Rational Design, Computational Analysis and Antibacterial Activities of Synthesized Peptide-Based Molecules Targeting Quorum Sensing-Dependent Biofilm Formation in Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Docking
2.2. Binding Interactions Between the Selected Compounds and LasR
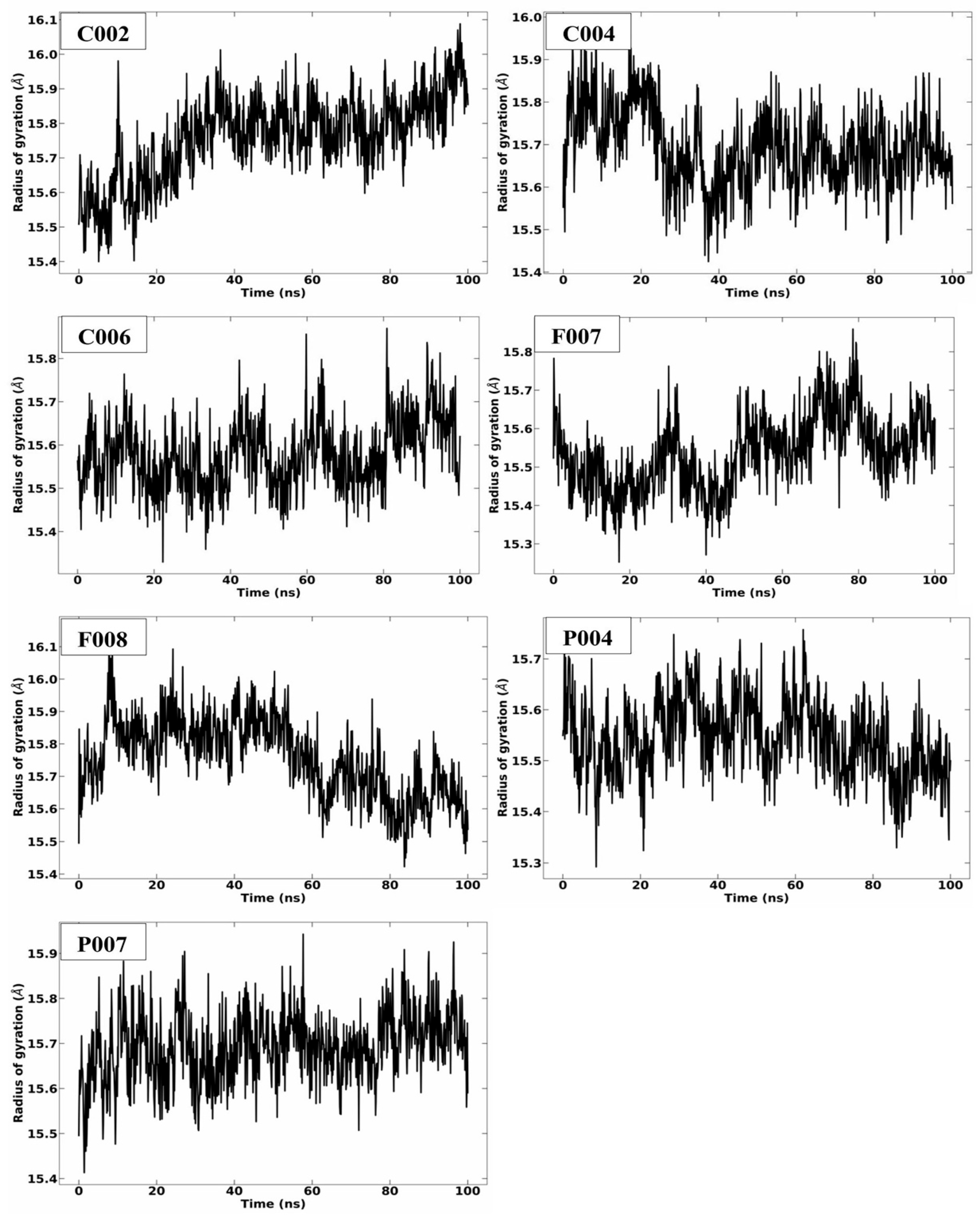
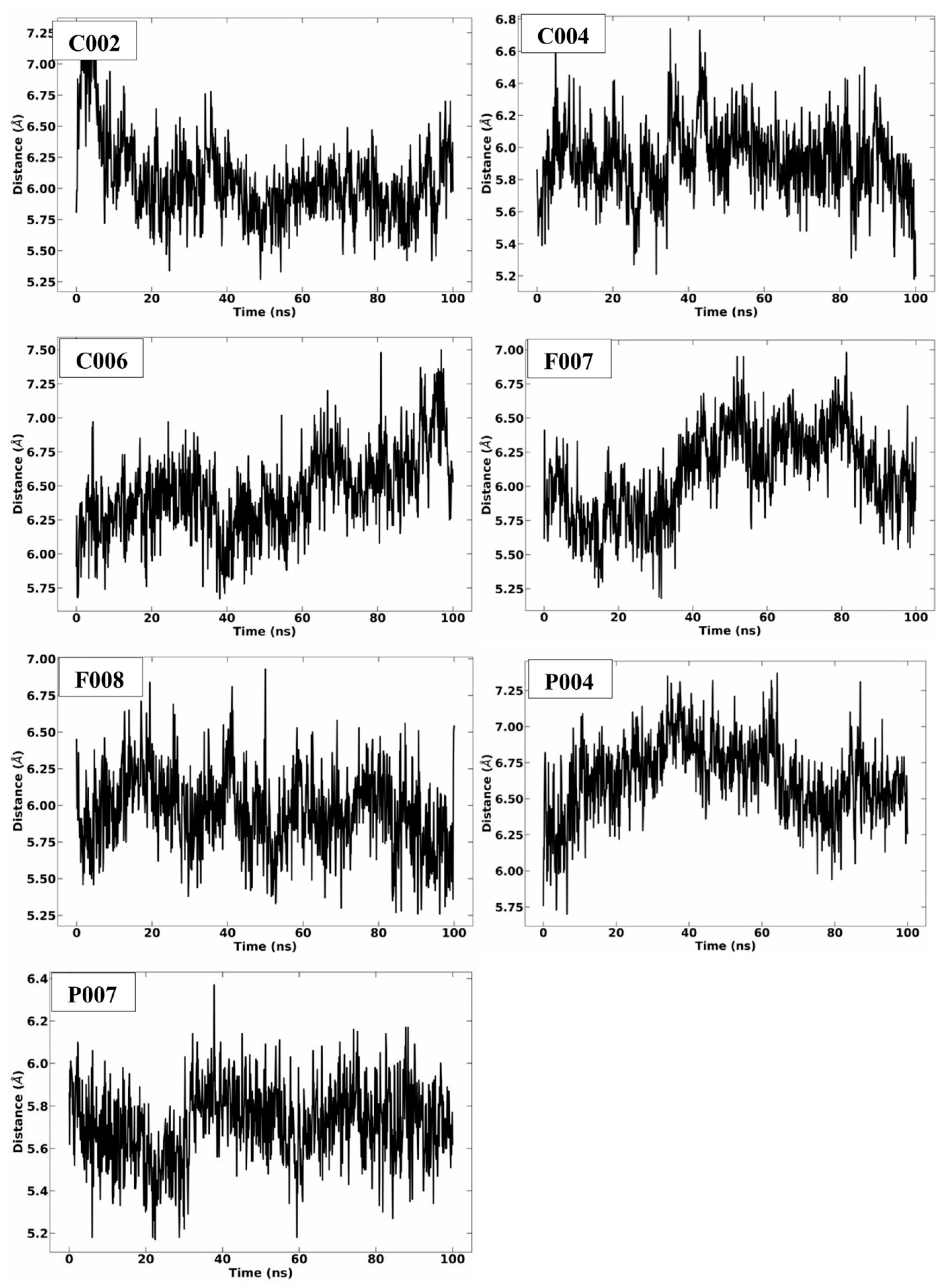
2.3. MD Simulation Analysis
2.4. ADMET Predictions
2.5. Compounds Chemistry and Characterization
- P004 (PGK)

- P007 (YGK)
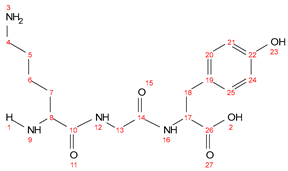
- C002 (IG-coumarin)

- C004 (LA-coumarin)
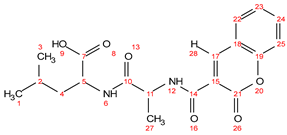
- C006 (PG-coumarin)
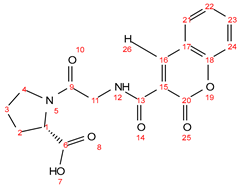
2.6. Antibacterial Test of the Synthesized Compounds
2.6.1. Minimum Inhibitory Concentration (MIC)
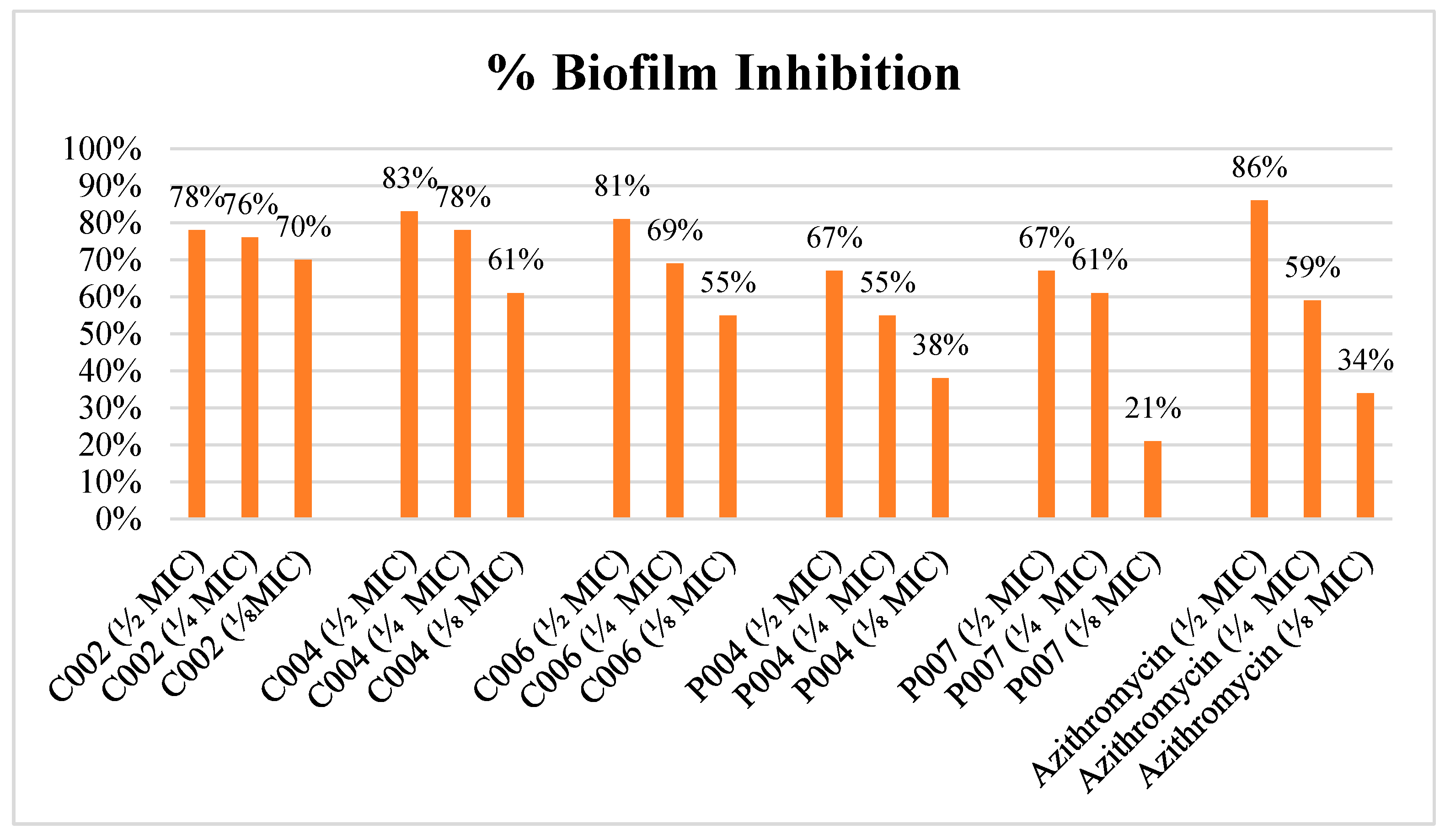
2.6.2. Antibiofilm Activity Assessment
3. Materials and Methods
3.1. Ligand and Target Protein Preparation
3.2. Molecular Docking Studies
3.3. MD Simulations of the Selected Ligands with LasR Protein
3.4. MM-PBSA Calculations and Post Simulation Analysis
3.5. Visualization and Plotting Software
3.6. ADMET Calculations
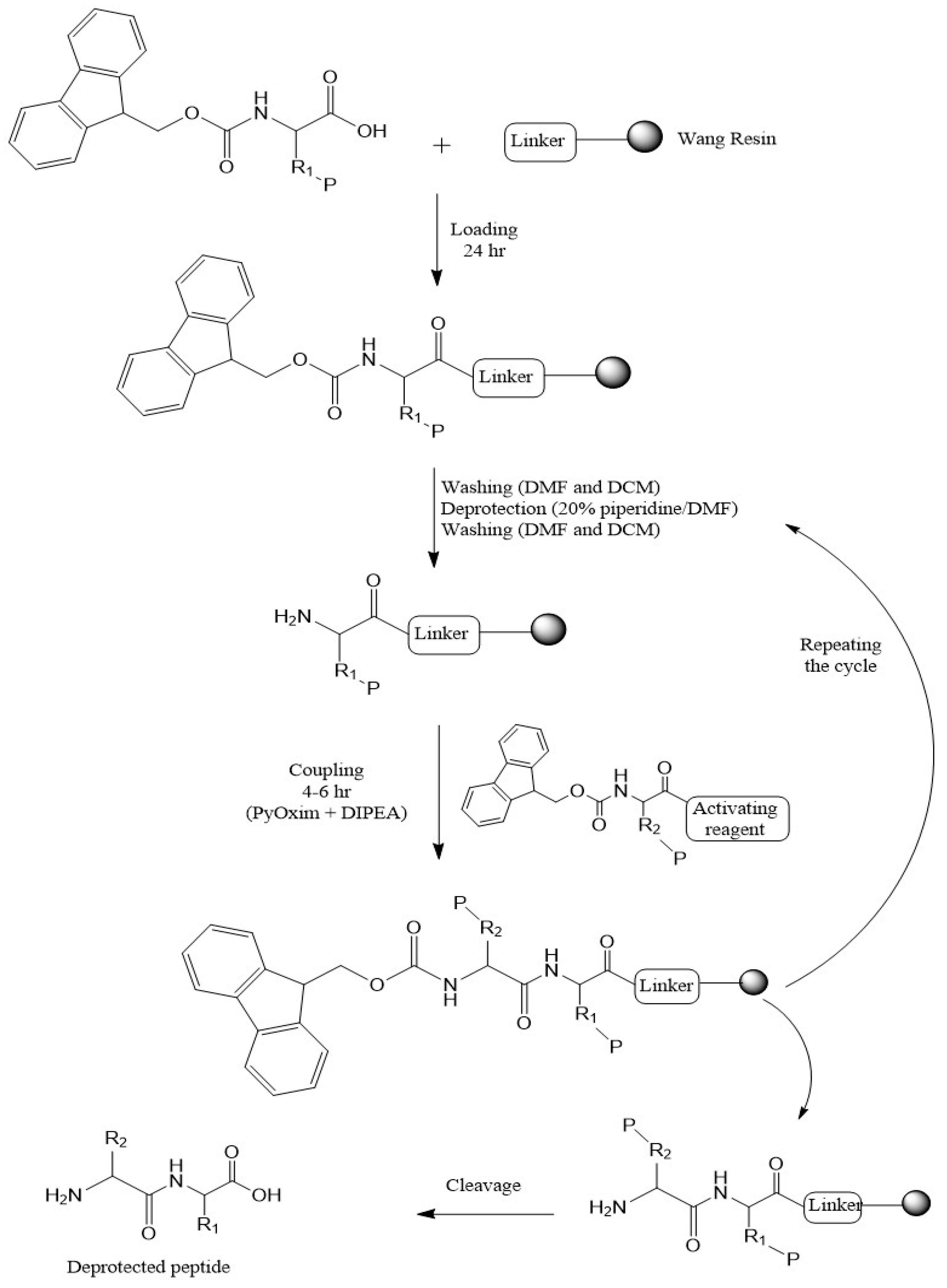
3.7. Synthesis Chemistry
3.8. Antibacterial Activities
3.8.1. Determination of Minimum Inhibitory Concentration
3.8.2. Antibiofilm Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moussaoui, O.; Bhadane, R.; Sghyar, R.; El Hadrami, E.M.; El Amrani, S.; Ben Tama, A.; Kandri Rodi, Y.; Chakroune, S.; Salo-Ahen, O.M.H. Novel Amino Acid Derivatives of Quinolines as Potential Antibacterial and Fluorophore Agents. Sci. Pharm. 2020, 88, 57. [Google Scholar] [CrossRef]
- Koesling, D.; Bozzaro, C. The post-antibiotic era: An existential threat for humanity. Intergener. Justice Rev. 2022, 8, 51–56. [Google Scholar]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Hancock, R.E.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M. Antimicrobial peptides—Mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Orsi, M.; Reymond, J.L. Can large language models predict antimicrobial peptide activity and toxicity? RSC Med. Chem. 2024, 15, 2030–2036. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, e2203291. [Google Scholar] [CrossRef]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martinez-de-Tejada, G. AMPs as anti-biofilm agents for human therapy and prophylaxis. In Antimicrobial Peptides: Basics for Clinical Application; Springer: Berlin/Heidelberg, Germany, 2019; pp. 257–279. [Google Scholar]
- Lopes, B.S.; Hanafiah, A.; Nachimuthu, R.; Muthupandian, S.; Md Nesran, Z.N.; Patil, S. The role of antimicrobial peptides as antimicrobial and antibiofilm agents in tackling the silent pandemic of antimicrobial resistance. Molecules 2022, 27, 2995. [Google Scholar] [CrossRef]
- Selvaraj, S.P.; Chen, J.-Y. Conjugation of antimicrobial peptides to enhance therapeutic efficacy. Eur. J. Med. Chem. 2023, 259, 115680. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Deo, S.; Turton, K.L.; Kainth, T.; Kumar, A.; Wieden, H.-J. Strategies for improving antimicrobial peptide production. Biotechnol. Adv. 2022, 59, 107968. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, H.; Chandarajoti, K.; Jiao, Z.; Nie, L.; Lv, S.; Zuo, J.; Zhou, W.; Han, X. Design and Synthesis of coumarin-based amphoteric antimicrobials with the biofilm interference and immunoregulation effects. RSC Med. Chem. 2024, 16, 1223–1234. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef]
- Brindhadevi, K.; LewisOscar, F.; Mylonakis, E.; Shanmugam, S.; Verma, T.N.; Pugazhendhi, A. Biofilm and Quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Biochem. 2020, 96, 49–57. [Google Scholar] [CrossRef]
- Folsom, J.P.; Richards, L.; Pitts, B.; Roe, F.; Ehrlich, G.D.; Parker, A.; Mazurie, A.; Stewart, P.S. Physiology of Pseudomonas aeruginosa in biofilms as revealed by transcriptome analysis. BMC Microbiol. 2010, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Reig, S.; Le Gouellec, A.; Bleves, S. What is new in the anti–Pseudomonas aeruginosa clinical development pipeline since the 2017 WHO alert? Front. Cell. Infect. Microbiol. 2022, 12, 909731. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.; Kergaravat, B.; Jacquet, P.; Billot, R.; Grizard, D.; Chabrière, É.; Plener, L.; Daudé, D. Disrupting quorum sensing as a strategy to inhibit bacterial virulence in human, animal, and plant pathogens. Pathog. Dis. 2024, 82, ftae009. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.C.; Dong, S.H.; Rossi, F.M.; Karlen, K.M.; Kumar, R.S.; Nair, S.K.; Blackwell, H.E. Structural and Biochemical Studies of Non-native Agonists of the LasR Quorum-Sensing Receptor Reveal an L3 Loop “Out” Conformation for LasR. Cell Chem. Biol. 2018, 25, 1128–1139 e3. [Google Scholar] [CrossRef]
- Gerdt, J.P.; Blackwell, H.E. Competition Studies Confirm Two Major Barriers That Can Preclude the Spread of Resistance to Quorum-Sensing Inhibitors in Bacteria. ACS Chem. Biol. 2014, 9, 2291–2299. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, X.; Zheng, J.; Zou, Y.; Qiu, S.; Dai, Y. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef]
- Taha, M.N.; Saafan, A.E.; Ahmedy, A.; El Gebaly, E.; Khairalla, A.S. Two novel synthetic peptides inhibit quorum sensing-dependent biofilm formation and some virulence factors in Pseudomonas aeruginosa PAO1. J. Microbiol. 2019, 57, 618–625. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mion, S.; Carriot, N.; Lopez, J.; Plener, L.; Ortalo-Magné, A.; Chabrière, E.; Culioli, G.; Daudé, D. Disrupting quorum sensing alters social interactions in Chromobacterium violaceum. NPJ Biofilms Microbiomes 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zou, H.; Li, J.; Song, T.; Lv, W.; Wang, W.; Wang, Z.; Tao, S. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: Current understanding and future perspectives. Gut Microbes 2022, 14, 2039048. [Google Scholar] [CrossRef]
- Asfahl, K.L.; Schuster, M. Additive Effects of Quorum Sensing Anti-Activators on Pseudomonas aeruginosa Virulence Traits and Transcriptome. Front. Microbiol. 2017, 8, 2654. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Turkina, M.V.; Yakymenko, O.; Magnusson, K.-E.; Vikström, E. The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration. PLoS Pathog. 2012, 8, e1002953. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Tucker, K.D.; Passador, L. LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J. Bacteriol. 2002, 184, 4912–4919. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer*. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, F.; Tabassum, N.; Kim, Y.-M. Natural and synthetic molecules with potential to enhance biofilm formation and virulence properties in Pseudomonas aeruginosa. Crit. Rev. Microbiol. 2024, 50, 830–858. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Salih, T.; Ali, P.G. Rational design of novel compounds to serve as potential NDM-1 inhibitors using molecular docking, molecular dynamics simulation, and physicochemical studies. Mol. Simul. 2023, 49, 1373–1387. [Google Scholar] [CrossRef]
- Shang, D.; Han, X.; Du, W.; Kou, Z.; Jiang, F. Trp-containing antibacterial peptides impair quorum sensing and biofilm development in multidrug-resistant Pseudomonas aeruginosa and exhibit synergistic effects with antibiotics. Front. Microbiol. 2021, 12, 611009. [Google Scholar] [CrossRef] [PubMed]
- Aflakian, F.; Rad, M.; Hashemitabar, G.; Lagzian, M.; Ramezani, M. Design and assessment of novel synthetic peptides to inhibit quorum sensing-dependent biofilm formation in Pseudomonas aeruginosa. Biofouling 2022, 38, 131–146. [Google Scholar] [CrossRef]
- Beaudoin, T.; Stone, T.A.; Glibowicka, M.; Adams, C.; Yau, Y.; Ahmadi, S.; Bear, C.E.; Grasemann, H.; Waters, V.; Deber, C.M. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci. Rep. 2018, 8, 14728. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, S.-Y.; Hu, J.-Y.; Chen, Q.-X.; Jiao, S.-M.; Xiao, H.-C.; Zhang, Q.; Xu, J.; Zhao, J.-F.; Zhou, H.-B. Novel coumarin derivatives inhibit the quorum sensing system and iron homeostasis as antibacterial synergists against Pseudomonas aeruginosa. J. Med. Chem. 2023, 66, 14735–14754. [Google Scholar] [CrossRef]
- El-Sawy, E.R.; Abdel-Aziz, M.S.; Abdelmegeed, H.; Kirsch, G. Coumarins: Quorum Sensing and Biofilm Formation Inhibition. Molecules 2024, 29, 4534. [Google Scholar] [CrossRef]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, type III secretion and C-di-GMP levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar] [CrossRef]
- Proctor, C.R.; McCarron, P.A.; Ternan, N.G. Furanone quorum-sensing inhibitors with potential as novel therapeutics against Pseudomonas aeruginosa. J. Med. Microbiol. 2020, 69, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, P.-C.; Ma, H.-M.; Chen, S.-Y.; Fu, Y.-H.; Liu, Y.-Y.; Wang, X.; Yu, G.-C.; Huang, T.; Hibbs, D.E. Design, synthesis and evaluation of halogenated furanone derivatives as quorum sensing inhibitors in Pseudomonas aeruginosa. Eur. J. Pharm. Sci. 2019, 140, 105058. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, J.; Park, H.-Y.; Park, H.-J.; Lee, J.H.; Kim, C.K.; Yoon, J. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 80, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Haleem, A.; Ammar, U.; Masci, D.; El-Ansary, S.; Abdel Rahman, D.; Abou-Elazm, F.; El-Dydamony, N. Discovery of Benzopyrone-Based Candidates as Potential Antimicrobial and Photochemotherapeutic Agents through Inhibition of DNA Gyrase Enzyme B: Design, Synthesis, In Vitro and In Silico Evaluation. Pharmaceuticals 2024, 17, 1197. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Wei, D.-Q.; Gu, K.-R.; Hassan, M.I.; Tabrez, S. Current updates on computer aided protein modeling and designing. Int. J. Biol. Macromol. 2016, 85, 48–62. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; Santana Gomes, J.N.; de Oliveira Viana, J.; de Medeiros e Silva, Y.M.; Barbosa, E.G.; de Moura, R.O. The power of molecular dynamics simulations and their applications to discover cysteine protease inhibitors. Mini Rev. Med. Chem. 2024, 24, 1125–1146. [Google Scholar] [CrossRef]
- Sargsyan, K.; Grauffel, C.; Lim, C. How Molecular Size Impacts RMSD Applications in Molecular Dynamics Simulations. J. Chem. Theory Comput. 2017, 13, 1518–1524. [Google Scholar] [CrossRef]
- Bibi, S.; Khan, M.S.; El-Kafrawy, S.A.; Alandijany, T.A.; El-Daly, M.M.; Yousafi, Q.; Fatima, D.; Faizo, A.A.; Bajrai, L.H.; Azhar, E.I. Virtual screening and molecular dynamics simulation analysis of Forsythoside A as a plant-derived inhibitor of SARS-CoV-2 3CLpro. Saudi Pharm. J. 2022, 30, 979–1002. [Google Scholar] [CrossRef]
- Weiss, M.S.; Brandl, M.; Sühnel, J.; Pal, D.; Hilgenfeld, R. More hydrogen bonds for the (structural) biologist. Trends Biochem. Sci. 2001, 26, 521–523. [Google Scholar] [CrossRef]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, L.; Kong, C.; Zhou, J.; Zhou, F. A review of biophysical strategies to investigate protein-ligand binding: What have we employed? Int. J. Biol. Macromol. 2024, 276, 133973. [Google Scholar] [CrossRef]
- Roseli, R.B.; Huang, Y.-H.; Henriques, S.T.; Kaas, Q.; Craik, D.J. Molecular dynamics simulations support a preference of cyclotide kalata B1 for phosphatidylethanolamine phospholipids. Biochim. Biophys. Acta (BBA) Biomembr. 2024, 1866, 184268. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Vollmayr-Lee, K. Introduction to molecular dynamics simulations. Am. J. Phys. 2020, 88, 401–422. [Google Scholar] [CrossRef]
- Arroyo-Mañez, P.; Bikiel, D.E.; Boechi, L.; Capece, L.; Di Lella, S.; Estrin, D.A.; Martí, M.A.; Moreno, D.M.; Nadra, A.D.; Petruk, A.A. Protein dynamics and ligand migration interplay as studied by computer simulation. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2011, 1814, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular mechanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J. Comput. Chem. 2011, 32, 866–877. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Kumar, A.; Kini, S.G.; Rathi, E. A recent appraisal of artificial intelligence and in silico ADMET prediction in the early stages of drug discovery. Mini Rev. Med. Chem. 2021, 21, 2788–2800. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef]
- Chantell, C.A.; Onaiyekan, M.A.; Menakuru, M. Fast conventional Fmoc solid-phase peptide synthesis: A comparative study of different activators. J. Pept. Sci. 2012, 18, 88–91. [Google Scholar] [CrossRef]
- Arujõe, M.; Ploom, A.; Mastitski, A.; Järv, J. Comparison of various coupling reagents in solid-phase aza-peptide synthesis. Tetrahedron Lett. 2017, 58, 3421–3425. [Google Scholar] [CrossRef]
- Antunes, B.; Zanchi, C.; Johnston, P.R.; Maron, B.; Witzany, C.; Regoes, R.R.; Hayouka, Z.; Rolff, J. The evolution of antimicrobial peptide resistance in Pseudomonas aeruginosa is severely constrained by random peptide mixtures. PLoS Biol. 2024, 22, e3002692. [Google Scholar] [CrossRef] [PubMed]
- ChemAxon. MarvinSketch, version 20.16; ChemAxon Ltd.: Budapest, Hungary, 2020.
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide protein data bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. AMDock: A versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol. Direct 2020, 15, 12. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Aqvist, J. Ion-Water Interaction Potentials Derived from Free Energy Perturbation Simulations. J. Phys. Chem. 1990, 94, 8021–8024. [Google Scholar] [CrossRef]
- Ross, G.A.; Rustenburg, A.S.; Grinaway, P.B.; Fass, J.; Chodera, J.D. Biomolecular Simulations under Realistic Macroscopic Salt Conditions. J. Phys. Chem. B 2018, 122, 5466–5486. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Román, F.; González, A.; Velasco, S. Periodic boundary conditions and the correct molecular-dynamics ensemble. Phys. A Stat. Mech. Its Appl. 2008, 387, 6705–6711. [Google Scholar] [CrossRef]
- Patodia, S.; Bagaria, A.; Chopra, D. Molecular dynamics simulation of proteins: A brief overview. J. Phys. Chem. Biophys. 2014, 4, 166. [Google Scholar] [CrossRef]
- Golo, V.L.; Shaĭtan, K.V. Dynamic attractor for the Berendsen thermostat an the slow dynamics of biomacromolecules. Biofizika 2002, 47, 611–617. [Google Scholar] [PubMed]
- Tuble, S.C.; Anwar, J.; Gale, J.D. An Approach to Developing a Force Field for Molecular Simulation of Martensitic Phase Transitions between Phases with Subtle Differences in Energy and Structure. J. Am. Chem. Soc. 2004, 126, 396–405. [Google Scholar] [CrossRef]
- Ensing, B.; Nielsen, S.O. Multiscale molecular dynamics and the reverse mapping problem. In Trends in Computational Nanomechanics: Transcending Length and Time Scales; Springer: Berlin/Heidelberg, Germany, 2010; pp. 25–59. [Google Scholar]
- Larsson, P.; Hess, B.; Lindahl, E. Algorithm improvements for molecular dynamics simulations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 93–108. [Google Scholar] [CrossRef]
- Kawata, M.; Nagashima, U. Particle Mesh Ewald Method for Three-Dimensional Systems with Two-Dimensional Periodicity. Chem. Phys. Lett. 2001, 340, 165–172. [Google Scholar] [CrossRef]
- Sagui, C.; Darden, T.A. Molecular dynamics simulations of biomolecules: Long-range electrostatic effects. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 155–179. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Jin, Z.; Xiu, Z.; Li, G. Binding free energy estimation for protein-ligand complex based on MM-PBSA with various partial charge models. Curr. Pharm. Des. 2013, 19, 2293–2307. [Google Scholar] [CrossRef]
- Yekeen, A.A.; Durojaye, O.A.; Idris, M.O.; Muritala, H.F.; Arise, R.O. CHAPERONg: A tool for automated GROMACS-based molecular dynamics simulations and trajectory analyses. Comput. Struct. Biotechnol. J. 2023, 21, 4849–4858. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.S.; Hu, Z. Using PyMOL as a platform for computational drug design. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Maestro, S. Maestro; Schrödinger, LLC: New York, NY, USA, 2020; Volume 2020, p. 682. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kar, S.; Leszczynski, J. Open access in silico tools to predict the ADMET profiling of drug candidates. Expert Opin. Drug Discov. 2020, 15, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.A.; Fields, G.B. Trifluoroacetic acid cleavage and deprotection of resin-bound peptides following synthesis by Fmoc chemistry. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1997; pp. 67–83. [Google Scholar]
- Chan, W.; White, P. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hussen, N.H.; Hussein, L.B.; Hasan, A.H.; Hamid, S.J.; Abdl, C.O.; Sarkar, B.; Muhammed, K.; Muhamad, D. Chalcone-related small molecules as potent antibacterial and antifungal agents: Design, synthesis, In vitro, and computational approaches. Asp. Mol. Med. 2025, 5, 100066. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Yu, X.; Cao, R.; Hong, M.; Xu, Z.; Ren Lu, J.; Wang, Y.; Zhu, H. Antimicrobial peptides fight against Pseudomonas aeruginosa at a sub-inhibitory concentration via anti-QS pathway. Bioorg. Chem. 2023, 141, 106922. [Google Scholar] [CrossRef]

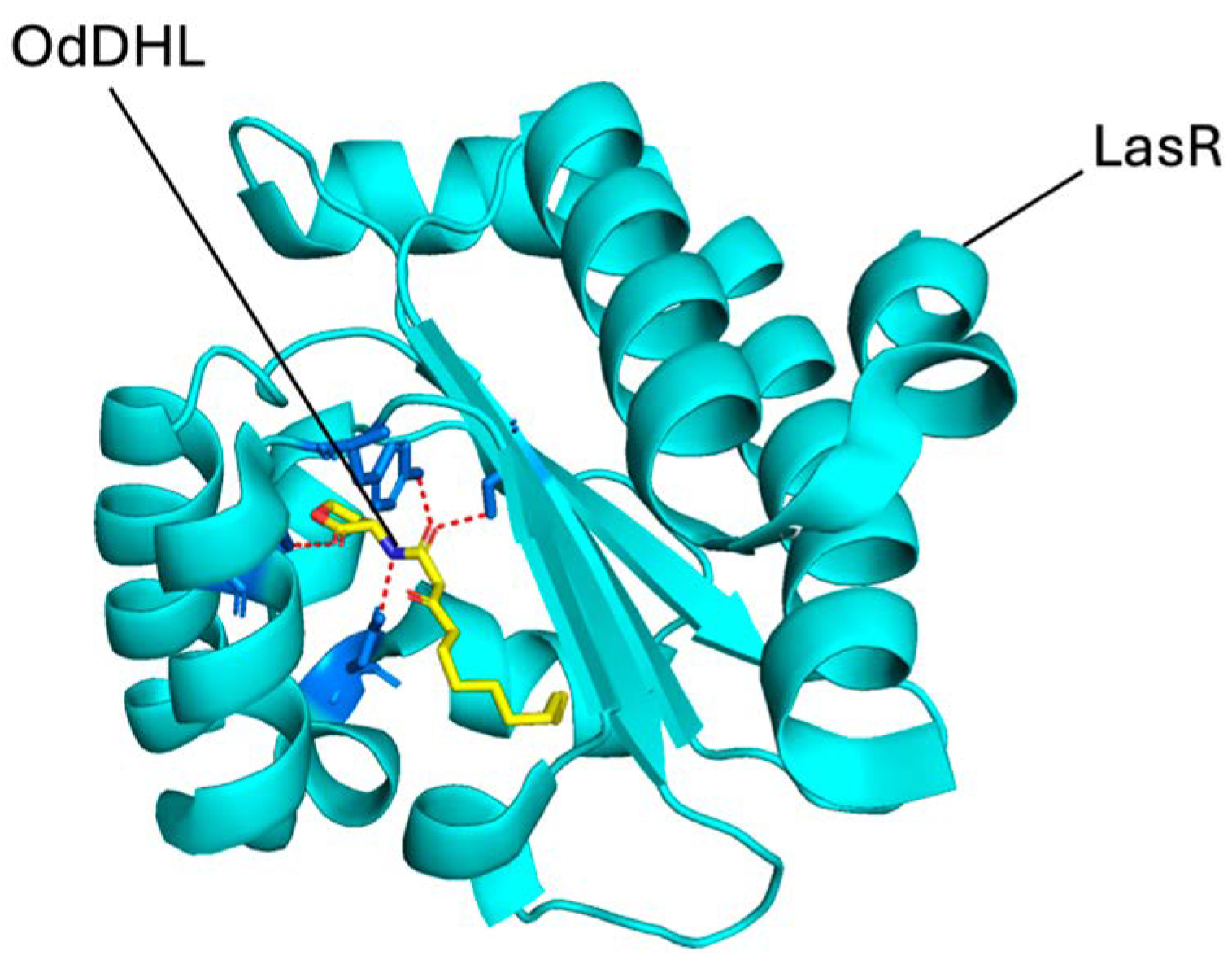
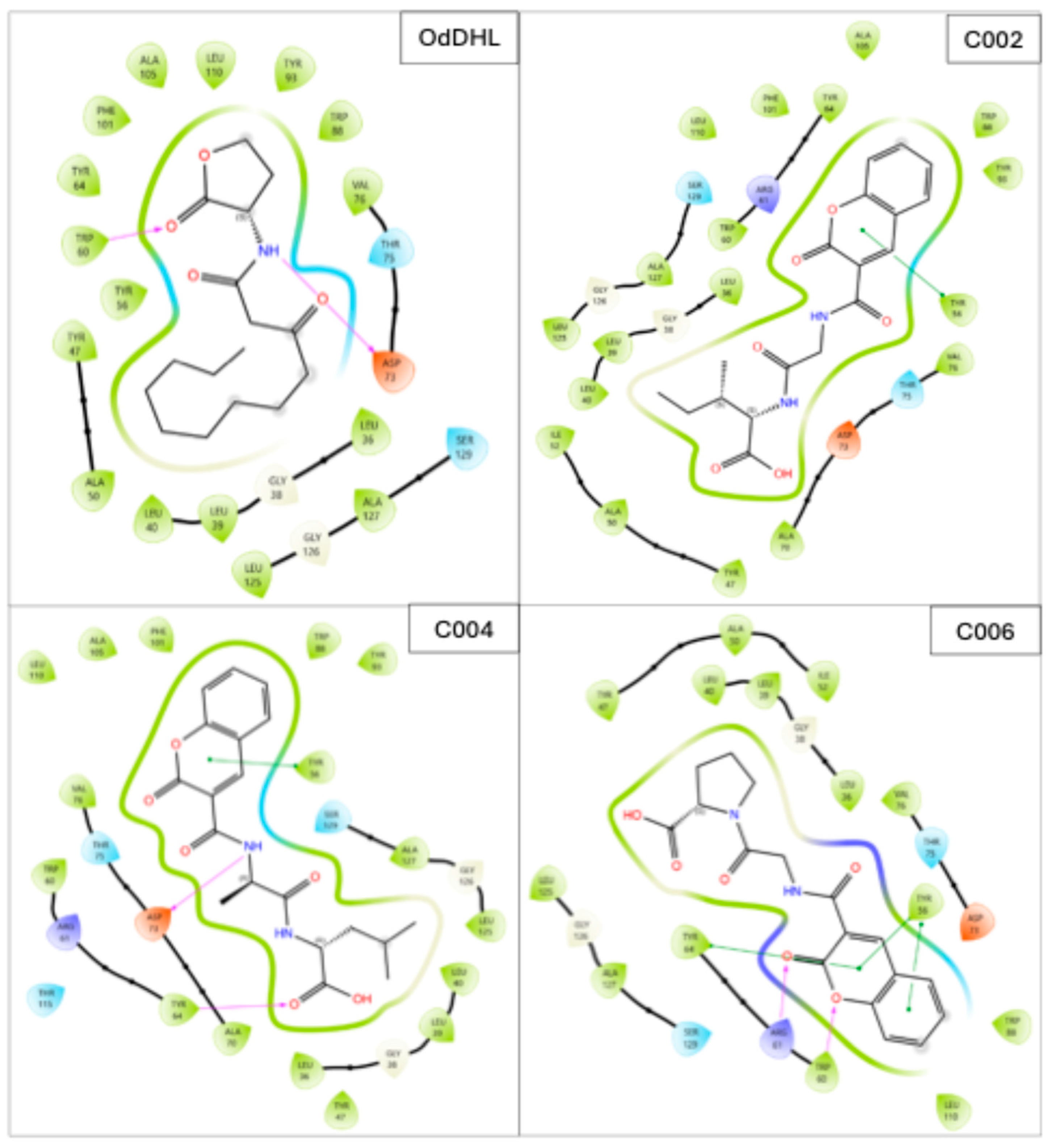
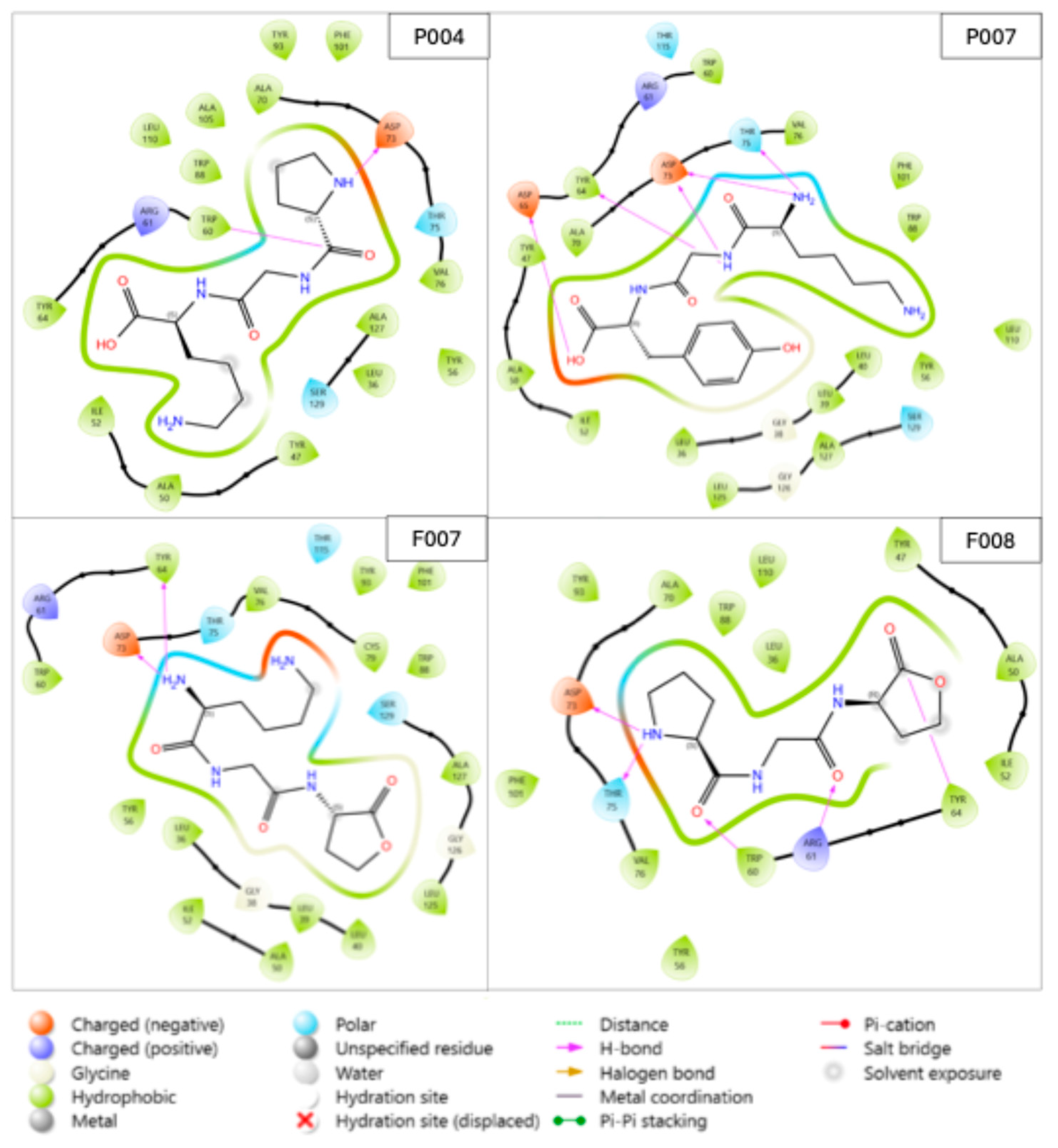
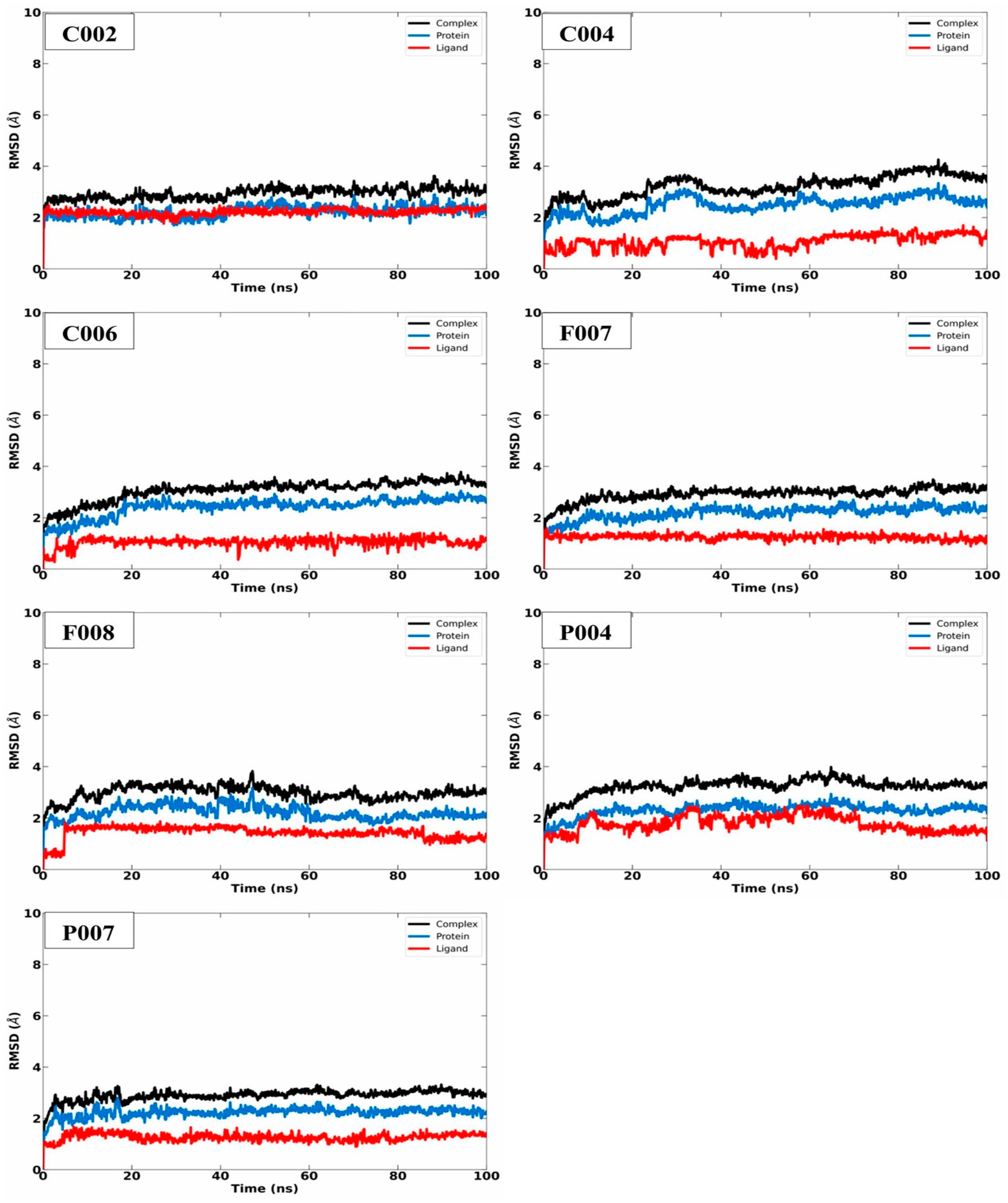
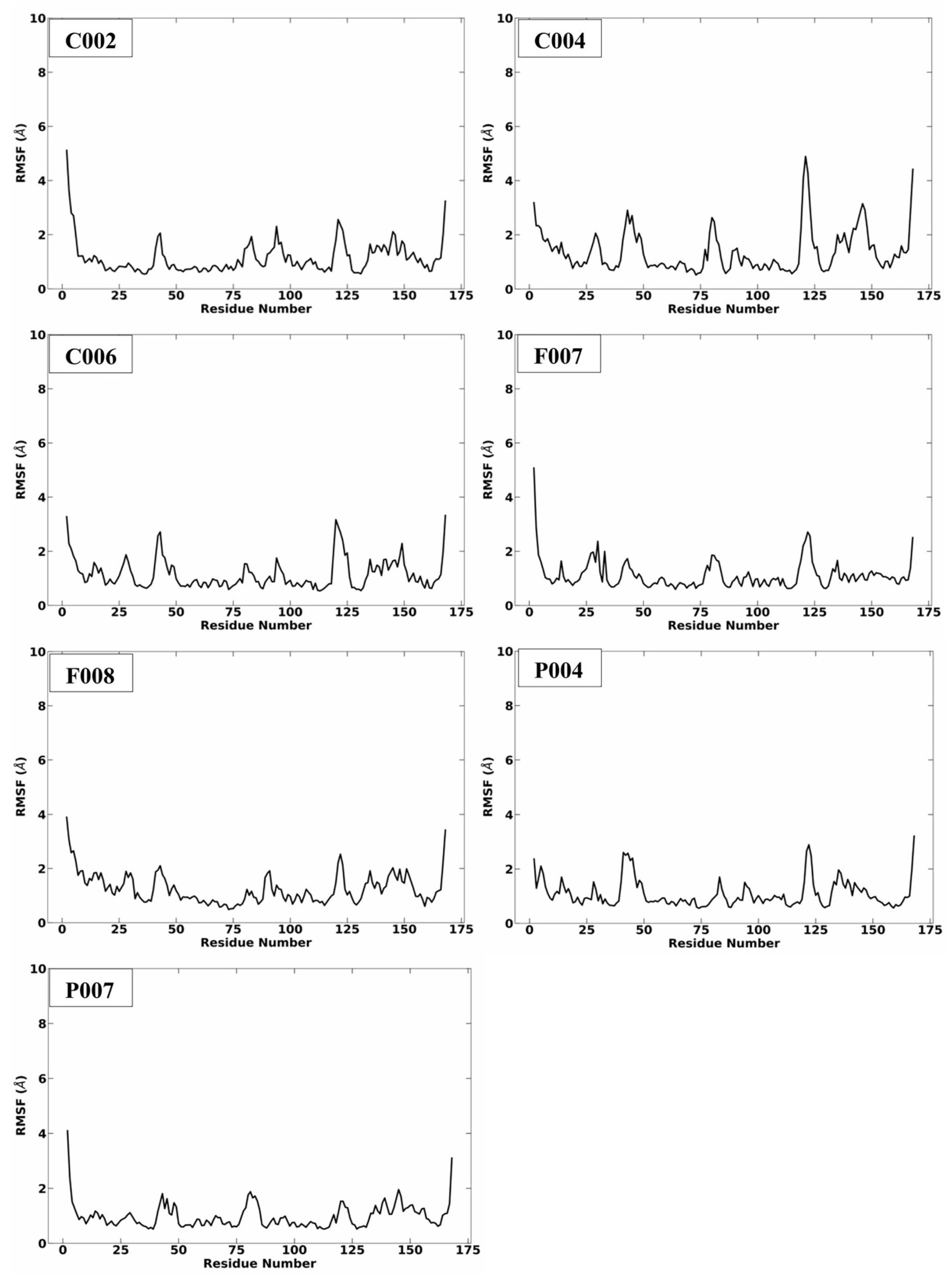



| Code | ∆G (kcal/mol) | MW (g/mol) | ClogP |
|---|---|---|---|
| OdDHL | −8.45 | 297.39 | 2.95 |
| P001 | −4.92 | 328.37 | −1.96 |
| P002 | −4.52 | 341.45 | 0.97 |
| P003 | −5.25 | 283.32 | −0.68 |
| P004 | −9.19 | 300.36 | −1.45 |
| P005 | −7.98 | 349.38 | −0.16 |
| P006 | −5.69 | 394.43 | −1.79 |
| P007 | −8.12 | 366.41 | −0.92 |
| P008 | −6.25 | 335.36 | −0.55 |
| C001 | −5.62 | 375.38 | 0.22 |
| C002 | −9.18 | 360.36 | 1.14 |
| C003 | −7.13 | 389.40 | 0.61 |
| C004 | −10.35 | 374.39 | 1.53 |
| C005 | −8.97 | 360.36 | 1.14 |
| C006 | −9.73 | 344.32 | 0.6 |
| C007 | −8.4 | 417.42 | −0.26 |
| C008 | −4.61 | 403.39 | −0.64 |
| C009 | −7.73 | 348.31 | −0.53 |
| C010 | −7.25 | 334.28 | −0.92 |
| C011 | −7.89 | 360.36 | 1.14 |
| F001 | −7.43 | 314.34 | −2.88 |
| F002 | −8.24 | 328.37 | −2.49 |
| F003 | −8.81 | 286.33 | −2.01 |
| F004 | −9.01 | 271.31 | −1.09 |
| F005 | −9.08 | 271.31 | −1.09 |
| F006 | −8.53 | 300.35 | −1.62 |
| F007 | −9.49 | 286.33 | −2.01 |
| F008 | −9.19 | 255.27 | −1.71 |
| F009 | −8.22 | 259.26 | −2.76 |
| F010 | −7.31 | 245.23 | −3.15 |
| F011 | −8.37 | 271.31 | −1.09 |
| Code | Sequence | MM-PBSA (kcal/mol) |
|---|---|---|
| C002 | IG-coumarin | −35.96 |
| C004 | LA-coumarin | −50.10 |
| C006 | PG-coumarin | −45.55 |
| F007 | KG-furanone | −43.27 |
| F008 | PG-furanone | −34.29 |
| P004 | PGK | −55.45 |
| P007 | YGK | −45.73 |
| Comp. | Absorption a HIA | Distribution | Metabolism | Excretion | |||||
|---|---|---|---|---|---|---|---|---|---|
| b PPB (%) | c BBB | CYP2D6-inh | CYP2D6-sub | CYP3A4-inh | CYP3A4-sub | CL-Plasma | t0.5 | ||
| C002 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C004 | 0.02 | 76.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.07 | 1.06 |
| C006 | 0.00 | 80.14 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 2.29 | 0.87 |
| F007 | 0.42 | 8.63 | 0.00 | 0.00 | 0.24 | 0.00 | 0.03 | 4.20 | 1.02 |
| F008 | 0.33 | 11.57 | 0.02 | 0.00 | 0.03 | 0.01 | 0.00 | 3.83 | 1.56 |
| P004 | 0.88 | 8.47 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 3.11 | 1.38 |
| P007 | 0.04 | 12.59 | 0.00 | 0.00 | 0.87 | 0.00 | 0.00 | 5.19 | 0.96 |
| Comp. | MW. | Lipinski | TPSA | logP | Toxicity | Carcinogenicity | |
|---|---|---|---|---|---|---|---|
| hERG-Blocker | H-HT | ||||||
| C002 | 360.13 | 0.00 | 125.71 | 1.47 | 0.03 | 0.26 | 0.34 |
| C004 | 374.15 | 0.00 | 125.71 | 2.57 | 0.02 | 0.43 | 0.25 |
| C006 | 344.1 | 0.00 | 116.92 | 0.86 | 0.05 | 0.71 | 0.54 |
| F007 | 286.16 | 0.00 | 136.54 | −2.67 | 0.19 | 0.48 | 0.40 |
| F008 | 255.12 | 0.00 | 96.53 | −2.07 | 0.18 | 0.43 | 0.24 |
| P004 | 300.36 | 0.00 | 133.55 | −2.84 | 0.15 | 0.48 | 0.07 |
| P007 | 366.19 | 0.00 | 167.77 | −2.02 | 0.17 | 0.69 | 0.02 |
| Code | MIC (μg/mL) |
|---|---|
| P004 | 1024 |
| P007 | 1024 |
| C002 | 512 |
| C004 | 512 |
| C006 | 512 |
| Azithromycin | 256 |
| Tripeptides | |
|---|---|
| P001 | RGP |
| P002 | LPL |
| P003 | PAP |
| P004 | PGK |
| P005 | YAP |
| P006 | YGR |
| P007 | YGK |
| P008 | YGP |
Coumarin-3-carboxylic Acid Conjugates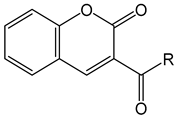 | |
| C001 | R = GK |
| C002 | R = IG |
| C003 | R = KA |
| C004 | R = LA |
| C005 | R = LG |
| C006 | R = PG |
| C007 | R = RA |
| C008 | R = RG |
| C009 | R = SA |
| C010 | R = SG |
| C011 | R = VA |
3-Aminodihydrofuran-2(3H)-one Conjugates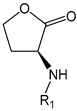 | |
| F001 | R1 = RG |
| F002 | R1 = RA |
| F003 | R1 = GK |
| F004 | R1 = IG |
| F005 | R1 = LG |
| F006 | R1 = KA |
| F007 | R1 = KG |
| F008 | R1 = PG |
| F009 | R1 = SA |
| F010 | R1 = SG |
| F011 | R1 = VA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, S.J.; Salih, T.M.; Aziz, T.A. Rational Design, Computational Analysis and Antibacterial Activities of Synthesized Peptide-Based Molecules Targeting Quorum Sensing-Dependent Biofilm Formation in Pseudomonas aeruginosa. Pharmaceuticals 2025, 18, 1572. https://doi.org/10.3390/ph18101572
Hamid SJ, Salih TM, Aziz TA. Rational Design, Computational Analysis and Antibacterial Activities of Synthesized Peptide-Based Molecules Targeting Quorum Sensing-Dependent Biofilm Formation in Pseudomonas aeruginosa. Pharmaceuticals. 2025; 18(10):1572. https://doi.org/10.3390/ph18101572
Chicago/Turabian StyleHamid, Shokhan Jamal, Twana Mohsin Salih, and Tavga Ahmed Aziz. 2025. "Rational Design, Computational Analysis and Antibacterial Activities of Synthesized Peptide-Based Molecules Targeting Quorum Sensing-Dependent Biofilm Formation in Pseudomonas aeruginosa" Pharmaceuticals 18, no. 10: 1572. https://doi.org/10.3390/ph18101572
APA StyleHamid, S. J., Salih, T. M., & Aziz, T. A. (2025). Rational Design, Computational Analysis and Antibacterial Activities of Synthesized Peptide-Based Molecules Targeting Quorum Sensing-Dependent Biofilm Formation in Pseudomonas aeruginosa. Pharmaceuticals, 18(10), 1572. https://doi.org/10.3390/ph18101572







