Targeting Polycystic Ovary Syndrome (PCOS) Pathophysiology with Flavonoids: From Adipokine–Cytokine Crosstalk to Insulin Resistance and Reproductive Dysfunctions
Abstract
1. Introduction
2. Pathophysiological Basis of Polycystic Ovary Syndrome (PCOS)
2.1. Insulin Resistance (IR) and Hyperinsulinemia
2.2. Hyperandrogenism and Ovarian Dysfunction
2.3. Adipokine–Cytokine Crosstalk
2.4. Oxidative Stress (OS) Induced Mitochondrial Dysfunction
3. Flavonoids: Structural Diversity and Medicinal Chemistry Aspects
3.1. Classification of Flavonoids
3.2. Pharmacokinetics and Bioavailability
3.3. Structure–Activity Relationship (SAR)
4. Flavonoids in Modulating Polycystic Ovary Syndrome (PCOS) Pathways
4.1. Effects on Insulin Resistance (IR) and Glucose Metabolism
4.2. Regulation of Adipokine Secretion
4.3. Cytokine Suppression and Anti-Inflammatory Effects
4.4. Antioxidant and Mitochondrial Protective Roles
4.5. Modulation of Hyperandrogenism and Hormonal Imbalance in PCOS
5. Translational and Clinical Evidence
5.1. Preclinical Evidence in Animal Models of PCOS
5.2. Human Clinical Studies and Trials
5.3. Safety and Toxicological Considerations
6. Integrative Therapeutic Perspective
6.1. Flavonoids as Adjunct to Standard Therapies
6.2. Nutraceutical and Functional Food Applications
6.3. Novel Formulations and Delivery Systems
7. Future Directions and Research Gaps
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AR | Androgen receptor |
| ARE | Antioxidant-responsive element |
| ATP | Adenosine triphosphate |
| COMT | Catechol-O-methyltransferase |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| CYP17A1 | Cytochrome P450 family 17 subfamily A member 1 |
| DHEA | Dehydroepiandrosterone |
| DNA | Deoxyribonucleic acid |
| EGCG | Epigallocatechin-3-gallate |
| ER | Estrogen receptor |
| GPx | Glutathione peroxidase |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IR | Insulin resistance |
| IRS-1 | Insulin receptor substrate-1 |
| LH | Luteinizing hormone |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OS | Oxidative stress |
| PCOS | Polycystic ovary syndrome |
| PI3K | Phosphatidylinositol 3-kinase |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| RCT | Randomized controlled trial |
| ROS | Reactive oxygen species |
| SAR | Structure–activity relationship |
| SERM | Selective estrogen receptor modulator |
| SHBG | Sex hormone-binding globulin |
| SOD | Superoxide dismutase |
| TFAM | Mitochondrial transcription factor A |
| TNF-α | Tumor necrosis factor-α |
| UGT | Uridine-5ʹ-diphosphate-glucuronosyltransferases |
References
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.S.; Dayel, S.B.; Abahussein, O. Polycystic ovary syndrome and reproductive health: A comprehensive review. Clin. Exp. Obstet. Gynecol. 2024, 51, 269. [Google Scholar] [CrossRef]
- Bates, G.W.; Legro, R.S. Longterm management of polycystic ovarian syndrome (pcos). Mol. Cell. Endocrinol. 2013, 373, 91–97. [Google Scholar] [CrossRef]
- Parker, J. Pathophysiological effects of contemporary lifestyle on evolutionary-conserved survival mechanisms in polycystic ovary syndrome. Life 2023, 13, 1056. [Google Scholar] [CrossRef]
- Stojanovic, O. From metabolism to immunity: The central role of adipose tissue inflammation in polycystic ovary syndrome. Curr. Res. Med. Sci. 2024, 3, 44–53. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Hassan, M.F. Polycystic ovary syndrome (pcos) and oxidative stress. J. Integr. Sci. Technol. 2024, 12, 752. [Google Scholar] [CrossRef]
- Rostamtabar, M.; Esmaeilzadeh, S.; Tourani, M.; Rahmani, A.; Baee, M.; Shirafkan, F.; Saleki, K.; Mirzababayi, S.S.; Ebrahimpour, S.; Nouri, H.R. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome. J. Cell. Physiol. 2021, 236, 824–838. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Adeniran, M.A.; Akinola, K.; Behera, H.; Kalaignazhal, G.; Egena, S.S.A. Unraveling the complexity of the molecular pathways associated with polycystic ovary syndrome (pcos) and identifying molecular targets for therapeutic development: A review of literature. Middle East Fertil. Soc. J. 2023, 28, 16. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Z.; Yu, Y.; Wang, X.; Zhao, Y. Natural compounds in the management of polycystic ovary syndrome: A comprehensive review of hormonal regulation and therapeutic potential. Front. Nutr. 2025, 12, 1520695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wu, Y.; Zhang, L.; Yu, Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with pi3k signaling pathway. Front. Endocrinol. 2022, 13, 1091147. [Google Scholar] [CrossRef]
- Manna, P.R.; Stocco, D.M. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J. Signal Transduct. 2011, 2011, 821615. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of insulin and insulin resistance in androgen excess disorders. World J. Diabetes 2021, 12, 616. [Google Scholar] [CrossRef]
- Wallace, I.R.; McKinley, M.C.; Bell, P.M.; Hunter, S.J. Sex hormone binding globulin and insulin resistance. Clin. Endocrinol. 2013, 78, 321–329. [Google Scholar] [CrossRef]
- Kwintkiewicz, J.; Giudice, L.C. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin. Reprod. Med. 2009, 27, 43–51. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Yan, H.; Wang, L.; Zhang, G.; Li, N.; Zhao, Y.; Liu, J.; Jiang, M.; Du, X.; Zeng, Q.; Xiong, D. Oxidative stress and energy metabolism abnormalities in polycystic ovary syndrome: From mechanisms to therapeutic strategies. Reprod. Biol. Endocrinol. 2024, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xing, C.; Zhang, J.; He, B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with pcos: A network meta-analysis. Reprod. Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Foda, A.M.; Ibrahim, S.S.; Ibrahim, S.M.; Elbaz, E.M. Pterostilbene ameliorates cognitive impairment in polycystic ovary syndrome rat model through improving insulin resistance via the irs-1/pi3k/akt/gsk-3β pathway: A comparative study with metformin. ACS Chem. Neurosci. 2024, 15, 3064–3077. [Google Scholar] [CrossRef]
- Sardana, K.; Muddebihal, A.; Sehrawat, M.; Bansal, P.; Khurana, A. An updated clinico-investigative approach to diagnosis of cutaneous hyperandrogenism in relation to adult female acne, female pattern alopecia & hirsutism a primer for dermatologists. Expert Rev. Endocrinol. Metab. 2024, 19, 111–128. [Google Scholar] [CrossRef]
- Jozkowiak, M.; Piotrowska-Kempisty, H.; Kobylarek, D.; Gorska, N.; Mozdziak, P.; Kempisty, B.; Rachon, D.; Spaczynski, R.Z. Endocrine disrupting chemicals in polycystic ovary syndrome: The relevant role of the theca and granulosa cells in the pathogenesis of the ovarian dysfunction. Cells 2022, 12, 174. [Google Scholar] [CrossRef]
- Kimura, S.; Matsumoto, T.; Matsuyama, R.; Shiina, H.; Sato, T.; Takeyama, K.-I.; Kato, S. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure. Trends Endocrinol. Metab. 2007, 18, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of androgen excess-related metabolic disturbances on granulosa cell function and follicular development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.N.M.; Amri, M.F.; Ugusman, A.; Hamid, A.A.; Wahab, N.A.; Mokhtar, M.H. Hyperandrogenism and its possible effects on endometrial receptivity: A review. Int. J. Mol. Sci. 2023, 24, 12026. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Jain, R.; Rais, M.; White, A.E.; Beer, T.M.; Kievit, P.; Winters-Stone, K.; Messaoudi, I.; Varlamov, O. Perpetuating effects of androgen deficiency on insulin resistance. Int. J. Obes. 2016, 40, 1856–1863. [Google Scholar] [CrossRef][Green Version]
- Vázquez, M.J.; Romero-Ruiz, A.; Tena-Sempere, M. Roles of leptin in reproduction, pregnancy and polycystic ovary syndrome: Consensus knowledge and recent developments. Metabolism 2015, 64, 79–91. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Kyrou, I.; Randeva, H.S. Polycystic ovary syndrome as a proinflammatory state: The role of adipokines. Curr. Pharm. Des. 2016, 22, 5535–5546. [Google Scholar] [CrossRef]
- Bai, H.; Ding, H.; Wang, M. Polycystic ovary syndrome (pcos): Symptoms, causes, and treatment. Clin. Exp. Obstet. Gynecol. 2024, 51, 126. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.; Deng, P.; Zhang, Y.; Liu, R.; Cai, D.; Xu, Q.; Jiang, X.; Sun, J.; Bai, W. The consequence and mechanism of dietary flavonoids on androgen profiles and disorders amelioration. Crit. Rev. Food Sci. Nutr. 2023, 63, 11327–11350. [Google Scholar] [CrossRef]
- Bril, F.; Ezeh, U.; Amiri, M.; Hatoum, S.; Pace, L.; Chen, Y.-H.; Bertrand, F.; Gower, B.; Azziz, R. Adipose tissue dysfunction in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2024, 109, 10–24. [Google Scholar] [CrossRef]
- Chen, L.; Liu, W.; Lai, S.; Li, Y.; Wang, X.; Zhang, H. Insulin resistance, serum visfatin, and adiponectin levels are associated with metabolic disorders in chronic hepatitis c virus-infected patients. Eur. J. Gastroenterol. Hepatol. 2013, 25, 935–941. [Google Scholar] [CrossRef]
- Wołodko, K.; Šentjurc, T.; Walewska, E.; Laniecka, E.; Jura, M.; Galvão, A. Increased susceptibility to diet-induced obesity in female mice impairs ovarian steroidogenesis: The role of elevated leptin signalling on nodal activity inhibition in theca cells. Mol. Metab. 2025, 91, 102062. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef]
- Deng, H.; Chen, Y.; Xing, J.; Zhang, N.; Xu, L. Systematic low-grade chronic inflammation and intrinsic mechanisms in polycystic ovary syndrome. Front. Immunol. 2024, 15, 1470283. [Google Scholar] [CrossRef]
- Oróstica, L.; Poblete, C.; Romero, C.; Vega, M. Pro-inflammatory markers negatively regulate irs1 in endometrial cells and endometrium from women with obesity and pcos. Reprod. Sci. 2020, 27, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Ye, Y.; Xia, L.; Feng, Y.; Zhang, A. Follicular fluid cytokine composition and oocyte quality of polycystic ovary syndrome patients with metabolic syndrome undergoing in vitro fertilization. Cytokine 2017, 91, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Immediata, V.; Ronchetti, C.; Spadaro, D.; Cirillo, F.; Levi-Setti, P.E. Oxidative stress and human ovarian response—From somatic ovarian cells to oocytes damage: A clinical comprehensive narrative review. Antioxidants 2022, 11, 1335. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on vegf & hif1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Fenkci, V.; Fenkci, S.; Yilmazer, M.; Serteser, M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. 2003, 80, 123–127. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (pcos): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Gharaei, R.; Mahdavinezhad, F.; Samadian, E.; Asadi, J.; Ashrafnezhad, Z.; Kashani, L.; Amidi, F. Antioxidant supplementations ameliorate pcos complications: A review of rcts and insights into the underlying mechanisms. J. Assist. Reprod. Genet. 2021, 38, 2817–2831. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Shi, C.; Xu, T.; Liu, B.; Zhou, L.; Jiang, L.; Liu, K. Efficacy and safety of dietary polyphenol administration as assessed by hormonal, glycolipid metabolism, inflammation and oxidative stress parameters in patients with pcos: A meta-analysis and systematic review. Crit. Rev. Food Sci. Nutr. 2024, 65, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Dar, I.H.; Bhat, S.A.; Ahmad, S. Flavonoids: Health benefits and their potential use in food systems. In Functional Food Products and Sustainable Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar]
- Feng, W.; Hao, Z.; Li, M. Isolation and structure identification of flavonoids. In Flavonoids, from Biosynthesis to Human Health; Justino, G.C., Ed.; Intech Open: London, UK, 2017; pp. 17–43. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Defining the role of flavonoid structure on cottonseed oil stabilization: Study of a-and c-ring substitution. J. Am. Oil Chem. Soc. 2007, 84, 129–136. [Google Scholar] [CrossRef]
- Sharma, A.; Singh Tuli, H.; Sharma, A.K. Chemistry and synthetic overview of flavonoids. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Springer: Singapore, 2019; pp. 23–38. [Google Scholar]
- Tunon, M.; Garcia-Mediavilla, M.; Sanchez-Campos, S.; González-Gallego, J. Potential of flavonoids as anti-inflammatory agents: Modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 2009, 10, 256–271. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Zhou, M.; Konigsberg, W.H.; Hao, C.; Pan, Y.; Sun, J.; Wang, X. Bioactivity and mechanisms of flavonoids in decreasing insulin resistance. J. Enzym. Inhib. Med. Chem. 2023, 38, 2199168. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.D.; Leal, A.E.B.P.; Silva, J.C.; Almeida, J.R.; de Oliveira, H.P. Influence of flavonoids on mechanism of modulation of insulin secretion. Pharmacogn. Mag. 2017, 13, 639. [Google Scholar] [CrossRef]
- Rajan, R.K.; M, S.S.K.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (pcos) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Ysrafil, Y.; Komara, N.K.; Kumalasari, M.R.; Nainu, F. Flavonoids and their advancement in pharmaceutical sciences. In Plant Secondary Metabolites; CRC Press: Boca Raton, FL, USA, 2025; pp. 310–340. [Google Scholar]
- Zhang, J.; Zhang, H.; Xin, X.; Zhu, Y.; Ye, Y.; Li, D. Efficacy of flavonoids on animal models of polycystic ovary syndrome: A systematic review and meta-analysis. Nutrients 2022, 14, 4128. [Google Scholar] [CrossRef]
- Spencer, J.P. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255S–3261S. [Google Scholar] [CrossRef]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free. Radic. Res. 2004, 38, 771–786. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M. Lipid-based delivery systems for flavonoids and flavonolignans: Liposomes, nanoemulsions, and solid lipid nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Kuche, K.; Bhargavi, N.; Dora, C.P.; Jain, S. Drug-phospholipid complex—A go through strategy for enhanced oral bioavailability. AAPS PharmSciTech 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Pardeshi, K.H.; Chavan, H.P.; Amrutkar, S.V. Pharmacotherapeutics and pharmacokinetics of herbal bioenhancers. Int. J. Food Eng. 2022, 21, 149–190. [Google Scholar]
- González-Paramás, A.M.; Ayuda-Durán, B.; Martínez, S.; González-Manzano, S.; Santos-Buelga, C. The mechanisms behind the biological activity of flavonoids. Curr. Med. Chem. 2019, 26, 6976–6990. [Google Scholar] [CrossRef]
- Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Bai, J.; Huang, L. Structural insights and biological activities of flavonoids: Implications for novel applications. Food Front. 2025, 6, 218–247. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Majee, C.; Mazumder, R.; Priya, N.; Atriya, A. Insight into the glycosylation methods of the flavonoids as an approach to enhance its bioavailability and pharmacological activities. Indian J. Pharm. Educ. Res. 2023, 57, 354–371. [Google Scholar] [CrossRef]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of nf-κb-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure–activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Yagasaki, K. Anti-diabetic phytochemicals that promote glut4 translocation via ampk signaling in muscle cells. Nutr. Aging 2014, 2, 35–44. [Google Scholar] [CrossRef]
- Bellavite, P.; Fazio, S.; Affuso, F. A descriptive review of the action mechanisms of berberine, quercetin and silymarin on insulin resistance/hyperinsulinemia and cardiovascular prevention. Molecules 2023, 28, 4491. [Google Scholar] [CrossRef]
- Bae, J.; Yang, Y.; Xu, X.; Flaherty, J.; Overby, H.; Hildreth, K.; Chen, J.; Wang, S.; Zhao, L. Naringenin, a citrus flavanone, enhances browning and brown adipogenesis: Role of peroxisome proliferator-activated receptor gamma. Front. Nutr. 2022, 9, 1036655. [Google Scholar] [CrossRef]
- Tang, G.; Xu, Y.; Zhang, C.; Wang, N.; Li, H.; Feng, Y. Green tea and epigallocatechin gallate (egcg) for the management of nonalcoholic fatty liver diseases (nafld): Insights into the role of oxidative stress and antioxidant mechanism. Antioxidants 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Hu, W.-S.; Hung, M.-Y.; Ou, H.-C.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Lin, K.-H.; Viswanadha, V.P.; Kuo, W.-W. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1032–1043. [Google Scholar] [CrossRef]
- Goswami, K.; Badruddeen, U.; Arif, M.; Akhtar, J.; Khan, M.I.; Ahmad, M. Flavonoids, isoflavonoids and others bioactives for insulin sensitizations. Curr. Diabetes Rev. 2024, 20, 92–102. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elfiky, A.M.; Abo-Zeid, F.S. The anti-androgenic effect of quercetin on hyperandrogenism and ovarian dysfunction induced in a dehydroepiandrosterone rat model of polycystic ovary syndrome. Steroids 2022, 177, 108936. [Google Scholar] [CrossRef]
- Ghafurniyan, H.; Azarnia, M.; Nabiuni, M.; Karimzadeh, L. The effect of green tea extract on reproductive improvement in estradiol valerate-induced polycystic ovarian syndrome in rat. Iran. J. Pharm. Res. IJPR 2015, 14, 1215–1233. [Google Scholar]
- Yao, J.; Zhang, Y.; Zhao, J.; Wang, X.-Z.; Lin, Y.-P.; Sun, L.; Lu, Q.-Y.; Fan, G.-J. Efficacy of flavonoids-containing supplements on insulin resistance and associated metabolic risk factors in overweight and obese subjects: A systematic review and meta-analysis of 25 randomized controlled trials. Front. Endocrinol. 2022, 13, 917692. [Google Scholar] [CrossRef]
- Merza, W.M.; Yaseen, A.K.; Mahmood, M.A. FSH, LH, lipid and adipokines in polycystic ovary syndrome: Clinical biochemistry insights for diagnosis and management. J. Steroid Biochem. Mol. Biol. 2025, 251, 106773. [Google Scholar] [CrossRef]
- Gulcelik, N.E.; Aral, Y.; Serter, R.; Koc, G. Association of hypoadiponectinemia with metabolic syndrome in patients with polycystic ovary syndrome. J. Natl. Med. Assoc. 2008, 100, 64–68. [Google Scholar] [CrossRef]
- Huang, H.; Park, P.H.; McMullen, M.R.; Nagy, L.E. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J. Gastroenterol. Hepatol. 2008, 23, S50–S53. [Google Scholar] [CrossRef]
- Mahmoudi, F. Evaluation of the effects of naringenin on the hypothalamic mrna levels of nesfatin-1 and crh in a rat model of pcos. Gene Cell Tissue 2024, 11, e154371. [Google Scholar]
- Lizcano, F.; Arroyave, F. Control of adipose cell browning and its therapeutic potential. Metabolites 2020, 10, 471. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of pcos: Possible involvement of ampk/sirt-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, Z.; Zhou, H.H.; Feng, Y.; Bu, Y.; Zhai, D.; Zhang, G.; Ding, S.; Wang, E.; Mi, Y. Effect of flavonoid intake on circulating levels of adiponectin and leptin: A systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2022, 36, 4139–4154. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Muthusami, S.; Periyasamy, L.; Stanley, J.A.; Gopalakrishnan, V.; Ramachandran, I. Effect of dht-induced hyperandrogenism on the pro-inflammatory cytokines in a rat model of polycystic ovary morphology. Medicina 2020, 56, 100. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 2025, 18, 34. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic effects of green tea polyphenol (–)-epigallocatechin-3-gallate (egcg) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Mahdiani, S.; Omidkhoda, N.; Heidari, S.; Hayes, A.W.; Karimi, G. Protective effect of luteolin against chemical and natural toxicants by targeting nf-κb pathway. Biofactors 2022, 48, 744–762. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín, M.Á.; Bravo, L.; Goya, L.; Ramos, S. Quercetin attenuates tnf-induced inflammation in hepatic cells by inhibiting the nf-κb pathway. Nutr. Cancer 2012, 64, 588–598. [Google Scholar] [CrossRef]
- Goleij, P.; Tabari, M.A.K.; Khandan, M.; Poudineh, M.; Rezaee, A.; Sadreddini, S.; Sanaye, P.M.; Khan, H.; Larsen, D.S.; Daglia, M. Genistein in focus: Pharmacological effects and immune pathway modulation in cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 3557–3571. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory roles of flavonoids on inflammasome activation during inflammatory responses. Mol. Nutr. Food Res. 2018, 62, 1800147. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Lima, J.L.; Fernandes, E. Proinflammatory pathways: The modulation by flavonoids. Med. Res. Rev. 2015, 35, 877–936. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Kayedpoor, P.; Karimzadeh-Bardei, L.; Nabiuni, M. The effect of curcumin on tnf-α, il-6 and crp expression in a model of polycystic ovary syndrome as an inflammation state. J. Reprod. Infertil. 2017, 18, 352–360. [Google Scholar] [PubMed]
- Lee, C.Y.; Sharma, A.; Uzarski, R.L.; Cheong, J.E.; Xu, H.; Held, R.A.; Upadhaya, S.K.; Nelson, J.L. Potent antioxidant dendrimers lacking pro-oxidant activity. Free Radic. Biol. Med. 2011, 50, 918–925. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Pereira, O.R.; Cardoso, S.M. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Curr. Top. Med. Chem. 2015, 15, 105–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, C.; Huang, P.; Cheng, Y.; Ma, Y.; Gao, J.; Ding, H. Luteolin alleviates muscle atrophy, mitochondrial dysfunction and abnormal fndc5 expression in high fat diet-induced obese rats and palmitic acid-treated c2c12 myotubes. J. Nutr. Biochem. 2025, 135, 109780. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, W.; Zhu, H.; Liu, C.; Qu, F.; Zhou, J. Flavonoid supplementation is beneficial for polycystic ovary syndrome: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e829–e837. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Kowalczyk, K.; Trybek, P.; Jarosz, T.; Radosz, P.; Setlak, M.; Madej, P. In search of new therapeutics—Molecular aspects of the pcos pathophysiology: Genetics, hormones, metabolism and beyond. Int. J. Mol. Sci. 2020, 21, 7054. [Google Scholar] [CrossRef]
- Jonard, S.; Dewailly, D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum. Reprod. Update 2004, 10, 107–117. [Google Scholar] [CrossRef]
- Dashputre, N.L.; Laddha, D.U.; Patil, M.N.K.; Dhopare, M.V.D. Nano Formulated Daidzein and Genistein as a Multi-Targeted Therapeutic Strategy for Letrozole-Induced PCOS in Rats. Available online: https://ssrn.com/abstract=5401775 (accessed on 14 October 2025).
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Medica 2008, 74, 1656–1665. [Google Scholar] [CrossRef]
- Martin, L.J.; Touaibia, M. Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Zilaee, M.; Mansoori, A.; Ahmad, H.S.; Mohaghegh, S.M.; Asadi, M.; Hormoznejad, R. The effects of soy isoflavones on total testosterone and follicle-stimulating hormone levels in women with polycystic ovary syndrome: A systematic review and meta-analysis. Eur. J. Contracept. Reprod. Health Care 2020, 25, 305–310. [Google Scholar] [CrossRef]
- Lee, J.; An, S.; Kim, Y.W.; Choi, L.Y.; Kim, D.Y.; Kim, M.H. Comparative advantage and efficacy of natural products for polycystic ovary syndrome. J. Ovarian Res. 2025, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xiang, Q.; Song, G.; Wang, X. Quercetin and polycystic ovary syndrome. Front. Pharmacol. 2022, 13, 1006678. [Google Scholar] [CrossRef] [PubMed]
- Pourteymour Fard Tabrizi, F.; Hajizadeh-Sharafabad, F.; Vaezi, M.; Jafari-Vayghan, H.; Alizadeh, M.; Maleki, V. Quercetin and polycystic ovary syndrome, current evidence and future directions: A systematic review. J. Ovarian Res. 2020, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, M.; Zhang, Y.; Jin, H.; Zhang, W.; Yang, J.; Yan, L.; Li, R.; Zhao, Y.; Qiao, J. High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of dhea-induced pcos. Reproduction 2016, 151, 431–441. [Google Scholar] [CrossRef]
- Liu, H.; Guan, H.; Tan, X.; Jiang, Y.; Li, F.; Sun-Waterhouse, D.; Li, D. Enhanced alleviation of insulin resistance via the irs-1/akt/foxo1 pathway by combining quercetin and egcg and involving mir-27a-3p and mir-96–5p. Free Radic. Biol. Med. 2022, 181, 105–117. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Chang, H.; Lu, F.; Guan, M.; Wu, X. Effect and mechanism of resveratrol against polycystic ovary syndrome: A review. Front. Endocrinol. 2025, 16, 1529231. [Google Scholar] [CrossRef]
- Ye, Y.-B.; He, K.-Y.; Li, W.-L.; Zhuo, S.-Y.; Chen, Y.-M.; Lu, W.; Wu, S.-L.; Liu, J.; Li, Y.-B.; Zeng, F.-F. Effects of daidzein and genistein on markers of cardiovascular disease risk among women with impaired glucose regulation: A double-blind, randomized, placebo-controlled trial. Food Funct. 2021, 12, 7997–8006. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Intharuksa, A.; Arunotayanun, W.; Na Takuathung, M.; Chaichit, S.; Prasansuklab, A.; Chaikhong, K.; Sirichanchuen, B.; Chupradit, S.; Koonrungsesomboon, N. Daidzein and genistein: Natural phytoestrogens with potential applications in hormone replacement therapy. Int. J. Mol. Sci. 2025, 26, 6973. [Google Scholar] [CrossRef]
- Meena, R.; Supriya, C.; Reddy, K.P.; Reddy, P.S. Altered spermatogenesis, steroidogenesis and suppressed fertility in adult male rats exposed to genistein, a non-steroidal phytoestrogen during embryonic development. Food Chem. Toxicol. 2017, 99, 70–77. [Google Scholar] [CrossRef]
- Legro, R.S. Ovulation induction in polycystic ovary syndrome: Current options. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 152–159. [Google Scholar] [CrossRef]
- Mazumder, A.; Singh, A.; Jha, S. A review on metformin: Clinical significance and side effects. Int. J. Pharm. Res. 2021, 13, 60–69. [Google Scholar] [CrossRef]
- Garthwaite, H.; Stewart, J.; Wilkes, S. Multiple pregnancy rate in patients undergoing treatment with clomifene citrate for who group ii ovulatory disorders: A systematic review. Hum. Fertil. 2022, 25, 618–624. [Google Scholar] [CrossRef]
- Beekmann, K.; de Haan, L.H.; Actis-Goretta, L.; Houtman, R.; van Bladeren, P.J.; Rietjens, I.M. The effect of glucuronidation on isoflavone induced estrogen receptor (er) α and erβ mediated coregulator interactions. J. Steroid Biochem. Mol. Biol. 2015, 154, 245–253. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Chemuturi, N.V.; Womble, S.W.; Sayre, C.L.; Davies, N.M. Flavonoids and drug interactions. In Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 281–319. [Google Scholar]
- Luo, E.-D.; Jiang, H.-M.; Chen, W.; Wang, Y.; Tang, M.; Guo, W.-M.; Diao, H.-Y.; Cai, N.-Y.; Yang, X.; Bian, Y. Advancements in lead therapeutic phytochemicals polycystic ovary syndrome: A review. Front. Pharmacol. 2023, 13, 1065243. [Google Scholar] [CrossRef]
- Guven, H.; Arici, A.; Simsek, O. Flavonoids in our foods: A short review. J. Basic Clin. Health Sci. 2019, 3, 96–106. [Google Scholar] [CrossRef]
- Adu, M.D.; Bondonno, C.P.; Parmenter, B.H.; Sim, M.; Davey, R.J.; Murray, K.; Radavelli-Bagatini, S.; Magliano, D.J.; Daly, R.M.; Shaw, J.E. Association between non-tea flavonoid intake and risk of type 2 diabetes: The australian diabetes, obesity and lifestyle study. Food Funct. 2022, 13, 4459–4468. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C.; Pizzorno, J. Consideration for flavonoid-containing dietary supplements to tackle deficiency and optimize health. Int. J. Mol. Sci. 2023, 24, 8663. [Google Scholar] [CrossRef]
- Asbaghi, O.; Kelishadi, M.R.; Larky, D.A.; Bagheri, R.; Amirani, N.; Goudarzi, K.; Kargar, F.; Ghanavati, M.; Zamani, M. The effects of green tea extract supplementation on body composition, obesity-related hormones and oxidative stress markers: A grade-assessed systematic review and dose–response meta-analysis of randomised controlled trials. Br. J. Nutr. 2024, 131, 1125–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Siddiqa, A.; Qureshi, R.; Yasmin, G.; Rafique, S.; Zafar, N.-U.-A.; Hussain, C.S.; Rehman, S.U.; Naheed, N. Unlocking the dual healing powers of plant-based metallic nanoparticles: Managing diabetes and tackling male infertility challenges. Front. Endocrinol. 2025, 16, 1482127. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.; Fernandes, A.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E. Lipid nanocarriers for the loading of polyphenols–a comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Asghar, A.; Randhawa, M.A.; Masood, M.M.; Abdullah, M.; Irshad, M.A. Nutraceutical formulation strategies to enhance the bioavailability and efficiency: An overview. In Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 329–352. [Google Scholar]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G. Nanodelivery of dietary polyphenols for therapeutic applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C. Flavonoid-based nanogels: A comprehensive overview. Gels 2025, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.; Wu, S.; Deng, Z.; Tang, Y.; Guan, Y.; Ye, Y.; He, Q.; Li, L. Multi-omics approach to reveal follicular metabolic changes and their effects on oocyte competence in pcos patients. Front. Endocrinol. 2024, 15, 1426517. [Google Scholar] [CrossRef] [PubMed]
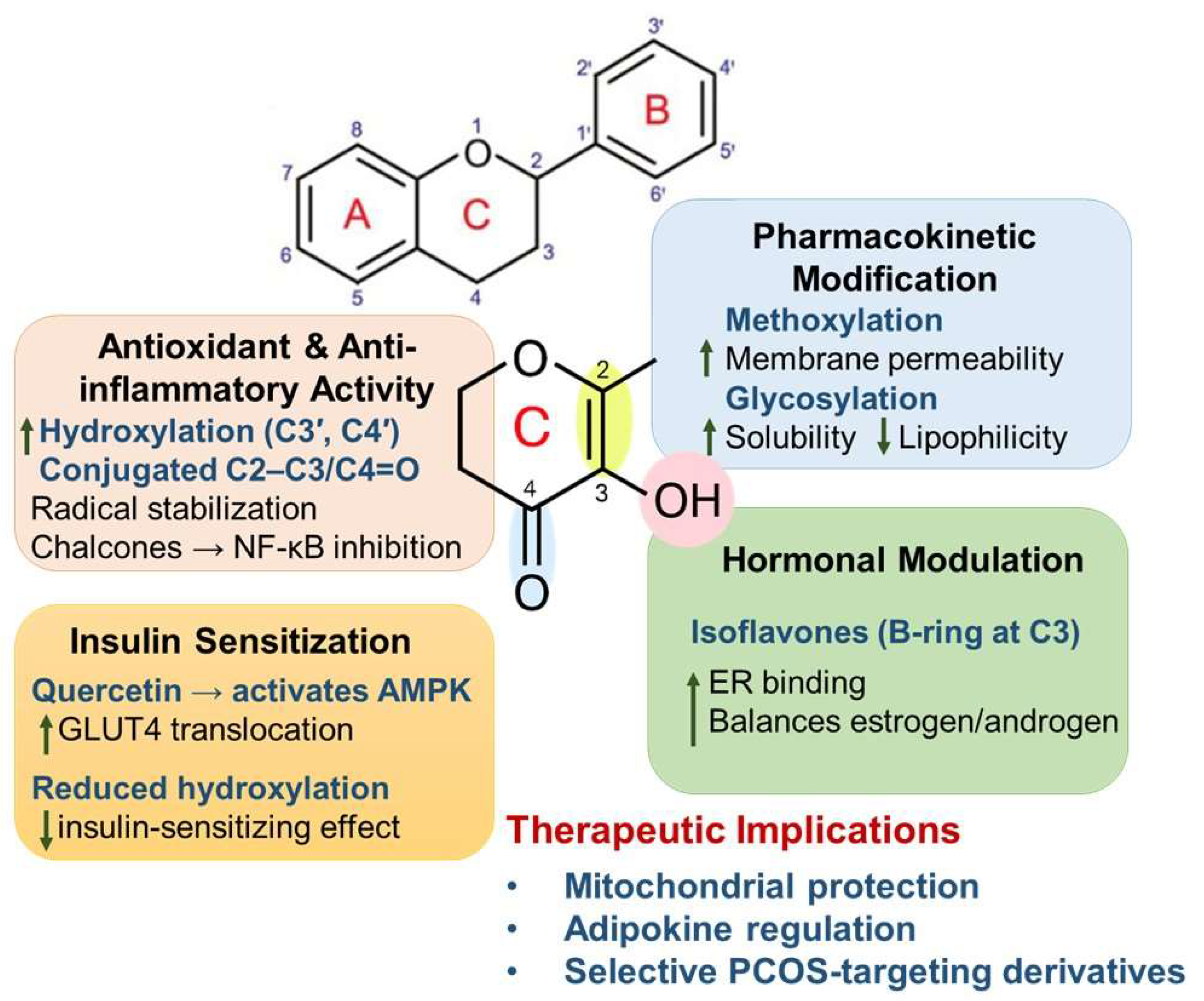
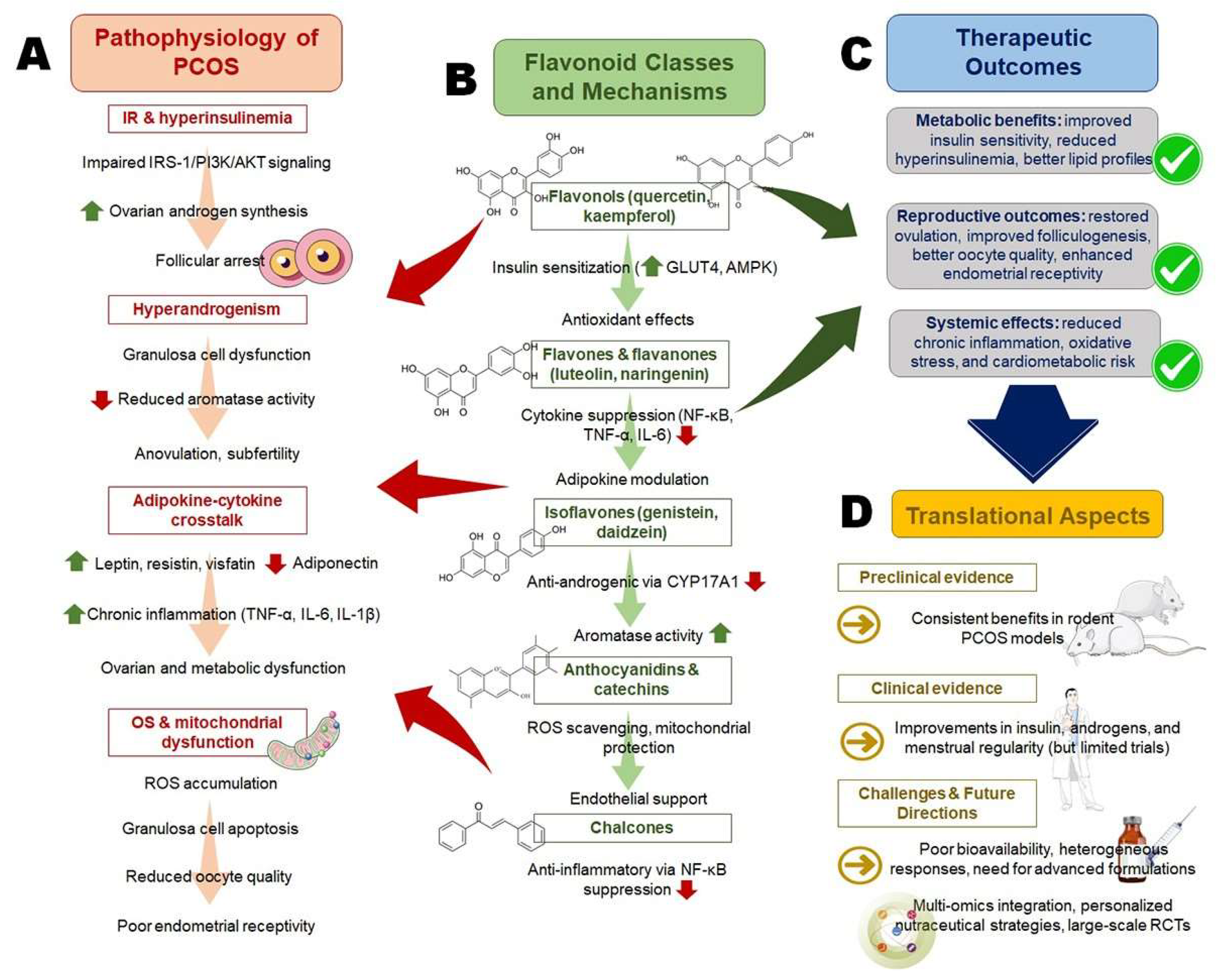
| Flavonoid Subclass (Examples) | Primary Molecular Targets | Pathophysiological Pathway in PCOS | Reported Clinical/Experimental Outcomes | References |
|---|---|---|---|---|
| Flavonols (Quercetin, Kaempferol) | IRS-1/PI3K/AKT, AMPK | Insulin resistance, hyperinsulinemia | ↓ Fasting insulin, improved HOMA-IR, ↓ serum testosterone, restored ovulation | [76,81,113,114] |
| Flavones (Luteolin, Apigenin) | NF-κB, COX-2, NLRP3 inflammasome | Chronic inflammation, cytokine excess | ↓ TNF-α, IL-6, IL-1β; restored ovarian folliculogenesis | [50,95,98,100] |
| Flavanones (Naringenin, Hesperidin) | PPARγ, adipokine regulation | Adipose tissue dysfunction, dyslipidemia | ↑ Adiponectin, ↓ leptin; improved lipid profiles, improved ovulation in models | [53,77,87] |
| Isoflavones (Genistein, Daidzein) | Estrogen receptors, CYP17A1 | Hyperandrogenism, follicular arrest | ↓ Serum androgens, ↑ estradiol, improved menstrual cyclicity, improved endometrial receptivity | [54,109,111] |
| Catechins/Anthocyanidins (EGCG, Cyanidin) | ROS scavenging, Nrf2 pathway, mitochondrial biogenesis | Oxidative stress, mitochondrial dysfunction | ↓ ROS, ↑ antioxidant enzymes, improved oocyte quality, reduced cystic follicles | [78,79,94,104] |
| Flavonoid | Model/Population Studied | Dose and Duration | Major Findings | Clinical Implications | References |
|---|---|---|---|---|---|
| Quercetin | Letrozole/DHEA rat models; women with PCOS | 50–150 mg/kg in rodents; 500–1000 mg/day in women for 8–12 weeks | ↓ Serum testosterone, improved insulin sensitivity, restored ovulation | Potential adjunct to metformin for metabolic + reproductive benefits | [81,89,113,114] |
| Naringenin | DHEA-induced PCOS rats | 50–100 mg/kg for 4–6 weeks | ↑ Adiponectin, ↓ systemic inflammation, improved estrous cycles | Targeting obese/insulin-resistant PCOS phenotypes | [77,87,90] |
| EGCG (green tea catechin) | PCOS rodent models; small RCTs | 50–100 mg/kg in rodents; 300–600 mg/day in humans | ↓ ROS, improved folliculogenesis, reduced BMI, improved ovulation | Nutraceutical for oxidative-stress-driven PCOS | [78,82,94,105] |
| Isoflavones (Genistein/Daidzein) | Rodent PCOS models; Asian PCOS women | 30–50 mg/day for 12 weeks | ↓ Total testosterone, ↑ estradiol, improved menstrual regularity | Dietary intervention with hormone-modulatory benefits | [54,108,111,118] |
| Resveratrol (flavonoid-like stilbene) | PCOS women; granulosa cell studies | 1000–1500 mg/day for 3 months | ↓ Theca cell androgen production, improved insulin sensitivity, ↓ inflammatory cytokines | Potential anti-androgenic nutraceutical, but bioavailability limits use | [41,73,117] |
| Luteolin | Rodent PCOS models; in vitro ovarian/adipose cells | 25–50 mg/kg (rodents), 4–6 weeks (typical preclinical) | ↓TNF-α, IL-6, IL-1β via NF-κB/NLRP3 inhibition; ↑AMPK activity; improved insulin signaling; reduced ovarian inflammatory infiltration; improved folliculogenesis | Anti-inflammatory + insulin-sensitizing candidate; supports ovulatory function and metabolic control in PCOS | [79,95,104] |
| Apigenin | Rodent PCOS models; granulosa/adipocyte cultures | 25–50 mg/kg (rodents), 4–8 weeks | Suppresses NF-κB/COX-2; mitigates oxidative stress; improves HOMA-IR surrogates in models; supports granulosa cell survival | Anti-inflammatory/antioxidant with potential to improve IR and ovarian microenvironment | [31,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Sengupta, P.; Rao, S.; Elgarawany, G.E.; Samrot, A.V.; Rosas, I.M.; Roychoudhury, S. Targeting Polycystic Ovary Syndrome (PCOS) Pathophysiology with Flavonoids: From Adipokine–Cytokine Crosstalk to Insulin Resistance and Reproductive Dysfunctions. Pharmaceuticals 2025, 18, 1575. https://doi.org/10.3390/ph18101575
Dutta S, Sengupta P, Rao S, Elgarawany GE, Samrot AV, Rosas IM, Roychoudhury S. Targeting Polycystic Ovary Syndrome (PCOS) Pathophysiology with Flavonoids: From Adipokine–Cytokine Crosstalk to Insulin Resistance and Reproductive Dysfunctions. Pharmaceuticals. 2025; 18(10):1575. https://doi.org/10.3390/ph18101575
Chicago/Turabian StyleDutta, Sulagna, Pallav Sengupta, Sowmya Rao, Ghada Elsayed Elgarawany, Antony Vincent Samrot, Israel Maldonado Rosas, and Shubhadeep Roychoudhury. 2025. "Targeting Polycystic Ovary Syndrome (PCOS) Pathophysiology with Flavonoids: From Adipokine–Cytokine Crosstalk to Insulin Resistance and Reproductive Dysfunctions" Pharmaceuticals 18, no. 10: 1575. https://doi.org/10.3390/ph18101575
APA StyleDutta, S., Sengupta, P., Rao, S., Elgarawany, G. E., Samrot, A. V., Rosas, I. M., & Roychoudhury, S. (2025). Targeting Polycystic Ovary Syndrome (PCOS) Pathophysiology with Flavonoids: From Adipokine–Cytokine Crosstalk to Insulin Resistance and Reproductive Dysfunctions. Pharmaceuticals, 18(10), 1575. https://doi.org/10.3390/ph18101575









