Solidification Materials and Technology for Solid Self-Emulsifying Drug Delivery Systems
Abstract
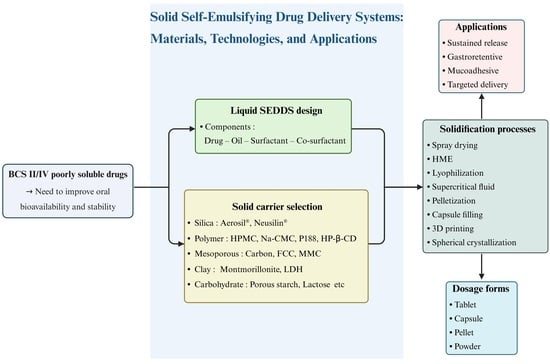
1. Introduction
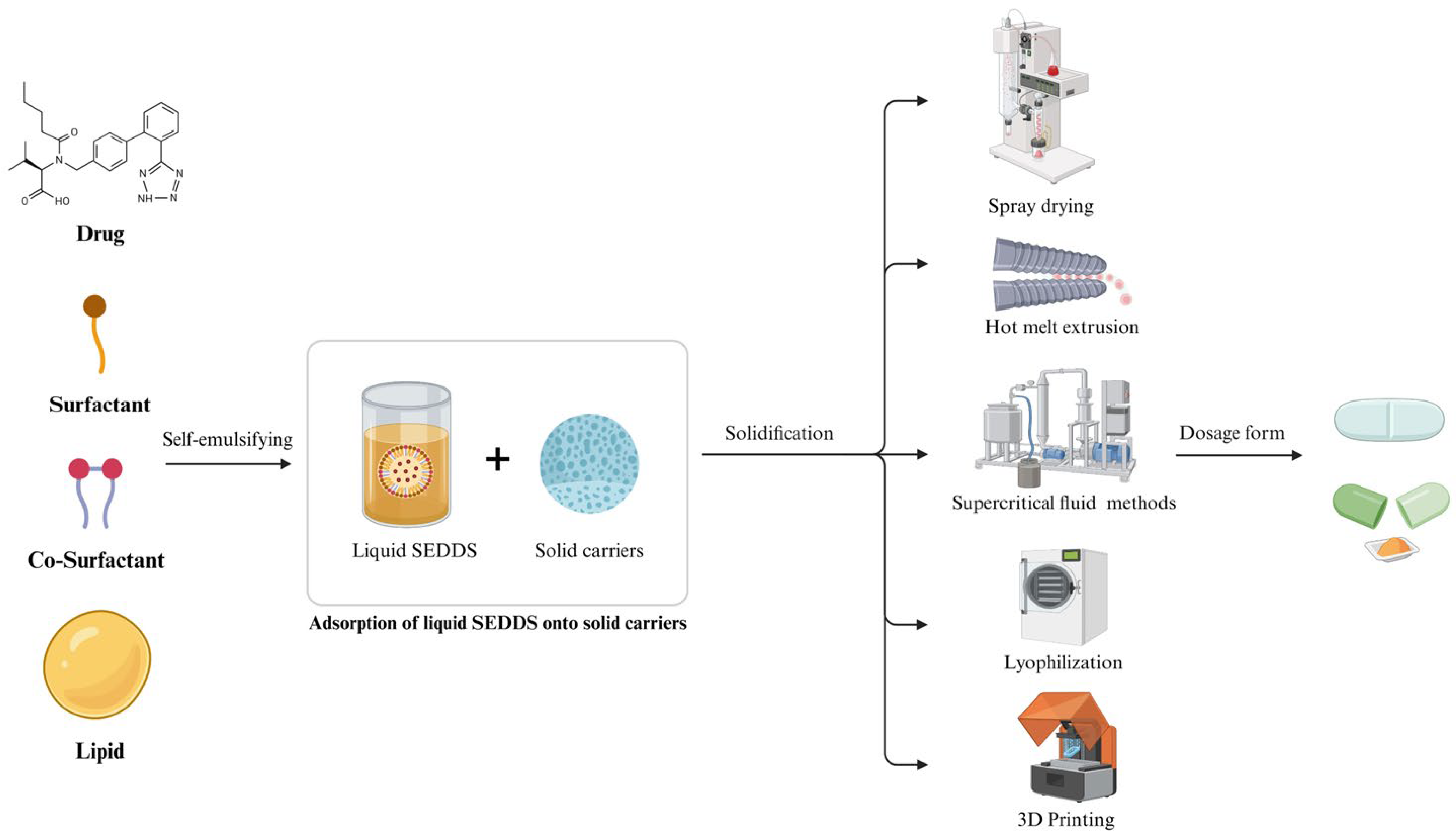
2. Liquid SEDDS
2.1. Lipids/Oils
2.2. Surfactants
2.3. Co-Surfactants/Co-Solvents
2.4. Characteristics of Suitable Drugs for SEDDS
3. Solid SEDDS
4. Role and Types of Solid Carriers for Solid SEDDS
4.1. Silica and Silicate-Based Carriers
4.2. Polymer-Based Carriers
4.3. Inorganic Mesoporous Materials
4.3.1. Mesoporous Carbon
4.3.2. Porous Carbonate Salts
4.3.3. Clay-Based Materials
4.4. Carbohydrate-Based Materials
5. Manufacturing Methods for Solid SEDDS
5.1. Spherical Crystallization Technology
5.2. Spray Drying
5.3. Supercritical Fluid (SCF)-Based Methods
5.4. Adsorption onto a Solid Carrier
5.5. Hot Melt Extrusion (HME)
5.6. Pellet Manufacturing Technology
5.7. Manufacturing Solid SEDDS Using Hard Gelatin Capsules
5.8. Lyophilization (Freeze-Drying)
5.9. Three-Dimensional Printing (Three-Dimensional Printing)
6. Applications of Solid SEDDS
6.1. Enhanced Bioavailability and Protein Delivery
6.2. Controlled Drug Release
6.3. Mucoadhesive Technology
6.4. Targeted Drug Delivery
6.5. Personalized Medicine
7. Challenges and Limitations of Solid SEDDS
7.1. Low Drug Loading
7.2. Stability and Compatibility Issues
7.3. Key Commercial Considerations
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEDDS | Self-Emulsifying Drug Delivery Systems |
| BCS | Biopharmaceutics Classification System |
| LDDS | Lipid-based Drug Delivery System |
| MCTs | Medium-chain triglycerides |
| LCTs | Long-chain triglycerides |
| HPMC | Hydroxypropyl methylcellulose |
| Na-CMC | Sodium carboxymethylcellulose |
| FCC | Functionalized calcium carbonate |
| LDHs | Layered Double Hydroxides |
| SCF | Supercritical Fluid |
| FDM | Fused Deposition Modeling |
References
- Rocha, B.; de Morais, L.A.; Viana, M.C.; Carneiro, G. Promising strategies for improving oral bioavailability of poor water-soluble drugs. Expert Opin. Drug Discov. 2023, 18, 615–627. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in solubilization approaches: A step towards bioavailability enhancement of poorly soluble drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, M.A.; Rautio, J.; Meanwell, N.A. Prodrugs as empowering tools in drug discovery and development: Recent strategic applications of drug delivery solutions to mitigate challenges associated with lead compounds and drug candidates. Chem. Soc. Rev. 2024, 53, 2099–2210. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Woo, M.R.; Baek, K.; Kim, J.S.; Kim, J.O.; Choi, Y.S.; Choi, H.G.; Jin, S.G. Novel rivaroxaban-loaded microsphere systems with different surface microstructure for enhanced oral bioavailability. Drug Deliv. Transl. Res. 2024, 14, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Woo, M.R.; Choi, Y.S.; Kang, M.J.; Kim, J.O.; Choi, H.G.; Jin, S.G. Engineering sodium alginate microparticles with different crystallinities for niclosamide repositioning and solubilization to improve solubility and oral bioavailability in rats. Int. J. Biol. Macromol. 2024, 283, 137471. [Google Scholar] [CrossRef]
- Patel, D.; Solanki, J.; Kher, M.M.; Azagury, A. A review: Surface engineering of lipid-based drug delivery systems. Small 2024, 20, 2401990. [Google Scholar] [CrossRef]
- Baek, K.; Woo, M.R.; ud Din, F.; Choi, Y.S.; Kang, M.J.; Kim, J.O.; Choi, H.G.; Jin, S.G. Comparison of Solid Self-Nanoemulsifying Systems and Surface-Coated Microspheres: Improving Oral Bioavailability of Niclosamide. Int. J. Nanomed. 2024, 19, 13857–13874. [Google Scholar] [CrossRef]
- Kesharwani, R.; Jaiswal, P.; Patel, D.K.; Yadav, P.K. Lipid-based drug delivery system (LBDDS): An emerging paradigm to enhance oral bioavailability of poorly soluble drugs. Biomed. Mater. Devices 2023, 1, 648–663. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Karpathak, S.; Rai, M.K.; Kumar, D.; Misra, D.P.; Agarwal, V. Lipid based drug delivery systems for oral, transdermal and parenteral delivery: Recent strategies for targeted delivery consistent with different clinical application. J. Drug Deliv. Sci. Technol. 2023, 85, 104526. [Google Scholar] [CrossRef]
- Zupančič, O.; Matić, J.; Doğan, A.; Gaggero, A.; Khinast, J.; Paudel, A. Comparing Low-Dose Carvedilol Continuous Manufacturing by Solid and Liquid Feeding in Self-Emulsifying Delivery Systems via Hot Melt EXtrusion (SEDEX). Pharmaceuticals 2024, 17, 1290. [Google Scholar] [CrossRef]
- Rehman, F.U.; Farid, A.; Shah, S.U.; Dar, M.J.; Rehman, A.U.; Ahmed, N.; Rashid, S.A.; Shaukat, I.; Shah, M.; Albadrani, G.M.; et al. Self-emulsifying drug delivery systems (SEDDS): Measuring energy dynamics to determine thermodynamic and kinetic stability. Pharmaceuticals 2022, 15, 1064. [Google Scholar] [CrossRef]
- Silva-Neto, A.F.; de Carvalho Amaral, A.R.; de Alencar Danda, L.J.; Chaves, L.L.; Alves, L.D.S.; Soares, M.F.D.L.R.; Soares-Sobrinho, J.L. Decoding excipients in lipid-based self-emulsifying drug delivery systems: Insights into physicochemical properties and therapeutic outcomes. Int. J. Pharm. 2025, 683, 126018. [Google Scholar] [CrossRef]
- Khairnar, H.; Jain, S.; Chatterjee, B. Lactoferrin reduces surfactant content in the self-emulsifying drug delivery system. ACS Omega 2024, 9, 13612–13620. [Google Scholar] [CrossRef]
- Friedl, J.D.; Jörgensen, A.M.; Le-Vinh, B.; Braun, D.E.; Tribus, M.; Bernkop-Schnürch, A. Solidification of self-emulsifying drug delivery systems (SEDDS): Impact on storage stability of a therapeutic protein. J. Colloid Interface Sci. 2021, 584, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.B.; du Plessis, L.H.; Viljoen, J.M. Solidification of self-emulsifying drug delivery systems as a novel approach to the management of uncomplicated malaria. Pharmaceuticals 2022, 15, 120. [Google Scholar] [CrossRef] [PubMed]
- Uttreja, P.; Karnik, I.; Adel Ali Youssef, A.; Narala, N.; Elkanayati, R.M.; Baisa, S.; Alshammari, N.D.; Banda, S.; Vemula, S.K.; Repka, M.A. Self-emulsifying drug delivery systems (SEDDS): Transition from liquid to solid—A comprehensive review of formulation, characterization, applications, and future trends. Pharmaceutics 2025, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, J.S.; Choi, M.J.; Baek, K.; Woo, M.R.; Kim, J.O.; Choi, H.G.; Jin, S.G. Effects of different physicochemical characteristics and supersaturation principle of solidified SNEDDS and surface-modified microspheres on the bioavailability of carvedilol. Int. J. Pharm. 2021, 597, 120377. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, J.S.; Yu, H.; Woo, M.R.; Choi, J.E.; Baek, K.; Kim, J.O.; Choi, Y.S.; Choi, H.G.; Jin, S.G. Comparison of the physicochemical properties, aqueous solubility, and oral bioavailability of rivaroxaban-loaded high-pressure homogenised and Shirasu porous glass membrane emulsified solid self-nanoemulsifying drug delivery systems. J. Mol. Liq. 2022, 346, 117057. [Google Scholar] [CrossRef]
- Jang, H.; Kim, N.; Jin, S.G. Development of a Carvedilol-Loaded Solid Self-Nanoemulsifying System with Increased Solubility and Bioavailability Using Mesoporous Silica Nanoparticles. Int. J. Mol. Sci. 2025, 26, 1592. [Google Scholar] [CrossRef]
- Govindan, I.; Rama, A.; Kailas, A.A.; Hebbar, S.; Naha, A. Transformative solidification techniques for self-emulsifying drug delivery and its foresight in modern-day drug delivery. J. Appl. Pharma. Sci. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Bruschi, M.L. Self-emulsifying systems for delivery of bioactive compounds from natural origin. AAPS PharmSciTech 2022, 23, 134. [Google Scholar] [CrossRef]
- Shahba, A.A.W.; Sherif, A.Y.; Elzayat, E.M.; Ali, S.; Kazi, M. Layer-by-Layer Engineering of Black Seed Oil Based SNEDDSs (BSO-SNEDDSs): Optimizing Chemical Stability and Bioavailability in Ramipril Formulations. Int. J. Nanomed. 2025, 20, 4415–4432. [Google Scholar] [CrossRef]
- Zulfakar, M.H.; Pubadi, H.; Ibrahim, S.I.; Hairul, N.M. Medium-Chain Triacylglycerols (MCTs) and Their Fractions in Drug Delivery Systems: A Systematic Review. J. Oleo Sci. 2024, 73, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Millotti, G.; Lagast, J. An overview of oral bioavailability enhancement through self-emulsifying drug delivery systems. Expert Opin. Drug Deliv. 2025, 22, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kojima, H.; Sako, K.; Kondo, H. Drug delivery to the intestinal lymph by oral formulations. Pharm. Dev. Technol. 2022, 27, 175–189. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Shegokar, R. Nanostructured lipid carriers (NLCs) for drug delivery: Role of liquid lipid (oil). Curr. Drug Deliv. 2021, 18, 249–270. [Google Scholar] [CrossRef]
- Le-Vinh, B.; Le, N.M.N.; Phan, T.N.Q.; Matuszczak, B.; Bernkop-Schnuerch, A. Effects of polymeric surfactants with low HLB values on drug and excipient release from self-emulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2024, 91, 105199. [Google Scholar] [CrossRef]
- Oliveira, L.T.; Castanheira, R.G.; Vilela, J.M.C.; Andrade, M.S.; de Oliveira, M.A.; Mosqueira, V.C.F. Impact of non-ionic surfactants on release kinetics, toxicity and colloidal characteristics of benznidazole self-emulsifying delivery system evidenced by flow field-flow fractionation. J. Chromatogr. A 2025, 1740, 465565. [Google Scholar] [CrossRef]
- Rathod, S.; Desai, H.; Patil, R.; Sarolia, J. Non-ionic surfactants as a P-glycoprotein (P-gp) efflux inhibitor for optimal drug delivery—A concise outlook. AAPS PharmSciTech 2022, 23, 55. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Patra, C.N.; Rao, M.B.; Jena, G.K.; Sahoo, L. SEDDS basic design and recent formulation advancement: A concurrent review. Pharm. Nanotechnol. 2022, 10, 289–298. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Sanchez-Lopez, E.; Santos, T.D.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and characterization of nanoemulsions for ophthalmic applications: Role of cationic surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef]
- Asad, M.; Rasul, A.; Abbas, G.; Shah, M.A.; Nazir, I. Self-emulsifying drug delivery systems: A versatile approach to enhance the oral delivery of BCS class III drug via hydrophobic ion pairing. PLoS ONE 2023, 18, e0286668. [Google Scholar] [CrossRef]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent advances in improving the bioavailability of hydrophobic/lipophilic drugs and their delivery via self-emulsifying formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Spleis, H.; Federer, C.; Claus, V.; Sandmeier, M.; Bernkop-Schnurch, A. Hydrophobic ion pairing of small molecules: How to minimize premature drug release from SEDDS and reach the absorption membrane in intact form. ACS Biomater. Sci. Eng. 2023, 9, 1450–1459. [Google Scholar] [CrossRef]
- Koehl, N.J.; Henze, L.J.; Holm, R.; Kuentz, M.; Keating, J.J.; De Vijlder, T.; Marx, A.; Griffin, B.T. Lipophilic salts and lipid-based formulations for bridging the food effect gap of venetoclax. J. Pharm. Sci. 2022, 111, 164–174. [Google Scholar] [CrossRef]
- Sethi, S.; Rana, V. Atazanavir-Concentrate Loaded Soft Gelatin Capsule for Enhanced Concentration in Plasma, Brain, Spleen, and Lymphatics. AAPS PharmSciTech 2022, 23, 270. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, J.S.; Lim, S.J.; Kim, J.O.; Yong, C.S.; Choi, H.G.; Jin, S.G. Comparison of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol-loaded self-emulsifying granule and solid self-nanoemulsifying drug delivery system: Powder property, dissolution and oral bioavailability. Pharmaceutics 2019, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Un Din, F.; Lee, H.I.; Kim, J.S.; Woo, M.R.; Cheon, S.; Park, S.; Woo, S.; Jin, S.G.; Choi, H.G. Physicochemical characterization and in vivo assessment of novel apixaban-loaded polymeric nano-aggregates. J. Pharm. Investig. 2025, 55, 707–719. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Seow, E.K.; Lim, S.C.; Yuen, K.H.; Khan, N.A.K. Formulation of oily tocotrienols as a solid self-emulsifying dosage form for improved oral bioavailability in human subjects. J. Drug Deliv. Sci. Technol. 2022, 76, 103752. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Kim, K.S.; Jin, S.G.; Choi, H.G. Development of novel D-cycloserine tablet with improvement of drug stability and dissolution-equivalence to the D-cycloserine-loaded commercial hard capsule. Bull. Korean Chem. Soc. 2020, 41, 603–608. [Google Scholar] [CrossRef]
- Gul, S.; Sridhar, S.B.; Jalil, A.; Akhlaq, M.; Arshad, M.S.; Sarwar, H.S.; Usman, F.; Shareef, J.; Thomas, S. Solid self-nanoemulsifying drug delivery systems of furosemide: In vivo proof of concept for enhanced predictable therapeutic response. Pharmaceuticals 2024, 17, 500. [Google Scholar] [CrossRef] [PubMed]
- Sanil, K.; Almotairy, A.; Uttreja, P.; Ashour, E.A. Formulation development and evaluation of cannabidiol hot-melt extruded solid self-emulsifying drug delivery system for oral applications. AAPS PharmSciTech 2024, 25, 136. [Google Scholar] [CrossRef] [PubMed]
- Uti, D.E.; Alum, E.U.; Atangwho, I.J.; Ugwu, O.P.C.; Egbung, G.E.; Aja, P.M. Lipid-based nano-carriers for the delivery of anti-obesity natural compounds: Advances in targeted delivery and precision therapeutics. J. Nanobiotechnol. 2025, 23, 336. [Google Scholar] [CrossRef]
- Kim, J.S.; Cheon, S.; Woo, M.R.; Woo, S.; Chung, J.E.; Youn, Y.S.; Oh, K.T.; Lim, S.J.; Ku, S.K.; Nguyen, B.L.; et al. Electrostatic spraying for fine-tuning particle dimensions to enhance oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2024, 19, 100953. [Google Scholar] [CrossRef]
- Appelhaus, J.; Steffens, K.E.; Wagner, K.G. Effect of Liquid Load Level and Binder Type on the Tabletability of Mesoporous Silica Based Liquisolids. AAPS PharmSciTech 2024, 25, 246. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Hong, Y.; Wu, F.; Shen, L.; Wang, Y.; Lin, X. Multifunctional role of silica in pharmaceutical formulations. AAPS PharmSciTech 2022, 23, 90. [Google Scholar] [CrossRef]
- Kim, J.S.; ud Din, F.; Cho, H.J.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Park, S.; Youn, Y.S.; Oh, K.T.; et al. Impact of carrier hydrophilicity on solid self nano-emulsifying drug delivery system and self nano-emulsifying granule system. Int. J. Pharm. 2023, 648, 123578. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Kim, J.S.; ud Din, F.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Park, S.; Kim, J.O.; Youn, Y.S.; Lim, S.J.; et al. Hydroxypropyl-β-cyclodextrin-based solid dispersed granules: A prospective alternative to conventional solid dispersion. Int. J. Pharm. 2022, 628, 122286. [Google Scholar]
- Kim, D.S.; Yang, E.S.; Yong, C.S.; Youn, Y.S.; Oh, K.T.; Li, D.X.; Kim, J.O.; Jin, S.G.; Choi, H.G. Effect of inorganic mesoporous carriers on 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol-loaded solid self-emulsifying drug delivery system: Physicochemical characterization and bioavailability in rats. Colloids Surf. B Biointerfaces 2017, 160, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, M.; German Ilić, I.; Bolko Seljak, K.; Zvonar Pobirk, A. High-shear wet granulation of SMEDDS based on mesoporous carriers for improved carvedilol solubility. Pharmaceutics 2022, 14, 2077. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Yoon, H.; Jin, S.G. Novel Saccharomyces cerevisiae-Loaded Polyvinylpyrrolidone/SiO2 Nanofiber for Wound Dressing Prepared Using Electrospinning Method. Materials 2024, 17, 2903. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.R.; Woo, S.; Bak, Y.W.; Cheon, S.; Kim, J.S.; Ji, S.H.; Park, S.; Kim, J.O.; Jin, S.G.; Choi, H.G. Comparison of two self-nanoemulsifying drug delivery systems using different solidification techniques for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib. Colloids Surf. B Biointerfaces 2024, 241, 114044. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, K.; Mohapatra, H.; Sindhu, R.K.; Bulzan, M.; Cavalu, S.; Paneshar, G.; Elansary, H.O.; El-Sabrout, A.M.; Mahmoud, E.; et al. Supersaturation-based drug delivery systems: Strategy for bioavailability enhancement of poorly water-soluble drugs. Molecules 2022, 27, 2969. [Google Scholar] [CrossRef]
- Kostelanská, K.; Prudilová, B.B.; Holešová, S.; Vlček, J.; Vetchý, D.; Gajdziok, J. Comparative study of powder carriers physical and structural properties. Pharmaceutics 2022, 14, 818. [Google Scholar] [CrossRef]
- Hate, S.S.; Reutzel-Edens, S.M.; Taylor, L.S. Influence of drug–silica electrostatic interactions on drug release from mesoporous silica-based oral delivery systems. Mol. Pharm. 2020, 17, 3435–3446. [Google Scholar] [CrossRef]
- Sri, B.U.; Muzib, Y.I.; Bhikshapathi, D.V.R.N.; Sravani, R. Enhancement of solubility and oral bioavailability of poorly soluble drug valsartan by novel solid self-emulsifying drug delivery system. Int. J. Drug Deliv. 2015, 7, 13–26. [Google Scholar]
- Truong, D.H.; Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Lee, H.H.; Moon, C.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of solid self-emulsifying formulation for improving the oral bioavailability of erlotinib. AAPS PharmSciTech 2016, 17, 466–473. [Google Scholar] [CrossRef]
- Joyce, P.; Dening, T.J.; Meola, T.R.; Schultz, H.B.; Holm, R.; Thomas, N.; Prestidge, C.A. Solidification to improve the biopharmaceutical performance of SEDDS: Opportunities and challenges. Adv. Drug Deliv. Rev. 2019, 142, 102–117. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Dalrymple, D.M.; Serajuddin, A.T.M. Development of solid SEDDS, V: Compaction and drug release properties of tablets prepared by adsorbing lipid-based formulations onto Neusilin® US2. Pharm. Res. 2013, 30, 3186–3199. [Google Scholar] [CrossRef]
- Meola, T.R.; Schultz, H.B.; Peressin, K.F.; Prestidge, C.A. Enhancing the oral bioavailability of simvastatin with silica-lipid hybrid particles: The effect of supersaturation and silica geometry. Eur. J. Pharm. Sci. 2020, 150, 105357. [Google Scholar] [CrossRef] [PubMed]
- Dening, T.J.; Rao, S.; Thomas, N.; Prestidge, C.A. Novel nanostructured solid materials for modulating oral drug delivery from solid-state lipid-based drug delivery systems. AAPS J. 2016, 18, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, M.; Gašperlin, M.; Zvonar Pobirk, A. Lipid-based systems with precipitation inhibitors as formulation approach to improve the drug bioavailability and/or lower its dose: A review. Acta Pharm. 2024, 74, 201–227. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, H.G.; Jin, S.G. Influence of hydroxypropylmethylcellulose and sodium lauryl sulfate on the solubility and dissolution of sirolimus in solvent-evaporated solid dispersions. Bull. Korean Chem. Soc. 2018, 39, 778–783. [Google Scholar] [CrossRef]
- Kim, J.S.; Ud Din, F.; Lee, S.M.; Kim, D.S.; Woo, M.R.; Cheon, S.; Ji, S.H.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. Comparison of Three Different Aqueous Microenvironments for Enhancing Oral Bioavailability of Sildenafil: Solid Self-Nanoemulsifying Drug Delivery System, Amorphous Microspheres and Crystalline Microspheres. Int. J. Nanomed. 2021, 16, 5797–5810. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, E.S.; Kim, D.S.; Kim, D.W.; Yoo, H.H.; Yong, C.S.; Youn, Y.S.; Oh, K.T.; Jee, J.P.; Kim, J.O.; et al. A novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for improved stability and oral bioavailability of an oily drug, 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol. Drug Deliv. 2017, 24, 1018–1025. [Google Scholar] [CrossRef]
- Cheon, S.; Kim, J.S.; Woo, M.R.; Ji, S.H.; Park, S.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; Lim, S.J.; et al. Establishment of nanoparticle screening technique: A pivotal role of sodium carboxymethylcellulose in enhancing oral bioavailability of poorly water-soluble aceclofenac. Int. J. Biol. Macromol. 2024, 277, 134246. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Elzayat, E.M. Development of bioresponsive poloxamer-based self-nanoemulsifying system for enhanced febuxostat bioavailability: Solidification strategy using I-optimal approach. Pharmaceutics 2025, 17, 975. [Google Scholar] [CrossRef]
- Shah, A.V.; Serajuddin, A.T.M. Development of solid self-emulsifying drug delivery system (SEDDS) I: Use of poloxamer 188 as both solidifying and emulsifying agent for lipids. Pharm. Res. 2012, 29, 2817–2832. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Un Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef]
- Kumar, M.; Chawla, P.A.; Faruk, A.; Chawla, V.; Thakur, S.; Jain, S.K. Development of superior chitosan–EDTA microparticles as an adsorbent base for solidifying the self-emulsifying drug delivery systems. Future J. Pharm. Sci. 2024, 10, 18. [Google Scholar] [CrossRef]
- Singh, K.; Tiwary, A.K.; Rana, V. Spray dried chitosan–EDTA superior microparticles as solid substrate for the oral delivery of amphotericin B. Int. J. Biol. Macromol. 2013, 58, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Chatterjee, B. Comparison of two grafted copolymers, Soluplus and Kollicoat IR, as solid dispersion carriers of artemether for oral delivery prepared by different solvent-based methods. ACS Omega 2023, 8, 45337–45347. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.; Jalil, A.; Nazir, I.; Matuszczak, B.; Kennedy, R.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: Hydrophobic drug polymer complexes provide a sustained release in vitro. Mol. Pharm. 2020, 17, 3709–3719. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, Q.; Sun, C.; Zhang, Z.; Jiang, T.; Sun, J.; Li, Y.; Wang, S. Mesoporous carbon with spherical pores as a carrier for celecoxib with needle-like crystallinity: Improve dissolution rate and bioavailability. Mater. Sci. Eng. C 2014, 39, 13–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Zhu, W.; Zhang, L.; Han, J.; Lin, Q.; Ai, F. Synthesis and evaluation of mesoporous carbon/lipid bilayer nanocomposites for improved oral delivery of the poorly water-soluble drug, nimodipine. Pharm. Res. 2015, 32, 2372–2383. [Google Scholar] [CrossRef]
- Niu, X.; Wan, L.; Hou, Z.; Wang, T.; Sun, C.; Sun, J.; Zhao, P.; Jiang, T.; Wang, S. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int. J. Pharm. 2013, 452, 382–389. [Google Scholar] [CrossRef]
- Gu, W.; Zhao, Q.; He, Y.; Wang, S.; Yang, Y.; Li, Y.; Feng, S.; Wang, S. Different mesoporous carbon carriers for the improvement of solubility and physical stability of poorly soluble drugs. Colloids Surf. B Biointerfaces 2025, 247, 114436. [Google Scholar] [CrossRef]
- Merchant, J.; Müllertz, A.; Rades, T.; Bannow, J. Functionalized calcium carbonate (FCC) as a novel carrier to solidify supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). Eur. J. Pharm. Biopharm. 2023, 193, 198–207. [Google Scholar] [CrossRef]
- Alvebratt, C.; Dening, T.J.; Åhlén, M.; Cheung, O.; Strømme, M.; Gogoll, A.; Prestidge, C.A.; Bergström, C.A. In vitro performance and chemical stability of lipid-based formulations encapsulated in a mesoporous magnesium carbonate carrier. Pharmaceutics 2020, 12, 426. [Google Scholar] [CrossRef]
- de la Torre, T.Z.G.; Lindmark, T.; Cheung, O.; Bergström, C.; Strømme, M. Bioavailability of Celecoxib Formulated with Mesoporous Magnesium Carbonate—An In Vivo Evaluation. Molecules 2022, 27, 6188. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Z.; Tan, S.; Zhu, Q. Clay nanoparticles as pharmaceutical carriers in drug delivery systems. Expert Opin. Drug Deliv. 2021, 18, 695–714. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Li, C.; Liu, S.; Sun, D. Double phase inversion of emulsions containing layered double hydroxide particles induced by adsorption of sodium dodecyl sulfate. Langmuir 2008, 24, 10054–10061. [Google Scholar] [CrossRef]
- Dening, T.J.; Thomas, N.; Rao, S.; Van Looveren, C.; Cuyckens, F.; Holm, R.; Prestidge, C.A. Montmorillonite and Laponite clay materials for the solidification of lipid-based formulations for the basic drug Blonanserin: In vitro and in vivo investigations. Mol. Pharm. 2018, 15, 4148–4160. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sevciuc, A.; Rijn, P.V. Layered double hydroxides as an intercalation system for hydrophobic molecules. Nanomaterials 2023, 13, 3145. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhang, H.; Dou, L. Layered double hydroxide-based nanocarriers for drug delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef]
- Di, X.; Liang, X.; Shen, C.; Pei, Y.; Wu, B.; He, Z. Carbohydrates used in polymeric systems for drug delivery: From structures to applications. Pharmaceutics 2022, 14, 739. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Jiang, S.; Liu, Z.; Gu, W.; Yu, H.; Li, Y. Porous starch based self-assembled nano-delivery system improves the oral absorption of lipophilic drug. Int. J. Pharm. 2013, 444, 162–168. [Google Scholar] [CrossRef]
- Sujka, M.; Pankiewicz, U.; Kowalski, R.; Nowosad, K.; Noszczyk-Nowak, A. Porous starch and its application in drug delivery systems. Polim. Med. 2018, 48, 25–29. [Google Scholar] [CrossRef]
- Hanmantrao, M.; Chaterjee, S.; Kumar, R.; Vishwas, S.; Harish, V.; Porwal, O.; Alrouji, M.; Alomeir, O.; Alhajlah, S.; Gulati, M.; et al. Development of guar gum-pectin-based colon targeted solid self-nanoemulsifying drug delivery system of xanthohumol. Pharmaceutics 2022, 14, 2384. [Google Scholar] [CrossRef] [PubMed]
- Teerapipattanapong, P.; Jaikon, P.; Ningsanonda, N.; Yonemochi, E.; Furuishi, T.; Hirun, N.; Kraisit, P. Evaluating Various Lactose Types as Solid Carriers for Improving Curcumin Solubility in Solid Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDSs) for Oral Administration. Sci 2024, 6, 69. [Google Scholar] [CrossRef]
- Singh, H.; Nathani, S.; Singh, N.; Roy, P.; Paul, S.; Sohal, H.S.; Jain, S.K. Development and characterization of Solid-SNEDDS formulation of DHA using hydrophilic carrier with improved shelf life, oxidative stability and therapeutic activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101326. [Google Scholar] [CrossRef]
- Sun, M.; Bi, J.; Zhao, Y.; Gong, J. Particle Design of Drugs via Spherical Crystallization: A Review from Fundamental Aspects to Technology Development. Cryst. Growth Des. 2024, 24, 2266–2287. [Google Scholar] [CrossRef]
- Manisha, E.; Harshavardhini, G.; Hyma, P. Spherical Crystallization: A Tool To Improve Solubility of Drugs. J. Adv. Sci. Res. 2022, 13, 28–34. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Mahdavi, H.; Rajabi, O. Recent Progress in Antisolvent Crystallization of Pharmaceuticals with a Focus on the Membrane-Based Technologies. Chem. Eng. Technol. 2024, 47, 750–763. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gupta, M.M.; Srivastava, B. Spherical crystallization: A technique use to reform solubility and flow property of active pharmaceutical ingredients. Int. J. Pharm. Investig. 2017, 7, 4–9. [Google Scholar] [CrossRef]
- Peña, R.; Jarmer, D.J.; Burcham, C.L.; Nagy, Z.K. Further understanding of agglomeration mechanisms in spherical crystallization systems: Benzoic acid case study. Cryst. Growth Des. 2019, 19, 1668–1679. [Google Scholar] [CrossRef]
- Xing, X.; Ouyang, J.; Guo, S.; Chen, M.; Gao, Z.; He, F.; Zhou, L.; Xie, Z. Spherical particles design of vanillin via crystallization method: Preparation, characterization and mechanism. Sep. Purif. Technol. 2023, 314, 123622. [Google Scholar] [CrossRef]
- Saini, S. Spherical Crystallization: An Overview. Int. J. Drug Deliv. Technol. 2014, 4, 72–80. [Google Scholar] [CrossRef]
- Baumann, J.M.; Adam, M.S.; Wood, J.D. Engineering advances in spray drying for pharmaceuticals. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 217–240. [Google Scholar] [CrossRef]
- Kim, W.; Kim, J.S.; Choi, H.G.; Jin, S.G.; Cho, C.W. Novel ezetimibe-loaded fibrous microparticles for enhanced solubility and oral bioavailability by electrospray technique. J. Drug Deliv. Sci. Technol. 2021, 66, 102877. [Google Scholar] [CrossRef]
- Choi, J.E.; Kim, J.S.; Kim, J.; Choi, M.J.; Baek, K.; Kim, J.O.; Choi, H.G.; Jin, S.G. A novel acidic microenvironment microsphere for enhanced bioavailability of carvedilol: Comparison of solvent evaporated and surface-attached system. J. Drug Deliv. Sci. Technol. 2022, 76, 103803. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, D.W.; Kim, K.S.; Choi, J.S.; Seo, Y.G.; Youn, Y.S.; Oh, K.T.; Yong, C.S.; Kim, J.O.; Jin, S.G.; et al. Development of a novel l-sulpiride-loaded quaternary microcapsule: Effect of TPGS as an absorption enhancer on physicochemical characterization and oral bioavailability. Colloids Surf. B Biointerfaces 2016, 147, 250–257. [Google Scholar] [CrossRef]
- Razmi, R.; Jubaer, H.; Krempski-Smejda, M.; Jaskulski, M.; Xiao, J.; Chen, X.D.; Woo, M.W. Recent initiatives in effective modeling of spray drying. Dry. Technol. 2021, 39, 1614–1647. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, J.S.; Kim, D.W.; Kim, K.S.; Seo, Y.G.; Cho, K.H.; Kim, J.O.; Yong, C.S.; Youn, Y.S.; Lim, S.J.; et al. Comparison of solvent-wetted and kneaded l-sulpiride-loaded solid dispersions: Powder characterization and in vivo evaluation. Int. J. Pharm. 2016, 511, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Essien, S.; Dell, K.; Woo, M.W.; Baroutian, S. Effects of spray-drying and freeze-drying on bioactive and volatile compounds of smoke powder food flavouring. Food Bioprocess Technol. 2022, 15, 785–794. [Google Scholar] [CrossRef]
- Patil, M.; Yadav, A.; Mogal, R.; Shaikh, M.; Lalsare, S.; Kshirsagar, S. Development and Evaluation of a Solid Self-Microemulsifying Drug Delivery System Containing Cilostazol Using the Spray Drying Technique. Biosci. Biotech. Res. Asia 2024, 21, 1415–1427. [Google Scholar] [CrossRef]
- Mohsin, K.; Alamri, R.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Hussain, M.D. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int. J. Nanomed. 2016, 11, 2829–2838. [Google Scholar]
- Steiner, D.; Schumann, L.V.; Bunjes, H. Processing of lipid nanodispersions into solid powders by spray drying. Pharmaceutics 2022, 14, 2464. [Google Scholar] [CrossRef]
- Li, W.; Huang, P.; Sun, X.; Zhang, Y.; Liu, Y.; Feng, N.; Wang, Z. Novel solid self-emulsifying drug delivery system to improve the physical performance and bioavailability of supercritical fluid extracts of frankincense and myrrh. J. Drug Deliv. Sci. Technol. 2025, 109, 107030. [Google Scholar] [CrossRef]
- Liu, H.; Liang, X.; Peng, Y.; Liu, G.; Cheng, H. Supercritical fluids: An innovative strategy for drug development. Bioengineering 2024, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Lee, D.; Kim, M.; Lee, S.; Shin, Y.; Ramsey, J.D.; Choi, H.G.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of a sorafenib-loaded solid self-nanoemulsifying drug delivery system: Formulation optimization and characterization of enhanced properties. J. Drug Deliv. Sci. Technol. 2023, 82, 104374. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jiang, Y.; Wang, X. Tailoring particle microstructures via supercritical CO2 processes for particular drug delivery. Curr. Pharm. Des. 2015, 21, 2543–2562. [Google Scholar] [CrossRef]
- Bagheri, H.; Notej, B.; Shahsavari, S.; Hashemipour, H. Supercritical carbon dioxide utilization in drug delivery: Experimental study and modeling of paracetamol solubility. Eur. J. Pharm. Sci. 2022, 177, 106273. [Google Scholar] [CrossRef]
- Aloisio, C.; Bueno, M.S.; Ponte, M.P.; Paredes, A.; Palma, S.D.; Longhi, M. Development of solid self-emulsifying drug delivery systems (SEDDS) to improve the solubility of resveratrol. Ther. Deliv. 2019, 10, 626–641. [Google Scholar] [CrossRef]
- Tan, A.; Rao, S.; Prestidge, C.A. Transforming lipid-based oral drug delivery systems into solid dosage forms: An overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm. Res. 2013, 30, 2993–3017. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.M.; Kim, D.S.; Kim, D.W.; Kim, K.S.; Cho, K.H.; Li, D.X.; Jin, S.G.; Choi, H.G. Enhanced chemical stability of D-cycloserine via tablet form containing magnesium oxide as an alkali stabilizer. Bull. Korean Chem. Soc. 2020, 41, 10–14. [Google Scholar] [CrossRef]
- Abou Assi, R.; Abdulbaqi, I.M.; Seok Ming, T.; Siok Yee, C.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and solid self-emulsifying drug delivery systems (SEDDs) as carriers for the oral delivery of azithromycin: Optimization, in vitro characterization and stability assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef]
- Mahajan, N.; Mujtaba, M.A.; Fule, R.; Thakre, S.; Akhtar, M.S.; Alavudeen, S.S.; Anwer, M.K.; Aldawsari, M.F.; Mahmood, D.; Alam, M.S. Self-emulsifying drug delivery system for enhanced oral delivery of tenofovir: Formulation, physicochemical characterization, and bioavailability assessment. ACS Omega 2024, 9, 8139–8150. [Google Scholar] [CrossRef]
- Mandić, J.; Pobirk, A.Z.; Vrečer, F.; Gašperlin, M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Seow, E.K.; Lim, S.C.; Yuen, K.H.; Abdul Karim Khan, N. Formulation and in vivo evaluation of a solid self-emulsifying drug delivery system using oily liquid tocotrienols as model active substance. Pharmaceutics 2021, 13, 1777. [Google Scholar] [CrossRef]
- Tran, P.H.; Lee, B.J.; Tran, T.T. Recent studies on the processes and formulation impacts in the development of solid dispersions by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2021, 164, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kallakunta, V.R.; Dudhipala, N.; Nyavanandi, D.; Sarabu, S.; Janga, K.Y.; Ajjarapu, S.; Bandari, S.; Repka, M.A. Formulation and processing of solid self-emulsifying drug delivery systems (HME S-SEDDS): A single-step manufacturing process via hot-melt extrusion technology through response surface methodology. Int. J. Pharm. 2023, 641, 123055. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, O.; Spoerk, M.; Paudel, A. Lipid-based solubilization technology via hot melt extrusion: Promises and challenges. Expert Opin. Drug Deliv. 2022, 19, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Sadeq, T.W.; Samad, A. Hot-Melt Extrusion (HME) Is Advanced Approach for Development of Solid Self Emulsifying Drug Delivery System. Int. J. Membr. Sci. Technol. 2023, 10, 3586–3598. [Google Scholar] [CrossRef]
- Zupančič, O.; Doğan, A.; Matić, J.; Kushwah, V.; Alva, C.; Spoerk, M.; Paudel, A. SEDEX—Self-Emulsifying Delivery Via Hot Melt Extrusion: A Continuous Pilot-Scale Feasibility Study. Pharmaceutics 2022, 14, 2617. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.S.; Jin, S.G.; Choi, H.G. Development of Novel Tamsulosin Pellet-Loaded Oral Disintegrating Tablet Bioequivalent to Commercial Capsule in Beagle Dogs Using Microcrystalline Cellulose and Mannitol. Int. J. Mol. Sci. 2023, 24, 15393. [Google Scholar] [CrossRef]
- Tian, W.; Li, X.; Li, W.; Xue, A.; Zheng, M.; Lin, X.; Hong, Y. Influence of Extrudate-Based Textural Properties on Pellet Molding Quality. Pharmaceuticals 2023, 16, 1505. [Google Scholar] [CrossRef]
- Nikolakakis, I.; Partheniadis, I. Self-emulsifying granules and pellets: Composition and formation mechanisms for instant or controlled release. Pharmaceutics 2017, 9, 50. [Google Scholar] [CrossRef]
- Araújo, G.P.; Martins, F.T.; Taveira, S.F.; Cunha-Filho, M.; Marreto, R.N. Effects of formulation and manufacturing process on drug release from solid self-emulsifying drug delivery systems prepared by high shear mixing. AAPS PharmSciTech 2021, 22, 254. [Google Scholar] [CrossRef]
- Thommes, M.; Kleinebudde, P. Properties of pellets manufactured by wet extrusion/spheronization process using κ-carrageenan: Effect of process parameters. AAPS PharmSciTech 2007, 8, 95. [Google Scholar] [CrossRef]
- Petrovick, G.F.; Pein, M.; Thommes, M.; Breitkreutz, J. Spheronization of solid lipid extrudates: A novel approach on controlling critical process parameters. Eur. J. Pharm. Biopharm. 2015, 92, 15–21. [Google Scholar] [CrossRef]
- Rhee, Y.S.; Lee, J.H.; Lee, B.J.; Park, E.S. Controlled-release pelletized dosage forms using the extrusion-spheronization process. J. Pharm. Investig. 2010, 40, 103–112. [Google Scholar] [CrossRef]
- Bhandari, V.; Avachat, A. Formulation and characterization of self emulsifing pellets of carvedilol. Braz. J. Pharm. Sci. 2015, 51, 663–671. [Google Scholar] [CrossRef]
- Jovičić-Bata, J.; Todorović, N.; Krstonošić, V.; Ristić, I.; Kovačević, Z.; Vuković, M.; Lalić-Popović, M. Liquid-and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements. Pharmaceutics 2024, 16, 892. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Etzler, F.M.; Ubben, J.; Birch, A.; Zhong, L.; Schwabe, R.; Dudhedia, M.S. Effects of lipophilic components on the compatibility of lipid-based formulations with hard gelatin capsules. J. Pharm. Sci. 2010, 99, 128–141. [Google Scholar] [CrossRef]
- Nirale, P.; Arora, S.; Solanki, A.; Bhat, J.; Singh, R.K.; Yadav, K.S. Liquid Filled Hard Shell Capsules: Current Drug Delivery Influencing Pharmaceutical Technology. Curr. Drug Deliv. 2022, 19, 238–249. [Google Scholar] [CrossRef]
- Nekkanti, V.; Karatgi, P.; Prabhu, R.; Pillai, R. Solid self-microemulsifying formulation for candesartan cilexetil. AAPS PharmSciTech 2010, 11, 9–17. [Google Scholar] [CrossRef]
- Qureshi, M.J.; Mallikarjun, C.; Kian, W.G. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: A study in diet induced hyperlipidemic rabbits. Asian J. Pharm. Sci. 2015, 10, 40–56. [Google Scholar] [CrossRef]
- Sonar, S.; Gondkar, S.; Saudagar, R.B. Liquid filled hard gelatin capsule. J. Drug Deliv. Ther. 2019, 9, 832–835. [Google Scholar] [CrossRef]
- Desai, M.M.; Nikalje, A.P.G. Formulation and Evaluation of Self Micro-Emulsifying drug delivery System of Carvedilol. Indian J. Pharm. Sci. 2023, 85, 426–434. [Google Scholar] [CrossRef]
- Amekyeh, H.; Billa, N. Lyophilized drug-loaded solid lipid nanoparticles formulated with beeswax and theobroma oil. Molecules 2021, 26, 908. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; More, M.; Patil, P.; Pardeshi, C.; Deshmukh, P.; Mujumdar, A.; Naik, J. A meticulous overview on drying-based (spray-, freeze-, and spray-freeze) particle engineering approaches for pharmaceutical technologies. Dry. Technol. 2021, 39, 1447–1491. [Google Scholar] [CrossRef]
- ElShagea, H.N.; ElKasabgy, N.A.; Fahmy, R.H.; Basalious, E.B. Freeze-dried self-nanoemulsifying self-nanosuspension (SNESNS): A new approach for the preparation of a highly drug-loaded dosage form. AAPS PharmSciTech 2019, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Nikam, A.P.; Meshram, P.D.; Vanjari, A.V.; Mundada, S.V. Comparative study of spray-drying and freeze-drying techniques for increasing fenofibrate’s solubility and dissolution rate. BIO Integr. 2024, 5, 985. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.; Alkhalidi, H.M.; Mahmood, S.; Almurisi, S.H.; Doolaanea, A.A.; Al-Sindi, T.A.; Chatterjee, B. Lyophilized amorphous dispersion of telmisartan in a combined carrier–alkalizer system: Formulation development and in vivo study. ACS Omega 2020, 5, 32466–32480. [Google Scholar] [CrossRef]
- Alqurshi, A.; Chan, K.A.; Royall, P.G. In-situ freeze-drying-forming amorphous solids directly within capsules: An investigation of dissolution enhancement for a poorly soluble drug. Sci. Rep. 2017, 7, 2910. [Google Scholar] [CrossRef]
- Woo, M.R.; Kim, J.S.; Cheon, S.; Ji, S.H.; Park, S.; Woo, S.; Kim, J.O.; Jin, S.G.; Choi, H.G. Microneedles integrated with crystallinity control for poorly water-soluble drugs: Enhanced bioavailability and innovative controlled release system. Mater. Des. 2024, 247, 113371. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, J. 3D printing technique in the development of self-nanoemulsifying drug delivery system: Scope and future prospects. Ther. Deliv. 2022, 13, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Than, Y.M.; Titapiwatanakun, V. Statistical design of experiment-based formulation development and optimization of 3D printed oral controlled release drug delivery with multi target product profile. J. Pharm. Investig. 2021, 51, 715–734. [Google Scholar] [CrossRef]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergström, C.A. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 120304. [Google Scholar] [CrossRef]
- Ahmad, J.; Garg, A.; Mustafa, G.; Mohammed, A.A.; Ahmad, M.Z. 3D printing technology as a promising tool to design nanomedicine-based solid dosage forms: Contemporary research and future scope. Pharmaceutics 2023, 15, 1448. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Abdullah, M.M.; Saleh, E. 3D printing of dapagliflozin containing self-nanoemulsifying tablets: Formulation design and in vitro characterization. Pharmaceutics 2021, 13, 993. [Google Scholar] [CrossRef]
- Barber, B.W.; Dumont, C.; Caisse, P.; Simon, G.P.; Boyd, B.J. A 3D-printed polymer–lipid-hybrid tablet towards the development of bespoke SMEDDS formulations. Pharmaceutics 2021, 13, 2107. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A proof of concept for 3D printing of solid lipid-based formulations of poorly water-soluble drugs to control formulation dispersion kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef]
- Englezos, K.; Wang, L.; Tan, E.C.; Kang, L. 3D printing for personalised medicines: Implications for policy and practice. Int. J. Pharm. 2023, 635, 122785. [Google Scholar] [CrossRef]
- Kim, J.S.; Ud Din, F.; Lee, S.M.; Kim, D.S.; Choi, Y.J.; Woo, M.R.; Kim, J.O.; Youn, Y.S.; Jin, S.G.; Choi, H.G. Comparative study between high-pressure homogenisation and Shirasu porous glass membrane technique in sildenafil base-loaded solid SNEDDS: Effects on physicochemical properties and in vivo characteristics. Int. J. Pharm. 2021, 592, 120039. [Google Scholar] [CrossRef]
- Woo, M.R.; Bak, Y.W.; Cheon, S.; Kim, J.S.; Ji, S.H.; Park, S.; Woo, S.; Kim, J.O.; Jin, S.G.; Choi, H.G. Modification of microenvironmental pH of nanoparticles for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib. Int. J. Pharm. 2024, 659, 124179. [Google Scholar]
- Lee, H.I.; Woo, M.R.; Kim, J.S.; Cheon, S.; Park, S.; Woo, S.; Jin, S.G.; Choi, H.G. Development of a novel apixaban-loaded solid self-emulsifying drug delivery system for oral administration: Physicochemical characterization and pharmacokinetics in rats. J. Pharm. Investig. 2025, 55, 575–588. [Google Scholar] [CrossRef]
- Choi, M.J.; Woo, M.R.; Baek, K.; Park, J.H.; Joung, S.; Choi, Y.S.; Choi, H.G.; Jin, S.G. Enhanced oral bioavailability of Rivaroxaban-Loaded microspheres by optimizing the polymer and surfactant based on molecular interaction mechanisms. Mol. Pharm. 2023, 20, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Woo, M.R.; Choi, H.G.; Jin, S.G. Effects of Polymers on the Drug Solubility and Dissolution Enhancement of Poorly Water-Soluble Rivaroxaban. Int. J. Mol. Sci. 2022, 23, 9491. [Google Scholar] [CrossRef]
- Ud Din, F.; Kim, D.S.; Kim, J.S.; Cheon, S.; Park, S.; Woo, S.; Woo, M.R.; Ali, Z.; Kim, J.O.; Jin, S.G.; et al. Comparative analysis of novel modified drug delivery systems for improving the oral bioavailability of water-insoluble tadalafil using copovidone, TPGS and hydroxypropyl-β-cyclodextrin. Biomed. Pharmacother. 2025, 186, 118039. [Google Scholar] [CrossRef]
- Jörgensen, A.M.; Steinbring, C.; Stengel, D.; To, D.; Schmid, P.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems (SEDDS) containing reverse micelles: Advanced oral formulations for therapeutic peptides. Adv. Healthc. Mater. 2023, 12, 2302034. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, Y.; Yue, H.; Chen, T.; Diao, Y.; Wei, W.; Zhang, S. Ionic liquids revolutionizing biomedicine: Recent advances and emerging opportunities. Chem. Soc. Rev. 2023, 52, 7262–7293. [Google Scholar] [CrossRef]
- Šahinović, M.; Hassan, A.; Kristó, K.; Regdon, G., Jr.; Vranić, E.; Sovány, T. Quality by design-based development of solid self-emulsifying drug delivery system (SEDDS) as a potential carrier for oral delivery of lysozyme. Pharmaceutics 2023, 15, 995. [Google Scholar] [CrossRef]
- Goo, Y.T.; Lee, S.; Choi, J.Y.; Kim, M.S.; Sin, G.H.; Hong, S.H.; Kim, C.H.; Choi, Y.W. Enhanced oral absorption of insulin: Hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Deliv. 2022, 29, 2831–2845. [Google Scholar] [CrossRef]
- Phan, T.N.Q.; Ismail, R.; Le-Vinh, B.; Zaichik, S.; Laffleur, F.; Bernkop-Schnurch, A. The effect of counterions in hydrophobic ion pairs on oral bioavailability of exenatide. ACS Biomater. Sci. Eng. 2020, 6, 5032–5039. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Al Hagbani, T.; Salawi, A.; Nazzal, S. Sustained release of curcumin self-emulsifying drug delivery system (SEDDS) from solvent-cast Soluplus® films. Pharm. Dev. Technol. 2021, 26, 1102–1109. [Google Scholar] [CrossRef]
- Omachi, Y. Gastroretentive sustained-release tablets combined with a solid self-micro-emulsifying drug delivery system adsorbed onto Fujicalin®. AAPS PharmSciTech 2022, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Bagul, N.; Patel, V.; Shahiwala, A.; Misra, M. Design and development of solid self emulsifying osmotic delivery system of nifedipine. Arch. Pharm. Pract. 2012, 3, 128–135. [Google Scholar]
- Zhang, X.; Yi, Y.; Qi, J.; Lu, Y.; Tian, Z.; Xie, Y.; Yuan, H.; Wu, W. Controlled release of cyclosporine A self-nanoemulsifying systems from osmotic pump tablets: Near zero-order release and pharmacokinetics in dogs. Int. J. Pharm. 2013, 452, 233–240. [Google Scholar] [CrossRef]
- Zheng, K.; Zhao, J.; Wang, Q.; Zhao, Y.; Yang, H.; Yang, X.; He, L. Design and evaluation of ginkgolides gastric floating controlled release tablets based on solid supersaturated self-nanoemulsifying. AAPS PharmSciTech 2023, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.; Abbas, G.; Ameer, N.; Mahmood, K.; Shah, S.; Hanif, M. Alginate raft as carrier for self-emulsifying drug delivery system of curcuminoid: In vitro and in vivo analysis. J. Mol. Liq. 2024, 393, 123427. [Google Scholar] [CrossRef]
- Komati, S.; Swain, S.; Rao, M.E.B.; Jena, B.R.; Dasi, V. Mucoadhesive multiparticulate drug delivery systems: An extensive review of patents. Adv. Pharm. Bull. 2019, 9, 521. [Google Scholar] [CrossRef]
- Han, A.S.; Kim, J.; Park, J.W.; Jin, S.G. Novel acyclovir-loaded film-forming gel with enhanced mechanical properties and skin permeability. J. Drug Deliv. Sci. Technol. 2022, 70, 103213. [Google Scholar] [CrossRef]
- Mujtaba, S.H.; Ghazy, E.; Arshad, R.; Aman, W.; Barkat, K.; Afzal, S.; Sadia, H.; Khan, S.A.; Rahdar, A.; Behzadmehr, R.; et al. Novel thiolated pluronic anchored gastro-retentive SEDDS of azithromycin against peptic ulcer. Inorg. Chem. Commun. 2024, 167, 112755. [Google Scholar] [CrossRef]
- Friedl, J.D.; Walther, M.; Vestweber, P.K.; Wächter, J.; Knoll, P.; Jörgensen, A.M.; Bernkop-Schnürch, A.; Windbergs, M. SEDDS-loaded mucoadhesive fiber patches for advanced oromucosal delivery of poorly soluble drugs. J. Control. Release 2022, 348, 692–705. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Narwade, M.; Shaikh, A.; Gajbhiye, K.R.; Kesharwani, P.; Gajbhiye, V. Advanced cancer targeting using aptamer functionalized nanocarriers for site-specific cargo delivery. Biomater. Res. 2023, 27, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Sun, J.; He, Z.G. Self-emulsifying drug delivery systems: Strategy for improving oral delivery of poorly soluble drugs. Curr. Drug Ther. 2007, 2, 85–93. [Google Scholar] [CrossRef]
- Gürsoy, R.N.; Çevik, Ö. Design, characterization and in vitro evaluation of SMEDDS containing an anticancer peptide, linear LyP-1. Pharm. Dev. Technol. 2014, 19, 486–490. [Google Scholar] [CrossRef]
- Gandhi, N.; Modi, S.; Soni, S.; Andey, T. Modular self-emulsifying drug delivery platform to enhance cellular uptake activity in triple-negative breast cancer. Eur. J. Pharm. Sci. 2025, 206, 106993. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming challenges in small-molecule drug bioavailability: A review of key factors and approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Hirunpanich, V.; Sato, H. Improvement of cyclosporine A bioavailability by incorporating ethyl docosahexaenoate in the microemulsion as an oil excipient. Eur. J. Pharm. Biopharm. 2009, 73, 247–252. [Google Scholar] [CrossRef]
- Neoral® (Cyclosporine) Soft Gelatin Capsules and Oral Solution: Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/050715s035,050716s038lbl.pdf (accessed on 18 June 2025).
- Lei, B.; Zha, W.; Wang, Y.; Wen, C.; Stude, E.J.; Wang, X.; Jin, F.; Wang, G.; Zhang, L.; Zhou, H. Development of a novel self-microemulsifying drug delivery system for reducing HIV protease inhibitor-induced intestinal epithelial barrier dysfunction. Mol. Pharm. 2010, 7, 844–853. [Google Scholar] [CrossRef]
- NORVIR® (Ritonavir) Capsules and Oral Solution: Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020945 (accessed on 21 October 2020).
- Thakkar, H.; Nangesh, J.; Parmar, M.; Patel, D. Formulation and characterization of lipid-based drug delivery system of raloxifene: Microemulsion and self-microemulsifying drug delivery system. J. Pharm. Bioallied Sci. 2011, 3, 442–448. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Fortovase 200 mg Soft Capsules: Summary of Product Characteristics (Annex I); European Medicines Agency: London, UK, 2006; Available online: https://ec.europa.eu/health/documents/community-register/html/h075.htm (accessed on 3 January 2006).
- AGENERASE® (Amprenavir) Capsules: Prescribing Information; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2005. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021007 (accessed on 11 April 2005).
- Park, H.; Ha, E.-S.; Kim, M.-S. Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics 2020, 12, 365. [Google Scholar] [CrossRef]
- Targretin® (Bexarotene) Gel 1%: Package Insert; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2024. Available online: https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/526861 (accessed on 29 February 2024).
- Mohsin, K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: Effects of digestion. AAPS PharmSciTech 2012, 13, 637–646. [Google Scholar] [CrossRef]
- Lipofen® (Fenofibrate Capsules, USP): Prescribing Information. Kowa Pharmaceuticals America, Inc.; Revised 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021612s007lbl.pdf (accessed on 18 September 2025).
- Hepion Pharmaceuticals, Inc.; Quotient Sciences. An Open Label, Single-Dose, Single-Period Study Designed to Assess the Mass Balance Recovery, Metabolite Profile and Metabolite Identification of [14C]-Rencofilstat in Healthy Male Subjects (Protocol HEPA-CRV431-105, Version 3.0); Hepion Pharmaceuticals, Inc.: Edison, NJ, USA; Quotient Sciences: Nottingham, UK, 2023. Available online: https://cdn.clinicaltrials.gov/large-docs/33/NCT05737433/Prot_000.pdf (accessed on 22 April 2023).
- NorthSea Therapeutics BV. A Randomised, Partially Double-Blind, Placebo- and Positive-Controlled, Four-Way Crossover Study to Evaluate the Effect of Icosabutate (NST-4016) on the QT/QTc Interval in Healthy Subjects (Protocol NST-01, Covance Study No. 8383557); NorthSea Therapeutics BV: Leeds, UK, 2018. Available online: https://cdn.clinicaltrials.gov/large-docs/75/NCT03577275/Prot_000.pdf (accessed on 11 April 2018).

| Manufacturing Method | Key Features | Advantages | Disadvantages | Materials | Critical Process Parameters |
|---|---|---|---|---|---|
| Spherical Crystallization | Single-step process forming spherical crystals from solvents and a bridging agent | Simple and cheap process, no high temperature, good flowability, and compressibility. | Use of organic solvents (safety concerns), potential instability at high storage temperatures. | Good solvent, poor solvent, bridging liquid; optional PVP/HPMC. | Solvent ratio, bridging-liquid addition rate, agitation speed, temperature. |
| Spray Drying | Liquid feed (SEDDS + carrier) is atomized and dried with hot gas. | Single-step continuous process, uniform particle size, improved dissolution rate, suitable for scale-up. | High-temperature process (unsuitable for heat-sensitive drugs), potential loss of volatile components, limited to certain carriers. | Hydrophilic carriers: Aerosil® 200, lactose(+SDS), mannitol, Ca-silicate; optional PVP. | Atomization/nozzle, inlet–outlet temperature, gas flow, feed solvent. |
| Supercritical Fluid (SCF) | Solubility change in SCF | Solvent-free, precise particle control | Expensive equipment, complex process | SC-CO2; drug/lipids in organic solvent. | Pressure, temperature, solvent choice, nozzle/expansion |
| Adsorption onto Solid Carrier | Liquid SEDDS absorbed by a porous solid carrier to form a free-flowing powder. | Economical, simple process (no complex equipment), improved stability and flowability. | Requires large carrier volume, risk of liquid exudation during compression, and potential for reduced drug release efficiency | Aerosil® 200, Neusilin® US2, Avicel® PH101, mannitol, Ca-silicate; PVP-treated silica optional. | Liquid/solid ratio, pore size, surface chemistry, mixing time/speed. |
| Hot Melt Extrusion (HME) | SEDDS components are blended and extruded through a die at high temperature and pressure. | Solvent-free, continuous, and efficient, enhanced drug uniformity, versatile shapes (strands, films). | High temperature/pressure risk, drug degradation, high energy consumption, and high shear stress can degrade the polymer matrix. | Solid carriers incl. Neusilin® US2; optional pH-responsive polymers. | Barrel T profile, screw design/speed, feed strategy (split feeding), die. |
| Pelletization | Liquid SEDDS incorporated into a binder, extruded, and spheronized. | Excellent flowability, narrow size distribution, easy to coat, efficient solvent-free process. | Complex process control (multiple variables) requires specific equipment, not suitable for all materials. | κ-Carrageenan; semi-solid lipids (Gelucire®, glyceryl behenate); silica/MCC. | Die size, spheronizer speed/time, binder viscosity/temperature. |
| Hard Gelatin Capsule Filling | Liquid SEDDS filled directly into hard gelatin capsules. | Simple, economical, no special equipment, addresses patient compliance issues with liquid dosage forms. | Risk of capsule leakage, limited drug loading capacity, and potential chemical/physical interactions with the capsule shell. | Liquid SEDDS (e.g., <50 nm on dispersion); optional spray-dried Mg aluminometasilicate; band sealing. | Fill volume ≦ ~90%, viscosity ~0.1–1 Pa·s, fill temperature, sealing. |
| Lyophilization | Liquid SEDDS frozen and dried by sublimation under vacuum. | Low-temperature process (suitable for heat-sensitive drugs), high stability (low moisture content), excellent redispersion. | High cost (equipment and energy), potential loss of volatile components under vacuum, and a time-consuming process. | Cryoprotectants (trehalose); PVP/HPMC matrices; in-capsule option. | Freezing protocol, shelf T ramp, chamber pressure (vacuum), primary/secondary drying time. |
| 3D Printing | Layer-by-layer fabrication using FDM or semi-solid extrusion. | Precise control over drug release, personalized medicine potential, complex dosage forms, multiple drug loading. | Slow manufacturing speed, high cost, limited choice of materials, and regulatory challenges. | Gelucire®-based solid SEDDS; polymer–lipid hybrids (PVA/PLA, poloxamer). | Nozzle diameter, infill %, wall thickness, extrusion speed/pressure; T control for FDM. |
| Category | Target/ Drug | Key Strategy | Dosage Form | Key Excipients | Key Outcomes | Refs. |
|---|---|---|---|---|---|---|
| Enhanced Bioavailability & Protein Delivery | Poorly water-soluble drugs | Self-emulsifying micro/nano-structuring | Solid SEDDS | PVP, SLS, Copovidone, Labrasol, Peceol, Mesoporous silica, HP-β-CD, etc. | Crystallization suppressed; supersaturation sustained; lower surfactant requirement | [161,162,163,164,165,166] |
| Lysozyme | SDS ion-pairing → lipophilicity ↑, leakage ↓ | Solid powder | Neusilin® UFL2, Syloid® 244 FP | Enzymatic activity retained; suitable for oral dosing | [169] | |
| Insulin | Ion-pairing with sodium n-octadecyl sulfate | Solidified after SEDDS loading | Capmul MCM, Labrasol, Tetraglycol | Enzymatic protection; intestinal permeability increased; stronger hypoglycemic effect (rat) | [170] | |
| Exenatide | Ion-pairing with long-chain anions | SEDDS-based oral delivery | Capmul MCM EP, Captex 355, Kolliphor RH40, sodium n-octadecyl sulfate | Relative oral bioavailability up to 19.6% vs. SC | [171] | |
| Controlled Drug Release | Nifedipine | SEDDS adsorption + semipermeable coating; laser-drilled orifice | Osmotic pump tablet | Gelucire®/Lutrol®/Transcutol®, Aerosil 200, etc. | ~84% released over 12 h; reconstituted droplet size preserved; release insensitive to agitation | [174] |
| Cyclosporine A | Cellulose acetate osmotic membrane; optimized osmotic/pore-former levels | Osmotic pump tablet | Labrafil M 1944CS, Cremophor EL, Polyethylene oxide, etc. | Sustained 12 h release; Tmax, MRT increased; Cmax reduced (dog) | [175] | |
| Ginkgolides | Swellable, gas-generating matrix | Floating tablet | HPMC 4KM/E15LV, NaHCO3, etc. | Floating lag < 1.5 s; total floating > 12 h; Zero-order release | [176] | |
| Fenofibrate | Swellable matrix | Gastro retentive tablet | Metolose® 90SH-100000SR, etc. | 12 h extended release | [173] | |
| Curcumin | gas-generating; improved bioavailability | Floating SEDDS powder | Sodium alginate, HPMC K100M, NaHCO3, etc. | 20-fold increase in anti-oxidant and 10-fold increase in anti-inflammatory activities | [177] | |
| Mucoadhesive Technology | Azithromycin | Covalent disulfide bonding with mucin | Gastro retentive SEDDS | Thiolated pluronic | 72 h extended release | [180] |
| Buccal mucosa | Mucoadhesive fiber | Patch | polyacrylic acid thiomer | ~200× buccal adhesion; sustained release 4 h | [181] | |
| Targeted Drug Delivery | Lysozyme | SDS ion-pairing + lipid carrier | Solid SEDDS (adsorbed) | Miglyol 812, Sodium lauryl sulfate, Tween 80, etc. | GI stability and residence increased; self-emulsification maintained (targeted uptake potential) | [169] |
| LyP-1 | Tumor-homing peptide-decorated SMEDDS | Nanoemulsion | Peceol, Labrasol, PEG 300 | ~20 nm droplets; receptor-mediated selective uptake increased | [186] | |
| Cisplatin + siRNA | W/O/W modular architecture; surface-charge optimization | Modular SEDDS | Labrafac PG, Labrasol, Gelucire 4414, Compritol 888 ATO | Uptake in TNBC cells increased; toxicity reduced; synergistic combination effect | [187] | |
| Personalized Medicine | Various APIs | 3D printing for precise control of dose/release/geometry | FDM/semisolid-extruded S-SEDDS tablets | PVA, HPMC, PEG, Capryol 90, etc. | Personalized dosing and release; improved treatment adherence | [188] |
| Brand Name/ Phase | Drug | Company | Dosage Form | Key Excipients | Stabilizer | Refs. |
|---|---|---|---|---|---|---|
| Neoral® | Cyclosporine A | Novartis | Soft gelatin capsule | Cremophor RH40, propylene glycol, dehydrated alcohol, corn oil mono/di-glycerides | DL-α-tocopherol | [189,190] |
| Norvir® | Ritonavir | AbbVie | Soft gelatin capsule | Cremophor EL, oleic acid | BHT | [191,192] |
| Fortovase® | Saquinavir | Roche | Soft gelatin capsule | Medium-chain mono/di-glycerides, povidone K30 | DL-α-tocopherol | [193,194] |
| Agenerase® | Amprenavir | Glaxo Wellcome/ GSK | Soft gelatin capsule | PEG 400, propylene glycol | TPGS | [21,195] |
| Targretin® | Bexarotene | Ligand | Soft gelatin capsule | PEG 400, polysorbate 20, povidone | BHT | [196,197] |
| Lipofen® | Fenofibrate | Cipher | Hard gelatin capsule | Gelucire® 44/14, PEG 20,000, PEG 8000, sodium starch glycolate | Hydroxypropylcellulose | [198,199] |
| Phase 1 | Rencofilstat (CRV431) | Hepion Pharmaceuticals | capsule | Polysorbate 80, PEG-400) | Medium Chain Triglycerides | [200] |
| Phase 1 | Icosabutate (NST-4016) | NorthSea Therapeutics BV | capsule | Not specified | Not specified | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, K.; Jin, S.G. Solidification Materials and Technology for Solid Self-Emulsifying Drug Delivery Systems. Pharmaceuticals 2025, 18, 1550. https://doi.org/10.3390/ph18101550
Baek K, Jin SG. Solidification Materials and Technology for Solid Self-Emulsifying Drug Delivery Systems. Pharmaceuticals. 2025; 18(10):1550. https://doi.org/10.3390/ph18101550
Chicago/Turabian StyleBaek, Kyungho, and Sung Giu Jin. 2025. "Solidification Materials and Technology for Solid Self-Emulsifying Drug Delivery Systems" Pharmaceuticals 18, no. 10: 1550. https://doi.org/10.3390/ph18101550
APA StyleBaek, K., & Jin, S. G. (2025). Solidification Materials and Technology for Solid Self-Emulsifying Drug Delivery Systems. Pharmaceuticals, 18(10), 1550. https://doi.org/10.3390/ph18101550