Rapid On-Demand Point-of-Care Monitoring of Clozapine and Its Metabolite Norclozapine Using Miniature Mass Spectrometry
Abstract
1. Introduction
2. Results
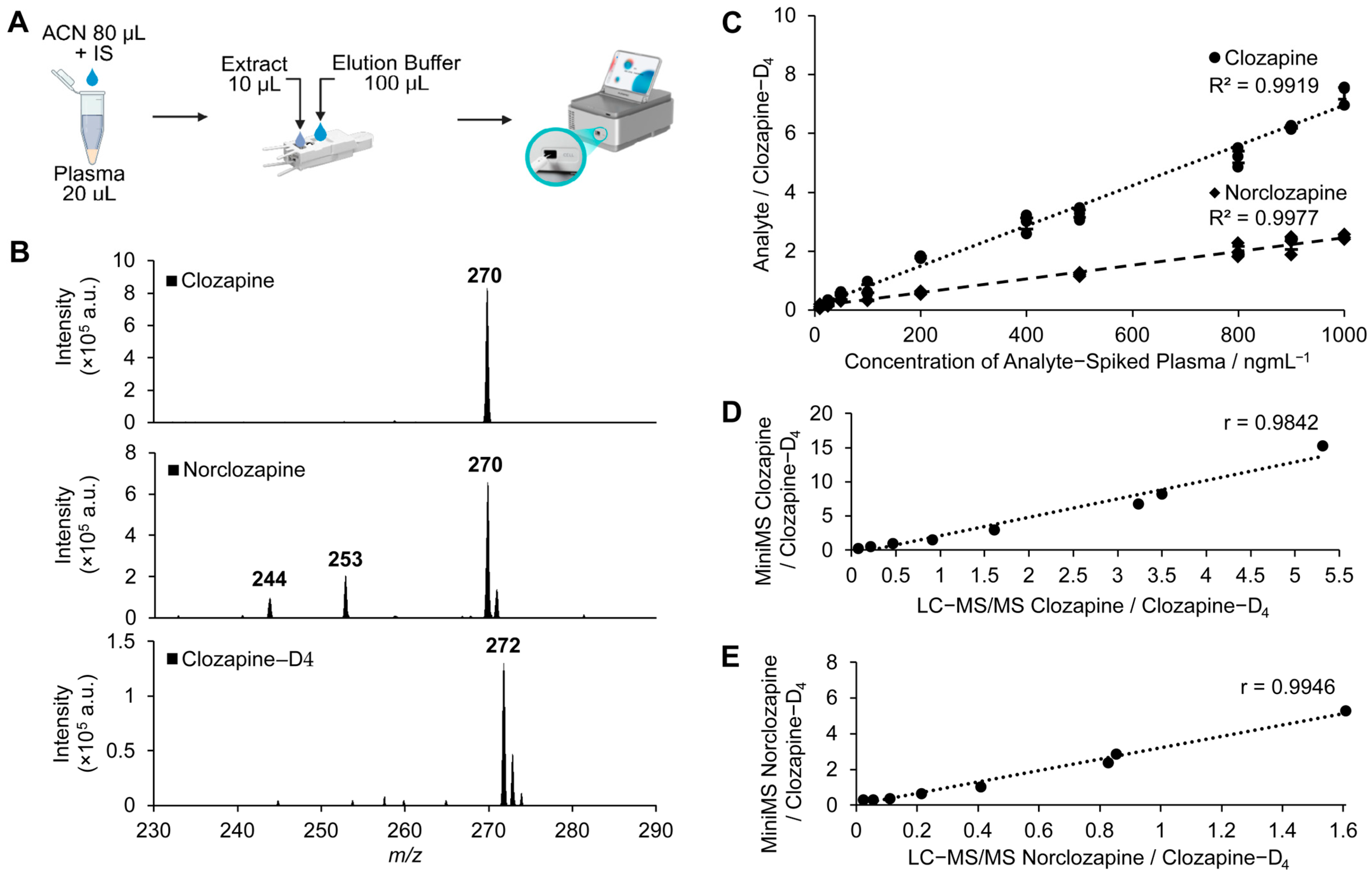
2.1. Development and Performance of Clozapine and Norclozapine Detection Using Mini-MS
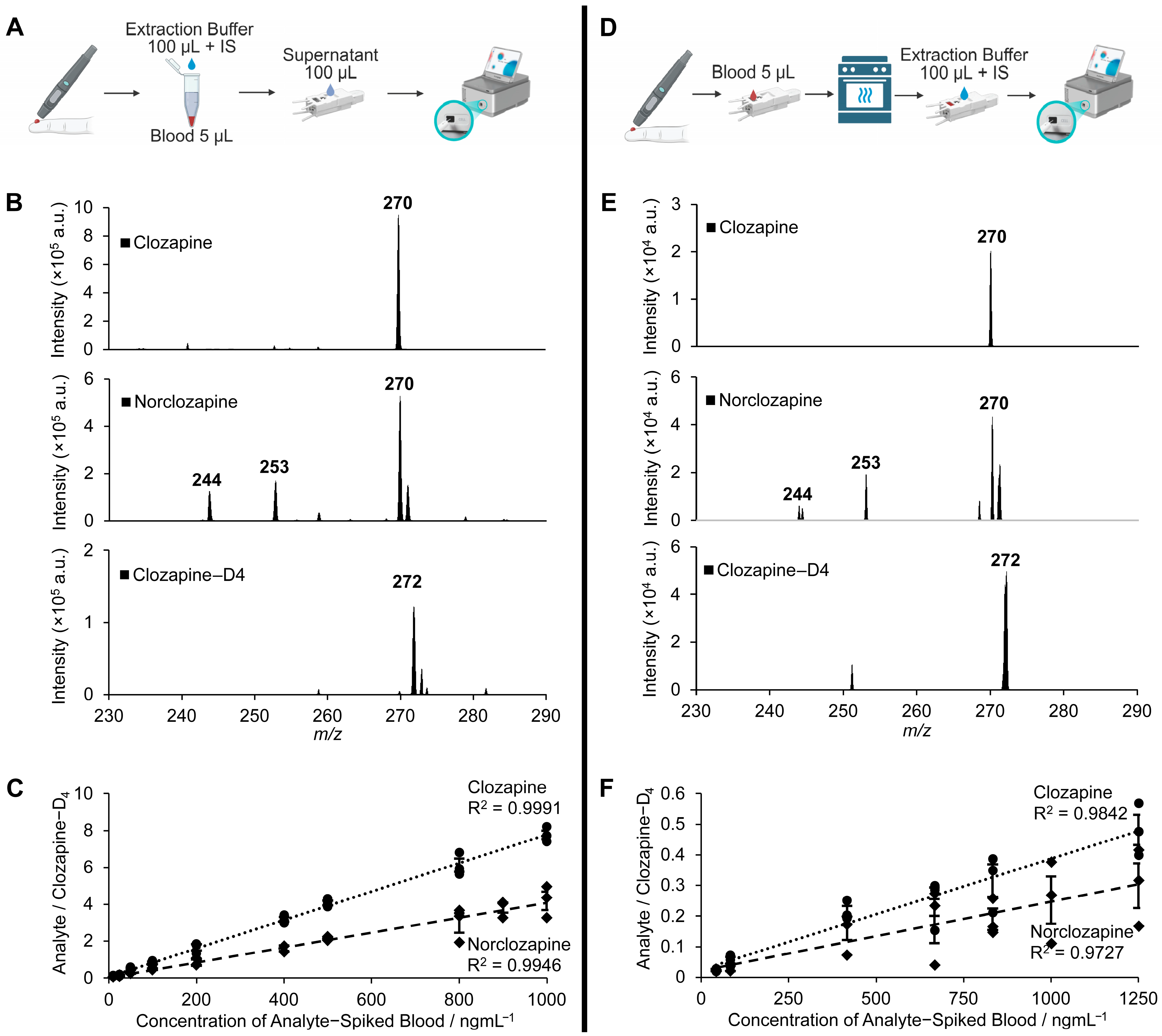
2.2. Clinical Applications in Confirmed Cases at Two Hospital Sites
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Blood and Plasma Collection
4.3. Preparation of Internal Standards, Calibration Curves, and Quality Control Samples
4.4. Preparations for Plasma, Whole Blood, and Dried Blood Spots
4.5. Miniature Mass Spectrometry and Workflow
4.6. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
4.7. Performance Evaluation of Mini-MS
4.8. Clozapine-Treated Patient Samples from Two Hospital Sites
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADR | Adverse Drug Reaction |
| DAPI | Discontinuous Atmospheric Pressure Interface |
| DBS | Dried Blood Spot |
| IS | Internal Standard |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| Mini-MS | Miniature Mass Spectrometry/Spectrometer |
| nESI | Nano-Electrospray Ionisation |
| NSWHP | New South Wales Health Pathology |
| PCS | Paper Capillary Spray |
| POC | Point-of-Care |
| TDM | Therapeutic Drug Monitoring |
| TRS | Treatment-Resistant Schizophrenia |
References
- Hippius, H. A historical perspective of clozapine. J. Clin. Psychiatry 1999, 60 (Suppl. S12), 22–23. [Google Scholar] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Crilly, J. The history of clozapine and its emergence in the US market: A review and analysis. Hist. Psychiatry 2007, 18, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Buchanan, R.W.; Kirkpatrick, B.; Davis, O.R.; Irish, D.; Summerfelt, A.; Carpenter, W.T., Jr. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am. J. Psychiatry 1994, 151, 20–26. [Google Scholar] [CrossRef]
- Sriretnakumar, V.; Huang, E.; Müller, D.J. Pharmacogenetics of clozapine treatment response and side-effects in schizophrenia: An update. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1709–1731. [Google Scholar] [CrossRef]
- Lappin, J.M.; Wijaya, M.; Watkins, A.; Morell, R.; Teasdale, S.; Lederman, O.; Rosenbaum, S.; Dick, S.; Ward, P.; Curtis, J. Cardio-metabolic risk and its management in a cohort of clozapine-treated outpatients. Schizophr. Res. 2018, 199, 367–373. [Google Scholar] [CrossRef]
- Siskind, D.; Sharma, M.; Pawar, M.; Pearson, E.; Wagner, E.; Warren, N.; Kisely, S. Clozapine levels as a predictor for therapeutic response: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2021, 144, 422–432. [Google Scholar] [CrossRef]
- Siskind, D.; Russell, A.; Gamble, C.; Baker, A.; Cosgrove, P.; Burton, L.; Kisely, S. Metabolic measures 12 months after a randomised controlled trial of treatment of clozapine associated obesity and diabetes with exenatide (CODEX). J. Psychiatr. Res. 2020, 124, 9–12. [Google Scholar] [CrossRef]
- Siskind, D.; Sidhu, A.; Cross, J.; Chua, Y.T.; Myles, N.; Cohen, D.; Kisely, S. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust. N. Z. J. Psychiatry 2020, 54, 467–481. [Google Scholar] [CrossRef]
- Siskind, D.J.; Russell, A.W.; Gamble, C.; Winckel, K.; Mayfield, K.; Hollingworth, S.; Hickman, I.; Siskind, V.; Kisely, S. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: A randomized controlled trial (CODEX). Diabetes Obes. Metab. 2018, 20, 1050–1055. [Google Scholar] [CrossRef]
- Mamakou, V.; Thanopoulou, A.; Gonidakis, F.; Tentolouris, N.; Kontaxakis, V. Schizophrenia and type 2 diabetes mellitus. Psychiatriki 2018, 29, 64–73. [Google Scholar] [CrossRef]
- Larsen, J.R.; Svensson, C.K.; Vedtofte, L.; Jakobsen, M.L.; Jespersen, H.S.; Jakobsen, M.I.; Koyuncu, K.; Schjerning, O.; Nielsen, J.; Ekstrom, C.T.; et al. High prevalence of prediabetes and metabolic abnormalities in overweight or obese schizophrenia patients treated with clozapine or olanzapine. CNS Spectr. 2018, 31, 441–452. [Google Scholar]
- Potkin, S.G.; Bera, R.; Gulasekaram, B.; Costa, J.; Hayes, S.; Jin, Y.; Richmond, G.; Carreon, D.; Sitanggan, K.; Gerber, B.; et al. Plasma clozapine concentrations predict clinical response in treatment-resistant schizophrenia. J. Clin. Psychiatry 1994, 55 (Suppl. B), 133–136. [Google Scholar]
- Zheng, Y.; Meyerowitz-Katz, G.; Bramwell, S.; Jayaballa, R.; Assur, Y.; Vasani, D.; Ganapathy, R.; Maberly, G.; Brakoulias, V. Evaluating the Effectiveness of Joint Specialist Case Conferences in Improving Diabetes Control in Patients With Schizophrenia on Clozapine. J. Nerv. Ment. Dis. 2023, 211, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Siafis, S.; Fernando, P.; Falkai, P.; Honer, W.G.; Röh, A.; Siskind, D.; Leucht, S.; Hasan, A. Efficacy and safety of clozapine in psychotic disorders—A systematic quantitative meta-review. Transl. Psychiatry 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; McMahon, L.; Falkai, P.; Hasan, A.; Siskind, D. Impact of smoking behavior on clozapine blood levels—a systematic review and meta-analysis. Acta Psychiatr. Scand. 2020, 142, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Molden, E. Therapeutic drug monitoring of clozapine in adults with schizophrenia: A review of challenges and strategies. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1211–1221. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Hunter, S.; Obee, S.J. Assessing Adherence to Clozapine: Practical Considerations. J. Clin. Psychopharmacol. 2023, 43, 417–421. [Google Scholar] [CrossRef]
- de Leon, J. A Critical Commentary on the 2017 AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology. Pharmacopsychiatry 2018, 51, 63–68. [Google Scholar] [CrossRef]
- Skokou, M.; Karavia, E.A.; Drakou, Z.; Konstantinopoulou, V.; Kavakioti, C.A.; Gourzis, P.; Kypreos, K.E.; Andreopoulou, O. Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review. Pharmaceuticals 2022, 15, 817. [Google Scholar] [CrossRef]
- de Leon, J.; Ruan, C.J.; Schoretsanitis, G.; De Las Cuevas, C. A Rational Use of Clozapine Based on Adverse Drug Reactions, Pharmacokinetics, and Clinical Pharmacopsychology. Psychother. Psychosom. 2020, 89, 200–214. [Google Scholar] [CrossRef]
- Kar, N.; Barreto, S.; Chandavarkar, R. Clozapine Monitoring in Clinical Practice: Beyond the Mandatory Requirement. Clin. Psychopharmacol. Neurosci. 2016, 14, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Kuzin, M.; Kane, J.M.; Hiemke, C.; Paulzen, M.; Haen, E. Elevated Clozapine Concentrations in Clozapine-Treated Patients with Hypersalivation. Clin. Pharmacokinet. 2021, 60, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, R.J.; Gee, S.; Belsey, S.; Couchman, L.; Lally, J. Therapeutic monitoring of plasma clozapine and N-desmethylclozapine (norclozapine): Practical considerations. BJPsych. Adv. 2023, 29, 92–102. [Google Scholar] [CrossRef]
- Lochhead, J.D.; Nelson, M.A.; Schneider, A.L. Risks and Benefits of Rapid Clozapine Titration. Ment. Illn. 2016, 8, 6457. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jiao, B.; Zhou, X.; Zhang, W.; Ouyang, Z. Miniaturization of Mass Spectrometry Systems: An Overview of Recent Advancements and a Perspective on Future Directions. Anal. Chem. 2025, 97, 9111–9125. [Google Scholar] [CrossRef]
- Xu, W.; Charipar, N.; Kirleis, M.A.; Xia, Y.; Ouyang, Z. Study of Discontinuous Atmospheric Pressure Interfaces for Mass Spectrometry Instrumentation Development. Anal. Chem. 2010, 82, 6584–6592. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, W.; Brown, H.; Wu, J.; Fang, X.; Shahi, M.; Chen, R.; Zhang, H.; Jiao, B.; Wang, N.; et al. Rapid detection of IDH mutations in gliomas by intraoperative mass spectrometry. Proc. Natl. Acad. Sci. USA 2024, 121, e2318843121. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Chen, J.; Wang, R.; Lu, M.; Yu, C. Miniature mass spectrometer-based point-of-care assay for quantification of metformin and sitagliptin in human blood and urine. Anal. Bioanal. Chem. 2024, 416, 3305–3312. [Google Scholar] [CrossRef]
- Oloyede, E.; Blackman, G.; Mantell, B.; Harris, E.; Williams, J.; Taylor, D.; MacCabe, J.; McGuire, P. What are the barriers and facilitators of clozapine use in early psychosis? A survey of UK early intervention clinicians. Schizophrenia 2023, 9, 26. [Google Scholar] [CrossRef]
- Shah, P.; Iwata, Y.; Plitman, E.; Brown, E.E.; Caravaggio, F.; Kim, J.; Nakajima, S.; Hahn, M.; Remington, G.; Gerretsen, P.; et al. The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: A systematic review. Psychiatry Res. 2018, 268, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Appley, M.G.; Robinson, E.L.; Thomson, A.; Russell, E.; Sisco, E. An analytical platform for near real-time drug landscape monitoring using paraphernalia residues. Forensic Chem. 2023, 34, 100489. [Google Scholar] [CrossRef]
- Tanner, R.; Smith, S.G.; van Meijgaarden, K.E.; Giannoni, F.; Wilkie, M.; Gabriele, L.; Palma, C.; Dockrell, H.M.; Ottenhoff, T.H.M.; McShane, H. Optimisation, harmonisation and standardisation of the direct mycobacterial growth inhibition assay using cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 2019, 469, 1–10. [Google Scholar] [CrossRef]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef]
- Volmer, D.A.; Jessome, L.L. Ion Suppression: A Major Concern in Mass Spectrometry. LCGC N. Am. 2006, 24, 498–510. [Google Scholar]
- Chen, C.H.; Lin, Z.; Tian, R.; Shi, R.; Cooks, R.G.; Ouyang, Z. Real-time sample analysis using a sampling probe and miniature mass spectrometer. Anal. Chem. 2015, 87, 8867–8873. [Google Scholar] [CrossRef]
- El Abdellati, K.; De Picker, L.; Morrens, M. Antipsychotic Treatment Failure: A Systematic Review on Risk Factors and Interventions for Treatment Adherence in Psychosis. Front. Neurosci. 2020, 14, 531763. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Bu, J.; Zhou, X.; Ouyang, Z. Tandem Analysis by a Dual-Trap Miniature Mass Spectrometer. Anal. Chem. 2019, 91, 1391–1398. [Google Scholar] [CrossRef]
- Kang, M.; Lian, R.; Zhang, X.; Li, Y.; Zhang, Y.; Zhang, Y.; Zhang, W.; Ouyang, Z. Rapid and on-site detection of multiple fentanyl compounds by dual-ion trap miniature mass spectrometry system. Talanta 2020, 217, 121057. [Google Scholar] [CrossRef]
- Paal, M.; Habler, K.; Vogeser, M. Estimation of inter-laboratory reference change values from external quality assessment data. Biochem. Med. 2021, 31, 030902. [Google Scholar] [CrossRef]


| Concentration (ngmL−1) | Inter CV % | Intra CV % | Accuracy % | Inter CV % | Intra CV % | Accuracy % |
|---|---|---|---|---|---|---|
| Clozapine in Plasma Extract | Norclozapine in Plasma Extract | |||||
| 100 (L) | 11.18% | 13.20% | 101% | 4.55% | 10.40% | 94% |
| 500 (M) | 12.95% | 2.90% | 108% | 7.10% | 4.80% | 94% |
| 1000 (H) | 8.48% | 4.60% | 106% | 1.60% | 3.60% | 101% |
| Clozapine in Blood Extract | Norclozapine in Blood Extract | |||||
| 100 (L) | 14.05% | 16.54% | 108% | 14.62% | 9.15% | 114% |
| 500 (M) | 11.05% | 5.20% | 104% | 4.41% | 5.10% | 104% |
| 1000 (H) | 7.90% | 5.20% | 100% | 12.87% | 2.78% | 102% |
| Clozapine in DBSs | Norclozapine in DBSs | |||||
| 167 (L) | 9.60% | 22.30% | 96% | 17.80% | 13.90% | 107% |
| 417 (M) | 8.30% | 14.20% | 108% | 18.70% | 10.90% | 85% |
| 833 (H) | 2.90% | 21.10% | 104% | 3.70% | 10.90% | 116% |
| 1250 (H2) | 3.70% | 17.60% | 101% | 4.50% | 2.50% | 117% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lew, W.Y.; Yang, Y.; Zhang, N.; Bu, J.; Li, Z.; Fitzpatrick, M.; Bonnitcha, P.; Sullivan, D.; Zhang, W.; et al. Rapid On-Demand Point-of-Care Monitoring of Clozapine and Its Metabolite Norclozapine Using Miniature Mass Spectrometry. Pharmaceuticals 2025, 18, 1549. https://doi.org/10.3390/ph18101549
Wang X, Lew WY, Yang Y, Zhang N, Bu J, Li Z, Fitzpatrick M, Bonnitcha P, Sullivan D, Zhang W, et al. Rapid On-Demand Point-of-Care Monitoring of Clozapine and Its Metabolite Norclozapine Using Miniature Mass Spectrometry. Pharmaceuticals. 2025; 18(10):1549. https://doi.org/10.3390/ph18101549
Chicago/Turabian StyleWang, Xiaosuo, Wei Yi Lew, Yang Yang, Nan Zhang, Jiexun Bu, Zhentao Li, Michael Fitzpatrick, Paul Bonnitcha, David Sullivan, Wenpeng Zhang, and et al. 2025. "Rapid On-Demand Point-of-Care Monitoring of Clozapine and Its Metabolite Norclozapine Using Miniature Mass Spectrometry" Pharmaceuticals 18, no. 10: 1549. https://doi.org/10.3390/ph18101549
APA StyleWang, X., Lew, W. Y., Yang, Y., Zhang, N., Bu, J., Li, Z., Fitzpatrick, M., Bonnitcha, P., Sullivan, D., Zhang, W., Zheng, Y., & O’Sullivan, J. F. (2025). Rapid On-Demand Point-of-Care Monitoring of Clozapine and Its Metabolite Norclozapine Using Miniature Mass Spectrometry. Pharmaceuticals, 18(10), 1549. https://doi.org/10.3390/ph18101549






