Adipokines at the Metabolic–Brain Interface: Therapeutic Modulation by Antidiabetic Agents and Natural Compounds in Alzheimer’s Disease
Abstract
1. Introduction
2. Literature Search Methods
3. General Concepts of Alzheimer’s Disease
- AD is the leading cause of dementia, with the burden rising sharply due to population aging.
- Core pathology involves Aβ plaques and tau tangles, shaped by genetics and amplified by chronic neuroinflammation.
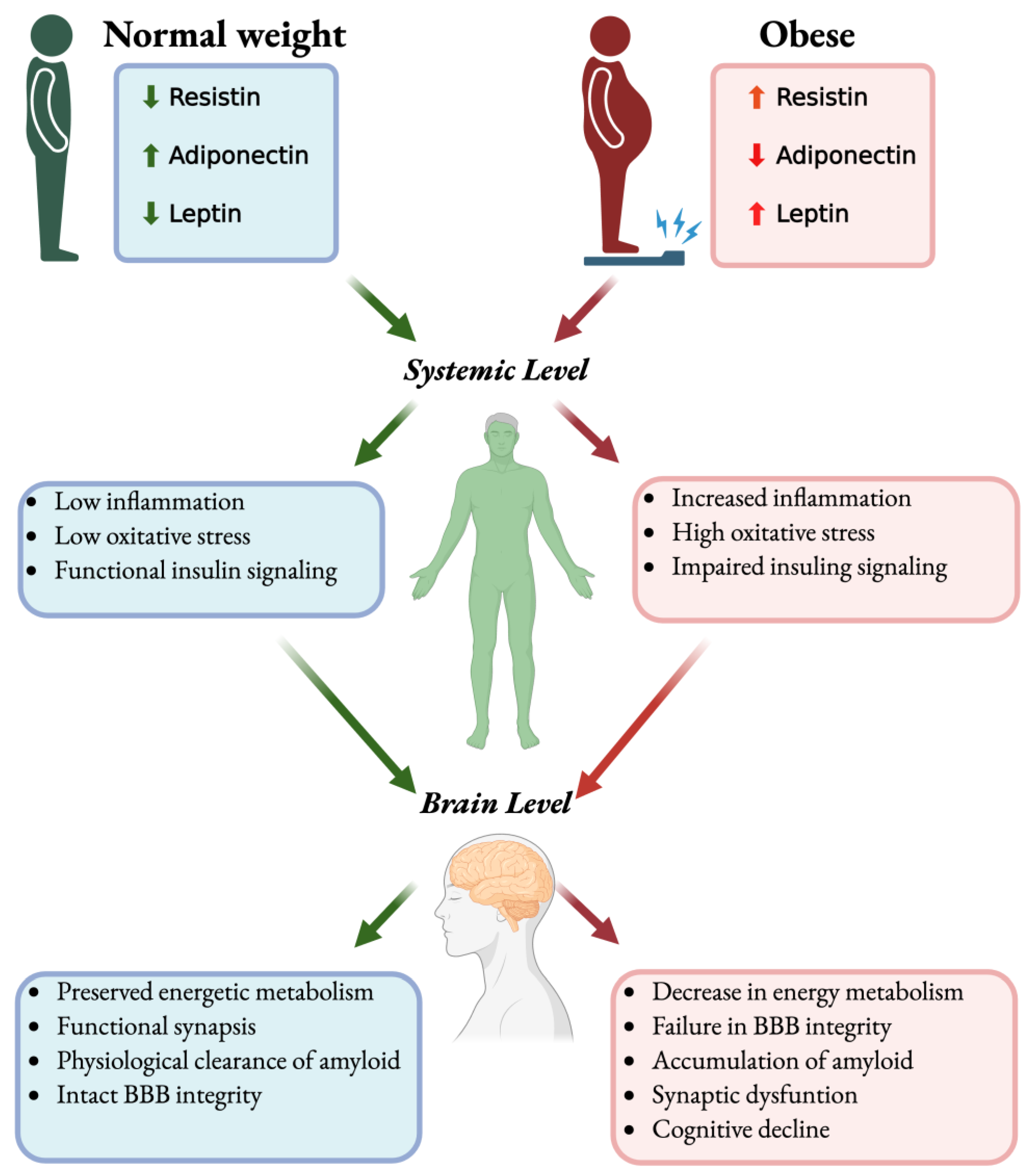
- Early cerebral glucose hypometabolism and brain insulin resistance occur, often independent of peripheral diabetes, linking AD to systemic metabolic dysfunction.
- Obesity-related cytokines and adipokine imbalance create a peripheral-to-central bridge that influences brain pathology.
4. Obesity as a Risk Factor for Alzheimer’s Disease
- Obesity in midlife strongly predicts later cognitive decline and higher amyloid/tau burden.
- Adipose-driven inflammation and insulin resistance activate microglia, astrocytes and impair neurovascular–glymphatic homeostasis.
- Imaging/biomarkers show obesity-linked cerebral hypometabolism, cortical/hippocampal atrophy, and adipokine imbalance, highlighting modifiable risk markers.
- Lifestyle and metabolic interventions can ameliorate these pathways and are plausible disease-modifying strategies.
5. Neuroinflammation in Alzheimer’s Disease and Its Link to Obesity
- Neuroinflammation is a disease driver: microglia/astrocyte activation accelerates Aβ/tau pathology and disrupts synapses and the BBB.
- Genetic signals underscore causal involvement of immune pathways in AD.
- Obesity amplifies neuroinflammation via systemic cytokines, insulin resistance, BBB leakage, and neurovascular–glymphatic dysfunction.
- Imaging/biomarker evidence converges on this nexus and identifies modifiable targets.
6. Adipokines and Their Relevance in the Brain
- Adipokines bridge peripheral metabolism and CNS function.
- Leptin is neuroprotective, but leptin resistance in obesity diminishes central signaling efficacy. Adiponectin enhances insulin sensitivity and is anti-inflammatory; dementia studies show heterogeneous levels. Resistin is pro-inflammatory, impairs insulin signaling, and compromises BBB integrity.
- Adipokine imbalance disrupts cerebral glucose metabolism, amplifies neuroinflammation, and promotes Aβ/tau pathology.
7. Resistin and Adiponectin: Opposing Adipokines Forces in Alzheimer’s Disease
- Resistin promotes NF-κB–dependent inflammation, insulin resistance, BBB disruption, and aggravation of Aβ/tau pathology. These actions accelerate synaptic dysfunction and cerebral metabolic failure.
- Adiponectin counters inflammation and insulin resistance; higher functional adiponectin is associated with neuroprotection. Stage-dependent “adiponectin resistance” may blunt these benefits in AD.
- The resistin–adiponectin balance/ratio reflects metabolic–neuroinflammatory risk and has biomarker potential.
- Therapeutic angle: boost adiponectin signaling and/or inhibit resistin pathways within precision, stage-aware interventions.
8. Natural Compounds Targeting Adipokines Signaling in Alzheimer’s Disease in the Context of Obesity
- Phytochemicals shift the adipokine axis toward higher adiponectin and lower resistin activity.
- Mechanistically: activate AMPK/PPAR signaling and suppress TLR4–NF-κB pathways, improving insulin sensitivity and neuronal energetics while dampening neuroinflammation. These effects can indirectly reduce Aβ/tau stress and support synaptic function.
- Evidence is preclinically strong but mixed in humans. Next steps: stage-/metabolic-stratified trials with adipokine endpoints and improved bioavailability/delivery strategies.
9. Therapeutic Modulation of Adipokines in AD: Antidiabetic Agents and Natural Compounds
- Antidiabetic agents slope the adipokine/insulin axis.
- Cognitive benefits to date are modest/heterogeneous and often confounded by weight loss and vascular–metabolic improvements.
- Intranasal insulin yields short-term memory gains with genotype/formulation-dependent responses.
- Trial priorities: patient stratification (stage/metabolic profile), rigorous safety monitoring, and standardized outcomes anchored to adipokine and insulin-signaling endpoints.
10. Future Directions
- Strong preclinical effects of leptin/adiponectin/resistin on Aβ/tau, neuroinflammation, and metabolism do not consistently replicate in humans due to species, obesity phenotype, leptin resistance, comorbidities, and sex/hormonal factors.
- Run longitudinal, powered studies linking peripheral adipokines with CSF/PET Aβ/tau, imaging, fluid biomarkers, and cognition, stratified by obesity phenotype, leptin resistance, APOE, and age.
- Use genetics, multimodal AI models, and sex-specific approaches; evaluate simple screening panels and integrate microbiome and single-cell/spatial omics to map cell-specific actions.
11. General Conclusions
- Closing the translational gap: There is strong preclinical evidence that adipokines shape insulin signaling, neuroinflammation, and Aβ/tau which does not translate consistently to humans, demanding rigorous, longitudinal, stratified trials to define who benefits, from what, and when.
- Dual clinical utility: Adipokines are both biomarkers and targets; integrating adipokine profiling with precision therapeutics (AdipoR agonists, resistin inhibitors, advanced delivery) plus lifestyle/nutraceutical strategies can enable early detection, patient stratification, and combinatorial treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Scheen, A.J.; Paquot, N. Obesity: A new paradigm for treating obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 196–198. [Google Scholar] [CrossRef]
- Despres, J.P.; Arsenault, B.J.; Cote, M.; Cartier, A.; Lemieux, I. Abdominal obesity: The cholesterol of the 21st century? Can. J. Cardiol. 2008, 24 (Suppl. SD), 7D–12D. [Google Scholar] [CrossRef]
- Naderali, E.K.; Ratcliffe, S.H.; Dale, M.C. Obesity and alzheimer’s disease: A link between body weight and cognitive function in old age. Am. J. Alzheimer’s Dis. Other Dement. 2009, 24, 445–449. [Google Scholar]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar]
- Ly, M.; Yu, G.Z.; Mian, A.; Cramer, A.; Meysami, S.; Merrill, D.A.; Samara, A.; Eisenstein, S.A.; Hershey, T.; Babulal, G.M.; et al. Neuroinflammation: A Modifiable Pathway Linking Obesity, Alzheimer’s disease, and Depression. Am. J. Geriatr. Psychiatry 2023, 31, 853–866. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar]
- Korczyn, A.D.; Grinberg, L.T. Is Alzheimer disease a disease? Nat. Rev. Neurol. 2024, 20, 245–251. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Rios, J.A.; Cisternas, P.; Arrese, M.; Barja, S.; Inestrosa, N.C.; Ríos Ja Cisternas, P.; Arrese, M.; Barja, S.; Inestrosa, N.C. Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog. Neurobiol. 2014, 121, 125–146. [Google Scholar]
- Lv, Y.; Xiang, Y.; Fu, W.; Li, B.; Li, X. Global burden trends of Alzheimer’s disease and other dementias attributable to smoking from 1990 to 2021. J. Alzheimer’s Dis. 2025, 107, 1154–1167. [Google Scholar] [CrossRef]
- Makin, S. The future of Alzheimer’s treatment. Nature 2025, 640, S4–S6. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Morris, J.K.; Burns, J.M. Insulin: An emerging treatment for Alzheimer’s disease dementia? Curr. Neurol. Neurosci. Rep. 2012, 12, 520–527. [Google Scholar] [CrossRef]
- Businaro, R.; Ippoliti, F.; Ricci, S.; Canitano, N.; Fuso, A. Alzheimer’s disease promotion by obesity: Induced mechanisms-molecular links and perspectives. Curr. Gerontol. Geriatr. Res. 2012, 2012, 986823. [Google Scholar]
- Craft, S. Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging 2005, 26 (Suppl. S1), 65–69. [Google Scholar] [PubMed]
- Mandrekar, S.; Landreth, G.E. Microglia and Inflammation in Alzheimers Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar]
- Kiliaan, A.J.; Arnoldussen, I.A.C.; Gustafson, D.R. Adipokines: A link between obesity and dementia? Lancet Neurol. 2014, 13, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Osei, S.Y. Adipokines in obesity. Front. Horm. Res. 2008, 36, 182–197. [Google Scholar]
- Aguilar-Valles, A.; Inoue, W.; Rummel, C.; Luheshi, G.N. Obesity, adipokines and neuroinflammation. Neuropharmacology 2015, 96, 124–134. [Google Scholar] [CrossRef]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar]
- He, D.; Liu, M.; Tang, Y.; Tian, X.; Zhou, L.; Chen, Y.; Liu, X. Systematic analysis and prediction of the burden of Alzheimer’s disease and other dementias caused by hyperglycemia. Front. Public Health 2025, 12, 1516267. [Google Scholar] [CrossRef]
- Mallapaty, S. One potent gene raises risk of Alzheimer’s, Parkinson’s and other brain diseases. Nature 2025. online ahead of print. [Google Scholar] [CrossRef]
- Jackson, K.; Barisone, G.A.; Diaz, E.; Jin, L.W.; DeCarli, C.; Despa, F. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann. Neurol. 2013, 74, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.M.; Ghura, S.; Koster, K.P.; Liakaite, V.; Maienschein-Cline, M.; Kanabar, P.; Collins, N.; Ben-Aissa, M.; Lei, A.Z.; Bahroos, N.; et al. APOE-modulated Aβ-induced neuroinflammation in Alzheimer’s disease: Current landscape, novel data, and future perspective. J. Neurochem. 2015, 133, 465–488. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hyman, B.T.; Serrano-Pozo, A. Multifaceted roles of APOE in Alzheimer disease. Nat. Rev. Neurol. 2024, 20, 457–474. [Google Scholar] [CrossRef]
- Bertram, L.; Lange, C.; Mullin, K.; Parkinson, M.; Hsiao, M.; Hogan, M.F.; Schjeide, B.M.; Hooli, B.; Divito, J.; Ionita, I.; et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am. J. Hum. Genet. 2008, 83, 623–632. [Google Scholar]
- Cisternas, P.; Inestrosa, N.C. Brain glucose metabolism: Role of Wnt signaling in the metabolic impairment in Alzheimer’s disease. Neurosci. Biobehav. Rev. 2017, 80, 316–328. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Z.H.; Kang, S.S.; Liu, X.; Xia, Y.; Chan, C.B.; Ye, K. High-fat diet-induced diabetes couples to Alzheimer’s disease through inflammation-activated C/EBPβ/AEP pathway. Mol. Psychiatry 2022, 27, 3396–3409. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 24, 276. [Google Scholar] [CrossRef]
- Godoy, J.A.; Rios, J.A.; Zolezzi, J.M.; Braidy, N.; Inestrosa, N.C. Signaling pathway cross talk in Alzheimer’s disease. Cell Commun. Signal. 2014, 12, 23. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Alshakhshir, N.; Zhao, L. Glycolytic Metabolism, Brain Resilience, and Alzheimer’s Disease. Front. Neurosci. 2021, 15, 662242. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef]
- Marcus, C.; Mena, E.; Subramaniam, R.M. Brain PET in the diagnosis of Alzheimer’s disease. Clin. Nucl. Med. 2014, 39, e413–e426. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin Resistance Exacerbates Alzheimer Disease via Multiple Mechanisms. Front. Neurosci. 2021, 15, 687157. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin Transl Imaging 2013, 1, 217–233. [Google Scholar] [CrossRef]
- Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease: FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 486–510. [Google Scholar] [CrossRef]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-mediated metabolic regulation in adipose tissue. Obes. Rev. 2024, 25, e13724. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, D.; Wang, J.; Luo, X.; Guo, R. Inflammation—Cause or consequence of late onset Alzheimer’s disease or both? A review of the evidence. Eur. J. Inflamm. 2022, 20, 1–10. [Google Scholar] [CrossRef]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef]
- Kazemeini, S.; Nadeem-Tariq, A.; Shih, R.; Rafanan, J.; Ghani, N.; Vida, T.A. From Plaques to Pathways in Alzheimer’s Disease: The Mitochondrial-Neurovascular-Metabolic Hypothesis. Int. J. Mol. Sci. 2024, 35, 11720. [Google Scholar] [CrossRef]
- Clark, T.A.; Lee, H.P.; Rolston, R.K.; Zhu, X.; Marlatt, M.W.; Castellani, R.J.; Nunomura, A.; Casadesus, G.; Smith, M.A.; Lee, H.G.; et al. Oxidative Stress and its Implications for Future Treatments and Management of Alzheimer Disease. Int. J. Biomed. Sci. 2010, 6, 225–227. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Pathology and Treatments of Alzheimer’s Disease Based on Considering Changes in Brain Energy Metabolism Due to Type 2 Diabetes. Molecules 2024, 29, 5936. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.G.; Katz, S.L. Clinicopathological Conference of the Framingham Union Hospital. BMQ 1965, 16, 65–71. [Google Scholar] [PubMed]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 2005, 26, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.S.; Beiser, A.; Vasan, R.S.; Au, R.; Auerbach, S.; Kiel, D.P.; Wolf, P.A.; Seshadri, S. Thyroid function and the risk of Alzheimer disease: The Framingham study. Arch. Intern. Med. 2008, 168, 1514–1520. [Google Scholar] [CrossRef]
- Stewart, R.; Masaki, K.; Xue, Q.L.; Peila, R.; Petrovitch, H.; White, L.R.; Launer, L.J. A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch. Neurol. 2005, 62, 55–60. [Google Scholar] [CrossRef]
- Walker, J.M.; Harrison, F.E. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer’s Disease: Impacts on Cognitive Decline. Nutrients 2015, 7, 7332–7357. [Google Scholar] [CrossRef]
- Stickel, A.M.; Tarraf, W.; Gonzalez, K.A.; Isasi, C.R.; Kaplan, R.; Gallo, L.C.; Zeng, D.; Cai, J.; Pirzada, A.; Daviglus, M.L.; et al. Central Obesity, Cardiometabolic Risk, and Cognitive Change in the Study of Latinos: Investigation of Neurocognitive Aging. J. Alzheimer’s Dis. 2021, 82, 1203–1218. [Google Scholar] [CrossRef]
- Chuang, Y.F.; An, Y.; Bilgel, M.; Wong, D.F.; Troncoso, J.C.; O’Brien, R.J.; Breitner, J.C.; Ferruci, L.; Resnick, S.M.; Thambisetty, M. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol. Psychiatry 2016, 21, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Pasco, J.A. Obesity and brain function: The brain–body crosstalk. Medicina 2020, 56, 499. [Google Scholar] [CrossRef]
- Uranga, R.M.; Keller, J.N. The complex interactions between obesity, metabolism and the brain. Front. Neurosci. 2019, 13, 513. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Gué, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid-protein, and the-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Folstein, M.F. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol. Aging 2006, 27, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Link, C.D. Decreased Insulin-Receptor Signaling Promotes the Autophagic Degradation of beta-amyloid peptide in C. elegans. Autophagy 2007, 3, 569–580. [Google Scholar]
- Pearson-Leary, J.; McNay, E.C. Intrahippocampal administration of amyloid-β1-42 oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J. Alzheimer’s Dis. 2012, 30, 413–422. [Google Scholar] [CrossRef]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Josephs, K.A.; Dickson, D.W.; Tosakulwong, N.; Weigand, S.D.; Murray, M.E.; Petrucelli, L.; Liesinger, A.M.; Senjem, M.L.; Spychalla, A.J.; Knopman, D.S.; et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: A longitudinal retrospective study. Lancet Neurol. 2017, 16, 917–924. [Google Scholar] [CrossRef]
- Jayaraman, A.; Pike, C.J. Alzheimer’s Disease and Type 2 Diabetes: Multiple Mechanisms Contribute to Interactions. Curr. Diabetes Rep. 2014, 14, 476. [Google Scholar] [CrossRef]
- Kullenberg, H.; Rossen, J.; Johansson, U.B.; Hagströmer, M.; Nyström, T.; Kumlin, M.; Svedberg, M.M. Correlations between insulin-degrading enzyme and metabolic markers in patients diagnosed with type 2 diabetes, Alzheimer’s disease, and healthy controls: A comparative study. Endocrine 2024, 84, 450–458. [Google Scholar] [CrossRef]
- Corraliza-Gomez, M.; Bermejo, T.; Lilue, J.; Rodriguez-Iglesias, N.; Valero, J.; Cozar-Castellano, I.; Arranz, E.; Sanchez, D.; Ganfornina, M.D. Insulin-degrading enzyme (IDE) as a modulator of microglial phenotypes in the context of Alzheimer’s disease and brain aging. J. Neuroinflamm. 2023, 20, 233. [Google Scholar] [CrossRef]
- Gong, X.; Liang, Z.; Liu, W.; Zhao, Y.; Yang, Y.; Wu, M.; Shang, J.; Xiao, Y.; Mei, Y.; Su, Q.; et al. High Fat Diet Aggravates AD-Related Pathogenic Processes in APP/PS1 Mice. Curr. Alzheimer Res. 2021, 18, 310–325. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Z.; Tian, Z.; Blanchard, J.; Dai, C.L.; Chalbot, S.; Iqbal, K.; Liu, F.; Gong, C.X. Intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol. Neurobiol. 2014, 49, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Musa, M.; Labeikovsky, W.; Pugazhenthi, S. Sirt3 deficiency induced down regulation of insulin degrading enzyme in comorbid Alzheimer’s disease with metabolic syndrome. Sci. Rep. 2022, 12, 19808. [Google Scholar] [CrossRef]
- Hatakawa, Y.; Takeuchi, Y.; Lee, S.H.; Oe, T. Tyrosine modifications of insulin-degrading enzyme enable favorable control of substrate specificity for both Alzheimer’s disease and type-2 diabetes mellitus. Bioorg. Chem. 2024, 153, 107916. [Google Scholar] [CrossRef]
- Dimache, A.M.; Șalaru, D.L.; Sascău, R.; Stătescu, C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients 2021, 13, 2118. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Glodzik, L.; Pupi, A.; De Santi, S.; de Leon, M.J. Pre-Clinical Detection of Alzheimer’s Disease Using FDG-PET, with or without Amyloid Imaging. J. Alzheimer’s Dis. 2010, 23, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Mchugh, P.F. FDG-and amyloid-PET in Alzheimer’s disease: Is the whole greater than the sum of the parts? Q. J. Nucl. Med. Mol. Imaging 2011, 55, 250–264. [Google Scholar]
- Mosconi, L.; Tsui, W.H.; De Santi, S.; Li, J.; Rusinek, H.; Convit, A.; Li, Y.; Boppana, M.; de Leon, M.J. Reduced hippocampal metabolism in MCI and AD: Automated FDG-PET image analysis. Neurology 2005, 64, 1860–1867. [Google Scholar] [CrossRef]
- Soltani, S.; Dolatshahi, M.; Soltani, S.; Khazaei, K.; Rahmani, M.; Raji, C.A. Relationships Between Brain Glucose Metabolism Patterns and Impaired Glycemic Status: A Systematic Review of FDG-PET Studies With a Focus on Alzheimer’s Disease. Hum. Brain Mapp. 2025, 46, e70180. [Google Scholar] [CrossRef] [PubMed]
- Cayir, S.; Volpi, T.; Toyonaga, T.; Gallezot, J.D.; Yang, Y.; Sadabad, F.E.; Mulnix, T.; Mecca, A.P.; Fesharaki-Zadeh, A.; Matuskey, D. Relationship between neuroimaging and cognition in frontotemporal dementia: An FDG-PET and structural MRI study. J. Neuroimaging 2024, 34, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef]
- Yonamine, C.Y.; Passarelli, M.; Suemoto, C.K.; Pasqualucci, C.A.; Jacob-Filho, W.; Alves, V.A.F.; Marie, S.K.N.; Correa-Giannella, M.L.; Britto, L.R.; Machado, U.F. Postmortem Brains from Subjects with Diabetes Mellitus Display Reduced GLUT4 Expression and Soma Area in Hippocampal Neurons: Potential Involvement of Inflammation. Cells 2023, 12, 1250. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ma, Y.H.; Hu, H.Y.; Ma, L.Z.; Tan, L.; Yu, J.T. Late-Life Obesity Associated with Tau Pathology in Cognitively Normal Individuals: The CABLE Study. J. Alzheimer’s Dis. 2022, 85, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Steppan, C.M.; Brown, E.J.; Wright, C.M.; Bhat, S.; Banerjee, R.R.; Dai, C.Y.; Enders, G.H.; Silberg, D.G.; Wen, X.; Wu, G.D.; et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 502–506. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta 2015, 438, 46–54. [Google Scholar] [CrossRef]
- Benomar, Y.; Amine, H.; Crépin, D.; Al Rifai, S.; Riffault, L.; Gertler, A.; Taouis, M. Central Resistin/TLR4 impairs adiponectin signaling, contributing to insulin and FGF21 resistance. Diabetes 2016, 65, 913–926. [Google Scholar] [CrossRef]
- Watson, L.S.; Wilken-Resman, B.; Williams, A.; DiLucia, S.; Sanchez, G.; McLeod, T.L.; Sims-Robinson, C. Hyperinsulinemia alters insulin receptor presentation and internalization in brain microvascular endothelial cells. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221118626. [Google Scholar] [CrossRef]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front. Endocrinol. 2018, 9, 359824. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Wang, X.; Wang, C.; Yang, J.; Guan, B. Change in Adipokines and Gastrointestinal Hormones After Bariatric Surgery: A Meta-analysis. Obes. Surg. 2023, 33, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef]
- Coucha, M.; Abdelsaid, M.; Ward, R.; Abdul, Y.; Ergul, A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr. Physiol. 2018, 8, 773–799. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Ding, G.; Davoodi-Bojd, E.; Li, Q.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 2016, 37, 1326. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Arunsak, B.; Suparan, K.; Sriwichaiin, S.; Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. Combined caloric restriction and exercise provides greater metabolic and neurocognitive benefits than either as a monotherapy in obesity with or without estrogen deprivation. J. Nutr. Biochem. 2022, 110, 109125. [Google Scholar] [CrossRef]
- Chakrabarti, S. Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 525–533. [Google Scholar]
- Ma, J.; Zhang, W.; Wang, H.-F.; Wang, Z.-X.; Jiang, T.; Tan, M.-S.; Yu, J.-T.; Tan, L. Peripheral Blood Adipokines and Insulin Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analysis. Curr. Alzheimer Res. 2016, 13, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Griciuc, A.; Tanzi, R.E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 228. [Google Scholar] [CrossRef] [PubMed]
- Daskoulidou, N.; Shaw, B.; Zelek, W.M.; Morgan, B.P. The Alzheimer’s disease-associated complement receptor 1 variant confers risk by impacting glial phagocytosis. Alzheimer’s Dement. 2025, 21, e70458. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Grinberg, L.T.; Boxer, A.; Ossenkoppele, R.; Jonsson, M.; Seeley, W.; Ehrenberg, A.; Spina, S.; Janelidze, S.; Rojas-Martinex, J.; et al. Cerebrospinal fluid biomarkers in autopsy-confirmed Alzheimer disease and frontotemporal lobar degeneration. Neurology 2022, 98, E1137–E1150. [Google Scholar] [CrossRef]
- Kac, P.R.; González-Ortiz, F.; Emeršič, A.; Dulewicz, M.; Koutarapu, S.; Turton, M.; An, Y.; Smirnov, D.; Kulczyńska-Przybik, A.; Varma, V.R.; et al. Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer’s disease neuropathology. Nat. Commun. 2024, 15, 2615. [Google Scholar] [CrossRef]
- Liu, Y.; Kwok, W.; Yoon, H.; Ryu, J.C.; Stevens, P.; Hawkinson, T.R.; Shedlock, C.J.; Ribas, R.A.; Medina, T.; Keohane, S.B.; et al. Imbalance in Glucose Metabolism Regulates the Transition of Microglia from Homeostasis to Disease-Associated Microglia Stage 1. J. Neurosci. 2024, 44, e1563232024. [Google Scholar] [CrossRef]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef]
- de Mello, J.E.; Teixeira, F.C.; dos Santos, A.; Luduvico, K.; Soares de Aguiar, M.S.; Domingues, W.B.; Campos, V.F.; Tavares, R.G.; Schneider, A.; Stefanello, F.M.; et al. Treatment with Blackberry Extract and Metformin in Sporadic Alzheimer’s Disease Model: Impact on Memory, Inflammation, Redox Status, Phosphorylated Tau Protein and Insulin Signaling. Mol. Neurobiol. 2024, 61, 7814–7829. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H.K. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van Gijsel-Bonnello, M.; Baranger, K.; Benech, P.; Rivera, S.; Khrestchatisky, M.; De Reggi, M.; Gharib, B. Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer’s disease: Alleviation by pantethine. PLoS ONE 2017, 12, e0175369. [Google Scholar] [CrossRef]
- van der Harg, J.M.; Eggels, L.; Ruigrok, S.R.; Jeroen, J.J.; La Fleur, S.E.; Scheper, W. Neuroinflammation is not a prerequisite for diabetes-induced Tau phosphorylation. Front. Neurosci. 2015, 9, 167835. [Google Scholar] [CrossRef]
- Kacířová, M.; Železná, B.; Blažková, M.; Holubová, M.; Popelová, A.; Kuneš, J.; Šedivá, B.; Maletínská, L. Aging and high-fat diet feeding lead to peripheral insulin resistance and sex-dependent changes in brain of mouse model of tau pathology THY-Tau22. J. Neuroinflamm. 2021, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi, M.; Commean, P.K.; Rahmani, F.; Liu, J.; Lloyd, L.K.; Nguyen, C.; Hantler, N.; Ly, M.; Yu, G.; Ippolito, J.E.; et al. Alzheimer Disease Pathology and Neurodegeneration in Midlife Obesity: A Pilot Study. Aging Dis. 2024, 15, 1843–1854. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Sousa, N.; Palha, J.A. Blood-brain-barriers in aging and in Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Winkler Ea Bell, R.D.; Deane, R.; Zlokovic, B.V. From the liver to the blood-brain barrier: An interconnected system regulating brain amyloid-β levels. J. Neurosci. Res. 2011, 89, 967–968. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef]
- Dorrance, A.M.; Matin, N.; Pires, P.W. The Effects of Obesity on the Cerebral Vasculature. Curr. Vasc. Pharmacol. 2014, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Montagnani, M.; Kwang, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Macvicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Rege, S.V.; Ramanathan, A.; Wang, Y.; Ahuja, A.; Lazic, D.; Tsai, P.S.; Zhao, Z.; Zhou, Y.; et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017, 20, 406–416. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood–Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions. Nutrients 2015, 7, 6719. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A High-Fat, Refined Sugar Diet Reduces Hippocampal Brain-Derived Neurotrophic Factor, Neuronal Plasticity, and Learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Tokunaga, M.; Zhang, M.R.; Ji, B.; Suhara, T.; Higuchi, M. Assessment of neuroinflammation in a mouse model of obesity and β-amyloidosis using PET. J. Neuroinflamm. 2016, 13, 221. [Google Scholar] [CrossRef]
- Rudge, J.D.A. A New Hypothesis for Alzheimer’s Disease: The Lipid Invasion Model. J. Alzheimer’s Dis. Rep. 2022, 6, 129. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, J.; Shen, Q.; Li, M.; Peng, Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2018, 15, 242. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Henn, R.E.; Elzinga, S.E.; Glass, E.; Parent, R.; Guo, K.; Allouch, A.A.; Mendelson, F.E.; Hayes, J.; Webber-Davis, I.; Murphy, G.G.; et al. Obesity-induced neuroinflammation and cognitive impairment in young adult versus middle-aged mice. Immun. Ageing 2022, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- González Olmo, B.M.; Bettes, M.N.; DeMarsh, J.W.; Zhao, F.; Askwith, C.; Barrientos, R.M. Short-term high-fat diet consumption impairs synaptic plasticity in the aged hippocampus via IL-1 signaling. NPJ Sci. Food 2023, 7, 35. [Google Scholar] [CrossRef]
- Képes, Z.; Aranyi, C.; Forgács, A.; Nagy, F.; Kukuts, K.; Hascsi, Z.; Esze, R.; Somodi, S.; Káplár, M.; Varga, J.; et al. Glucose-level dependent brain hypometabolism in type 2 diabetes mellitus and obesity. Eur. J. Hybrid Imaging 2021, 5, 3. [Google Scholar] [CrossRef]
- Pegueroles, J.; Pané, A.; Vilaplana, E.; Montal, V.; Bejanin, A.; Videla, L.; Carmona-Iragui, M.; Barroeta, I.; Ibarzabal, A.; Casajoana, A.; et al. Obesity impacts brain metabolism and structure independently of amyloid and tau pathology in healthy elderly. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12052. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kulas, J.A.; Wang, C.; Holtzman, D.M.; Ferris, H.A.; Hansen, S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA 2021, 118, e2102191118. [Google Scholar] [CrossRef]
- Montague-Cardoso, K. Beta-amyloid production in neurons is regulated by astrocyte-derived cholesterol. Commun. Biol. 2021, 4, 1116. [Google Scholar] [CrossRef]
- Boccara, E.; Golan, S.; Beeri, M.S. The association between regional adiposity, cognitive function, and dementia-related brain changes: A systematic review. Front. Med. 2023, 10, 1160426. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Grodstein, F.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Kelly, J.F.; Evans, D.A.; Bennett, D.A. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 2008, 70, 2219–2225. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Vieira, V.J.; Valentine, R.J.; Wilund, K.R.; Antao, N.; Baynard, T.; Woods, J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1164–E1171. [Google Scholar] [CrossRef]
- Ng, R.C.L.; Jian, M.; Ma, O.K.F.; Bunting, M.; Kwan, J.S.C.; Zhou, G.J.; Senthilkumar, K.; Iyaswamy, A.; Chan, P.K.; Li, M.; et al. Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer’s disease mouse model. Mol. Psychiatry 2021, 26, 5669–5689. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, I.A.C.; Kiliaan, A.J.; Gustafson, D.R. Obesity and dementia: Adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014, 24, 1982–1999. [Google Scholar] [CrossRef]
- Calió, M.L.; Mosini, A.C.; Marinho, D.S.; Salles, G.N.; Massinhani, F.H.; Ko, G.M.; Porcionatto, M.A. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105219. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Kim, E.; Kim, C.H.; Song, H.T.; Lee, J.E. Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: An in vivo and in vitro study. Cell Death Dis. 2015, 6, e1844. [Google Scholar] [CrossRef]
- Abdelwahed, O.M.; Tork, O.M.; Gamal el Din, M.M.; Rashed, L.; Zickri, M. Effect of glucagon-like peptide-1 analogue; Exendin-4, on cognitive functions in type 2 diabetes mellitus; possible modulation of brain derived neurotrophic factor and brain Visfatin. Brain Res. Bull. 2018, 139, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, P.; Gherardelli, C.; Gutierrez, J.; Salazar, P.; Mendez-Orellana, C.; Wong, G.W.; Inestrosa, N.C. Adiponectin and resistin modulate the progression of Alzheimer’s disease in a metabolic syndrome model. Front. Endocrinol. 2023, 14, 1237796. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.W.; Zerfass, K.M.; Heckstall, E.M.; Evans, K.A. Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther. Adv. Endocrinol. Metab. 2015, 6, 189. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Gong, P.; Zhang, H.; Chen, Y.; Yao, S.; Li, W.; Zhang, Y.; Yu, Y. The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway. Int. J. Mol. Sci. 2022, 23, 9041. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yang, X.; Huang, Y.X.; Qiu, H.; Huang, G.; Xiao, H.; Kuai, J. The neuroprotective effect of apelin-13 in a mouse model of intracerebral hemorrhage. Neurosci. Lett. 2016, 628, 219–224. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Qian, G.; Zhou, J. Omentin-1 attenuates adipose tissue inflammation via restoration of TXNIP/NLRP3 signaling in high-fat diet-induced obese mice. Fundam. Clin. Pharmacol. 2020, 34, 721–735. [Google Scholar]
- Yang, E.; Cai, Y.; Yao, X.; Liu, J.; Wang, Q.; Jin, W.; Wu, Q.; Fan, W.; Qiu, L.; Kang, C.; et al. Tissue plasminogen activator disrupts the blood-brain barrier through increasing the inflammatory response mediated by pericytes after cerebral ischemia. Aging 2019, 11, 10167–10182. [Google Scholar] [CrossRef]
- Wołoszynowska-Fraser, M.U.; Rossi, S.L.; Long, J.M.; McCaffery, P.J.; Rapp, P.R. Differential retinoic acid signaling in the hippocampus of aged rats with and without memory impairment. eNeuro 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Lee, Y.L.; Yoon, H.K.; Kang, S.W.; Lee, W.J.; Park, J.Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Diabetol. 2014, 13, 41. [Google Scholar] [CrossRef]
- Gaweda-Walerych, K.; Aragona, V.; Lodato, S.; Sitek, E.J.; Narożańska, E.; Buratti, E. Progranulin deficiency in the brain: The interplay between neuronal and non-neuronal cells. Transl. Neurodegener. 2025, 14, 18. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of Leptin Action and Leptin Resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef]
- Flak, J.N.; Myers, M.G. Minireview: CNS Mechanisms of Leptin Action. Mol. Endocrinol. 2016, 30, 3–12. [Google Scholar] [CrossRef]
- Nazarians-Armavil, A.; Menchella, J.A.; Belsham, D.D. Cellular Insulin Resistance Disrupts Leptin-Mediated Control of Neuronal Signaling and Transcription. Mol. Endocrinol. 2013, 27, 990–1003. [Google Scholar] [CrossRef]
- Tezapsidis, N.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Casadesus, G.; Robakis, N.K.; Wolozin, B.; Perry, G.; Zhu, X.; Greco, S.J.; et al. Leptin: A novel therapeutic strategy for Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Stone, J.G.; Torres, S.L.; Siedlak, S.L.; Perry, G.; Kryscio, R.; Jicha, G.; Casadesus, G.; Smith, M.A.; Zhu, X.; et al. Dysregulation of leptin signaling in Alzheimer disease: Evidence for neuronal leptin resistance. J. Neurochem. 2014, 128, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, C.A.; Carvalho, M.G.; Sousa, L.P.; Caramelli, P.; Gomes, K.B. Leptin in Alzheimer’s disease. Clin. Chim. Acta 2015, 450, 162–168. [Google Scholar] [PubMed]
- Bulló, M.; García-Lorda, P.; Megias, I.; Salas-Salvadó, J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes. Res. 2003, 11, 525–531. [Google Scholar] [CrossRef]
- Ahima, R.S. Connecting Leptin and Alzheimer Disease. Arch. Neurol. 2010, 67, 873–875. [Google Scholar] [CrossRef]
- Lee, S.; Byun, M.S.; Yi, D.; Ahn, H.; Jung, G.; Jung, J.H.; Chang, Y.Y.; Kim, K.; Choi, H.; Choi, J.; et al. Plasma Leptin and Alzheimer Protein Pathologies Among Older Adults. JAMA Netw. Open 2024, 7, e249539. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Lam, K.S.L.; Cheng, O.Y.; Kwan, J.S.C.; Ho, P.W.L.; Cheng, K.K.Y.; Chung, S.K.; Ho, J.W.M.; Guo, V.Y.; Xu, A. Adiponectin is Protective against Oxidative Stress Induced Cytotoxicity in Amyloid-Beta Neurotoxicity. PLoS ONE 2012, 7, e52354. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Kwan, J.S.C.; Bunting, M.; Ng, R.C.L.; Chan, K.H. Adiponectin suppresses amyloid-β oligomer (AβO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-κB signaling pathway. J. Neuroinflamm. 2019, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.L.; Chan, K.H. Potential neuroprotective effects of adiponectin in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 592. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, N.; Qiu, Y.; Tan, J.; Wang, F.; Qin, L.; Dai, A. Resistin-like molecules: A marker, mediator and therapeutic target for multiple diseases. Cell Commun. Signal. 2023, 21, 18. [Google Scholar] [CrossRef]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an Adipokine with Potent Proinflammatory Properties. J. Immunol. 2005, 174, 5789–5795. [Google Scholar] [CrossRef]
- Schwartz, D.R.; Lazar, M.A. Human resistin: Found in translation from mouse to man. Trends Endocrinol. Metab. 2011, 22, 259–265. [Google Scholar] [CrossRef]
- Taouis, M.; Benomar, Y. Is resistin the master link between inflammation and inflammation-related chronic diseases? Mol. Cell. Endocrinol. 2021, 533, 111341. [Google Scholar] [CrossRef]
- Amine, H.; Benomar, Y.; Taouis, M. Palmitic acid promotes resistin-induced insulin resistance and inflammation in SH-SY5Y human neuroblastoma. Sci. Rep. 2021, 11, 5427. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Song, J.; Dong, M.; Xie, T. Mechanism of Action and Risk Prediction of Adiponectin in Cardiovascular Diseases. Front. Biosci.—Landmark 2024, 29, 286. [Google Scholar] [CrossRef]
- Jamaluddin, M.S.; Yan, S.; Lü, J.; Liang, Z.; Yao, Q.; Chen, C. Resistin Increases Monolayer Permeability of Human Coronary Artery Endothelial Cells. PLoS ONE 2013, 8, e84576. [Google Scholar] [CrossRef] [PubMed]
- Salameh, T.S.; Mortell, W.; Banks, W.A. Resistin Is Associated with Blood-Brain Barrier Disruption in Mice Resistant to Diet-Induced Obesity and Treated with Topiramate. Diabetes 2018, 67, 2043-P. [Google Scholar] [CrossRef]
- Mounien, L.; Marty, N.; Tarussio, D.; Metref, S.; Genoux, D.; Preitner, F.; Foretz, M.; Thorens, B. Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. FASEB J 2010, 24, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Minokoshi, Y. Leptin, GABA, and glucose control. Cell Metab. 2013, 18, 304–306. [Google Scholar] [CrossRef]
- Varela, L.; Horvath, T.L. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012, 13, 1079–1086. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Lu, X.Y. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Yan, X.D.; Qu, X.S.; Yin, J.; Qiao, J.; Zhang, J.; Qi, J.S.; Wu, M.N. Adiponectin Ameliorates Cognitive Behaviors and in vivo Synaptic Plasticity Impairments in 3xTg-AD Mice. J. Alzheimer’s Dis. 2022, 85, 343–357. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef]
- Kastin, A.J.; Pan, W. Intranasal Leptin: Blood-Brain Barrier Bypass (BBBB) for Obesity? Endocrinology 2006, 147, 2086–2087. [Google Scholar] [CrossRef]
- Prévost, M.; Crépin, D.; Rifai SAl Poizat, G.; Gonçalves, M.; van Barneveld, F.; Shadpay, R.; Taouis, K.; Riffault, L.; Benomar, Y.; Taouis, M. The Resistin/TLR4/miR-155-5p axis: A novel signaling pathway in the onset of hypothalamic neuroinflammation. J. Neuroinflamm. 2025, 22, 198. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, N.; Shan, X.; Deng, G.; Xu, Q.; Ye, H.; Sun, Y. Celastrol targets adenylyl cyclase-associated protein 1 to reduce macrophages-mediated inflammation and ameliorates high fat diet-induced metabolic syndrome in mice. Acta Pharm. Sin. B 2021, 11, 1200–1212. [Google Scholar] [CrossRef]
- Li, R.; Lau, W.B.; Ma, X.L. Adiponectin resistance and vascular dysfunction in the hyperlipidemic state. Acta Pharmacol. Sin. 2010, 31, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Kaiyrlykyzy, A.; Umbayev, B.; Masoud, A.R.; Baibulatova, A.; Tsoy, A.; Olzhayev, F.; Alzhanova, D.; Zholdasbekova, G.; Davletov, K.; Akilzhanova, A.; et al. Circulating adiponectin levels, expression of adiponectin receptors, and methylation of adiponectin gene promoter in relation to Alzheimer’s disease. BMC Med. Genom. 2022, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.K.; Mukadam, N.; Kohl, G.; Livingston, G. Effect of diabetes medications on the risk of developing dementia, mild cognitive impairment, or cognitive decline: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2025, 104, 627–648. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Daly, R.M.; Suetta, C. Weighing the risk of GLP-1 treatment in older adults: Should we be concerned about sarcopenic obesity? J. Nutr. Health Aging 2025, 29, 100652. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Rocha, D.Q.C.; McIntyre, R.S.; Mesquita, L.M.; Köhler, C.A.; Hyphantis, T.N.; Sales, P.M.G.; Machado-Vieira, R.; Berk, M. Adipokines as emerging depression biomarkers: A systematic review and meta-analysis. J. Psychiatr. Res. 2014, 59, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Letra, L.; Matafome, P.; Rodrigues, T.; Duro, D.; Lemos, R.; Baldeiras, I.; Patrcio, M.; Castelo-Branco, M.; Caetano, G.; Seica, R.; et al. Association between adipokines and biomarkers of Alzheimer’s disease: A cross-sectional study. J. Alzheimer’s Dis. 2019, 67, 725–735. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ha, J.; Kim, M.; Cho, S.Y.; Kim, H.; Kim, E. Plasma adiponectin levels predict cognitive decline and cortical thinning in mild cognitive impairment with beta-amyloid pathology. Alzheimers Res. Ther. 2022, 14, 165. [Google Scholar] [CrossRef]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Borkowska, A.; Ciebiada, M.; Loba, J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab. Brain Dis. 2015, 31, 257. [Google Scholar] [CrossRef]
- Zeki Al Hazzouri, A.; Stone, K.L.; Haan, M.N.; Yaffe, K. Leptin, mild cognitive impairment, and dementia among elderly women. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2013, 68, 175–180. [Google Scholar] [CrossRef]
- Olszanecka-Glinianowicz, M.; Owczarek, A.; Bozentowicz-Wikarek, M.; Brzozowska, A.; Mossakowska, M.; Zdrojewski, T.; Grodzicki, T.; Więcek, A.; Chudek, J. Relationship between circulating visfatin/NAMPT, nutritional status and insulin resistance in an elderly population—Results from the PolSenior substudy. Metabolism 2014, 63, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yuan, Y.; Huang, R.; Tian, S.; Wang, J.; Lin, H.; An, K.; Han, J.; Wang, S. Association between plasma adipsin level and mild cognitive impairment in Chinese patients with type 2 diabetes: A cross-sectional study. BMC Endocr. Disord. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, M.; Vacínová, G.; Včelák, J.; Vejražková, D.; Lukášová, P.; Rusina, R.; Holmerová, I.; Jarolímová, E.; Vaňková, H.; Bendlová, B. Plasma Levels of Adipokines in Patients with Alzheimer’s Disease—Where Is the “Breaking Point” in Alzheimer’s Disease Pathogenesis? Physiol. Res. 2020, 69, S339. [Google Scholar] [CrossRef]
- Cisternas, P.; Martinez, M.; Ahima, R.S.; William Wong, G.; Inestrosa, N.C. Modulation of Glucose Metabolism in Hippocampal Neurons by Adiponectin and Resistin. Mol. Neurobiol. 2019, 56, 3024–3037. [Google Scholar] [CrossRef]
- Fernández, C.M.; Moltó, E.; Gallardo, N.; del Arco, A.; Martínez, C.; Andrés, A.; Ros, M.; Carrascosa, J.M.; Arribas, C. The expression of rat resistin isoforms is differentially regulated in visceral adipose tissues: Effects of aging and food restriction. Metabolism 2009, 58, 204–211. [Google Scholar] [CrossRef]
- Kos, K.; Harte, A.L.; da Silva, N.F.; Tonchev, A.; Chaldakov, G.; James, S.; Snead, D.R.; Hoggart, B.; O’Hare, J.P.; McTernan, P.G.; et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J. Clin. Endocrinol. Metab. 2007, 92, 1129–1136. [Google Scholar] [CrossRef]
- Benomar, Y.; Gertler, A.; De Lacy, P.; Crépin, D.; Hamouda, H.O.; Riffault, L.; Taouis, M. Central resistin overexposure induces insulin resistance through toll-like receptor 4. Diabetes 2013, 62, 102–144. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118664. [Google Scholar]
- Lehmann, S.; Schraen-Maschke, S.; Buée, L.; Vidal, J.S.; Delaby, C.; Hirtz, C.; Blanc, F.; Paquet, C.; Allinquant, B.; Bombois, S.; et al. Clarifying the association of CSF Aβ, tau, BACE1, and neurogranin with AT(N) stages in Alzheimer disease. Mol. Neurodegener. 2024, 19, 66. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix Metalloproteinase-Mediated Neuroinflammation in Vascular Cognitive Impairment of the Binswanger Type. Cell. Mol. Neurobiol. 2016, 36, 195. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef]
- Samant, N.P.; Gupta, G.L. Adiponectin: A potential target for obesity-associated Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1565–1572. [Google Scholar] [CrossRef]
- Vu, V.; Bui, P.; Eguchi, M.; Xu, A.; Sweeney, G. Globular adiponectin induces LKB1/AMPK-dependent glucose uptake via actin cytoskeleton remodeling. J. Mol. Endocrinol. 2013, 51, 155–165. [Google Scholar] [CrossRef]
- Takahashi, J.; Takahashi, N.; Tadaishi, M.; Shimizu, M.; Kobayashi-Hattori, K. Valerenic Acid Promotes Adipocyte Differentiation, Adiponectin Production, and Glucose Uptake via Its PPARγLigand Activity. ACS Omega 2022, 7, 48113–48120. [Google Scholar] [CrossRef]
- Novinbahador, T.; Abbasi, A.; Molani-Gol, R.; Aghebati-Maleki, L.; Pouraghaei, A.; Soleimanpour, H. Neuroprotection through adiponectin receptor agonist: An updated meta-analysis of preclinical Alzheimer’s disease studies. BMC Neurol. 2025, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.L.; Cheng, O.Y.; Jian, M.; Kwan, J.S.C.; Ho, P.W.L.; Cheng, K.K.Y.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.C.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Nie, L.; Ali, T.; Wang, S.; Chen, X.; Liu, Z.; Li, W.; Zhang, K.; Xu, J.; Liu, J.; et al. Adiponectin alleviated Alzheimer-like pathologies via autophagy-lysosomal activation. Aging Cell 2021, 20, e13514. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Shu, Y.; Zeng, Y. Links Between Adiponectin and Dementia: From Risk Factors to Pathophysiology. Front. Aging Neurosci. 2020, 11, 491775. [Google Scholar] [CrossRef]
- Sindzingre, L.; Bouaziz-Amar, E.; Mouton-Liger, F.; Cognat, E.; Dumurgier, J.; Vrillon, A.; Paquet, C.; Lilamand, M. The role of adiponectin in Alzheimer’s disease: A translational review. J. Nutr. Health Aging 2024, 28, 100166. [Google Scholar] [CrossRef]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum Resistin (FIZZ3) Protein Is Increased in Obese Humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef]
- Inadera, H. The usefulness of circulating adipokine levels for the assessment of obesity-related health problems. Int. J. Med. Sci. 2008, 5, 248. [Google Scholar] [CrossRef]
- Clain, J.; Couret, D.; Planesse, C.; Krejbich-Trotot, P.; Meilhac, O.; Lefebvre D’Hellencourt, C.; Viranaicken, W.; Diotel, N. Distribution of Adiponectin Receptors in the Brain of Adult Mouse: Effect of a Single Dose of the Adiponectin Receptor Agonist, AdipoRON, on Ischemic Stroke. Brain Sci. 2022, 12, 680. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.C.; Kwon, Y.W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.Y.; Lee, J.; Yang, H.M.; et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-κB signaling pathway. Front. Cell. Neurosci. 2017, 11, 309417. [Google Scholar] [CrossRef]
- Al Hannan, F.; Culligan, K.G. Human resistin and the RELM of Inflammation in diabesity. Diabetol. Metab. Syndr. 2015, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Song, J.; Choi, S.M.; Whitcomb, D.J.; Kim, B.C. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017, 8, e3102. [Google Scholar] [CrossRef]
- Ali, T.; Rehman, S.U.; Khan, A.; Badshah, H.; Abid NBin Kim, M.W.; Jo, M.H.; Chung, S.S.; Lee Hgon Rutten, B.P.F.; Kim, M.O. Adiponectin-mimetic novel nonapeptide rescues aberrant neuronal metabolic-associated memory deficits in Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 23. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Schaar, A.E.; Gustafson, G.E.; Smith, A.B.; Howell, P.R.; Greenman, A.; Baum, S.; Colman, R.J.; Lamming, D.W.; Diffee, G.M.; et al. Adiponectin receptor agonist AdipoRon improves skeletal muscle function in aged mice. Elife 2022, 11, e71282. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.M.V.; Gustafson, D.; Hagen, C.E.; Roberts, R.O.; Knopman, D.; Jack, C.; Petersen, R.C.; Mielke, M.M. Serum Adiponectin Levels, Neuroimaging, and Cognition in the Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2016, 53, 573. [Google Scholar] [CrossRef]
- Habib, S.S.; Al-Khlaiwi, T.; Butt, M.A.; Habib, S.M.; Al-Khliwi, H.; Al-Regaiey, K. Novel Adiponectin-Resistin Indices and Ratios Predict Increased Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus. J. Saudi Heart Assoc. 2023, 35, 59. [Google Scholar] [CrossRef]
- Zhou, J.; Kim, Y.K.; Li, C.; Park, S. Natural compounds for Alzheimer’s prevention and treatment: Integrating SELFormer-based computational screening with experimental validation. Comput. Biol. Med. 2025, 185, 109523. [Google Scholar] [CrossRef]
- Aktary, N.; Jeong, Y.; Oh, S.; Shin, Y.; Sung, Y.; Rahman, M.; Ramos Santiago, L.; Choi, J.; Song, H.G.; Nurkolis, F.; et al. Unveiling the therapeutic potential of natural products in Alzheimer’s disease: Insights from in vitro, in vivo, and clinical studies. Front. Pharmacol. 2025, 16, 1601712. [Google Scholar] [CrossRef]
- Yang, J.; Shi, X.; Wang, Y.; Ma, M.; Liu, H.; Wang, J.; Xu, Z. Multi-Target Neuroprotection of Thiazolidinediones on Alzheimer’s Disease via Neuroinflammation and Ferroptosis. J. Alzheimer’s Dis. 2023, 96, 927–945. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, J.; Huang, L.; Yang, X. Phytochemicals targeting Alzheimer’s disease via the AMP-activated protein kinase pathway, effects, and mechanisms of action. Biomed. Pharmacother. 2024, 173, 116373. [Google Scholar] [CrossRef] [PubMed]
- Deepika Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yuan, C.; Ju, Y.; Liu, Y.; Shi, B.; Yang, Y.; Jin, S.; He, X.; Zhang, L.; Min, D. Quercetin Attenuates Oxidative Stress and Apoptosis in Brain Tissue of APP/PS1 Double Transgenic AD Mice by Regulating Keap1/Nrf2/HO-1 Pathway to Improve Cognitive Impairment. Behav. Neurol. 2024, 2024, 5698119. [Google Scholar] [CrossRef]
- Rezvan, N.; Moini, A.; Gorgani-Firuzjaee, S.; Hosseinzadeh-Attar, M.J. Oral quercetin supplementation enhances adiponectin receptor transcript expression in polycystic ovary syndrome patients: A randomized placebo-controlled double-blind clinical trial. Cell J. 2018, 19, 627–633. [Google Scholar]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Jiao, L.; Ma, G.; Wei, B.; Wei, M. Epigallocatechin-3-Gallate Attenuates Microglial Inflammation and Neurotoxicity by Suppressing the Activation of Canonical and Noncanonical Inflammasome via TLR4/NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, e1801230. [Google Scholar] [CrossRef]
- Shimada, M.; Mochizuki, K.; Sakurai, N.; Goda, T. Dietary supplementation with epigallocatechin gallate elevates levels of circulating adiponectin in non-obese type-2 diabetic Goto-Kakizaki rats. Biosci. Biotechnol. Biochem. 2007, 71, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Kang JBin Koh, P.O. Epigallocatechin gallate improves neuronal damage in animal model of ischemic stroke and glutamate-exposed neurons via modulation of hippocalcin expression. PLoS ONE 2024, 19, e0299042. [Google Scholar] [CrossRef] [PubMed]
- Quadros Gomes, B.A.; Bastos Silva, J.P.; Rodrigues Romeiro, C.F.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Santos Mendes, P.F.; Pompeu Varela, E.L.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef] [PubMed]
- Veronica Witte, A.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef]
- Ege, D. Action Mechanisms of Curcumin in Alzheimer’s Disease and Its Brain Targeted Delivery. Materials 2021, 14, 3332. [Google Scholar] [CrossRef]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer pathogenesis. Neurobiol. Dis. 2019, 127, 432. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus. Curr. Clin. Pharmacol. 2018, 12, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665. [Google Scholar] [CrossRef]
- Yu, X.; Ye, L.; Zhang, H.; Zhao, J.; Wang, G.; Guo, C.; Shang, W. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J. Ginseng Res. 2014, 39, 199. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Zhu, Z.; Zhou, R.; Xu, P.; Zhou, L.; Kan, Y.; Li, J.; Zhao, J.; Fang, P.; et al. Upregulation of adiponectin by Ginsenoside Rb1 contributes to amelioration of hepatic steatosis induced by high fat diet. J. Ginseng Res. 2022, 46, 561–571. [Google Scholar] [CrossRef]
- Babalola, J.A.; Lang, M.; George, M.; Stracke, A.; Tam-Amersdorfer, C.; Itxaso, I.; Lucija, D.; Tadic, J.; Schilcher, I.; Loeffler, T.; et al. Astaxanthin enhances autophagy, amyloid beta clearance and exerts anti-inflammatory effects in in vitro models of Alzheimer’s disease-related blood brain barrier dysfunction and inflammation. Brain Res. 2023, 1819, 148518. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Nishida, Y.; Takikawa, A.; Fujisaka, S.; Kado, T.; Aminuddin, A.; Bilal, M.; Jeelani, I.; Aslam, M.R.; Nishimura, A.; et al. Astaxanthin, a marine carotenoid, maintains the tolerance and integrity of adipose tissue and contributes to its healthy functions. Nutrients 2021, 13, 4374. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xiang, W.; Chen, Y.; Peng, N.; Du, X.; Lu, S.; Zuo, Y.; Li, B.; Hu, Y.; Li, X. DHA Ameliorates Cognitive Ability, Reduces Amyloid Deposition, and Nerve Fiber Production in Alzheimer’s Disease. Front. Nutr. 2022, 9, 852433. [Google Scholar] [CrossRef]
- de Barbosa, M.M.A.L.; de Melo, A.L.T.R.; Damasceno, N.R.T. The benefits of ω-3 supplementation depend on adiponectin basal level and adiponectin increase after the supplementation: A randomized clinical trial. Nutrition 2017, 34, 7–13. [Google Scholar] [CrossRef]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, P.; Wang, X. Berberine Exerts Neuroprotective Effects in Alzheimer’s Disease by Switching Microglia M1/M2 Polarization Through PI3K-AKT Signaling. Physiol. Res. 2025, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhou, W.; Dou, F.; Wang, C.; Yu, Z. TRPV1 sustains microglial metabolic reprogramming in Alzheimer’s disease. EMBO Rep. 2021, 22, e52013. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.L.; Xiang, Y.; Tian, D.Y.; Zhu, C.; Li, W.W.; Liu, Y.H.; Bu XLe Shen, L.L.; Jin, W.S.; Wang, Z.; et al. Capsaicin consumption reduces brain amyloid-beta generation and attenuates Alzheimer’s disease-type pathology and cognitive deficits in APP/PS1 mice. Transl. Psychiatry 2020, 10, 230. [Google Scholar] [CrossRef]
- Jalili, C.; Kiani, A.; Gholami, M.; Bahrehmand, F.; Fakhri, S.; Kakehbaraei, S.; Kakebaraei, S. Brain targeting based nanocarriers loaded with resveratrol in Alzheimer’s disease: A review. IET Nanobiotechnol. 2023, 17, 154. [Google Scholar] [CrossRef]
- Pei, J.J.; Palanisamy, C.P.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Srinivasan, G.P.; Panagal, M.; Jayaraman, S. Curcumin-loaded polymeric nanomaterials as a novel therapeutic strategy for Alzheimer’s disease: A comprehensive review. Ageing Res. Rev. 2024, 99, 102393. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Monney, M.; Jornayvaz, F.R.; Gariani, K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 diabetes. Diabetes Metab. 2023, 49, 101470. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Riera-Guardia, N.; Rothenbacher, D. The effect of thiazolidinediones on adiponectin serum level: A meta-analysis. Diabetes Obes. Metab. 2008, 10, 367–375. [Google Scholar] [CrossRef]
- Saunders, A.M.; Burns, D.K.; Gottschalk, W.K. Reassessment of Pioglitazone for Alzheimer’s Disease. Front. Neurosci. 2021, 15, 666958. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.; Baker, L.D.; Wilkinson, C.W.; Trittschuh, E.H.; Chapman, D.; Watson, G.S.; Cholerton, B.; Plymate, S.R.; Arbuckle, M.; Craft, S. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 35, 789–797. [Google Scholar] [CrossRef]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; Van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Meng, X.; Gong, Y.; Xiao, F.; Cao, Z.; Zhuang, Z.; Yi, X.; Wang, J.; Feng, R.; Gong, C.; Ni, P. Curcumin’s multi-target mechanisms in the treatment of Alzheimer’s disease and creative modification techniques. J. Alzheimer’s Dis. 2025, 106, 410–426. [Google Scholar] [CrossRef]
- Kaur, K.; Kulkarni, Y.A.; Wairkar, S. Exploring the potential of quercetin in Alzheimer’s Disease: Pharmacodynamics, Pharmacokinetics, and Nanodelivery systems. Brain Res. 2024, 1834, 148905. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhuang, Y.; Lin, H.; Li, Y.; Liu, L.; Meng, Q.; Cui, T.; Liu, J.; Li, Z. Activation of AMPK by berberine promotes adiponectin multimerization in 3T3-L1 adipocytes. FEBS Lett. 2011, 585, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, F.; Chen, Y.; Li, G. Astaxanthin attenuates cognitive deficits in Alzheimer’s disease models by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Cell Biosci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Gray, B.; Steyn, F.; Davies, P.S.W.; Vitetta, L. Omega-3 fatty acids: A review of the effects on adiponectin and leptin and potential implications for obesity management. Eur. J. Clin. Nutr. 2013, 67, 1234–1242. [Google Scholar] [CrossRef]
- Kang, J.H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.M.; Tu, T.H.; Noh, H.J.; Kim, C.S.; Choe, S.Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Forny-Germano, L.; De Felice, F.G.; Do Nascimento Vieira, M.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019, 13, 430286. [Google Scholar] [CrossRef] [PubMed]
- Cente, M.; Zorad, S.; Smolek, T.; Fialova, L.; Paulenka Ivanovova, N.; Krskova, K.; Balazova, L.; Skrabana, R.; Filipcik, P. Plasma Leptin Reflects Progression of Neurofibrillary Pathology in Animal Model of Tauopathy. Cell. Mol. Neurobiol. 2020, 42, 125. [Google Scholar] [CrossRef]
- McGuire, M.J.; Ishii, M. Leptin Dysfunction and Alzheimer’s Disease: Evidence from Cellular, Animal, and Human Studies. Cell. Mol. Neurobiol. 2016, 36, 203. [Google Scholar] [CrossRef]
- Ueda, H.; Howson, J.M.M.; Esposito, L.; Heward, J.; Snook, H.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.D.; Smith, A.N.; Di Genova, G.; et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bea, F.J.; Solas, M.; Solomon, A.; Mugueta, C.; Winblad, B.; Kivipelto, M.; Ramirez, M.J.; Cedazo-Mínguez, A. Insulin levels are decreased in the cerebrospinal fluid of women with prodomal alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 405–413. [Google Scholar] [CrossRef]
- Kempers, M.J.E.; Van Der Sluijs Veer, L.; Nijhuis-Van Der Sanden, R.W.G.; Lanting, C.I.; Kooistra, L.; Wiedijk, B.M.; Last, B.F.; De Vijlder, J.J.M.; Grootenhuis, M.A.; Vulsma, T. Neonatal screening for congenital hypothyroidism in The Netherlands: Cognitive and motor outcome at 10 years of age. J. Clin. Endocrinol. Metab. 2007, 92, 919–924. [Google Scholar] [CrossRef]
- Ishii, M.; Iadecola, C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 966–974. [Google Scholar] [CrossRef]

| Adipokine | Main Cerebral Effect | Experimental Model | Reference |
|---|---|---|---|
| Leptin | Enhances synaptic plasticity (LTP), neurogenesis; reduces Aβ | Transgenic mice (db/db, APP/PS1), hippocampal slices, neuronal cultures | [141] |
| Adiponectin | Anti-inflammatory, neuroprotective, promotes neurogenesis; crosses BBB | Obese and ischemic mice, CSF from humans, neuronal and glial cultures | [142] |
| Visfatin (NAMPT) | Enhances BDNF, neuronal survival, modulates neuroinflammation | High-fat diet (HFD) mice, glial and hippocampal cultures, inflammatory models | [143] |
| Resistin | Increases IL-6 and TNF-α; promotes neuroinflammation | Obese mice, Alzheimer’s models, clinical plasma/CSF studies | [144] |
| Chemerin | Modulates neuroinflammation and oxidative stress | HFD mice, endothelial cultures; CMKLR1 expression in brain | [145,146] |
| Apelin | Neurotrophic effects; improves feeding behavior and neurovascular function | Obese mice, neuronal cultures, intracerebral injection studies | [147] |
| Omentin-1 | Anti-inflammatory, potential neuroprotective action | HFD and insulin-resistant models; indirect brain associations | [148] |
| PAI-1 | Disrupts BBB integrity; contributes to neurodegeneration | Stroke models in mice, endothelial BBB models | [149] |
| RBP4 | Associated with cognitive decline, affects hippocampal function | Clinical obesity/cognition studies; in vitro BBB and neuronal assays | [150] |
| Vaspin | Anti-inflammatory effects, possible neuroprotection | HFD mice, glial cultures (brain-specific evidence limited) | [151] |
| Progranulin | Modulates microglial activity, neuroinflammatory regulation | ALS and EAE models, human CSF analysis | [152] |
| Adipokine | Sample and Population | Main Findings in Elderly | Observed Discordance | Reference |
|---|---|---|---|---|
| Adiponectin | Plasma (older adults with MCI, AD, depression) | Low levels associated with MCI and inflammation- High levels in Aβ individuals predict cortical thinning | Appears neuroprotective in some studies, yet high levels predict atrophy in Aβ MCI | [191,192] |
| Leptin | Plasma and CSF (cognitively healthy, MCI, AD) | Lower plasma leptin linked to lower CSF Aβ and worse cognition- Some studies find no clear association | Inconsistent link between plasma/CSF leptin and cognitive outcomes or biomarkers | [161,193] |
| Visfatin | Plasma (elderly with obesity or diabetes) | Suggested neuroprotective and inflammatory roles, but inconsistent concentrations across cohorts | No consensus on whether it rises or falls in aging; mechanisms remain unclear | [194] |
| Adipsin | Plasma (early AD cohorts, middle-aged and older adults) | Proposed as early biomarker with leptin and adiponectin, but limited age-specific trend data | Preliminary findings; lack of consistent replication in large elderly cohorts | [195,196] |
| Feature | Adiponectin | Resistin | References |
|---|---|---|---|
| Primary Source | Adipocytes (mainly subcutaneous WAT) | Macrophages and mononuclear immune cells (in humans) | [19] |
| Levels in Obesity | ↓ Decreased | ↑ Increased | [214,215] |
| Receptors in Brain | AdipoR1 (neurons), AdipoR2 (astrocytes, endothelial cells) | TLR4, CAP1 (microglia, astrocytes) | [216,217] |
| Main CNS Effects | ↑ Glucose uptake, ↓ ROS, ↑ mitochondrial biogenesis, ↓ neuroinflammation, ↑ synaptic plasticity | ↑ NF-κB activation, ↑ ROS, ↑ cytokine release, ↓ insulin signaling, ↑ BACE1 and tau phosphorylation | [218,219] |
| Mechanistic Pathways | AMPK, PPARα, ERK1/2, PGC1α | TLR4–NF-κB, ERK1/2, GSK3β | [217,220] |
| Impact on Aβ and Tau | ↓ Aβ deposition, ↓ tau phosphorylation | ↑ Aβ generation (↑ BACE1), ↑ tau phosphorylation (↑ GSK3β) | [144,211] |
| Effect on BBB Integrity | Preserves BBB (↑ eNOS, ↓ endothelial inflammation) | Disrupts BBB (↓ tight junctions, ↑ MMP activity) | [174,221] |
| Observed in AD Models | Improves memory, reduces pathology | Correlates with cognitive decline, hippocampal atrophy | [144,222] |
| Therapeutic Strategies | AdipoR agonists (AdipoRon), PPAR-γ agonists (pioglitazone), AMPK activators (metformin) | TLR4 antagonists, CAP1 inhibitors, siRNA/antisense therapies | [217,223] |
| Compounds | Primary Adipokine Effect | Reported Brain Effect | Reference |
|---|---|---|---|
| Resveratrol (polyphenol) | ↑ Adiponectin; ↓ inflammatory tone | ↓ Aβ/tau stress; ↓ microglial activation; improved memory and glucose metabolism in models | [264,265] |
| Curcumin (polyphenol) | ↑ Adiponectin; ↓ Resistin (preclinical) | ↓ Neuroinflammation; ↓ Aβ; support synapses in models | [266] |
| Quercetin (flavonol) | ↑ Adiponectin signaling (AdipoR↑) | ↓ Aβ deposition; ↑ synaptic plasticity; antioxidant neuroprotection | [267] |
| Berberine (alkaloid) | ↑ Adiponectin; ↓ Resistin | ↑ Glucose uptake; ↓ oxidative stress; cognitive benefit in models | [268,269] |
| Astaxanthin (carotenoid) | ↑ Adiponectin; ↓ Resistin (models) | ↑ Aβ clearance; ↓ BBB inflammation; memory benefit (models) | [270,271] |
| Omega-3 PUFAs (EPA/DHA) | ↑ Adiponectin; ↓ Resistin (immune cells) | ↓ Aβ; ↑ synaptic integrity; better cognition (models); mixed human cognition | [272] |
| Capsaicin (vanilloid) | ↑ Adiponectin secretion | ↓ Aβ generation; improved plasticity in models | [253,273] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormazabal, P.; Bastías-Pérez, M.; Inestrosa, N.C.; Cisternas, P. Adipokines at the Metabolic–Brain Interface: Therapeutic Modulation by Antidiabetic Agents and Natural Compounds in Alzheimer’s Disease. Pharmaceuticals 2025, 18, 1527. https://doi.org/10.3390/ph18101527
Ormazabal P, Bastías-Pérez M, Inestrosa NC, Cisternas P. Adipokines at the Metabolic–Brain Interface: Therapeutic Modulation by Antidiabetic Agents and Natural Compounds in Alzheimer’s Disease. Pharmaceuticals. 2025; 18(10):1527. https://doi.org/10.3390/ph18101527
Chicago/Turabian StyleOrmazabal, Paulina, Marianela Bastías-Pérez, Nibaldo C. Inestrosa, and Pedro Cisternas. 2025. "Adipokines at the Metabolic–Brain Interface: Therapeutic Modulation by Antidiabetic Agents and Natural Compounds in Alzheimer’s Disease" Pharmaceuticals 18, no. 10: 1527. https://doi.org/10.3390/ph18101527
APA StyleOrmazabal, P., Bastías-Pérez, M., Inestrosa, N. C., & Cisternas, P. (2025). Adipokines at the Metabolic–Brain Interface: Therapeutic Modulation by Antidiabetic Agents and Natural Compounds in Alzheimer’s Disease. Pharmaceuticals, 18(10), 1527. https://doi.org/10.3390/ph18101527






