Cyclodextrins as Active Therapeutic Agents: Beyond Their Role as Excipients
Abstract
1. Introduction
2. Cyclodextrins: Physicochemical Properties and Biological Mechanisms
2.1. Physicochemical Properties
2.2. Inclusion Complex Formation
3. Engineering and Design of Cyclodextrin Derivatives
3.1. Monosubstitution of CDs
3.2. Two-Position Modification
3.3. Three-Position Displacement
3.4. Mechanistic Basis of Cyclodextrin Biological and Therapeutic Effects
4. Safety Profile and Toxicity
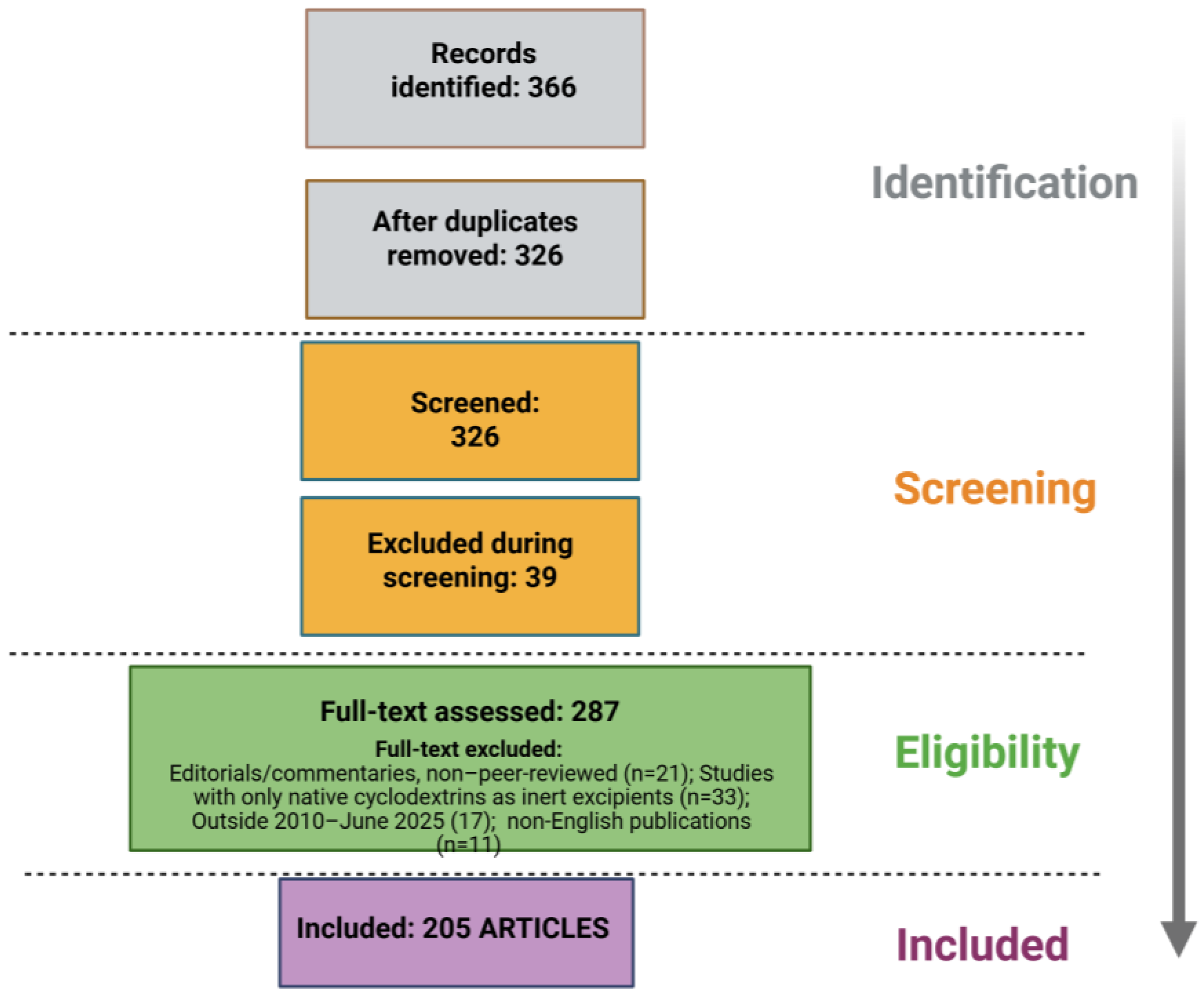
5. Methods
6. Application in Therapy
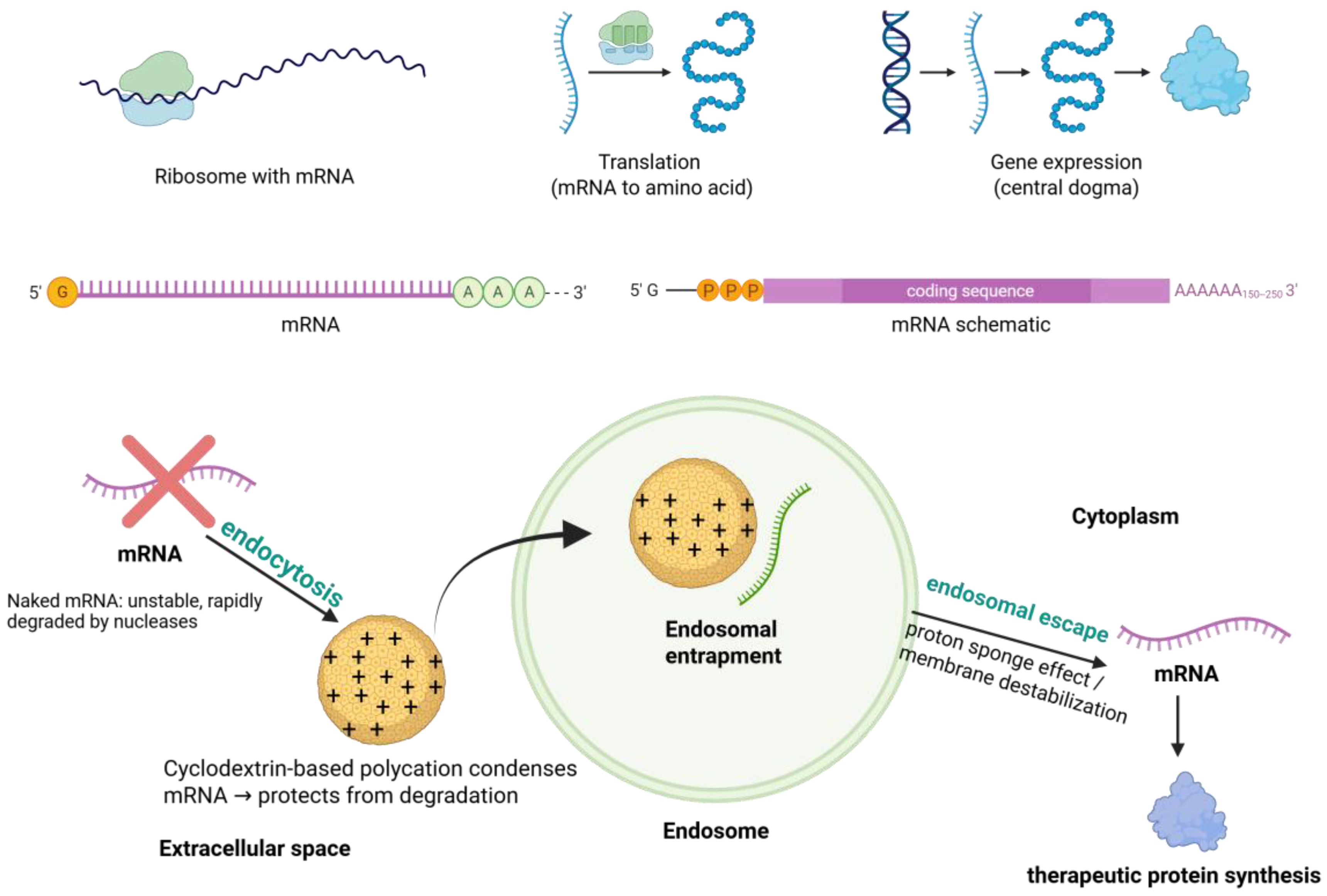
6.1. Cyclodextrin-Based Systems for Gene and mRNA Delivery
- Encapsulation and Protection from Nucleases. Cyclodextrins can encapsulate or complex nucleic acids to shield them from enzymatic degradation. CD-based polycations form polyplexes with DNA, siRNA, or mRNA, prolonging nucleic acid half-life and ensuring that a meaningful fraction of the dose reaches target cells [105,106,107,108].
- Enhanced Cellular Uptake and Endosomal Escape. When conjugated with cationic molecules, CDs condense nucleic acids into nanoparticles that cells can internalize via endocytosis. CDs may also transiently modulate membranes to facilitate uptake. Once inside endosomes, CD-based carriers can promote cytosolic release through mechanisms such as the proton sponge effect or pH-sensitive linkages, preventing degradation in endo-lysosomal compartments [109,110,111].
- Chemically Modified CDs and Polyrotaxane Architectures. Native CDs have limited affinity for large nucleic acids, but cationic modifications or polyrotaxane structures enhance binding and condensation. These supramolecular architectures increase complex stability while enabling controlled disassembly and release [112,113,114,115].
- Stimuli-Responsive and Targeted Delivery. A crucial advantage of cyclodextrin carriers is their favorable safety profile. CDs are derived from starch, and many derivatives (e.g., hydroxypropyl-β-CD) are already FDA-approved excipients with low toxicity and immunogenicity. Incorporating CDs into cationic polymers can mitigate polymer-induced cytotoxicity, and biodegradable CD-based polymers degrade into non-toxic sugars. This supports repeat administration, an essential requirement for chronic gene therapy or mRNA vaccination [116,117,118]. CD-based systems can be designed with pH- or enzyme-sensitive linkages that trigger release in the acidic endosome or protease-rich tumor microenvironment. Ligands such as folate, transferrin, or antibodies can be attached for cell-specific targeting, improving therapeutic precision (Figure 4) [119,120].
6.2. Cyclodextrin-Based Delivery of siRNA
6.3. Clinical Relevance and Translational Outlook
6.4. Cystic Fibrosis (CF)
6.5. Duchenne Muscular Dystrophy (DMD)
6.6. Infectious Diseases (Vaccines)
6.7. Cyclodextrins and Lipid Homeostasis
6.8. Cyclodextrins in Neurodegenerative Disease Therapy: Roles as Delivery Enhancers and Therapeutic Facilitators
6.8.1. Exploring Intranasal Administration as a Brain-Targeting Route for HPbCD
6.8.2. Niemann–Pick Disease
6.8.3. Alzheimer’s Disease
6.8.4. Parkinson’s Disease
6.9. Cyclodextrin-Based Drug Delivery Systems for Cancer Therapy
Lung Cancer
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J.; Sohajda, T. Analytical Characterization of Cyclodextrins: History, Official Methods and Recommended New Techniques. J. Pharm. Biomed. Anal. 2016, 130, 347–365. [Google Scholar] [CrossRef]
- Spiridon, I.; Anghel, N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules 2025, 30, 3044. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. The Contribution of Franz Schardinger to Cyclodextrins: A Tribute on the Occasion of the Centenary of His Death. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 19–28. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- French, D. The Schardinger Dextrins. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1957; Volume 12, pp. 189–260. [Google Scholar]
- Martin, J.; Díaz-Montaña, E.J.; Asuero, A.G. Cyclodextrins: Past and Present; IntechOpen: London, UK, 2018; ISBN 978-1-78923-069-7. [Google Scholar]
- Kfoury, M.; Lichtfouse, E.; Fourmentin, S. The revival of cyclodextrins as active pharmaceutical ingredients. Environ. Chem. Lett. 2025, 23, 1–6. [Google Scholar] [CrossRef]
- Cramer, F. Einschlussverbindungen; Springer: Berlin/Heidelberg, Germany, 1954. [Google Scholar]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A. Thirty Years with Cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef]
- di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2016, 22, 1. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Ali, R. (Ed.) Cyclodextrins—Core Concepts and New Frontiers; IntechOpen: London, UK, 2023. [Google Scholar]
- Gotsev, M.G.; Ivanov, P.M.; Jaime, C. Molecular dynamics study of the conformational dynamics and energetics of some large-ring cyclodextrins (CDn, n = 24, 25, 26, 27, 28, 29). Chirality 2007, 19, 203–213. [Google Scholar] [CrossRef]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. α-Cyclodextrin: How Effectively Can Its Hydrophobic Cavity Be Hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-Cyclodextrin Cavity: Amount, Stability and Mechanism of Binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β–Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef]
- Galindres, D.M.; Espitia-Galindo, N.; Valente, A.J.M.; Sofio, S.P.C.; Rodrigo, M.M.; Cabral, A.M.T.D.P.V.; Esteso, M.A.; Zapata-Rivera, J.; Vargas, E.F.; Ribeiro, A.C.F. Interactions of Sodium Salicylate with β–Cyclodextrin and an Anionic Resorcin[4]arene: Mutual Diffusion Coefficients and Computational Study. Int. J. Mol. Sci. 2023, 24, 3921. [Google Scholar] [CrossRef]
- Sierpe, R.; Donoso-González, O.; Lang, E.; Noyong, M.; Simon, U.; Kogan, M.J.; Yutronic, N. Solid-State Formation of a Potential Melphalan Delivery Nanosystem Based on β–Cyclodextrin and Silver Nanoparticles. Int. J. Mol. Sci. 2023, 24, 3990. [Google Scholar] [CrossRef] [PubMed]
- Sangkhawasi, M.; Kerdpol, K.; Ismail, A.; Nutho, B.; Hanpiboon, C.; Wolschann, P.; Krusong, K.; Rungrotmongkol, T.; Hannongbua, S. In Vitro and In Silico Study on the Molecular Encapsulation of α–Tocopherol in a Large-Ring Cyclodextrin. Int. J. Mol. Sci. 2023, 24, 4425. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, G.; Zhang, X.; Shi, R.; Chen, R.; Zhang, X.; Peng, Y.; Yang, H.; Lu, Y.; Song, C. β-Cyclodextrin Polymer-Based Fluorescence Enhancement Strategy via Host–Guest Interaction for Sensitive Assay of SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 7174. [Google Scholar] [CrossRef] [PubMed]
- Araj, S.K.; Szeleszczuk, Ł. A Review on Cyclodextrins/Estrogens Inclusion Complexes. Int. J. Mol. Sci. 2023, 24, 8780. [Google Scholar] [CrossRef]
- Escobedo-González, R.G.; Moyers-Montoya, E.D.; Martínez-Pérez, C.A.; García-Casillas, P.E.; Miranda-Ruvalcaba, R.; Nicolás-Vázquez, M.I.N. In Silico Study of Novel Cyclodextrin Inclusion Complexes of Polycaprolactone and Its Correlation with Skin Regeneration. Int. J. Mol. Sci. 2023, 24, 8932. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Najlaoui, F.; McGlinchey, M.J.; Sanz García, J.; Jaouen, G.; Gibaud, S. Unravelling the Role of Uncommon Hydrogen Bonds in Cyclodextrin Ferrociphenol Supramolecular Complexes: A Computational Modelling and Experimental Study. Int. J. Mol. Sci. 2023, 24, 12288. [Google Scholar] [CrossRef] [PubMed]
- Pilipović, A.; Tepavčević, V.; Kumar, D.; Poša, M. Cyclodextrins, Surfactants and Their Inclusion Complexes. Molecules 2025, 30, 3944. [Google Scholar] [CrossRef]
- Wenz, G. Cyclodextrins as building blocks for supramolecular structures and functional units. Angew. Chem. Int. Ed. 1994, 33, 803–822. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Arslan, M.; Sayin, S.; Yilmaz, M. Enantioselective sorption of some chiral carboxylic acids by various cyclodextringrafted iron oxide magnetic nanoparticles. Tetrahedron Asymmetry 2013, 24, 982–989. [Google Scholar] [CrossRef]
- Li, S.; Taura, D.; Hashidzume, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Photocontrolled size changes of doubly-threaded dimer based on an α-cyclodextrin derivative with two recognition sites. Chem. Lett. 2010, 39, 242–243. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, C.; Zhang, H.; Zhao, Y. Enantioselective recognition of aliphatic amino acids by β-cyclodextrin derivatives bearing aromatic organoselenium moieties on the primary or secondary side. Eur. J. Org. Chem. 2003, 2003, 1415–1422. [Google Scholar] [CrossRef]
- Casas-Solvas, J.M.; Vargas-Berenguel, A. Synthesis of a β-cyclodextrin derivative bearing an azobenzene group on the secondary face. Tetrahedron Lett. 2008, 49, 6778–6780. [Google Scholar] [CrossRef]
- Pitha, J.; Harman, S.M.; Michel, M.E. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J. Pharm. Sci. 1986, 75, 165–167. [Google Scholar] [CrossRef]
- Zhao, M.; Hao, A.; Li, J.; Wei, Y.; Guo, P. New cyclomaltoheptaose (β-cyclodextrin) derivative 2-O-(2-hydroxybutyl)cyclomaltoheptaose: Preparation and its application for the separation of enantiomers of drugs by capillary electrophoresis. Carbohydr. Res. 2005, 340, 1563–1565. [Google Scholar] [CrossRef]
- Zheng, L.; Xiong, L.; Li, J.; Li, X.; Sun, J.; Yang, S.; Xia, J. Synthesis of a novel β-cyclodextrin derivative with high solubility and the electrochemical properties of ferrocene-carbonyl-β-cyclodextrin inclusion complex as an electron transfer mediator. Electrochem. Commun. 2008, 10, 340–345. [Google Scholar] [CrossRef]
- Martina, K.; Trotta, F.; Robaldo, B.; Belliardi, N.; Jicsinszky, L.; Cravotto, G. Efficient regioselective functionalizations of cyclodextrins carried out under microwaves or power ultrasound. Tetrahedron Lett. 2007, 48, 9185–9189. [Google Scholar] [CrossRef]
- Suzuki, I.; Ueno, A.; Osa, T. Marked differences in molecular association behavior between two regioisomers of γ-cyclodextrin derivatives bearing a pyrenecarbonyl moiety at C-2 and C-3. Chem. Lett. 1989, 18, 2013–2016. [Google Scholar] [CrossRef]
- Sallas, F.; Leroy, P.; Marsura, A.; Nicolas, A. First selective synthesis of thio-β-cyclodextrin derivatives by a direct Mitsunobu reaction on free β-cyclodextrin. Tetrahedron Lett. 1994, 35, 6079–6082. [Google Scholar] [CrossRef]
- Delahousse, G.; Peulon-Agasse, V.; Debray, J.C.; Vaccaro, M.; Cravotto, G.; Jabin, I.; Cardinael, P. The incorporation of calix[6]arene and cyclodextrin derivatives into sol-gels for the preparation of stationary phases for gas chromatography. J. Chromatogr. A 2013, 1318, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Schurig, V. Enantiomer separation using mobile and immobile cyclodextrin derivatives with electromigration. Electrophoresis 1994, 15, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ong, T.; Ng, S.C. Chemically bonded cationic β-cyclodextrin derivatives as chiral stationary phases for enantioseparation applications. Tetrahedron Lett. 2012, 53, 2312–2315. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Tan, T.T.Y.; Ng, S.C. Click chemistry for facile immobilization of cyclodextrin derivatives onto silica as chiral stationary phases. Tetrahedron Lett. 2008, 49, 5190–5191. [Google Scholar] [CrossRef]
- Lopez, O.L.; Marinescu, L.; Bols, M. New cup-shaped α-cyclodextrin derivatives and a study of their catalytic properties in oxidation reactions. Tetrahedron 2007, 63, 8872–8880. [Google Scholar] [CrossRef]
- Li, Y.; Song, C.; Zhang, L.; Zhang, W.; Fu, H. Fabrication and evaluation of chiral monolithic column modified by β-cyclodextrin derivatives. Talanta 2010, 80, 1378–1384. [Google Scholar] [CrossRef]
- Zhang, X.; Sasaki, K.; Kuroda, Y. Syntheses and photophysical studies of cyclodextrin derivatives with two proximate anthracenyl groups. J. Org. Chem. 2006, 71, 4872–4877. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.; Xu, D.; Zhang, J. Enantiomeric separation in high-performance liquid chromatography using novel β-cyclodextrin derivatives modified by R-configuration groups as chiral stationary phases. Talanta 2011, 84, 1080–1092. [Google Scholar] [CrossRef]
- Le, H.T.; Jeon, H.M.; Lim, C.W.; Kim, T.W. 6-Triazolyl-6-deoxy-β-cyclodextrin derivatives: Synthesis, cellular toxicity, and phase-solubility study. Carbohydr. Res. 2014, 391, 22–28. [Google Scholar] [CrossRef]
- Stepniak, P.; Lainer, B.; Chmurski, K.; Jurczak, J. The effect of urea moiety in amino acid binding by β–cyclodextrin derivatives: A 1000-fold increase in efficacy comparing to native β-cyclodextrin. Carbohydr. Polym. 2017, 164, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Seguin, C.; Brion, A.; Lavalle, P.; Schaaf, P.; Fournel, S.; Bourel-Bonnet, L.; Frisch, B.; De Giorgi, M. β-Cyclodextrin-functionalized chitosan/alginate compact polyelectrolyte complexes (CoPECs) as functional biomaterials with anti-inflammatory properties. ACS Appl. Mater. Interfaces 2018, 10, 29347–29356. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Hong, S.; Martin, K.A.; Czarnik, A.W. A general method for the synthesis of cyclodextrinyl aldehydes and carboxylic acids. J. Org. Chem. 1995, 60, 2792–2795. [Google Scholar] [CrossRef]
- Di Fabio, G.; Malgieri, G.; Isernia, C.; D’Onofrio, J.; Gaglione, M.; Messere, A.; Zarrelli, A.; De Napoli, L. A novel synthetic strategy for monosubstituted cyclodextrin derivatives. Chem. Commun. 2012, 48, 3875–3877. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, H.; Liu, S.; Xu, H.; Huang, J.; Yu, J. Hollow nanosphere fabricated from β-cyclodextrin-grafted α,β-poly(aspartic acid) as the carrier of camptothecin. Colloids Surf. B Biointerfaces 2013, 105, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Singh, A.P.; Ray, B.; Aswal, V.; Kar, A.G.; Maiti, P. Efficacy of polyurethane graft on cyclodextrin to control drug release for tumor treatment. J. Colloid Interface Sci. 2019, 534, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Karginov, V.A.; Nestorovich, E.M.; Schmidtmann, F.; Robinson, T.M.; Yohannes, A.; Fahmi, N.E.; Bezrukov, S.M.; Hecht, S.M. Inhibition of S. aureus α-hemolysin and B. anthracis lethal toxin by β-cyclodextrin derivatives. Bioorg. Med. Chem. 2007, 15, 5424–5431. [Google Scholar] [CrossRef]
- Martina, K.; Caporaso, M.; Tagliapietra, S.; Heropoulos, G.; Rosati, O.; Cravotto, G. Synthesis of water-soluble multidentate aminoalcohol β-cyclodextrin derivatives via epoxide opening. Carbohydr. Res. 2011, 346, 2677–2682. [Google Scholar] [CrossRef]
- Grasso, G.I.; Bellia, F.; Arena, G.; Vecchio, G.; Rizzarelli, E. Noncovalent interaction-driven stereoselectivity of copper(II) complexes with cyclodextrin derivatives of L- and D-carnosine. Inorg. Chem. 2011, 50, 4917–4924. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Yang, X.; Liao, X.; Yang, J.; Zhang, J.-H.; Gao, C.-Z. Scutellarin-cyclodextrin conjugates: Synthesis, characterization and anticancer activity. Carbohydr. Polym. 2013, 92, 1308–1314. [Google Scholar] [CrossRef]
- Li, Y.; Ha, Y.; Guo, Q.; Li, Q. Synthesis of two β-cyclodextrin derivatives containing a vinyl group. Carbohydr. Res. 2015, 404, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Moutard, S.; Djedaïni-Pilard, F.; Meudal, S.; Luijten, W.; Perly, B.; Pilard, S. Structural identification of new glycolipids based on cyclodextrin using high-resolution positive and negative electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Bjerre, J.; Hauch Fenger, T.; Marinescu, L.G.; Bols, M. Synthesis of some trifluoromethylated cyclodextrin derivatives and analysis of their properties as artificial glycosidases and oxidases. Eur. J. Org. Chem. 2007, 2007, 704–710. [Google Scholar] [CrossRef]
- Takenaka, Y.; Nakashima, H.; Yoshida, N. Fluorescent amino-β-cyclodextrin derivative as a receptor for various types of alcohols having cyclic and macrocyclic rings. J. Mol. Struct. 2007, 871, 149–155. [Google Scholar] [CrossRef]
- Wang, R.; Ong, T.; Ng, S.C. Chemically bonded cationic β-cyclodextrin derivatives and their applications in supercritical fluid chromatography. J. Chromatogr. A 2012, 1224, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Casas-Solvas, J.M.; Martos-Maldonado, M.C.; Vargas-Berenguel, A. Synthesis of β-cyclodextrin derivatives functionalized with azobenzene. Tetrahedron 2008, 64, 10919–10923. [Google Scholar] [CrossRef]
- Alvarez-Dorta, D.; León, E.I.; Kennedy, A.R.; Martín, A.; Pérez-Martín, I.; Suárez, E. Easy access to modified cyclodextrins by an intramolecular radical approach. Angew. Chem. Int. Ed. 2015, 54, 3674–3678. [Google Scholar] [CrossRef]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Masson, M.; Jarvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Varan, G. Cyclodextrin in Vaccines: Enhancing Efficacy and Stability. Future Pharmacol. 2023, 3, 597–611. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
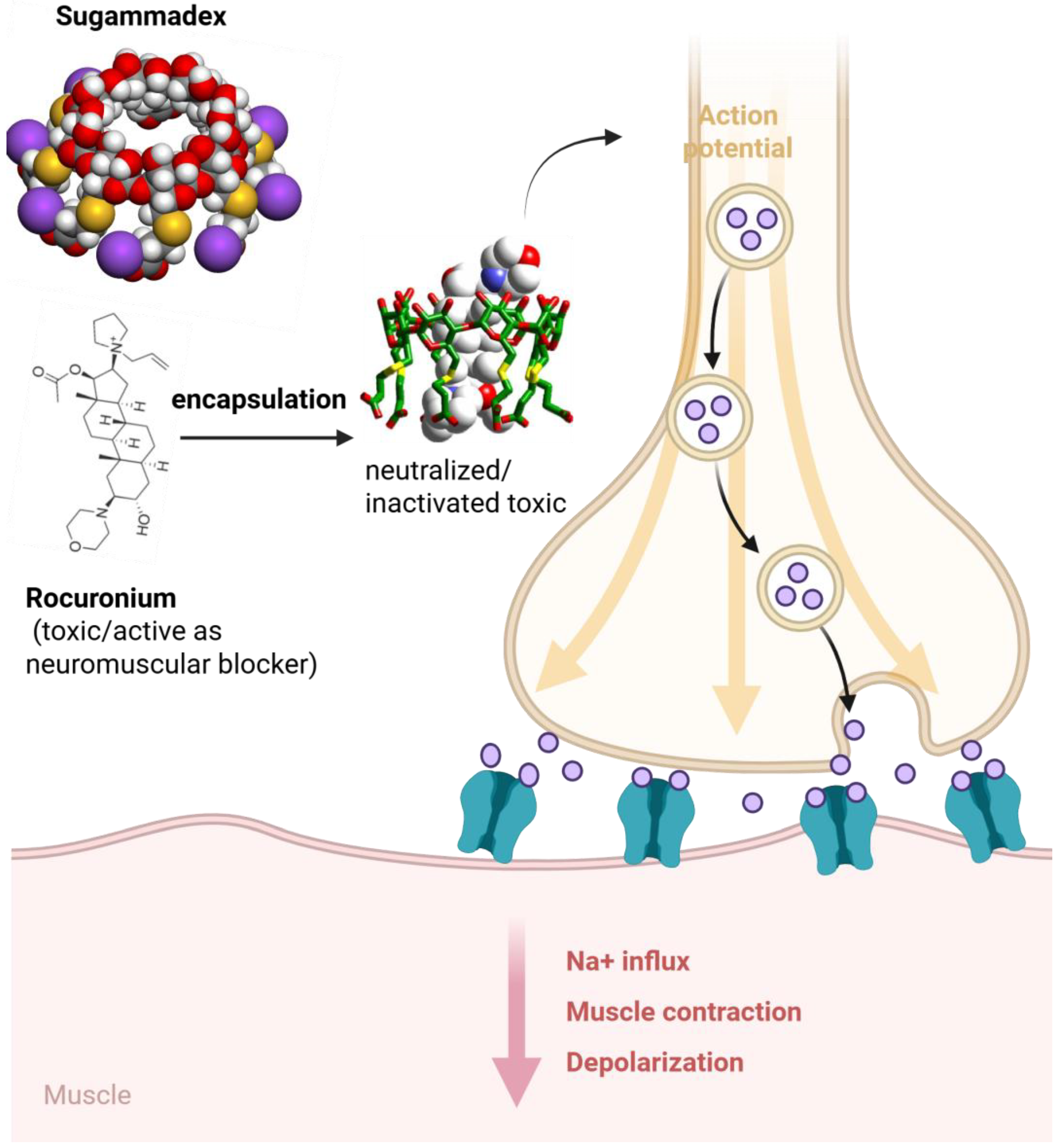
- Baldo, B.A.; McDonnell, N.J.; Pham, N.H. Drug-specific cyclodextrins with emphasis on sugammadex, the neuromuscular blocker rocuronium and perioperative anaphylaxis: Implications for drug allergy. Clin. Exp. Allergy. 2011, 41, 1663–1678. [Google Scholar] [CrossRef]
- Nag, K.; Singh, D.R.; Shetti, A.N.; Kumar, H.; Sivashanmugam, T.; Parthasarathy, S. Sugammadex: A revolutionary drug in neuromuscular pharmacology. Anesth. Essays Res. 2013, 7, 302–306. [Google Scholar]
- Khalid, F.M.; Ijaz, M.; Mahmood, A.; Waqas, M.K.; Hussain, T.; Asim, M.H.; Ahmad, N.; Arshad, S.; Rehman, M.U.; Nazir, I. Mucoadhesive, Fluconazole-Loaded Nanogels Complexed with Sulfhydryl-β-cyclodextrin for Oral Thrush Treatment. AAPS PharmSciTech 2023, 24, 194. [Google Scholar] [CrossRef]
- Clemence, B.F.; Xiao, L.; Yang, G. Oral Administration of Berberine Hydrochloride Based on Chitosan/Carboxymethyl-β-Cyclodextrin Hydrogel. Polymers 2024, 16, 27. [Google Scholar] [CrossRef]
- Badilli, U.; Amasya, G.; Sen, T.; Tarimci, N. Topical emulgel formulation containing inclusion complex of calcipotriol with cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 249–255. [Google Scholar] [CrossRef]
- Singh, R.M.; Kumar, A.; Pathak, K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS PharmSciTech 2013, 14, 412–424. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Gill, E.J. Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery. Gels 2025, 11, 177. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.M.; Oliveri, V.; McGown, A.; Kaya, E.; Chen, Y.L.; Austin, C.; Walker, M.; Platt, F.M.; Vecchio, G.; Spencer, J. Synthesis and Study of Multifunctional Cyclodextrin-Deferasirox Hybrids. ChemMedChem 2019, 14, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use; EMA: London, UK, 2017; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-and-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 30 September 2025).
- U.S. Food and Drug Administration (FDA). Pharmacology/Toxicology Review: Sulfobutylether-β-Cyclodextrin (SBECD), NDA 214787; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/214787Orig1s000PharmR.pdf (accessed on 30 September 2025).
- U.S. Food and Drug Administration (FDA). Drug Approval Package: Bridion (Sugammadex). Clinical Pharmacology and Biopharmaceutics Review(s); FDA: Silver Spring, MD, USA, 2015. [Google Scholar]
- Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39 (Suppl. S1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Kraszni, M.; Ágh, F.; Horváth, D.; Mirzahosseini, A.; Horváth, P. Effect of Substitution Degree and Homogeneity on Cyclodextrin-Ligand Complex Stability: Comparison of Fenbufen and Fenoprofen Using CD and NMR Spectroscopy. Int. J. Mol. Sci. 2023, 24, 7544. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Kopnova, T.Y.; Le, N.T.; Belogurova, N.G.; Kudryashova, E.V. Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin’s Antibacterial Activity In Vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Chen, Q.; Huo, K.G.; Ji, S.M.; Pang, S.D.; Sun, T.Y.; Niu, Y.; Jiang, Z.H.; Zhang, P.; Han, S.X.; Li, J.Y. Unleashing the potential of mRNA: Overcoming delivery challenges with nanoparticles. Bioeng. Transl. Med. 2024, 10, e10713. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the messenger: Advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Gómez-Aguado, I.; Rodríguez-Castejón, J.; Vicente-Pascual, M.; Rodríguez-Gascón, A.; Solinís, M.Á.; del Pozo-Rodríguez, A. Nanomedicines to deliver mRNA: State of the art and future perspectives. Nanomaterials 2020, 10, 364. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef]
- Wright, K.J.; Badwaik, V.D.; Samaddar, S.; Hyun, S.-H.; Glauninger, K.; Eom, T.; Thompson, D.H. Organocatalytic synthesis and evaluation of polycarbonate pendant polymer: β-Cyclodextrin-based nucleic acid delivery vectors. Macromolecules 2018, 51, 670–678. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Garcia Fernandez, J.M.; Benito, J.M. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011, 40, 1586–1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, X.; Dou, Y.; He, B.; Liu, L.; Wei, Z.; Li, J.; Wang, C.; Mao, C.; Zhang, J.; et al. A pH-responsive cyclodextrin-based hybrid nanosystem as a nonviral vector for gene delivery. Biomaterials 2013, 34, 4159–4172. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Wu, Y.-L. Cationic star copolymers based on β-cyclodextrins for efficient gene delivery to mouse embryonic stem cell colonies. Chem. Commun. 2015, 51, 10815–10818. [Google Scholar] [CrossRef]
- Davis, M.E. The first targeted delivery of siRNA in humans via a Self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Nature. HIV vaccine failure prompts Merck to halt trial. Nature 2007, 449, 390. [Google Scholar] [CrossRef]
- Ledford, H. HIV vaccine may raise risk. Nature 2007, 450, 325. [Google Scholar] [CrossRef]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and Immunological Effects of MRNA Vaccines in Malignant Diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic MRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Revdekar, A.; Salvi, B.V.; Shende, P. Active Transfection of Genetic Materials Using Cyclodextrin-Anchored Nanovectors. Mater. Adv. 2024, 5, 9548–9564. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Godinho, B.M.D.C.; Ogier, J.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Cyclodextrin-Based siRNA Delivery Nanocarriers: A State-of-the-Art Review. Expert Opin. Drug Deliv. 2012, 9, 1293–1304. [Google Scholar]
- Chaturvedi, K.; Ganguly, K.; Kulkarni, A.R.; Kulkarni, V.H.; Koppolu, B.P.; Chalasani, K.B.; Shah, J.C.; Patil, P. Folate Appended Cyclodextrins for Drug, DNA, and siRNA Delivery. Eur. J. Pharm. Biopharm. 2018, 132, 45–60. [Google Scholar]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in Humans from Systemically Administered siRNA via Targeted Nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Wang, X.; Zhou, Z.; Zhang, T.; Liu, J.; Song, C. Cyclodextrin-Based Polyplexes for siRNA Delivery with High Gene-Silencing Efficiency and Low Toxicity. Mol. Pharm. 2016, 13, 1729–1739. [Google Scholar]
- Godinho, B.M.D.C.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Self-Assembling Modified β-Cyclodextrin Nanoparticles as Neuronal siRNA Delivery Vectors: Focus on Huntington’s Disease. Mol. Pharm. 2013, 10, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Gazzali, A.M.; Bakhtiar, A. Clinical Advances of siRNA-Based Nanotherapeutics for Cancer Treatment. Pharmaceutics 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.; Pei, Y.; Xu, J.; Yeo, Y. Pharmacokinetics and Biodistribution of Recently-Developed siRNA Nanomedicines. Adv. Drug Deliv. Rev. 2016, 104, 93–109. [Google Scholar] [CrossRef]
- Drouin, M.; Saenz, J.; Chiffoleau, E. C-Type Lectin-Like Receptors: Head or Tail in Cell Death Immunity. Front. Immunol. 2020, 11, 251. [Google Scholar] [CrossRef]
- Yan, H.; Kamiya, T.; Suabjakyong, P.; Tsuji, N.M. Targeting C-Type Lectin Receptors for Cancer Immunity. Front. Immunol. 2015, 6, 408. [Google Scholar] [CrossRef]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy-A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef]
- Yin, J.J.; Zhou, Z.W.; Zhou, S.F. Cyclodextrin-based targeting strategies for tumor treatment. Drug Deliv. Transl. Res. 2013, 3, 364–374. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Lee, A.; Dadhwal, S.; Gamble, A.; Hook, S. Liposomes with cyclodextrin channels and polyethyleneimine (PEI) improves cytoplasmic vaccine delivery and induces anti-cancer immune activity in mice. J. Liposome Res. 2022, 32, 22–31. [Google Scholar] [CrossRef]
- Li, B.L.; Zhang, J.; Jin, W.; Chen, X.Y.; Yang, J.M.; Chi, S.M.; Ruan, Q.; Zhao, Y. Oral administration of pH-responsive polyamine modified cyclodextrin nanoparticles for controlled release of anti-tumor drugs. React. Funct. Polym. 2022, 172, 105175–105187. [Google Scholar] [CrossRef]
- Nazli, A.; Malanga, M.; Sohajda, T.; Béni, S. Cationic Cyclodextrin-Based Carriers for Drug and Nucleic Acid Delivery. Pharmaceutics 2025, 17, 81. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, R.; Koeppen, K.; Stanton, B.A. Cyclodextrins reduce the ability of Pseudomonas aeruginosa outer-membrane vesicles to reduce CFTR Cl− secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L206–L215. [Google Scholar] [CrossRef] [PubMed]
- Cerri, L.; Migone, C.; Vizzoni, L.; Grassiri, B.; Fabiano, A.; Piras, A.M.; Zambito, Y. Cross-Linked Thiolated Hydroxypropil-β-Cyclodextrin for Pulmonary Drug Delivery. Int. J. Mol. Sci. 2024, 25, 9394. [Google Scholar] [CrossRef] [PubMed]
- Cutrignelli, A.; Sanarica, F.; Lopalco, A.; Lopedota, A.; Laquintana, V.; Franco, M.; Boccanegra, B.; Mantuano, P.; De Luca, A.; Denora, N. Dasatinib/HP-β-CD Inclusion Complex Based Aqueous Formulation as a Promising Tool for the Treatment of Paediatric Neuromuscular Disorders. Int. J. Mol. Sci. 2019, 20, 591. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Homami, S.S.; Monzavi, A.; Olyai, M.R.T.B. Synthesis and characterization of ataluren-cyclodextrins complexes. J. Mol. Struct. 2023, 1272, 134053. [Google Scholar] [CrossRef]
- Mantuano, P.; Boccanegra, B.; Conte, E.; De Bellis, M.; Cirmi, S.; Sanarica, F.; Cappellari, O.; Arduino, I.; Cutrignelli, A.; Lopedota, A.A.; et al. β-Dystroglycan Restoration and Pathology Progression in the Dystrophic mdx Mouse: Outcome and Implication of a Clinically Oriented Study with a Novel Oral Dasatinib Formulation. Biomolecules 2021, 11, 1742. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Sergeeva, O.V.; Koteliansky, V.E.; Zatsepin, T.S. mRNA-Based Therapeutics—Advances and Perspectives. Biochemistry 2016, 81, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics 2020, 10, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; An, T.; Hong, K.-J. Revolutionizing mRNA Vaccines Through Innovative Formulation and Delivery Strategies. Biomolecules 2025, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J. Pharm. Investig. 2023, 53, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Calias, P.; Hansen, K.M.; Bullock, K.M.; Engelke, K. Brain uptake and distribution patterns of 2-hydroxypropyl-β-cyclodextrin after intrathecal and intranasal administration. J. Pharm. Pharmacol. 2022, 74, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Soddu, E.; Bayrakdar, E.T.; Uyanikgil, Y.; Kanit, L.; Armagan, G.; Rassu, G.; Gavini, E.; Giunchedi, P. Neuroprotective effects of engineered polymeric nasal microspheres containing hydroxypropyl-β-cyclodextrin on β-amyloid (1-42)–induced toxicity. J. Pharm. Sci. 2016, 105, 2372–2380. [Google Scholar] [CrossRef]
- Orphanet. Hydroxy-Propyl-Beta-Cyclodextrin. Available online: https://www.orpha.net/en/drug/substance/327849?name=&mode=®ion=&status= (accessed on 18 September 2025).
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Chin, J.; Hoffman, A.; Winston, A.; Stoner, R.; LaGorio, L.; Friedmann, K.; Hernandez, M.; Ory, D.S.; Porter, F.D.; et al. Long-Term Treatment of Niemann-Pick Type C1 Disease with Intrathecal 2-Hydroxypropyl-β-Cyclodextrin. Pediatr. Neurol. 2018, 80, 24–34. [Google Scholar] [CrossRef]
- Yuan, T.L.; Fellmann, C.; Lee, C.S.; Ritchie, C.D.; Thapar, V.; Lee, L.C.; Hsu, D.J.; Grace, D.; Carver, J.O.; Zuber, J.; et al. Development of siRNA Payloads to Target KRAS-Mutant Cancer. Cancer Discov. 2014, 4, 1182–1197. [Google Scholar] [CrossRef]
- Symens, N.; Méndez-Ardoy, A.; Díaz-Moscoso, A.; Sánchez-Fernández, E.; Remaut, K.; Demeester, J.; García Fernández, J.M.; De Smedt, S.C.; Rejman, J. Efficient Transfection of Hepatocytes Mediated by mRNA Complexed to Galactosylated Cyclodextrins. Bioconjug. Chem. 2012, 23, 1276–1289. [Google Scholar] [CrossRef]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the Cholesterol-Lowering Compound Methyl-β-Cyclodextrin in Models of α-Synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Nam, J.M.; Sabe, H. Intracellular Trafficking of Folate-Conjugated Cyclodextrin Nanoparticles Delivering Therapeutic Oligonucleotides in Cancer Cells. Sci. Rep. 2013, 3, 1104. [Google Scholar]
- Farmer, C.A.; Thurm, A.; Farhat, N.; Bianconi, S.; Keener, L.A.; Porter, F.D. Long-Term Neuropsychological Outcomes from an Open-Label Phase I/IIa Trial of 2-Hydroxypropyl-β-Cyclodextrins (VTS-270) in Niemann-Pick Disease, Type C1. CNS Drugs 2019, 33, 677–683. [Google Scholar] [CrossRef] [PubMed]
- VTS-270 to Treat Niemann-Pick Type C1 (NPC1) Disease—NCT02534844. Available online: https://clinicaltrials.gov/study/NCT02534844 (accessed on 18 September 2025).
- Van Meter, S.; Mallinckrodt Pharmaceuticals. Clinical update on intrathecal VTS-270 for the treatment of Niemann-Pick Disease. Michael, Marcia, and Christa Parseghian Scientific Conference for Niemann-Pick Type C Research, 3–6 June 2017. Available online: https://www.parseghian.org/scientificmeeting.html (accessed on 18 September 2025).
- Ding, D.; Jiang, H.; Salvi, R. Cochlear spiral ganglion neuron degeneration following cyclodextrin-induced hearing loss. Hear. Res. 2021, 400, 108125. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Molecular Mind Games: The Medicinal Action of Cyclodextrins in Neurodegenerative Diseases. Biomolecules 2023, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Yergey, A.L.; Blank, P.S.; Cologna, S.M.; Backlund, P.S.; Porter, F.D.; Darling, A.J. Characterization of Hydroxypropyl-β-Cyclodextrins Used in the Treatment of Niemann–Pick Disease Type C1. PLoS ONE 2017, 12, e0175478. [Google Scholar] [CrossRef]
- Message from Mallinckrodt Concerning Adrabetadex Transition. Available online: https://cdn.clinicaltrials.gov/large-docs/42/NCT04958642/Prot_000.pdf (accessed on 20 September 2025).
- Quandt, K. The FDA Needs to be more Flexible in Assessing Treatments for Rare Diseases, Like the One that Seemed to Help My Son. Stat News, 7 September 2022. Available online: https://www.statnews.com/2022/09/07/the-fda-needs-to-be-more-flexible-in-assessing-treatments-for-rare-diseases-like-the-one-that-seemed-to-help-my-son/ (accessed on 20 September 2025).
- Mandos Health Monthly Communication. May 2022. Available online: https://mandoshealth.com/communications/May_1_2022 (accessed on 20 September 2025).
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, J.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef]
- Banks, W.A.; Engelke, K.; Hansen, K.; Bullock, K.; Calias, P. Modest blood-brain barrier permeability of the cyclodextrin Kleptose: Modification by efflux and luminal surface binding. J. Pharmacol. Exp. Ther. 2019, 371, 121–129. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhao, J.; Fu, Y.; Inoue, Y.; Ren, Y.; Chen, Y.; Doss, S.V.; Shue, F.; Jeevaratnam, S.; Bastea, L.; et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat. Neurosci. 2022, 25, 1020–1033. [Google Scholar] [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Beal, M.F. Neuroprotective effects of cyclodextrin in Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, P714–P715. [Google Scholar] [CrossRef]
- Borsche, M.; Pereira, S.L.; Klein, C.; Grünewald, A. Mitochondria and Parkinson’s disease: Clinical, molecular, and translational aspects. J. Park. Dis. 2021, 11, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Emin, D.; Zhang, Y.P.; Lobanova, E.; Miller, A.; Li, X.; Xia, Z.; Dakin, H.; Sideris, D.I.; Lam, J.Y.; Ranasinghe, R.T.; et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat. Commun. 2022, 13, 5512. [Google Scholar] [CrossRef] [PubMed]
- Jarazo, J.; Barmpa, K.; Modamio, J.; Saraiva, C.; Sabaté-Soler, S.; Rosety, I.; Griesbeck, A.; Skwirblies, F.; Zaffaroni, G.; Smits, L.M.; et al. Parkinson’s disease phenotypes in patient neuronal cultures and brain organoids improved by 2-hydroxypropyl-cyclodextrin treatment. Mov. Disord. 2022, 37, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, K.; Zeng, Y.; Hancock, T.; Segatori, L. Genetic and chemical activation of TFEB mediates clearance of aggregated-synuclein. PLoS ONE 2015, 10, e0120819. [Google Scholar] [CrossRef] [PubMed]
- Jarazo, J.M.; Walter, J.; Schwamborn, J.C. (Inventors) 2-Hydroxypropyl-Beta-Cyclodextrin for Use in a Method of Treatment of a Parkinsonian Condition. WIPO Patent WO2019219741A1, 21 November 2019. [Google Scholar]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular Drug-Delivery Systems Based on Polymeric Core–Shell Architectures. Angew. Chem. Int. Ed. 2004, 43, 278–282. [Google Scholar] [CrossRef]
- Haag, R.; Kratz, F. Polymer Therapeutics: Concepts and Applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef]
- Ion, D.; Niculescu, A.-G.; Paduraru, D.N.; Andronic, O.; Mușat, F.; Grumezescu, A.M.; Bolocan, A. An Up-to-Date Review of Natural Nanoparticles for Cancer Management. Pharmaceutics 2022, 14, 18. [Google Scholar] [CrossRef]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, P.; Pérez-Lorenzo, M.J.; Alcázar-Garrido, Á.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Nokhodchi, A. Micro- and nanoformulations of paclitaxel based on micelles, liposomes, cubosomes, and lipid nanoparticles: Recent advances and challenges. Drug Discov. Today 2022, 27, 576–584. [Google Scholar] [CrossRef]
- Menezes, P.D.P.; Andrade, T.A.; Frank, L.A.; de Souza, E.; Trindade, G.; Trindade, I.A.S.; Serafini, M.R.; Guterres, S.S.; Araújo, A.A.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int. J. Pharm. 2019, 559, 312–328. [Google Scholar] [CrossRef]
- Qiu, N.; Li, X.; Liu, J. Application of cyclodextrins in cancer treatment. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 229–246. [Google Scholar] [CrossRef]
- Bognanni, N.; Viale, M.; Distefano, A.; Tosto, R.; Bertola, N.; Loiacono, F.; Ponassi, M.; Spinelli, D.; Pappalardo, G.; Vecchio, G. Cyclodextrin Polymers as Delivery Systems for Targeted Anti-Cancer Chemotherapy. Molecules 2021, 26, 6046. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Sun, W.; Yang, Y.; Jin, H.; Qiu, L.; Chen, J.; Chen, J. Co-responsive smart cyclodextrin-gated mesoporous silica nanoparticles with ligand-receptor engagement for anti-cancer treatment. Mater. Sci. Eng. C 2019, 103, 109831. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr. Polym. 2022, 276, 118747. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater. Chem. Phys. 2018, 218, 62–69. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Popovic, S.L.; Todorovic, D.V.; Djurdjevic, P.M.; Matic, S.D.; Mitrovic, M.M.; Popovic, A.M.; Kacaniova, M.M.; Baskic, D.D. Effect of β-cyclodextrin encapsulation on cytotoxic activity of acetylshikonin against HCT-116 and MDA-MB-231 cancer cell lines. Saudi Pharm. J. 2020, 28, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Lai, Y.-C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion complex of saikosaponin-d with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-skin cancer activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Elbatanony, R.S.; Shukla, S.K.; Kulkarni, N.S.; Kanabar, D.D.; Chauhan, G.; Ayehunie, S.; Chen, Z.-S.; Muth, A.; Gupta, V. Bypassing P-glycoprotein mediated efflux of afatinib by cyclodextrin complexation—Evaluation of intestinal absorption and anti-cancer activity. J. Mol. Liq. 2021, 327, 114866. [Google Scholar] [CrossRef]
- Xu, J.; Ren, X.; Guo, T.; Sun, X.; Chen, X.; Patterson, L.H.; Li, H.; Zhang, J. NLG919/cyclodextrin complexation and anti-cancer therapeutic benefit as a potential immunotherapy in combination with paclitaxel. Eur. J. Pharm. Sci. 2019, 138, 105034. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Yao, S.; Yao, C.; Li, Y.; Ke, M.; Zhang, S.; Qian, L.; Hu, X.; Ren, F. Cyclodextrin single isomer-based vesicle for chlorin e6 delivery and enhanced efficiency of photodynamic therapy for cancer treatment. J. Mol. Liq. 2022, 352, 118683. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Tok, T.T.; Ozkan, S.A. The redox mechanism investigation of non-small cell lung cancer drug: Erlotinib via theoretical and experimental techniques and its host–guest detection by β-Cyclodextrin nanoparticles modified glassy carbon electrode. Sens. Actuators B Chem. 2019, 278, 172–180. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Mohammed, A.; Makia, R.; Ali, M.; Raheem, R.; Yousif, E. Cytotoxic Effects of Valsartan Organotin(IV) Complexes on Human Lung Cancer Cells. Biointerface Res. Appl. Chem. 2021, 11, 8156–8164. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, B.; Zhou, W.; Liu, Y. High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy. Biomacromolecules 2020, 21, 4998–5007. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, P.P.G.; Tan, M.; Tammela, T.; Wu, K.; Chung, A.; Oberli, M.; Wang, K.; Spektor, R.; Riley, R.S.; Viana, C.T.R.; et al. Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J. Control. Release 2018, 290, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Lin, X.; Bai, Y.; Jiang, Q. Precise fabrication of folic acid-targeted therapy on metformin encapsulated β-cyclodextrin nanomaterials for treatment and care of lung cancer. Process Biochem. 2022, 118, 74–83. [Google Scholar] [CrossRef]
- Hyun, H.; Lee, S.; Lim, W.; Jo, D.; Jung, J.S.; Jo, G.; Kim, S.Y.; Lee, D.-W.; Um, S.; Yang, D.H.; et al. Engineered beta-cyclodextrin-based carrier for targeted doxorubicin delivery in breast cancer therapy in vivo. J. Ind. Eng. Chem. 2019, 70, 145–151. [Google Scholar] [CrossRef]
- Farrokhi, F.; Karami, Z.; Esmaeili-Mahani, S.; Heydari, A. Delivery of DNAzyme targeting c-Myc gene using β-cyclodextrin polymer nanocarrier for therapeutic application in human breast cancer cell line. J. Drug Deliv. Sci. Technol. 2018, 47, 477–484. [Google Scholar] [CrossRef]
- Mihanfar, A.; Targhazeh, N.; Sadighparvar, S.; Darband, S.G.; Majidinia, M.; Yousefi, B. Doxorubicin loaded magnetism nanoparticles based on cyclodextrin dendritic-graphene oxide inhibited MCF-7 cell proliferation. Biomol. Concepts 2021, 12, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Mostafa, E.M.; Musa, A.; Gilani, S.J.; Ghoneim, M.M.; Alshehri, S.; et al. Formulation of ternary genistein β-cyclodextrin inclusion complex: In vitro characterization and cytotoxicity assessment using breast cancer cell line. J. Drug Deliv. Sci. Technol. 2022, 67, 102932. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jo, J.; Jo, D.; Kim, J.; Min, J.-J.; Yang, D.H.; Hyun, H. Supramolecular assembly based on host–guest interaction between beta-cyclodextrin and adamantane for specifically targeted cancer imaging. J. Ind. Eng. Chem. 2018, 57, 37–44. [Google Scholar] [CrossRef]
- Panagiotakis, S.; Mavroidi, B.; Athanasopoulos, A.; Charalambidis, G.; Coutsolelos, A.G.; Paravatou-Petsotas, M.; Pelecanou, M.; Mavridis, I.M.; Yannakopoulou, K. Unsymmetrical, monocarboxyalkyl meso-arylporphyrins in the photokilling of breast cancer cells using permethyl-β-cyclodextrin as sequestrant and cell uptake modulator. Carbohydr. Polym. 2022, 275, 118666. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Arkan, E.; Derakhshankhah, H.; Haghshenas, B.; Jahanban-Esfahlan, R.; Jaymand, M. A novel bioreducible and pH-responsive magnetic nanohydrogel based on β-cyclodextrin for chemo/hyperthermia therapy of cancer. Carbohydr. Polym. 2021, 252, 117229. [Google Scholar] [CrossRef]
- Ercan, A.; Çelebier, M.; Varan, G.; Öncül, S.; Nenni, M.; Kaplan, O.; Bilensoy, E. Global omics strategies to investigate the effect of cyclodextrin nanoparticles on MCF-7 breast cancer cells. Eur. J. Pharm. Sci. 2018, 123, 377–386. [Google Scholar] [CrossRef]
- Kasinathan, K.; Marimuthu, K.; Murugesan, B.; Pandiyan, N.; Pandi, B.; Mahalingam, S.; Selvaraj, B. Cyclodextrin functionalized multi-layered MoS2 nanosheets and its biocidal activity against pathogenic bacteria and MCF-7 breast cancer cells: Synthesis, characterization and in-vitro biomedical evaluation. J. Mol. Liq. 2021, 323, 114631. [Google Scholar] [CrossRef]
- Baskar, G.; Supria Sree, N. Synthesis, characterization and anticancer activity of β-cyclodextrin-Asparaginase nanobiocomposite on prostate and lymphoma cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101417. [Google Scholar] [CrossRef]
- Trindade, G.G.G.; Thrivikraman, G.; Menezes, P.P.; França, C.M.; Lima, B.S.; Carvalho, Y.M.B.G.; Souza, E.P.B.S.S.; Duarte, M.C.; Shanmugam, S.; Quintans-Júnior, L.J.; et al. Carvacrol/β-cyclodextrin inclusion complex inhibits cell proliferation and migration of prostate cancer cells. Food Chem. Toxicol. 2019, 125, 198–209. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Cieślak, M.; Królewska-Golińska, K.; Makowski, T.; Socka, M.; Biela, T. Stereocomplexed micelles based on polylactides with β-cyclodextrin core as anti-cancer drug carriers. Eur. Polym. J. 2019, 120, 109271. [Google Scholar] [CrossRef]
- Reis, C.A.; Rodrigues, C.F.; Moreira, A.F.; Jacinto, T.A.; Ferreira, P.; Correia, I.J. Development of gold-core silica shell nanospheres coated with poly-2-ethyl-oxazoline and β-cyclodextrin aimed for cancer therapy. Mater. Sci. Eng. C 2019, 98, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Russo Spena, C.; De Stefano, L.; Palazzolo, S.; Salis, B.; Granchi, C.; Minutolo, F.; Tuccinardi, T.; Fratamico, R.; Crotti, S.; D’Aronco, S.; et al. Liposomal delivery of a Pin1 inhibitor complexed with cyclodextrins as new therapy for high-grade serous ovarian cancer. J. Control. Release 2018, 281, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.L.; Turner, S.P. Bone cancer: Diagnosis and treatment principles. Am. Fam. Physician 2018, 98, 205–213. [Google Scholar] [PubMed]
- Badila, A.E.; Radulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Radulescu, M.; Radulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Ahmadi, D.; Zarei, M.; Rahimi, M.; Khazaie, M.; Asemi, Z.; Mir, S.M.; Sadeghpour, A.; Karimian, A.; Alemi, F.; Rahmati-Yamchi, M.; et al. Preparation and in-vitro evaluation of pH-responsive cationic cyclodextrin coated magnetic nanoparticles for delivery of methotrexate to the Saos-2 bone cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101584. [Google Scholar] [CrossRef]
- Khelghati, N.; Rasmi, Y.; Farahmandan, N.; Sadeghpour, A.; Mir, S.M.; Karimian, A.; Yousefi, B. Hyperbranched polyglycerol β-cyclodextrin as magnetic platform for optimization of doxorubicin cytotoxic effects on Saos-2 bone cancerous cell line. J. Drug Deliv. Sci. Technol. 2020, 57, 101741. [Google Scholar] [CrossRef]
- Plesselova, S.; Garcia-Cerezo, P.; Blanco, V.; Reche-Perez, F.J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F.; Giron-Gonzalez, M.D.; Salto-Gonzalez, R. Polyethylenimine–Bisphosphonate–Cyclodextrin Ternary Conjugates: Supramolecular Systems for the Delivery of Antineoplastic Drugs. J. Med. Chem. 2021, 64, 12245–12260. [Google Scholar] [CrossRef]
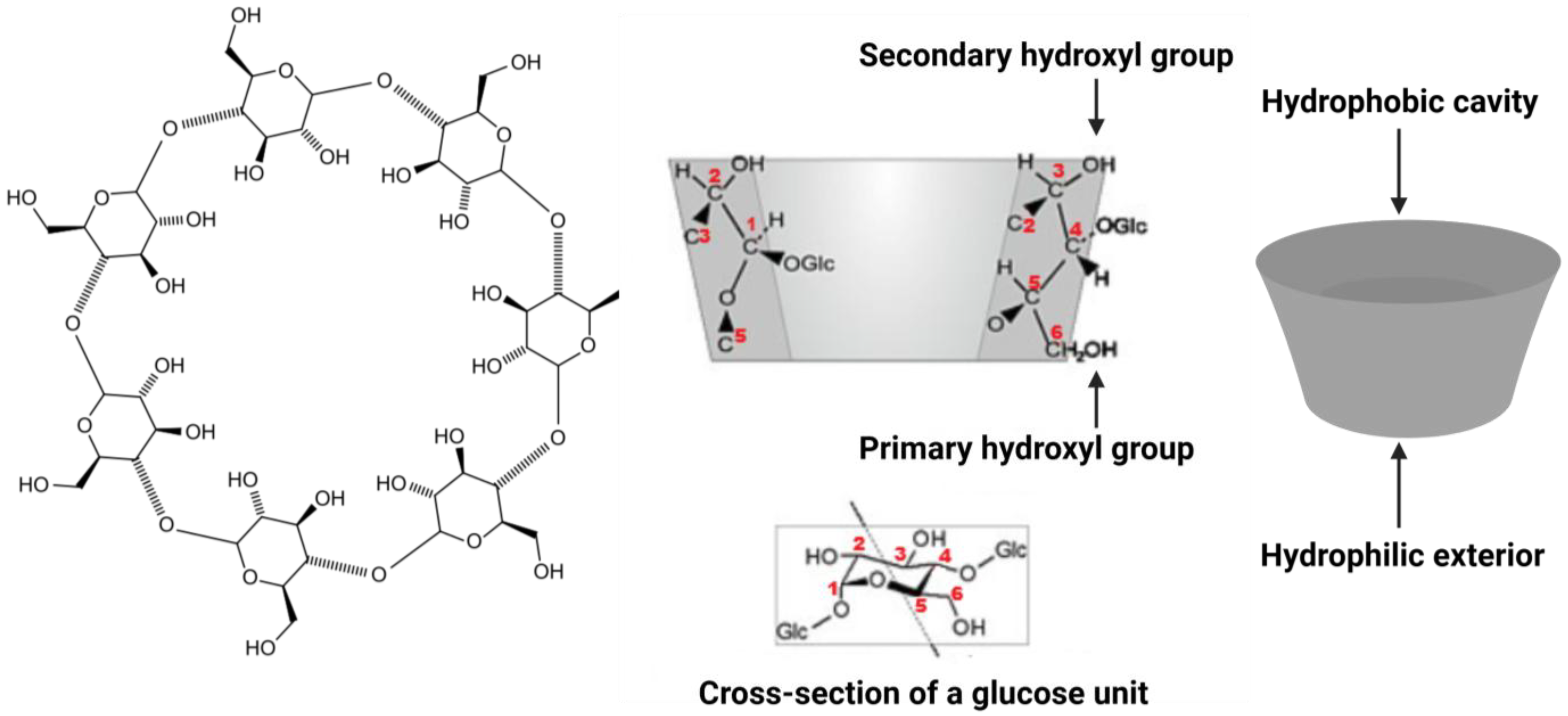
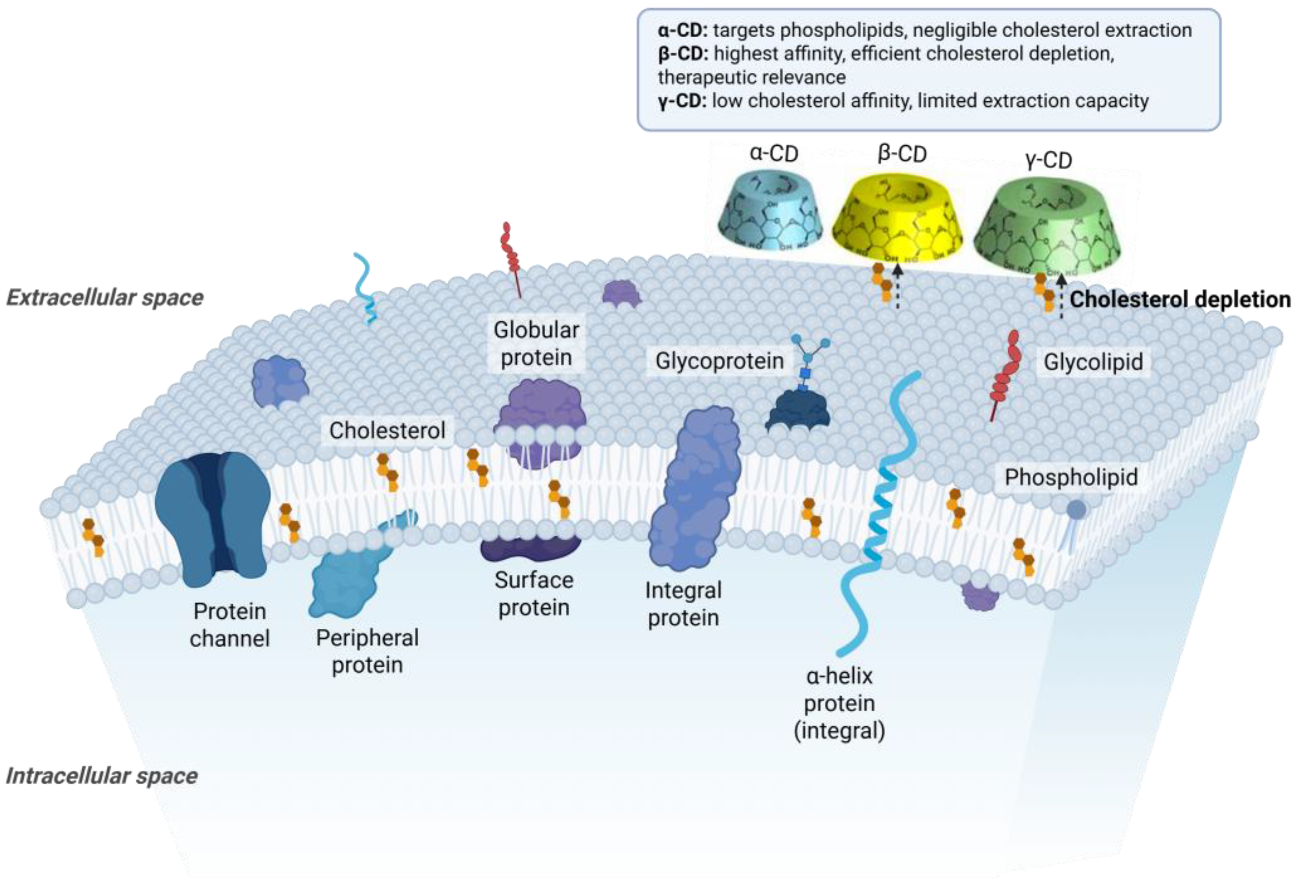
| Cyclodextrin | Glucose Units in Ring | Cavity Diameter (Å) | Water Solubility (g/L) |
|---|---|---|---|
| α-CD | 6 | 4.7–5.3 | ~145 |
| β-CD | 7 | 6.0–6.5 | ~18 |
| γ-CD | 8 | 7.5–8.3 | ~232 |
| Derivative and Modification | Preparation Method | Analytical Application | Ref. |
|---|---|---|---|
| α-CD grafted via PEG-sol–gel | Polyethylene glycol–based sol–gel | Stationary phase for aromatic isomer separation | [51] |
| Mono-6-deoxy-benzimide-β-CD | Direct amide coupling | Enantioseparation of rigid analytes | [52] |
| Cationic β-CDs (4 variants) | Quaternization; coated on silica | CSP tested with various alcohol eluents | [53] |
| Cationic β-CDs (4 variants; pyCDCl best) | Quaternization | Enantiomer resolution | [54] |
| Mono-azido-β-CD “clicked” onto silica | Cu-catalyzed azide–alkyne cycloaddition | Stable CSP with high enantioseparation | [55] |
| R-configured β-CD derivatives | Stereospecific substitution | CSP; molecular docking to probe chiral recognition | [56] |
| Vinylene-functionalized cationic β-CD on vinylized silica | Vinyl click-chemistry | Novel CSP | [57] |
| Chiral monolithic phases from novel CD derivatives | In situ polymerization on capillary | Monolithic CSP for multiple chiral compounds | [58] |
| Derivative and Modification | Key Feature/Application | Ref. |
|---|---|---|
| Thio-β-CD (high yield) | Mitsunobu reaction; easy thio-functionalization | [52] |
| Cup-shaped α-CD with aldehyde | Enhanced catalytic performance | [54] |
| β-CD–triazole hybrids | Rigid, water-soluble; increased prednisolone solubility; non-cytotoxic | [59] |
| Urea-substituted β-CD | Amphiphilic anion receptors; stable, water-soluble | [60] |
| Chitosan-functionalized β-CD | Anti-inflammatory activity | [61] |
| Mono-aldehyde & carboxyl β-CD derivatives | NaBH4/NaCNBH3 reductions; general route to tosyl-derived CDs | [62] |
| Solid-phase C-6 mono-substitution | Mild detachment on resin | [63,64,65] |
| Hollow CD nanospheres | Improved CPT stability; sustained release; high loading | [66,67] |
| β-CD blockers of anthrax toxin pores | Pore-blocking antivirulence agents | [68] |
| C6-aminated permethyl-CDs | Epoxide opening; aminoalcohol linkers; microwave-optimized yield | [69] |
| D-Carnosine-β-CD conjugates | Enzyme-resistant peptide delivery; enhanced stereoselective binding | [70] |
| Scutellarin-β-CD conjugates | Increased solubility, stability, cytotoxicity; antitumor activity | [71] |
| Maleic/itaconic acid–esterified β-CD | Phosphate-catalyzed esterification; 70/21% yields | [72] |
| Phospholipidyl-β-CD | Self-organizing amphiphiles; characterized by ESI-MS/MS | [73] |
| Trifluoromethylated β-CD | Artificial enzyme activity | [74] |
| Naphthalene-fluorophore β-CD | Fluorescent host–guest sensing; van der Waals–driven inclusion | [75,76] |
| Anthracene-β-CD | Good solubility; fluorescence profiling | [77] |
| Azobenzene-triazole-β-CD | Click-linked photoresponsive derivative | [78] |
| Primary-face modified CDs | Novel routes to C-6 functionalization | [79] |
| CD Derivative Used | Genetic Cargo | Target Cells/Tissue | Outcome/Key Findings | Ref. |
|---|---|---|---|---|
| HP-β-CD nanoparticle | siRNA targeting KRAS | Lung cancer cells | Enhanced cellular uptake, 60% knockdown efficiency; minimal toxicity | [142] |
| Methylated β-CD | mRNA encoding GFP | Hepatocytes | Increased transfection efficiency and protein expression in vitro and in vivo | [143] |
| Cationic Amphiphilic Cyclodextrin | mRNA for CFTR protein | Airway epithelial cells | Improved mRNA stability, efficient endosomal escape, restoration of CFTR function in cystic fibrosis model | [144] |
| Modified γ-CD nanoparticle | miRNA for oncogene silencing | Glioblastoma cells | Significant reduction in tumor growth and increased survival in animal models | [145] |
| Neurodegenerative Disease | CD Derivative | Target Lipid/Protein | Model/System Used | Observed Effects | Ref. |
|---|---|---|---|---|---|
| Alzheimer’s Disease | HP-β-CD | Cholesterol/Amyloid-β | Transgenic mouse model | Reduced amyloid deposition, improved memory | [150] |
| Parkinson’s Disease | HP-β-CD | α-Synuclein/Cholesterol | In vitro neuronal cultures | Decreased aggregation, enhanced neuronal survival | [150] |
| Huntington’s Disease | Methylated β-CD | Mutant huntingtin protein | Cellular models | Reduced aggregation, lowered cytotoxicity | [151] |
| Authors et al. | CD-Based System | Key Findings | Ref. |
|---|---|---|---|
| Dai et al. | Supramolecular nanoparticles: reduction-sensitive permethyl-β-CD–Camptothecin prodrug + adamantane–porphyrin photosensitizer + HA-TPP + β-CD | Mitochondrial uptake in A549 cells; in situ CPT release; ROS generation under light; synergistic chemo-photodynamic efficacy against lung cancer | [198] |
| Guimarães et al. | Cyclodextrin complexes of LGK974 (porcupine inhibitor) | ↑ Solubility & bioavailability of LGK974; enabled safer, repeated oral/parenteral dosing; ↓ toxicity in Wnt-dependent tissues | [199] |
| Vaidya et al. | Β-CD–erlotinib inclusion coated with PLGA | Enhanced NSCLC cell uptake; lowered IC50; suppressed colony formation; ↑ apoptosis; inhibition of autophagy | [200] |
| Wang et al. | Sulfobutylether-β-CD–resveratrol complexes loaded on polymeric nanoparticles | ↑ Cellular uptake, cytotoxicity & apoptosis in NSCLC models; maintained resveratrol’s antioxidant activity; superior efficacy vs. Free drug | [201] |
| Shukla et al. | CD-derivative–Celastrol complex | Improved intestinal permeability & physiological stability; enhanced cytotoxicity in human lung cancer cells | [202] |
| Lin et al. | Β-CD–polycaprolactone block copolymer conjugated with folic acid—metformin carrier | pH-responsive metformin release (faster at pH 6.4); folate-receptor-mediated uptake in A549; controlled release and targeted anti-tumor efficacy with low toxicity | [203] |
| Authors | Cancer Type | CD-Based System | Key Findings | Ref. |
|---|---|---|---|---|
| Hyun et al. | Breast cancer | β-CD + polyethylene glycol + folic acid nanocarrier loaded with doxorubicin (IV administration in animals) | Decreased tumor volume after IV dosing; no systemic toxicity or cardiotoxicity—targeted, safer DOX delivery | [204] |
| Farrokhi et al. | Breast cancer | β-CD polymer nanocarrier delivering an RNA-cleaving DNAzyme targeting c-Myc (tested in MCF-7 cells) | Synergistic inhibition of MCF-7 proliferation when combined with doxorubicin—enhanced anticancer activity | [205] |
| Mihanfar et al. | Breast cancer | β-CD-functionalized dendrimeric graphene-oxide magnetic nanoparticles loaded with doxorubicin | Increased proliferation inhibition and apoptosis; reduced off-target DOX side effects in vivo; GO may sensitize cancer cells | [206] |
| Zafar et al. | Breast cancer | β-CD complexes of genistein with D-α-Tocopherol PEG1000 succinate (TPGS) | Improved genistein solubility → significantly greater antioxidant and cytotoxic activities vs. free genistein | [207] |
| Lee et al. | Breast cancer | β-CD + polyethylene glycol + folic acid carrier for adamantane–NIRF conjugate | Highly efficient tumor targeting and excellent breast-tumor targetability | [208] |
| Panagiotakis et al. | Breast cancer | Permethyl-β-CD complexes with water-insoluble photosensitizers (meso-tetraphenylporphyrin & analog) | Photostability, strong intracellular fluorescence, high photokilling efficiency and low dark toxicity in MCF-7 cells | [209] |
| Soleimani et al. | Breast cancer | Magnetic nanogel: β-CD + poly(2-ethyl-2-oxazoline) + iron-oxide NPs, loaded with doxorubicin HCl | High drug loading, slow/stimuli-triggered release, good cytocompatibility; enables combined chemotherapy + hyperthermia | [210] |
| Ercan et al. | Breast cancer | Blank 6-O-Caproyl-β-CD nanoparticles (administered to MCF-7 cells) | Increased levels of apoptosis-related proteins and prevention of cell proliferation (intrinsic apoptotic effect) | [211] |
| Kasinathan et al. | Breast cancer | Hybrid nanocomposite of β-CD and molybdenum disulfide (MoS2) | Potent inhibition of MCF-7 cells plus antibacterial properties—promising for cancer therapy | [212] |
| Baskar & Supria Sree | Prostate cancer | β-CD–chitosan nanobiocomposite loaded with l-asparaginase | Good anticancer activity vs. prostate cell lines; IC50 ≈ 125 µg/mL (≈ half the concentration of free enzyme) | [213] |
| Trindade et al. | Prostate cancer | Inclusion complexes of β-CD with carvacrol | Dose-dependent inhibition of tumor cells in 2D/3D cultures; potent antiproliferative effects against PC-3 cells | [214] |
| Kost et al. | Cervical cancer | β-CD core complexed with doxorubicin, loaded into stereocomplexed polylactide micelles (SCMs) | Controlled DOX release and more efficient tumor cell suppression vs. free drug | [215] |
| Reis et al. | Cervical cancer | Gold-core/silica-shell (AuMSS) NPs coated with poly-2-ethyl-2-oxazoline (PEOZ) and β-CD (various ratios) | Improved biological performance, enhanced cytocompatibility and increased internalization in HeLa cells | [216] |
| Russo Spena et al. | Ovarian cancer | Modified CD encapsulating a Pin1 inhibitor, remotely loaded into PEGylated liposomes | Preferential tumor accumulation, favorable PK; induced proteasome-dependent degradation of Pin1—promising antitumor effects | [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, A.S.; Varut, R.-M.; Trasca, D.-M.; Stoica, G.A.; Radivojevic, K.; Carmen, S.; Arsenie, C.C.; Popescu, C. Cyclodextrins as Active Therapeutic Agents: Beyond Their Role as Excipients. Pharmaceuticals 2025, 18, 1592. https://doi.org/10.3390/ph18101592
Pirvu AS, Varut R-M, Trasca D-M, Stoica GA, Radivojevic K, Carmen S, Arsenie CC, Popescu C. Cyclodextrins as Active Therapeutic Agents: Beyond Their Role as Excipients. Pharmaceuticals. 2025; 18(10):1592. https://doi.org/10.3390/ph18101592
Chicago/Turabian StylePirvu, Andreea Silvia, Renata-Maria Varut, Diana-Maria Trasca, George Alin Stoica, Kristina Radivojevic, Sirbulet Carmen, Cristian Cosmin Arsenie, and Cristina Popescu. 2025. "Cyclodextrins as Active Therapeutic Agents: Beyond Their Role as Excipients" Pharmaceuticals 18, no. 10: 1592. https://doi.org/10.3390/ph18101592
APA StylePirvu, A. S., Varut, R.-M., Trasca, D.-M., Stoica, G. A., Radivojevic, K., Carmen, S., Arsenie, C. C., & Popescu, C. (2025). Cyclodextrins as Active Therapeutic Agents: Beyond Their Role as Excipients. Pharmaceuticals, 18(10), 1592. https://doi.org/10.3390/ph18101592







