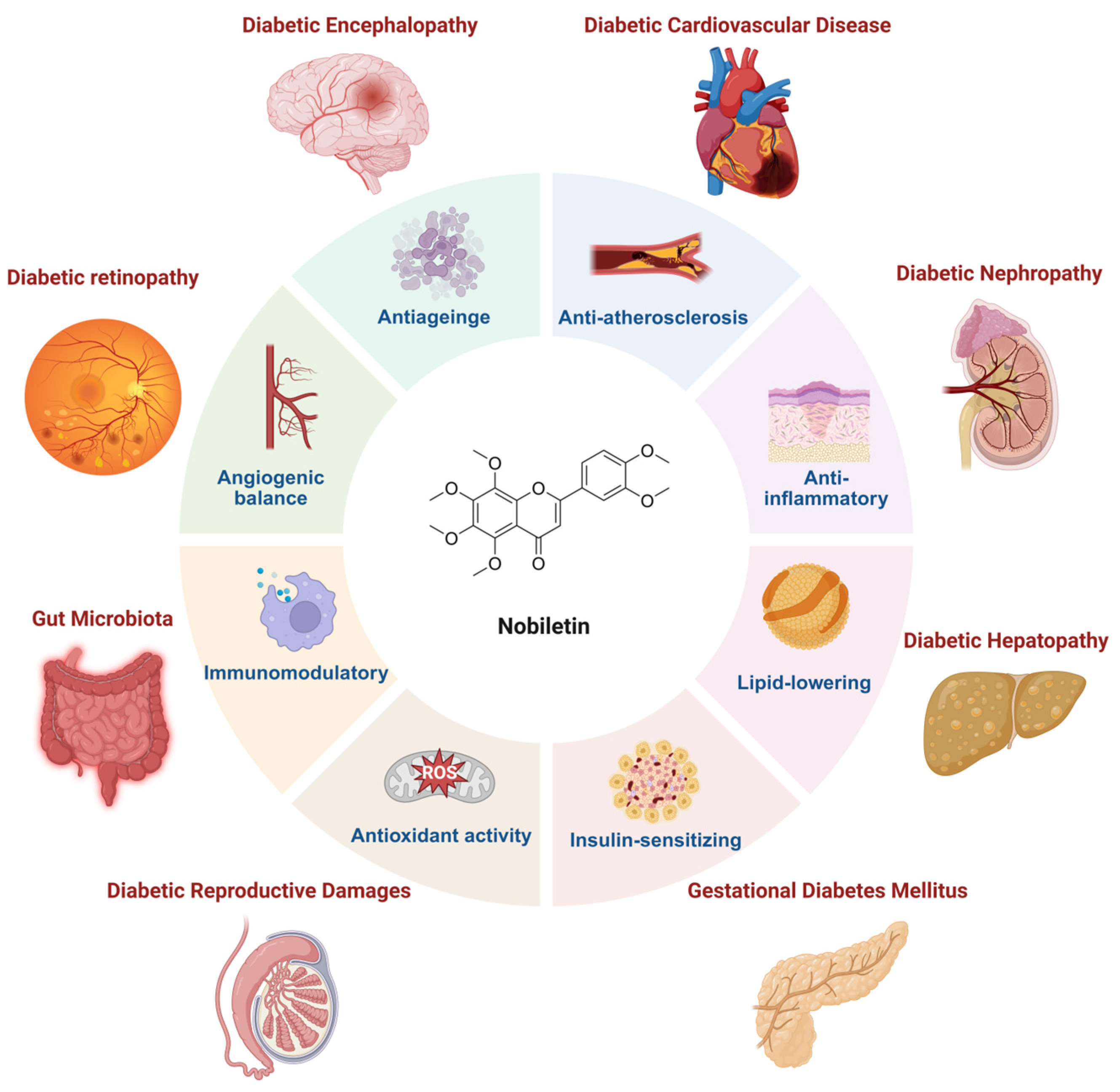
Potential and Mechanism of Nobiletin in Diabetes Mellitus and Associated Complications
Abstract
1. Introduction
2. Basic Information on Nobiletin
2.1. Chemical Property of Nobiletin
2.2. Bioactivity of Nobiletin
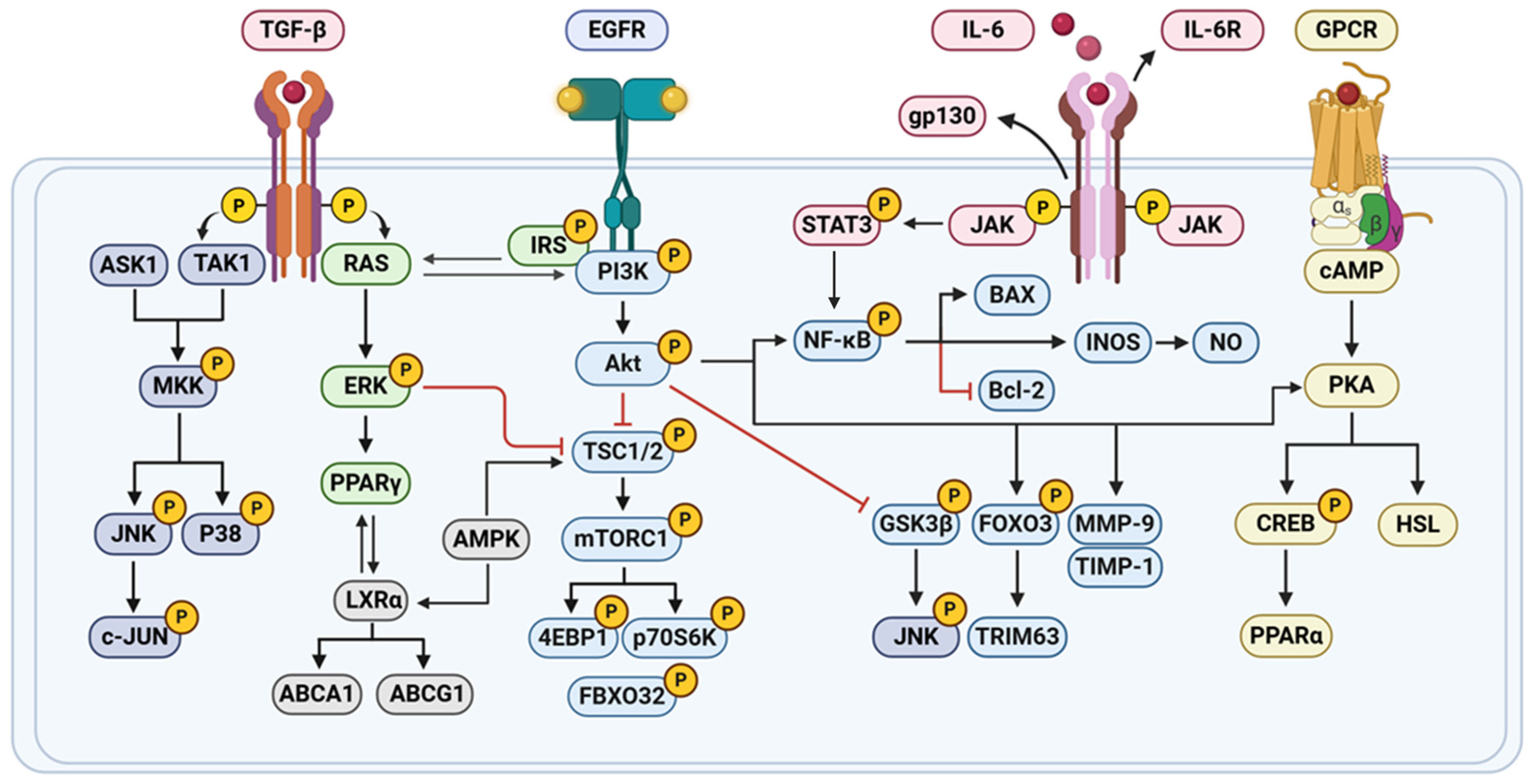
3. Therapeutic Potential and Mechanism of Nobiletin on Diabetes and Its Complications
3.1. Nobiletin Attenuates Gut Microbiota Dysbiosis
3.2. Nobiletin Modulates Metabolic Rhythms
3.3. Nobiletin Ameliorates Insulin Resistance
| Type of Study | Study Subject | Dose/Dosing Method/Period | Effect and Molecular Mechanisms | References |
|---|---|---|---|---|
| In vivo | HFD-induced C57BL/6J mice | Nobiletin; 10 or 100 mg/kg; 5 weeks; p.o. | NOB altered the expression levels of several lipid metabolism-related and adipokine genes, increased the mRNA expression of Pparg, sterol regulatory element-binding protein-1c, fatty acid synthase, stearoyl-CoA desaturase-1, Ppara, Cpt1a, Ucp2 and adiponectin, and decreased the mRNA expression of Tnf-α and monocyte chemoattractant protein-1 in WAT. NOB also up-regulated GLUT4 protein expression and AKT phosphorylation and suppressed IκBα degradation in WAT. | [34] |
| In vitro | LPS; RAW264.7 cells | Nobiletin; 10 μM, 25 μM and 50 μM | Nobiletin also inhibits LPS-induced COX-2, iNOS, and IL-6 expression, suppresses NO production, inhibits phosphorylated STAT3 protein expression, and upregulates p-FOXO3a expression. | [42] |
| In vitro | 3T3-F442 preadipocytes | Nobiletin; 5, 20 or 50 μM | Nobiletin treatment significantly increased the uptake of [(3)H]-deoxyglucose by differentiated 3T3-F442A adipocytes, and this increase is concentration-dependent and related to the PI3K/AKT/PKA pathway. | [55] |
| In vivo | ob/ob mice | Nobiletin; 200 mg/kg; 5 weeks; p.o. | Nobiletin significantly reduced the mRNA expression levels of inflammatory adipokines such as Il-6 and Mcp-1 and increased the mRNA expression levels of adiponectin and Pparg, and increased glucose utilization in WAT and muscle. | [49] |
| In vitro | Insulin, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone; 3T3-L1 cells | Nobiletin; 16, 32 or 64 μM [57]; 1, 10 or 100 μM [58]; 10 μM [78] | Nobiletin increases the secretion of the insulin-sensitizing factor adiponectin and reduces the secretion of the insulin-resistant factor MCP-1 in 3T3-L1 adipocytes. Nobiletin significantly suppressed the differentiation of 3T3-L1 preadipocytes into adipocytes, upon induction with insulin together with a cAMP elevator such as IBMX, by downregulating the expression of the gene encoding Pparg2 [57]. In addition, nobiletin decreased the phosphorylation of CREB and strongly enhanced the phosphorylation of STAT5 [58]. Nobiletin induced lipolysis in adipocytes by the activation of the cAMP/PKA/HSL pathway [54]. NOB enhanced the oscillation of core clock genes (Bmal1, Cry1, Dec1, and Dec2) in differentiated 3T3-L1 adipocytes, inhibited lipid accumulation in 3T3-L1 and SVF cells, upregulated the expression of IκBα, a target of RORs to inhibit NF-κB activation and proinflammatory cytokine expression [78]. | [54,57,58,78] |
| In vivo | High-fat diet; Ampkβ1−/−, AccDKI, iβ1β2AKO mice | Nobiletin; 0.3%, w/w; 12 or 18 weeks; p.o. | Nobiletin increased phosphorylation of AMPK and ACC in primary mouse hepatocytes, which is associated with increased FA oxidation and attenuated FA synthesis. Despite loss of ACC phosphorylation in Ampkβ1−/− hepatocytes, nobiletin suppressed FA synthesis and enhanced FA oxidation. In mice fed a high-fat diet, nobiletin robustly prevented obesity, hepatic steatosis, dyslipidemia, and insulin resistance, and it improved energy expenditure in Ampkβ1−/−, AccDKI, and iβ1β2AKO mice to the same extent as in WT controls. | [46] |
| In vivo | High-fat diet; C57BL/6J | Nobiletin; 0.02%, w/w; 16 weeks; p.o. | NOB significantly reduced hepatic lipid droplets and triglyceride levels and the expression of inflammatory factors (IFN-γ, TNF-α, IL-6, IL-1β, NF-κB, TLR2, and TLR4). It also improved glucose tolerance and insulin resistance, and decreased plasma insulin, free fatty acids, TC, non-HDL-C, and apolipoprotein B levels. | [45] |
| In vivo | High-fat diet; Ldlr−/− mice | Nobiletin; 0.3%, w/w; 10 or 12 weeks; p.o. [64]. 0.1 or 0.3%, w/w; 8 or 26 weeks; p.o. [83] | Nobiletin reduced fasting jejunal triglyceride accumulation through accelerated TRL secretion and lower jejunal fatty acid synthesis with no impact on fatty acid oxidation. Nobiletin led to higher levels of p-AKT and FoxO1 and normal Srebf1c expression indicating increased insulin sensitivity [64]. Nobiletin attenuated dyslipidemia through a reduction in VLDL-triglyceride (TG) secretion. Nobiletin prevented hepatic TG accumulation, increased expression of Pgc1α and Cpt1α, and enhanced fatty acid β-oxidation. Nobiletin increased hepatic and peripheral insulin sensitivity and glucose tolerance and dramatically attenuated atherosclerosis in the aortic sinus [83]. | [64,83] |
| In vivo | High-fat diet; ApoE−/− mice | Nobiletin; 50, 100, and 200 mg/kg; p.o. | The levels of FBG and GSP in hyperglycemic mice are effectively reduced. The secretory function of pancreas is improved. Meanwhile, Nobiletin treatment restored the gut microbial composition and affected metabolic function. | [66] |
| In vitro | Human pancreatic islet cells | Nobiletin; 20 μM [76] and 10 μM [77] | Nobiletin, an agonist of the core-clock proteins RORα/γ, boosted both circadian amplitude of T2DM islet clocks and insulin secretion by these islets [76]. Nobiletin increased islet circadian clock amplitude and augmented glucose-stimulated insulin secretion (GSIS) in isolated human islets in a Bmal1-dependent manner [77]. | [76,77] |
| In vivo | Bmal1flox/flox and Bmal1L KO mice | Nobiletin; 200 mg/kg; p.o. | Nobiletin inhibited hepatic DNL and decreased liver TG in HFD-fed mice independently of liver Bmal1, whereas liver-specific Bmal1 depletion reversed the beneficial effects of Nobiletin on liver cholesterol homeostasis, increased serum VLDL levels in Bmal1L KO mice, lower liver and serum TC and TG in Bmal1flox/flox mice. | [79] |
3.3.1. Preservation of Islet β-Cells and Release of Insulin from β-Cells
3.3.2. Suppression of α-Glucosidase to Reduce Intestinal Glucose Absorption
3.4. Nobiletin and Diabetic Complications
3.4.1. Nobiletin and Diabetic Hepatopathy
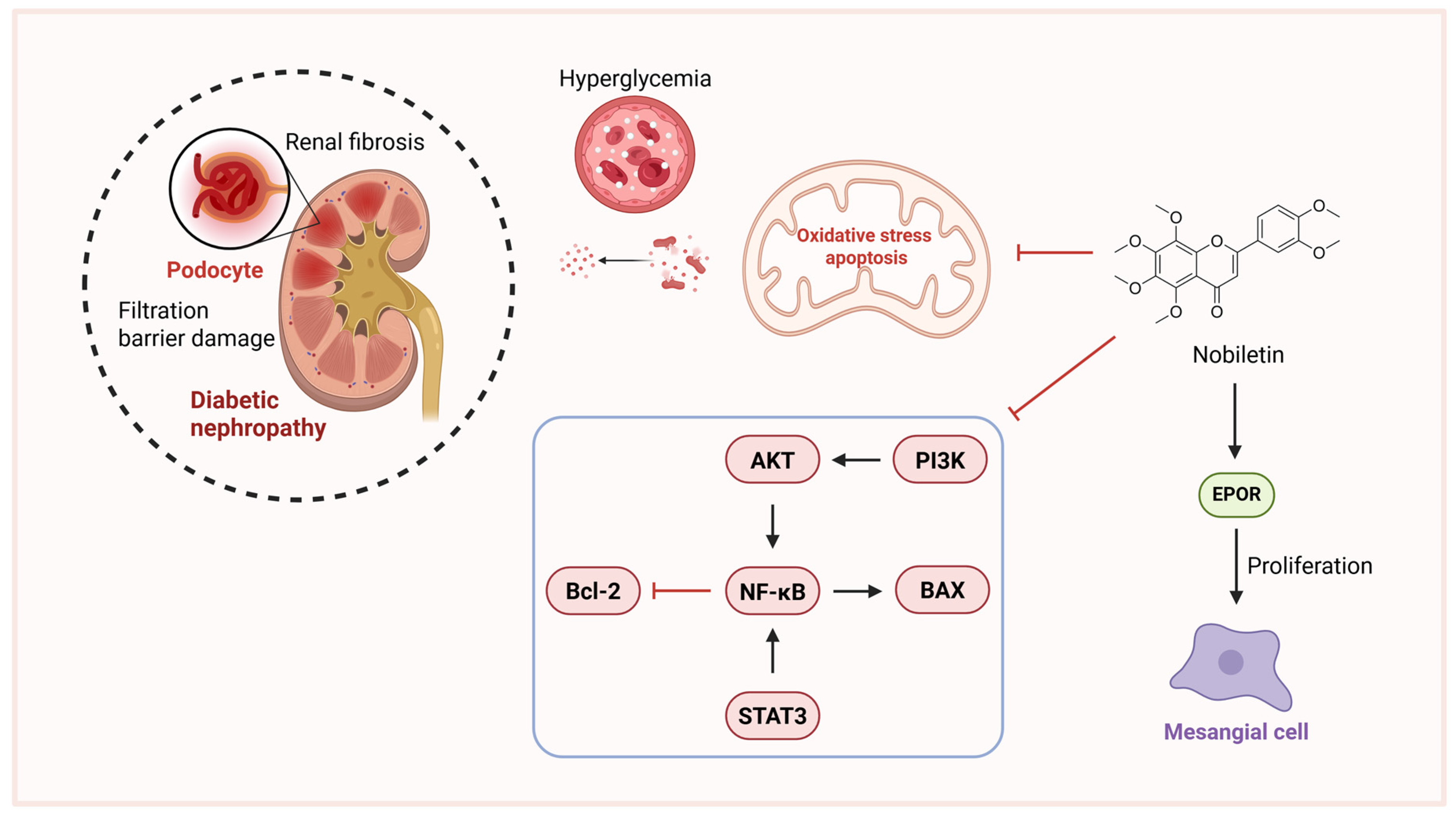
3.4.2. Nobiletin and Diabetic Nephropathy
3.4.3. Nobiletin and Diabetic Encephalopathy
3.4.4. Nobiletin and Diabetic Retinopathy
3.4.5. Nobiletin and Diabetic Cardiovascular Disease
3.4.6. Nobiletin and Diabetic Reproductive Damages
3.4.7. Nobiletin and Gestational Diabetes Mellitus
| Type of Study | Study Subject | Dose/Dosing Method/Period | Diabetic Complications | Effect and Molecular Mechanisms | References |
|---|---|---|---|---|---|
| In vitro | LPS; BV-2 cells | Nobiletin; 0, 25, 50, 100 μM | Diabetic encephalopathy | NOB inhibited microglial activation and the production of proinflammatory cytokines COX-2, IL-1β, TNF-α, and iNOS. Inhibition of MAPK, PI3K/AKT, and NF-κB signaling pathways alleviated LPS-induced redox imbalance, mitochondrial membrane potential disturbances, and the suppression of mitochondrial respiration-related protein expression. | [106] |
| In vitro | High glucose (25 mM); Müller glial cells (MIO-M1) | Nobiletin; 0.25, 4, 64 μM | Diabetic retinopathy | Nobiletin inhibits the PI3K/Akt signaling pathway, suppresses MMP-9 gene expression and enhances TIMP-1 production. | [122] |
| In vitro | Tm or Tg; MIO-M1 cell | Nobiletin; 64 μM | Diabetic retinopathy | Nobiletin has a protective effect on ER stress-induced Müller cell death and enhances the expression of PEDF in Müller cells, thereby potentially protecting the integrity of the BRB. | [123] |
| In vivo | STZ; Wistar rat | Nobiletin; 10, 25 mg/kg; 4 weeks; p.o. | Diabetic cardiovascular disease | nobiletin ameliorated the hemodynamic parameters, oxidative stress, collagen level, MMP-2 and MMP-9 levels, and vascular reactivity significantly compared with vehicle treated diabetic group. | [125] |
| In vivo | STZ; C57BL/6 mice | Nobiletin; 50 mg/kg; 11 weeks; i.g. | Diabetic cardiovascular disease | Nobiletin treatment ameliorated cardiac dysfunction in the DCM group, blunted the mRNA expression of NADPH oxidase isoforms p67phox, p22phox and p91phox, and abated oxidative stress, decreased the Tgfb1, Ctgf, fibronectin, and collagen Iα expressions and blunted cardiac fibrosis. | [126] |
| In vivo | High-fat diet, STZ; SD rats | Nobiletin; 5 mg/kg, i.p. | Diabetic cardiovascular disease | Nobiletin reduced ACSL4 and NCOA4 expression and inhibited the effect of Erastin or oe-ACSL4 in increasing ACSL4 expression. Alleviation of myocardial ischemia–reperfusion injury in T2DM rats | [127] |
| In vitro and In vivo | HepG2 cells; High-fat diet; Ldlr−/− mice | Nobiletin; 10 μM; 0.1 or 0.3%, w/w; 8 or 26 weeks; p.o. | Diabetic hepatopathy | Nobiletin inhibits apoB100 secretion from HepG2 cells through activation of MAPK/ERK. attenuated dyslipidemia through a reduction in VLDL-triglyceride secretion. Nobiletin prevented hepatic TG accumulation, increased expression of Pgc1α and Cpt1α, and enhanced fatty acid β-oxidation. Nobiletin increased hepatic and peripheral insulin sensitivity and glucose tolerance and dramatically attenuated atherosclerosis in the aortic sinus. | [83] |
| In vitro | High glucose; glomerular mesangial cell | Nobiletin; 5, 10, 20, 30 μM | Diabetic nephropathy | Nobiletin inhibits IL-1β, IL-6, TNF-α, STAT and NF-κB, reducing ECM accumulation. | [96] |
| In vivo | STZ/cadmium (Cd)-induced; rats | Diabetic nephropathy | Nobiletin improved pathological damage and reduced renal tubular neutrophil infiltration, inhibited the expression of NF-κB p65 protein, downregulated Bax protein expression, and increased Bcl-2 protein expression. | [97] | |
| In vivo | STZ; albino rats | Nobiletin; 10, 25 mg/kg; 30 days; p.o. | Diabetic reproductive damages | Nobiletin decreased glucose, glycosylated hemoglobin (HbA1c), Homeostatic Model of Insulin Resistance (HOMA-IR), and pro-inflammatory cytokines expression, increased insulin, testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) expression, improved hyperglycemia, reduced pro-inflammatory cytokines, and augmented insulin, testosterone, LH, FSH and CYP17A1, reduced lipid peroxidation and improved the activity of the antioxidant enzymes and AR in testicular tissues of the diabetic group. | [128] |
| In vivo | TNF; Human placenta, VAT and skeletal muscle cells; Leprdb/+ (db/+) mice | Nobiletin; 100, 200 μM; 50 mg/kg; 17 days; p.o. | Gestational diabetes mellitus | Nobiletin increased insulin sensitivity in human skeletal muscle impaired by inflammation and decreased inflammation-induced pro-inflammatory mediators in human placenta and adipose tissue, improved fasting glucose levels, and reduced inflammation in mice placenta and maternal adipose tissue. These effects may be elicited through the NF-κB, Akt and MAPK pathways. | [132] |
4. Nobiletin in Food Applications
4.1. Nanoemulsion Delivery Systems
4.2. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS)
4.3. Nanoparticles
4.4. Plant-Derived Carriers
4.5. Vesicular Systems
| Delivery Systems | Particle Size | Loading Efficiency/Drug Loading | Encapsulation Efficiency | Release Kinetics | Bioavailability | Reference |
|---|---|---|---|---|---|---|
| Nanoemulsion delivery systems | The averaged droplet size is 325.7 ± 28.1 nm | 1% w/v nobiletin | / | The bioaccessibility of nobiletin delivered by the oil suspension and emulsion is 17.9 ± 1.8% and 81.3 ± 3.0%, respectively. | The bioavailability of nobiletin in oil suspension and emulsion is 19.93 ± 3.93% and 46.20 ± 5.03%, respectively. | [146] |
| The average droplet size distribution of the nanoemulsion is 264 nm | The aged citrus peel extract-loaded oil suspension was prepared by suspending 8% of aged citrus extract in pure MCT. | / | The lipids in the nanoemulsion were almost hydrolyzed into free fatty acids at 90 min. | The bioaccessibility of nobiletin in nanoemulsion is 32.3%. | [147] | |
| The minimum average droplet diameter (about 226 nm) | The nobiletin loading capacity of 1,3-diacylglycerol (1,3-DAG) oil is 14.53 ± 0.44 mg/g. | 1,3-DAG oil nanoemulsion showed a high encapsulation efficiency (above 95%). | 1,3-DAG releases >80% of free fatty acids (FFA) within 60 min. | The bioaccessibility of nobiletin (above 80%). | [148] | |
| The average droplet size of the nobiletin emulsion is 196.10 ± 1.16 nm | 0.5% w/w nobiletin | / | The bioaccessibility of naringenin in the oily suspension and emulsion was 13.0 ± 0.2% and 58.6 ± 2.5%, respectively. | The serum Cmax of nobiletin for the emulsion and oil suspension are 1.29 ± 0.03 μg/mL and 0.92 ± 0.04 μg/mL, respectively, and Tmax is 0.5 h. | [149] | |
| Nanoparticles | The size distribution of the nanoparticles is approximately 126 ± 30.18 nm | The drug loading efficiency of nobiletin is 14.93%. | The drug encapsulation efficiency of nobiletin is 74.68%. | The release rate of nobiletin was 10% in 12 h, saturated in 5 days, and 91.4% in 8 days. | / | [154] |
| The average particle size of NTFe is 351.2 ± 2.42 nm, and the average particle size of NTAl is 508.6 ± 4.85 nm. | The loading efficiency of NTFe is 286.492%, and that of NTAl is 214.803%. | The encapsulation efficiency of NTFe is 99.914%, and that of NTAl is 99.883%. | The drug release performance of NTFe and NTAl was not much different and was better than that of uncoated nobiletin. | / | [155] | |
| The particle size of NOB/zein/TA NPs (NZT NPs) is 100 nm. | The nobiletin loading capacity is 48.46 ± 1.62%. | The encapsulation efficiency of NZT48 NPs was 92.37 ± 0.12%. | NZTSZ: Stomach (2 h): Release <10% (resistant to gastric acid degradation). Intestinal (48 h): Slow and sustained release. | / | [156] | |
| The nobiletin micelles displayed the particle sizes of 68.64 ± 4.37 nm | / | / | NOB/SD releases 95% of nobiletin within 5 min, and maintains supersaturation for at least 120 min. | In the NOB/SD group, Cₘₐₓ is 7.23 ± 1.62 μg/mL and AUC is 9.68 ± 1.74 μg·h/mL. | [157] | |
| Plant-Derived carriers | / | The nobiletin loading capacity of sunflower pollen grains (SPG) is 770 ± 40 mg/g | / | NSGA has a protective sustained release in the stomach (2%) and a long-term sustained release in the intestine (>100 h). | / | [134] |
| The particle size of NOB/Tea is around 400 nm | The loading efficiency of nobiletin is over 75% | Encapsulation Efficiency: >95% | / | / | [159] | |
| Vesicular systems | The particle size of composite PEVs is 126.70 ± 11.80 nm | The loading efficiency of nobiletin is 15.08 ± 0.82%. | Entrapment Efficiency: 93.50 ± 3.60% | / | Skin local bioavailability: total skin deposition 95.30 ± 3.40%. | [161] |
5. Nobiletin and Clinical Trail
6. Nobiletin and Commercial Value
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Ekim Üstünel, B. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv. Sci. 2021, 8, e2100275. [Google Scholar] [CrossRef]
- The World Health Organization. Global report on diabetes: Executive summary. In Global Report on Diabetes: Executive Summary; The World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef] [PubMed]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Wei, K.J.; Pan, B.H.; Du, Y.Z.; Hu, Y.T.; Chen, S.H.; Lyu, G.Y.; Yu, J.J. Effect of polymethoxylated flavonoids on glucose and lipid metabolism in rat model of obesity induced by a high-fat diet. Zhongguo Zhong Yao Za Zhi 2024, 49, 3270–3279. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Xiao, P.T.; Cai, Y.Y.; Chu, C.; Chen, B.Z.; Li, P.; Li, J.; Liu, E.H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef]
- Li, R.W.; Theriault, A.G.; Au, K.; Douglas, T.D.; Casaschi, A.; Kurowska, E.M.; Mukherjee, R. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006, 79, 365–373. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Xu, S.; Ren, J.; Tang, L.; Gong, J.; Lin, Y.; Fang, H.; Su, D. Hesperetin, a Promising Treatment Option for Diabetes and Related Complications: A Literature Review. J. Agric. Food Chem. 2022, 70, 8582–8592. [Google Scholar] [CrossRef]
- Mirzaei, A.; Mirzaei, A.; Najjar Khalilabad, S.; Askari, V.R.; Baradaran Rahimi, V. Promising influences of hesperidin and hesperetin against diabetes and its complications: A systematic review of molecular, cellular, and metabolic effects. Excli J. 2023, 22, 1235–1263. [Google Scholar] [CrossRef]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liang, J.J.; Wang, D.L.; Chen, J.B.; Cao, J.P.; Wang, Y.; Sun, C.D. Nobiletin as a chemopreventive natural product against cancer, a comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 6309–6329. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, S.; Sheng, C.; Yang, C.; Li, Y. Nobiletin from citrus peel: A promising therapeutic agent for liver disease-pharmacological characteristics, mechanisms, and potential applications. Front. Pharmacol. 2024, 15, 1354809. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J.; Li, H.; Li, J. Recent advances in the therapeutic potential of nobiletin against respiratory diseases. Phytomedicine 2024, 128, 155506. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, B.; Li, J.; Wang, X.; Li, B.; Liang, H. Coordination-Driven Metal-Polyphenolic Nanoparticles toward Effective Anticancer Therapy. Adv. Healthc. Mater. 2022, 11, e2200559. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.X.H.; Tan, L.T.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. [Google Scholar] [CrossRef]
- Hattori, T.; Tagawa, H.; Inai, M.; Kan, T.; Kimura, S.I.; Itai, S.; Mitragotri, S.; Iwao, Y. Transdermal delivery of nobiletin using ionic liquids. Sci. Rep. 2019, 9, 20191. [Google Scholar] [CrossRef]
- Onoue, S.; Nakamura, T.; Uchida, A.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Hiza, A.; Tsukaguchi, Y.; Asakawa, T.; Kan, T.; et al. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur. J. Pharm. Sci. 2013, 49, 453–460. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Mallya, P.; Kumar, V.A.; Jain, V.; Sharma, S.; Dey, S. Nobiletin as a Molecule for Formulation Development: An Overview of Advanced Formulation and Nanotechnology-Based Strategies of Nobiletin. AAPS PharmSciTech 2020, 21, 226. [Google Scholar] [CrossRef]
- Zhu, Y.; Zuo, F.; Ouyang, H.; Chen, L.; Zhang, M.; Shang, Y.; Lv, Z.; Chang, Y.; He, J. Determination of eleven components in rat plasma by UPLC-MS/MS and GC-MS for pharmacokinetic studies after oral administration of Citri Reticulatae Pericarpium extract. J. Pharm. Biomed. Anal. 2024, 248, 116315. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, Y.; Lu, S.; Xia, Y.; Cheng, B. Polymethoxylated flavonoids in citrus fruits: Absorption, metabolism, and anticancer mechanisms against breast cancer. PeerJ 2024, 12, e16711. [Google Scholar] [CrossRef] [PubMed]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Chee, W.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and enhancements to improve efficiency and predictive performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (-)epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Ai, Z.; Su, J. Nobiletin-loaded micelles reduce ovariectomy-induced bone loss by suppressing osteoclastogenesis. Int. J. Nanomed. 2019, 14, 7839–7849. [Google Scholar] [CrossRef]
- Lin, S.C.; Chen, M.C.; Li, S.; Lin, C.C.; Wang, T.T. Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir. Ther. 2017, 22, 689–697. [Google Scholar] [CrossRef]
- Delaney, B.; Phillips, K.; Vasquez, C.; Wilson, A.; Cox, D.; Wang, H.B.; Manthey, J. Genetic toxicity of a standardized mixture of citrus polymethoxylated flavones. Food Chem. Toxicol. 2002, 40, 617–624. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, Y.; Chen, L.; Zhu, Y.; Xiao, X.; Wang, D.; Zhu, Y. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol. Res. 2018, 40, 211–220. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Li, M.; Cao, D.; Yang, X.; Gong, J. Nobiletin ameliorates ischemia-reperfusion injury by suppressing the function of Kupffer cells after liver transplantation in rats. Biomed. Pharmacother. 2017, 89, 732–741. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Choi, S.S.; Choi, B.K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 2013, 24, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Nemoto, K.; Yoshida, C.; Miyata, S.; Mori, J.; Soejima, S.; Yokosuka, A.; Mimaki, Y.; Ohizumi, Y.; Degawa, M. Suppressive effect of nobiletin, a citrus polymethoxyflavonoid that downregulates thioredoxin-interacting protein expression, on tunicamycin-induced apoptosis in SK-N-SH human neuroblastoma cells. Neurosci. Lett. 2013, 549, 135–139. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-Ameliorated Lipopolysaccharide-Induced Inflammation in Acute Lung Injury by Suppression of NF-κB Pathway In Vivo and Vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Yu, K.Y.; Guo, L.; Bai-Zhong, C.; Li, P.; Liu, E.H. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017, 234, 254–261. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, M.S.; Woo, J.T.; Jeong, M.J.; Kim, S.R.; Jung, U.J. Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity. Mol. Nutr. Food Res. 2017, 61, 1600889. [Google Scholar] [CrossRef]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid Res. 2020, 61, 387–402. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.G. AMPK-Dependent Metabolic Regulation by PPAR Agonists. PPAR Res. 2010, 2010, 549101. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, S.; Zhao, Z.; Zhao, P.; Chen, L.; Mei, Z. Antidiabetic activities of entagenic acid in type 2 diabetic db/db mice and L6 myotubes via AMPK/GLUT4 pathway. J. Ethnopharmacol. 2018, 211, 366–374. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef]
- Namkoong, S.; Sung, J.; Yang, J.; Choi, Y.; Jeong, H.S.; Lee, J. Nobiletin Attenuates the Inflammatory Response Through Heme Oxygenase-1 Induction in the Crosstalk Between Adipocytes and Macrophages. J. Med. Food 2017, 20, 873–881. [Google Scholar] [CrossRef]
- Shen, W.; Xu, Y.; Lu, Y.H. Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef]
- Abe, T.; Sato, T.; Murotomi, K. Sudachitin and Nobiletin Stimulate Lipolysis via Activation of the cAMP/PKA/HSL Pathway in 3T3-L1 Adipocytes. Foods 2023, 12, 1947. [Google Scholar] [CrossRef]
- Onda, K.; Horike, N.; Suzuki, T.; Hirano, T. Polymethoxyflavonoids tangeretin and nobiletin increase glucose uptake in murine adipocytes. Phytother. Res. 2013, 27, 312–316. [Google Scholar] [CrossRef]
- Kunimasa, K.; Kuranuki, S.; Matsuura, N.; Iwasaki, N.; Ikeda, M.; Ito, A.; Sashida, Y.; Mimaki, Y.; Yano, M.; Sato, M.; et al. Identification of nobiletin, a polymethoxyflavonoid, as an enhancer of adiponectin secretion. Bioorg. Med. Chem. Lett. 2009, 19, 2062–2064. [Google Scholar] [CrossRef]
- Miyata, Y.; Tanaka, H.; Shimada, A.; Sato, T.; Ito, A.; Yamanouchi, T.; Kosano, H. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sci. 2011, 88, 613–618. [Google Scholar] [CrossRef]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Zhang, Y.; Qu, T.Q.; Sang, X.Q.; Li, Y.X.; Ren, F.Z.; Wen, P.C.; Sun, Y.N. Nobiletin Improves D-Galactose-Induced Aging Mice Skeletal Muscle Atrophy by Regulating Protein Homeostasis. Nutrients 2023, 15, 1801. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Pakdeechote, P.; Maneesai, P.; Prasarttong, P. Nobiletin alleviates high-fat diet-induced nonalcoholic fatty liver disease by modulating AdipoR1 and gp91(phox) expression in rats. J. Nutr. Biochem. 2021, 87, 108526. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Lu, R.; Yonezawa, T.; Watanabe, A.; Woo, J.T.; Abe-Dohmae, S.; Yokoyama, S. Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis 2020, 297, 32–39. [Google Scholar] [CrossRef]
- Weiss, J.; Gattuso, G.; Barreca, D.; Haefeli, W.E. Nobiletin, sinensetin, and tangeretin are the main perpetrators in clementines provoking food-drug interactions in vitro. Food Chem. 2020, 319, 126578. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Nakatani, Y.; Ishida, M.; Kadota, A.; Sugahara, T. Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect. Nutrients 2024, 16, 2080. [Google Scholar] [CrossRef]
- Morrow, N.M.; Trzaskalski, N.A.; Hanson, A.A.; Fadzeyeva, E.; Telford, D.E.; Chhoker, S.S.; Sutherland, B.G.; Edwards, J.Y.; Huff, M.W.; Mulvihill, E.E. Nobiletin Prevents High-Fat Diet-Induced Dysregulation of Intestinal Lipid Metabolism and Attenuates Postprandial Lipemia. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 127–144. [Google Scholar] [CrossRef]
- Koh, Y.C.; Liu, C.P.; Leung, S.Y.; Lin, W.S.; Ho, P.Y.; Ho, C.T.; Pan, M.H. Nobiletin Enhances Skeletal Muscle Mass and Modulates Bile Acid Composition in Diet-Induced Obese Mice. J. Agric. Food Chem. 2025, 73, 9076–9087. [Google Scholar] [CrossRef]
- Liao, X.; Zou, J.; Wu, M.; Deng, Y.; Shi, J.; Hao, Y.; Deng, H.; Liao, W. Hypoglycemic Effect of Nobiletin via Gut Microbiota-Metabolism Axis on Hyperglycemic Mice. Mol. Nutr. Food Res. 2023, 67, e2200289. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, J.; Du, C.; Xu, Y. Modulation of Fat Deposition-Gut Interactions in Obese Mice by Administrating with Nobiletin. Genes 2023, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Liao, J.A.; Lin, C.T.; Wei, G.J.; Tung, Y.C. Citrus depressa peel extract acts as a prebiotic to reduce lipid accumulation and modulate gut microbiota in obese mice. J. Food Drug Anal. 2024, 32, 213–226. [Google Scholar] [CrossRef]
- Yang, J.; Xia, X.; Du, M.; Cheng, S.; Zhu, B.; Xu, X. Highly Effective Nobiletin-MPN in Yeast Microcapsules for Targeted Modulation of Oxidative Stress, NLRP3 Inflammasome Activation, and Immune Responses in Ulcerative Colitis. J. Agric. Food Chem. 2024, 72, 13054–13068. [Google Scholar] [CrossRef]
- Zhan, M.; Yang, X.; Zhao, C.; Han, Y.; Xie, P.; Mo, Z.; Xiao, J.; Cao, Y.; Xiao, H.; Song, M. Dietary nobiletin regulated cefuroxime- and levofloxacin-associated "gut microbiota-metabolism" imbalance and intestinal barrier dysfunction in mice. Food Funct. 2024, 15, 1265–1278. [Google Scholar] [CrossRef]
- Yang, N.; Pang, Y.S.; Zheng, Y.; Gong, Y.J.; Ding, W.J. Nobiletin restores the intestinal barrier of HFD-induced obese mice by promoting MHC-II expression and lipid metabolism. Mol. Med. 2025, 31, 26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Ye, Z.; Li, Y.; Zou, J.; Wu, M.; Wang, K.; Liao, W.; Shen, J. Hypoglycemic Effect of Nobiletin via Regulation of Islet β-Cell Mitophagy and Gut Microbiota Homeostasis in Streptozocin-Challenged Mice. J. Agric. Food Chem. 2022, 70, 5805–5818. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Guo, R.; Tian, H.; Li, L.; Liu, H.; Mi, Y.; Liu, X. Nobiletin protects against insulin resistance and disorders of lipid metabolism by reprogramming of circadian clock in hepatocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 549–562. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef]
- Rakshit, K.; Matveyenko, A.V. Induction of Core Circadian Clock Transcription Factor Bmal1 Enhances β-Cell Function and Protects Against Obesity-Induced Glucose Intolerance. Diabetes 2021, 70, 143–154. [Google Scholar] [CrossRef]
- Kim, E.; Mawatari, K.; Yoo, S.H.; Chen, Z. The Circadian Nobiletin-ROR Axis Suppresses Adipogenic Differentiation and IκBα/NF-κB Signaling in Adipocytes. Nutrients 2023, 15, 3919. [Google Scholar] [CrossRef]
- Lu, Z.; Li, X.; Wang, M.; Zhang, X.; Zhuang, R.; Wu, F.; Li, W.; Zhu, W.; Zhang, B. Liver-Specific Bmal1 Depletion Reverses the Beneficial Effects of Nobiletin on Liver Cholesterol Homeostasis in Mice Fed with High-Fat Diet. Nutrients 2023, 15, 2547. [Google Scholar] [CrossRef]
- Xiong, X.; Kiperman, T.; Li, W.; Dhawan, S.; Lee, J.; Yechoor, V.; Ma, K. The Clock-modulatory Activity of Nobiletin Suppresses Adipogenesis Via Wnt Signaling. Endocrinology 2023, 164, bqad096. [Google Scholar] [CrossRef]
- Abd El-Khalik, S.R.; AbuoHashish, N.A.; Nasif, E.; Arakeep, H.M.; El-Anwar, N.; Yousef, D.M.; Ayad, N.H. Possible Mitigating Effect of Nobiletin on Rotenone-Induced Parkinsonism in Male Albino Rat Model: Targeting Bmal1/Nrf2 Mediated Ferroptosis and Restoring Mitochondrial Mitophagy. Neurochem. Res. 2025, 50, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Li, X.P.; Gao, L.F.; Liu, J.; Bi, Z.J.; Miao, Y.H.; Shan, Y.; Yu, H.L. Nobiletin, an activator of the pyruvate kinase isozyme M1/M2 protein, upregulated the glycolytic signalling pathway and alleviated depressive-like behaviour caused by artificial light exposure at night in zebrafish. Food Chem. 2025, 463 Pt 2, 141328. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef]
- Tahmasbi, M.; Karimpour, A.; Rashidi, M.; Zangooei, M.; Khedri, A.; Panahi, G. Nobiletin can play a role in improving inflammation by inhibiting the NF-kB and MAPK pathways in muscle cells. J. Diabetes Metab. Disord. 2025, 24, 166. [Google Scholar] [CrossRef]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Kaji, M.; Kaneko, Y.K.; Ihim, S.A.; Kanoh, R.; Yamamoto, M.; Yamaguchi, M.; Ishikawa, T. Oral ingestion of Shiikuwasha extract suppresses diabetes progression in db/db mice by preserving β-cell mass. Front. Nutr. 2023, 10, 1336133. [Google Scholar] [CrossRef]
- Y, K.K.; Kan, T.; Ishikawa, T. Citrus flavonoids as a target for the prevention of pancreatic β-cells dysfunction in diabetes. Nihon Yakurigaku Zasshi 2020, 155, 209–213. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dellacassa, E.; Curbelo, R.; Nardin, T.; Larcher, R.; Medrano-Fernandez, A.; Del Castillo, M.D. Health-Promoting Potential of Mandarin Pomace Extracts Enriched with Phenolic Compounds. Nutrients 2024, 16, 2370. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Holstein, A.; Hinze, S.; Thiessen, E.; Plaschke, A.; Egberts, E.H. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 2002, 17, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Ran, Q.; Gan, Q.; Zhu, Y.; Song, L.; Shen, L.; Duan, X.; Zhu, X.; Huang, W. Mechanism insights into the pleiotropic effects of nobiletin as a potential therapeutic agent on non-alcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2024, 173, 116322. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Z.; Ma, Y.; Yang, X.; Zhu, Z.; Zhang, Z.; Hu, J.; Liang, W.; Ding, G. AKAP1 contributes to impaired mtDNA replication and mitochondrial dysfunction in podocytes of diabetic kidney disease. Int. J. Biol. Sci. 2022, 18, 4026–4042. [Google Scholar] [CrossRef]
- Ruan, Z.; Liu, J.; Liu, W.; Huang, W.-J. Qufeng Tongluo Decoction May Alleviate Podocyte Injury Induced by High Glucose and Hydrogen Peroxide by Regulating Autophagy. Integr. Med. Nephrol. Androl. 2024, 11, e24-00023. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Cao, A. The Potential of Huangqi Decoction for Treating Diabetic Kidney Disease. Integr. Med. Nephrol. Androl. 2024, 11, e00020. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Zhao, F.; Zhao, Z.; Tian, J.; Jia, K. Nobiletin suppresses high-glucose-induced inflammation and ECM accumulation in human mesangial cells through STAT3/NF-κB pathway. J. Cell Biochem. 2019, 120, 3467–3473. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Fan, H.; Ni, Z. Nobiletin ameliorates streptozotocin-cadmium-induced diabetic nephropathy via NF-κB signalling pathway in rats. Arch. Physiol. Biochem. 2024, 130, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, T.; Zhang, X.Q.; Gong, Y.; Su, H.; Fan, J.; Wang, L.; Hu, Q.D.; Tan, R.Z. Screening of active components in Astragalus mongholicus Bunge and Panax notoginseng formula for anti-fibrosis in CKD: Nobiletin inhibits Lgals1/PI3K/AKT signaling to improve renal fibrosis. Ren. Fail. 2024, 46, 2375033. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Y.; Liu, J.; Zhang, Y.; Yi, X.; Yan, W.; Cui, X.; Guo, T.; Zhao, W.; Han, S.; et al. Nobiletin: A potential erythropoietin receptor activator protects renal cells against hypoxia. Apoptosis 2025, 30, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Iampanichakul, M.; Poasakate, A.; Potue, P.; Rattanakanokchai, S.; Maneesai, P.; Prachaney, P.; Settheetham-Ishida, W.; Pakdeechote, P. Nobiletin resolves left ventricular and renal changes in 2K-1C hypertensive rats. Sci. Rep. 2022, 12, 9289. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yu, J. Integrating Network Pharmacology, Bioinformatics, and Mendelian Randomization Analysis to Identify Hub Targets and Mechanisms of Kunkui Baoshen Decoction in Treating Diabetic Kidney Disease. Curr. Pharm. Des. 2024, 30, 3367–3393. [Google Scholar] [CrossRef]
- Dolatshahi, M.; Sanjari Moghaddam, H.; Saberi, P.; Mohammadi, S.; Aarabi, M.H. Central nervous system microstructural alterations in Type 1 diabetes mellitus: A systematic review of diffusion Tensor imaging studies. Diabetes Res. Clin. Pract. 2023, 205, 110645. [Google Scholar] [CrossRef]
- Zang, Y.; Jiang, D.; Zhuang, X.; Chen, S. Changes in the central nervous system in diabetic neuropathy. Heliyon 2023, 9, e18368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef] [PubMed]
- Stykel, M.G.; Ryan, S.D. Nitrosative stress in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-κB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef]
- Olofinsan, K.A.; Salau, V.F.; Erukainure, O.L.; Islam, M.S. Harpephyllum caffrum fruit (wild plum) facilitates glucose uptake and modulates metabolic activities linked to neurodegeneration in isolated rat brain: An in vitro and in silico approach. J. Food Biochem. 2022, 46, e14177. [Google Scholar] [CrossRef]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchi, S.; Nakajima, A.; Yokosuka, A.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y.; et al. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009, 1295, 218–229. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Liu, B.B.; Liu, Q.; Geng, D.; Weng, L.J.; Yi, L.T. Nobiletin Ameliorates the Deficits in Hippocampal BDNF, TrkB, and Synapsin I Induced by Chronic Unpredictable Mild Stress. Evid. Based Complement. Altern. Med. 2013, 2013, 359682. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Miyazaki, K.; Sakai, S.; Yawo, H.; Nakata, N.; Moriguchi, S.; Fukunaga, K.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. Eur. J. Pharmacol. 2008, 578, 194–200. [Google Scholar] [CrossRef]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 2014, 259, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Nagayach, A.; Bhaskar, R.; Ghosh, S.; Singh, K.K.; Han, S.S.; Sinha, J.K. Advancing the understanding of diabetic encephalopathy through unravelling pathogenesis and exploring future treatment perspectives. Ageing Res. Rev. 2024, 100, 102450. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, Y.; Jia, Z.; Shi, S. Risk factors and prevalence of diabetic retinopathy: A protocol for meta-analysis. Medicine 2020, 99, e22695. [Google Scholar] [CrossRef]
- Ruta, L.M.; Magliano, D.J.; Lemesurier, R.; Taylor, H.R.; Zimmet, P.Z.; Shaw, J.E. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet. Med. 2013, 30, 387–398. [Google Scholar] [CrossRef]
- Navaratna, D.; McGuire, P.G.; Menicucci, G.; Das, A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes 2007, 56, 2380–2387. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert. Opin. Investig. Drugs 2012, 21, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Mohammad, G.; Nawaz, M.I.; Siddiquei, M.M.; Van den Eynde, K.; Mousa, A.; De Hertogh, G.; Opdenakker, G. Relationship between vitreous levels of matrix metalloproteinases and vascular endothelial growth factor in proliferative diabetic retinopathy. PLoS ONE 2013, 8, e85857. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J. Cell Physiol. 2012, 227, 1052–1061. [Google Scholar] [CrossRef]
- Limb, G.A.; Daniels, J.T.; Pleass, R.; Charteris, D.G.; Luthert, P.J.; Khaw, P.T. Differential expression of matrix metalloproteinases 2 and 9 by glial Müller cells: Response to soluble and extracellular matrix-bound tumor necrosis factor-alpha. Am. J. Pathol. 2002, 160, 1847–1855. [Google Scholar] [CrossRef]
- Miyata, Y.; Nagase, T.; Katsura, Y.; Takahashi, H.; Natsugari, H.; Oshitari, T.; Kosano, H. In vitro studies on nobiletin isolated from citrus plants and the bioactive metabolites, inhibitory action against gelatinase enzymatic activity and the molecular mechanisms in human retinal Müller cell line. Biomed. Pharmacother. 2017, 93, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsumoto, K.; Kusano, S.; Kusakabe, Y.; Katsura, Y.; Oshitari, T.; Kosano, H. Regulation of Endothelium-Reticulum-Stress-Mediated Apoptotic Cell Death by a Polymethoxylated Flavone, Nobiletin, Through the Inhibition of Nuclear Translocation of Glyceraldehyde 3-Phosphate Dehydrogenase in Retinal Müller Cells. Cells 2021, 10, 669. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Liu, Z.; Xing, Q.; Liu, R.; Wu, Q.; Hu, Y.; Zhang, J. The signaling pathways of selected traditional Chinese medicine prescriptions and their metabolites in the treatment of diabetic cardiomyopathy: A review. Front. Pharmacol. 2024, 15, 1416403. [Google Scholar] [CrossRef] [PubMed]
- Parkar, N.A.; Bhatt, L.K.; Addepalli, V. Efficacy of nobiletin, a citrus flavonoid, in the treatment of the cardiovascular dysfunction of diabetes in rats. Food Funct. 2016, 7, 3121–3129. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Xiang, S.Z.; Jin, Y.G.; Wei, W.Y.; Bian, Z.Y.; Deng, W.; Tang, Q.Z. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: Induced diabetic cardiomyopathy. Mol. Cell Biochem. 2016, 417, 87–96. [Google Scholar] [CrossRef]
- Huang, Q.; Tian, L.; Zhang, Y.; Qiu, Z.; Lei, S.; Xia, Z.Y. Nobiletin alleviates myocardial ischemia-reperfusion injury via ferroptosis in rats with type-2 diabetes mellitus. Biomed. Pharmacother. 2023, 163, 114795. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Ismail, K.A.; Khadrawy, S.M. Nobiletin protects against diabetes-induced testicular injury via hypophysis-gonadal axis upregulation and amelioration of oxidative stress. Mol. Biol. Rep. 2022, 49, 189–203. [Google Scholar] [CrossRef]
- Li, R.X.; Fan, C.L.; Xu, W.Y.; Wei, W.; Wang, X.X.; Li, Z.T.; Zhao, P.C.; Su, Z.J.; Tang, X.Y.; Yao, Z.H.; et al. Simultaneous determination of multiple constituents of Qi-Lin pill by UPLC-MS/MS: Applications to pharmacokinetics and testicular tissue distribution in rats. J. Pharm. Biomed. Anal. 2023, 223, 115157. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef]
- Nakajima, A.; Nemoto, K.; Ohizumi, Y. An evaluation of the genotoxicity and subchronic toxicity of the peel extract of Ponkan cultivar ‘Ohta ponkan’ (Citrus reticulata Blanco) that is rich in nobiletin and tangeretin with anti-dementia activity. Regul. Toxicol. Pharmacol. 2020, 114, 104670. [Google Scholar] [CrossRef]
- Wu, D.; Liang, Y.; Pei, Y.; Li, B.; Liang, H. Plant exine capsules based encapsulation strategy: A high loading and long-term effective delivery system for nobiletin. Food Res. Int. 2020, 127, 108691. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.T. The biological fate and bioefficacy of citrus flavonoids: Bioavailability, biotransformation, and delivery systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Chen, J.; Tian, G.; McClements, D.J.; Xiao, H.; Zheng, J. Encapsulation of Polymethoxyflavones in Citrus Oil Emulsion-Based Delivery Systems. J. Agric. Food Chem. 2017, 65, 1732–1739. [Google Scholar] [CrossRef]
- Li, P.; Yao, X.; Zhou, Q.; Meng, X.; Zhou, T.; Gu, Q. Citrus Peel Flavonoid Extracts: Health-Beneficial Bioactivities and Regulation of Intestinal Microecology in vitro. Front. Nutr. 2022, 9, 888745. [Google Scholar] [CrossRef]
- Iwashita, M.; Shioi, R.; Sugiyama, M.; Hashizume, K.; Kan, T.; Naito, S.; Takai, H.; Kawase, Y.; Hamabe-Horiike, T.; Katanasaka, Y.; et al. Monodemethylated Metabolites of Orally Administered Nobiletin: Identification and Quantitation in Rat Plasma and Tissues. J. Agric. Food Chem. 2023, 71, 10028–10036. [Google Scholar] [CrossRef]
- Fan, K.; Kurihara, N.; Abe, S.; Ho, C.T.; Ghai, G.; Yang, K. Chemopreventive effects of orange peel extract (OPE). I: OPE inhibits intestinal tumor growth in ApcMin/+ mice. J. Med. Food 2007, 10, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cajas, Y.N.; Cañón-Beltrán, K.; Mazzarella, R.; Nuñez-Puente, C.; González, E.M.; Rodriguez-Martinez, H.; Rizos, D.; Martinez-Serrano, C.A. Nobiletin as a novel agent to enhance porcine in vitro embryo development and quality. Theriogenology 2024, 223, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, Y.; Wang, H.; He, H. Synergistic modification of hot-melt extrusion and nobiletin on the multi-scale structures, interactions, thermal properties, and in vitro digestibility of rice starch. Front. Nutr. 2024, 11, 1398380. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Uchida, A.; Takahashi, H.; Seto, Y.; Kawabata, Y.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Yamada, S. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J. Pharm. Sci. 2011, 100, 3793–3801. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, M.; Feng, K.; Xiao, J.; Huang, Q.; Ho, C.T.; Liu, J. Natural product nobiletin-loaded Pickering emulsion stabilized by bovine serum albumin/carboxymethyl inulin complexes: Preparation and digestive characteristics. Front. Pharmacol. 2024, 15, 1375779. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Huang, S.; Xie, W.; Huang, W.; Chen, Y.; Li, Q.; Zeng, F.; Liu, X. Antisolvent precipitation for the synergistic preparation of ultrafine particles of nobiletin under ultrasonication-homogenization and evaluation of the inhibitory effects of α-glucosidase and porcine pancreatic lipase in vitro. Ultrason. Sonochem. 2024, 105, 106865. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Wang, L.; Wang, F.; Xiong, L.; Shen, X.; Song, H. Pickering high internal phase emulsions stabilized by soy protein isolate/κ-carrageenan complex for enhanced stability, bioavailability, and absorption mechanisms of nobiletin. Carbohydr. Polym. 2025, 351, 123117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, K.; Huang, G.; Xin, Y.; Xiao, J.; Cao, Y.; Ludescher, R.; Ho, C.T.; Huang, Q. Assessment of Oral Bioavailability and Biotransformation of Emulsified Nobiletin Using In Vitro and In Vivo Models. J. Agric. Food Chem. 2020, 68, 11412–11420. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Zheng, T.; Liu, Q.; Zhu, J.; Huang, Q. Evaluation of Oral Bioaccessibility of Aged Citrus Peel Extracts Encapsulated in Different Lipid-Based Systems: A Comparison Study Using Different in Vitro Digestion Models. J. Agric. Food Chem. 2020, 68, 97–105. [Google Scholar] [CrossRef]
- Feng, K.; Duan, Y.; Zhang, H.; Xiao, J.; Ho, C.T.; Huang, Q.; Cao, Y. Influence of 1,3-diacylglycerol on physicochemical and digestion properties of nanoemulsions and its enhancement of encapsulation and bioaccessibility of hydrophobic nobiletin. Food Funct. 2023, 14, 6212–6225. [Google Scholar] [CrossRef]
- Ju, S.N.; Shi, H.H.; Yang, J.Y.; Zhao, Y.C.; Xue, C.H.; Wang, Y.M.; Huang, Q.R.; Zhang, T.T. Characterization, stability, digestion and absorption of a nobiletin nanoemulsion using DHA-enriched phosphatidylcholine as an emulsifier in vivo and in vitro. Food Chem. 2022, 397, 133787. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Z.; Zhang, T.; Sun, S.; Ye, J.; Li, Z.; Mao, L.; Ren, J. Enhancement of Anti-Inflammatory Properties of Nobiletin in Macrophages by a Nano-Emulsion Preparation. J. Agric. Food Chem. 2018, 66, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Lei, L.; Chen, H.; Li, B.; Cao, Y.; Li, Y. Tailoring of structured hydroxypropyl methylcellulose-stabilized emulsions for encapsulation of nobiletin: Modification of the oil and aqueous phases. Food Funct. 2018, 9, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Wang, X.L.; Zheng, D.C.; Mai, C.T.; Liu, Z.Q.; Zhou, H.; Xie, Y. Novel treatment for refractory rheumatoid arthritis with total glucosides of paeony and nobiletin codelivered in a self-nanoemulsifying drug delivery system. Acta Pharmacol. Sin. 2022, 43, 2094–2108. [Google Scholar] [CrossRef]
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef]
- Zhang, H.H.; Kuo, W.S.; Tu, P.Y.; Lee, C.T.; Wang, H.C.; Huang, Y.T.; Shen, M.C.; Lin, T.S.; Su, P.L.; Tsai, J.S.; et al. Enhancing Lung Recovery: Inhaled Poly(lactic-co-glycolic) Acid Encapsulating FTY720 and Nobiletin for Lipopolysaccharide-Induced Lung Injury, with Advanced Inhalation Tower Technology. ACS Nano 2025, 19, 7634–7649. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B.; Wu, D.; Chen, X.; Li, B.; Wang, L.; Liang, H. Ultrasound-based one-step fabrication of nobiletin particle: A facile stabilization strategy. Food Chem. 2022, 369, 130896. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Wang, S.; Li, B.; Liang, H. Nanoparticle Encapsulation Strategy: Leveraging Plant Exine Capsules Used as Secondary Capping for Oral Delivery. J. Agric. Food Chem. 2019, 67, 8168–8176. [Google Scholar] [CrossRef]
- Ning, J.; Zheng, G.; Cai, Y.; Hu, Y.; Liu, Y.; Lai, E.; Chen, B.; Liu, Y.; Liang, Z.; Fu, J.; et al. The Self-Assembly Soluplus Nanomicelles of Nobiletin in Aqueous Medium Based on Solid Dispersion and Their Increased Hepatoprotective Effect on APAP-Induced Acute Liver Injury. Int. J. Nanomed. 2023, 18, 5119–5140. [Google Scholar] [CrossRef]
- Wu, D.; Liang, Y.; Huang, K.; Jing, X.; Li, B.; Liang, H. Leveraging plant exine capsules as pH-responsive delivery vehicles for hydrophobic nutraceutical encapsulation. Food Funct. 2018, 9, 5436–5442. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ma, W.; Chen, X.; Ji, S.; Li, T.; Shi, Y.; Yue, P.; Zhou, B.; Ren, J.; Li, B.; et al. The in situ stability of nobiletin in green tea infusion regulating by the assembly of small molecules. Food Chem. 2025, 487, 144427. [Google Scholar] [CrossRef]
- Neubert, R.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- Bayoumi, M.; Arafa, M.G.; Nasr, M.; Sammour, O.A. Nobiletin-loaded composite penetration enhancer vesicles restore the normal miRNA expression and the chief defence antioxidant levels in skin cancer. Sci. Rep. 2021, 11, 20197. [Google Scholar] [CrossRef]
- Fujioka, K.; Greenway, F.; Sheard, J.; Ying, Y. The effects of grapefruit on weight and insulin resistance: Relationship to the metabolic syndrome. J. Med. Food 2006, 9, 49–54. [Google Scholar] [CrossRef]
- Dallas, C.; Gerbi, A.; Elbez, Y.; Caillard, P.; Zamaria, N.; Cloarec, M. Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (Sinetrol-XPur) on weight management and metabolic parameters in healthy overweight individuals. Phytother. Res. 2014, 28, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Navajas-Porras, B.; Bosch-Sierra, N.; Valle, C.G.; Salazar, J.D.; Marqués-Cardete, R.; Sáez, G.; Morillas, C.; Bañuls, C. Effects of a flavonoid-enriched orange juice on antioxidant capacity, lipid profile, and inflammation in obese patients: A randomized placebo-controlled trial. Food Res. Int. 2025, 217, 116759. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wu, D.; Li, B.; Li, Y.; Li, J. Stable Nobiletin Liquid Preparation and Preparation Method Thereof. CN 107998073, 12 June 2020. [Google Scholar]
- Zhu, C.; Sun, C.; Wang, Y.; Li, X. Method for Separating and Purifying Seven Flavonoid Compounds from Ougan Flavedo by Combined Use of Solvent Extraction, Sephadex Gel Filtration and High-Speed Counter-Current Chromatography. ZA 2021010928, 20 March 2022. [Google Scholar]
- Huang, J.; Liu, S.; Gu, Y.; Lai, R. Method for Separating and Preparing Nobiletin from Citrus Depressa. CN 109369590, 22 February 2019. [Google Scholar]
- Iwashita, M.; Umehara, M.; Onishi, S.; Yamamoto, M.; Yamagami, K.; Ishigami, T. Method for Manufacturing Nobiletin-Containing Solid Dispersion. WO 2018025871, 8 February 2018. [Google Scholar]
- Yang, W.; Song, Y.; Chen, H.; Luo, X.; Yuan, J. A Technique Based on Multi-Solvents for Preparing Nobiletin. CN 105669626, 13 April 2018. [Google Scholar]
- Taguchi, S.; Hoshi, R.; Mitsumaki, Y.; Sashita, Y.; Yano, A. Flavonoids of Citrus Fruits for Health Food with Antihypertensive and Antidiabetic Activities. JP 2001240539, 4 September 2001. [Google Scholar]
- Guthrie, N. Compositions Comprising at Least One Polymethoxyflavone, Flavonoid, Liminoid, and/or Tocotrienol Useful in Combination Therapies for Treating Diabetes. WO 2014203059, 24 December 2014. [Google Scholar]
- Wu, X.; Mei, Z.; Zheng, D.; Liu, Z.; Zhu, X.; Zhou, Y.; Zeng, L.; Liang, Z. Application of Nobiletin in Preparation or Screening of Diabetic Cardiomyopathy Drug. CN 108403684, 17 August 2018. [Google Scholar]
- Zhang, X.; Chen, S.; Wang, X.; Xie, F.; Liu, X.; Wang, J.; Yan, A.; Gao, N.; Li, F. A Snap Bean Preservative. CN 106172719, 16 April 2019. [Google Scholar]
- Sasaki, T. Peroxisome Proliferator-Activated Receptor (PPAR) Activator and Drug, Supplement, Functional Food and Food Additive Using the Same. WO 2006049234, 16 April 2014. [Google Scholar]
- Krohn, M.; Seibert, S.; Kleber, A.; Wonschik, J. Sweetener and/or Sweetness Enhancer, Sweetener Composition, Methods of Making the Same and Consumables Containing the Same. WO 2012107203, 16 August 2012. [Google Scholar]
- Shin, J.-E. Preparation and Incorporation of Co-Products into Beverages to Achieve Metabolic and Gut Health Benefits. US 20170119024, 4 May 2017. [Google Scholar]
- Zhou, H.; Xie, B.; Zang, X.; Cheng, L.; Liang, G. A Multiple Index Component Content Determination, Fingerprint Construction and Preparation Method for Liver-Tonifying Eyesight-Improving Oral Liquid. CN 105510452, 14 November 2017. [Google Scholar]
- Kusano, S.; Tamasu, S. Composition Containing 4′-Demethylnobiletin for Skin Whitening Cosmetics, Medicines, Foods and Drinks. JP 2017226612, 28 October 2020. [Google Scholar]


| Treatment | Clinical Trial Program/Identifier | Indication | Development Stage | Clinical Result | Reason for Terminal or Negative Result | Reference |
|---|---|---|---|---|---|---|
| Orange juice (14%: nobiletin, sinensetin, tangeretin) | NCT06680635 | Obesity Adult Onset/Diabetes Mellitus Type 2 | / | No Results Posted | No results. | NCT06680635 |
| Orange juice (nobiletin, 22.8 mg/L) | NCT06279780 | Obesity Adult Onset | / | Consuming a juice containing 22.8 mg/L nobiletin combined with a low-calorie diet significantly improved anthropometric measures and metabolic biomarkers in obese individuals, including lower LDL-C, ApoB/ApoA1 ratio, and A1c levels, enhanced antioxidant capacity and GPX1 expression, accompanied by decreased inflammatory markers such as TNFα and IFNγ, and enhanced adipokines (leptin, PAI-1, and adiponectin). | The short intervention period of 6 weeks; the lack of consideration of other bioactive compounds in the juice; the lack of blood or urine markers to confirm adherence | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Lai, W.; Li, Y.; Hong, K.; Xu, Y. Potential and Mechanism of Nobiletin in Diabetes Mellitus and Associated Complications. Pharmaceuticals 2025, 18, 1528. https://doi.org/10.3390/ph18101528
Zhao C, Lai W, Li Y, Hong K, Xu Y. Potential and Mechanism of Nobiletin in Diabetes Mellitus and Associated Complications. Pharmaceuticals. 2025; 18(10):1528. https://doi.org/10.3390/ph18101528
Chicago/Turabian StyleZhao, Chuyun, Wenjie Lai, Yu Li, Kinfong Hong, and Youhua Xu. 2025. "Potential and Mechanism of Nobiletin in Diabetes Mellitus and Associated Complications" Pharmaceuticals 18, no. 10: 1528. https://doi.org/10.3390/ph18101528
APA StyleZhao, C., Lai, W., Li, Y., Hong, K., & Xu, Y. (2025). Potential and Mechanism of Nobiletin in Diabetes Mellitus and Associated Complications. Pharmaceuticals, 18(10), 1528. https://doi.org/10.3390/ph18101528









