phase I | [212Pb]Pb-VMT-α-NET | Dose escalation: Injected activity calculated based on kidney dose Interval of 8–10 weeks 2 cycles | Inoperable or metastatic, well differentiated, SSTR+ NETs with previous PRRT treatment | 27 | Safety, optimal IA, dosimetry |
| Author (Year) | n | Thrombocytopenia, % (n) | Lymphopenia, % (n) | Anemia, % (n) | Kidney Toxicity, % (n) | Liver Toxicity, % (n) | Clinical Adverse Events | Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade of AE Severity | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | |||
| Ballal et al. [33] | 91 | 17.6% (16) | 1.1% (1) | 5.5% (5) | 0% (0) | 56.0% (51) | 0% (0) | 12.1% (11) | 0% (0) | N/A | 0% (0) | Nausea, vomiting, abdominal pain and distention, diarrhea, gastritis, fatigue, musculoskeletal pain, loss of appetite, headache, flushing, myalgia | Malignant ascites and pleural effusion, no tumor lysis syndrome |
| Ballal et al. [32] | 32 | 6.2% (2) | 0% (0) | 34.4% (11) | 0% (0) | 59.3% (19) | 0% (0) | 3.1% (1) | 0% (0) | N/A | 0% (0) | Nausea, vomiting, abdominal pain and distention, diarrhea, gastritis, fatigue, musculoskeletal pain, loss of appetite, headache, flushing | No tumor-lysis syndrome |
| Delpassand et al. [34] | 10 * | * | * | * | * | * | * | * | * | * | * | Nausea, fatigue * NB. Toxicity reported for the entire population of 20 patients. In the total cohort, 170 AE were reported of which 49 (29%) were grade 2, 7 (5%) grade 3, and none grade 4. | Transient alopecia, worsening achalasia, CVA, hypoglycemia, dyspnea |
| Demirci et al. [35] | 11 | ** | 0% (0) | ** | 0% (0) | ** | 0% (0) | 9.1% (1) | 0% (0) | NR | 0% (0) | NO ** NB. One unspecified grade 2 hematologic toxicity was reported | Ileus |
| Kratochwil et al. [36] | 7 | 14.3% (1) | 0% (0) | 14.3% (1) | 0% (0) | 42.8% (3) | 0% (0) | 14.3% (1) | 0% (0) | NR | NR | NR | Graves’ disease, MDS |
| Michler et al. [39] | 12 | 0% (0) | 0% (0) | 62.5% (5) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | Nausea, vomiting, fatigue | |||
| Yadav et al. [38] | 9 | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 77.8% (7) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | Nausea, stomach discomfort, diarrhea | Palpitations, no tumor-lysis syndrome, no life-threatening hypertension |
| Yang et al. [37] | 10 | 0% (0) | 0% (0) | 20% (2) | 0% (0) | 70% (7) | 0% (0) | 10% (1) | 0% (0) | 0% (0) | 0% (0) | Loss of appetite, nausea and vomiting | No tumor-lysis syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leupe, H.; Cauwenbergh, M.; Cleeren, F.; Dekervel, J.; Verslype, C.; Deroose, C.M. Clinical Experience with Targeted Alpha-Emitter Peptide Receptor Radionuclide Therapy (α-PRRT) for Somatostatin Receptor-Positive Neuroendocrine Tumors. Pharmaceuticals 2025, 18, 1608. https://doi.org/10.3390/ph18111608
Leupe H, Cauwenbergh M, Cleeren F, Dekervel J, Verslype C, Deroose CM. Clinical Experience with Targeted Alpha-Emitter Peptide Receptor Radionuclide Therapy (α-PRRT) for Somatostatin Receptor-Positive Neuroendocrine Tumors. Pharmaceuticals. 2025; 18(11):1608. https://doi.org/10.3390/ph18111608
Chicago/Turabian StyleLeupe, Hannes, Merel Cauwenbergh, Frederik Cleeren, Jeroen Dekervel, Chris Verslype, and Christophe M. Deroose. 2025. "Clinical Experience with Targeted Alpha-Emitter Peptide Receptor Radionuclide Therapy (α-PRRT) for Somatostatin Receptor-Positive Neuroendocrine Tumors" Pharmaceuticals 18, no. 11: 1608. https://doi.org/10.3390/ph18111608
APA StyleLeupe, H., Cauwenbergh, M., Cleeren, F., Dekervel, J., Verslype, C., & Deroose, C. M. (2025). Clinical Experience with Targeted Alpha-Emitter Peptide Receptor Radionuclide Therapy (α-PRRT) for Somatostatin Receptor-Positive Neuroendocrine Tumors. Pharmaceuticals, 18(11), 1608. https://doi.org/10.3390/ph18111608